ABSTRACT
Subclinical mastitis is an asymptomatic inflammatory condition that can be difficult to define and diagnose. In the dairy industry, subclinical mastitis is diagnosed by milk somatic cell counts (SCCs) of ≥250,000 cells mL−1. In this pilot study, we assessed the efficacy of this index to identify human subclinical mastitis by comparing SCC levels with the inflammatory response [interleukin-8 (IL-8) levels] in 37 samples from asymptomatic and 10 clinical mastitis (CM) lactating women. The milk microbiota was determined by 16S rRNA gene sequencing. The SCC of CM samples ranged from 310,000 to 6,600,000 cells mL−1. However, 14 of 37 (37.8%) asymptomatic samples had high SCC (250,000–460,000 cells mL−1), indicating subclinical mastitis. SCC levels significantly (P < 0.001) and positively correlated with milk IL-8 levels reflecting the escalating inflammatory response across subclinical and clinical mastitis samples. Samples with an SCC of ≥250,000 cells mL−1 showed significant increases in IL-8 responses when compared with milk samples from healthy women. The milk microbiome of CM samples was dominated by streptococcal and staphylococcal species (89.9% combined median relative abundance). In contrast, the combined median streptococcal/staphylococcal relative levels were 75.4% and 66.3% in milks from asymptomatic (subclinical mastitis) and healthy groups, respectively. The Streptococcus genus was increased in samples with an SCC of ≥250,000, although this should be interpreted with caution. Thus, the index of ≥250,000 somatic cells mL−1 could be a reliable indicator of subclinical mastitis in humans and should aid future studies investigating the impact of subclinical mastitis on maternal health, breastfeeding behaviors, infant health, and development.
IMPORTANCE
This pilot study suggests that SCC at a level of (greater than or equal to) 250,000 cells mL−1, as used in the dairy industry, is a suitable index to identify asymptomatic subclinical mastitis in lactating women since it reflects a significant increase in the inflammatory response compared to milk samples from healthy women. Using this index should aid studies into the short- and long-term consequences of subclinical mastitis for mother and infant.
KEYWORDS: subclinical mastitis, clinical mastitis, somatic cells, somatic cell counts, breastfeeding, breast milk, lactation, milk microbiota, inflammation, interleukin-8
INTRODUCTION
Breastfeeding is the optimal regime for nearly all feeding newborn infants (1, 2) and simultaneously benefits the health of the mother-child dyad (3). Nonetheless, breastfeeding can be ceased due to breast inflammation, a condition known as mastitis (4), which has a frequency of up to 33% (5), with milk stasis and infection being the major causes (6). Lactational mastitis is classified into clinical and subclinical mastitis based on clinical manifestations (7). Clinical mastitis symptoms include breast redness, pain, pyrexia, flu-like symptoms, engorgement, and reduced milk secretion (7) with Staphylococcus aureus being the major pathogen identified. In contrast, subclinical mastitis occurs more frequently than clinical mastitis and is an often misidentified entity (8). It is prevalent during early lactation, often with absence of symptoms (9), and is usually associated with coagulase-negative staphylococci (5). However, when symptoms are reported, these include sharp, needling pain and a burning sensation (7). Importantly, subclinical mastitis in humans is associated with dramatic changes in the inflammatory/anti-inflammatory profiles of breast milk (8). Diagnosis of subclinical mastitis in the absence of symptoms can be achieved by measuring sodium/potassium (Na+:K+) ratio and interleukin-8 (IL-8) levels in breast milk (9). The proinflammatory cytokine IL-8 is produced within the mammary gland and can be easily measured in milk (10, 11). Along with other cytokines, IL-8 mediates tight junction-permeabilization between the epithelial cells, thus allowing plasma constituents, including Na+, to cross into milk (12). Thus, a Na+:K+ ratio of >0.6 and increased IL-8 levels are considered indicators of subclinical mastitis in humans (9, 13). However, subclinical mastitis in humans remains challenging to identify and therefore define. Despite this, subclinical mastitis has been identified as a risk factor for HIV transmission between mother and infant (9) and poor weight gain/growth in infants (10, 11, 14–17). In a European multicenter cohort, subclinical mastitis has also been shown to alter the composition of breast milk by significantly reducing the concentrations of lactose, docosahexaenoic acid (DHA), linolenic acid, calcium, and phosphorus while significantly increasing total protein, α-lactalbumin, albumin, arachidonic acid to DHA ratio, n-6 to n-3 fatty acid ratio and several minerals (18). The consequences of these alterations on breast milk output of the mother, breastfeeding behaviors, and infant growth and development are unknown. A reliable indicator of subclinical mastitis could help in the progression of such research.
In a study investigating the associations between human clinical mastitis and common indices of mammary gland inflammation, namely, IL-8, Na+ levels, and somatic cell counts (SCCs), Hunt et al. (19) concluded that IL-8 and SCC may be better indicators of mammary inflammation. Indeed, while IL-8 and SCC levels were both significantly elevated in milk from symptomatic breasts (IL-8 = 2,960 pg mL−1; SCC = 1,564,000 cells mL−1) compared with those from non-symptomatic breasts (IL-8 = 302 pg mL−1; SCC = 120,000 cells mL−1), Na+ only “tended” to be greater in milk from symptomatic breasts (7.3 mM versus 5.0 mM in asymptomatic samples). SCC is the standard method used in the dairy industry to diagnose mastitis and to assess the immunological status of lactating cows (20), where subclinical mastitis in cows is associated with changes in milk quality (21). As in humans, IL-8 is also higher in milk from cows with subclinical mastitis (21). In another study, the positive linear relationship observed between SCC and total bacterial counts led the authors to conclude that SCC should be used as a diagnostic tool for preliminary assessment of lactating breast health (22). However, Wren et al. (23) concluded that SCC may not be a suitable biomarker of subclinical mastitis in lactating women, given that it did not correlate with the Na+:K+ ratio or proinflammatory cytokines at two stages of lactation.
Given the paucity of data on SCC levels in human subclinical mastitis, we set out to assess the suitability of using the dairy index for subclinical mastitis, ≥250,000 cells mL−1 (24, 25), to identify subclinical mastitis in asymptomatic women. We, therefore, analyzed the SCC levels in breast milk samples from asymptomatic lactating women along with samples from women diagnosed with clinical mastitis (CM). After stratifying the samples as healthy (H), subclinical mastitis (SM), and CM, we evaluated the IL-8 levels across all milk samples to assess the extent of the inflammatory response and the suitability of using SCC to identify samples with potential subclinical mastitis. Finally, we examined the microbiota profiles of all milk samples to determine microbiological differences/similarities between them.
