Abstract
This study aimed to investigate the association between non-traditional lipid profiles and the risk of 1-year vascular events in patients who were already using statins before stroke and had admission LDL-C < 100 mg/dL. This study was an analysis of a prospective, multicenter, nationwide registry of consecutive patients with acute ischemic stroke patients who treated with statin before index stroke and LDL-C < 100 mg/dL on admission. Non-traditional lipid profiles including non-HDL, TC/HDL ratio, LDL/HDL ratio, and TG/HDL ratio were analyzed as a continuous or categorical variable. The primary vascular outcome within one year was a composite of recurrent stroke (either hemorrhagic or ischemic), myocardial infarction (MI) and all-cause mortality. Hazard ratios (95% Cis) for 1-year vascular outcomes were analyzed using the Cox PH model for each non-traditional lipid profiles groups. A total of 7028 patients (age 70.3 ± 10.8years, male 59.8%) were finally analyzed for the study. In unadjusted analysis, no significant associations were observed in the quartiles of LDL/HDL ratio and 1-year primary outcome. However, after adjustment of relevant variables, compared with Q1 of the LDL/HDL ratio, Q4 was significantly associated with increasing the risk of 1-year primary outcome (HR 1.48 [1.19–1.83]). For the LDL/HDL ratio, a linear relationship was observed (P for linearity < 0.001). Higher quartiles of the LDL/HDL ratio were significantly and linearly associated with increasing the risk of 1-year primary vascular outcomes. These findings suggest that even during statin therapy with LDL-C < 100 mg/dl on admission, there should be consideration for residual risk based on the LDL/HDL ratio, following stroke.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73851-5.
Keywords: Non-traditional lipid profiles, Lipid ratio, LDL/HDL ratio, LDL-cholesterol, Acute ischemic stroke, Vascular outcome, Residual cardiovascular risk
Subject terms: Endocrinology, Neurology
Introduction
Stroke constitutes a significant cause of morbidity and mortality, especially among the elderly population. Lowering low-density lipoprotein cholesterol (LDL-C) through statin treatment is crucial for preventing atherosclerotic cardiovascular diseases, including stroke1. For secondary prevention, the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial emphasized the benefit of high-dose statin therapy for stroke patients with admission LDL-C > 100 mg/dL2. Additionally, the Treat Stroke to Target (TST) trial demonstrated that maintaining LDL-C < 70 mg/dL significantly reduces the risk of long-term vascular events compared to keeping LDL-C in the range of 90-110 mg/dL in atherosclerotic ischemic stroke3.
In addition to LDL-C, non-traditional lipid profiles, such as non-high density lipoprotein (non-HDL) cholesterol and lipid ratios, have been recognized as contributors to residual vascular risk4. Previous studies have considered non-HDL cholesterol, triglyceride (TG)/HDL ratio, and LDL/HDL ratio as residual risk factors for cardiovascular diseases in patients with coronary artery diseases5,6. Elevated lipid ratios in patients with general risk factors have been also associated with an increased risk of stroke7.
Among ischemic stroke patients, with the escalating use of cholesterol-lowering therapy for primary and secondary prevention, there is an increasing proportion of patients who were already on statin treatment before the index event and had well-controlled LDL-C levels (< 100 mg/dL) upon admission. In a previous study, there was a relationship between admission LDL-C levels and early vascular outcomes for patients not taking a statin at the time of the index event but not for patients already on statins8. Therefore, the best post-stroke target lipid level for patients who were already on statins and had well-controlled LDL-C levels at the time of their index stroke remains unclear. In such cases, the impact of non-traditional lipid profiles on outcome might need consideration. Research on the prognostic implications and targets for treatment of non-traditional lipid profiles for patients with statin pretreatment and baseline LDL-C < 100 mg/dL remains limited. While statin therapy may be associated with the reduced risk of vascular outcome for these patients with low LDL-C on admission9, understanding the clinical significance of non-traditional lipid profiles beyond LDL-C could hold importance.
Therefore, this study aimed to investigate the association between non-traditional lipid profiles and the risk of 1-year vascular events in patients who were already using statins before index stroke and had admission LDL-C < 100 mg/dL.
Methods
Subjects
This study was an analysis of a prospective, multicenter, nationwide registry of consecutive patients with acute stroke or transient ischemic attack (TIA) admitted to 18 academic hospitals in South Korea, the Clinical Research Center for Stroke-Korea (CRCS-K) registry. Detailed methodologic information about the CRCS-K registry has been reported previously10,11. We identified patients with acute cerebrovascular events admitted between January 2011 and July 2020 (N = 75690). Among the patients with acute cerebrovascular events, we included ischemic stroke or TIA with lesion-positive on diffusion weighted imaging (DWI) within 7 days of onset (N = 68468), patients classified as non-cardioembolic ischemic stroke according to the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria (N = 52878), statin pretreatment before index stroke (N = 10189) and admission LDL-C < 100 mg/dl(N = 7063). Patients without information on fasting lipid profiles at admission were excluded. A detailed patient selection flowchart is shown in Supplemental Fig. 1.
