Abstract
Biomaterial-associated thrombus formation and bacterial infection remain major challenges for blood-contacting devices. For decades, titanium-based implants have been largely used for different medical applications. However, titanium can neither suppress blood coagulation, nor prevent bacterial infections. To address these challenges, tanfloc/heparin polyelectrolyte multilayers on titania nanotubes array surfaces (NT) were developed. The surfaces were characterized by scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), and water contact angle measurements. To evaluate the hemocompatibility of the surfaces, fibrinogen adsorption, Factor XII activation, and platelet adhesion and activation were analyzed. The antibacterial activity was investigated against Gram-negative P. aeruginosa and Gram-positive S. aureus. Bacterial adhesion and morphology, as well as biofilm formation, were analyzed using fluorescence microscopy and SEM. The anti-thrombogenic properties of the surfaces were demonstrated by significant decreases in fibrinogen adsorption, Factor XII activation, and platelet adhesion and activation. Modifying NT with tanfloc/heparin also reduces the adhesion and proliferation of P. aeruginosa and S. aureus bacteria after 24 hr of incubation, with no biofilm formation. The modified NT surfaces with tanfloc/heparin polyelectrolyte multilayers are a promising biomaterial for use on implant surfaces because of their enhanced blood biocompatibility and antibacterial properties.
Keywords: antibacterial, antithrombogenic, polyelectrolyte multilayer, tanfloc, titania nanotubes
1 |. INTRODUCTION
The use of blood-contacting implants, such as heart valves, catheters, and stents to treat cardiovascular diseases has increased remarkably in recent years (Jaffer, Fredenburgh, Hirsh, & Weitz, 2015). However, catastrophic complications from these biomaterials still occur, such as thrombus formation and bacterial infections, which are the most common causes for device failure (Xu, Meyerhoff, & Siedlecki, 2019). The processes leading to bacterial adhesion and coagulation are related, and microbial infections may become worse due to the activation of the coagulation cascade (van Gorp et al., 1999). Therefore, it is vital to develop multifunctional surfaces that can simultaneously prevent clot formation and bloodstream infections. Among the biomaterials used for blood-contacting applications, titanium and its alloys are widely employed due to their excellent mechanical properties, corrosion resistance, and remarkable biocompatibility with many body tissues (Navarro, Michiardi, Castaño, & Planell, 2008). Nevertheless, titanium’s compatibility with blood and its ability to inhibit bacterial infection are still far from ideal (Movafaghi et al., 2017; Zhang et al., 2016).
Several approaches have been investigated to prevent both bacterial infections and thrombosis on titanium surfaces, including modification of the surface chemistry, wettability, and topography (Sperling, Fischer, Maitz, & Werner, 2009). Protein adsorption and activation is a critical initial step that can lead to adverse biological responses that prevent healthy tissue healing. Therefore, the use of natural antifouling coatings has emerged as a strategy for improving biocompatibility of surfaces. One promising technique recently investigated is the layer-by-layer (LbL) deposition of polyelectrolyte multilayers (PEMs). LbL assembly is used to change the surface chemistry of materials by alternately adsorbing/depositing polycationic and polyanionic layers onto a solid substrate (da Câmara et al., 2019). PEMs can be easily and reproducibly prepared, to achieve control over coating thickness and surface chemistry, without the use of hazardous organic solvents (Almodóvar, Place, Gogolski, Erickson, & Kipper, 2011). Among the polycation-polyanion polymer pairs recently used, heparin and chitosan PEMs have generated particular interest for biological activation of surfaces (Almodóvar et al., 2013; Lin et al., 2018; Zomer Volpato et al., 2012). Heparin (HP) is a highly negatively charged polymer and a natural glycosaminoglycan (GAG), which in the body prevents blood coagulation by inhibiting the thrombin activation (Zhang et al., 2018). Although heparinized materials have been used clinically in blood-contacting biomaterials, there are still some limitations, such as loss of bioactivity over long periods of time, especially due to its high solubility (Liu et al., 2014). Chitosan (CS) and its cationic derivatives allow the formation of multilayers with the negatively charged heparin (Almodóvar et al., 2013). CS-based PEMs can be used to improve the tissue compatibility of surfaces and inhibit bacterial infections (de Oliveira et al., 2020). Another naturally occurring GAG that has been employed in biomedical materials is hyaluronic acid (HA). This anionic biopolymer has great cytocompatibility and biodegradability. Because of these properties, it has been used in PEM coatings on biomaterials (Koenig, Neumann, Schlensak, Wendel, & Nolte, 2019).
Recently, tanfloc (TA) has attracted considerable interest due to its promising cytocompatibility, biodegradability, and antimicrobial properties (Facchi et al., 2017). TA is a hydrophilic, cationic (pKa ≈ 6.0), and condensed amino-functionalized tannin derivative (Martins et al., 2018). TA is synthetized from condensed tannins (from polyphenols with repeat flavan-3-ol units) found in plants that act to inhibit pathogen occurrences (Graham, Gang, Fowler, & Watts, 2008). Condensed tannins are often extracted from the Acacia decurrens (Black wattle), and they are then used to obtain the TA derivative from acid catalysis performed with ammonium chloride and formic acid (Martins et al., 2018). TA has strong antimicrobial activity due to the presence of ammonium moieties (−NH3+) stabilized by chloride counterions and phenolic moieties in its structure. Because of its cationic behavior in aqueous solutions, it can replace CS to provide PEMs with HP or HA. Previous studies have shown TA-based materials presented cytocompatibility and antimicrobial activities (Facchi et al., 2017; Martins et al., 2018); however, no studies report the blood compatibility, antiadhesive, and antibacterial traits of TA-based PEMs.
In this work, TA/HP PEMs on titania nanotubes (NT) were successfully developed. First, the surface topography was modified by making NT, as it has been shown that NT has better antibacterial properties and reduces thrombogenic effects on titanium (Puckett, Taylor, Raimondo, & Webster, 2010; Smith, Capellato, Kelley, Gonzalez-Juarrero, & Popat, 2013). Then, NT surface was modified with PEMs using TA or CS as polycations, and HP or HA as polyanions. The PEMs were fabricated using five layers, terminating with the polycation. The surfaces were characterized using SEM, XPS, and contact angle measurements. Fibrinogen adsorption, FXII activation, and static platelet adhesion and activation on the surfaces were investigated. The adhesion and morphology of S. aureus and P. aeruginosa were also evaluated. Previous study has shown that CS/HP PEMs on NT can reduce platelet adhesion (Simon-Walker et al., 2017); however, titanium modification with TA has never been investigated. In this study, biological responses of TA and CS-based PEMs deposited on the NT surface were compared. The results indicated that TA-based PEMs enhance the antithrombogenic, antibacterial, and antiadhesive properties of the NT when compared to CS-based PEMs.
2 |. MATERIALS AND METHODS
2.1 |. Fabrication of titania nanotubes (NT) with PEMs
NT surfaces were fabricated from titanium sheets (0.5-mm thick) via the anodization process reported elsewhere (Sabino, Kauk, Movafaghi, Kota, & Popat, 2019). Prior to the surface modification with PEMs, the NT surfaces were treated with oxygen plasma at 200 V in 10 cm3/min of oxygen gas for 15 min. Tanfloc (TA, Tanac SA, Brazil) (Martins et al., 2018) and chitosan (CS, Golden Shell Biochemical, China, 85% deacetylated and 87 kDa) (Ramin et al., 2019) were purified by first preparing a 10% w/v TA solution in DI water and 1.0% w/v CS solution in aqueous acetic acid (1.0% v/v). Dialysis was then conducted with 7,000 MWCO membrane for 2 days, with exchange of water twice a day. The polymeric solutions were then frozen and lyophilized for 3 days. To prepare the PEMs, solutions of TA, CS, HP (Celsus Laboratories), and HA (Sigma Aldrich) were prepared in an acetic acid-acetate buffer (0.2 M sodium acetate and acetic acid at pH 5.0) at a concentration of 1 mg/mL. After that, the solutions were filtered with 0.22-μm syringe filters. An aqueous acetic acid solution (pH 4.0) was used as a rinse solution. The oxidized NT surface was rinsed with 0.5 mL rinse solution under shaking (50 rpm) for 4.0 min before the TA or CS deposition. The rinse solution was aspirated, and LbL deposition was carried out on the oxidized NT surface. Then, 0.5 mL TA or CS solutions (polycations) were added to the oxidized NT surface under shaking (50 rpm). After 5.0 min, the polycation solution was aspirated and the surface was rinsed under shaking for 4.0 min. Then, the rinse solution was aspirated and HP or HA polyanion solutions (0.5 mL) were deposited onto the oxidized NT surface containing one layer of polycation (TA or CS). This method was repeated until the fifth layer was deposited. The surfaces with five layers (polycation terminated) were rinsed for 4 min and incubated in DI water for further use. Figure 1 presents the process of PEMs fabrication, as well as the polyelectrolytes’ chemical structures. The CS/HP, CS/HA, TA/HP, and TA/HA PEMs deposited on NT surfaces are referred to as NT(CS-HP), NT(CS-HA), NT(TA-HP), and NT(TA-HA), respectively.
FIGURE 1.
