ABSTRACT
The COVID-19 pandemic persists despite the availability of vaccines, and it is, therefore, crucial to develop new therapeutic and preventive approaches. In this study, we investigated the potential role of oral microbiome in SARS-CoV-2 infection. Using an in vitro SARS-CoV-2 pseudovirus infection assay, we found a potent inhibitory effect exerted by Porphyromonas gingivalis on SARS-CoV-2 infection mediated by known P. gingivalis compounds such as phosphoglycerol dihydroceramide (PGDHC) and gingipains as well as by unknown bacterial factors. We found that the gingipain-mediated inhibition of infection is likely due to cytotoxicity, whereas PGDHC inhibited virus infection by an unknown mechanism. Unidentified factors present in P. gingivalis supernatant inhibited SARS-CoV-2 likely via the fusion step of the virus life cycle. We addressed the role of other oral bacteria and found certain periodontal pathogens capable of inhibiting SARS-CoV-2 pseudovirus infection by inducing cytotoxicity on target cells. In the human oral cavity, we observed that the modulatory activity of oral microbial communities varied among individuals, in that some saliva-based cultures were capable of inhibiting while others were enhancing infection. These findings contribute to our understanding of the complex relationship between the oral microbiome and viral infections, offering potential avenues for innovative therapeutic strategies in combating COVID-19.
IMPORTANCE
The oral microbiome is important in health and disease, and in this study, we addressed the potential role of the oral microbiome in COVID-19 infection. Our in vitro studies suggest that certain bacteria of the oral microbiome such as P. gingivalis produce compounds that could potentially inhibit SARS-CoV-2 infection. These findings elucidating the interactions between the oral microbiome and SARS-CoV-2 infection will be important in our understanding of COVID-19 pathogenesis and the development of innovative therapeutic and preventive strategies against COVID-19 infection.
KEYWORDS: COVID-19, SARS-CoV-2, Porphyromonas gingivalis, oral microbiome, oral microbiology, coronavirus, antiviral agents
INTRODUCTION
As of August 2023, World Health Organization estimated 770 million cases and 7 million deaths due to COVID-19 around the world. COVID-19 remains a major global public health problem despite the availability of effective vaccines. This can be attributed largely to the continuous emergence of SARS-CoV-2 variants and socioeconomic factors including vaccine hesitancy and lack of access to healthcare (1, 2). It is therefore critically necessary to constantly find new avenues to treat and prevent infection.
Human mouth contains a tremendously large and diverse microbiota that is second only to the gut, with over 700 species of resident bacteria. Evidence continues to accrue supporting the essential role that the oral and gastrointestinal microbiome plays in health and disease (3, 4), including COVID-19 (5–8). A number of studies have attempted to establish an association between the oral and gastrointestinal microbiome with COVID-19 as well as other viral infections (5, 8–10). For example, microbiome dysbiosis in both gut and mouth as a result of an altered microbial composition was associated with a more severe COVID-19 infection (8, 10). In addition, people with periodontitis were found to be more likely to have severe disease or die from COVID-19 (11–13). Collectively, these studies strongly suggest that oral microbiome dysbiosis or potentially specific oral pathogens that are associated with periodontitis could play a role in COVID-19 infection and disease severity.
It is speculated that the oral microbiome could impact SARS-CoV-2 infection through its interaction with the host. The oral microbiome has been shown previously to modulate innate and adaptive immunity, which could exacerbate SARS-CoV-2 infection and disease (14, 15). Lipopolysaccharides (LPS)-producing bacteria found in the oral cavity of COVID-19 patients exert an inflammatory effect (16). Oral bacteria species such as Prevotella and Veillonella, which are also commonly found in the oral cavity of COVID-19 patients, may stimulate the production of IL-6, IL-23, and IL-1, inducing unwanted inflammation (17). There is evidence to suggest that in people with respiratory conditions such as pneumonia and chronic obstructive pulmonary disease, translocation of periodontal pathogens to the lungs may facilitate SARS-CoV-2 replication in lung cells (18, 19).
Alternatively, oral microbes could directly interact with SARS-CoV-2 and modulate infection. The potential for oral bacteria to contain anti-COVID-19 properties is possible since bacteria are known to possess antiviral compounds including LPS, polyketones, alkaloids, peptides, polyphenols, pyrones, quinones, sterols, and terpenoids (20, 21). Depending on the chemical properties of the antiviral compound and the specific virus, the mechanisms of viral inhibition have been shown to target virtually all the stages of the viral life cycle such as viral attachment and penetration, genome integration, replication, and transcription (20).
In the present study, we began to address the role of the oral microbiome in COVID-19 infection. We assessed the role of Porphyromonas gingivalis based on the observation that patients with periodontitis, which is an oral disease associated with P. gingivalis infection, had a higher risk of complications from SARS-CoV-2 infection (3, 22–24). A previous study showed P. gingivalis LPS was able to induce the expression of the SARS-CoV-2 receptor ACE2 and the accessory protease TMPRSS2 in human gingival fibroblasts (23), suggesting a possible mechanism that could explain the increased severity of CoV-2 infection among periodontitis patients.
We also investigated the possible impact of the oral salivary microbiome from healthy human volunteers as well as laboratory strains of various commensal and pathogenic oral bacteria on SARS-CoV-2 infection. Important results of these studies are described herein, showing that certain known compounds as well as unidentified factors are capable of markedly inhibiting SARS-CoV-2 infection in vitro. Interestingly, oral bacterial communities cultured from healthy human volunteers exhibited variable modulatory activities toward SARS-CoV-2. Altogether, these findings suggest a possible role for the oral microbiome in COVID-19 infection, and further studies are needed to validate these observations in vivo or in the clinical setting.
RESULTS
P. gingivalis factors inhibited SARS-CoV-2 pseudoviral infection
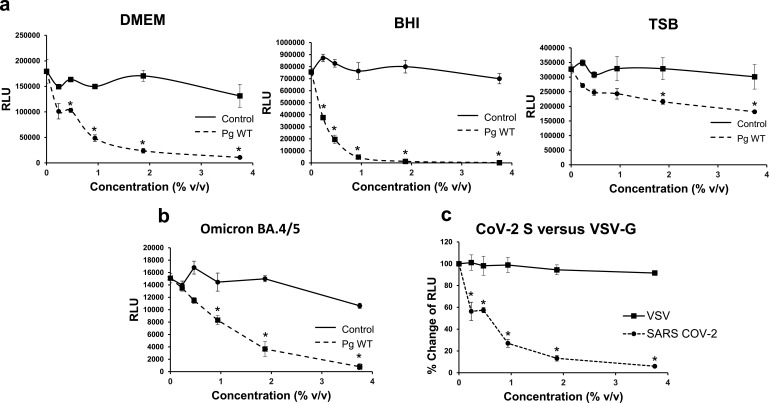
Certain factors produced by P. gingivalis have previously been shown to have antiviral properties (25, 26). We tested whether P. gingivalis contained anti-SARS-CoV-2 properties and found that bacterial factors present in P. gingivalis supernatants markedly inhibited lentiviral vectors pseudotyped with SARS-CoV-2 spike protein (Fig. 1a). Infection of ACE2 + 293 T cells with pseudoviruses containing either spike (S) protein cloned from either the original Wuhan strain or the Omicron BA.4/5 variant was significantly inhibited (Fig. 1a and b). The antiviral effect observed was specifically mediated by SARS-CoV-2 spike proteins, in that VSV-G pseudotyped viruses were unaffected (Fig. 1c). The anti-SARS-CoV-2 effect exerted by P. gingivalis supernatant was substantial with an IC50 of 0.3% vol/vol. Using the WST-1 assay, we found that higher concentrations (≥7.5% vol/vol) were cytotoxic to the target cells (Fig. 4c). P. gingivalis supernatants produced with 10% FBS DMEM and BHI cultures were significantly more inhibitory than those produced with TSB. In addition to P. gingivalis supernatants, we also tested P. gingivalis lysates for inhibition and found that P. gingivalis lysates were highly cytotoxic even at low concentrations (less than 5%) (data not shown).
Fig 1.
P. gingivalis factors inhibit SARS-CoV-2 pseudovirus infection. ACE2-overexpressing 293T cells were infected with a single-round HIV-1 lentiviral vector pseudotyped with SARS-CoV-2 (CoV-2) spike (Wuhan strain) or VSV-G. The viruses contain luciferase reporter to allow for the measurement of infection in RLU. (a) Inhibition of infection by various concentrations (% vol/vol) of supernatants of P. gingivalis W83 WT (Pg WT) grown in brain-heart infusion (BHI), tryptic soy broth (TSB), and 10% FCS DMEM medium and medium control (Control) is shown. (b) The inhibitory effect of supernatant of P. gingivalis grown in 10% FCS DMEM on CoV-2 Omicron BA.4/5 pseudotyped lentivirus was measured. (c) Comparison of CoV-2 pseudovirus versus VSV control inhibition by P. gingivalis supernatant (grown in DMEM/10%FCS) is shown and inhibition of infection (%Change of RLU) is indicated. The mean luciferase activity ± SD (N = 3) of infected cells was determined. Difference between infection with treatment and control as well as between infection with CoV-2 and VSV pseudovirus was considered significant (*P < 0.05) using Student’s t-test.
