Abstract
Background and objective
Acute pancreatitis (AP) is a frequent cause of hospitalization for gastrointestinal issues, with a significant proportion of cases requiring intensive care. Although various scoring systems are available to predict AP severity, they often involve inconvenience and can be time-consuming and expensive. Hematocrit, a simple, cost-effective, readily available hematological test, has been used to predict AP severity. However, its effectiveness has been inconsistent across different studies. In light of this, we aimed to analyze the role of hematocrit levels in determining AP severity.
Methods
We conducted a prospective study at Patan Hospital in Lalitpur, Nepal, from June 8, 2022, to June 27, 2023. Sixty-five AP patients were evaluated to determine the prognostic value of hematocrit at admission. The severity of AP was classified per the Revised Atlanta Classification.
Results
Among the patients, 52 (80%) had mild AP (MAP), five (7.69%) had moderately severe AP (MSAP), and eight (12.31%) had severe AP (SAP). The receiver operating characteristic (ROC) curve for admission hematocrit levels yielded an area under the curve (AUC) of 0.551 (95% CI: 0.423-0.675). A hematocrit cutoff value of 42% resulted in a sensitivity of 69.23% and a specificity of 46.15% for predicting severe AP (MSAP + SAP).
Conclusions
Based on our findings, hematocrit at admission is not a strong predictor of the severity of AP.
Keywords: prognostic value, severity prediction, revised atlanta classification, acute pancreatitis, hematocrit
Introduction
Acute pancreatitis (AP) is a significant global health issue, ranking high among gastrointestinal conditions that require hospitalization [1]. In 2019, the age-standardized incidence and mortality rates per 100,000 people with AP were 34.8 and 1.4 globally, 55.3 and 1.8 for South Asia, and 25.9 and 2.5 for Nepal [2]. Over the past two decades, The global incidence and hospitalization rates of AP have been on the rise. While the overall mortality for AP is approximately 1%, it can rise to a staggering 30-40% in hospitalized patients with organ failure or pancreatic necrosis [1,3-5].
AP is generally not a severe condition, with most patients (80-85%) experiencing a mild disease. However, around 20% of patients develop moderate to severe AP [6]. The severity of pancreatitis has been classified based on the Revised Atlanta Classification, as follows: mild acute pancreatitis (MAP) - does not involve organ failure or local or systemic complications; moderately severe acute pancreatitis (MSAP) - characterized by organ failure that resolves within 48 hours, local or systemic complications without persistent organ failure, or both; and severe acute pancreatitis (SAP) - marked by persistent (>48 h) organ failure that can involve either single or multiple organs [7,8,9].
Various scoring systems have been developed to predict the severity of AP, but they often prove to be cumbersome, time-consuming, or expensive. In contrast, hematocrit is a simple, cost-effective, and readily available hematological test that has shown promise in predicting the severity of AP. However, the results from different studies on hematocrit have been inconsistent [10-14]. This study, therefore, holds significant potential for contributing to the understanding of the severity of AP and the potential role of hematocrit levels in its diagnosis.
Materials and methods
Study design and setting
This was a cross-sectional (prospective, analytical, single hospital-based) study conducted at the Patan Academy of Health Sciences, Nepal, from June 8, 2022, to June 27, 2023. Patients older than 14 years with a diagnosis of AP were included. Patients with chronic liver disease, chronic kidney disease, chronic obstructive pulmonary disease, or hematological malignancies and those unwilling to participate in the study were excluded. The study was conducted after obtaining approval from the Institutional Review Committee (IRC) of PAHS (IRC no: PMM2206031633). Informed written consent was obtained from the participants or their guardians. Participants were informed of the study's details, benefits, and potential risks and were allowed to withdraw without citing any reason; patient confidentiality was ensured, and the data were collected on a structured proforma (Appendices)
Acute pancreatitis diagnosis
AP was diagnosed if two of the following criteria were present: severe, persistent epigastric pain (often radiating to the back); serum lipase or amylase levels three times above normal; or characteristic findings on ultrasound, CT, or MRI [8]. AP patients were classified as mild, moderately severe, or severe based on the Revised Atlanta Classification [8,9].