Ethics statement
Clinical information was collected from the CRCS-K registry with approval from the local institutional review boards of all the participating centers, including Seoul National University Bundang Hospital and Chonnam National University Hospital. A waiver for informed consent was provided by the Institutional Review Board of Seoul National University Bundang Hospital and Chonnam National University Hospital due to study subject anonymity and minimal risk to the participants. The data used in this study are available upon reasonable request following the submission of a legitimate academic research proposal to be assessed by the CRCS-K steering committee and all research was performed in accordance with relevant guidelines and regulations.
Ethics approval
The current study was approved by local institutional review boards at all participating centers, including Chonnam National University Hospital (CNUH-2024-032).
Data collection
Demographic, clinical, imaging, and laboratory data were prospectively collected. Lipid profiles, including LDL-C, total cholesterol (TC), HDL-C, and TG levels, were obtained during the first fasting period after admission. Four non-traditional lipid profile parameters, non-HDL, TC/HDL ratio, LDL/HDL ratio, and TG/HDL ratio, were analyzed as continuous and categorical variables. The patients were classified into quartiles, with each quartile representing 25% of the distribution, for each non-traditional lipid profile for comparison: Q1 (0–25.0%), Q2 (25.1-50.0%), Q3 (50.1–75.0%) and Q4 (75.1–100%). Ischemic stroke subtypes were classified according to the TOAST criteria, which were refined to incorporate additional information based on modern imaging studies12,13. We analyzed non-cardioembolic etiologies, including large artery atherosclerosis (LAA), small vessel occlusion (SVO), other determined etiology (OD), and undetermined etiology (UD), according to each lipid profiles.
Outcomes
The primary vascular outcome within one year was a composite of recurrent stroke (either hemorrhagic or ischemic), myocardial infarction (MI) and all-cause mortality. The secondary vascular outcomes were the individual outcomes of (a) all-cause mortality, (b) stroke (either ischemic or hemorrhagic), and (c) MI. Detailed definitions of the vascular outcome events and methods of outcome capture used in the current study are described in the Supplemental Methods and previous reports10,11.
Statistical analysis
Baseline characteristics and outcomes were compared among each non-traditional lipid profiles quartiles by using the chi-square test, ANOVA, or Kruskal‒Wallis test according to the type of variable. The event probability of 1-year vascular outcomes according to the non-traditional lipid profiles quartiles in all patients and in patients by stroke subtype was calculated by using the Kaplan–Meier method, and the log-rank test was performed to analyze differences among the groups. Hazard ratios (HRs) and 95% confidence intervals (95% Cis) for 1-year vascular outcomes were analyzed using the Cox proportional hazards model for each non-traditional lipid profiles groups. Adjustments were made for the following 18 predetermined variables with clinically relevant associations with the outcome variables; age, male sex, BMI, NIHSS score, history of stroke, history of coronary artery diseases, HTN, DM, dyslipidemia, smoking, prior antiplatelet, in-hospital anti-diabetic treatment, in-hospital antihypertensive treatment, statin, glucose, creatinine, LDL-C and SBP. In the analysis of the LDL/HDL ratio, although LDL-C has been restricted to less than 100 mg/dl, it remains a crucial risk marker with significant variability. To investigate whether the LDL/HDL ratio has independent clinical significance, LDL-C was included as an adjusted variable. The modifying effect of stroke subtype on the relationships between each non-traditional lipid profiles groups and clinical outcomes was explored by separately introducing an interaction term of ischemic stroke subtype and LDL/HDL ratio quartile groups (and TC/HDL ratio) into the models.
Two-sided p values < 0.05 were considered indicative of significance. Given the known insensitivity of interaction testing, evidence of heterogeneity was considered present with p values ≤ 0.10. In addition, the goodness of fit of the four models were compared using the Akaike (AIC) and Bayesian information criterion (BIC). Lower AIC and BIC indicate a better fit. Statistical analyses were performed with R software using the “rms” package (version 3.6.0, R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
General characteristics
A total of 7,028 patients (mean age 70.3 ± 10.8 years, male 59.8%) met study entry criteria. The median NIHSS score was 3 (IQR 1–6) and the mean LDL-C level on admission was 69.7 ± 17.3 mg/dL. For lipid profiles at admission, the mean levels of HDL-C, TG, TC, and non-HDL were 43.2 ± 12.5 mg/dL, 116.4 ± 72.1 mg/dL, 130.4 ± 24.8 mg/dL, and 87.2 ± 22.3 mg/dL, respectively. The mean LDL-C/HDL-C ratio, TG/HDL ratio, and TC/HDL ratio was 1.72 ± 0.61, 3.06 ± 2.63, and 3.20 ± 1.08, respectively. Non-statin lipid-lowering agents, in addition to statins, were used in 6.5% of patients before index stroke. At discharge, 92.2% of the study subjects received statin treatment.
The demographic and clinical characteristics of subjects according to the quartiles of non-traditional lipid profiles (LDL/HDL ratio, TC/HDL ratio, TG/HDL ratio, and non-HDL) are presented in Table 1 and Supplemental Tables 1–3. As the quartile of the LDL/HDL ratio increased, there were significant trends of decreasing age and increasing body weight and BMI. Additionally, the frequency of DM, HTN, and history of CAD significantly increased as the quartile of LDL/HDL ratio increased (Table 1).