Host airway epithelial cells from the lungs are the primary target cells for SARS-CoV-2 infection. Furthermore, periodontal pathogens found in the lungs of patients with certain respiratory conditions could play a role in lung cell infection by SARS-CoV-2 (18). We, therefore, tested the effects of P. gingivalis factors on infection of lung cells, using lung tumor epithelial cell line H838 overexpressing ACE2 as target cells. We found that P. gingivalis supernatants at 2% and 4% concentration of the total media volume inhibited infection by roughly 50% (Fig. 2). Altogether, these viral inhibition studies strongly suggest that P. gingivalis secretes factors capable of inhibiting SARS-CoV-2 infection that was mediated by the spike protein.
Fig 2.
P. gingivalis factors inhibit lung cancer cell infection by SARS-CoV-2 pseudovirus. The lung cancer cell line H838 was transiently transfected to overexpress ACE2. Supernatants of P. gingivalis wt grown in DMEM/10% FBS at 2% and 4% vol/vol were able to inhibit infection by SARS-CoV-2 pseudovirus transducing luciferase. Infection was measured by luciferase activity (RLU) and the mean RLU ± SD (N = 3) of infected cells with P. gingivalis supernatant or control supernatant (DMEM/10% FBS only) was determined. Difference between infection with treatment and control was considered significant (*P < 0.05) using Student’s t-test.
P. gingivalis phosphoglycerol dihydroceramides (PGDHC inhibited SARS-CoV-2 pseudovirus infection
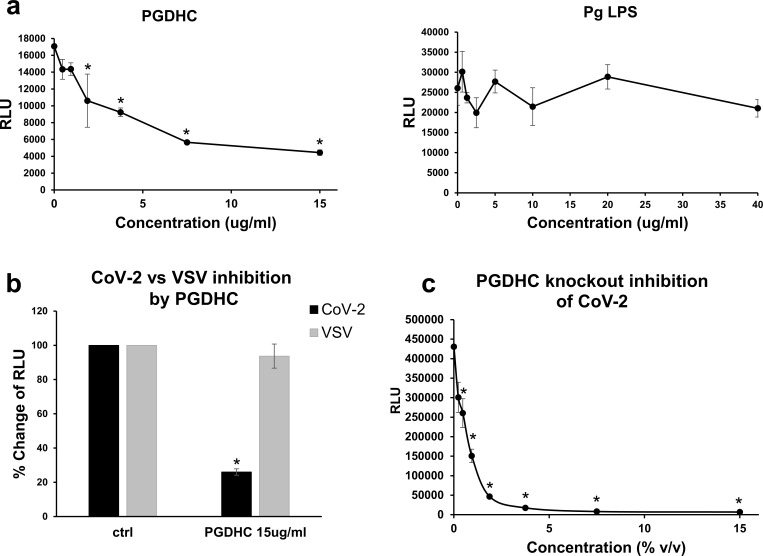
To investigate which secreted factors were inhibiting SARS-CoV-2 pseudovirus infection, we tested known compounds that have been shown to be immunomdulatory including PGDHC and LPS. We found that PGDHC strongly inhibited SARS-CoV-2 pseudoviruses containing the spike protein (IC50 ~2 µg/mL) (Fig. 3a), whereas the LPS had no effect on the pseudoviral infection (Fig. 3a). PGDHC did not inhibit VSV pseudoviruses, which suggests that PGDHC inhibition of SARS-CoV-2 was mediated by the spike protein (Fig. 3b). Interestingly, supernatants from the SPT-mutant (27), which does not produce PGDHCs or phosphoethanoloamine dihydroceramides (PEDHCs) had similar inhibitory activity as the parent strain (Fig. 1a and 3c). Taken together, these results suggest that PGDHC and other unknown factors produced by P. gingivalis are strong inhibitors of SARS-CoV-2 infection that is mediated by the spike protein.
Fig 3.
P. gingivalis phosphoglycerol dihydroceramide (PGDHC) inhibits SARS-CoV-2 pseudovirus infection. (a) ACE2 + 293 T cells were pre-incubated for 6 h with various concentrations of P. gingivalis PGDHC or LPS and subsequent infection in the presence of these P. gingivalis factors with SARS-CoV-2 pseudovirus was assessed. (b) Inhibition by PGDHC on infection (indicated as % Change of RLU) was specific to SARS-CoV-2 (CoV-2) but not VSV-pseudovirus (VSV). Infected ACE2 + 293 T cells with PGDHC (15 µg/mL) or without (ctrl) are shown. (c) Effects of PGDHC gene knockout on SARS-CoV-2 pseudoviral inhibition. Infected ACE2 + 293 T cells in the presence of various concentrations of PGDHC knockout supernatant are shown. Infection was measured by luciferase activity (RLU) and the mean RLU ± SD (N = 3) of infected cells with or without P. gingivalis supernatant was determined. Difference between infection with treatment and control as well as between CoV-2 and VSV pseudovirus infection was considered significant (*P < 0.05) using Student’s t-test.
P. gingivalis gingipains inhibited SARS-CoV-2 pseudovirus infection
Gingipains, which are a family of cysteine proteases secreted by P. gingivalis, have previously been shown to have antiviral effects toward HIV (25) and have been associated with increased metapneumovirus infection (28). To assess the possible role of gingipains in COVID-19 infection, we used the triple gingipain knockout P. gingivalis strain (RGPA, RGPB, and KGP knockout). We found that the antiviral effects of P. gingivalis wild-type supernatants were dramatically reduced in the P. gingivalis gingipain knockout supernatants (Fig. 4a and b). In addition, the triple gingipain knockout supernatant at higher concentrations (7.5% and 15% vol/vol) still significantly inhibited SARS-CoV-2 pseudoviral infection of ACE2 + 293 T cells (Fig. 4b) despite the loss of cytotoxic effects at these high supernatant concentrations (Fig. 4c). Taken together, these results suggest that gingipains mediate the inhibition of SARS-CoV-2 pseudovirus through its cytotoxic effects. Also, in addition to gingipains, there are other possible anti-SARS-CoV-2 mediators present in P. gingivalis.
Fig 4.
Effects P. gingivalis gingipains in SARS-CoV-2 pseudovirus infection. (a) For SARS-CoV-2 pseudovirus inhibition of ACE2 + 293T infection, various concentrations (%vol/vol) of supernatants from wildtype P. gingivalis (Pg WT) and triple gingipain knockout P. gingivalis mutant (Pg W83ΔkgpΔrgpAΔrgpB), and 10% FCS DMEM only control (DMEM ctrl) were used. (b) Inhibition of ACE2 + 293T infection at higher bacterial supernatant concentrations (7.5% and 15%) is shown. (c) Cytotoxicity of ACE2 + 293T (measured by WST) at high concentrations of Pg WT supernatant but not triple gingipain knockout is shown. Infection was measured by luciferase activity (RLU) and the mean RLU ± SD (N = 3) of infected cells with or without P. gingivalis supernatant was determined. Difference between infection with treatment and control was considered significant (*P < 0.05) using Student’s t-test.
P. gingivalis antiviral factors inhibited SARS-CoV-2 infection independent of spike protein binding to ACE2 receptor
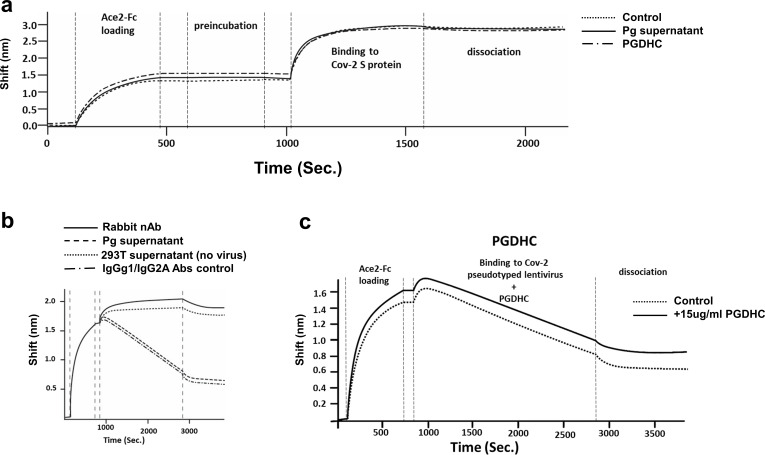
We evaluated whether the inhibition of SARS-CoV-2 pseudoviral infection occurred during the early steps of the viral life cycle. To assess the effects on viral entry, we determined the ability of P. gingivalis supernatant and purified PGDHCs to interfere with the binding of SARS-CoV-2 spike protein to ACE2 receptor protein using Biolayer Interferometry analysis. As expected, the spike protein bound to ACE2 efficiently (Fig. 5a). Neither the P. gingivalis supernatant (15% vol/vol) nor PGDHCs (15 µg/mL) affected the binding of the purified spike protein to purified ACE2 receptor protein (Fig. 5a).
Fig 5.