MATERIALS AND METHODS
Design
This is a cross-sectional descriptive study that was designed to assess the microbiome and immunological profiles of milk from healthy, subclinical, and clinical mastitic lactating mothers.
Setting
The pilot study was carried out from June 2017 to May 2019. The participating women were recruited by Cork University Maternity Hospital, Cork, Ireland.
Data collection
Demographic data were not collected since the aim of the pilot study was to determine the microbiome and immunological profiles of milk from healthy, subclinical, and clinical mastitic lactating mothers. The inclusion criteria were that the samples should be collected during the fourth week post-partum and the mothers who were diagnosed with mastitis (CM) should display local (breast redness, engorgement of breast, and pain) and/or systemic symptoms (pyrexia and flu-like symptoms). The exclusion criterion was the administration of antibiotics before sample collection in the previous 4 weeks. Forty-seven lactating mothers were recruited, from which 10 were diagnosed with mastitis.
Sample collection
A detailed explanation of the sampling procedure was given to the participating women by the research nurses and samples were self-collected. Nipples and mammary areola were cleaned with sterile alcohol-free aqueous solution wipes (Ted Kelleher, First Aid & Hygiene Supplies Ltd, Macroom, Ireland). Hand expression was recommended, but pumping was also acceptable. Approximately, 10 mL of milk was aseptically collected using sterile gloves in a sterile tube with the first few drops (~500 µL) being discarded. Within 24 hours after the receipt of samples, 125 µL of each milk sample was used for SCC enumeration. Samples were stored below 4°C until processing and remaining volumes were immediately frozen at –20°C for subsequent DNA extraction and measurement of IL-8.
Measurement
SCC enumeration
We employed a direct microscopic method (DMSCC) using the single-strip counting procedure (26) allowing for enumeration of SCC in the limited volume of the acquired samples. In brief, three smears (10 µL each) were prepared on somatic cell slides (5638–01930; Bellco Glass Inc., Vineland, NJ, USA) and air-dried overnight. Smears were stained by adding two drops of modified Newman-Lampert Stain solution (Sigma-Aldrich, Germany). Following 2-min incubation, excess stain was removed by resting the edge of the slide on absorbent paper and air-drying. The slides were dipped thrice in water at 37°C–45°C. Somatic cells were enumerated on each of the smears using a light microscope at ×100 magnification. The field diameter (fd) was measured with a stage micrometer to calculate the single-strip factor (SSF) according to the formula SSF = 10,000/(11.28 × fd). The SSF was multiplied by each strip count to determine SCC mL−1.
To validate this method, we analyzed the SCC of 12 fresh bovine milk samples using a Somacount 300 (Bentley Instruments, Inc., Chaska, MN, USA) and the DMSCC method, as described above.
Criteria for sample classification
Samples were classified as H if their SCC was ˂ 250,000 cells mL−1. Milk samples were classified as subclinical (SM) when they were originating from an asymptomatic donor and their SCC was ≥250,000 cells mL−1 following the classification criteria used in the dairy industry (24, 25). For comparative purposes, a subset of 10 H and 10 SM samples (matching the number of CM samples) was selected using a random number generator.
IL-8
Two milliliters of the milk samples was centrifuged once at 10,000 × g for 30 min at 4°C. The fat layer was bypassed using a thin needle (Sterican, B. Braun, Ireland) and the liquid phase underneath was extracted for further processing. The IL-8 human ELISA kit (Invitrogen, ThermoFisher Scientific, Waltham, MA, USA) was used according to the manufacturer’s instructions. Absorbance was read at 450 nm using a microtiter plate reader (Spectramax M3; Molecular Devices, San Jose, CA, USA). Based on the standard curve, the IL-8 levels in each test sample were quantitated. IL-8 was quantified for 7 out of 10 CM samples due to limited volume of these samples.
DNA extraction and 16S rRNA gene sequencing
DNA was purified from milk samples using the PowerFood Microbial DNA Isolation Kit (MoBio Laboratories, Carlsbad, USA) as previously described (27). PCR amplification of the V3–V4 region was performed using the forward primer 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′ and the reverse primer 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′. Each 30-µL PCR reaction contained up to 5 ng µL−1 microbial genomic DNA, 1 µL of each primer (6 µM), and 15-µL Phusion High-Fidelity PCR Master Mix (ThermoFisher Scientific). The PCR conditions used were identical to the ones used by Angelopoulou et al. (27). The Agencourt AMPure XP system (Beckman Coulter, UK) was used to purify the amplicons. A subsequent limited‐cycle amplification step was performed to add multiplexing indices and Illumina sequencing adapters. Amplicons were quantified, normalized, and pooled using the Qubit dsDNA HS Assay Kit (Life Technologies, Carlsbad, CA, USA). Library preparation was carried out by GATC Biotech (Ebersberg, Germany) prior to 2 × 300 bp sequencing on the Illumina MiSeq platform.
Bioinformatic analysis of high-throughput sequencing data
Raw reads were quality checked using fastqc (v.0.11.5, http://www.bioinformatics.babraham.ac.uk/projects/fastqc). Primers were removed using cutadapt (v) (28). Amplicon sequence variants (ASVs) were inferred using the dada2 pipeline (29) implemented in R (https://www.r-project.org/). Taxonomy assignment was performed using the SILVA reference database (v.138.1) (30). ASVs not assigned to a phylum were removed.
Data analysis
All statistics for bioinformatic analysis were performed in R (v.3.6.1). The phyloseq package (v.1.40.0) (31) was used for storing and managing microbiome data. Alpha diversity was calculated using the number of observed species and the Shannon entropy. Beta diversity was calculated on total sample sum normalized data using the Bray-Curtis divergence. Permutational multivariate analysis of variance was used to test grouping variables using 999 simulations. The vegan package (v.2.6–2) was used for calculating diversity metrics. Differential abundance was calculated using linear discriminant analysis with effect size (LEfSe) using default settings (32). Pairwise differences between levels within grouping variables (asymptomatic, subclinical mastitis and clinical mastitis; SCC of <250,000 and >250,000 were compared using the Wilcoxon signed-rank test. Plotting was performed using the ggplot2 (33) and ggpubr (34) packages. IL-8 and SCC data were not normally distributed, as assessed by the Shapiro-Wilk test, and were compared by pairwise Wilcoxon tests. Correlation of IL-8 and SCC data was performed with the Spearman method. Correlations between DMSCC and automated methods were performed with the Pearson method. For all tests, differences were considered significant at a P value of ≤0.05.
