Abstract
Background
Despite advances made in curbing the global malaria burden since the 2000s, progress has stalled, in part due to a plateauing of the financing available to implement needed interventions. In 2020, approximately 3.3 billion USD was invested globally for malaria interventions, falling short of the targeted 6.8 billion USD set by the GTS, increasing the financial gap between desirable and actual investment. Models for malaria control optimization are used to disentangle the most efficient interventions or packages of interventions for inherently constrained budgets. This systematic review aimed to identify and characterise models for malaria control optimization for resource allocation in limited resource settings and assess their strengths and limitations.
Methods
Following the Prospective Register of Systematic Reviews and Preferred reporting Items for Systematic Reviews and Meta-Analysis guidelines, a comprehensive search across PubMed and Embase databases was performed of peer-reviewed literature published from inception until June 2024. The following keywords were used: optimization model; malaria; control interventions; elimination interventions. Editorials, commentaries, opinion papers, conference abstracts, media reports, letters, bulletins, pre-prints, grey literature, non-English language studies, systematic reviews and meta-analyses were excluded from the search.
Results
The search yielded 2950 records, of which 15 met the inclusion criteria. The studies were carried out mainly in countries in Africa (53.3%), such as Ghana, Nigeria, Tanzania, Uganda, and countries in Asia (26.7%), such as Thailand and Myanmar. The most used interventions for analyses were insecticide-treated bed nets (93.3%), IRS (80.0%), Seasonal Malaria Chemoprevention (33.3%) and Case management (33.3%). The methods used for estimating health benefits were compartmental models (40.0%), individual-based models (40.0%), static models (13.0%) and linear regression model (7%). Data used in the analysis were validated country-specific data (60.0%) or non-country-specific data (40.0%) and were analysed at national only (40.0%), national and subnational levels (46.7%), or subnational only levels (13.3%).
Conclusion
This review identified available optimization models for malaria resource allocation. The findings highlighted the need for country-specific analysis for malaria control optimization, the use of country-specific epidemiological and cost data in performing modelling analyses, performing cost sensitivity analyses and defining the perspective for the analysis, with an emphasis on subnational tailoring for data collection and analysis for more accurate and good quality results. It is critical that the future modelling efforts account for fairness and target at risk malaria populations that are hard-to-reach to maximize impact.
Trial registration: PROSPERO Registration number: CRD42023436966
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-024-05118-3.
Keywords: Optimization, Malaria, Resource allocation, Limited resource setting
Background
Malaria persists as a global health challenge, particularly in low- and middle-income countries (LMICs). The burden of malaria is concentrated in sub-Saharan Africa, contributing to over 95% of global cases [1]. Ten African countries were labelled as "High Burden to High Impact" (HBHI) in 2017 due to their substantial contribution to the global burden [1, 2]. Despite progress in reducing the disease burden from 81 cases per 1000 population at risk in 2000 to 59 cases in 2015, advancements have stalled following the plateauing of deployed resources [1].
The World Health Organization (WHO) has set ambitious targets outlined in the Global Technical Strategy (GTS), aiming to reduce malaria cases and deaths by at least 75% by 2025 and 90% by 2030, compared to 2015 [3]. To meet these targets, the WHO recommends: Prevention, involving interventions such as mass distribution of insecticide-treated bed nets (ITNs), indoor residual spraying (IRS), larviciding, intermittent preventive treatment in pregnancy (IPTp) and infants (IPTi, now PMC), seasonal malaria chemoprevention (SMC); as well as case management, focusing on the diagnosis and treatment of malaria cases at the health facility and community levels [3–5]. Countries through their national control or elimination programmes try to align with these global strategies through country-specific national strategic plans, adopting and implementing country-specific interventions at the national and subnational levels [6, 7].
Scaling up malaria interventions to achieve GTS targets necessitates significant financial support globally and domestically. In 2020, approximately 3.3 billion USD were invested globally for malaria interventions [1], falling short of the targeted 6.8 billion USD set by the GTS, increasing the financial gap between desirable and actual investment [1, 3]. The financial gap poses a significant risk of resurgence, potentially leading to billions of avertable malaria cases and deaths, and costing over 5 billion USD to health systems and communities by 2030 [8]. In 2020, although majority of the global investment for malaria came from international donors, about 33% of investments within countries came from domestic (government) funding [1]. These financial constraints are felt most in LMICs, and a lack of sufficient evidence on country-specific financial costs and effects of different interventions makes it difficult to determine the true efficiency of these interventions [9]. Also, in order to ensure epidemiological and economic efficiency, subnational tailoring needs to be taken into consideration to improve efficiency of interventions [10, 11]. The most efficient interventions or packages of interventions refer to strategies or combinations of strategies that are chosen based on careful analysis of costs, benefits, and contextual factors to achieve the maximum possible reduction in malaria burden with the resources available [12]. It would, therefore, be imperative that models for malaria control optimization are used to disentangle the most efficient interventions or packages of interventions for inherently constrained budget(s). Disease-specific models for optimization have been systematically reviewed for other diseases such as HIV/AIDS [13], but not for malaria, showing the need to understand what models for malaria control optimization are available in the literature.
Mathematical models in malaria research serve several key purposes and are extensively employed to simulate and understand the transmission dynamics of the disease [14]; by simulating the effects of various interventions to assess their impact and inform strategic planning for control and elimination efforts [15], for example in evaluating the cost-effectiveness of different strategies and identifying the most promising combinations of interventions [16]. One significant application of mathematical modelling in malaria is the optimization of intervention strategies. Optimization in the context of malaria modelling refers to the use of mathematical techniques to identify the best possible strategies or determine the optimal mix and coverage levels of interventions or the geographic targeting of resources for achieving specific objectives, such as reducing malaria transmission or minimizing costs [10, 16–18]. The optimization process typically involves defining the following: an objective function that represents the goal of the optimization such as to minimize the number of malaria cases or deaths; constraints such as a budget constraint which are the limitations or restrictions considered in the optimization process; and optimization techniques such as linear programming, or integer programming that are used to solve the optimization problem given the objective function and constraints [13, 19]. While mathematical models provide valuable insights and theoretical optimization solutions, implementing these solutions in the real world requires consideration of several practical factors:
-
(i)
The implementation of optimized malaria strategies often needs to align with national health policies, priorities, and political realities. For instance, there might be political resistance to certain interventions or a preference for locally developed strategies over those recommended by external modelling efforts [2].