P. gingivalis PGDHC and factors do not affect the binding of SARS-CoV2 S protein to ACE2. (a) BLI binding assay was performed to determine if PGDHCs, P. gingivalis supernatant, or medium alone (Control) inhibited the binding of S protein to ACE2-Fc. (b) SARS-CoV-2 pseudovirions binding to the ACE2 loaded biosensor treated with rabbit neutralizing antibody (Rabbit nAb), P. gingivalis supernatant, non-neutralizing IgGg1/IgG2A Abs control or 293T supernatant only (no virus). (c) SARS-CoV-2 pseudovirions binding to the ACE2 loaded biosensor treated with 15 µg/mL PGDHC or without PGDHC (Control). The BLI curves of the binding as well as the loading, preincubation, binding, and dissociation steps are shown. The sensogram shows the binding shift (nm) over time (seconds).
Using BLI analysis, we also assessed the ability of P. gingivalis secreted factors to inhibit the binding of pseudoviruses containing the spike protein to purified ACE2 protein. Binding of the pseudovirus to ACE2 caused a negative shifting of the BLI curve similar to prior observations with virus binding (Fig. 5b) (29, 30). Using an anti-spike rabbit neutralizing antibody (nAb), the binding of pseudovirus containing the spike protein to ACE2 receptor was inhibited based on the lack of negative shifting of the BLI curve (Fig. 5b). By contrast, the P. gingivalis supernatant, PGDHC, and non-neutralizing antibodies did not inhibit SARS-CoV-2 pseudovirus binding to ACE2 (Fig. 5b and c). Thus, these results altogether suggest that the observed inhibition by P. gingivalis is not mediated by the binding of the spike protein on the virus to ACE2 receptor.
P. gingivalis inhibited SARS-CoV-2 infection at the fusion step of the virus life cycle
We assessed whether the secreted factors produced by P. gingivalis interfered with the virus life cycle, specifically after the binding of spike protein to ACE2. A syncytium formation assay we developed was used to assess whether P. gingivalis is inhibiting SARS-CoV-2 pseudoviral infection at the viral-host cell fusion step of the virus life cycle. To develop the syncytium-formation assay, we generated TZM.bl expressing SARS-CoV-2 S protein and 293T cells expressing a Tat-P2A-Rev (Fig. 6a, left panel). Once co-cultured, the spike/ACE2 interaction resulted in membrane fusion and the formation of syncytium (Fig. 6a, left and middle panels). In the fused cells, Tat transactivates the LTR-Luc cassette present in the TZM.bl cells, which results in the expression of the luciferase reporter gene (Fig. 6a, left and right panels). As expected, the addition of anti-spike protein mouse-neutralizing antibody inhibited syncytium formation at an IC50 of 0.5 µg/mL (Fig. 6a, right panel).
Fig 6.
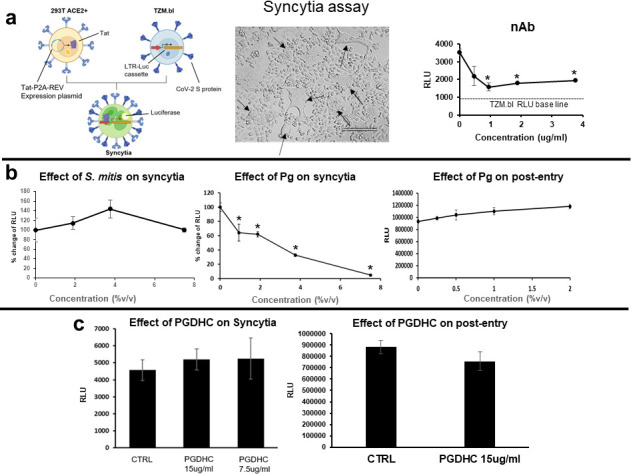
Effect of P. gingivalis factors on fusion and postentry steps of the viral life cycle. A syncytium formation assay was used to assess the effects of P. gingivalis factors on the fusion step of the viral life cycle. (a) Luc activity (RLU) indicates a fusion between 293T ACE2 + and Tzm-bl cells expressing the S protein (left panel). Light microscopy of syncytia formed (indicated by arrows) is shown (middle panel). Inhibition of syncytium formation by rabbit anti-S neutralizing antibody (nAb) is shown (right panel). (b and c) The effects of P. gingivalis supernatant at various concentrations (%vol/vol) and PGDHC at 7.5 µg/mL or 15 µg/mL on syncytium formation (left panels) and postentry (right panels) are shown. Inhibition of syncytium formation by P. gingivalis supernatant is shown as a % change of RLU. Ten percent FCS DMEM only was used as control (CTRL). To measure the effects of P. gingivalis factors on postentry, ACE2 + 293T were infected, and 24 h later, the P. gingivalis factors were added and RLU was measured 2 days later. Infection was measured by luciferase activity (RLU) and the mean RLU ± SD (N = 3) of infected cells was determined. Difference between infection with treatment and control was considered significant (*P < 0.05) using Student’s t-test.
We observed that P. gingivalis supernatant but not PGDHCs was capable of inhibiting the formation of syncytium (Fig. 6b and c, respectively). P. gingivalis supernatant potently inhibited syncytium formation with an IC50 ~2.7% vol/vol. A post-entry assay showed that neither the P. gingivalis supernatant nor PGDHC had an effect on pseudoviral infection at the post-entry step (Fig. 6b and c, respectively). Altogether, these results strongly suggest that P. gingivalis anti-COVID-19 factors, but not PGDHC, inhibit SARS-CoV-2 infection mediated by the spike protein at the fusion step of the viral life cycle. Additionally, the secreted factors had no effect on viral post-entry process, specifically once the virus had already entered the host cell.
SARS-CoV-2 pseudovirus infection is inhibited by P. gingivalis and other oral bacteria
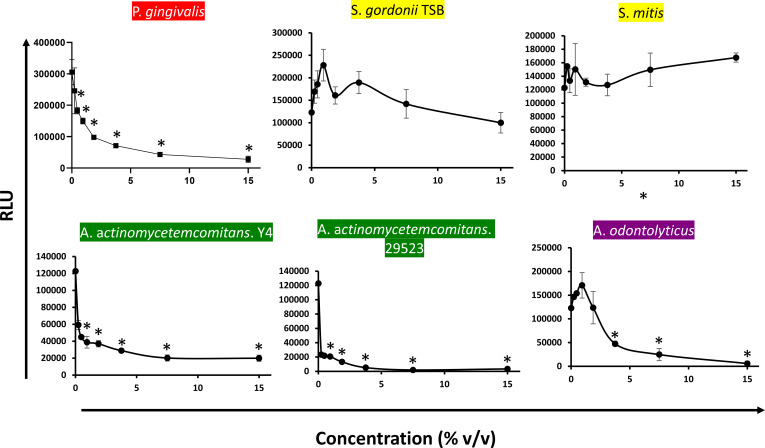
Because the oral pathogen P. gingivalis showed some anti-SARS-CoV-2 properties, it is possible that other oral bacteria, either pathogens or commensals, would exhibit similar properties. To explore this possibility, oral bacterial supernatants from different species belonging to various microbial complexes classified by Dr. Socransky were generated and tested for anti-SARS-CoV-2 properties (31). We observed that supernatants from oral pathogens Actinobacillus actinomycetemcomitans (Y4 strain) and Actinobacillus actinomycetemcomitans (29523 strain) as well as the oral commensal Actinomyces odontolyticus inhibited pseudoviral infection (32). Strong inhibition by these oral bacteria was observed at very low supernatant concentrations (Fig. 7). Cytotoxicity analysis using WST-1 assay indicated that these oral pathogens inhibited SARS-CoV-2 pseudoviral infection due to the highly cytotoxic effects of the supernatants on ACE2 293T target cells. In contrast, supernatants from cultures of other oral commensals including S. mitis and S. gordonii had a poor antiviral effect (Fig. 7). These results suggest that SARS-CoV-2 infection can be inhibited by certain oral bacteria including P. gingivalis, A. actinomycetemcomitans, and A. odontilyticus; however, not all oral microbiota exhibit this inhibitory activity.
Fig 7.
Oral bacteria belonging to various microbial complexes can inhibit SARS-CoV-2 pseudoviruses. The red complex (P. gingivalis), yellow (S. gordoniand S. mitis), green (A. actinomycetemcomitans Y4 and A. actinomycetemcomitans 29523), and purple (A. odontilyticus) grown in 10% FCS DMEM except for S. gordonii (grown in TSB) were tested for modulatory activity against SARS-CoV-2 pseudovirus infection. Oral bacterial supernatants at various concentrations were added to SARS-CoV-2 pseudovirus infection assay using ACE2 +293T target cells. Infection was measured by luciferase activity (RLU) and the mean RLU ± SD (N = 3) of infected cells was determined. Difference between infection with treatment and control was considered significant (*P < 0.05) using Student’s t-test.