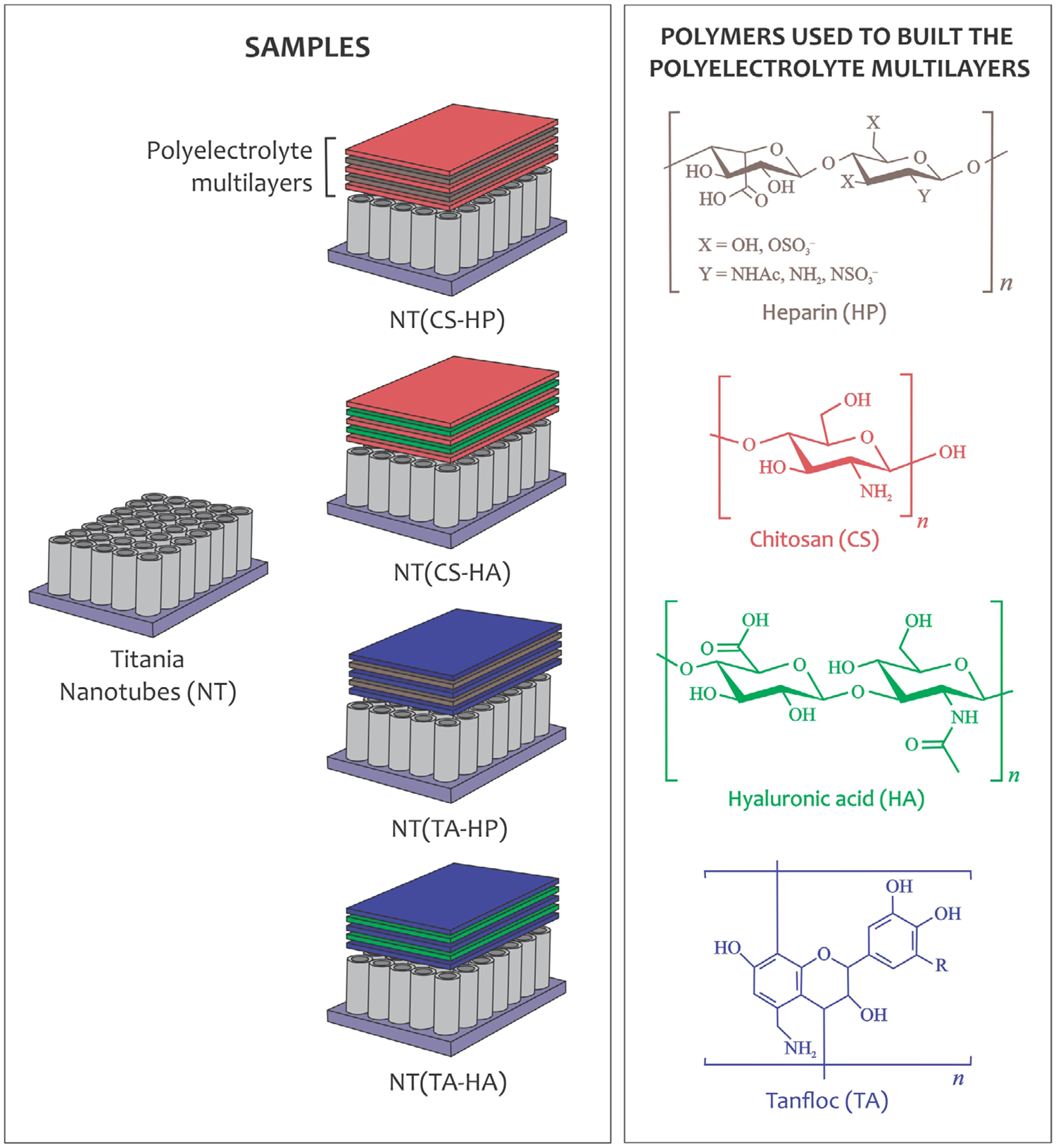
Schematic representation of the PEM construction on titania nanotubes (NT) and chemical structures of the polyelectrolytes
2.2 |. Surface characterization
The morphology of each surface was characterized by SEM. Before imaging at 15 kV, the surfaces were coated with 20-nm gold. Static contact angles of different surfaces were obtained using a Ramé-Hart goniometer. Approximately 10 μL of DI water were placed on the surface to obtain the water contact angles. The composition of different surfaces was investigated using XPS. Survey spectra were collected from 0 to 1,100 eV (pass energy of 187 eV), and high-resolution spectra were collected for carbon at a pass energy of 10 eV. Spectral analysis was performed using Origin and MultiPak. Prior to all studies, the surfaces were sterilized by rinsing in 70% ethanol, followed by rinse and incubation with sterile PBS for 30 min.
2.3 |. Hemocompatibility studies
To analyze the antithrombogenic properties of the surfaces, the adsorption of fibrinogen, a key protein involved in thrombus formation (Gorbet & Sefton, 2006), and the activation of Factor XII (FXII), that is responsible for the activation of the intrinsic pathway of the coagulation cascade were investigated (Vogler & Siedlecki, 2010). Additionally, platelet adhesion and activation that also mediates blood clotting on different surfaces were evaluated (Reviakine et al., 2017).
2.3.1 |. Fibrinogen adsorption on different surfaces
To investigate the fibrinogen adsorption, the surfaces were incubated in a 48-well plate with 100 μg/mL of human fibrinogen solution in PBS on a horizontal shaker plate (100 rpm) at 37°C and 5% CO2. After 2 hr of incubation, the protein solution was aspirated, and the surfaces were rinsed twice with PBS and once with DI water to remove any non-adherent proteins. The protein adsorption on different surfaces was investigated by XPS analysis, as described in Section 2.2.
2.3.2 |. Isolation of human platelet poor plasma (PPP) and platelet rich plasma (PRP)
Whole human blood was obtained from healthy donors, who signed formal consents for this study. All practices were approved by the Colorado State University Institutional Review Board, which agrees with the National Institutes of Health’s “Guiding Principles for Ethical Research”. To obtain PPP, whole blood was drawn into 2.7 mL vacuum tubes coated with sodium citrate and centrifuged at 1500 g for 10 min. To obtain PRP, whole blood was drawn into 10 mL vacuum tubes coated with ethylenediaminetetraacetic acid (EDTA) and centrifuged at 150 g for 15 min.
2.3.3 |. FXII activation on different surfaces
To investigate the activation of the intrinsic pathway of the coagulation cascade, FXII activation on different surfaces was characterized by an in vitro plasma coagulation time (CT) assay. CT is defined as the time needed from activation of the intrinsic pathway of the coagulation cascade to the appearance of a visible clot (Bauer, Xu, Vogler, & Siedlecki, 2017). A mathematical model was developed to correlate CT with FXIIa concentration through three adjustable parameters (Guo et al., 2006). This method is reported in detail elsewhere (Sabino et al., 2019). Briefly, purified human FXII (Haematologic Technologies) was incubated with different surfaces at a concentration of 30 μg/mL for 30 min. After, 100 μL aliquots were taken and added to a cuvette with 0.5 mL of PPP, 0.3 mL of 0.01 M PBS, and 0.1 mL of 0.1 M CaCl2. The CaCl2 was the last reagent added to ensure a common zero-time CT. The cuvettes were then covered with parafilm and rotated at 20 rpm on a hematology mixer, and the corresponding CT was recorded. The CT was then used to calculate the equivalent FXIIa activity by referencing back to the FXIIa titration curve obtained in previous study (Sabino et al., 2019).
2.3.4 |. Platelet adhesion and activation on different surfaces
Platelet adhesion under static conditions was investigated using fluorescence microscopy. The surfaces were incubated in a 48-well plate with 500 μL of PRP at 37°C and 5% CO2 on a horizontal shaker plate (100 rpm). After 2 hr of incubation, PRP was aspirated, and the surfaces were rinsed twice with sterile PBS to remove non-adherent platelets. The surfaces were then incubated with 2 μM calcein-AM solution in PBS for 30 min in a dark environment. After that, the surfaces were rinsed with PBS and imaged using a fluorescence microscope. ImageJ was used to count the total number of platelets attached.
Platelet activation under static conditions on different surfaces was characterized using SEM. After 2 hr incubation in PRP and rinse with PBS, the surfaces were incubated in a primary fixative solution (3% glutaraldehyde, 0.1 M sucrose, and 0.1 M sodium cacodylate in DI water) for 45 min. The surfaces were then incubated in a buffer solution (fixative without glutaraldehyde) for 10 min. After that, the surfaces were dehydrated in ethanol solutions (35, 50, 70, and 100%, respectively) for 10 min each. Prior to SEM imaging, the surfaces were gold-coated, as described in Section 2.2.
2.4 |. Antibacterial activity studies
The antibacterial activity of different surfaces was evaluated against Gram-negative Pseudomonas aeruginosa (P. aeruginosa, P01) and Gram-positive Staphylococcus aureus (S. aureus, ATCC 6538) cultures obtained from agar plate culture and pellet, respectively. A nutrient broth media solution (NBM, LB-Miller) was prepared at a concentration of 25 mg/mL. The S. aureus pellet was resuspended in NBM solution and an aliquot of P. aeruginosa was taken from the agar plate and dissolved in NBM. Both bacteria solutions were incubated at 37°C for 24 hr until the optical density at 600 nm was approximately 1 (Bartlet, Movafaghi, Dasi, Kota, & Popat, 2018).
2.4.1 |. Growth inhibition in solution
The bacteria solutions were diluted to obtain a concentration of 106 CFU/mL. The surfaces were then incubated in 500 μL of bacteria solution for 6 and 24 hr. A 100 μL aliquot of the solution was taken, and the absorbance was read at 600 nm using a plate reader. Bacteria solutions under the same conditions without exposure to surfaces were used as control. The values were then compared to the control, and the inhibition percentage was calculated.
2.4.2 |. Bacteria adhesion on different surfaces
Fluorescence microscopy was used to measure the amount of live and dead bacteria that adhered to the surfaces. After incubation for 6 and 24 hr, the bacteria solution was removed, and the surfaces were rinsed three times with PBS to remove any non-adhered bacteria. The surfaces were then incubated in stain solution (3 μL/mL of propidium iodide and Syto 9 stain 1:1 in PBS) for 20 min at room temperature and in a dark environment. After incubation, the stain solution was aspirated, and the surfaces were rinsed once with PBS. The surfaces were then immediately imaged using a fluorescence microscope. ImageJ was used to calculate the percentage of live and dead bacteria on the surfaces.
2.4.3 |. Bacteria morphology and biofilm formation on different surfaces
Bacteria morphology and biofilm formation on different surfaces were characterized by SEM. The surfaces were incubated in bacteria solution as described in Section 2.4a for 6 and 24 hr. After the incubation, the surfaces were rinsed twice with PBS, fixed, and dehydrated, as described in Section 2.3d. Prior to SEM imaging, the surfaces were gold coated as described in Section 2.2.
2.5 |. Statistical analysis
SEM images and XPS analysis were reconfirmed on at least two different samples of each surface. Contact angle measurements were taken using two droplets per sample on three different samples of each surface (nmin = 6). All other experiments were conducted at least twice with three samples of each surface (nmin = 6). The quantitative results were analyzed via analysis of variance (ANOVA) and Tukey tests using Origin software (p < .05).
3 |. RESULTS
3.1 |. Surface characterization
The SEM images indicate that NTs are uniform and vertically oriented, and the PEMs with five layers do not entirely coat the surface and still maintain the nanotube topography (Figure 2). The results also show no apparent changes in morphology for NT(CS-HP), NT(CS-HA), and NT(TA-HP) in comparison with NT. NT(TA-HA) shows some changes in morphology, although the nanotubes are still visible. No significant difference was observed in nanotubes’ diameter (110 ± 14 nm) even after PEM deposition.
FIGURE 2.