-
(ii)
Optimized strategies derived from models must also be feasible from a logistical standpoint. This includes the availability of resources, infrastructure, and personnel to carry out the interventions effectively. Real-world constraints such as supply chain issues, geographical barriers, and weather conditions can significantly affect the feasibility and effectiveness of optimized plans [20, 21].
-
(iii)
Community acceptance and adherence to interventions are crucial for their success. Optimized strategies must consider local cultural practices, beliefs, and social dynamics that could influence the uptake of interventions like ITNs or IRS [22, 23].
-
(iv)
Real-world conditions are often dynamic and unpredictable. Optimization strategies need to be flexible and adaptable to changing conditions, such as shifts in malaria transmission patterns due to climate change or evolving resistance to anti-malarial drugs [24].
To optimize resource allocation for malaria control, various mathematical models have been employed in combination with economic/cost models. These include individual-based mathematical models [25–27], a geospatial dynamic transmission epidemic model [16] and compartmental transmission models [28, 29]. Although one study in Senegal demonstrated the impact of improving allocative efficiency to scale up malaria intervention packages, no model was used. Rather, they aggregated annual cost estimates of the considered intervention packages to provide data for better programmatic decision-making [30]. While some studies implemented their models across multiple countries in Africa [25, 26] and Asia [29], others were country specific [16, 28]. Within the latter subset, some performed subnational data analysis, emphasising subnational tailoring in order to account for context-specific drivers of intervention efficacy and thus achieving more precise results.
A systematic literature review is presented here, to identify available models for malaria control optimization for resource allocation in limited resource settings, determine how they have been used and to assess them for quality and utility.
The research question for this review was “Are there English-language publications in peer-reviewed scientific journals describing how models have been used to allocate resources for malaria interventions in limited resource settings?” The main aim of this review was to identify and characterize models for malaria control optimization for resource allocation in malaria control and elimination settings and to identify their strengths and limitations.
Methods
A systematic search of peer-reviewed literature published from inception until June 2024 was conducted. The Preferred reporting Items for Systematic Reviews and Meta-Analysis protocols (PRISMA) 2020 guidelines were used to report the findings (Additional file 1). The protocol for the systematic review was registered with the international Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42023436966. Amendments made to the protocol were documented and justified accordingly.
Search strategy
The databases PubMed and Embase (OVID) were searched for relevant studies using the following keywords: optimization model; malaria; control interventions; elimination interventions; and MeSH terms: resource allocation, models, linear programming, malaria, Plasmodium, communicable disease control, disease eradication. A detailed list of all search terms and results are available in Additional file 2. The search was run by the principal investigator. For each MeSH term and corresponding keyword, articles were sought by performing a title and abstract search on associated search terms. The results from the search of each MeSH term and corresponding keyword were combined exclusively using the Boolean operator ‘OR’. The final products of each keyword search were then combined using the Boolean operator ‘AND’ (Table 1). Records returned by the search were saved using the EndNote reference management software. Each record was screened by two independent reviewers using the Rayyan software. The screening process involved a review of the titles and abstracts of each record to identify potentially eligible records and exclude the records which were out of the scope of this review. The two reviewers then reviewed the full texts of the remaining records to identify eligible records for inclusion in the review. At the end of each stage, the reviewers discussed their findings to ensure uniformity and reviewed any discordances. A third reviewer was consulted in case of failure to resolve any discordances between the two reviewers. A list of all studies excluded at each stage of the screening process and the reasons for exclusion was made using the Rayyan software [31].
Table 1.
Search strategy – models for malaria control optimization
| # | Block | Search words |
|---|---|---|
| 1 | Optimization model | Resource allocat* OR allocative efficiency OR investment case OR dynamic model* OR programming OR dynamic programming OR dynamic analysis OR linear model* OR linear programming OR nonlinear model* OR nonlinear programming OR integer model* OR integer programming OR optimization AND model* OR optimization model* OR decision model* OR mathematical model* OR compartmental model* OR transmission model* OR agent-based model* OR individual based model* |
| 2 | Malaria | Malaria OR Plasmodium |
| 3 | Interventions | Control OR control interventions OR elimination OR pre-elimination OR elimination interventions OR eradication |
| 4 | #1 AND #2 AND #3 |
Inclusion criteria
Each record in the search were included if they met all of the following criteria: (1) Were scientific peer-reviewed journals written in English; (2) Were published before 30th June 2024; (3) Studies were included irrespective of the targeted geographical regions; (4) Studies were included irrespective of the population subgroups; (5) Studies containing an optimization model (mathematical or statistical); (6) All studies with human Plasmodium species; (7) Studies with two or more interventions; (8) Cost data were used in combination with the model outputs; (9) Outcomes/health benefits were clearly stated and/or measured.
Exclusion criteria
The following records were excluded: (1) Editorials, commentaries, opinion papers, conference abstracts, media reports, letters, bulletins, pre-prints, grey literature; (2) Non-English language studies; (3) Systematic reviews, meta-analyses.
Data extraction and synthesis
Data including (1) the first author and publication year; (2) geographic focus; (3) interventions used in analysis; (4) administrative level in the analysis; (5) populations considered; (6) time horizon of analysis; (7) method used to estimate health benefits (model structure); (8) Plasmodium species; (9) types of constraints; (10) data sources; (11) optimization goal; (12) epidemiological optimization, (13) cost optimization, (14) estimated time to elimination; (15) equity considerations in resource allocation; (16) conclusion of the article; were extracted from the selected studies into a Microsoft Excel Office 365 spreadsheet (Additional file 3). Missing or unclear data which were considered relevant triggered an email query to the corresponding authors of the respective studies and any additional information was included in the data extraction sheet if provided. All extracted data were double checked for errors by a second independent reviewer (SP) and discrepancies in entries were settled by appropriate discussion among both reviewers (RN and SP). A narrative synthesis of the study characteristics was performed.
Quality assessment
The quality of all included studies was assessed using the joint International Society for Pharmacoeconomics and Outcomes Research-Society for Medical Decision Making Modelling Good Research Practices Task Force (ISPOR) [19]. The following criteria from ISPOR were used for quality assessment: conceptualising the model, dynamic transmission models, parameter estimation and uncertainty, and model transparency and validation (Additional file 4).