Oral bacteria from healthy individuals modulated SARS-CoV-2 infection
Since we observed that secreted factors from certain types of strains propagated in the laboratory had anti-SARS-CoV-2 effect, we explored whether oral bacteria enriched from saliva samples from healthy human volunteers exhibited any modulatory activity toward SARS-CoV-2. Interestingly, out of all cultures grown from 37 individuals, those from nine individuals exhibited antiviral activity, whereas culture supernatants from 17 individuals enhanced infection (Fig. 8a). Moreover, 11 of the 37 healthy volunteers harbored oral bacteria with no effect on pseudovirus infection (Fig. 8a). Also, the anti-SARS-CoV-2 property of bacterial supernatants from three individuals was associated with strong cytotoxicity to target cells (data not shown). We observed significant differences in viral activity among groups that have either inhibitory, enhancing, or no effect on viral infection (Fig. 8b). Collectively, these results strongly suggest that oral bacteria present in healthy human individuals have the capacity to modulate SARS-CoV-2 pseudoviral infection. Specifically, the modulatory activity of oral bacteria present in the saliva can either be inhibitory, enhancing, or neutral to SARS-CoV-2 infection.
Fig 8.
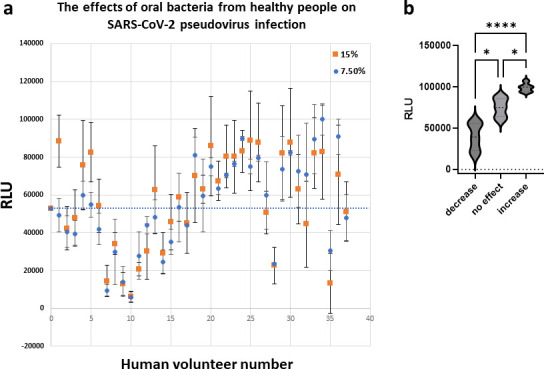
Oral bacteria from healthy people modulate SARS-CoV-2 pseudovirus Infection. (a) Oral bacteria were isolated from the saliva of 37 healthy human volunteers and tested for modulatory activity against SARS-CoV-2 pseudovirus infection. Oral bacteria were cultured aerobically in BHI, and supernatants at either 7.5% or 15% vol/vol concentration were tested for the ability to inhibit SARS-CoV-2 pseudovirus infection of ACE2 + 293 T cells. Infection was measured by luciferase activity (RLU) with the mean RLU ± SD (N = 3) of infected cells. The bacterial supernatant activity toward SARS-CoV-2 from each volunteer is shown. The baseline level of infection is indicated by the dotted line at approximately 55,000 RLU. (b) The samples were divided into three equal groups that exhibited inhibition of (decrease), enhancement of (increase), or no effect on virus infection. The three groups passed the normality tests (D’Agostino/Pearson, Anderson/Darling, and KS) but showed difference in SD as determined by both Brown/Frosythe and Bartlett homoscedasticity tests. The descriptive parameters suggested to proceed to analyze the data set using a non-parametric one-way ANOVA (Welch coupled with Dunnett’s T3 multiple comparisons post-Hoc test). P-values: * <0.05, **** <0.0001.
DISCUSSION
The role the oral microbiome plays in COVID-19 infection has not been elucidated, and in this study, we demonstrated the potential influence of the oral microbiome on SARS-CoV-2 infection. Interestingly, using an in vitro pseudovirus infection assay, we demonstrated that certain oral bacteria, specifically two oral pathogens (P. gingivalis and A. actinomycetemcomitans) and a commensal (A. odontilyticus) were capable of inhibiting SARS-CoV-2. In contrast, two oral commensals that were tested, specifically S. mitis and S. gordonii, had no detectible antiviral properties. Interestingly, supernatants from enrichment cultures generated with saliva samples from healthy human individuals were either capable of inhibiting or enhancing while some had no effect on SARS-CoV-2 pseudovirus infection.
The anti-SARS-CoV-2 effect of Pg was noteworthy, in that we observed a marked antiviral effect exerted by P. gingivalis supernatant on SARS-CoV-2 pseudoviral infection of ACE2 + 293 T cells with an IC50 of 0.3% vol/vol. P. gingivalis was not only capable of inhibiting pseudoviruses expressing the spike protein from the original Wuhan strain but also the BA.4/5 omicron variant. In addition, we found that P. gingivalis also inhibited the infection of relevant lung cells (adenocarcinoma cell line H838), which is important since airway lung epithelial cells are the in vivo target cells of SARS-CoV-2. Periodontal pathogen infections of the lungs have been observed, and it is proposed that translocation of periodontal pathogens from the oral cavity into the lungs could influence SARS-CoV-2 infection of lung epithelial cells (18, 19). If P. gingivalis were to be found in the lungs of patients with COVID-19, P. gingivalis could potentially inhibit SARS-CoV-2 infection of lung epithelial cells.
Inhibition of ACE2 + H838 was significant but not as potent as the 293T cell target cells, which suggests that there are differences in the expression of cellular factors in the lung versus 293T cells that are important for infection. The use of primary lung cultures/organoids or lung infection in animal models is needed to assess fully the antiviral properties of P. gingivalis. In addition to cellular factors expressed on target cells, the expression of bacterial factors with antiviral properties will be key. We found that bacteria grown in DMEM/10%BSF and BHI but not TSB are capable of inhibiting SARS-CoV-2 pseudovirus, suggesting that perhaps, the growth conditions in the oral cavity could play a role in the production of antiviral factors by P. gingivalis.
We determined that purified PGDHCs from P. gingivalis strongly inhibited SARS-CoV-2 pseudovirus infection. In contrast, P. gingivalis LPS, known to activate several cells of the immune system interacting with TLR4, had no anti-SARS-CoV-2 property (33). To provide context, the different subtypes of P. gingivalis dihydroceramides (PGDHCs and PEDHCs) have been shown to be secreted into the environment on outer membrane vesicles (OMVs) and be immunomodulatory (34, 35). In addition, these dihdyroceramides are thought to be virulence factors that are associated with bone loss in periodontitis patients as well as with the pathogenesis of Alzheimer’s (36–38). Although the underlying mechanisms involved in the PGDHC-mediated viral inhibition still need to be determined, it is possible that the purified PGDHCs because of their sphingolipid structures are inhibiting the virus membrane-to-cell membrane fusion or the virus decapsulation by modifying the lipid bilayer biophysical properties, by acting on the ACE2-viral membrane glycoprotein complex or acting on accessory proteins required for the successful virus entry. PGDHCs have also been shown to trigger signal transduction pathways involved in osteoclastogenesis and amyloidogeneis, which may inhibit SARS-CoV-2 infection (36–38).
It is important to note that like the wild-type, the SPT mutant was also strongly inhibitory, which is contradictory, suggesting that synthesis of PGDHCs by P. gingivalis is not required for viral inhibition. However, although it is true that this mutant does not synthesize PGDHCs, the SPT mutant is also defective in the synthesis of other subtypes of DHCs, including the phosphoethanolamine dihydroceramides (PEDHCs). Therefore, it is possible that the different DHC types may promote or constrain invasion and act so by different mechanisms. Finally, it may be that in the purified form, PGDHCs are inhibitory, but they are not inhibitory when delivered as an integral part of an OMV membrane. Hence, further studies are required to elucidate the mechanisms involved in the observed PGDHC-mediated viral inhibition.
P. gingivalis gingipains also inhibited SARS-CoV-2 pseudovirus infection based on our observation that the triple gingipain knockout P. gingivalis strain had a significantly reduced ability to inhibit infection. Gingipains were previously shown to be critical in processing fimbriae and several bacterial proteins that contribute to hemagglutination, coaggregation, and hemoglobin binding [reviewed in reference (39)]. Since we did not test purified gingipains, it is possible that other proteins regulated by gingipain aminopeptidase activity could be responsible for inhibiting SARS-CoV-2 pseudovirus infection (40). The aminopeptidase gingipain could also cleave ACE2 preventing virus infection. Based on our observation, gingipain-mediated inhibition is also likely due to its known host cell cytotoxicity property (41, 42), consistent with our observation. RGPA and KGP were shown to inhibit HIV by binding the HGP44 domain to the HIV envelope glycoprotein (25) and the ability of catalytically active gingipains to bind to the spike protein to inhibit infection should be explored.
In addition to the known compounds such as PGDHC and gingipains, there are other unidentified anti-SARS-CoV-2 factors produced by P. gingivalis based on our observation that PGDHC and triple gingipain knockout mutants retained significant antiviral activity. Our entry and post-entry assays revealed that these unknown factors present in P. gingivalis supernatants inhibited SARS-CoV-2 pseudovirus at the fusion step of the virus life cycle. Inhibition of fusion was likely through the spike protein since the supernatant had no effect on infection of 293T ACE2 + cells by control VSV pseudovirus. Although fusion was inhibited, entry through binding of spike protein or pseudovirus to ACE2 was not inhibited by the supernatants nor by purified PGDHCs. The antiviral factors present in P. gingivalis supernatant could interfere with a fusion of the viral membrane with the cellular membrane following S trimer-ACE2 interaction but by what mechanism needs to be determined.