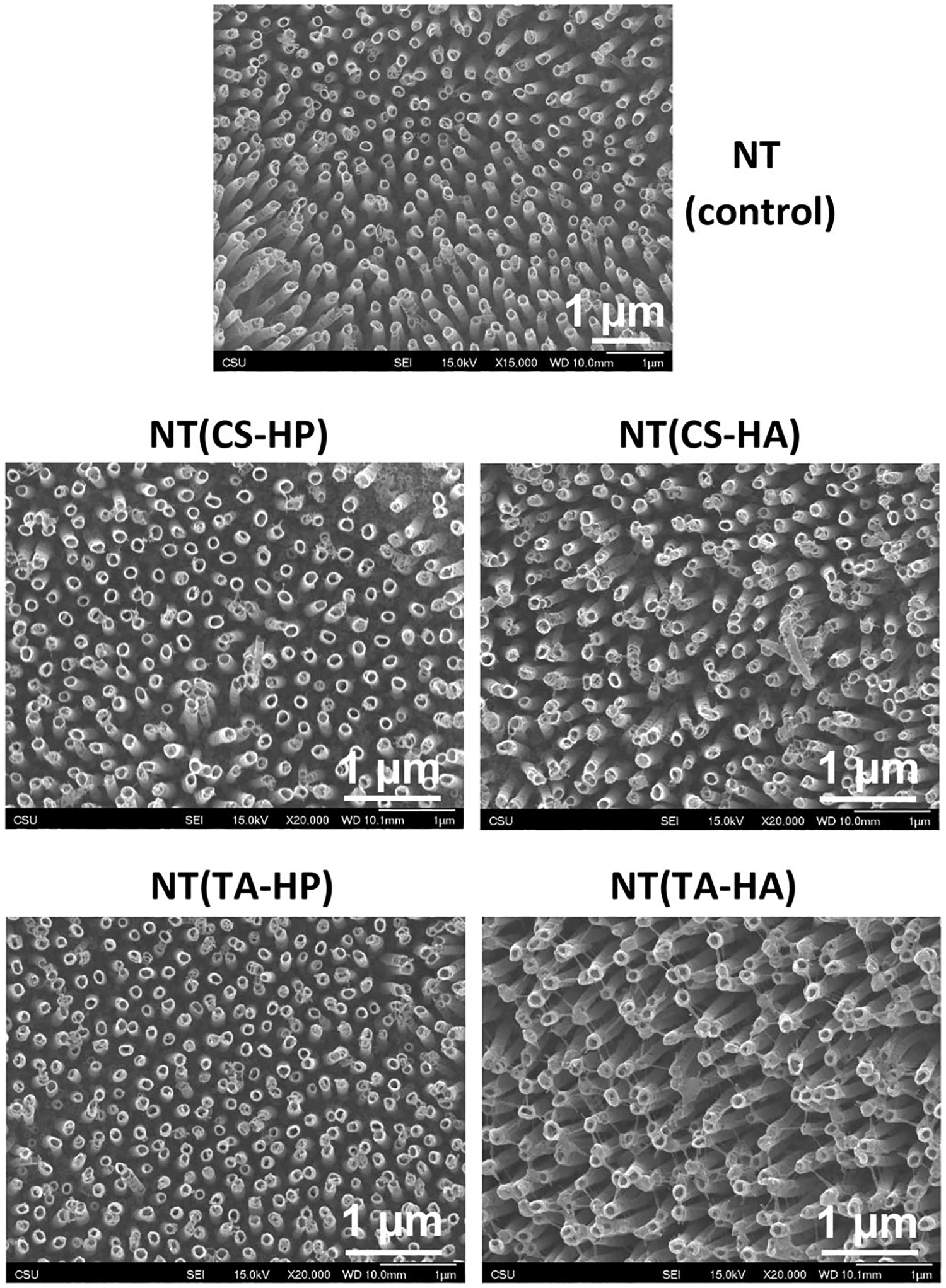
Representative SEM images of titania nanotubes (NT) before and after suface modification with PEMs
Contact angle measurements show that all surfaces are hydrophilic (Table 1). A surface is considered hydrophilic when the static contact angle (θ) between the surface and water is less than 90°, and hydrophobic when θ is greater than 90°. NT surfaces modified with HP were significantly more hydrophilic than the ones modified with HA. The NT modified with TA are slightly more hydrophilic in comparison with the ones modified with CS, although no significant difference was observed between NT(CS-HP) and NT(TA-HP); and between NT(CS-HA) and NT(TA-HA).
TABLE 1.
Static water contact angle measurements for different surfaces
| Surface | Contact angle (degrees) |
|---|---|
| NT | <10 |
| NT(CS-HP) | 13 ± 1 |
| NT(CS-HA) | 19 ±2 |
| NT(TA-HP) | 11 ±2 |
| NT(TA-HA) | 16 ±2 |
XPS survey spectra indicate that all surfaces have oxygen (O1s), titanium (Ti2p3/2), and carbon (C1s) elements (Figure 3a). Surfaces modified with HP have S2p and S2s peaks. As expected, all surfaces modified with PEMs have N1s peaks, which is a characteristic of the polyelectrolytes’ composition. The elemental composition of different surfaces was also obtained from survey XPS scans using MultiPak software (Table 2). All modified NT surfaces show an increase in the carbon content, as well as a decrease in the titanium signal in comparison with the unmodified NT surface. However, although the oxygen is present in all polyelectrolytes’ chemical structure, its percentage concentration on the surfaces has decreased compared to NT. This occurs due to the significant increase in carbon content. The titanium concentration on surfaces modified with TA is smaller than the ones modified with CS, indicating that the TA-based PEMs achieve greater coverage of the NT surface than CS-based PEMs. High-resolution C1s spectra were also obtained from XPS analysis (Figure 3b). Table 3 shows the contribution of different peaks in the overall C1s spectra. The modified NT surfaces have a significant increase in both C—O and C—N groups when compared to the unmodified NT surface. NT(TA-HP) and NT(TA-HA) also show a higher concentration of C—C peaks and smaller presence of O—C=O peaks compared with NT(CS-HP) and NT(CS-HA).
FIGURE 3.
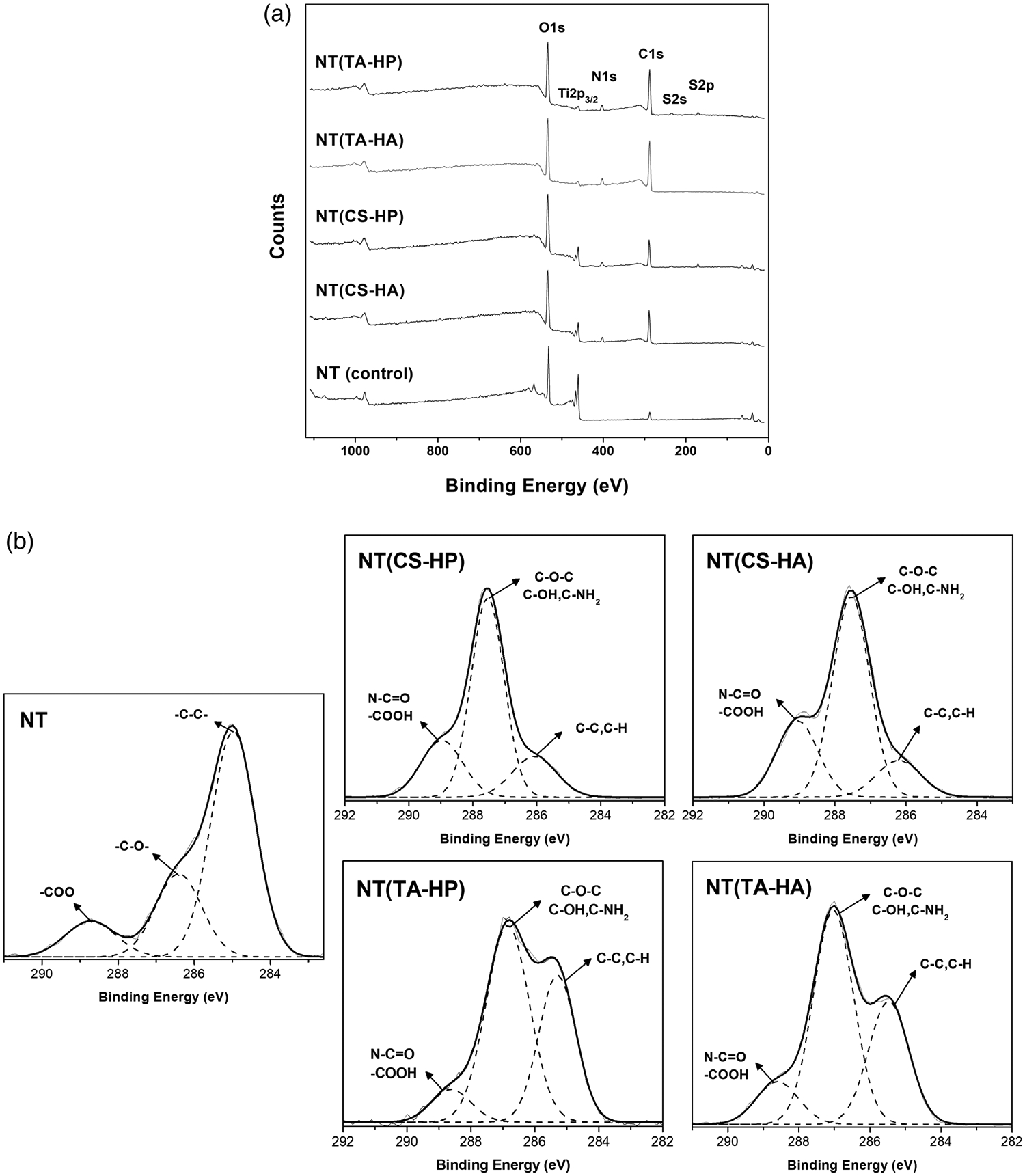
(a) XPS survey scans for different surfaces; (b) High-resolution XPS spectra for C1s obtained from the different surfaces
TABLE 2.
Elemental composition of the different surfaces determined by survey XPS analysis
| % C1s | % O1s | % ti2p | % N1s | % S2p | |
|---|---|---|---|---|---|
| nt | 11.7 | 68.3 | 20.0 | 0 | 0 |
| nt(cs-hp) | 47.2 | 41.8 | 5.3 | 4.2 | 1.5 |
| nt(cs-ha) | 48.1 | 44.5 | 3.6 | 3.8 | 0 |
| nt(ta-hp) | 59.0 | 34.4 | 0.9 | 4.5 | 1.2 |
| nt(ta-hA) | 62.1 | 32.2 | 0.6 | 5.1 | 0 |
TABLE 3.
The relative percentages of C—C/C—H, C—O/C—N, and N—C=O/COOH determined from the peak areas from the C1s envelopes for the different surfaces
| C—C, C—h | C—O, C—N | N—C=O, COOH | |
|---|---|---|---|
| nt | 63.5 | 25.7 | 10.8 |
| nt(cs-hp) | 16.8 | 61.1 | 22.1 |
| nt(cs-ha) | 25.9 | 60.6 | 13.5 |
| nt(tA-hp) | 35.7 | 55.8 | 8.5 |
| nt(tA-ha) | 32.4 | 55.9 | 11.7 |
3.2 |. Hemocompatibility studies
Survey and high-resolution XPS spectra for C1s were collected for the surfaces after incubation in human fibrinogen solution. The elemental composition was used to investigate the amount of fibrinogen adsorbed on the material surfaces (Table 4). Since proteins contain high nitrogen levels, the increase in the nitrogen content on the surfaces is due to the fibrinogen adsorption. The results show that all modified NT surfaces significantly decrease the amount of fibrinogen adsorbed compared to NT. However, the NT(TA-HA) surface presents the lowest adsorption, followed by the NT(TA-HP) surface. High-resolution C1s spectra indicate three peaks for all surfaces: N—C=O/COOH, C—N/C—O, and C—C/C—H (Figure 4). An accurate way to determine fibrinogen adhered on the surface is to identify the contribution of the amide peak (N—C=O) in the overall C1s peak as the amide group is characteristic of proteins.(Leszczak, Smith, & Popat, 2013) Figure 3b shows that all surfaces already indicated the N—C=O and/or COOH groups before fibrinogen adsorption assay. Therefore, to determine the amount of protein adsorbed, the contribution of the amide peak after protein adsorption was subtracted by the contribution of the same peak before the protein adsorption study. The NT surfaces modified with TA-based PEMs have significantly less fibrinogen adsorbed compared to the other surfaces (Table 5). The NT surfaces modified with CS also have a slight decrease in fibrinogen adsorption when compared to unmodified NT; however, the TA-based PEMs promoted better fibrinogen resistance. NT(TA-HA) has the lowest amount of fibrinogen adsorption.
TABLE 4.
Nitrogen content found on the material surfaces determined after the fibrinogen adsorption assay
| % N (BEFORE) | % N (after) | Increase (%) | |
|---|---|---|---|
| nt | 0 | 13.1 | 13.1 |
| nt(cs-hp) | 4.2 | 9.7 | 5.5 |
| nt(cs-ha) | 3.8 | 13.1 | 9.3 |
| nt(ta-hp) | 4.5 | 9.2 | 4.7 |
| nt(tA-hA) | 5.1 | 7.1 | 2.0 |
Note: The difference corresponds to the amount of fibrinogen adsorbed to each surface. The results were obtained from survey XPS analysis.