Table 1.
General characteristics of subjects according to the quartiles of LDL/HDL ratio.
| All | Q1 (0.20–1.29) | Q2 (1.30–1.66) | Q3 (1.67–2.08) | Q4 (2.09–7.29) | P † | P trend ‡ | |
|---|---|---|---|---|---|---|---|
| N | 7028 | 1767 | 1738 | 1751 | 1772 | ||
| LDL/HDL ratio | 0.20–1.29 | 1.30–1.66 | 1.67–2.08 | 2.09–7.29 | |||
| Age, mean ± SD | 70.3 ± 10.8 | 70.9 ± 11.0 | 70.4 ± 10.6 | 70.5 ± 10.6 | 69.5 ± 11.1 | 0.001 | 0.001 |
| Male, n (%) | 4206 (59.8) | 1000 (56.6) | 962 (55.4) | 1055 (60.3) | 1189 (67.1) | < 0.0001 | < 0.0001 |
| Onset-to-arrival | |||||||
| Within 24 h, n (%) | 4432 (63.1) | 1151 (65.1) | 1125 (64.7) | 1108 (63.3) | 1048 (59.1) | 0.001 | 0.0002 |
| Height, cm, mean ± SD | 161.8 ± 8.8 | 161.3 ± 8.9 | 160.9 ± 8.7 | 161.9 ± 8.8 | 163.0 ± 8.9 | < 0.0001 | < 0.0001 |
| Weight, kg, mean ± SD | 63.3 ± 11.3 | 61.4 ± 11.0 | 62.3 ± 10.7 | 63.9 ± 11.2 | 65.3 ± 11.8 | < 0.0001 | < 0.0001 |
| BMI, % | 24.1 ± 3.4 | 23.5 ± 3.4 | 24.0 ± 3.3 | 24.3 ± 3.3 | 24.5 ± 3.4 | < 0.0001 | < 0.0001 |
| NIHSS, med (IQR) | 3 (1–6) | 3 (1–6) | 3 (1–6) | 3 (1–6) | 3 (1–6) | 0.82 | 0.06 |
| Premorbid mRS 0–1 | 5629 (80.1) | 1376 (77.9) | 1417 (81.5) | 1419 (81.0) | 1417 (80.0) | 0.033 | 0.17 |
| History of stroke | 3222 (45.8) | 900 (50.9) | 826 (47.5) | 742 (42.4) | 754 (42.6) | < 0.0001 | < 0.0001 |
| History of TIA | 285 (4.1) | 71 (4.0) | 69 (4.0) | 71 (4.1) | 74 (4.2) | 0.99 | 0.79 |
| History of PAD | 98 (1.4) | 18 (1.0) | 25 (1.4) | 29 (1.7) | 26 (1.5) | 0.43 | 0.21 |
| History of CAD | 1476 (21.0) | 333 (18.8) | 352 (20.3) | 391 (22.3) | 400 (22.6) | 0.02 | 0.002 |
| HTN | 5851 (83.3) | 1450 (82.1) | 1449 (83.4) | 1475 (84.2) | 1477 (83.4) | 0.38 | 0.23 |
| DM | 3637 (51.8) | 820 (46.4) | 858 (49.4) | 921 (52.6) | 1038 (58.6) | < 0.0001 | < 0.0001 |
| Dyslipidemia | 5108 (72.7) | 1262 (71.4) | 1270 (73.1) | 1310 (74.8) | 1266 (71.4) | 0.07 | 0.71 |
| Smoking | < 0.0001 | 0.003 | |||||
| Never | 4559 (64.9) | 1199 (67.9) | 1175 (67.6) | 1133 (64.7) | 1052 (59.4) | ||
| Current | 1228 (17.5) | 244 (13.8) | 273 (15.7) | 316 (18.0) | 395 (22.3) | ||
| Ex (beyond 5 year) | 821 (11.7) | 202 (11.4) | 209 (12.0) | 198 (11.3) | 212 (12.0) | ||
| Recent (within 5 year) | 420 (6.0) | 122 (6.9) | 81 (4.7) | 104 (5.9) | 113 (6.4) | ||
| Medication history | |||||||
| Antihypertensive | 5402 (76.9) | 1306 (73.9) | 1324 (76.2) | 1394 (79.6) | 1378 (77.8) | 0.001 | 0.001 |
| Andi-diabetics | 3226 (45.9) | 748 (42.3) | 771 (44.4) | 806 (46.0) | 901 (50.8) | < 0.0001 | < 0.0001 |
| Lipid lowering agents | 455 (6.5) | 128 (7.2) | 120 (6.9) | 88 (5.0) | 119 (6.7) | 0.04 | 0.19 |
| Antiplatelet | 4909 (69.8) | 1263 (71.5) | 1218 (70.1) | 1190 (68.0) | 1238 (69.9) | 0.16 | 0.16 |