The potential for oral bacteria to modulate SARS-CoV-2 infection is entirely possible, in that bacteria are known to possess antiviral compounds that have been shown to disrupt the viral life cycle by interfering with viral attachment and penetration, genome integration, replication, and transcription (20, 21). The ability of P. gingivalis supernatants to inhibit SARS-CoV-2 suggests that SARS-CoV-2 inhibitor(s) are likely soluble secreted factors that can inhibit the fusion step of the SARS-CoV-2 life cycle. Metabolomic, proteomic, and biochemical studies are currently underway to identify the antiviral compounds produced by P. gingivalis.
A previous study showing the association between periodontitis and COVID-19 disease severity suggests that the periodontal pathogens including P. gingivalis may enhance infection or exacerbate disease (43–45). This observation seems inconsistent with our present findings, showing that P. gingivalis could inhibit SARS-CoV-2 infection. One plausible explanation to reconcile these two seemingly contradictory findings is that the association between periodontitis and severe disease may not be due to the direct effect of P. gingivalis on SARS-CoV-2 infection, but rather, due to the inflammation associated with periodontitis. Inflammation elicited by P. gingivalis has been showed to trigger apoptosis in H1N1-infected lung cells (26). Thus, P. gingivalis could directly inhibit SARS-CoV-2 infection per our observation, but it could also promote severe disease by its ability to induce inflammation in people with COVID-19. Indeed, excessive inflammation seen in patients experiencing a “cytokine storm” is strongly associated with severe disease (43, 46, 47).
Accruing evidence suggests that the oral cavity is an important site of SARS-CoV-2 infection. Oral and salivary gland epithelial cells were found to be infected by the virus (45, 48–50). However, we found that the oral gingival epithelial cells are resistant to infection (48). With P. gingivalis residing in the oral cavity, antiviral compounds produced by P. gingivalis would be highly significant in not only preventing the infection of oral cells but also preventing the transmission of the virus present in the saliva. Because of the importance of the oral cavity in infection and transmission of SARS-CoV-2, we will assess whether P. gingivalis will inhibit the infection of salivary gland epithelial cells.
In addition to P. gingivalis, other oral bacteria could play a role in modulating SARS-CoV-2 infection. We tested supernatants isolated from oral bacteria clones representing the various microbial complexes determined by Socransky and coworkers that classify oral commensals and pathogens (31). We found that the oral pathogen A. actinomycetemcomitans and the commensal bacterium A. odontolyticus inhibited pseudoviral infection mainly due to their highly cytotoxic effects on ACE2 + 293T target cells. By contrast, oral streptococci such as S. mitis and S. gordonii had no antiviral effect. It is possible that oral microbiota could also exert their antiviral effects indirectly through their ability to alter gene expression and hence the susceptibility of target cells (47, 51).
Finally, we explored the possible role of the oral microbiome in COVID-19 by assessing the modulatory effect of supernatants generated from microbial communities directly enriched from the saliva of healthy human volunteers. Surprisingly, we found that oral bacteria present in healthy human individuals have differing capacities to modulate SARS-CoV-2 pseudoviral infection. Individuals harbor oral bacteria that were either inhibitory, enhancing, or inactive to SARS-CoV-2 infection. Since the data are preliminary, we are currently investigating what explains this phenomenon. Since aerobic cultures were tested, the anaerobic P. gingivalis is likely not contributing to the modulatory effect on the virus. It could be due to differences in oral microbiome composition and host-microbiome interactions that could regulate the production of these virus-modulatory compounds (51).
Given the importance of the oral microbiome in health and disease, we began to address the role of the oral microbiome in COVID-19, a disease that clearly remains a global public health problem (52). Our in vitro studies revealed striking differences between oral bacteria species, in that certain periodontal pathogens, especially P. gingivalis, produce known and unknown compounds that are capable of inhibiting SARS-CoV-2. The P. gingivalis W83 strain was used based on numerous important host-microbe interaction studies that employed this widely used strain and its association with periodontal disease (53). However, we recognize the in vivo relevance of the P. gingivalis-mediated inhibition of SARS-CoV-2. Hence, we will begin to ascertain whether the antiviral properties are also being produced in vivo by testing various P. gingivalis strains especially primary strains directly isolated from the oral cavity of healthy individuals and periodontitis patients.
Another conclusion from this study is that differences in the oral microbiome composition could explain why certain people may have modulating activities. It can be proposed that these inhibiting or enhancing activities could contribute significantly to either the resistance or susceptibility of people to SARS-CoV-2 infection. More studies are needed to explore these interesting questions regarding the role of the oral microbiome in COVID-19. Ultimately, a clear understanding of how the oral microbiome plays a role in COVID-19 will lead to an effective therapy to treat or prevent infection.
MATERIALS AND METHODS
Saliva collection
De-identified unstimulated saliva was collected from 37 healthy human volunteers, ages 18 and older. All participants understood the nature of the study and provided informed consent. The Nova Southeastern University Institutional Review Board approved the study design as well as the procedure for obtaining informed consent (NSU IRB protocol number 2020–607). All experiments were performed in accordance with the approved guidelines.
Mammalian cell cultures
293T (ATCC CRL-3216), ACE2 + 293, H838 (ATCC CRL-5844), and TZM.bl cells were cultured in DMEM complemented with 10% SBF and 2% Penicillin (5,000 U/mL)/Streptomycin (5 mg/mL) (GIBCO, Waltham, MA, USA), and maintained at 37°C in 5% CO2 atmosphere. The TZM.bl cells were obtained from the NIH HIV Reagent Program, Division of AIDS, NIAID, NIH. TZM-bl cells and ARP-8129 were contributed by Dr. John C. Kappes, Dr. Xiaoyun Wu and Tranzyme Inc.
Bacterial cultures
P. gingivalis (Pg WT) W83 BAA-308 was purchased from American Type Culture Collection (Manassas, VA, USA). P. gingivalis RGPA/RGPB/KGP triple knockout bacteria were provided by Jan S. Potempa, University of Louisville, KY, USA. A serine palmitoyl transferase (SPT) knockout strain of P. gingivalis W83, which does not produce any dihydroceramides (sphingolipid-null strain), was provided by Mary Ellen Davey, The ADA Forsyth Institute, Cambridge, MA, USA. Bacteria were cultured on a sheep blood agar plate at 37°C under anaerobic conditions using the Anaeropack system (Mitsubishi Gas Chemical, Tokyo, Japan). Once colonies were detected, the bacteria were transferred to a 10 mL liquid growing medium in the same anaerobic conditions. To grow bacteria from saliva, cell debris was first removed by brief centrifugation, and subsequently, 10 μL of saliva was cultured in a 10 mL liquid medium. After 24 h, bacteria supernatants were collected, filtered, and stored at −80°C.
The following oral bacterial strains were purchased from American Type Culture Collection (Manassas, VA, USA) and used in this study: S. gordonii (Sg) 10558, A. actinomycetemcomitans (Aa) 29523, Aa Y4 43718, A. odontolytica (Ao)17982, S. mitis (Sm) 49456. Aa 29523, Aa Y4, and Sm were on a sheep blood agar plate at 37°C under 5% CO2 using the Anaeropack CO2 (Mitsubishi Gas Chemical, Tokyo, Japan). Sg was cultured on a sheep blood agar plate at 37°C with aerobic conditions. Once colonies were detected, Pg W83, Sg, Ao, and Sm were transferred in trypticase soy broth (TSB; BD, San Jose, CA, USA) medium supplemented with yeast extract (BD, San Jose, CA, USA), L-cysteine HCL, hemine, and menadoine (Sigma-Aldrich, St. Louis, MO, USA). Aa 29523 and Aa Y4 were transferred in brain heart infusion (BHI; BD, San Jose, CA, USA) broth medium. After 7 days, bacteria were removed by centrifugation at 2,500 rpm for 10 minutes at room temperature. 0.22 μm filtered supernatants were used in the study.
Isolation of PGDHC from P. gingivalis
PGDHC lipid class from P. gingivalis (ATCC #33277, type strain) was isolated as previously described (54). Briefly, P. gingivalis was grown in basal (peptone, trypticase, and yeast extract, [BBL]) medium supplemented with hemin and menadione (Sigma-Aldrich, St. Louis, MO, USA) and BHI. Total lipids were extracted from bacterial pellet samples using the phospholipid extraction procedure of Bligh and Dyer as modified by Garbus (55, 56). Total lipids were fractionated by a two-step HPLC method as previously described (54, 57). Total lipids were separated first by semi-preparative HPLC separation using a neutral HPLC solvent (hexane:isopropanol:water, 6:8:0.75, vol/vol/vol) followed by LC-MS evaluation of each fraction (see below), and pooling of fractions enriched for PG DHC lipids. The LC-MS analysis was performed using a Sciex500 UHPLC-qTOF instrument, and lipid classes were quantified using the multiple reaction monitoring detection modes for negative ions. The pooled fractions were then re-fractionated using the same HPLC solvent supplemented with 0.1% acetic acid. The fractions containing PGDHC were identified by LC-MS and were pooled to yield a highly enriched PG DHC lipid class as previously described (57). This method provided highly enriched lipid fractions (>97% purity as determined by mass spectrometric and NMR analysis) (57), and the purified lipid fractions were devoid of lipoprotein contaminants.