Sampling and statistical analysis
The sample size calculation was based on the statistical analysis of Obuchowski et al. [15]. In the study conducted by Bohara et al., the area under the ROC curve for hematocrit at admission was 0.713 (95% CI: 0.536 - 0.889); the exact value of the area under the ROC curve was used to calculate the sample size [16]. The sample size calculated using the above formula through easy ROC statistical software was 65: 13 patients and 52 controls. Cases were defined as patients diagnosed with either MSAP or SAP and controls were defined as patients with MAP according to the Revised Atlanta Classification.
The data were entered and analyzed using EPI-INFO. ROC analysis was performed using the software EZR. ROC curves were generated to assess admission hematocrit levels as a predictor of acute pancreatitis severity, with the revised Atlanta Classification serving as the gold standard for severity. Categorical variables (sex, age) are presented as numbers and percentages (%), and continuous variables (length of hospital stay) are presented as the mean ± standard deviation (SD), and median and interquartile range (IQR).
Results
Sixty-five patients meeting the inclusion criteria were enrolled in the study. Among them, 52 (80%) were diagnosed with MAP, five (7.69%) with MSAP, and eight (12.31%) with SAP. The patients' ages ranged from 21 to 79, with a median age of 40 (95% CI: 38.00-44.87 years). Regarding sex distribution, 56 patients were male, while nine were female. The median duration of hospital stay for AP patients in our study was four days (95% CI: 3-4 days). The median duration of hospital stay for patients with mild, moderately severe, and severe pancreatitis were 3.5 days, 11 days, and 7.5 days, respectively (Table 1).
Table 1. Characteristics and findings in the study population.
AP: acute pancreatitis; MAP: mild acute pancreatitis; MSAP: moderately severe acute pancreatitis; SAP: severe acute pancreatitis
| Severity of AP | No. of patients | Sex | Median hematocrit, % | Median duration of hospital stay, days | |
| Male | Female | ||||
| MAP | 52 | 45 | 7 | 42 | 3.5 |
| MSAP | 5 | 4 | 1 | 35 | 11.0 |
| SAP | 8 | 7 | 1 | 42 | 7.5 |
| Total | 65 | 56 | 9 | ||
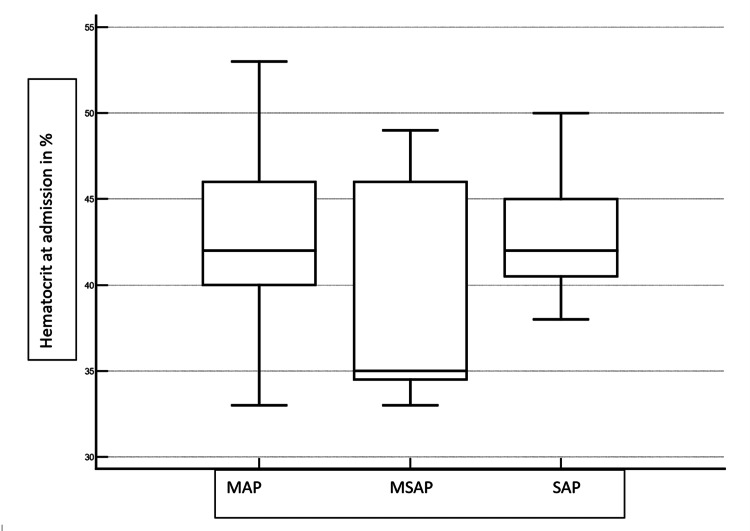
The median hematocrit levels for mild, moderately severe, and severe pancreatitis in the present study were 42%, 35%, and 42%, respectively (Figure 1).
Figure 1. Hematocrit levels with severity of AP.