| Aspirin | 3332 (47.4) | 825 (46.7) | 827 (47.6) | 803 (45.9) | 877 (49.5) | 0.16 | 0.21 |
| Clopidogrel | 2426 (34.5) | 628 (35.5) | 599 (34.5) | 576 (32.9) | 623 (35.2) | 0.36 | 0.59 |
| Reperfusion therapy | 0.07 | 0.01 | |||||
| No | 6215 (88.4) | 1537 (87.0) | 1531 (88.1) | 1553 (88.7) | 1594 (90.0) | ||
| IVT | 441 (6.3) | 120 (6.8) | 107 (6.2) | 111 (6.3) | 103 (5.8) | ||
| EVT | 216 (3.1) | 69 (3.9) | 61 (3.5) | 41 (2.3) | 45 (2.5) | ||
| IV + EVT | 156 (2.2) | 41 (2.3) | 39 (2.2) | 46 (2.6) | 30 (1.7) | ||
| Laboratory findings | |||||||
| WBC counts | 8.1 ± 3.1 | 8.0 ± 3.3 | 8.0 ± 3.2 | 8.0 ± 3.0 | 8.3 ± 3.1 | 0.02 | 0.0062 |
| Platelet counts | 227.4 ± 77.6 | 220.0 ± 81.5 | 227.4 ± 78.5 | 227.5 ± 72.5 | 234.6 ± 76.9 | < 0.0001 | < 0.0001 |
| Hemoglobin | 13.0 ± 2.0 | 12.9 ± 1.9 | 13.0 ± 2.0 | 13.1 ± 2.0 | 13.2 ± 2.2 | 0.002 | 0.0001 |
| Glucose | 152.2 ± 68.2 | 147.5 ± 68.0 | 147.8 ± 61.5 | 153.0 ± 67.3 | 160.2 ± 74.6 | < 0.0001 | < 0.0001 |
| BUN | 18.6 ± 10.0 | 18.6 ± 10.2 | 18.2 ± 9.0 | 18.6 ± 9.6 | 19.2 ± 10.9 | 0.03 | 0.0439 |
| Creatinine | 1.2 ± 1.3 | 1.1 ± 1.0 | 1.1 ± 0.9 | 1.1 ± 1.1 | 1.3 ± 1.9 | < 0.0001 | < 0.0001 |
| LDL-cholesterol | 69.7 ± 17.3 | 54.4 ± 15.2 | 67.9 ± 14.5 | 74.6 ± 13.4 | 81.7 ± 12.8 | < 0.0001 | < 0.0001 |
| TC | 130.4 ± 24.8 | 122.5 ± 26.8 | 129.3 ± 25.2 | 133.0 ± 22.9 | 136.9 ± 21.9 | < 0.0001 | < 0.0001 |
| TG | 116.4 ± 72.1 | 91.5 ± 57.4 | 106.0 ± 59.9 | 121.6 ± 71.5 | 146.2 ± 84.4 | < 0.0001 | < 0.0001 |
| HDL | 43.2 ± 12.5 | 53.9 ± 14.0 | 45.8 ± 9.8 | 40.3 ± 7.5 | 32.9 ± 6.3 | < 0.0001 | < 0.0001 |
| Non-HDL | 87.2 ± 22.3 | 68.6 ± 18.1 | 83.4 ± 17.6 | 92.7 ± 17.7 | 103.9 ± 19.1 | < 0.0001 | < 0.0001 |
| SBP | 146.5 ± 25.9 | 146.0 ± 25.8 | 146.8 ± 26.4 | 147.5 ± 25.7 | 145.6 ± 25.8 | 0.14 | 0.83 |
| In-hospital treatment | |||||||
| Aspirin | 5934 (84.4) | 1444 (81.7) | 1496 (86.1) | 1481 (84.6) | 1513 (85.4) | 0.002 | 0.01 |
| Clopidogrel | 4183 (59.5) | 1028 (58.2) | 1054 (60.6) | 1040 (59.4) | 1061 (59.9) | 0.51 | 0.46 |
| Other antiplatelet | 743 (10.6) | 226 (12.8) | 171 (9.8) | 174 (9.9) | 172 (9.7) | 0.01 | 0.01 |
| Anti-diabetics | 2710 (38.6) | 616 (34.9) | 649 (37.3) | 693 (39.6) | 752 (42.4) | < 0.0001 | < 0.0001 |
| Anti-hypertensives | 3645 (51.9) | 890 (50.4) | 882 (50.7) | 943 (53.9) | 930 (52.5) | 0.14 | 0.08 |
| Lipid lowering agents other than statin | 165 (2.3) | 47 (2.7) | 34 (2.0) | 35 (2.0) | 49 (2.8) | 0.24 | 0.82 |
| Statin | 6483 (92.2) | 1606 (90.9) | 1613 (92.8) | 1628 (93.0) | 1636 (92.3) | 0.08 | 0.12 |
†P-value by Chi-square test, ANOVA and Kruskal-Wallis test. ‡P-value by Cochran-Armitage trend test, Cochran-Mantel-Haenszel test and linear contrasts test in ANOVA.