RESULTS
Based on SCC, 37.8% of asymptomatic samples were subclinical
For SCC enumeration, we initially compared the automated method and the DMSCC method using fresh bovine milk samples. As both methods provided similar counts with the two methods strongly correlating with each other (R = 0.98, Fig. 1), we proceeded with DMSCC to analyze our human milk samples.
Fig 1.
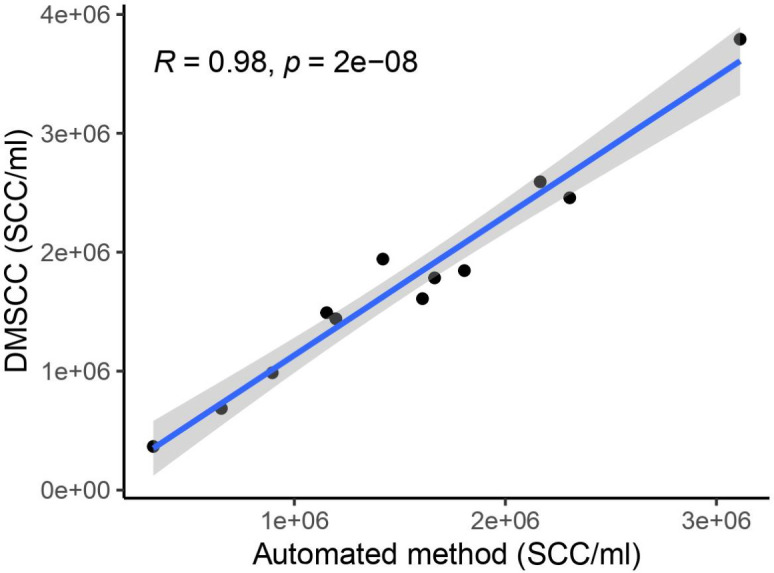
Pearson correlation of automated method versus DMSCC for the enumeration of somatic cells in 12 fresh bovine milk samples (R = 0.98). DMSCC, direct microscopy somatic cell count; SCC, somatic cell count.
In total, 47 breast milk samples were analyzed, 37 of which were collected from asymptomatic women and 10 of which were from women diagnosed with clinical mastitis. The SCC of the asymptomatic samples (A samples, Fig. 2) ranged from 35,000 to 490,000 cells mL−1, whereas the SCCs of the clinical mastitis samples were between 310,000 and 6,600,000 cells mL−1 (CM samples, Fig. 2). Applying the criteria for subclinical mastitis (SCC ≥250,000 cells mL−1) to the asymptomatic samples revealed that 37.8% (n = 14) of the asymptomatic samples were potentially subclinical (Table 1). Hereafter, the samples were referred to as H, SM, and CM. Median SCC levels (cells mL−1) with interquartile range (IQR) are presented for each group in Fig. 3a. A significant difference (P < 0.001) was observed between the SCC for H samples (median 140,000 IQR 85,000) and SM samples (median 330,000, IQR 123,334). Moreover, a significant difference (P < 0.001) was detected between H and the CM group (median 353,614; IQR 1,171,306). No significant difference was observed between SM and CM samples.
Fig 2.
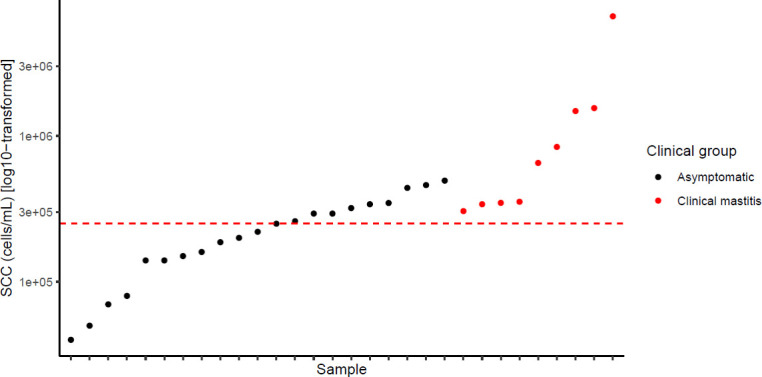
SCC of 37 asymptomatic (●) milk samples and 10 clinical mastitic (●) milk samples. The red line indicates the cut-off for SCC of ≥250,000 cells mL−1.
TABLE 1.
SCC-based classification of milk samples from asymptomatic women (A), women with subclinical mastitis (SM), and those with clinical mastitis (CM)
| State | SCC | Sample classification based on SCC |
|---|---|---|
| A | 190,000 | H1 |
| A | 140,000 | H2 |
| A | 70,000 | H3 |
| A | 40,000 | H4 |
| A | 140,000 | H5 |
| A | 80,000 | H6 |
| A | 50,000 | H7 |
| A | 200,000 | H8 |
| A | 160,000 | H9 |
| A | 150,000 | H10 |
| A | 220,000 | H11 |
| A | 40,000 | H12 |
| A | 120,000 | H13 |
| A | 35,000 | H14 |
| A | 50,000 | H15 |
| A | 50,000 | H16 |
| A | 160,000 | H17 |
| A | 49,000 | H18 |
| A | 40,000 | H19 |
| A | 190,000 | H20 |
| A | 160,000 | H21 |
| A | 80,000 | H22 |
| A | 230,000 | H23 |
| A | 260,000 | SM1 |
| A | 440,000 | SM2 |
| A | 340,000 | SM3 |
| A | 250,000 | SM4 |
| A | 490,000 | SM5 |
| A | 250,000 | SM6 |
| A | 350,000 | SM7 |
| A | 460,000 | SM8 |
| A | 320,000 | SM9 |
| A | 290,000 | SM10 |
| A | 420,000 | SM11 |
| A | 360,000 | SM12 |
| A | 310,000 | SM13 |
| A | 430,000 | SM14 |
| CM1 | 1,600,000 | CM1 |
| CM2 | 340,000 | CM2 |
| CM3 | 840,000 | CM3 |
| CM4 | 6,600,000 | CM4 |
| CM5 | 1,500,000 | CM5 |
| CM6 | 650,000 | CM6 |
| CM7 | 310,000 | CM7 |
| CM8 | 350,000 | CM8 |
| CM9 | 800,000 | CM9 |
| CM10 | 920,000 | CM10 |
Fig 3.