Results
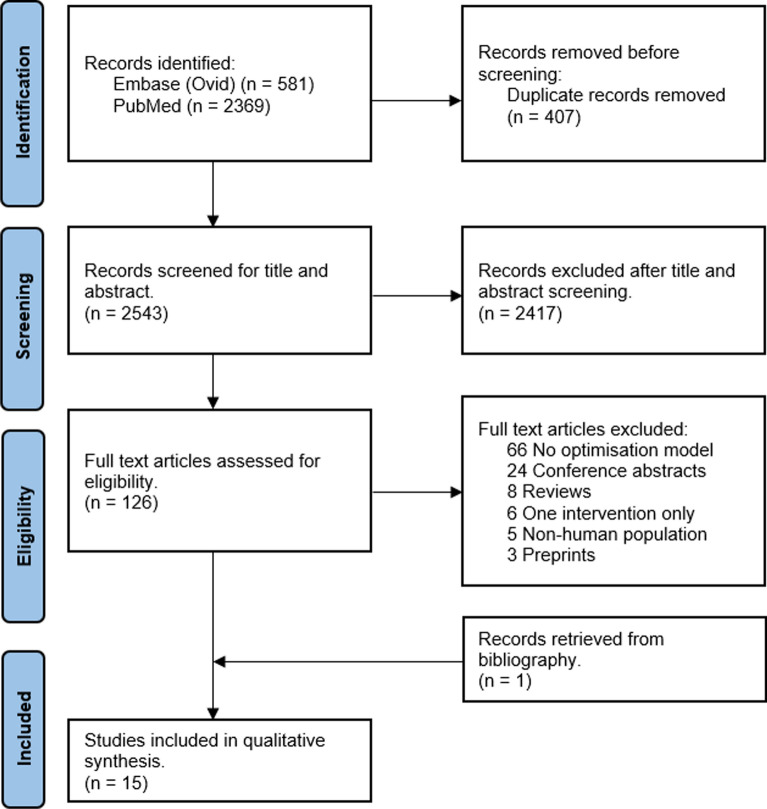
A total of 2950 articles were identified from the search. When duplicates were removed, 2543 articles were screened for titles and abstracts, with 126 full-text articles assessed for eligibility. In total, 14 articles from the database search and 1 article from bibliography were included in the final analysis (Fig. 1).
Fig. 1.
PRISMA diagram
The majority of modelling analyses (Table 2) were performed for countries in Africa [16–18, 25, 28, 32–34], with a single study (6.7%) spanning several countries in Africa and Asia [35]. Four studies (26.7%) focused solely on countries in Asia [10, 11, 29, 36], while two studies were done at the global scale [37, 38]. Myanmar and Thailand were the prominent Asian countries studied individually [10, 11, 36]. Among the African countries involved, two studies were from West Africa [16, 28], the others from East Africa [17, 34], and one from South Africa [32].
Table 2.
Summary of models for malaria control optimization studies
| Field | Frequency (%) | References |
|---|---|---|
| Total number of articles reviewed | 15 (100) | [10, 11, 16–18, 25, 28, 29, 32–38] |
| Region of focus | ||
| Africa | 8 (53.3) | [16–18, 25, 28, 32–34] |
| Asia | 4 (26.7) | [10, 11, 29, 36] |
| Africa and Asia | 1 (6.7) | [35] |
| Global | 2 (13.3) | [37, 38] |
| Number of interventions per article | ||
| 2 | 3 (20.0) | [10, 11, 17] |
| 3 | 3 (20.0) | [34–36] |
| 4 | 1 (6.7) | [33] |
| 5 | 5 (33.3) | [18, 25, 29, 32, 38] |
| 6 or more | 3 (20.0) | [16, 28, 37] |
| Plasmodium species | ||
| P. falciparum | 13(86.7) | [10, 11, 16–18, 25, 28, 32–35, 37, 38] |
| P. falciparum + P. vivax | 2 (13.3) | [29, 36] |
| Types of interventions | ||
| ITNs/LLINs | 14 (93.3) | [10, 11, 16–18, 25, 28, 29, 33–38] |
| IRS | 12 (80.0) | [16–18, 28, 29, 32–38] |
| SMC | 6 (33.3) | [16, 18, 25, 28, 33, 37] |
| Treatment | 6 (33.3) | [25, 29, 35–38] |
| IPTp | 5 (26.7) | [16, 25, 28, 37, 38] |
| MDA | 3 (20.0) | [16, 18, 29] |
| Vaccine (RTS,S) | 3 (20.0) | [25, 33, 38] |
| Surveillance | 3 (20.0) | [29, 32, 37] |
| Community health workers | 2 (13.3) | [10, 11] |
| IPTi (PMC) | 2 (13.3) | [25, 38] |
| Social and behaviour change communication | 2 (13.3) | [16, 28] |
| Larval source management | 2 (13.3) | [16, 37] |
| Passive case detection | 2 (13.3) | [28, 32] |
| 2nd generation ITNs | 1 (6.7) | [17] |
| Proactive case detection | 1 (6.7) | [32] |
| Active case detection | 1 (6.7) | [32] |
| Health system strengthening | 1 (6.7) | [28] |
| Mass screen and treatment | 1 (6.7) | [18] |
| Intermittent screen and treat | 1 (6.7) | [34] |
| Population used in optimization | ||
| General population | 14 (93.3) | [10, 11, 16, 18, 25, 28, 29, 32–38] |
| Children under five years | 1 (6.7) | [17] |
| Administrative level of data analysis | ||
| National | 13 (86.7) | [10, 11, 16–18, 25, 28, 29, 33, 35–38] |
| Subnational | 9 (66.7) | [10, 11, 16, 18, 32, 34, 35, 37, 38] |
All studies investigated WHO-recommended interventions for control or elimination targets (Table 2), such as insecticide-treated nets [10, 11, 16–18, 25, 28, 29, 33–38], seasonal malaria chemoprevention [16, 18, 25, 28, 33, 37], intermittent preventive treatment [16, 25, 28, 37, 38], indoor residual spraying [16–18, 28, 29, 32–38], mass drug administration [16, 18, 29], and diagnosis and treatment through community health workers or health facilities [10, 11, 25, 28, 29, 32, 35–38]. Other interventions investigated were surveillance [29, 32, 37], social and behaviour change communication [16, 28], larval source management [16, 37], active [32], passive [28, 32] and proactive case detection [32], health system strengthening [28], mass screen and treatment [18], and intermittent screen and treat [34]. Although not yet implemented in malaria-endemic countries at the time, the hypothetical implementation of the RTS,S vaccine was modelled in three studies, taking into account the epidemiological and cost components [25, 33, 38]. A full summary of all included articles can be found in Table 3.