One-year vascular outcomes
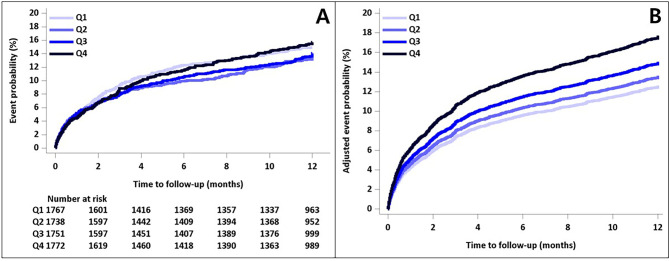
The mean follow-up duration was 330 ± 88.9 days, and 91.5% of the study subjects completed 1-year of follow-up. The 1-year cumulative incidences of the composite of stroke, MI and all-cause mortality was 14.5%; all-cause mortality, 8.9%; stroke (either ischemic or hemorrhagic), 7.2%; and MI, 0.5%. In crude analysis, the 1-year cumulative incidences of the composite of stroke, MI, and all-cause mortality did not significantly differ among the quartiles of the LDL/HDL ratio: 14.9% in the lowest quartile (Q1) of the LDL/HDL ratio, 13.4% in the second quartile (Q2), 14.1% in the third quartile (Q3), and 15.7% in the highest quartile (Q4)(Table 2, p for trend = 0.59). The 1-year cumulative incidences of stroke, all-cause mortality, and MI as individual outcomes also did not show significant differences according to the quartiles of LDL/HDL ratio. Similar observations with no significant differences were made for 1-year cumulative incidences of vascular events according to the quartiles of TG/HDL ratio and TC/HDL ratio (Supplemental Table 4). However, non-HDL showed a significant association of increasing quartile levels with decreased event rates of 1-year composite vascular events and secondary outcome variables (Supplemental Table 4).
Table 2.
One-year vascular outcomes according to the quartiles of the LDL/HDL ratio.
| All | Q1 (0.20–1.29) | Q2 (1.30–1.66) | Q3 (1.67–2.08) | Q4 (2.09–7.29) | P trend b | |
|---|---|---|---|---|---|---|
| N | 7028 | 1767 | 1738 | 1751 | 1772 | |
| Primary outcome | ||||||
| No. of events | 963 | 249 | 221 | 231 | 262 | |
| 1-yar event rate (%, 95% CI)a | 14.52 (13.67–15.38) | 14.88 (13.17–16.59) | 13.42 (11.77–15.08) | 14.05 (12.36–15.74) | 15.70 (13.94–17.45) | 0.59 |
| Stroke | ||||||
| No. of events | 463 | 111 | 113 | 123 | 116 | |
| 1-yar event rate (%, 95% CI)a | 7.24 (6.60–7.88) | 6.88 (5.64–8.12) | 6.95 (5.70–8.19) | 7.80 (6.47–9.14) | 7.30 (6.01–8.60) | 0.68 |
| All-cause mortality | ||||||
| No. of events | 572 | 155 | 122 | 131 | 164 | |
| 1-yar event rate (%, 95% CI)a | 8.91 (8.21–9.61) | 9.55 (8.11–10.99) | 7.80 (6.46–9.13) | 8.18 (6.83–9.52) | 10.09 (8.62–11.56) | 0.60 |
| MI | ||||||
| No. of events | 33 | 6 | 8 | 9 | 10 | |
| 1-yar event rate (%, 95% CI)a | 0.54 (0.35–0.72) | 0.38 (0.08–0.69) | 0.52 (0.16–0.89) | 0.57 (0.20–0.94) | 0.68 (0.26–1.10) | 0.33 |
aBased on the Kaplan-Meier estimates. bP-value by log-rank test for trend.
The unadjusted and adjusted associations of LDL/HDL ratio with 1-year vascular outcomes are shown in Table 3. In unadjusted analysis, no significant associations were observed in the quartiles of LDL/HDL ratio and 1-year primary outcome and secondary outcomes. However, after adjustment for the 18 prespecified clinically relevant variables, compared with Q1 of the LDL/HDL ratio, Q4 of the LDL/HDL ratio was significantly associated with increasing the risk of 1-year composite of stroke, MI, and all-cause mortality (HR 1.48 [1.19–1.83]). Similarly, compared with Q1 of the TC/HDL ratio, Q2, Q3, and Q4 of the TC/HDL ratio were significantly associated with increasing the risk of 1-year primary outcome (aHR 1.21 [1.00-1.45], 1.26 [1.04–1.53], and 1.40 [1.15–1.70], respectively)(Supplemental Table 5). For TG/HDL ratio, Q4 of the TG/HDL ratio, compared with Q1, was more likely to occur to 1-year primary outcome (aHR 1.30 [1.08–1.57]) (Supplemental Table 6). However, there were no significant associations between the quartiles of non-HDL and 1-year primary outcome (Supplemental Table 7). Kaplan-Meier survival plots for these are presented in Fig. 1 and Supplemental Fig. 2A–C.
Table 3.