Soluble SARS-CoV-2 spike protein production
Plasmid containing the SARS-CoV-2 S ectodomain with mutated Furin cutting site and two prolines to enhance stability that was described previously (58) was kindly donated by Dr. Jason S. McLellan from The University of Texas at Austin, TX. Two hundred milliliters of Expi293 cells suspension (GIBCO, Waltham, MA, USA) were transfected with the plasmid encoding the stabilized SARS-CoV-2 soluble S protein with the Expifectamine kit (GIBCO, Waltham, MA, USA) following manufacturer’s protocol. After 6 days of incubation, the cell suspension was centrifuged, and the supernatant was clarified by 0.22 µm filtration. Supernatant was then processed through a His-tag cobalt resin (ThermoFisher, Waltham, MA, USA) following manufacturer’s protocol. The His-tag S protein was purified by gel filtration using the Akta Explorer 100 system with a high-load Superdex pg 200 16/600 column (GE Life Sciences, USA). Main peak fraction was collected, and protein purity was verified by BN PAGE.
Generation of ACE2+ 293T cells
The 293T cells were seeded in a 6-well plate and infected with a hACE2 lentivirus. Infected cells were incubated for 72 h. After the incubation period, the cells were treated with 500 μg/mL Hygromycin B (Fisher Scientific, Waltham, MA, USA) for about 14 days to select stable ACE2-expressing cells. Surviving cells were grown to confluency in a T75 flask, and aliquots of cells were cryopreserved in complete DMEM/10%DMSO in liquid nitrogen.
Pseudovirus and lentivirus production
Five million 293T cells were seeded in a T75 flask and let adhere overnight. The day after, the medium was replaced with 1:1 optiMEM (GIBCO, Waltham, MA) and 10% FBS DMEM (GIBCO, Waltham, MA, USA). Seventy-five microliters of Lipofectamine2000 (Invitrogen, Waltham, MA, USA) were added to 1.8 mL optiMEM. To produce lentivirus carrying ACE2 expression cassette 14.2 μg of pLENTI-ACE2 (ADDGENE, Watertown, MA, USA), 16 μg of pCMVdR 8.2 (ADDGENE, Watertown, MA), and 6.4 μg of pCMV-VSV-G (ADDGENE, Watertown, MA, USA) were mixed in 1.8 mL optiMEM. To produce SARS-CoV-2 pseudotyped lentivirus 16 μg of pCMDdR8.2, 14.2 μg of pHAGE-Luc-zGFP (BEI resources, Manassas, VA, USA), and 6.4 μg of CoV-2 glycoprotein expression plasmid (Wuhan version: pHDM BEI resources; for the BA.4/5 pLV-Spike V13 invivoGen plv-spike-v13) were mixed in 1.8 mL complete optiMEM. Both Lipofectamine-optiMEM and Plasmid-optiMEM solutions were incubated for 5 minutes at RT. After incubation, plasmid solution and lipofectamine2000 were mixed and incubated for 15 minutes at RT. After incubation, the final solution was added dropwise to the cells while gently shaking. The day after, the medium was replaced with 15 mL complete DMEM and 1× non-essential amino acids (GIBCO, Waltham, MA, USA). Two supernatant batches were collected after 24 and 48 h. The supernatant was collected and filtered with 0.45 μm PVDF Millex-GV filter (Millipore,Burlington, MA, USA). The cleared medium was supplemented with 1:5 Lenti Concentrator (OriGene, Rockville, MD, USA) and incubated 2–4 h at 4°C. The cold solution was then centrifuged at 4,000 RPM at 4°C for 40 minutes. Supernatant was removed, and the pellet was resuspended in 1/75 of the initial volume with complete DMEM. Seventy microliters of aliquots of the virus were stored at −80°C.
Pseudovirus infection inhibition assay
The ACE2 + 293 T cells or H838 cells were trypsinized and seeded in a 96-well plate at a concentration of 5000 cells per well in a volume of 100 μL of DMEM complete medium. Cells were incubated and allowed to attach for 2 h at 37C/5% CO2. In a separate plate, treatment curves (treatment concentration + complete DMEM) were prepared by serial dilution in a final volume of 50 μL. After incubation, the treatment was added to the cells and incubated overnight. The treatments were with the following: P. gingivalis, P. gingivalis triple-gingipain knockout, or SPT knockout supernatants in 10% FBS DMEM, TSB, or BHI as well as P. gingivalis LPS or PGDHC compounds. Treatment with supernatants from bacteria grown from human saliva samples was also performed.
Pseudoviruses expressing the spike protein were added the next day. In another 96-well plate, the virus and treatments of either 293T or H838 cells were mixed in another serial dilution and left to incubate for 4 h before 50 μL were added to the cells in a final volume of 200 μL. Seventy-two hours after infection, luciferase was measured using BriteLite Plus (Perkinelmer, Waltham, MA, USA) and SynergyH1 plate reader.
Postentry virus inhibition assay
To assess if our compounds have post-entry effect, the infection was carried out by spinoculation. In brief, the cells were exposed to the virus for 4 h followed by 40 minutes of centrifugation at 3,000 RPM. The medium was removed, the cell was washed with PBS, and supplemented with fresh medium. The day after, appropriate treatments were applied. Luciferase activity was measured at 72 h after using BriteLite as described above.
Syncytium formation assay
ACE2 + 293 were transiently transfected with pALPS expressing tat-p2a-rev cassette (Addgene, Watertown, MA, USA). The TZM.bl cells were transiently transfected with Wuhan CoV-2 spike expression plasmid pHDM (BEI resources, Manassas, VA, USA). ACE2 + 293 expressing tat/rev cells were treated with P. gingivalis supernatant, PGDHC, or medium alone overnight. The day after, TZM.bl expressing spikes were added to the 293 ACE2 +expressing Tat/rev cells and incubated for 48 h. After 2 days, the co-cultures were assessed for the formation of syncytia and luciferase activity as described above.
WST-1 cytotoxicity assay
293 ACE2 +cells, at ~90% confluency, were trypsinized and seeded in a 96-well plate at a concentration of 5,000 per well in a volume of 100 μL of DMEM complete medium. Cells were incubated and allowed to attach for 2 h at 37C at 5% CO2. In a separate plate, treatment curves were prepared by serial dilution in a final volume of 50 μL per well. After the incubation, the treatment was added to the cells and incubated for 72 h. The treatments used are as follows: Pg Supernatant in TSB, BHI, and 10% FBS DMEM, Pg LPS, Pg RGPA/RGPB/KGP Knockout Supernatant in 10% FBS in DMEM, TSB, BHI, 10% FBS DMEM, PGDHC. On the third day, toxicity was assessed by WST-1 (Cayman chemicals, Ann Arbor, MI, USA). The medium was removed and replaced with 100 μL of our complete DMEM plus 10 μL of WST-1. In total, 450 nm absorbance was then measured using the SynergyH1 plate reader (BioTek, Winooski, VT, USA) every 5 minutes over the course of 2 h.
Biolayer interferometry assay
To assess the ability of P. gingivalis supernatant and PGDHC to interfere with the ACE2-CoV-2 Spike protein interaction, we loaded anti-human IgG Fc biosensors (Gatorbio, Palo Alto, CA, USA) for 360 seconds in a solution of 5 μg/mL ACE2 Fc-tagged protein (ACRO Biosystems, Newark, DE, USA) in Buffer K (Gatorbio, Palo Alto, CA, USA). After a 120-minute baseline, the biosensors were incubated for 300 seconds in a solution containing 15% P. gingivalis supernatant, 15 μg/mL PGHDC, or medium alone control. Following another 120-second baseline, the biosensors were immersed with a solution containing 10 μg/mL of soluble CoV-2 Spike protein (see Supplementary Information) for 600 seconds. As the last step, a 600-second dissociation step was performed in pure Buffer K.
We developed a method to analyze the inhibitory effect of PGDHC directly on pseudotyped lentiviruses. In brief, hFc biosensors (Gatorbio, Palo Alto, CA, USA) were loaded with fc tagged ACE2 protein (ACRO biosystems, Newark, DE, USA) until a 1–1.5 nm shift was reached. Following the 120-second baseline, the sensors were dipped in a solution containing CoV-2 pseudotyped lentivirus, lentivirus with 5 μg/mL neutralizing antibody, and virus with non-specific rabbit or non-specific IgG1/IgG2A antibodies.
Statistical analysis
Differences in viral infection susceptibility (measured by luciferase activity) were statistically evaluated using one-way ANOVA and Tukey post-hoc test for multiple comparisons. Student t-test was used for single group comparison. The experiments were performed in triplicate from the same treatment preparations, and the means ± standard deviations were calculated. Differences with a probability of 5% (P < 0.05) were considered statistically significant.
ACKNOWLEDGMENTS
The authors thank Roodelyne Pierrelus, Sunniva Ruiz, and Margarita Uribe for assisting in saliva collection. The authors are grateful to Shin Nakamura for providing P. gingivalis cultures and supernatants. The authors thank Ms. Caitlin Dargue for excellent administrative support.