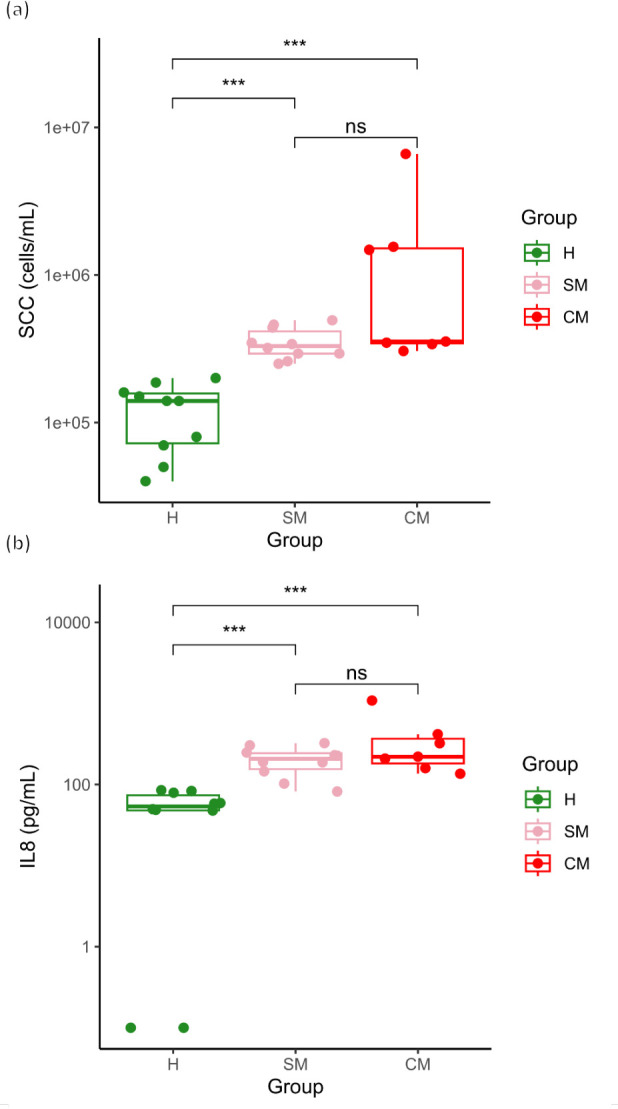
Boxplots of SCC (a, cells mL−1) enumeration and IL-8 (b, pg mL−1) concentration in healthy (H, green), subclinical (SM, pink), and clinical (CM, red) mastitis milk samples (pairwise Wilcoxon test; ***P < 0.001). SCC, somatic cell count.
IL-8 levels in breast milk samples correlate with SCC results
Given that IL-8 levels in breast milk are considered a reliable indicator of subclinical mastitis, we next measured IL-8 levels in all milk samples and determined if the levels observed correlated with our classification of milk samples based on SCC enumeration. We detected a significant positive correlation between the two (rho = 0.71; P < 0.001; Fig. 4). Moreover, after removing the highest outlier, we still observed a significant correlation (rho = 0.68; P < 0.001) between SCC and IL-8.
Fig 4.
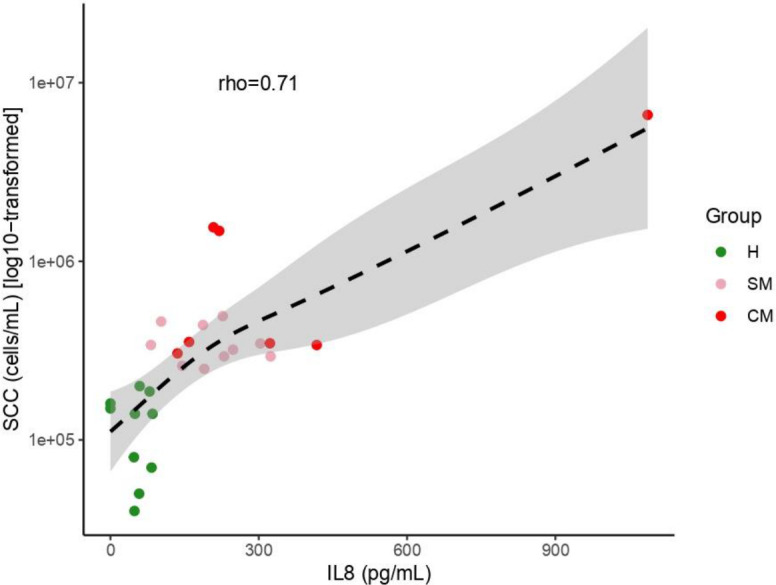
Relationship between SCC (cells/mL) and IL-8 (pg/mL) in milk samples with H (green, n = 10) being healthy samples, SM (pink, n = 10) subclinical mastitis samples and CM, clinical mastitic samples (red, n = 7). Regression line was plotted using the “gam” function, and the Spearman correlation coefficient is reported.
Median IL-8 levels (pg mL−1) with IQR are presented in Fig. 3b. The concentrations of IL-8 for two of the tested H samples were below the detection limit and thus are presented as 0.1 pg mL−1 (Fig. 3b). A significant difference (P < 0.001) was observed between H (median 53.9, IQR 26.4) and SM samples (median 208.5, IQR 87.7). Furthermore, we detected a significant difference (P < 0.001) between H and CM samples (median 219.99, IQR 186.2) (Fig. 3b). No significant difference was observed between SM and CM samples.
16S rRNA gene sequencing identified a common milk microbiota in more than 50% of samples
Amplicon sequencing of human milk samples was available for 30 samples [H (n = 11), SM (n = 10), CM (n = 9)]. One CM sample failed sequencing. Seven samples were re-sequenced on a second sequencing run due to low read counts on the first run. Sequencing yielded a total of 1,062,261 reads post-processing with median reads per sample of 16,869.5 reads (IQR 5390). Breast milk samples were dominated by Streptococcus, Acinetobacter, Staphylococcus, Pseudomonas, Rothia, Corynebacterium, Cutibacterium, and Gemella, with these genera in greater than 50% of samples (Fig. S1). We did not observe significant differences for the alpha (Fig. S2a and b) and beta diversity metrics (Fig. 5a and b) for comparisons between the H, SM, and CM groups or the groups based on a cut-off of 250,000 SCCs. There was also no significant difference between sequencing runs (Fig. S2c).
Fig 5.