FIGURE 4.
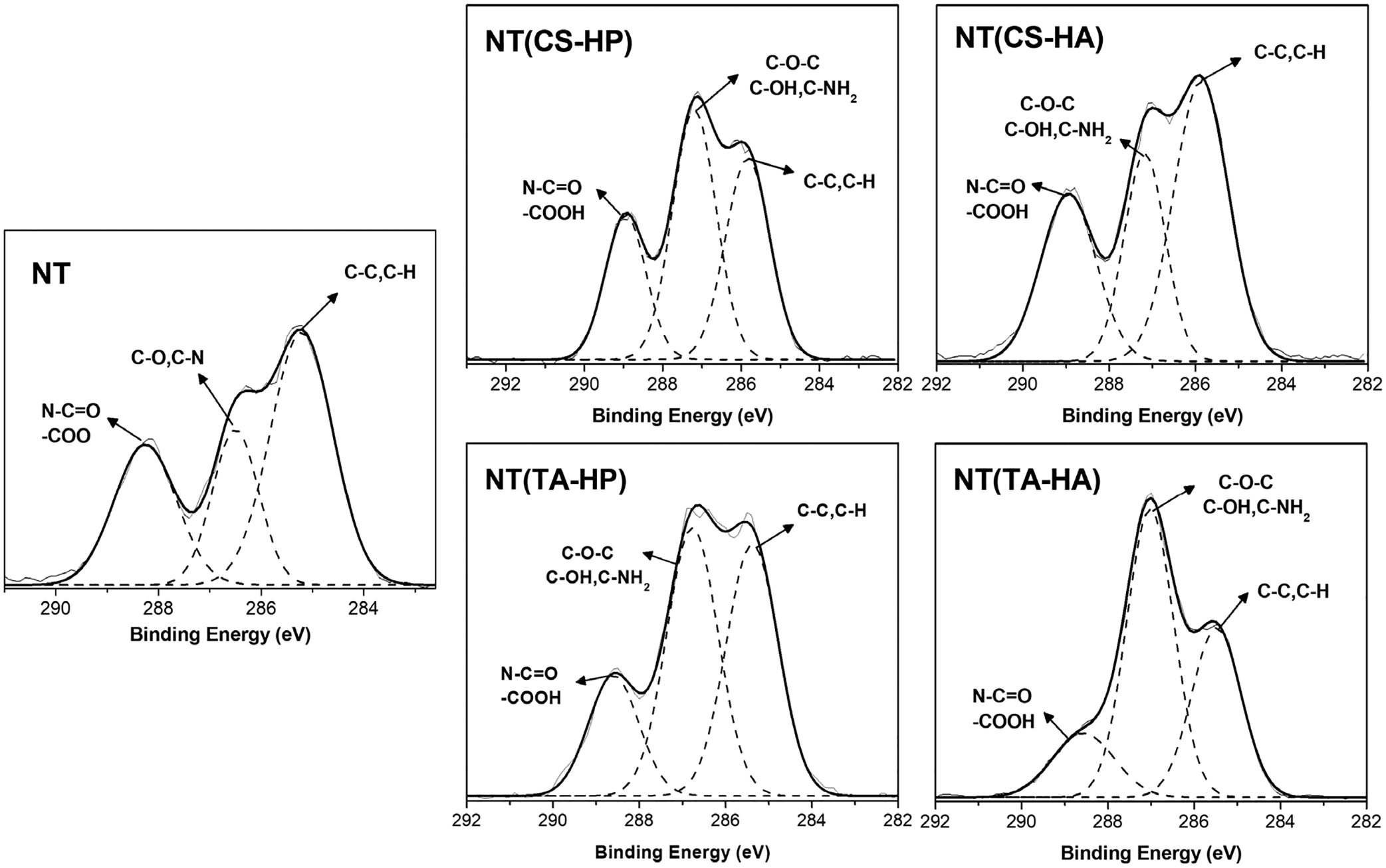
High-resolution XPS spectra for C1s obtained the different surfaces after 2 hr incubation in fibrinogen solution
TABLE 5.
The contribution of amide (N—C=O) peaks in the C1s envelopes are given in percentages after the fibrinogen adsorption assay
| N—C=O (before) | N—C=O (after) | Increase (%) | |
|---|---|---|---|
| nt | 10.8 | 27.5 | 16.7 |
| nt(cs-hp) | 22.1 | 34.7 | 12.6 |
| nt(cs-ha) | 13.5 | 27.8 | 14.3 |
| nt(tA-hp) | 8.5 | 18.3 | 9.8 |
| nt(tA-hA) | 11.7 | 15.2 | 3.5 |
Note: The difference corresponds to the amount of fibrinogen adsorbed on the material surfaces.
FXII activation on surfaces was determined by the CT assay. The calibration curve obtained in the previous study was used to convert the CT measured into FXIIa concentration (Figure 5) (Sabino et al., 2019). NT(TA-HP) was the only surface that significantly decreases the FXII activation when compared to the unmodified NT. This surface dramatically decreases FXII activation, reaching about one-eighth of the FXIIa amount produced on unmodified NT.
FIGURE 5.

FXII activation on different surfaces. Experiments were conducted at least twice with three samples of each surface (nmin = 6). NT(TA-HP) has significantly lower FXII activation than the NT surfaces (p < .05)
The fluorescence images of cells adhered to surfaces under static conditions show that NT(TA-HP) and NT(TA-HA) significantly decrease the platelet adhesion compared to the unmodified NT (Figure 6). The NT(TA-HP) promotes the lowest amount of platelet adhesion, approximately 10-fold less than that imparted on NT. NT(CS-HP) also decreases platelet adhesion compared to NT, but not significantly. After attachment to the biomaterial surface, the platelets can activate, which is indicated by a morphological membrane change (Simon-Walker et al., 2017). When platelets are unactivated, they exhibit a compact round shape. As they start to activate, dendritic extensions are formed and platelets can be considered partially activated (short-dendritic morphology) or fully activated (long dendritic morphology) (Movafaghi et al., 2017). The SEM results show that NT and NT(CS-HA) surfaces present partially activated platelets with some short dendritic extensions (Figure 7). The formation of platelet aggregates on NT and NT(CS-HA) surfaces is another evidence of platelet activation (Movafaghi et al., 2017). NT(CS-HP) surface also exhibits platelet aggregation; however, no dendritic extension was observed. NT(TA-HA) surface presents a small amount of platelet aggregation, with a very low content of partially activated platelets. NT(TA-HP) was the only surface with no platelet aggregation or dendritic formation. SEM results also agree with the fluorescence images, as NT and NT(CS-HA) surfaces present the highest number of adhered cells, followed by NT(CS-HP). Both TA-modified surfaces have very low amounts of platelets.
FIGURE 6.
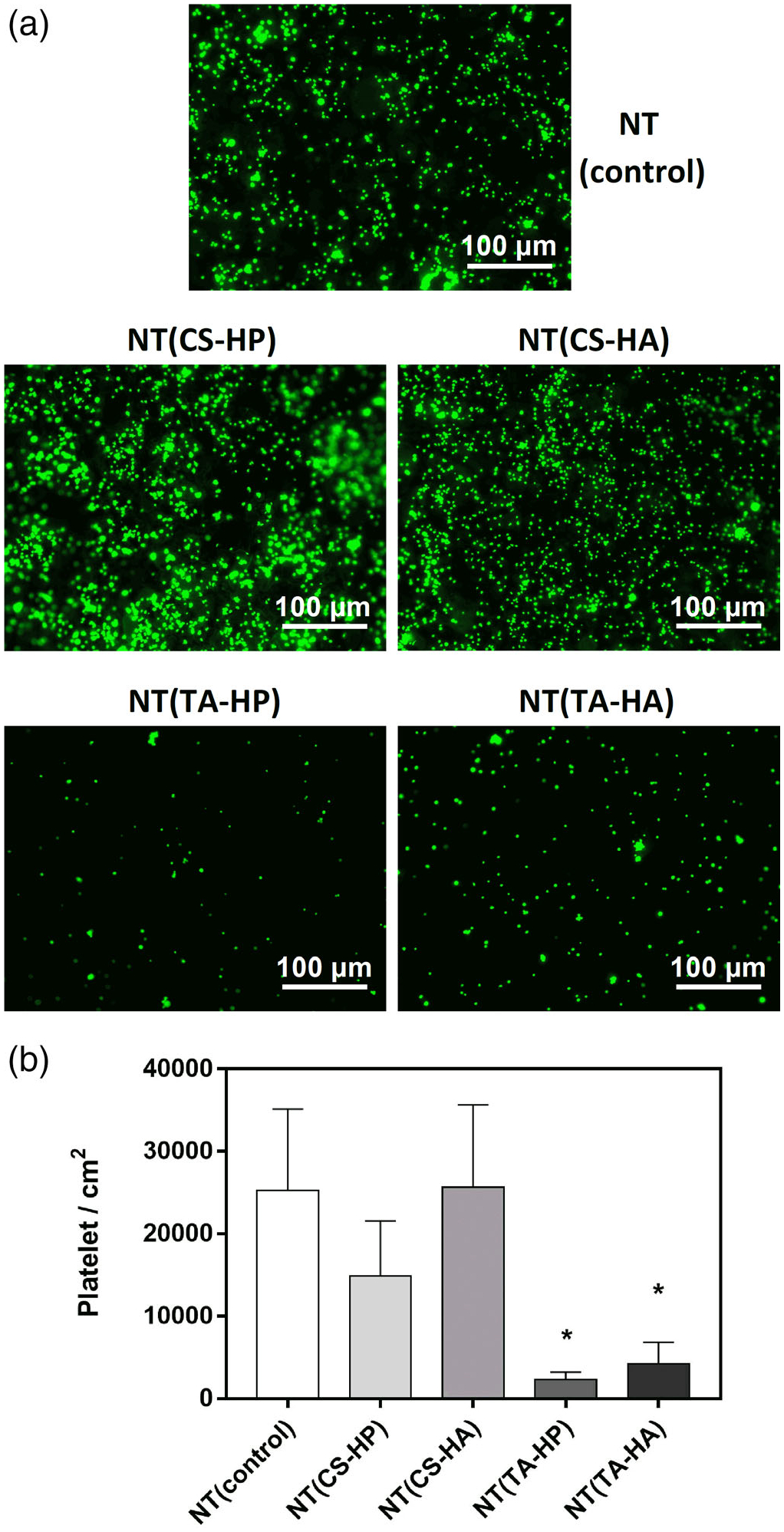
(a) Representative fluorescence microscopy images of platelet adhesion on different surfaces after 2 hr of incubation in PRP; (b) Number of adhered platelets per area (cm2) on the different surfaces stained with calcein-AM. Experiments were conducted at least twice with three samples of each surface (nmin = 6). NT(TA-HP) and NT(TA-HA) have significantly lower cell adhesion than the NT surfaces (p < .05)
FIGURE 7.