AP: acute pancreatitis; MAP: mild acute pancreatitis; MSAP: moderately severe acute pancreatitis; SAP: severe acute pancreatitis
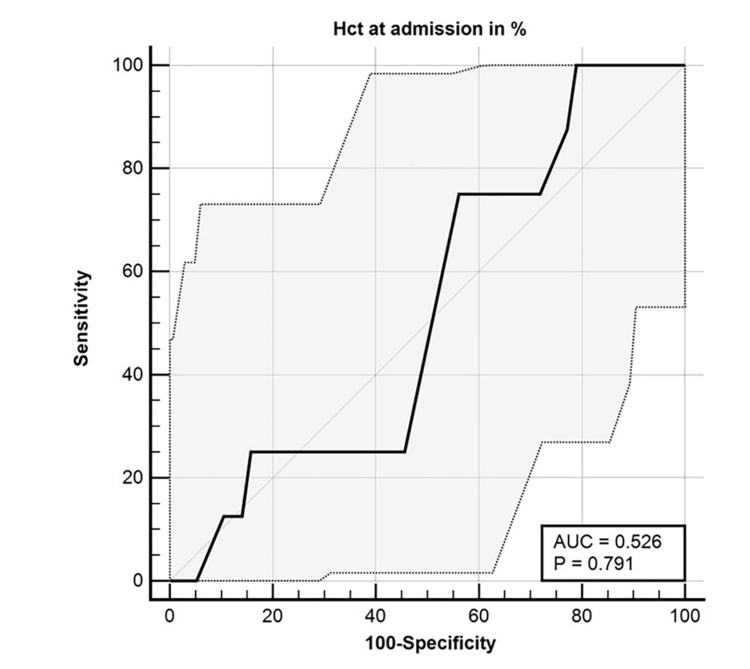
The ROC curve analysis indicated an area of 0.526 for admission hematocrit, with a 95% CI of 0.399 to 0.652. The hematocrit cutoff value was 37%, the sensitivity was 100%, and the specificity was 21.05% for using hematocrit at admission as a predictor of SAP (Figure 2).
Figure 2. ROC curve for hematocrit at admission as a predictor of SAP.
AUC: area under the ROC curve; Hct: hematocrit; ROC: receiver operating characteristic; SAP: severe acute pancreatitis
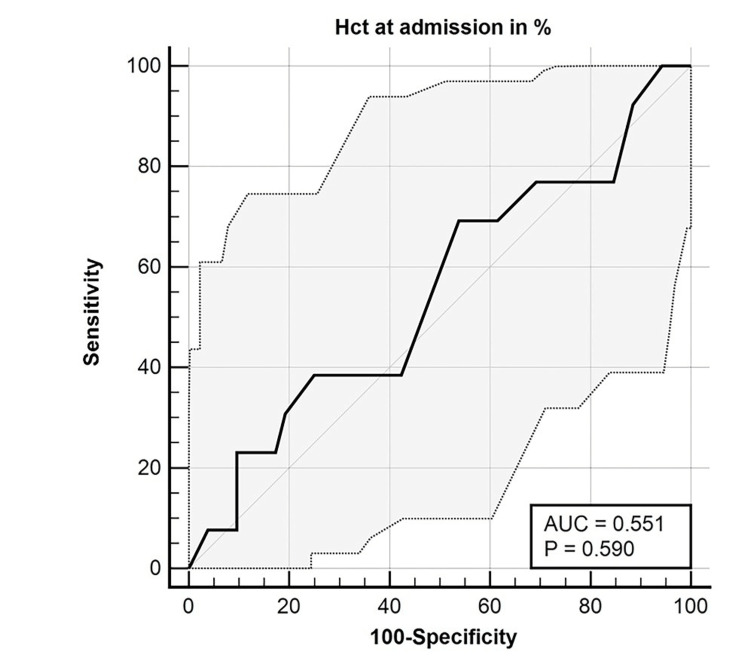
The ROC curve analysis revealed an area of 0.551 for admission hematocrit, with a 95% CI of 0.423 to 0.675. The hematocrit cutoff value was 42%, the sensitivity was 69.23%, and the specificity was 46.15% for using hematocrit at admission as a predictor of severity of AP (MSAP + SAP) (Figure 3).
Figure 3. ROC curve for admission hematocrit predicting AP severity (MSAP + SAP).