Associations of LDL/HDL ratio with one-year vascular outcomes.
| Unadjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | ||
|---|---|---|---|---|---|
| Primary outcomes | Q1 | 1(Ref) | 1(Ref) | ||
| Q2 | 0.89 (0.74–1.06) | 0.19 | 1.09 (0.90–1.32) | 0.39 | |
| Q3 | 0.92 (0.77–1.10) | 0.37 | 1.22 (1.00-1.49) | 0.05 | |
| Q4 | 1.04 (0.87–1.23) | 0.68 | 1.48 (1.19–1.83) | 0.0004 | |
| Stroke | Q1 | 1(Ref) | 1(Ref) | ||
| Q2 | 1.02 (0.78–1.32) | 0.89 | 1.14 (0.86–1.51) | 0.36 | |
| Q3 | 1.10 (0.85–1.42) | 0.46 | 1.34 (1.00-1.80) | 0.05 | |
| Q4 | 1.03 (0.80–1.34) | 0.81 | 1.33 (0.95–1.84) | 0.09 | |
| All-cause mortality | Q1 | 1(Ref) | 1(Ref) | ||
| Q2 | 0.79 (0.62-1.00) | 0.05 | 1.03 (0.80–1.32) | 0.81 | |
| Q3 | 0.84 (0.66–1.06) | 0.14 | 1.16 (0.89–1.50) | 0.27 | |
| Q4 | 1.04 (0.84–1.30) | 0.72 | 1.50 (1.15–1.97) | 0.003 | |
| MI | Q1 | 1(Ref) | 1(Ref) | ||
| Q2 | 1.33 (0.46–3.83) | 0.60 | 1.16 (0.38–3.51) | 0.80 | |
| Q3 | 1.48 (0.53–4.17) | 0.45 | 1.06 (0.34–3.37) | 0.92 | |
| Q4 | 1.64 (0.60–4.51) | 0.34 | 1.06 (0.30–3.69) | 0.93 | |
Adjusted variable: age, male, NIHSS, BMI, LDL-C, history of stroke, history of CAD, HTN, DM, dyslipidemia, smoking status, prior antiplatelet, creatinine, glucose, SBP, in-hospital anti-hypertensive, in-hospital antidiabetics, in-hospital lipid lowering agents.
Fig. 1.
Kaplan-Meier survival plots for 1-year primary outcome according to the LDL/HDL ratio, unadjusted (A) and adjusted plots (B).
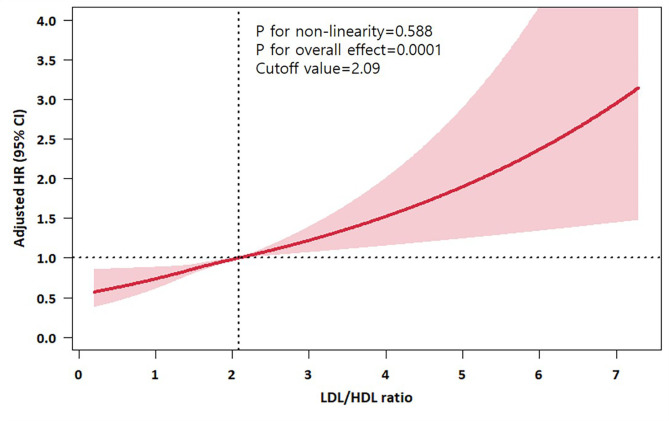
Adjusted HR plots for each non-traditional lipid profiles as continuous variables are presented in Fig. 2 and Supplemental Fig. 3A–C. For the LDL/HDL ratio, a linear relationship was observed (P for linearity < 0.001), and the cut-off value associated with significant increase in the composite outcome within 1-year was 2.09 of LDL/HDL ratio. For other lipid profiles of TC/HDL ratio and TG/HDL ratio, non-linear associations were observed. Among the four lipid profiles models, the model for the LDL/HDL ratio had the lowest Akaike Information Criteria and Bayes Information Criterion (Supplemental Table 8).
Fig. 2.
Adjusted HR of continuous LDL/HDL ratio for primary outcome within 1 year.
TOAST subgroup analysis
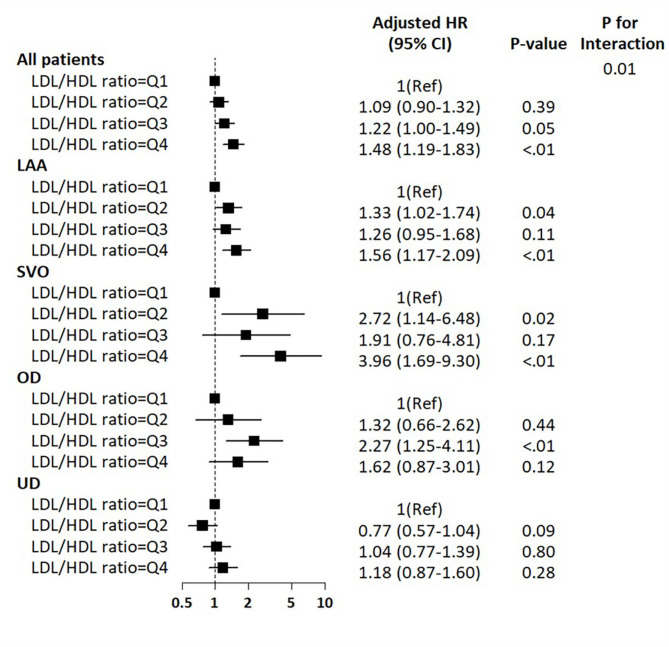
There were significant interactions between LDL/HDL ratio and TOAST subgroups with 1-year primary outcome (P interaction = 0.01). Among the LAA and SVO subtypes, compared with Q1 of LDL/HDL ratio, higher quartiles were more likely to be associated with increasing the risk of 1-year primary vascular outcomes, while no associations were observed among UD subtype (Fig. 3). For other lipid ratios and non-HDL cholesterol, there were no potential interactions of stroke subtypes with 1-year primary outcome (Supplemental Fig. 4).