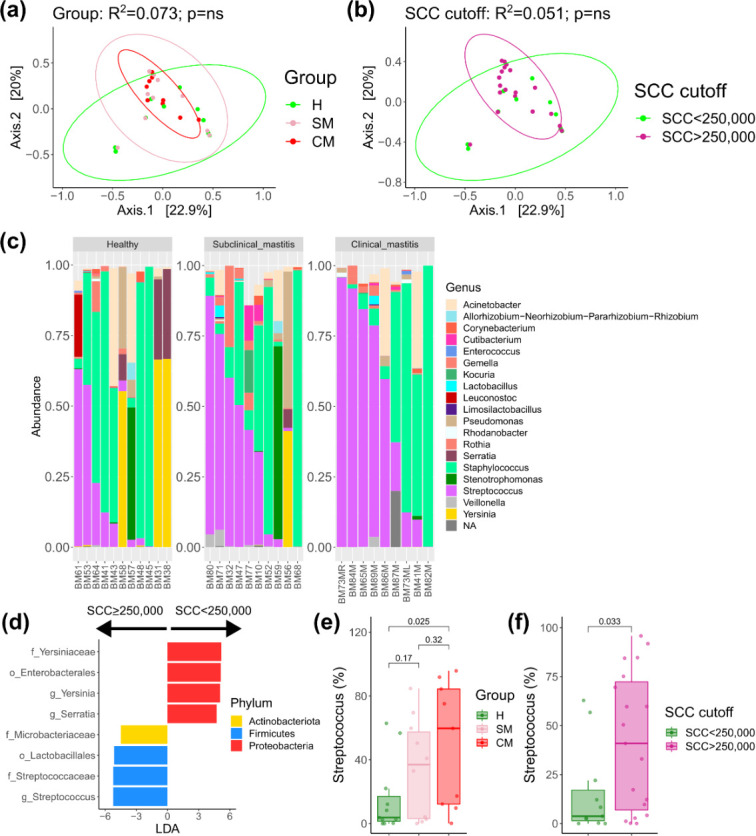
Beta diversity PCoA plots of Bray-Curtis divergence for (a) groups H, SM, and CM and (b) SCC cut-off of 250,000 cells. (c) Taxonomic barplots at the genus level of the top 20 most abundant genera. (d) LEfSe results comparing SCC cut-off of 250,000 cells. Boxplots of Streptococcus genus abundance between groups of H, SM, and CM (e) and SCC cut-off (f). CM, clinical mastitis; H, health; SCC, somatic cell count; SM-Subclinical mastitis.
However, a trend for increased Streptococcus in subjects with clinical and subclinical mastitis was suggested (Fig. 5c), with an increase in the combined percentage of Streptococcus and Staphylococcus from H [66.3% (IQR 90%)], SM [75.4% (IQR 38.3%)], and CM [89.9% (IQR 22.9%)]. However, as can be seen by the interquartile ranges, there was very large variability between samples, particularly in the H group, so we performed differential abundance testing, comparing subjects with an SCC of ≥250,000 (equivalent to combining SM and CM) versus those with an SCC of <250,000 (equivalent to the H group). This demonstrated an increase in the genus Streptococcus in subjects with an SCC of ≥250,000 (Fig. 5d). Interestingly, Yersinia and Serratia were increased in subjects with an SCC of <250,000, although this was driven by three samples in the H group. When looking specifically at Streptococcus percentage, there was an increasing trend from H to SM, to CM (Fig. 5e) which accounted for the difference based on SCC cut-off (Fig. 5f). Correlating Streptococcus counts with SCC demonstrated a positive correlation which did not pass the significance threshold (Spearman rho 0.359, P = 0.051). There was no correlation between Streptococcus and IL-8 (Spearman rho 0.18, P = 0.33).
DISCUSSION
International health organizations encourage women to breastfeed during the first 6 months of life (35). However, mastitis is a major cause of breastfeeding cessation. Subclinical mastitis is generally an asymptomatic inflammatory condition that is more common than clinical mastitis yet difficult to define. Despite this, subclinical mastitis can negatively impact infant health (9–11, 14–18). Thus, in this pilot study, we used SCC to stratify human milk samples into H, SM, and CM based on the dairy index for subclinical mastitis, ≥250,000 cells mL−1 (24, 25). Of the 47 milk samples, 10 were from mothers with diagnosed clinical mastitis.
Subclinical mastitis was observed in 37.8% of asymptomatic women based on SCC enumeration, which is in agreement with the results of Samuel et al. (18), who measured the Na+:K+ ratio in the milk of 305 women with 35.4% of the women having at least one episode of subclinical mastitis. The SCC for SM and CM samples were significantly different from the H samples, though no significant difference was observed between SM and CM samples. To determine if the increased somatic cells were indicative of increased inflammation, we measured levels of IL-8, a cytokine produced to elicit the infiltration of immune cells and commonly used as an inflammation index of mammary gland inflammation (8, 19) in both bovine and human milk (10, 19, 36). In our pilot study, we detected significant differences in IL-8 levels between H and SM samples (P < 0.001) and H and CM samples (P < 0.001) (though not between SM and CM samples), indicating the presence of inflammation in SM and CM samples. Indeed, SCC correlated significantly and positively with IL-8 levels in our samples with the increase of SCC in SM and CM samples implying a significant immune response. This is in agreement with the findings of Hunt et al. (19), who detected a 10-fold increase of IL-8 and SCC in mastitic milk samples compared to healthy milk samples. Thus, an SCC of ≥250,000 cells mL−1 in human breast milk could potentially be used as a diagnostic tool for subclinical mastitis. Given the unreliability of using Na+ levels in milk for identifying mammary inflammation (19), the significant correlation between SCC and IL-8 levels identified in this study and that of Hunt et al. (19), as well as the universal use of the dairy index for identifying subclinical mastitis in the dairy industry (24, 25), SCC may prove more advantageous than previous indicators of human mastitis.
We next assessed the microbiota profiles of the H, SM, and CM milk samples. Amplicon 16S rRNA sequencing revealed that eight genera (Streptococcus, Acinetobacter, Staphylococcus, Pseudomonas, Rothia, Corynebacterium, Cutibacterium, and Gemella) were present in over 50% of the samples. Three of these have been identified as members of the “core” milk microbiota (Streptococcus, Staphylococcus, and Pseudomonas) by Murphy et al. (37), who reported 12 core genera present in 90% or more of the study subjects (n = 10). Likewise, Hunt et al. (38) reported nine core OTUs (present in 100% of subjects, n = 9) constituting the core microbiota that also included Streptococcus, Staphylococcus, Pseudomonas, as well as Corynebacterium. The observed differences between the three studies could reflect the diversity of milk samples due to geographical location (39, 40) or other factors.