Table 3.
Summary of included articles
| Study identifier (Year of publication) | Geographic focus | Interventions or Scenarios | Administrative Level | Population | Time horizon of analysis (years) | Method of estimating health benefits | Species | Constraint | Data sources | Optimization goal | Optimization technique | Equity considerations in resource allocation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sherrard-Smith et al. [17] | Tanzania, Uganda | Pyrethroid-only ITNs; Pyrethroid-PBO ITNs; IRS | National | Children under 5 years | 3 | Dynamic mathematical model (MINT tool); Scenario-based | P. falciparum | Minimum budget | Peer-reviewed literature (RCT) | Reducing annual cost per case averted | Stochastic programming | No |
| 2 | Njau et al. [32] | South Africa | Passive case detection; IRS; Active case detection; Proactive case detection; Border surveillance | Subnational (Province) | General population | 11 | Dynamic mathematical transmission model; Scenario-based | P. falciparum | Maximum budget | Local data (DHIS) | Achieve malaria elimination within a 10-year period | Stochastic programming | Yes, Malaria Surveillance Agents (MSAs) |
| 3 | Shretta et al. [28] | Ghana | Passive case detection; LLINs; IRS; Health system strengthening; Social and behavioural change; SMC; IPTp | National | General population | 10 | Dynamic compartmental transmission model; Scenario-based | P. falciparum | Minimum and maximum budget | Local data; World Malaria Reports; Peer-reviewed literature; Expert opinion | Provide economic evidence on risks of withdrawing financing as a strategy for resource mobilization | Metaheuristic method (Particle swarm optimization) | No |
| 4 | Shretta et al. [29] | Asia Pacific (22 countries) | LLINs; IRS; MDA; Treatment; Surveillance | Multi-national; National | General population | 12 | Dynamic compartmental transmission model; Scenario-based (METCAP) | P. falciparum; P. vivax | Minimum budget | World Malaria Reports; Peer-reviewed literature | Malaria elimination by 2030 | Stochastic programming | No |
| 5 | Winskill et al. [25] | Sub-Saharan Africa | Treatment; LLINs; SMC; IPTi (PMC); RTS,S vaccine | Multi-national | General population | Not specified | Individual-based model | P. falciparum | Minimum and maximum budget | Peer-reviewed literature; Country level reports; WHO-CHOICE framework; Global Fund Price Reference Report | Maximise reduction in malaria transmission, case incidence and mortality with the least marginal cost | Non-linear programming | No |
| 6 | Sudathip et al. [36] | Thailand | Treatment; IRS; ITNs | National | General population | 20 | Two epidemiological models. Model A: Log-normal generalised linear regression model; Model B:; Scenario-based | P. falciparum; P. vivax | Minimum and maximum budget | Historical data; Expert opinion; Privately shared data | To measure the cost–benefit of a complete implementation of the NMES and thus assess the justification to invest in malaria elimination in Thailand | Linear programming | No |
| 7 | Drake et al. [10] | Myanmar | ITNs; CHWs | National; Subnational | General population | 1 | Geographically targeted resource allocation framework; Scenario-based | P. falciparum | Minimum budget | Local data; Reports | Using a geographic budget allocation network to maximise health benefits | Linear programming (knapsack) | Yes, Community Health Workers (CHWs) |
| 8 | Scott et al. [16] | Nigeria | LLINs; IRS; IPTp; SMC; Larval source management; MDA; Behavioural change communication | National; Subnational | General population | 5 | Geospatial epidemic (dynamic transmission) model; Optimization algorithm (Optima Malaria model); Scenario-based | P. falciparum | Maximum and minimum budget | Malaria Atlas Project (MAP); UN Population Division | Optimizing the allocation of scarce funding in targeted geographical regions to maximize reductions in malaria morbidity and mortality | Stochastic programming | No |
| 9 | Winskill et al. [33] | Sub-Saharan Africa | LLINs; IRS, SMC; RTS,S vaccine | Multi-national | General population | 10 | Individual-based model | P. falciparum | Cost-effectiveness threshold | Peer-reviewed literature; PMI, CHAI, MSF estimates | To derive the most cost-effective pathways for scaling-up malaria interventions in order to inform decisions about the introduction of the RTS,S malaria vaccine | Non-linear programming (gradient descent) | No |
| 10 | Winskill et al. [35]) | Sub-Saharan Africa (19 countries); Greater Mekong Subregion | LLINs; IRS; ACTs | Multi-national; Subnational | General population | 15 | Individual-based model; Scenario-based | P. falciparum | Maximum and minimum budget | PMI reports; WHO World Malaria Reports; NMCPs; DHS; MICS; Peer-reviewed literature | To estimate the impact of PMI investments to date in reducing malaria burden and to explore the potential negative impact on malaria burden should a proposed 44% reduction in PMI funding occur | Linear programming | No |
| 11 | Patouillard et al. [37] | Global (All 97 malaria endemic countries) | All control interventions recommended by the WHO* | Multi-national; Subnational | General population | 15 | Individual-based model | P. falciparum | Maximum budget | World Malaria Reports; Global Rural–Urban Mapping Project; DHS; Procurement databases; Peer-reviewed literature; National malaria strategic plans; NMCP reports; WHO-CHOICE project; Key informant interviews | To estimate the financing required for malaria control and elimination over the 2016–2030 period | Stochastic programming | No |
| 12 | Walker et al. [18] | Sub-Saharan Africa | LLINs; IRS; SMC; MDA; Mass screen and treatment (MSAT) | Multi-national; Subnational; Pixel (Fine-scale) | General population | 20 | Individual-based model; Scenario-based | P. falciparum | Minimum budget | WHO Pesticide Evaluation Scheme (WHOPES); Peer-reviewed literature; PMI reports; Malaria Atlas Project (MAP) | To estimate the most cost-efficient strategies to achieve goals for reducing burden and transmission | Non-linear programming | No |
| 13 | Dudley et al. [38] | NA | LLINs; IRS; IPT; ACT; RTS,S vaccine | Multi-national; Subnational | General population | 5 | Integer linear program and compartment model; Scenario-based | P. falciparum | Maximum and minimum budget | Peer-reviewed literature; Country specific data; WHO Pesticide Evaluation Scheme (WHOPES) | Minimise person-days of malaria infection | Integer linear programming | No |
| 14 | Drake et al. [11] | Myanmar | ITNs; CHWs | National; Subnational | General population | 1 | Decision tree; Spatially explicit resource allocation model; Scenario-based | P. falciparum | Minimum budget | Three Millenium Development Goal (3MDG); Peer-reviewed literature; Routine health system surveillance records | To maximize impact from investment in ITN use and early diagnosis and treatment through malaria CHWs | Linear programming | Yes, Community Health Workers (CHWs) |
| 15 | Stuckey et al. [34] | Kenya | LLINs; IRS; Intermittent screen and treat (IST) | Subnational | General population | 5 | Microsimulation individual-based model (OpenMalaria); Scenario-based | P. falciparum | Cost-effectiveness threshold | Local survey data (MTC); WHO-CHOICE; Global Fund to Fight AIDS, Tuberculosis and Malaria Price and Quality Reporting Tool; Peer-reviewed literature | To address the cost effectiveness of feasible malaria control interventions | Stochastic programming | No |
Of the twelve studies that used dynamic transmission models in combination with economic/cost models for optimization, six (40.0%) used compartmental models [16, 17, 28, 29, 32, 38], and the other six (40.0%) used individual-based models [18, 25, 33–35, 37]. Two (13.0%) studies used static models [10, 11] and one (7.0%) study used a linear regression model for their analysis [36] (Fig. 2).