Fig. 3.
Associations of LDL/HDL ratio with one-year vascular outcomes according to the ischemic stroke subtypes.
Discussion
Our study, which focused on over 7,000 patients with acute ischemic stroke who were treated with statins before the index stroke and had LDL-cholesterol levels < 100 mg/dl upon admission, demonstrated an association between non-traditional lipid profiles and an increased risk of 1-year vascular outcomes. Higher quartiles of the LDL/HDL ratio, TC/HDL ratio, and TG/HDL ratio, though not non-HDL levels, were significantly associated with increasing the risk of 1-year composite vascular events. These findings suggest that even during statin therapy with LDL-C < 100 mg/dl on admission, there should be consideration for residual risk based on non-traditional lipid profiles.
Among the parameters examined in this study, the best performance model was the LDL/HDL ratio, which is the most widely used in clinical practice. Our study is noteworthy of finding a linear relationship between the LDL/HDL ratio and the increasing risk of 1-year vascular events in ischemic stroke patients under statin treatment and admission LDL-C < 100 mg/dl. Compared with the lowest quartile of the LDL/HDL ratio, the highest quartile of was more likely to be associated with increasing the risk of 1-year composite of vascular events and all-cause mortality by relatively 48% and 50%, respectively. In a prior study, when statins had already been taken before index stroke and LDL-C levels were well controlled at admission, LDL-C levels had little association with early vascular outcomes in ischemic stroke8. Therefore, our study provides important insights that even in patients undergoing appropriate LDL-C lowering treatment before index stroke, there could still be residual risk, which may be predicted through lipid ratio like the LDL/HDL ratio. While the reduction of LDL-C through statin therapy diminishes a patient’s cardiovascular risk, a residual risk persists even when LDL-C levels are adequately controlled14. In such cases, non-traditional lipid profiles may serve as clinical targets for this residual risk. Emerging evidence from various studies indicates that the assessment of cardiovascular residual risk through non-traditional lipid parameters is gaining increasing importance15–18. For instance, when LDL-C levels are well controlled with statins, it may be advisable to consider additional lipid-lowering therapy, targeting non-HDL as a therapeutic goal19.
This finding is consistent with findings in other cardiovascular diseases. Several studies have found associations between the LDL/HDL ratio, and cardiovascular event risk in patients with coronary artery diseases20,21. Additionally, a previous study found that an elevated LDL/HDL ratio could be a positive predictor of aortogenic cerebral embolism22. In contrast, some studies found an opposing finding that a high LDL/HDL ratio protected against death, recurrence, and moderate disability within 3 months following stroke onset23. However, the population in these studies differed from the current investigation as were not confined to patients who were already taking statins and had their LDL-C levels appropriately controlled. In a previous study of general population without DM or cardiovascular diseases, when LDL-C was controlled below 100 mg/dL by statin therapy, the LDL/HDL ratio had an HR for cardiovascular diseases event and death of 1.43 and 1.34, respectively24. In our study, beyond LDL-C, the risk of 1-year composite of stroke, MI, and all-cause mortality significantly and linearly increased when the LDL/HDL ratio surpassed 2.09. Currently, guidelines for dyslipidemia in stroke patients specify a target goal for LDL-C levels, but do not clearly define targets for other lipid profiles25.
We included patients with the LDL-C level at < 100 mg/dl on admission, not < 70 mg/dl, based on current stroke guidelines that use LDL-C > 100 mg/dl as the criterion for high-intensity statin therapy25. The SPARCL study included patients with acute ischemic stroke and LDL-C > 100 mg/dl3. Recent guidelines mention LDL targets of < 70 mg/dl or even < 55 mg/dl19,25, but these targets primarily guide atherosclerotic stroke management. The applicability to other stroke mechanism or etiologies such as SVO or UD remains uncertain and requires further research. Initiating lipid-lowering treatment in non-CE stroke may be considered when LDL-C is > 100 mg/dl.
In a previous meta-analysis, the TC/HDL ratio demonstrated a linear correlation with stroke outcomes26. For each 1-unit increase in the TC/HDL ratio, the risk of stroke increased by 16%. In contrast, we found a non-linear relationship of the TC/HDL ratio and 1-year composite of stroke, MI, and all-cause mortality, with lower risk with a lower TC/HDL ratio. In addition, compared with Q1 of the TC/HDL ratio, higher quartiles were significantly associated with increasing risk of 1-year composite of stroke, MI, and all-cause mortality (adjusted HR 1.21, 1.26, and 1.40 in Q2, Q3, and Q4, respectively) after adjustments of relevant variables. When the TC/HDL ratio was 3.65 or lower, there was a reduced HR for a one-year composite of stroke, MI, and all-cause mortality.