This work was supported by the Florida Blue Foundation (M.C. and S.S.A.), NIH NIDCR R01-DE027249 (M.C. and A.B.), R01-DE031159 (M.E.D.), and NIGMS R16GM150469) (A.H.).
Conceptualization, A.B., M.J.C, T.K., and A.H.; methodology, A.B., A.C., M.J.C., C.G.G., A.L., M.R.P., F.J.N., J.P., R.M., and M.E.D.; validation, A.B., M.J.C., A.H., C.G., and A.C.; resources, M.J.C., T.K., and S.S.A.; data curation, A.B., M.J.C., and A.H.; writing/original draft preparation, A.B, M.J.C., A.C., T.K, M.E.D., and F.C.H.; supervision, A.B., M.J.C., and T.K.; project administration, M.J.C., S.A.A., T.K., A.B., and C.G. All authors have read and agreed to the published version of the manuscript.
Contributor Information
Mark J. Cayabyab, Email: mcayabya@nova.edu.
Justin R. Kaspar, The Ohio State University College of Dentistry, Columbus, Ohio, USA
DATA AVAILABILITY
The data sets used and analyzed in this study are available from the corresponding author (mcayabya@nova.edu) upon request.
REFERENCES
- 1. McGowan VJ, Bambra C. 2022. COVID-19 mortality and deprivation: pandemic, syndemic, and endemic health inequalities. Lancet Public Health 7:e966–e975. doi: 10.1016/S2468-2667(22)00223-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed F, Ahmed N, Pissarides C, Stiglitz J. 2020. Why inequality could spread COVID-19. Lancet Public Health 5:e240. doi: 10.1016/S2468-2667(20)30085-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silvestre FJ, Márquez-Arrico CF. 2021. COVID-19 and periodontitis: a dangerous association? Front Pharmacol 12:789681. doi: 10.3389/fphar.2021.789681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tinsley N, Cook N. 2020. The gut microbiome and its interaction with health, disease, treatment response and toxicity in patients advanced cancer: focus on lung cancer and immunotherapy. Transl Lung Cancer Res 9:2305–2307. doi: 10.21037/tlcr-20-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bao L, Zhang C, Dong J, Zhao L, Li Y, Sun J. 2020. Oral microbiome and SARS-CoV-2: beware of lung co-infection. Front Microbiol 11:1840. doi: 10.3389/fmicb.2020.01840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bucci V, Ward DV, Bhattarai S, Rojas-Correa M, Purkayastha A, Holler D, Qu MD, Mitchell WG, Yang J, Fountain S, Zeamer A, Forconi CS, Fujimori G, Odwar B, Cawley C, Moormann AM, Wessolossky M, Maldonado-Contreras A. 2023. The intestinal microbiota predicts COVID-19 severity and fatality regardless of hospital feeding method. mSystems 8:e0031023. doi: 10.1128/msystems.00310-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shreiner AB, Kao JY, Young VB. 2015. The gut microbiome in health and in disease. Curr Opin Gastroenterol 31:69–75. doi: 10.1097/MOG.0000000000000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yeoh YK, Zuo T, Lui G-Y, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui D-C, Chow KM, Ng SSS, Li T-M, Ng RW, Yip TC, Wong G-H, Chan FK, Wong CK, Chan PK, Ng SC. 2021. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70:698–706. doi: 10.1136/gutjnl-2020-323020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harper A, Vijayakumar V, Ouwehand AC, Ter Haar J, Obis D, Espadaler J, Binda S, Desiraju S, Day R. 2020. Viral infections, the microbiome, and probiotics. Front Cell Infect Microbiol 10:596166. doi: 10.3389/fcimb.2020.596166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rafiqul Islam SM, Foysal MJ, Hoque MN, Mehedi HMH, Rob MA, Salauddin A, Tanzina AY, Biswas S, Noyon SH, Siddiki A, Tay A, Mannan A. 2022. Dysbiosis of oral and gut microbiomes in SARS-CoV-2 infected patients in Bangladesh: elucidating the role of opportunistic gut microbes. Front Med (Lausanne) 9:821777. doi: 10.3389/fmed.2022.821777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andrews M, Gao H, Datta S, Katz J. 2023. Increased odds for COVID-19 infection among individuals with periodontal disease. Clin Oral Investig 27:5925–5933. doi: 10.1007/s00784-023-05204-x [DOI] [PubMed] [Google Scholar]
- 12. Moradi Haghgoo J, Torkzaban P, Farhadian M, Moosavi Sedeh SA. 2023. Association between the severity of periodontitis, COVID-19, C-reactive protein and interleukin-6 levels in hospitalized patients: a case‒control study. BMC Oral Health 23:556. doi: 10.1186/s12903-023-03270-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song J, Wu Y, Yin X, Zhang J. 2023. Relationship between periodontitis and COVID-19: a bidirectional two-sample Mendelian randomization study. Health Sci Rep 6:e1413. doi: 10.1002/hsr2.1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moreno CM, Boeree E, Freitas CMT, Weber KS. 2023. Immunomodulatory role of oral microbiota in inflammatory diseases and allergic conditions. Front Allergy 4:1067483. doi: 10.3389/falgy.2023.1067483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Qv W, Ma Y, Zhang Y, Ding C, Chu M, Chen F. 2022. The interplay between oral microbes and immune responses. Front Microbiol 13. doi: 10.3389/fmicb.2022.1009018 [DOI] [Google Scholar]
- 16. Haran JP, Bradley E, Zeamer AL, Cincotta L, Salive M-C, Dutta P, Mutaawe S, Anya O, Meza-Segura M, Moormann AM, Ward DV, McCormick BA, Bucci V. 2021. Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms and long COVID. JCI Insight 6:e152346. doi: 10.1172/jci.insight.152346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan L, Zhong M-M, Liu Q, Chen Y, Zhao Y-Q, Zhao J, Dusenge MA, Feng Y, Ye Q, Hu J, Ou-Yang Z-Y, Zhou Y-H, Guo Y, Feng Y-Z. 2023. Potential interaction between the oral microbiota and COVID-19: a meta-analysis and bioinformatics prediction. Front Cell Infect Microbiol 13:1193340. doi: 10.3389/fcimb.2023.1193340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mojon P. 2002. Oral health and respiratory infection. J Can Dent Assoc 68:340–345. [PubMed] [Google Scholar]
- 19. Aquino-Martinez R, Hernández-Vigueras S. 2021. Severe COVID-19 lung infection in older people and periodontitis. J Clin Med 10:279. doi: 10.3390/jcm10020279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raihan T, Rabbee MF, Roy P, Choudhury S, Baek K-H, Azad AK. 2021. Microbial metabolites: the emerging hotspot of antiviral compounds as potential candidates to avert viral pandemic alike COVID-19. Front Mol Biosci 8:732256. doi: 10.3389/fmolb.2021.732256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chaisuwan W, Phimolsiripol Y, Chaiyaso T, Techapun C, Leksawasdi N, Jantanasakulwong K, Rachtanapun P, Wangtueai S, Sommano SR, You S, Regenstein JM, Barba FJ, Seesuriyachan P. 2021. The antiviral activity of bacterial, fungal, and algal polysaccharides as bioactive ingredients: potential uses for enhancing immune systems and preventing viruses. Front Nutr 8:772033. doi: 10.3389/fnut.2021.772033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghosh A, Joseph B, Anil S. 2022. Does periodontitis influence the risk of COVID-19? A scoping review. Clin Exp Dent Res 8:1011–1020. doi: 10.1002/cre2.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sena K, Furue K, Setoguchi F, Noguchi K. 2021. Altered expression of SARS-CoV-2 entry and processing genes by Porphyromonas gingivalis-derived lipopolysaccharide, inflammatory cytokines and prostaglandin E2 in human gingival fibroblasts. Arch Oral Biol 129:105201. doi: 10.1016/j.archoralbio.2021.105201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Griffen AL, Becker MR, Lyons SR, Moeschberger ML, Leys EJ. 1998. Prevalence of Porphyromonas gingivalis and periodontal health status. J Clin Microbiol 36:3239–3242. doi: 10.1128/JCM.36.11.3239-3242.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xie H, Belogortseva NI, Wu J, Lai W-H, Chen C. 2006. Inhibition of human immunodeficiency virus type 1 entry by a binding domain of Porphyromonas gingivalis gingipain. Antimicrob Agents Chemother 50:3070–3074. doi: 10.1128/AAC.01578-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Y, Zhou R, Yi Z, Li Y, Fu Y, Zhang Y, Li P, Li X, Pan Y. 2018. Porphyromonas gingivalis induced inflammatory responses and promoted apoptosis in lung epithelial cells infected with H1N1 via the Bcl‑2/Bax/Caspase‑3 signaling pathway. Mol Med Rep 18:97–104. doi: 10.3892/mmr.2018.8983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moye ZD, Valiuskyte K, Dewhirst FE, Nichols FC, Davey ME. 2016. Synthesis of sphingolipids impacts survival of Porphyromonas gingivalis and the presentation of surface polysaccharides. Front Microbiol 7:1919. doi: 10.3389/fmicb.2016.01919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pyrc K, Strzyz P, Milewska A, Golda A, Schildgen O, Potempa J. 2011. Porphyromonas gingivalis enzymes enhance infection with human metapneumovirus in vitro. J Gen Virol 92:2324–2332. doi: 10.1099/vir.0.032094-0 [DOI] [PubMed] [Google Scholar]