No statistically significant differences were observed in alpha diversity or beta diversity based on clinical groups or cut-off points. Using LEfSe, we did observe an increase in the genus Streptococcus in samples with an SCC of ≥250,000, with increasing abundance from H to SM, to CM. Streptococcus is a core member of the human milk microbiota, and Streptococcus spp. are dominant commensals in the oral cavity of infants (41). Due to the relatively small numbers in each group and the weak and non-significant correlation of Streptococcus with SCC counts, this finding must be interpreted with caution. Moreover, challenges related to analyzing low biomass samples such as human milk (38, 42) and the polymicrobial nature of mastitis need to be considered. However, Bolte et al. (43) reported that Streptococcus spp. were associated with a diet high in animal protein and fat and were increased in subjects with inflammatory bowel disease and colon cancer, among other diseases. Future studies with larger sample sizes are needed to verify our findings to determine if increased Streptococcus abundance is a feature of the subclinical milk microbiome and if this is contributing to the proinflammatory state.
Interestingly, samples with an SCC of ≥250,000 had lower levels of Serratia/Yersinia compared to H samples. Serratia was a member of the core genera in healthy human milk samples in the study conducted by Hunt et al. (38). In contrast, other studies have identified Serratia in bovine (44) and human mastitis (45). In line with our observations, Pseudomonas has been reported as a dominant member of the human milk microbiome in several studies (38, 46, 47), and the same applies to Staphylococcus (38, 45, 48–51) and Streptococcus (5, 45, 52). Interestingly, Staphylococcus and Streptococcus species constituted 82% of species in CM milk samples, but their combined relative levels were less in SM and H samples, at 60% and 45%, respectively.
Limitations
We must acknowledge some limitations in this pilot study, the first being the heterogeneity of the symptoms of the cases when the samples were collected. In addition, data are also limited in that only basic information regarding the symptomatology was collected. Therefore, information about a mother’s background and demographics was not available to single out other factors that might affect breastfeeding practices and SCC levels during mastitis such as parity, lactation status, age, sampling time, feeding frequency, and season. However, this pilot study should form the basis for follow-up studies with larger sample sizes.
Conclusions
Using the dairy index for subclinical mastitis (≥250,000 cells mL−1), we stratified human milk samples from women who were asymptomatic for mastitis or diagnosed with clinical mastitis into H, SM, and CM. IL-8 analysis of the milk samples verified our approach as the increasing SCC in SM and CM samples correlated with an escalating immune response. A high prevalence (37.8%) of subclinical mastitis was found in the population of 37 asymptomatic lactating women. More research into the consequences of subclinical mastitis for infant and maternal health is clearly warranted. The significance of this pilot study is that SCC at a level of ≥250,000 cells mL−1 is a suitable index to identify asymptomatic subclinical mastitis in lactating women since it reflects a significant increase in the inflammatory response compared to milk samples from healthy women and should aid studies into the short- and long-term consequences of subclinical mastitis for mother and infant.
ACKNOWLEDGMENTS
The authors thank the mothers involved in this pilot study for the kind donation of their milk. Also, the authors extend their most grateful thanks to Ms. Grainne Meehan and Mrs. Clare Boyle for assisting with sample collection.
This work was funded by APC Microbiome Ireland, a Centre for Science and Technology funded by the Science Foundation Ireland (SFI) (grant number SFI/12/RC/2273-P2), as well as by the European Union (European Research Council
(ERC), BACtheWINNER; project no. 101054719). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.
A.A. designed the study, performed the experiments, analyzed the data, and wrote the manuscript. H.M.B.H. and A.L. conducted bioinformatic and statistical analyses on the 16S data and also reviewed the manuscript. A.K.W. supported the design of the study and interpretation of the data, and critically revised the manuscript. C.-A.O’S. designed the study, acquired ethics approval, participated in manuscript revision, recruited suitable subjects, obtained informed consent, and collected clinical data on mothers. C.A.R. and E.D. designed the study, acquired ethics approval and participated in manuscript revision. C.S.and C.H. designed the study, was involved in data interpretation and manuscript corrections and revision. R.P.R. conceptualized and designed the study and was involved in data interpretation and manuscript corrections and revision. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
The authors have no financial relationships relevant to this article to disclose.
Contributor Information
R. Paul Ross, Email: p.ross@ucc.ie.
Rosemary C. She, City of Hope Department of Pathology, Duarte, California, USA
DATA AVAILABILITY
Sequencing data are available at the Sequence Read Archive under the BioProject ID PRJNA1141436. All analysis codes, a phyloseq object with processed sequencing data, and all other data presented in the paper are accessible at https://github.com/ajlavelle/human_mastitis_study.
ETHICS APPROVAL
This pilot study was approved by the Cork Clinical Research Ethics Committee of the Cork Teaching Hospitals [Cork, Ireland: ECM 3 (rr) 21/03/2017]. All experiments were performed in accordance with the relevant guidelines and regulations. Informed written consent was obtained from all participants.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.04051-23.
Prevalence and mean abundance of genera in breast milk.