AP: acute pancreatitis; AUC: area under the ROC curve; MSAP: moderately severe acute pancreatitis; ROC: receiver operating characteristic; SAP: severe acute pancreatitis
Discussion
In our study, we observed a median age of 40 years among AP patients, with a notable male predominance of 6.22:1. This finding contrasts with the study by Bohara et al., which reported a mean age of 50.16 years and a nearly equal gender distribution (male-to-female ratio: 1.06:1) [16]. Similarly, Bhattarai et al. found a mean age of 44 and a lower male-to-female ratio of 2.3:1 [17]. The male dominance noted in our study and Bhattarai et al.'s work may be attributed to higher alcohol consumption among males in Nepal [18]. In contrast, studies conducted outside Nepal, such as those at the University Hospital of the West Indies and the Mayo Clinic, have reported a female predominance and nearly equal gender distribution, respectively [19,20]. These regional variations suggest that factors such as alcohol consumption significantly influence the demographics of AP.
Our findings indicate that the median hospital stay for AP patients was four days, with a mean stay of 5.14 days. The duration of hospitalization varied by disease severity, as mild cases experienced shorter stays. This is consistent with findings from the Mayo Clinic study, which reported a comparable mean hospital stay, although severe AP cases had longer durations [20]. Conversely, Bhattarai et al. noted a higher mean stay of 12 days, which could be due to differences in patient management or disease severity [17]. A similar trend was observed in a study from the West Indies, indicating that regional healthcare practices, patient severity, and treatment protocols play crucial roles in determining hospital stay length [19].
Our study found that 80% of patients were diagnosed with MAP, with fewer cases of MSAP (7.69%) and SAP (12.31%). These findings align closely with Bhattarai et al., where 72.6% of patients had MAP, 9.7% had MSAP, and 17.7% had SAP [17]. In contrast, Bohara et al. reported a lower prevalence of MAP (48.4%) and a higher proportion of moderately severe cases (38.7%) [16]. The study by Reid et al. from the West Indies observed mild disease in 61.1% of patients, with moderately severe and severe cases comprising 26.7% and 12.2%, respectively [19]. The Mayo Clinic reported an even higher incidence of mild cases (84%) and lower rates of severe cases (3%), reflecting a more favorable patient profile [20].
Regarding the predictive value of hematocrit at admission, our study found that it was not a strong predictor of SAP. The area under the ROC curve (AUC) for hematocrit at admission was lower than that reported by Bohara et al., who found it to be a more reliable predictor of severity in their cohort [16]. Notably, Bohara et al. indicated that hematocrit at 24 hours was a much stronger predictor of severity, a parameter not evaluated in our study [16]. This comparison underscores the variability in the predictive value of hematocrit across different studies, suggesting that other factors may influence its reliability as a predictor of disease severity.
The findings from a post hoc analysis of three large prospective databases indicate that an admission hematocrit level of ≥44% is a significant predictor of persistent organ failure (POF) in patients with SAP, with an AUC of 0.67, a sensitivity of 59.16%, and a specificity of 74.24% [11]. These results align with the association observed between elevated hematocrit levels and adverse outcomes, such as pancreatic necrosis and prolonged hospitalization. The literature notes a range of proposed cutoff values for hematocrit across different studies, with 44% being the most prevalent, highlighting the importance of standardized thresholds for clinical decision-making [21-23]. Furthermore, the emphasis on adequate fluid resuscitation to reduce mortality and promote hematocrit reduction at 48 hours underscores the need for goal-directed therapy in managing AP [24]. While our study focuses solely on admission hematocrit levels, the post hoc analysis suggests that combining admission hematocrit with other severity predictors, such as blood urea nitrogen (BUN), could enhance the accuracy of predicting complications.
Our study offers valuable insights into the demographic characteristics and disease severity of acute pancreatitis in a specific population in Nepal, addressing a notable gap in the existing literature. By comparing our findings with previous studies, such as those by Bohara et al. and Bhattarai et al., we contextualize our results and highlight the influence of regional factors on disease presentation and management. Additionally, our investigation into the role of hematocrit as a predictor of severe acute pancreatitis, despite its limited predictive value, contributes to understanding this parameter and opens new avenues for future research on other predictive factors.
Despite providing these insightful findings, our study has several limitations. The reliance on admission hematocrit alone may overlook rapid disease progression in some patients. Additionally, the single-center study design with a small sample size limits the generalizability of our results. Furthermore, we did not assess changes in hematocrit over time or evaluate morbidity and mortality related to acute pancreatitis, both of which are essential for understanding the full impact of the disease. Addressing these limitations in future research could provide a more comprehensive understanding of acute pancreatitis and its clinical implications.