- 29. Dzimianski JV, Lorig-Roach N, O’Rourke SM, Alexander DL, Kimmey JM, DuBois RM. 2020. Rapid and sensitive detection of SARS‑CoV‑2 antibodies by biolayer interferometry. Sci Rep 10:21738. doi: 10.1038/s41598-020-78895-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carson Cameron BP, Nathan O, Andrew C, Smedley J, John L, David OA. (Ed ForteBio) (ForteBio, KBI Biopharma, Fremont, CA, 2019)
- 31. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. 1998. Microbial complexes in subgingival plaque. J Clin Periodontol 25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x [DOI] [PubMed] [Google Scholar]
- 32. Henderson B, Ward JM, Ready D. 2000. Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen? Periodontol 2000 54:78–105. doi: 10.1111/j.1600-0757.2009.00331.x [DOI] [PubMed] [Google Scholar]
- 33. Nativel B, Couret D, Giraud P, Meilhac O, d’Hellencourt CL, Viranaïcken W, Da Silva CR. 2017. Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Sci Rep 7:15789. doi: 10.1038/s41598-017-16190-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rocha FG, Ottenberg G, Eure ZG, Davey ME, Gibson FC. 2021. Sphingolipid-containing outer membrane vesicles serve as a delivery vehicle to limit macrophage immune response to Porphyromonas gingivalis. Infect Immun 89:e00614-20. doi: 10.1128/IAI.00614-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rocha FG, Moye ZD, Ottenberg G, Tang P, Campopiano DJ, Gibson FC, Davey ME. 2020. Porphyromonas gingivalis sphingolipid synthesis limits the host inflammatory response. J Dent Res 99:568–576. doi: 10.1177/0022034520908784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamada C, Akkaoui J, Ho A, Duarte C, Deth R, Kawai T, Nichols F, Lakshmana MK, Movila A. 2020. Potential role of phosphoglycerol dihydroceramide produced by periodontal pathogen Porphyromonas gingivalis in the pathogenesis of Alzheimer’s disease. Front Immunol 11:591571. doi: 10.3389/fimmu.2020.591571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duarte C, Yamada C, Garcia C, Akkaoui J, Ho A, Nichols F, Movila A. 2022. Crosstalk between dihydroceramides produced by Porphyromonas gingivalis and host lysosomal cathepsin B in the promotion of osteoclastogenesis. J Cell Mol Med 26:2841–2851. doi: 10.1111/jcmm.17299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanzaki H, Movila A, Kayal R, Napimoga MH, Egashira K, Dewhirst F, Sasaki H, Howait M, Al-Dharrab A, Mira A, Han X, Taubman MA, Nichols FC, Kawai T. 2017. Phosphoglycerol dihydroceramide, a distinctive ceramide produced by Porphyromonas gingivalis, promotes RANKL-induced osteoclastogenesis by acting on non-muscle myosin II-A (Myh9), an osteoclast cell fusion regulatory factor. Biochim Biophys Acta Mol Cell Biol Lipids 1862:452–462. doi: 10.1016/j.bbalip.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lunar Silva I, Cascales E. 2021. Molecular strategies underlying Porphyromonas gingivalis virulence. J Mol Biol 433:166836. doi: 10.1016/j.jmb.2021.166836 [DOI] [PubMed] [Google Scholar]
- 40. Veillard F, Potempa B, Poreba M, Drag M, Potempa J. 2012. Gingipain aminopeptidase activities in Porphyromonas gingivalis. Biol Chem 393:1471–1476. doi: 10.1515/hsz-2012-0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morioka M, Hinode D, Nagata A, Hayashi H, Ichimiya S, Ueda M, Kido R, Nakamura R. 1993. Cytotoxicity of Porphyromonas gingivalis toward cultured human gingival fibroblasts. Oral Microbiol Immunol 8:203–207. doi: 10.1111/j.1399-302x.1993.tb00560.x [DOI] [PubMed] [Google Scholar]
- 42. Shah HN, Gharbia SE, O’Toole CM. 1992. Assessment of the relative cytotoxicity of Porphyromonas gingivalis cells, products, and components on human epithelial cell lines. J Periodontol 63:44–51. doi: 10.1902/jop.1992.63.1.44 [DOI] [PubMed] [Google Scholar]
- 43. Tamimi F, Altigani S, Sanz M. 2022. Periodontitis and coronavirus disease 2019. Periodontol 2000 89:207–214. doi: 10.1111/prd.12434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Takahashi Y, Watanabe N, Kamio N, Kobayashi R, Iinuma T, Imai K. 2021. Aspiration of periodontopathic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID-19. J Oral Sci 63:1–3. doi: 10.2334/josnusd.20-0388 [DOI] [PubMed] [Google Scholar]
- 45. Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, Conde CD, Gasmi B, Stein S, Beach M, et al. 2021. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med 27:892–903. doi: 10.1038/s41591-021-01296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marouf N, Cai W, Said KN, Daas H, Diab H, Chinta VR, Hssain AA, Nicolau B, Sanz M, Tamimi F. 2021. Association between periodontitis and severity of COVID-19 infection: a case–control study. J Clin Periodontol 48:483–491. doi: 10.1111/jcpe.13435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bachtiar BM, Bachtiar EW, Kusumaningrum A, Sunarto H, Soeroso Y, Sulijaya B, Apriyanti E, Fragrantia Theodorea C, Putra Pratomo I, Efendi D, Yudhistira . 2023. Porphyromonas gingivalis association with inflammatory markers and exosomal miRNA-155 in saliva of periodontitis patients with and without diabetes diagnosed with COVID-19. Saudi Dent J 35:61–69. doi: 10.1016/j.sdentj.2022.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bontempo A, Chirino A, Heidari A, Boparai S, Arora S, Ruiz S, Antonson SA, Kawai T, Cayabyab MJ. 2023. Assessment of SARS-CoV-2 entry in gingival epithelial cells expressing CD147. Eur J Oral Sci 131:e12906. doi: 10.1111/eos.12906 [DOI] [PubMed] [Google Scholar]
- 49. Chen L, Zhao J, Peng J, Li X, Deng X, Geng Z, Shen Z, Guo F, Zhang Q, Jin Y, Wang L, Wang S. 2020. Detection of SARS-CoV-2 in saliva and characterization of oral symptoms in COVID-19 patients. Cell Prolif 53:e12923. doi: 10.1111/cpr.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhong M, Lin B, Pathak JL, Gao H, Young AJ, Wang X, Liu C, Wu K, Liu M, Chen J-M, Huang J, Lee L-H, Qi C-L, Ge L, Wang L. 2020. ACE2 and furin expressions in oral epithelial cells possibly facilitate COVID-19 infection via respiratory and fecal-oral routes. Front Med (Lausanne) 7:580796. doi: 10.3389/fmed.2020.580796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li N, Ma WT, Pang M, Fan QL, Hua JL. 2019. The commensal microbiota and viral infection: a comprehensive review. Front Immunol 10:1551. doi: 10.3389/fimmu.2019.01551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McVernon J, Liberman J. 2023. WHO keeps covid-19 a public health emergency of international concern. BMJ 380:504. doi: 10.1136/bmj.p504 [DOI] [PubMed] [Google Scholar]
- 53. Murugaiyan V, Utreja S, Hovey KM, Sun Y, LaMonte MJ, Wactawski-Wende J, Diaz PI, Buck MJ. 2024. Defining Porphyromonas gingivalis strains associated with periodontal disease. Sci Rep 14:6222. doi: 10.1038/s41598-024-56849-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nichols FC, Clark RB, Liu Y, Provatas AA, Dietz CJ, Zhu Q, Wang Y-H, Smith MB. 2020. Glycine lipids of Porphyromonas gingivalis are agonists for toll-like receptor 2. Infect Immun 88:e00877-19. doi: 10.1128/IAI.00877-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. doi: 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- 56. Garbus J, Deluca HF, Loomans ME, Strong FM. 1963. The rapid incorporation of phosphate into mitochondrial lipids. J Biol Chem 238:59–63. [PubMed] [Google Scholar]
- 57. Nichols FC, Clark RB, Maciejewski MW, Provatas AA, Balsbaugh JL, Dewhirst FE, Smith MB, Rahmlow A. 2020. A novel phosphoglycerol serine-glycine lipodipeptide of Porphyromonas gingivalis is a TLR2 ligand. J Lipid Res 61:1645–1657. doi: 10.1194/jlr.RA120000951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Amanat F, Strohmeier S, Rathnasinghe R, Schotsaert M, Coughlan L, García-Sastre A, Krammer F. 2021. Introduction of two prolines and removal of the polybasic cleavage site lead to higher efficacy of a recombinant spike-based SARS-CoV-2 vaccine in the mouse model. mBio 12:e02648-20. doi: 10.1128/mBio.02648-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and analyzed in this study are available from the corresponding author (mcayabya@nova.edu) upon request.