Observed ASVs and Shannon entropy.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Ballard O, Morrow AL. 2013. Human milk composition. Pediatr Clin North Am 60:49–74. doi: 10.1016/j.pcl.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lyons KE, Ryan CA, Dempsey EM, Ross RP, Stanton C. 2020. Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients 12:1039. doi: 10.3390/nu12041039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berti C, Agostoni C, Davanzo R, Hyppönen E, Isolauri E, Meltzer HM, Steegers-Theunissen RPM, Cetin I. 2017. Early-life nutritional exposures and lifelong health: immediate and long-lasting impacts of probiotics, vitamin D, and breastfeeding. Nutr Rev 75:83–97. doi: 10.1093/nutrit/nuw056 [DOI] [PubMed] [Google Scholar]
- 4. Contreras GA, Rodríguez JM. 2011. Mastitis: comparative etiology and epidemiology. J Mammary Gland Biol Neoplasia 16:339–356. doi: 10.1007/s10911-011-9234-0 [DOI] [PubMed] [Google Scholar]
- 5. Marín M, Arroyo R, Espinosa-Martos I, Fernández L, Rodríguez JM. 2017. Identification of emerging human mastitis pathogens by MALDI-TOF and assessment of their antibiotic resistance patterns. Front Microbiol 8:1258. doi: 10.3389/fmicb.2017.01258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angelopoulou A, Field D, Ryan CA, Stanton C, Hill C, Ross RP. 2018. The microbiology and treatment of human mastitis. Med Microbiol Immunol 207:83–94. doi: 10.1007/s00430-017-0532-z [DOI] [PubMed] [Google Scholar]
- 7. Fernández L, Arroyo R, Espinosa I, Marín M, Jiménez E, Rodríguez JM. 2014. Probiotics for human lactational mastitis. Benef Microbes 5:169–183. doi: 10.3920/BM2013.0036 [DOI] [PubMed] [Google Scholar]
- 8. Tuaillon E, Viljoen J, Dujols P, Cambonie G, Rubbo P-A, Nagot N, Bland RM, Badiou S, Newell M-L, Van de Perre P. 2017. Subclinical mastitis occurs frequently in association with dramatic changes in inflammatory/anti-inflammatory breast milk components. Pediatr Res 81:556–564. doi: 10.1038/pr.2016.220 [DOI] [PubMed] [Google Scholar]
- 9. Willumsen JF, Filteau SM, Coutsoudis A, Uebel KE, Newell M-L, Tomkins AM. 2002. Subclinical mastitis as a risk factor for mother-infant HIV transmission, p 211–223. In Koletzko B, Michaelsen KF, Hernell O (ed), Short and long term effects of breast feeding on child health. Kluwer Academic Publishers, Boston. [DOI] [PubMed] [Google Scholar]
- 10. Filteau SM, Lietz G, Mulokozi G, Bilotta S, Henry CJ, Tomkins AM. 1999. Milk cytokines and subclinical breast inflammation in Tanzanian women: effects of dietary red palm oil or sunflower oil supplementation. Immunology 97:595–600. doi: 10.1046/j.1365-2567.1999.00834.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Filteau SM, Rice AL, Ball JJ, Chakraborty J, Stoltzfus R, de Francisco A, Willumsen JF. 1999. Breast milk immune factors in Bangladeshi women supplemented postpartum with retinol or β-carotene. Am J Clin Nutr 69:953–958. doi: 10.1093/ajcn/69.5.953 [DOI] [PubMed] [Google Scholar]
- 12. Peaker M. 1975. Recent advances in the study of monovalent ion movements across the mammary epithelium: relation to onset of lactation. J Dairy Sci 58:1042–1047. doi: 10.3168/jds.s0022-0302(75)84677-7 [DOI] [PubMed] [Google Scholar]
- 13. Rasmussen L-BW, Hansen DH, Kaestel P, Michaelsen KF, Friis H, Larsen T. 2008. Milk enzyme activities and subclinical mastitis among women in Guinea-Bissau. Breastfeed Med 3:215–219. doi: 10.1089/bfm.2007.0035 [DOI] [PubMed] [Google Scholar]
- 14. Morton JA. 1994. The clinical usefulness of breast milk sodium in the assessment of lactogenesis. Pediatrics 93:802–806. [PubMed] [Google Scholar]
- 15. Li C, Solomons NW, Scott ME, Koski KG. 2019. Anthropometry before day 46 and growth velocity before 6 months of Guatemalan breastfed infants are associated with subclinical mastitis and milk cytokines, minerals, and trace elements. J Nutr 149:1651–1659. doi: 10.1093/jn/nxz109 [DOI] [PubMed] [Google Scholar]
- 16. Wren-Atilola HM, Solomons NW, Scott ME, Koski KG. 2021. Infant anthropometry and growth velocity before 6 months are associated with breastfeeding practices and the presence of subclinical mastitis and maternal intestinal protozoa in indigenous communities in Guatemala. Curr Dev Nutr 5:zab086. doi: 10.1093/cdn/nzab086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wren-Atilola HM, Solomons NW, Scott ME, Koski KG. 2019. Infant growth faltering linked to subclinical mastitis, maternal faecal–oral contamination, and breastfeeding. Matern Child Nutr 15:e12756. doi: 10.1111/mcn.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samuel TM, De Castro CA, Dubascoux S, Affolter M, Giuffrida F, Billeaud C, Picaud J-C, Agosti M, Al-Jashi I, Pereira AB, Costeira MJ, Silva MG, Marchini G, Rakza T, Haaland K, Stiris T, Stoicescu S-M, Martínez-Costa C, Vanpee M, Domellöf M, Euridice C-G, Thakkar SK, Silva-Zolezzi I. 2019. Subclinical mastitis in a European multicenter cohort: prevalence, impact on human milk (HM) composition, and association with infant HM intake and growth. Nutrients 12:105. doi: 10.3390/nu12010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunt KM, Williams JE, Shafii B, Hunt MK, Behre R, Ting R, McGuire MK, McGuire MA. 2013. Mastitis is associated with increased free fatty acids, somatic cell count, and interleukin-8 concentrations in human milk. Breastfeed Med 8:105–110. doi: 10.1089/bfm.2011.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamphuis C, Sherlock R, Jago J, Mein G, Hogeveen H. 2008. Automatic detection of clinical mastitis is improved by in-line monitoring of somatic cell count. J Dairy Sci 91:4560–4570. doi: 10.3168/jds.2008-1160 [DOI] [PubMed] [Google Scholar]
- 21. Karthikeyan A, Radhika G, Aravindhakshan TV, Anilkumar K. 2016. Expression profiling of innate immune genes in milk somatic cells during subclinical mastitis in crossbred dairy cows. Anim Biotechnol 27:303–309. doi: 10.1080/10495398.2016.1184676 [DOI] [PubMed] [Google Scholar]
- 22. Vaidya Y, Patel S, Joshi C, Nauriyal D, Kunjadia A. 2017. Somatic cell count: a human breast wellbeing indicator. J Breast Health 13:88–93. doi: 10.5152/tjbh.2017.3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wren H, Li C, Solomons N, Chomat AM, Scott M, Koski K. 2014. Comparison of breast milk sodium‐potassium ratio, pro‐inflammatory cytokines, and somatic cell count as potential biomarkers of subclinical mastitis (623.21). FASEB J 28. doi: 10.1096/fasebj.28.1_supplement.623.21 [DOI] [Google Scholar]
- 24. Lievaart JJ, Kremer WDJ, Barkema HW. 2007. Short communication: comparison of bulk milk, yield-corrected, and average somatic cell counts as parameters to summarize the subclinical mastitis situation in a dairy herd. J Dairy Sci 90:4145–4148. doi: 10.3168/jds.2006-871 [DOI] [PubMed] [Google Scholar]