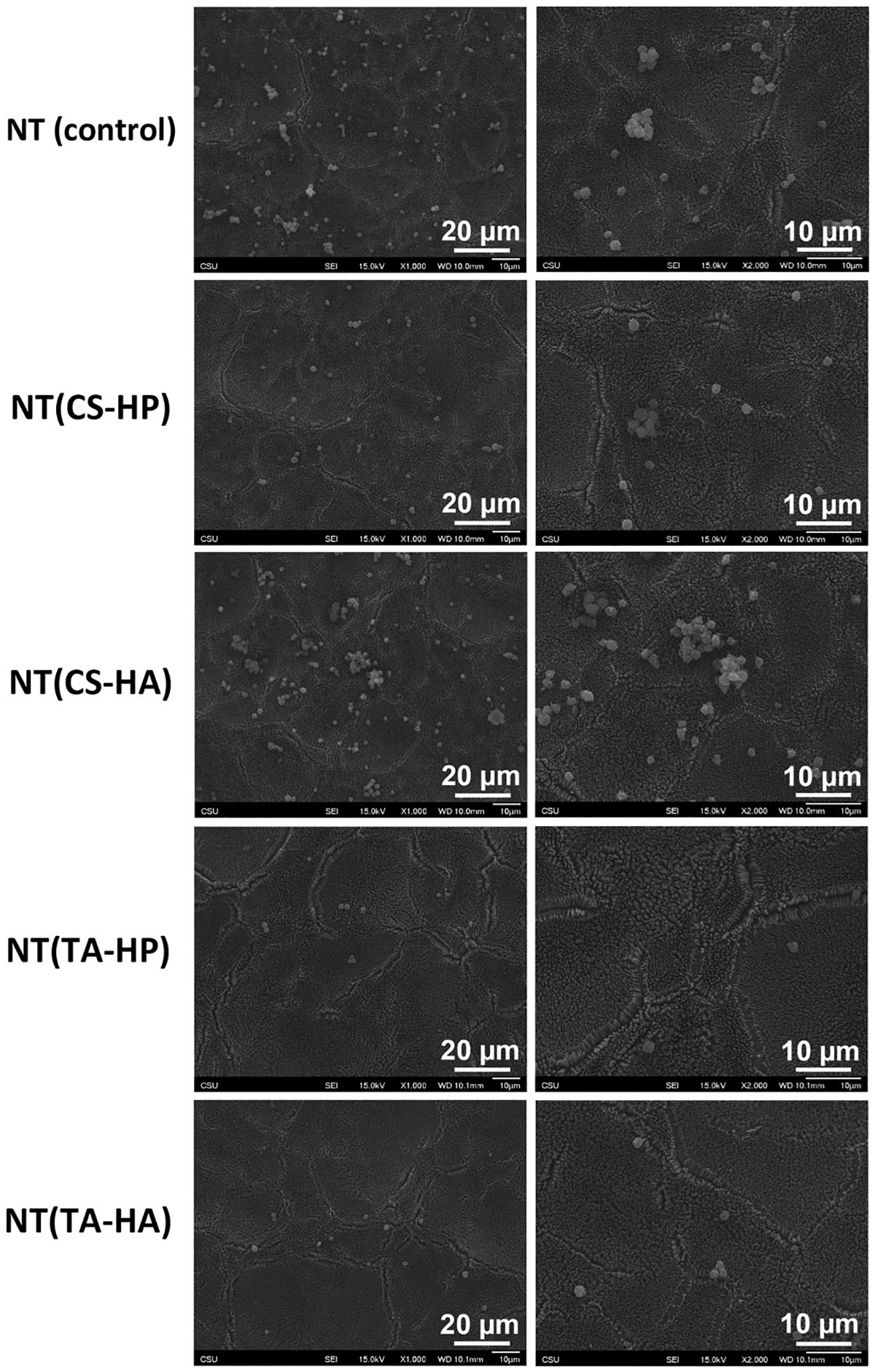
Representative SEM images of adhered platelets on different surfaces after 2 hr of incubation in PRP
3.3 |. Antibacterial activity studies
Bacterial growth inhibition percentage was calculated for different surfaces in comparison with the control (Figure 8). All modified NT surfaces promote higher growth inhibition toward P. aeruginosa significantly after 24 hr of incubation when compared to NT. However, there is no significant difference in the growth inhibition imparted on S. aureus.
FIGURE 8.
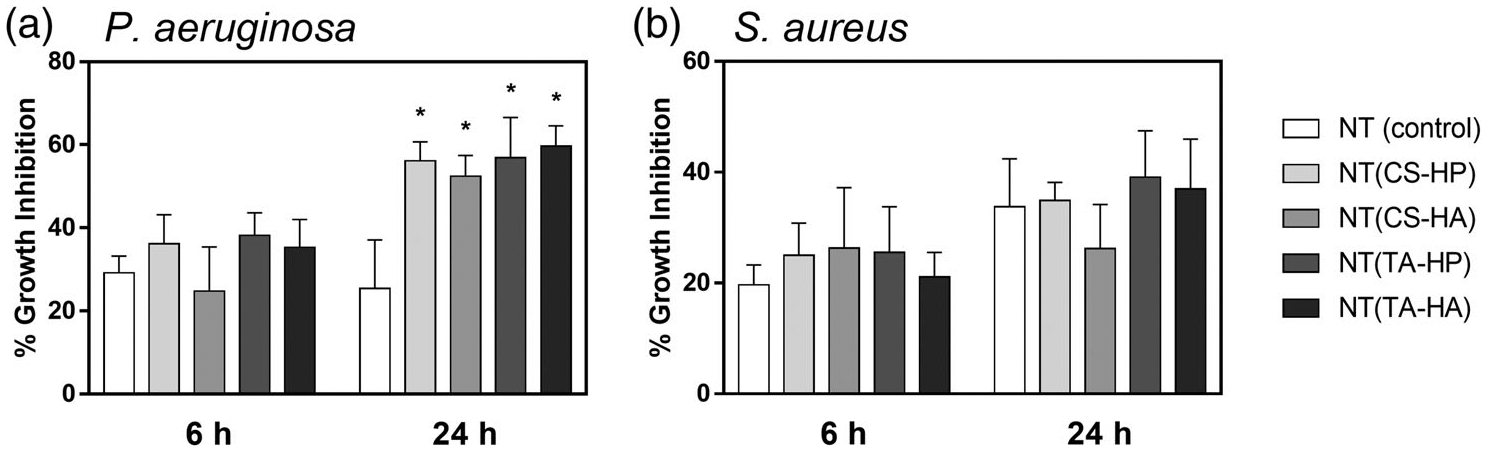
Percentage growth inhibition in solution promoted by the different surfaces against (a) P. aeruginosa and (b) S. aureus after 6 and 24 hr of incubation in bacteria solution. Experiments were conducted at least twice with three samples of each surface (nmin = 6). All NT-modified surfaces with PEMs present significantly higher growth inhibition toward P. aeruginosa after 24 hr than the NT surfaces (p < .05)
NT(CS-HA), NT(TA-HP), and NT(TA-HA) significantly decrease the adhesion of live P. aeruginosa bacteria after 24 hr when compared to NT (Figure 9a,b). After 24 hr, the bacteria attached on NT(TA-HP) surface are mostly dead, while NT(TA-HA) has almost no bacteria attached. NT(TA-HP) and NT(TA-HA) also foster better antiadhesive property than the NT(CS-HP), although no significant difference was observed between them. Both TA-modified surfaces dramatically reduce the bacteria adhesion on NT (approximately 80%). The amount of the dead bacteria adhered significantly increase on NT(CS-HA) when compared to NT.
FIGURE 9.
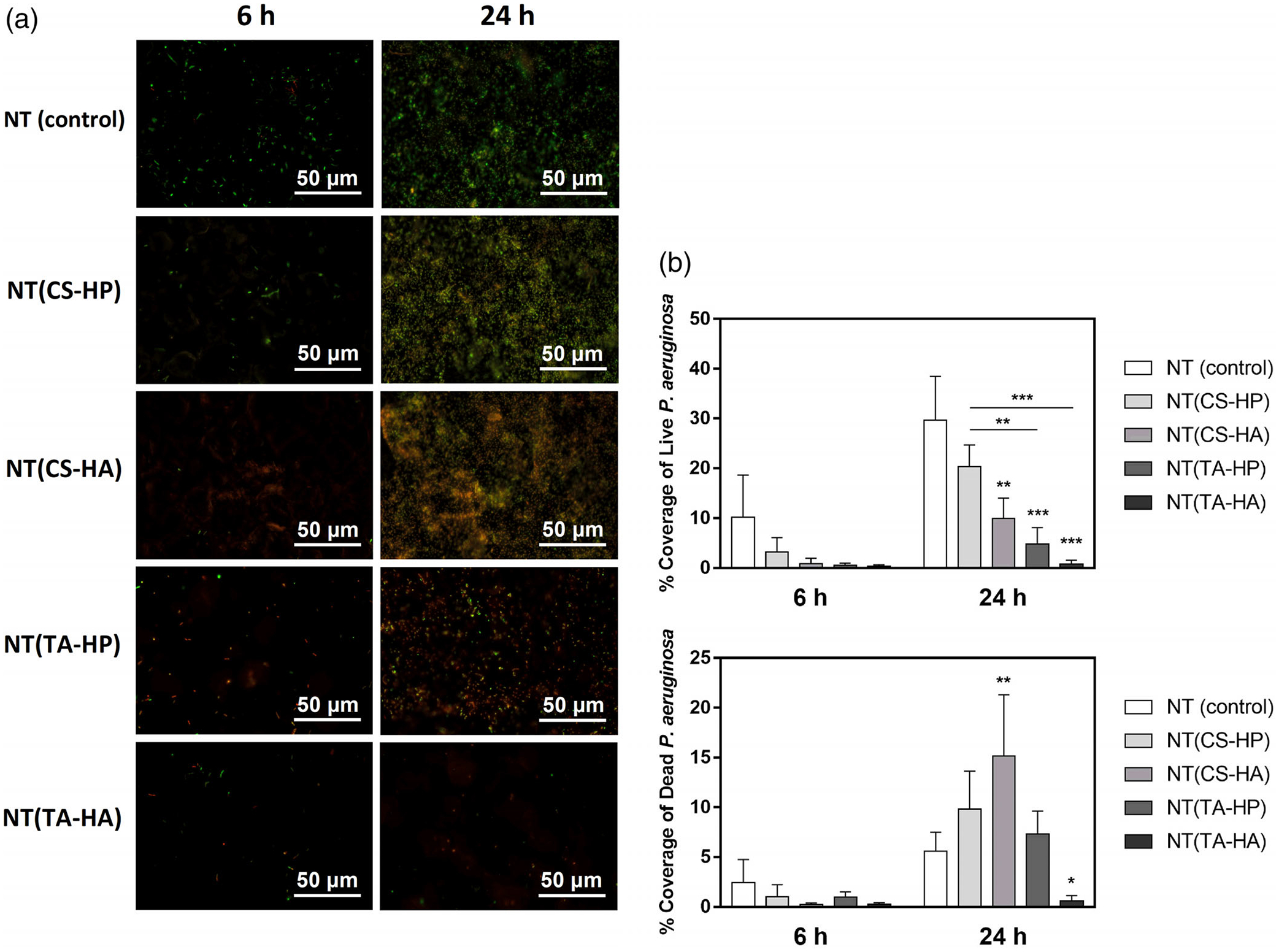
(a) Representative fluorescence microscopy images of P. aeruginosa on the different surfaces after 6 and 24 hr of incubation in bacteria solution. Green stain indicates live bacteria, and red stain indicates dead bacteria. (b) Live/Dead P. aeruginosa adhesion on different surfaces after 6 and 24 hr of incubation in bacteria solution. Experiments were conducted at least twice with three samples of each surface (nmin = 6). NT(CS-HA), NT(TA-HP), and NT(TA-HA) have significantly lower live bacteria adhesion than the NT surfaces (* indicates p ≤ .05; ** indicates p ≤ .01; *** indicates p ≤ .001)
Both CS-based PEMs significantly decrease the adhesion of live S. aureus bacteria after 24 hr when compared to the NT (Figure 10a,b). However, no significant difference in the percentage area of live bacteria was observed between TA-modified surfaces and NT. On the other hand, the NT(TA-HP) and NT(TA-HA) surfaces significantly increase the antimicrobial activity toward both bacteria, imparting a higher number of damaged bacterial cells in comparison with the NT surface.