Fig. 2.
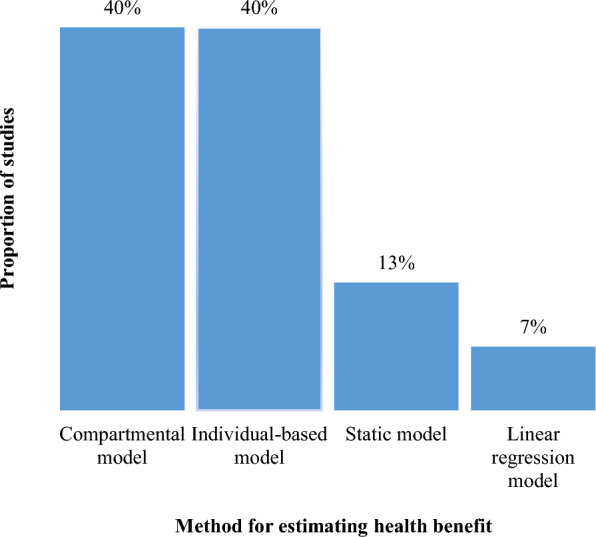
Method for estimating health benefits
The administrative level of data analysis (Table 4) varied between national/multinational only (40.0%), national/multinational and subnational (46.7%), and subnational only (13.3%). A total of 9 (60.0%) studies used at least one source of country-specific epidemiologic or cost data or both for the transmission model parameterization, model calibration or economic analysis, while 6 (40.0%) studies used non-country-specific epidemiologic and cost data for the transmission model parameterization, model calibration or economic analysis. The country-specific data were sourced from district health information system (DHIS) databases, country level reports, NMCP reports, national malaria strategic plans, demographic and health survey (DHS) data, malaria indicator cluster survey (MICS) data, expert opinion and routine health system surveillance records [10, 11, 25, 28, 32, 34, 35, 37, 38]. The non-country-specific data were sourced from peer-reviewed literature, WHO reports, USAID reports, Global Fund reports, Malaria Atlas Project (MAP), procurement databases [16–18, 29, 33, 36]
Table 4.
Administrative level and data used in analysis
| Subgroup | Frequency (%) | References | |
|---|---|---|---|
| Level of analysis | National/multinational only | 6 (40.0%) | [17, 25, 28, 29, 33, 36] |
| National/multinational and subnational | 7 (46.7%) | [10, 11, 16, 18, 35, 37, 38] | |
| Subnational only | 2 (13.3%) | [32, 34] | |
| Data used in analysis | Country-specific data | 9 (60.0%) | [10, 11, 25, 28, 32, 34, 35, 37, 38] |
| Non-country-specific data | 6 (40.0%) | [16–18, 29, 33, 36] |
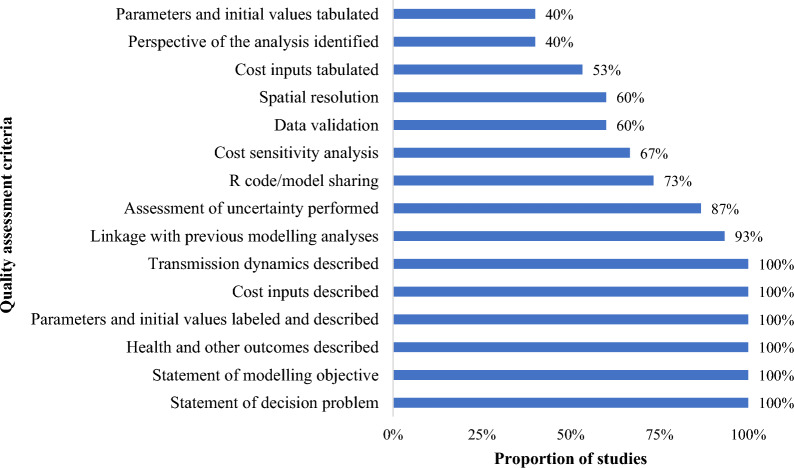
Regarding the quality of the studies included in this review, all the studies included the statement of decision problem, the statement of modelling objective, describing health and other outcomes, labelling and describing parameters and initial values, describing cost inputs and transmission dynamics (Fig. 3). A few parameters that were not well explored by most studies were: the perspective of the analysis; and tabulating the parameters and cost inputs. Most studies shared the R code or model description either through an open-source platform or a previously published article.
Fig. 3.
Quality assessment of included articles
Discussion
A total of 15 articles on models for malaria control optimization were identified from the literature. The majority of modelling analyses focused on countries in Africa and in the Asia Pacific regions. The interventions most commonly found in the analyses were ITNs, IRS, SMC and improved clinical case management. The data sources were country specific for some of the studies, although all studies had to rely on non-country-specific data to complete the analysis. The administrative level of analysis was at both the national and subnational levels, with a few studies having only subnational data analysis. There was a significant number of studies that had a budget constraint. However, very few carried out resource allocation within their constrained budgets. The studies included in this review exhibit various strengths and limitations, which will be outlined and examined below.