Conclusions
Our study showed that hematocrit at admission is not a strong predictor of the severity of acute pancreatitis. However, multicenter studies with large sample sizes are recommended to validate our findings. Studies using admission hematocrit combined with other predictors of the severity of AP would be relevant and provide deeper insights.
Acknowledgments
The authors would like to thank the staff at the Department of Internal Medicine, Patan Hospital, and all the participants in this study for their assistance in the research project.
Appendices
Data collection form
Name
Age of the patient
Gender
Hospital number
Date of admission (DD/MM/YYYY)
Date of discharge/death (DD/MM/YYYY)
Duration of hospital stay (days)
Vitals (at first encounter at the emergency department)
a) Blood pressure (systolic/diastolic, mmHg)
b) Respiratory rate (breaths/min)
c) Temperature (°F)
d) Heart rate (beats/min)
e) SpO2 (%)
Laboratory Parameters
a) Total leucocyte count (TLC)
b) Hct at admission (%)
c) Creatinine (mg/dl)
Atrial Blood Gas Analysis (ABG)
a) pH
b) paO2
c) FiO2
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Institutional Review Committee (IRC)-PAHS issued approval PMM2206031633.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Milan Dhungana, Subodh Kumar Bidari, Ram Chandra Panthi, Kushal Raj Joshi, Mipsang Lama, Gyan Krishna Kayastha, Ritika Shrestha, Dinesh Neupane, Gurbi Khanal
Acquisition, analysis, or interpretation of data: Milan Dhungana, Subodh Kumar Bidari, Ram Chandra Panthi, Kushal Raj Joshi, Ritika Shrestha, Dinesh Neupane, Gurbi Khanal
Drafting of the manuscript: Milan Dhungana, Subodh Kumar Bidari, Ram Chandra Panthi, Kushal Raj Joshi, Mipsang Lama, Ritika Shrestha, Dinesh Neupane, Gurbi Khanal
Critical review of the manuscript for important intellectual content: Milan Dhungana, Subodh Kumar Bidari, Ram Chandra Panthi, Kushal Raj Joshi, Gyan Krishna Kayastha, Dinesh Neupane, Gurbi Khanal
Supervision: Milan Dhungana, Subodh Kumar Bidari, Ram Chandra Panthi, Kushal Raj Joshi, Mipsang Lama, Gyan Krishna Kayastha, Ritika Shrestha
References
- 1.Acute pancreatitis. Forsmark CE, Vege SS, Wilcox CM. N Engl J Med. 2016;375:1972–1981. doi: 10.1056/NEJMra1505202. [DOI] [PubMed] [Google Scholar]
- 2.The global, regional, and national burden of acute pancreatitis in 204 countries and territories, 1990-2019. Li CL, Jiang M, Pan CQ, Li J, Xu LG. BMC Gastroenterol. 2021;21:332. doi: 10.1186/s12876-021-01906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A population-based cohort study on risk factors for acute pancreatitis: a comparison by age group. Park JM, Park N, Lee SH, et al. Pancreatology. 2023;23:321–329. doi: 10.1016/j.pan.2023.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Iannuzzi JP, King JA, Leong JH, et al. Gastroenterology. 2022;162:122–134. doi: 10.1053/j.gastro.2021.09.043. [DOI] [PubMed] [Google Scholar]
- 5.The incidence and aetiology of acute pancreatitis across Europe. Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. Pancreatology. 2017;17:155–165. doi: 10.1016/j.pan.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Executive summary: WSES Guidelines for the management of severe acute pancreatitis. Leppäniemi A, Tolonen M, Tarasconi A, et al. J Trauma Acute Care Surg. 2020;88:888–890. doi: 10.1097/TA.0000000000002691. [DOI] [PubMed] [Google Scholar]
- 7.Acute pancreatitis. Boxhoorn L, Voermans RP, Bouwense SA, et al. Lancet. 2020;396:726–734. doi: 10.1016/S0140-6736(20)31310-6. [DOI] [PubMed] [Google Scholar]
- 8.Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Banks PA, Bollen TL, Dervenis C, et al. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 9.Acute pancreatitis: a review. Mederos MA, Reber HA, Girgis MD. JAMA. 2021;325:382–390. doi: 10.1001/jama.2020.20317. [DOI] [PubMed] [Google Scholar]
