Abstract
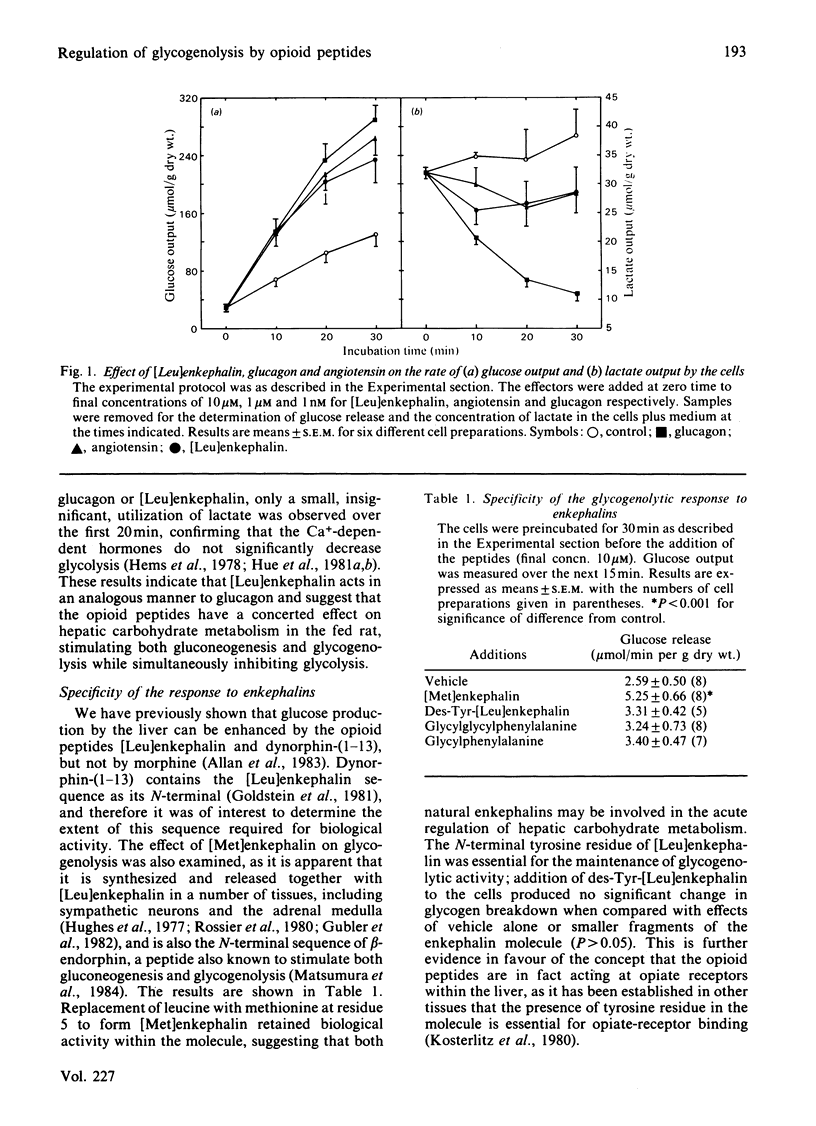
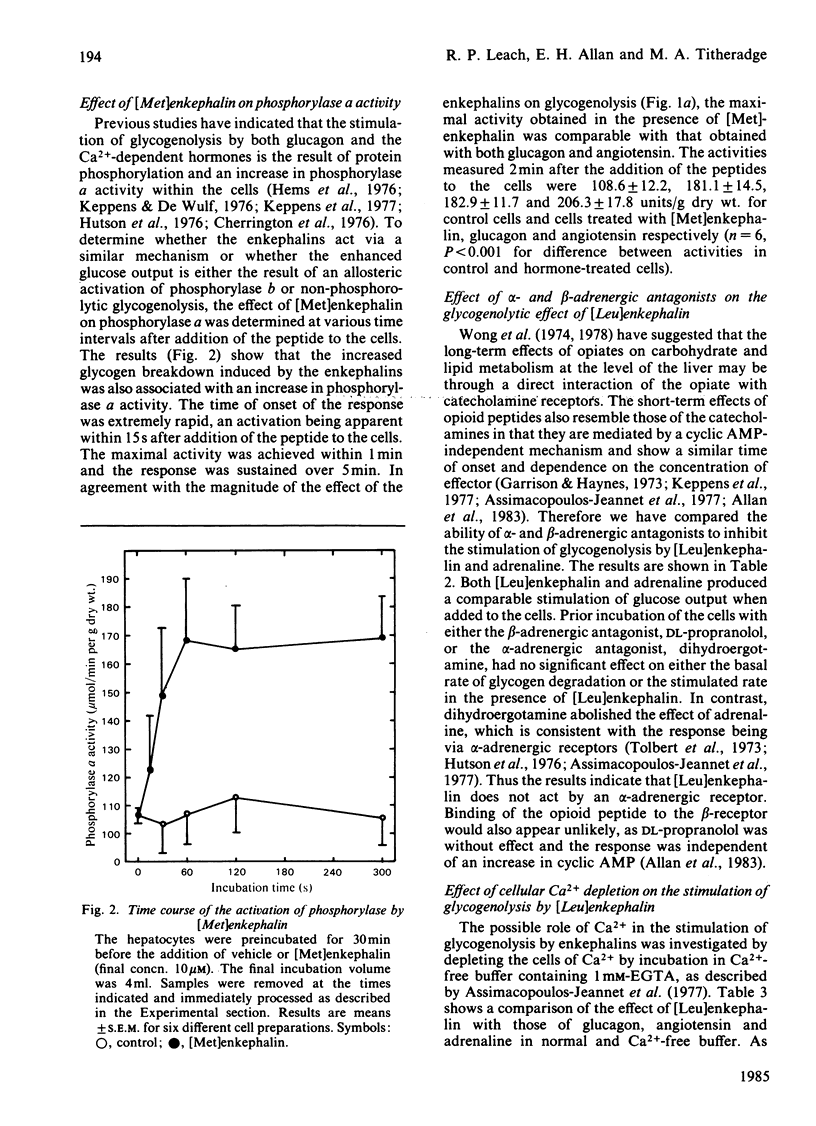
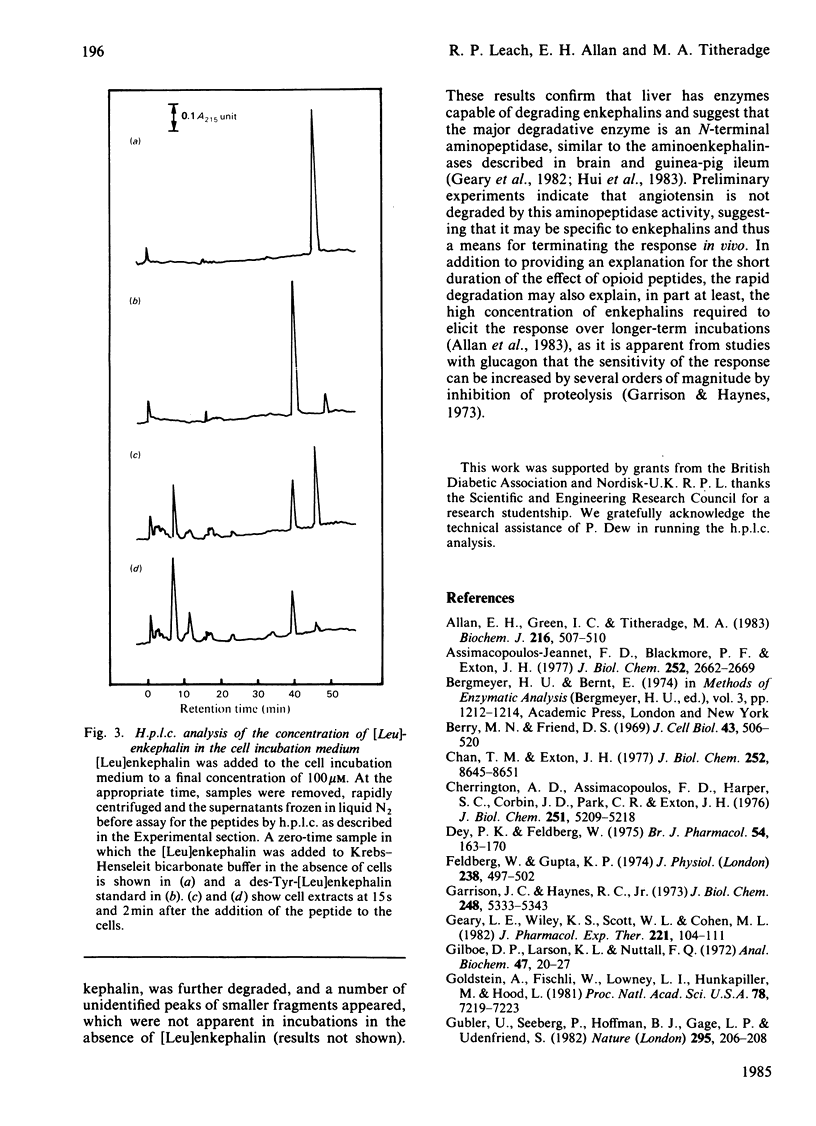
Addition of the opioid peptides, [Leu]enkephalin and [Met]enkephalin, to isolated hepatocytes was shown to produce a stimulation of glycogenolysis comparable with that observed in the presence of maximal concentrations of glucagon, adrenaline or angiotensin. This stimulation was demonstrated to be the result of an activation of phosphorylase by a rapid Ca2+-dependent mechanism and was not decreased by the presence or either alpha- or beta-adrenergic antagonists, although it was dependent on the presence of the N-terminal tyrosine residue in the enkephalin molecule. It is suggested that this may be further evidence for specific opioid receptors in the liver. Addition of [Leu]enkephalin also inhibited lactate formation, indicating that the opioid peptides exert a concerted effect on hepatic carbohydrate metabolism to enhance glucose output. The transient nature of the effect of the enkephalins was shown to be the result of a rapid breakdown of the peptides in the incubation as a result of aminopeptidase activity, the initial product being the inactive des-tyrosine derivative.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan E. H., Green I. C., Titheradge M. A. The stimulation of glycogenolysis and gluconeogenesis in isolated hepatocytes by opioid peptides. Biochem J. 1983 Nov 15;216(2):507–510. doi: 10.1042/bj2160507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F. D., Blackmore P. F., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Studies on role of calcium in alpha-adrenergic activation of phosphorylase. J Biol Chem. 1977 Apr 25;252(8):2662–2669. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. M., Exton J. H. alpha-Adrenergic-mediated accumulation of adenosine 3':5' monophosphate in calcium-depleted hepatocytes. J Biol Chem. 1977 Dec 10;252(23):8645–8651. [PubMed] [Google Scholar]
- Cherrington A. D., Assimacopoulos F. D., Harper S. C., Corbin J. D., Park C. R., Exton J. H. Studies on the alpha-andrenergic activation of hepatic glucose output. II. Investigation of the roles of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in the actions of phenylephrine in isolated hepatocytes. J Biol Chem. 1976 Sep 10;251(17):5209–5218. [PubMed] [Google Scholar]