Data quality and availability
While most studies used country-specific data for their analysis, they all had to complement their data sources with non-country-specific data for a more comprehensive analysis [10, 11, 25, 28, 32, 34, 35, 37, 38]. Moreso, there is an observed lower quality of the respective studies, as they did not meet all the criteria of the quality assessment such as not performing a cost sensitivity analysis and not defining the perspective for the analysis. There is a need for the accessibility of country-specific epidemiological and cost data, performing cost sensitivity analysis, and defining the perspective for the analysis in order to improve on the quality of the studies and render the results of these studies fit for purpose. There is limited evidence in peer-reviewed literature on modelling for malaria optimization in limited resource settings. The country focus of the modelling studies included in the review were not representative of the burden of malaria in sub-Saharan Africa. For instance, modelling analyses were performed in countries in East Africa [17, 34], West Africa [16, 28], and Southern Africa [32], with none performed in Central Africa. Multinational modelling analyses were performed in countries at risk of malaria in sub-Saharan Africa [18, 25, 33, 35]. However, there is a trade-off of doing these multinational analyses at scale, as subnational tailoring is needed for policy within NMCPs. In Asia, the two main countries that had modelling analyses performed were Myanmar [10, 11] and Thailand [36], while another study focused on 22 countries in the Asia Pacific for the modelling analyses [29]. The limited number of modelling analyses for malaria control optimization specific to countries within Africa and Asia is as a consequence of the lack or inaccessibility of country-specific epidemiologic and cost data at the national and subnational levels. This data gap limits future studies within the respective countries, limits the build-up of a critical mass of modellers within these countries, and makes it challenging for policymakers to make an evidence-based informed case to potential donors for future funding. There is a dire need to carry out more representative and country-specific modelling studies for resource allocation across Africa and the Asia Pacific, for malaria control or elimination.
Model reproducibility and translational elements
There is a marked heterogeneity across all studies in the optimization modelling analyses used to. Specifically, as some studies use dynamic transmission models [16, 17, 28, 32], individual-based models [18, 25, 33–35, 37], or decision tree models [10, 11], the disparities in these optimization modelling analyses across studies make the comparison of the methods used to measure outcomes across these studies difficult. Also, the applications or software used in the development of these models for malaria control optimization analysis are varied [16, 17, 34], with limited knowledge or accessibility of the source code to the public. This limitation makes the reproducibility of the model across similar or neighbouring countries challenging and to some extent inaccessible. It is important to note that there are known current modelling efforts for informing allocation of malaria interventions in collaboration with country NMCPs [30, 39, 40]. These efforts on the use of non-optimization modelling techniques have gotten stakeholders involved in discussions surrounding the application of these models within countries, and in the development of policy engagement tools such as open access applications to facilitate the translation of these models [39–43].
Interventions
All studies included vector control interventions such as the use of ITNs or IRS, most studies included improved clinical case management [10, 11, 18, 25, 28, 29, 34, 36–38], and some included surveillance [29, 32] in their analysis. The use of ITNs in combination with other prevention or treatment packages of interventions for optimal malaria control is usually recommended for use within the respective countries. Studies were identified that included a hypothetical implementation of the RTS, S vaccine in combination with standard interventions within the respective countries of interest [25, 33, 38]. There is a need for a consensus between countries to draw a clear path to malaria elimination, with country NMCPs driving the discussions around this consensus. There are key interventions that countries seeking elimination need to incorporate within their specific models for an eventual implementation. Some of these interventions are surveillance including active case detection and the implementation of the RTS,S vaccine. Accounting for these interventions would allow an analysis involving all possible intervention mixes, and provide more comprehensive outputs and outcomes, hence, a more realistic budget for the expected outcomes to lead to malaria elimination within the respective country.
Equity considerations and subnational tailoring
For treatment interventions, only three studies considered targeting all high risk malaria populations including those that are hard-to-reach, by diagnosing and treating individuals in the most rural of communities with the help of community health workers [10, 11] or malaria surveillance agents [32]. With the most vulnerable or hard to reach populations falling within those at risk for malaria, the integration of community health workers within a community is a key aspect in maximising coverage [10, 11] for malaria control and elimination interventions within the population at risk. Also, the intervention of these community health workers is primordial in reducing mortality [44]. There is, therefore, the need to take into account equity considerations in the implementation of malaria interventions for impact. Finally, while three studies used resource allocation in their analyses [10, 11, 16], most studies used a health systems approach and did not tailor their analyses to subnational levels through the use of resource allocation techniques. National level analysis in the absence of subnational tailoring does not account for much heterogeneity in resource allocation, hence, inherently not providing optimal results. We recognise, however, that employing the same methodologies for a national level analysis across a large set of countries would be valuable as a means to equitably set budgets in a given endemic region.
Subnational allocation of resources provides a more specific attribution of the most effective interventions to the specific needs of each country or community, as can be seen in a recent study in Senegal [30]. Although there was no model used in the analysis, the authors present an aggregation of annual cost estimates of the intervention packages to provide data for better programmatic decision-making [30]. Overall, more efficient allocation of interventions within a country would mean dropping less efficacious interventions to focus more resources on other more relevant ones and thus ensure greater impact. Resource allocation is therefore invaluable in the establishment of informed and sustainable National Strategic Plans for endemic countries looking to control malaria. It is also a critical tool for the decision-making process in countries making a push towards elimination [32, 36], especially considering that interventions become more cost-ineffective as they near elimination.
Limitations
This manuscript provides a systematic review of existing literature to identify models for malaria control optimization. However, the review has certain limitations, which are outlined below:
The review did not include abstracts from scientific conferences or other scientific meetings, potentially omitting important modelling studies conducted by national malaria control programs (NMCPs) to inform resource allocation decisions. And so, although the findings from this review shows that there are relatively few published modelling studies or examples that incorporate cost constraints and describe how to optimize limited budgets, the findings from this manuscript may not fully represent the actual use of models for malaria control optimization worldwide. The absence of these studies from the review limits the comprehensiveness of the findings, suggesting a need for the development of additional approaches to better capture and reflect the full range of modelling activities being undertaken globally.
The review specifically focused on models for malaria control optimization for resource allocation and their use. However, it did not account for non-optimization models that are also crucial for understanding the full spectrum of modelling tools available and their application in strategic planning for malaria control and resource allocation. Consequently, the review does not provide a complete picture of all modelling approaches that could be utilized by NMCPs for decision-making and planning.
These limitations suggest that while the review provides valuable insights into the current use of optimization models in malaria control, it may not fully capture the diversity of modelling efforts and their practical applications in real-world settings. Further research, including unpublished studies and non-optimization models, is necessary to obtain a more comprehensive understanding of the role of modelling in malaria control optimization.