- 10.IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:0–15. doi: 10.1016/j.pan.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 11.Admission hematocrit and rise in blood urea nitrogen at 24 h outperform other laboratory markers in predicting persistent organ failure and pancreatic necrosis in acute pancreatitis: a post hoc analysis of three large prospective databases. Koutroumpakis E, Wu BU, Bakker OJ, et al. Am J Gastroenterol. 2015;110:1707–1716. doi: 10.1038/ajg.2015.370. [DOI] [PubMed] [Google Scholar]
- 12.The predictive value of C-reactive protein (CRP) in acute pancreatitis - is interval change in CRP an additional indicator of severity? Stirling AD, Moran NR, Kelly ME, Ridgway PF, Conlon KC. HPB (Oxford) 2017;19:874–880. doi: 10.1016/j.hpb.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Admission hematocrit: a simple, useful and early predictor of severe pancreatitis. Gan SI, Romagnuolo J. Dig Dis Sci. 2004;49:1946–1952. doi: 10.1007/s10620-004-9598-8. [DOI] [PubMed] [Google Scholar]
- 14.Early prediction of severity of acute pancreatitis using hematocrit level on admission. Bajec A, Radenkovic D, Bajec D, Gregoric P, Levicanin M. Pancreatology. 2014;14:61–62. [Google Scholar]
- 15.ROC curves in clinical chemistry: uses, misuses, and possible solutions. Obuchowski NA, Lieber ML, Wians FH Jr. Clin Chem. 2004;50:1118–1125. doi: 10.1373/clinchem.2004.031823. [DOI] [PubMed] [Google Scholar]
- 16.Hemoconcentration as a predictor of severity in acute pancreatitis. Bohara TP, Karki D, Parajuli A, Rupakheti S, Joshi MR. J Kathmandu Med Coll. 2015;4:3–7. [Google Scholar]
- 17.Clinical profile and outcomes in patients with acute pancreatitis attending a teaching hospital at Gandaki Province, Nepal. Bhattarai S, Gyawali M. JCMS Nepal. 2020;4:16–18. [Google Scholar]
- 18.Prevalence of non-communicable diseases risk factors and their determinants: results from STEPS survey 2019, Nepal. Bista B, Dhimal M, Bhattarai S, et al. PLoS One. 2021;16:0. doi: 10.1371/journal.pone.0253605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acute pancreatitis: a 7 year retrospective cohort study of the epidemiology, aetiology and outcome from a tertiary hospital in Jamaica. Reid GP, Williams EW, Francis DK, Lee MG. Ann Med Surg (Lond) 2017;20:103–108. doi: 10.1016/j.amsu.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Predictors and outcomes of moderately severe acute pancreatitis - evidence to reclassify. Kwong WT, Ondrejková A, Vege SS. Pancreatology. 2016;16:940–945. doi: 10.1016/j.pan.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Hemoconcentration as an early risk factor for necrotizing pancreatitis. Baillargeon JD, Orav J, Ramagopal V, Tenner SM, Banks PA. Am J Gastroenterol. 1998;93:2130–2134. doi: 10.1111/j.1572-0241.1998.00608.x. [DOI] [PubMed] [Google Scholar]
- 22.Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Brown A, Orav J, Banks PA. Pancreas. 2000;20:367–372. doi: 10.1097/00006676-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Hemoconcentration: an early marker of severe and/or necrotizing pancreatitis? A critical appraisal. Lankisch PG, Mahlke R, Blum T, Bruns A, Bruns D, Maisonneuve P, Lowenfels AB. Am J Gastroenterol. 2001;96:2081–2085. doi: 10.1111/j.1572-0241.2001.03966.x. [DOI] [PubMed] [Google Scholar]
- 24.Revisiting the Ranson score in acute pancreatitis: Is the drop in hematocrit a worrisome sign? Acehan F, Tez M, Kalkan C, et al. J Hepatobiliary Pancreat Sci. 2023;30:315–324. doi: 10.1002/jhbp.1200. [DOI] [PubMed] [Google Scholar]