- 25. Runciman DJ, Malmo J, Deighton M. 2010. The use of an internal teat sealant in combination with cloxacillin dry cow therapy for the prevention of clinical and subclinical mastitis in seasonal calving dairy cows. J Dairy Sci 93:4582–4591. doi: 10.3168/jds.2009-2956 [DOI] [PubMed] [Google Scholar]
- 26. Fitts JE, Laird D. 2004. Chapter 10 Direct microscopic methods for bacteria or somatic cells. In Wehr HM, Frank JF (ed), Standard methods for the examination of dairy products. American Public Health Association. [Google Scholar]
- 27. Angelopoulou A, Holohan R, Rea MC, Warda AK, Hill C, Ross RP. 2019. Bovine mastitis is a polymicrobial disease requiring a polydiagnostic approach. Int Dairy J 99:104539. doi: 10.1016/j.idairyj.2019.104539 [DOI] [Google Scholar]
- 28. Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet j 17:10. doi: 10.14806/ej.17.1.200 [DOI] [Google Scholar]
- 29. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–6. doi: 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McMurdie PJ, Holmes S. 2013. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. doi: 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. doi: 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wickham H. 2009. ggplot2: Elegant graphics for data analysis. Springer Publishing Company, Incorporated. [Google Scholar]
- 34. Kassambara A. 2019. ggpubr: “ggplot2” based publication ready plots. R package version 0.2.3
- 35. WHO, World Health Organization . 2000. Mastitis: causes and management
- 36. Riollet C, Rainard P, Poutrel B. 2000. Differential induction of complement fragment C5a and inflammatory cytokines during intramammary infections with Escherichia coli and Staphylococcus aureus. Clin Diagn Lab Immunol 7:161–167. doi: 10.1128/CDLI.7.2.161-167.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murphy K, Curley D, O’Callaghan TF, O’Shea C-A, Dempsey EM, O’Toole PW, Ross RP, Ryan CA, Stanton C. 2017. The composition of human milk and infant faecal microbiota over the first three months of life: a pilot study. Sci Rep 7:40597. doi: 10.1038/srep40597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hunt KM, Foster JA, Forney LJ, Schütte UME, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA. 2011. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One 6:e21313. doi: 10.1371/journal.pone.0021313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gómez-Gallego C, Morales JM, Monleón D, du Toit E, Kumar H, Linderborg KM, Zhang Y, Yang B, Isolauri E, Salminen S, Collado MC. 2018. Human breast milk NMR metabolomic profile across specific geographical locations and its association with the milk microbiota. Nutrients 10:1355. doi: 10.3390/nu10101355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kumar H, du Toit E, Kulkarni A, Aakko J, Linderborg KM, Zhang Y, Nicol MP, Isolauri E, Yang B, Collado MC, Salminen S. 2016. Distinct patterns in human milk microbiota and fatty acid profiles across specific geographic locations. Front Microbiol 7:1619. doi: 10.3389/fmicb.2016.01619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xiao J, Fiscella KA, Gill SR. 2020. Oral microbiome: possible harbinger for children’s health. Int J Oral Sci 12:12. doi: 10.1038/s41368-020-0082-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Glassing A, Dowd SE, Galandiuk S, Davis B, Chiodini RJ. 2016. Inherent bacterial DNA contamination of extraction and sequencing reagents may affect interpretation of microbiota in low bacterial biomass samples. Gut Pathog 8:24. doi: 10.1186/s13099-016-0103-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bolte LA, Vich Vila A, Imhann F, Collij V, Gacesa R, Peters V, Wijmenga C, Kurilshikov A, Campmans-Kuijpers MJE, Fu J, Dijkstra G, Zhernakova A, Weersma RK. 2021. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 70:1287–1298. doi: 10.1136/gutjnl-2020-322670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Di Guardo G, Battisti A, Agrimi U, Forletta R, Reitano ME, Calderini P. 1997. Pathology of Serratia marcescens mastitis in cattle. Zentralbl Vet Med B 44:537–546. doi: 10.1111/j.1439-0450.1997.tb01005.x [DOI] [PubMed] [Google Scholar]
- 45. Patel SH, Vaidya YH, Patel RJ, Pandit RJ, Joshi CG, Kunjadiya AP. 2017. Culture independent assessment of human milk microbial community in lactational mastitis. Sci Rep 7:7804. doi: 10.1038/s41598-017-08451-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, Adisetiyo H, Zabih S, Lincez PJ, Bittinger K, Bailey A, Bushman FD, Sleasman JW, Aldrovandi GM. 2017. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr 171:647–654. doi: 10.1001/jamapediatrics.2017.0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Delgado S, Arroyo R, Jiménez E, Marín ML, del Campo R, Fernández L, Rodríguez JM. 2009. Staphylococcus epidermidis strains isolated from breast milk of women suffering infectious mastitis: potential virulence traits and resistance to antibiotics. BMC Microbiol 9:82. doi: 10.1186/1471-2180-9-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mediano P, Fernández L, Jiménez E, Arroyo R, Espinosa-Martos I, Rodríguez JM, Marín M. 2017. Microbial diversity in milk of women with mastitis: potential role of coagulase-negative staphylococci, viridans group streptococci, and corynebacteria. J Hum Lact 33:309–318. doi: 10.1177/0890334417692968 [DOI] [PubMed] [Google Scholar]
- 49. Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. 2012. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr 96:544–551. doi: 10.3945/ajcn.112.037382 [DOI] [PubMed] [Google Scholar]
- 50. Wang J, Xu H, Li Z, Li F, Yang Y, Yu X, Jiang D, Xing L, Sun H, Shao M. 2019. Pathogens in patients with granulomatous lobular mastitis. Int J Infect Dis 81:123–127. doi: 10.1016/j.ijid.2019.01.034 [DOI] [PubMed] [Google Scholar]
- 51. Martín R, Heilig HGHJ, Zoetendal EG, Jiménez E, Fernández L, Smidt H, Rodríguez JM. 2007. Cultivation-independent assessment of the bacterial diversity of breast milk among healthy women. Res Microbiol 158:31–37. doi: 10.1016/j.resmic.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 52. Jost T, Lacroix C, Braegger C, Chassard C. 2013. Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br J Nutr 110:1253–1262. doi: 10.1017/S0007114513000597 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prevalence and mean abundance of genera in breast milk.
Observed ASVs and Shannon entropy.
Data Availability Statement
Sequencing data are available at the Sequence Read Archive under the BioProject ID PRJNA1141436. All analysis codes, a phyloseq object with processed sequencing data, and all other data presented in the paper are accessible at https://github.com/ajlavelle/human_mastitis_study.