Conclusion
This review identified available optimization models for malaria resource allocation. The findings highlighted the need for country-specific modelling analysis for malaria control optimization, country-specific epidemiological and cost data for analysis, performing cost sensitivity analyses and defining the perspective for the analysis, with an emphasis on subnational tailoring for data collection and analysis for more accurate and good quality results. Such efforts should include all efficient prevention and treatment interventions, and surveillance and vaccination to inform context-specific control and elimination efforts respectively. It is critical that the future modelling efforts account for equity considerations and target at risk malaria populations that are hard-to-reach to maximize impact. Efforts towards developing publicly available applications of the models and sharing source codes to facilitate translation for policy engagement will enhance transparency, reproducibility and adaptability, and pave a way towards more harmonized models for malaria control optimization in the future.
Supplementary Information
Additional File 1. PRISMA 2020 Checklist
Additional File 2. Search strategy and results from each database
Additional File 3. Complete data extraction of all studies included in final review
Additional File 4. Quality assessment of included articles
Acknowledgements
The authors would like to thank Elinor Harriss, Outreach and Enquiry Services Manager, Bodleian Health Care Libraries, University of Oxford for her help in developing the search strategies and providing training on how to conduct the searches. The authors also acknowledge Ainura Moldokmatova, Rachel Hounsell and Caroline Franco, all from the University of Oxford, for their technical assistance in the early stages of the literature search.
Abbreviations
- 3MDG
Three Millenium Development Goal
- CHAI
Clinton health access initiative
- DHS
Demographic and health surveys
- GTS
Global Technical Strategy
- HBHI
High burden to high impact
- HIV/AIDS
Human immunodeficiency virus/acquired immunodeficiency syndrome
- IPTi
Intermittent preventive treatment in infants
- IPTp
Intermittent preventive treatment in pregnancy
- IRS
Indoor residual spraying
- ISPOR
International society for pharmacoeconomics and outcomes research
- ITN
Insecticide-treated bed nets
- LLIN
Long-lasting insecticidal nets
- LMIC
Low- and middle-income countries
- MAP
Malaria atlas project
- MDA
Mass drug administration
- MeSH
Medical subject heading
- MICS
Multiple indicator cluster surveys
- MSF
Médecins Sans Frontières
- NMCP
National malaria control program
- PMC
Perennial malaria chemoprevention
- PMI
President’s malaria initiative
- PRISMA
Preferred reporting items for systematic reviews and meta-analysis
- PROSPERO
Prospective register of systematic reviews
- SMC
Seasonal malaria chemoprevention
- UN
United Nations
- USD
US Dollars
- WHO
World Health Organization
- WHO-CHOICE
World Health Organization Choosing Interventions that are Cost-Effective
- WHOPES
WHO pesticide evaluation scheme
Author contributions
RN performed the initial search and screening, carried out the data extraction and analysis, and assessed the data for quality. SP performed the screening and reviewed the extracted data. RA, LW and RS conceptualised the project. RN wrote the main manuscript text. All authors read, edited, and approved the final manuscript.
Funding
This work was supported, in whole or in part, by the Li Ka Shing grant, University of Oxford and the Bill & Melinda Gates Foundation [INV047-048]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
Availability of data and materials
The data that support the findings of this study were deposited into the Dataverse database and are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Randolph Ngwafor, Email: randolph.ngwaforanye@ndm.ox.ac.uk.
Rima Shretta, Email: rima.shretta@ndm.ox.ac.uk.
References
- 1.WHO. World Malaria Report 2023. Geneva: World Health Organization; 2023. [Google Scholar]
- 2.WHO. High burden to high impact: a targeted malaria response. Geneva: World Health Organization; 2019. [Google Scholar]
- 3.WHO. Global technical strategy for malaria 2016–2030 2021 update. Geneva: World Health Organization; 2021. [Google Scholar]
- 4.WHO. WHO Guidelines for Malaria, 16 October 2023. Geneva: World Health Organization; 2023. [Google Scholar]
- 5.WHO. Seasonal malaria chemoprevention with sulfadoxine-pyrimethamine plus amodiaquine in children: a field guide. Geneva: World Health Organization; 2013. [Google Scholar]
- 6.NMCP. Cameroon malaria strategic plan 2019–2023. Yaounde, Cameroon: Cameroon National Malaria Control Programme; 2019. [Google Scholar]
- 7.WHO. A framework for malaria elimination. Geneva: World Health Organization; 2017. [Google Scholar]
- 8.RBM. Action and investment to deft malaria 2016–2030 for a Malaria-Free World. Geneva: World Health Organization on behalf of the Roll Back Malaria Partnership Secretariat; 2015. [Google Scholar]
- 9.Mills A, Lubell Y, Hanson K. Malaria eradication: the economic, financial and institutional challenge. Malar J. 2008;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake TL, Lubell Y, Kyaw SS, Devine A, Kyaw MP, Day NPJ, et al. Geographic resource allocation based on cost effectiveness: an application to malaria policy. Appl Health Econ Health Policy. 2017;15:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake TL, Kyaw SS, Kyaw MP, Smithuis FM, Day NP, White LJ, et al. Cost effectiveness and resource allocation of Plasmodium falciparum malaria control in Myanmar: a modelling analysis of bed nets and community health workers. Malar J. 2015;14:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conteh L, Shuford K, Agboraw E, Kont M, Kolaczinski J, Patouillard E. Costs and cost-effectiveness of malaria control interventions: a systematic literature review. Value Health. 2021;24:1213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avancena ALV, Hutton DW. Optimization models for HIV/AIDS resource allocation: a systematic review. Value Health. 2020;23(11):1509–21. [DOI] [PubMed] [Google Scholar]
- 14.Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, McKenzie FE. Ross, macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog. 2012;8: e1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton M, Mahiane G, Werst E, Sanders R, Briët O, Smith T, et al. Spectrum-Malaria: a user-friendly projection tool for health impact assessment and strategic planning by malaria control programmes in sub-Saharan Africa. Malar J. 2017;16:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott N, Hussain SA, Martin-Hughes R, Fowkes FJI, Kerr CC, Pearson R, et al. Maximizing the impact of malaria funding through allocative efficiency: using the right interventions in the right locations. Malar J. 2017;16:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherrard-Smith E, Winskill P, Hamlet A, Ngufor C, N’Guessan R, Guelbeogo MW, et al. Optimising the deployment of vector control tools against malaria: a data-informed modelling study. Lancet Planet Health. 2022;6:e100–9. [DOI] [PubMed] [Google Scholar]