FIGURE 10.
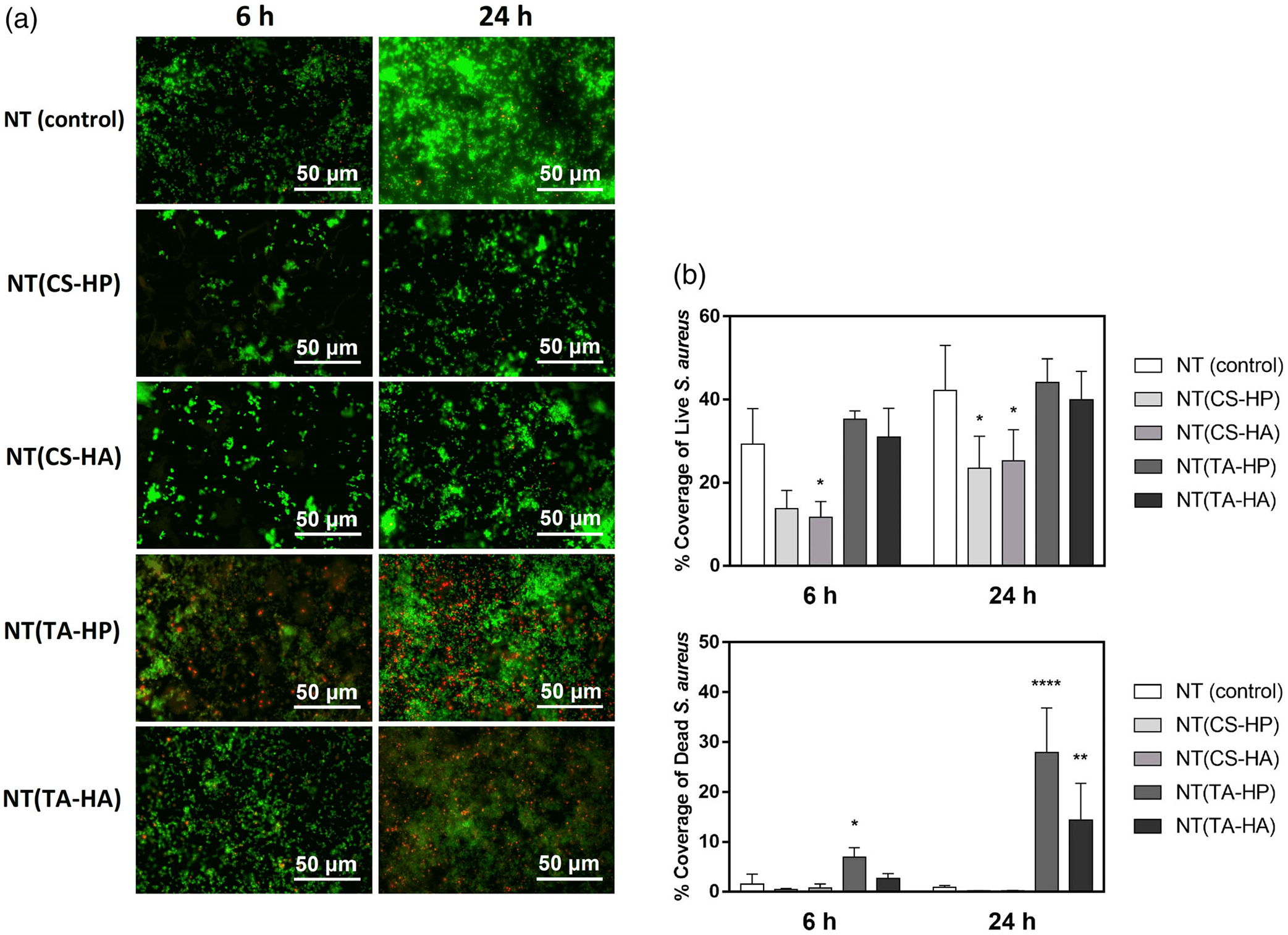
(a) Representative fluorescence microscopy images of S. aureus on different surfaces after 6 and 24 hr of incubation in bacteria solution. Green stain indicates live bacteria, and red stain indicates dead bacteria. (b) Live/Dead S. aureus adhesion on different surfaces after 6 and 24 hr of incubation in bacteria solution. Experiments were conducted at least twice with three samples of each surface (nmin = 6). NT(CS-HP) and NT(CS-HA) have significantly lower live bacteria adhesion than the NT surfaces. NT(TA-HP) and NT(TA-HA) have significantly higher dead bacteria adhesion than the NT surfaces (* indicates p ≤ .05; ** indicates p ≤ .01; **** indicates p ≤ .0001)
SEM images of the different surfaces seeded with P. aeruginosa show that NT and NT(CS-HP) have the highest bacteria adhesion, with some colony formation (Figure 11). The NT(CS-HP) also exhibit biofilm formation after 24 hr of incubation. NT(CS-HA) presents some damaged bacteria adhered after 24 hr, with no agglomeration. The NT(TA-HP) and NT(TA-HA) promoted better antiadhesive activity with more damaged cells compared to unmodified NT. This finding agrees with the fluorescence microscopy. No biofilm or colony formation was observed on either NT(TA-HP) and NT(TA-HA).
FIGURE 11.
Representative SEM images of P. aeruginosa on different surfaces after 6 and 24 hr of incubation
The NT imparted the highest S. aureus agglomeration, followed by NT(CS-HA) and NT(CS-HP) surfaces (Figure 12). The TA-modified surfaces show less bacterial attachment than NT, with little aggregation. This is not in agreement with the fluorescence microscopy images, likely because the fluorescence microscopy only allows a two-dimensional percentage coverage calculation. Because the bacteria adhered to NT(TA-HP) and NT(TA-HA) are more spaced out and the ones adhered to NT are agglomerated (also in three dimensions), the percentage coverage areas obtained from fluorescence images seem similar, even though NT presents significantly more bacterial adhesion. SEM results also show no biofilm formation on the surfaces.
FIGURE 12.
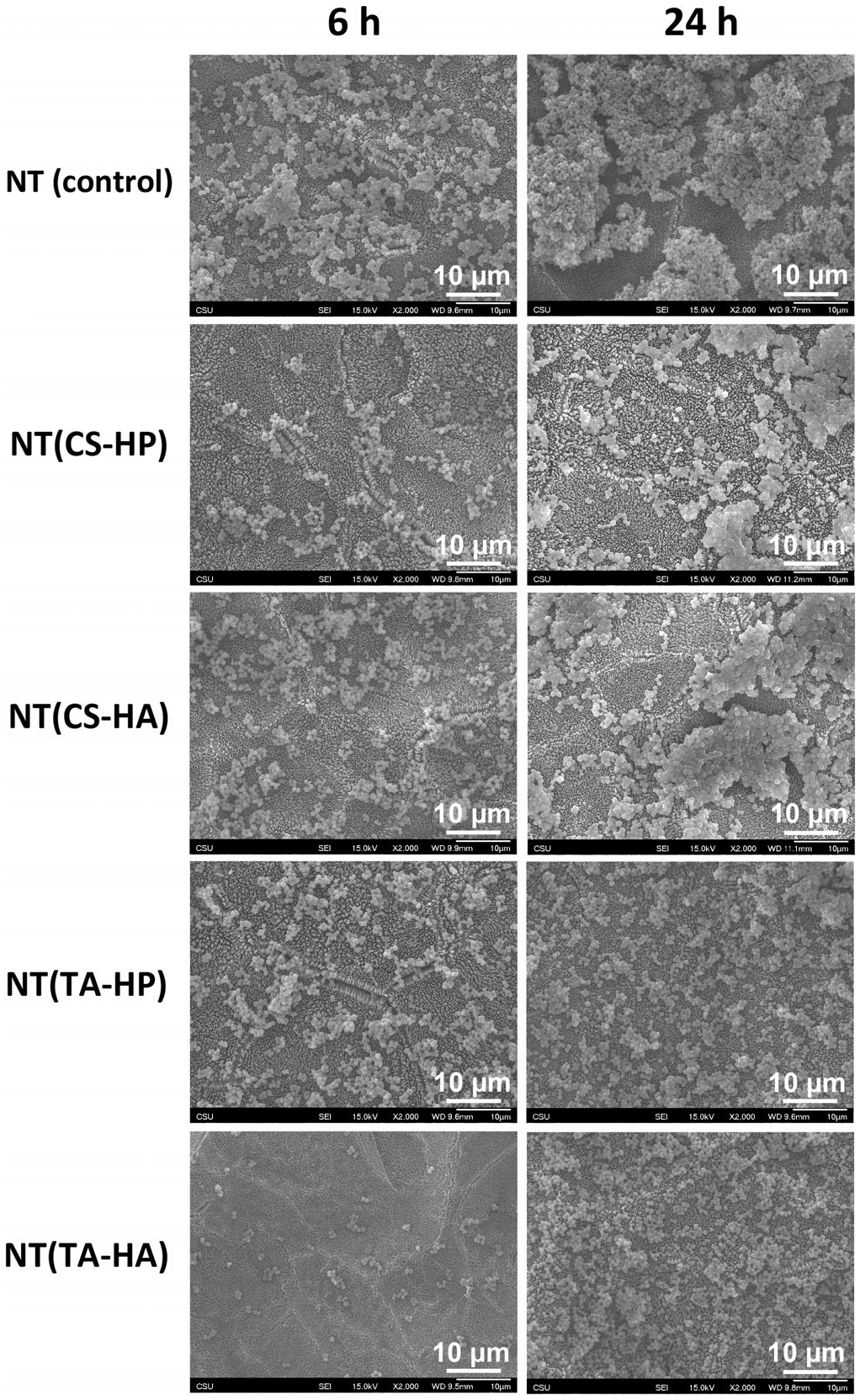
Representative SEM images of S. aureus on different surfaces after 6 and 24 hr of incubation
4 |. DISCUSSION
Preventing biomaterials-associated thrombus formation and bacterial infection are still challenges for the successful development of blood-contacting implants. The first event that happens when biomaterials come in contact with blood is the adsorption of blood plasma proteins, which mediate subsequent biological phenomena, such as the attachment of platelets and other blood cells to the surface (Xu, Bauer, & Siedlecki, 2014). Among the blood proteins that adsorb, fibrinogen plays a central role in the coagulation cascade and is primarily responsible for platelet adhesion (Wells, Guo, Emili, & Sefton, 2017). The blood clotting induced by biomaterial surfaces occurs primarily by the intrinsic pathway of the coagulation cascade (Yan, Xu, Vogler, & Siedlecki, 2018). This intrinsic pathway is initiated by the conversion of the protein Factor XII (FXII) into its activated form Factor XIIa (FXIIa), when blood encounters a foreign surface (Bauer et al., 2017). FXIIa then activates a complex sequence of reactions resulting in the conversion of fibrinogen to fibrin, platelet adhesion and activation, and finally thrombus formation (Sabino et al., 2019). Therefore, blood-compatible surfaces should be able to prevent the blood protein adsorption and FXII activation, as well as platelet attachment, in order to avoid blood clotting.