- Dey P. K., Feldberg W. Hyperglycaemia produced by drugs with analgesic properties introduced into the cerebral ventricles of cats. Br J Pharmacol. 1975 Jun;54(2):163–170. doi: 10.1111/j.1476-5381.1975.tb06925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Gupta K. P. Morphine hyperglycaemia. J Physiol. 1974 May;238(3):487–502. doi: 10.1113/jphysiol.1974.sp010539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison J. C., Haynes R. C., Jr Hormonal control of glycogenolysis and gluconeogenesis in isolated rat liver cells. J Biol Chem. 1973 Aug 10;248(15):5333–5343. [PubMed] [Google Scholar]
- Geary L. E., Wiley K. S., Scott W. L., Cohen M. L. Degradation of exogenous enkephalin in the guinea-pig ileum: relative importance of aminopeptidase, enkephalinase and angiotensin converting enzyme activity. J Pharmacol Exp Ther. 1982 Apr;221(1):104–111. [PubMed] [Google Scholar]
- Gilboe D. P., Larson K. L., Nuttall F. Q. Radioactive method for the assay of glycogen phosphorylases. Anal Biochem. 1972 May;47(1):20–27. doi: 10.1016/0003-2697(72)90274-6. [DOI] [PubMed] [Google Scholar]
- Goldstein A., Fischli W., Lowney L. I., Hunkapiller M., Hood L. Porcine pituitary dynorphin: complete amino acid sequence of the biologically active heptadecapeptide. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7219–7223. doi: 10.1073/pnas.78.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Seeburg P., Hoffman B. J., Gage L. P., Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982 Jan 21;295(5846):206–208. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Rodrigues L. M., Whitton P. D. Glycogen phosphorylase, glucose output and vasoconstriction in the perfused rat liver. Concentration-dependence of actions of adrenaline, vasopressin and angiotensin II. Biochem J. 1976 Nov 15;160(2):367–374. doi: 10.1042/bj1600367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Rodrigues L. M., Whitton P. D. Rapid stimulation by vasopressin, oxytocin and angiotensin II of glycogen degradation in hepatocyte suspensions. Biochem J. 1978 May 15;172(2):311–317. doi: 10.1042/bj1720311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Van Schaftingen E., Blackmore P. F. Stimulation of glycolysis and accumulation of a stimulator of phosphofructokinase in hepatocytes incubated with vasopressin. Biochem J. 1981 Mar 15;194(3):1023–1026. doi: 10.1042/bj1941023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Kosterlitz H. W., Smith T. W. The distribution of methionine-enkephalin and leucine-enkephalin in the brain and peripheral tissues. Br J Pharmacol. 