- 18.Walker PG, Griffin JT, Ferguson NM, Ghani AC. Estimating the most efficient allocation of interventions to achieve reductions in Plasmodium falciparum malaria burden and transmission in Africa: a modelling study. Lancet Glob Health. 2016;4:e474–84. [DOI] [PubMed] [Google Scholar]
- 19.Caro JJ, Briggs AH, Siebert U, Kuntz KM, Force I-SMGRPT. Modeling good research practices–overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–1. Value Health. 2012;15:796–803. [DOI] [PubMed] [Google Scholar]
- 20.Brady OJ, Slater HC, Pemberton-Ross P, Wenger E, Maude RJ, Ghani AC, et al. Role of mass drug administration in elimination of Plasmodium falciparum malaria: a consensus modelling study. Lancet Glob Health. 2017;5:e680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silal SP, Little F, Barnes KI, White LJ. Towards malaria elimination in Mpumalanga, South Africa: a population-level mathematical modelling approach. Malar J. 2014;13:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okello G, Jones C, Bonareri M, Ndegwa SN, Mcharo C, Kengo J, et al. Challenges for consent and community engagement in the conduct of cluster randomized trial among school children in low income settings: experiences from Kenya. Trials. 2013;14:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minakawa N, Dida GO, Sonye GO, Futami K, Kaneko S. Unforeseen misuses of bed nets in fishing villages along Lake Victoria. Malar J. 2008;7:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caminade C, McIntyre KM, Jones AE. Impact of recent and future climate change on vector-borne diseases. Ann N Y Acad Sci. 2019;1436:157–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winskill P, Walker PG, Cibulskis RE, Ghani AC. Prioritizing the scale-up of interventions for malaria control and elimination. Malar J. 2019;18:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker PGT, Griffin JT, Ferguson NM, Ghani AC. Estimating the most efficient allocation of interventions to achieve reductions in Plasmodium falciparum malaria burden and transmission in Africa: a modelling study. Lancet Global Health. 2016;4:e474–84. [DOI] [PubMed] [Google Scholar]
- 27.Sherrard-Smith E, Winskill P, Hamlet A, Ngufor C, N’Guessan R, Guelbeogo MW, et al. Optimising the deployment of vector control tools against malaria: a data-informed modelling study. Lancet Planetary Health. 2022;6:e100–9. [DOI] [PubMed] [Google Scholar]
- 28.Shretta R, Silal SP, Malm K, Mohammed W, Narh J, Piccinini D, et al. Estimating the risk of declining funding for malaria in Ghana: the case for continued investment in the malaria response. Malar J. 2020;19:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shretta R, Silal SP, Celhay OJ, Gran Mercado CE, Kyaw SS, Avancena A, et al. Malaria elimination transmission and costing in the Asia-Pacific: Developing an investment case. Wellcome Open Res. 2019;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faye S, Cico A, Gueye AB, Baruwa E, Johns B, Ndiop M, et al. Scaling up malaria intervention “packages” in Senegal: using cost effectiveness data for improving allocative efficiency and programmatic decision-making. Malar J. 2018;17:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan — a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Njau J, Silal SP, Kollipara A, Fox K, Balawanth R, Yuen A, et al. Investment case for malaria elimination in South Africa: a financing model for resource mobilization to accelerate regional malaria elimination. Malar J. 2021;20:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winskill P, Walker PG, Griffin JT, Ghani AC. Modelling the cost-effectiveness of introducing the RTS, S malaria vaccine relative to scaling up other malaria interventions in sub-Saharan Africa. BMJ Glob Health. 2017;2: e000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuckey EM, Stevenson J, Galactionova K, Baidjoe AY, Bousema T, Odongo W, et al. Modeling the cost effectiveness of malaria control interventions in the highlands of western Kenya. PLoS ONE. 2014;9: e107700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winskill P, Slater HC, Griffin JT, Ghani AC, Walker PGT. The US President’s Malaria Initiative, Plasmodium falciparum transmission and mortality: a modelling study. PLoS Med. 2017;14: e1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sudathip P, Kongkasuriyachai D, Stelmach R, Bisanzio D, Sine J, Sawang S, et al. The investment case for malaria elimination in Thailand: a cost-benefit analysis. Am J Trop Med Hyg. 2019;100:1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patouillard E, Griffin J, Bhatt S, Ghani A, Cibulskis R. Global investment targets for malaria control and elimination between 2016 and 2030. BMJ Glob Health. 2017;2: e000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dudley HJ, Goenka A, Orellana CJ, Martonosi SE. Multi-year optimization of malaria intervention: a mathematical model. Malar J. 2016;15:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diallo OO, Diallo A, Toh KB, Diakité N, Dioubaté M, Runge M, et al. Subnational tailoring of malaria interventions to prioritize the malaria response in Guinea. Medrxiv. 2024. 10.1101/2024.06.26.24309532v1.39072042 [Google Scholar]
- 40.Ozodiegwu ID, Ambrose M, Galatas B, Runge M, Nandi A, Okuneye K, et al. Application of mathematical modelling to inform national malaria intervention planning in Nigeria. Malar J. 2023;22:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awine T, Silal SP. Assessing the effectiveness of malaria interventions at the regional level in Ghana using a mathematical modelling application. PLoS Glob Public Health. 2022;2: e0000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Runge M, Snow RW, Molteni F, Thawer S, Mohamed A, Mandike R, et al. Simulating the council-specific impact of anti-malaria interventions: a tool to support malaria strategic planning in Tanzania. PLoS ONE. 2020;15: e0228469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Runge M, Molteni F, Mandike R, Snow RW, Lengeler C, Mohamed A, et al. Applied mathematical modelling to inform national malaria policies, strategies and operations in Tanzania. Malar J. 2020;19:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landier J, Parker DM, Thu AM, Lwin KM, Delmas G, Nosten FH, et al. Effect of generalised access to early diagnosis and treatment and targeted mass drug administration on Plasmodium falciparum malaria in Eastern Myanmar: an observational study of a regional elimination programme. Lancet. 2018;391:1916–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File 1. PRISMA 2020 Checklist
Additional File 2. Search strategy and results from each database
Additional File 3. Complete data extraction of all studies included in final review
Additional File 4. Quality assessment of included articles
Data Availability Statement
The data that support the findings of this study were deposited into the Dataverse database and are available from the corresponding author upon reasonable request.