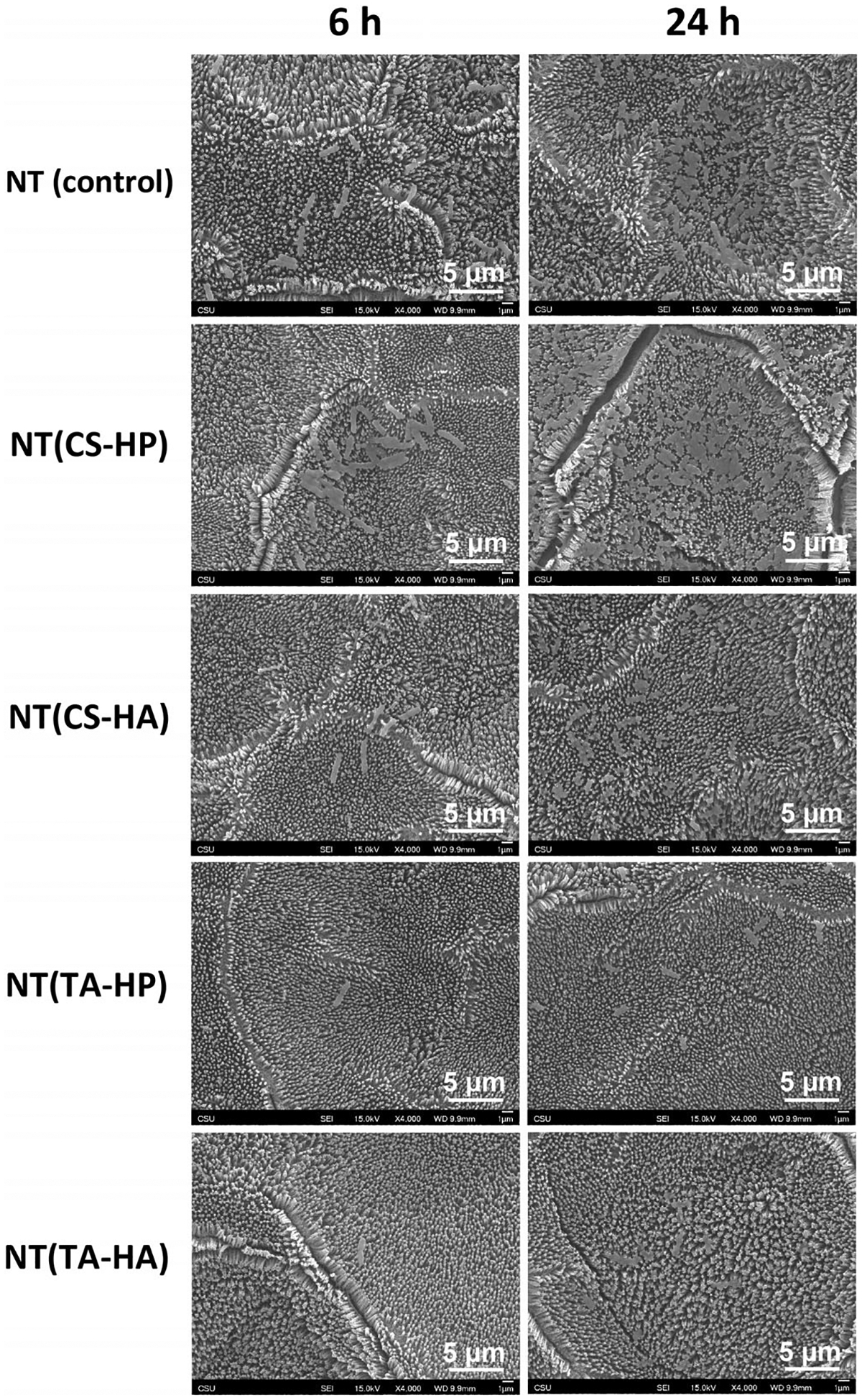
In this work, TA/HP PEMs on NT were fabricated with the goal to simultaneously prevent thrombus formation and bacterial infection. Firstly, the topography was changed by producing titania nanotube arrays surfaces because it has been shown that NT nanostructure reduces platelet and bacteria adhesion (Puckett et al., 2010; Smith & Popat, 2012). NT was fabricated from titanium sheets via an anodization process in HF solution, and its formation is caused by the field-assisted dissolution process (Ramin et al., 2019). Before NT modification with PEMs, the surfaces were treated with oxygen plasma to form hydroxyl groups, which allow the surface binding with polycations (Sabino et al., 2019). The PEMs were then constructed via layer-by-layer approach to modify the surface chemistry. PEMs were constructed using five layers to change the chemistry of NT surface, without modifying the topography (da Câmara et al., 2019). SEM and XPS results show that PEMs do not coat the nanotube surface (Figures 2 and 3a). Maintaining the nanoscale topography is desired because it mimics the natural tissue hierarchy, thus enhancing biocompatibility (Smith et al., 2013). XPS results also indicate that NT was successfully modified with PEMs since the surfaces present nitrogen peaks, which is characteristic of the cationic polyelectrolytes’ (TA and CS). The presence of S2s and S2p peaks was also expected on surfaces treated with HP, as sulfur occurs in HP chemical structure (Figure 3a). Contact angles measurements also indicate the successful modification of the surfaces. As expected, NT surfaces modified with HP are more hydrophilic than the ones with HA because HP is highly negatively charged, possessing high solubility (Table 1).
Fibrinogen is a key protein involved in thrombosis that promotes platelet attachment to the biomaterial surface, as well as platelet aggregation (Xu et al., 2014). Both TA-modified surfaces significantly decrease the fibrinogen adsorption (Tables 4 and 5). TA is reported as a biocompatible cationic tannin polymer derivative synthesized from condensed tannins (Facchi et al., 2017). Condensed tannins have a flavonoid type structures, that is, they comprise structures with two aromatic rings often linked to three-carbon chain oxygen heterocycles (Guil-Guerrero et al., 2016). The TA structure is based on flavan-3-ol repeat units (Figure 1). Therefore, the TA chemical networks are very different when compared to the CS and blood proteins. This may help to prevent both protein and platelet adhesion. Also, TA monomers contain both amine and phenolic -OH substituents. The amine groups are weakly cationic, whereas the phenolic groups are weakly anionic, possibly imparting the TA with polyzwitterion-like properties. Zwitterionic polymers have attracted interest due to their ability to resist both protein adsorption and bacterial adhesion (Lin, Zhang, Wang, & Chen, 2011). These materials possesses natural antifouling properties as the strong hydration shell prevents the surface interaction with biofoulants (Jhong et al., 2014). Similar behavior may be responsible for the protein resistance of the TA-modified surfaces reported here.
Biomaterial-associated thrombosis is due to plasma coagulation and platelet-mediated reactions (Xu et al., 2014). Plasma coagulation happens through the intrinsic pathway, which is initiated by the conversion of the blood protein Factor XII to its activated form FXIIa. NT(TA-HP) significantly decreases the FXII activation, and this can be due to the TA antifouling properties (Figure 5). To autoactivate, FXII needs to bind to the biomaterial surface and undergo a conformational change (Sabino et al., 2019). However, NT(TA-HA) does not significantly decrease FXIIa concentration, indicating that HP may also play an important role in inhibiting the FXII activation. Although NT(TA-HA) reduces more fibrinogen adsorption than NT(TA-HP), NT(TA-HP) was the only surface, which significantly decreases the FXII activation. This result is more relevant because decreasing protein adsorption is not enough for preventing blood clotting, and inhibition of protein activation is required to repress the coagulation cascade.
The platelet-mediated reactions, such as platelet adhesion, aggregation, and activation, also mediate blood clotting and depend on the fibrinogen adsorption to the surface (Xu et al., 2014). Therefore, the reduction in fibrinogen adsorption typically decreases both platelet adhesion and activation. The results agree with this statement for both TA-modified surfaces, which significantly decrease the platelet adhesion under static conditions (Figure 6). However, NT(TA-HP) was the only surface that presents no sign of platelet activation or aggregation, showing that HP also prevents platelet activation (Figure 7). Additionally, the use of HP in PEMs as the non-terminated layer has the advantage of reducing its loss of bioactivity. Since HP binds to TA or CS, its high solubility is effectively minimized, thus enhancing the biomaterial surface stability.
Another barrier for successful implementation of biomedical devices is bacterial infections. When bacteria colonize on the implant surface, biofilms can be formed, which protect the bacteria from being attacked by antibiotics (Bartlet et al., 2018). Gram-negative bacteria, such as P. aeruginosa, have a thin peptidoglycan layer covered by an outer lipid membrane, while Gram-positive only possess a thicker layer of glycoprotein (Follmann et al., 2012). However, the anionic phospholipid dipalmitoyl phosphatidylglycerol is the major component of both Gram-negative and Gram-positive bacteria (Martins et al., 2014). A Gram-positive bacteria commonly related to medical device infections is S. aureus, which is typically located on human skin (Bartlet et al., 2018). P. aeruginosa is highly involved with hospital infections and increase the oxidant and inflammatory processes (Xu et al., 2019). Because the mechanisms to combat these two types of bacteria are different, it is important to investigate how the biomaterial surface interacts with both.
The results show that PEMs on NT promote significant higher growth inhibition in solution only for Gram-negative when compared to the control group (Figure 8). This could be explained by the positively charged terminated PEMs that interacts with the negatively charged bacterial membrane, eventually causing the cell death. No significant difference was observed for Gram-positive bacteria, although all surfaces promote some growth inhibition. It is important to notice that NT already showed improved antibacterial properties when compared to pure titanium, and because Gram-positive bacteria is usually less resistant than Gram-negative, less effort is required to inhibit its growth. Both TA-based PEMs on NT significantly decrease the bacteria adhesion, promoting antibacterial effect as well (Figures 9b, 11, and 12). The antiadhesive and bactericidal properties can be assigned to cationic and phenolic moieties. Flavonoid-based materials have demonstrated great antimicrobial activities toward both Gram-negative and Gram-positive bacterial cells. The mechanism is associated with the formation of complexes with both extracellular and microbial proteins (Guil-Guerrero et al., 2016; Martins et al., 2018). TA also contains ammonium moieties that can electrostatically interact with the negatively charged microbial cell membrane that contains the anionic phospholipid dipalmitoyl phosphatidylglycerol. This interaction disrupts the microbial membrane promoting permeability, leakage of intracellular materials (lactate dehydrogenase, nucleic acid, and glucose) and suppressing the nutrient transport. All these events cause the death of bacteria (Facchi et al., 2017). Of note is that CS-modified surfaces also decrease bacterial adhesion; however, compared to the TA-based PEMs, the effect was lower. Also, compared to the TA-based PEMs, CS-modified surfaces have no antithrombogenic properties. The NT(TA-HP) surface enhances the hemocompatibility, antiadhesive, and antibacterial activities of the NT surface.