1977 Dec;61(4):639–647. doi: 10.1111/j.1476-5381.1977.tb07557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K. S., Wang Y. J., Lajtha A. Purification and characterization of an enkephalin aminopeptidase from rat brain membranes. Biochemistry. 1983 Mar 1;22(5):1062–1067. doi: 10.1021/bi00274a010. [DOI] [PubMed] [Google Scholar]
- Hutson N. J., Brumley F. T., Assimacopoulos F. D., Harper S. C., Exton J. H. Studies on the alpha-adrenergic activation of hepatic glucose output. I. Studies on the alpha-adrenergic activation of phosphorylase and gluconeogenesis and inactivation of glycogen synthase in isolated rat liver parenchymal cells. J Biol Chem. 1976 Sep 10;251(17):5200–5208. [PubMed] [Google Scholar]
- Ipp E., Dhorajiwala J., Pugh W., Moossa A. R., Rubenstein A. H. Effects of an enkephalin analog on pancreatic endocrine function and glucose homeostasis in normal and diabetic dogs. Endocrinology. 1982 Dec;111(6):2110–2116. doi: 10.1210/endo-111-6-2110. [DOI] [PubMed] [Google Scholar]
- Ipp E., Dobbs R., Unger R. H. Morphine and beta-endorphin influence the secretion of the endocrine pancreas. Nature. 1978 Nov 9;276(5684):190–191. doi: 10.1038/276190a0. [DOI] [PubMed] [Google Scholar]
- Ipp E., Schusdziarra V., Harris V., Unger R. H. Morphine-induced hyperglycemia: role of insulin and glucagon. Endocrinology. 1980 Aug;107(2):461–463. doi: 10.1210/endo-107-2-461. [DOI] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. The activation of liver glycogen phosphorylase by angiotensin II. FEBS Lett. 1976 Oct 1;68(2):279–282. doi: 10.1016/0014-5793(76)80453-x. [DOI] [PubMed] [Google Scholar]
- Keppens S., Vandenheede J. R., De Wulf H. On the role of calcium as second messenger in liver for the hormonally induced activation of glycogen phosphorylase. Biochim Biophys Acta. 1977 Feb 28;496(2):448–457. doi: 10.1016/0304-4165(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W., Lord J. A., Paterson S. J., Waterfield A. A. Effects of changes in the structure of enkephalins and of narcotic analgesic drugs on their interactions with mu- and delta-receptors. Br J Pharmacol. 1980 Feb;68(2):333–342. doi: 10.1111/j.1476-5381.1980.tb10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M., Fukushima T., Saito H., Saito S. In vivo and in vitro effects of beta-endorphin on glucose metabolism in the rat. Horm Metab Res. 1984 Jan;16(1):27–31. doi: 10.1055/s-2007-1014686. [DOI] [PubMed] [Google Scholar]
- Reid R. L., Yen S. S. beta-Endorphin stimulates the secretion of insulin and glucagon in humans. J Clin Endocrinol Metab. 1981 Mar;52(3):592–594. doi: 10.1210/jcem-52-3-592. [DOI] [PubMed] [Google Scholar]
- Rossier J., Audigier Y., Ling N., Cros J., Udenfriend S. Met-enkephalin-Arg6-Phe7, present in high amounts in brain of rat, cattle and man, is an opioid agonist. Nature. 1980 Nov 6;288(5786):88–90. doi: 10.1038/288088a0. [DOI] [PubMed] [Google Scholar]
- Shaw J. S., Miller L., Turnbull M. J., Gormley J. J., Morley J. S. Selective antagonists at the opiate delta-receptor. Life Sci. 1982 Sep 20;31(12-13):1259–1262. doi: 10.1016/0024-3205(82)90356-3. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Kirk C. J., Hems D. A. Role of extracellular calcium in the action of vasopressin on hepatic glycogenolysis. FEBS Lett. 1976 Oct 15;69(1):199–202. doi: 10.1016/0014-5793(76)80686-2. [DOI] [PubMed] [Google Scholar]
- Tolbert M. E., Butcher F. R., Fain J. N. Lack of correlation between catecholamine effects on cyclic adenosine 3':5'-monophosphate and gluconeogenesis in isolated rat liver cells. J Biol Chem. 1973 Aug 25;248(16):5686–5692. [PubMed] [Google Scholar]
- Wong S. C., Yeung D., Au K. S. Effects of morphine on the gluconeogenic enzymes from rat liver in vivo. Biochem Pharmacol. 1974 Feb 15;23(4):829–833. doi: 10.1016/0006-2952(74)90213-5. [DOI] [PubMed] [Google Scholar]
- Wong S. C., Yeung Y. G., Yeung D. Effects of morphine on isoenzymes of pyruvate kinase and tyrosine aminotransferase in rat. Biochem Pharmacol. 1978 May 1;27(9):1347–1351. doi: 10.1016/0006-2952(78)90118-1. [DOI] [PubMed] [Google Scholar]
- van de Werve G., Hue L., Hers H. G. Hormonal and ionic control of the glycogenolytic cascade in rat liver. Biochem J. 1977 Jan 15;162(1):135–142. doi: 10.1042/bj1620135. [DOI] [PMC free article] [PubMed] [Google Scholar]