5 |. CONCLUSIONS
Improving blood compatibility and antibacterial properties of medical devices is still a major concern. In this study, TA/HP PEMs on NT were fabricated, and the surfaces show excellent antifouling, antithrombogenic, and antibacterial properties. When compared to CS-based PEMs, the NT modified with TA-based PEMs impart much better biological responses. These responses are due to the chemical structure of TA (cationic tannin derivative) that is based on flavan-3-ol repeat units. Flavonoids are well-known polyphenolic materials with strong antimicrobial activities. The ammonium moieties in TA also maximize the antiadhesive and antimicrobial properties. Hemocompatibility studies demonstrated that TA/HP PEMs decrease protein adsorption and FXII activation as well as reduce platelet adhesion and activation on NT. This occurs because the TA comprises both anionic (phenolic) and cationic (ammonium) sites in its chemical structure. Modified NT surfaces with tanfloc/heparin polyelectrolyte multilayers are therefore a promising approach to enhance biocompatibility of blood-contacting implants. Future work is now directed toward investigating the dynamic interaction with blood, as well as the cell response to the surface.
ACKNOWLEDGMENTS
Research reported was supported by National Heart, Lung and Blood Institute of the National Institutes of Health under award number R01HL135505 and R21HL139208.
REFERENCES
- Almodóvar J, Mower J, Banerjee A, Sarkar AK, Ehrhart NP, & Kipper MJ (2013). Chitosan-heparin polyelectrolyte multilayers on cortical bone: Periosteum-mimetic, cytophilic, antibacterial coatings. Biotechnology and Bioengineering, 110, 609–618. [DOI] [PubMed] [Google Scholar]
- Almodóvar J, Place LW, Gogolski J, Erickson K, & Kipper MJ (2011). Layer-by-layer assembly of polysaccharide-based polyelectrolyte multilayers: A spectroscopic study of hydrophilicity, composition, and ion pairing. Biomacromolecules, 12, 2755–2765. [DOI] [PubMed] [Google Scholar]
- Bartlet K, Movafaghi S, Dasi LP, Kota AK, & Popat KC (2018). Antibacterial activity on superhydrophobic titania nanotube arrays. Colloids Surfaces B Biointerfaces, 166, 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JW, Xu L-C, Vogler EA, & Siedlecki CA (2017). Surface dependent contact activation of factor XII and blood plasma coagulation induced by mixed thiol surfaces. Biointerphases, 12, 02D410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Câmara PCF, Balaban RC, Hedayati M, Popat KC, Martins AF, & Kipper MJ (2019). Novel cationic tannin/glycosaminoglycan-based polyelectrolyte multilayers promote stem cells adhesion and proliferation. RSC Advances, 9, 25836–25846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira AC, Sabino RM, Souza PR, Muniz EC, Popat KC, Kipper MJ, … Martins AF (2020). Chitosan/gellan gum ratio content into blends modulates the scaffolding capacity of hydrogels on bone mesenchymal stem cells. Materials Science and Engineering C, 106, 110258. [DOI] [PubMed] [Google Scholar]
- Facchi DP, Lima AC, de Oliveira JH, Lazarin-Bidóia D, Nakamura CV, Canesin EA, … Martins AF (2017). Polyelectrolyte complexes based on alginate/tanfloc: Optimization, characterization and medical application. International Journal of Biological Macromolecules, 103, 129–138. [DOI] [PubMed] [Google Scholar]
- Follmann HDM, Martins AF, Gerola AP, Burgo TAL, Nakamura CV, Rubira AF, … Brazil P (2012). Antiadhesive and antibacterial multilayer films via layer-by-layer assembly of TMC/-heparin complexes. UTC, 13, 29. [DOI] [PubMed] [Google Scholar]
- Gorbet MB, & Sefton MV (2006). Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. The Biomaterials: Silver Jubilee Compendium, 25, 219–241. [DOI] [PubMed] [Google Scholar]
- Graham N, Gang F, Fowler G, & Watts M (2008). Characterisation and coagulation performance of a tannin-based cationic polymer: A preliminary assessment. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 327, 9–16. [Google Scholar]
- Guil-Guerrero JL, Ramos L, Moreno C, Zúñiga-Paredes JC, Carlosama-Yepez M, & Ruales P (2016). Antimicrobial activity of plant-food by-products: A review focusing on the tropics. Livest Sci, 189, 32–49. [Google Scholar]
- Guo Z, Bussard KM, Chatterjee K, Miller R, Vogler EA, & Siedlecki CA (2006). Mathematical modeling of material-induced blood plasma coagulation. Biomaterials, 27, 796–806. [DOI] [PubMed] [Google Scholar]
- Jaffer IH, Fredenburgh JC, Hirsh J, & Weitz JI (2015). Medical device-induced thrombosis: What causes it and how can we prevent it? Journal of Thrombosis and Haemostasis, 13, S72–S81. [DOI] [PubMed] [Google Scholar]
- Jhong J-F, Venault A, Liu L, Zheng J, Chen S-H, Higuchi A, Huang J, Chang Y. (2014). Introducing mixed-charge copolymers as wound dressing biomaterials. 6, 12, 9858–9870. [DOI] [PubMed] [Google Scholar]
- Koenig O, Neumann B, Schlensak C, Wendel HP, & Nolte A (2019). Hyaluronic acid/poly(ethylenimine) polyelectrolyte multilayer coatings for siRNA-mediated local gene silencing. Griebenow K editor. PLoS One, 14, e0212584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczak V, Smith BS, & Popat KC (2013). Hemocompatibility of polymeric nanostructured surfaces. Journal of Biomaterials Science, 24, 1529–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Romero R, Sorokina LV, Ballinger KR, Place LW, Kipper MJ, & Khetani SR (2018). A polyelectrolyte multilayer platform for investigating growth factor delivery modes in human liver cultures. Journal of Biomedical Materials Research Part A, 106, 971–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Zhang J, Wang Z, & Chen S (2011). Development of robust biocompatible silicone with high resistance to protein adsorption and bacterial adhesion. Acta Biomaterialia, 7, 2053–2059. [DOI] [PubMed] [Google Scholar]
- Liu X, Yuan L, Li D, Tang Z, Wang Y, Chen G, … Brash JL (2014). Blood compatible materials: State of the art. Journal of Materials Chemistry B, 2, 5718–5738. [DOI] [PubMed] [Google Scholar]
- Martins A, Facchi S, Follmann H, Pereira A, Rubira A, & Muniz E (2014). Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: A review. International Journal of Molecular Sciences, 15, 20800–20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins AF, Facchi SP, da Câmara PCF, Camargo SEA, Camargo CHR, Popat KC, & Kipper MJ (2018). Novel poly (ε-caprolactone)/amino-functionalized tannin electrospun membranes as scaffolds for tissue engineering. Journal of Colloid and Interface Science, 525, 21–30. [DOI] [PubMed] [Google Scholar]
- Movafaghi S, Leszczak V, Wang W, Sorkin JA, Dasi LP, Popat KC, & Kota AK (2017). Hemocompatibility of superhemophobic titania surfaces. Advanced Healthcare Materials, 6(4), 1600717. [DOI] [PubMed] [Google Scholar]
- Navarro M, Michiardi A, Castaño O, & Planell J (2008). Biomaterials in orthopaedics. Journal of the Royal Society Interface, 5, 1137–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett SD, Taylor E, Raimondo T, & Webster TJ (2010). The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials, 31, 706–713. [DOI] [PubMed] [Google Scholar]
- Ramin BBS, Rufato KB, Sabino RM, Popat KC, Kipper MJ, Martins AF, & Muniz EC (2019). Chitosan/iota-carrageenan/curcumin-based materials performed by precipitating miscible solutions prepared in ionic liquid. Journal of Molecular Liquids, 290, 111199. [Google Scholar]
- Reviakine I, Jung F, Braune S, Brash JL, Latour R, Gorbet M, & van Oeveren W (2017). Stirred, shaken, or stagnant: What goes on at the blood–biomaterial interface. Blood Reviews, 31, 11–21. [DOI] [PubMed] [Google Scholar]
- Sabino RM, Kauk K, Movafaghi S, Kota A, & Popat KC (2019). Interaction of blood plasma proteins with superhemophobic titania nanotube surfaces. Nanomedicine, 21, 102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Walker R, Romero R, Staver JM, Zang Y, Reynolds MM, Popat KC, & Kipper MJ (2017). Glycocalyx-inspired nitric oxide-releasing surfaces reduce platelet adhesion and activation on titanium. ACS Biomaterials Science & Engineering, 3, 68–77. [DOI] [PubMed] [Google Scholar]
- Smith BS, Capellato P, Kelley S, Gonzalez-Juarrero M, & Popat KC (2013). Reduced in vitro immune response on titania nanotube arrays compared to titanium surface. Biomaterials Science, 1, 322–332. [DOI] [PubMed] [Google Scholar]
- Smith BS, & Popat KC (2012). Titania nanotube arrays as interfaces for blood-contacting implantable devices. A study evaluating the nanotopography-associated activation and expression of blood plasma components. Journal of Biomedical Nanotechnology, 8(4), 642–658. [DOI] [PubMed] [Google Scholar]
- Sperling C, Fischer M, Maitz MF, & Werner C (2009). Blood coagulation on biomaterials requires the combination of distinct activation processes. Biomaterials, 30, 4447–4456. [DOI] [PubMed] [Google Scholar]
- van Gorp ECM, Suharti C, ten Cate H, Dolmans WMV, van der Meer JWM, ten Cate JW, & Brandjes DPM (1999). Review: Infectious diseases and coagulation disorders. The Journal of Infectious Diseases, 180, 176–186. [DOI] [PubMed] [Google Scholar]
- Vogler EA, & Siedlecki CA (2010). Contact activation of blood plasma coagulation: A contribuition from the hematology at biomaterial Interferances. Biomaterials, 30, 1857–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells LA, Guo H, Emili A, & Sefton MV (2017). The profile of adsorbed plasma and serum proteins on methacrylic acid copolymer beads: Effect on complement activation. Biomaterials, 118, 74–83. [DOI] [PubMed] [Google Scholar]
- Xu LC, Bauer JW, & Siedlecki CA (2014). Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surfaces B Biointerfaces., 124, 49–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L-C, Meyerhoff ME, & Siedlecki CA (2019). Blood coagulation response and bacterial adhesion to biomimetic polyurethane biomaterials prepared with surface texturing and nitric oxide release. Acta Biomaterialia, 84, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Xu L-C, Vogler EA, & Siedlecki CA (2018). Contact activation by the intrinsic pathway of blood plasma coagulation. Hemocompatibility of Biomaterials for Clinical Applications, 3, 28. [Google Scholar]
- Zhang K, ying CJ, Qin W, an LJ, xia GF, & Huang N (2016). Constructing bio-layer of heparin and type IV collagen on titanium surface for improving its endothelialization and blood compatibility. Journal of Materials Science. Materials in Medicine, 27, 81. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Li J, Yao X, & Tang B (2018). Surface immobilization of heparin and chitosan on titanium to improve hemocompatibility and antibacterial activities. Colloids Surfaces B Biointerfaces, 172, 338–345. [DOI] [PubMed] [Google Scholar]
- Zomer Volpato F, Almodóvar J, Erickson K, Popat KC, Migliaresi C, & Kipper MJ (2012). Preservation of FGF-2 bioactivity using heparin-based nanoparticles, and their delivery from electrospun chitosan fibers. Acta Biomaterialia, 8, 1551–1559. [DOI] [PubMed] [Google Scholar]