Our study also revealed a non-linear relationship for the TG/HDL ratio, with the highest risk observed at 3.6. The highest quartile of the TG/HDL ratio, compared with lowest quartile, was associated with relative 30% increased risk of composite vascular events within 1 year. In a meta-analysis investigating the TG/HDL ratio and stroke risk, it was found that the highest category had a 1.24 times greater risk of stroke compared to the lowest category26. However, it’s worth noting that other studies have reported inconsistent findings, with some suggesting that higher TG/HDL ratio is linked to favorable outcomes27.
In our study, we found that non-HDL does not have a strong association with predicting residual risk, similar to LDL-C. While dyslipidemia guidelines recommend maintaining non-HDL below 100 mg/dl for ASCVD patients, when stroke patients are already on statins and have LDL levels below 100 mg/dl, it appears that lipid ratios may be more helpful in predicting the risk of vascular events than non-HDL.
There are several limitations of this study. First, information regarding aspects of statin pretreatment were lacking. Details such as intensity, duration, type of statin, or dose of statin pretreatment were not available. Second, it was not possible to determine the medication status or lipid profiles and ratio during the follow-up periods. Third, as a registry-based retrospective study, there are inherent limitations of observational data to consider. Despite adjustment for various variables, the potential impact of unmeasured or residual confounding variables may not be entirely eliminated. Fourth, this study was conducted in only South Korea, which could introduce an additional element of confounding when considering differences in lipid profiles among different ethnic groups. Fourth, there is a possibility of index event bias in the analysis of stroke patients who received statin treatment with LDL-C levels of 100 or lower28. This might affect the relationship between baseline risk factors and the outcome of interest. However, the findings of this study would still be applicable to patients matching those analyzed.
In conclusion, our study found that, in ischemic stroke patients whose LDL-C levels were already controlled with statin, higher LDL/HDL ratio, TC/HDL ratio, and TG/HDL ratio, though not non-HDL levels, were associated with residual risk of 1-year composite of stroke, MI, and all-cause mortality. The risk with LDL/HDL particularly increased when the ratio value reached 2.09 or higher and showed a linear association with the 1-year primary vascular outcome. Our results suggest that non-traditional lipid profiles, particularly the LDL/HDL ratio, may be helpful in predicting the risk of subsequent vascular events for patients with ischemic stroke occurring despite well-controlled LDL with statin pretreatment. Based on our research findings, the patients with abnormal non-traditional lipid profiles may warrant more stringent lipid management and tighter cardiovascular risk control.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Study concept and design: JT Kim, H Kim, HJ Bae. Acquisition of data: JT Kim, MS Park, BJ Kim, J Kang, KJ Lee, JM Park, K Kang, SJ Lee, JG Kim, JK Cha, DH Kim, TH Park, K Lee, J Lee, KS Hong, YJ Cho, HK Park, BC Lee, KY Yu, MS Oh, DE Kim, WS Ryu, JC Choi, JH Kwon, WJ Kim, DI Shin, KS Yum, SI Sohn, JH Hong, J Lee, KY Park, HJ Bae. Analysis and interpretation of data: JT Kim, HJ Bae, JS Lee. Drafting of the manuscript: JT Kim, H Kim, JLS. All authors read and approved the final manuscript.
Funding
This research was supported by funding (2023-ER1006-00) from Research of Korea Centers for Disease Control and Prevention. This study was supported by a grant (BCRI24042) of Chonnam National University Hospital Biomedical Research Institute.
Data availability
Data used in this study are available upon reasonable request following submission of a legitimate academic research proposal to be assessed by the CRCS-K steering committee. One may request to contact the corresponding author regarding data availability.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joon-Tae Kim, Email: alldelight2@jnu.ac.kr.
Hee-Joon Bae, Email: braindoc@snu.ac.kr.
References
- 1.Cholesterol Treatment Trialists, C. et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 380, 581–590 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarenco, P. et al. High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 355, 549–559. 10.1056/NEJMoa061894 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Amarenco, P. et al. A comparison of two LDL cholesterol targets after ischemic stroke. N. Engl. J. Med. 382, 9. 10.1056/NEJMoa1910355 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Nesto, R. W. Beyond low-density lipoprotein: addressing the atherogenic lipid triad in type 2 diabetes mellitus and the metabolic syndrome. Am. J. Cardiovasc. Drugs 5, 379–387. 2005/11/02 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Di Giorgi, N. et al. A specific plasma lipid signature associated with high triglycerides and low HDL cholesterol identifies residual CAD risk in patients with chronic coronary syndrome. Atherosclerosis 339, 1–11. 10.1016/j.atherosclerosis.2021.11.013 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Johannesen, C. D. L. et al. Apolipoprotein B and non-HDL cholesterol better reflect residual risk than LDL cholesterol in statin-treated patients. J. Am. Coll. Cardiol. 77, 1439–1450. 2021/03/20 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Liu, X., Yan, L. & Xue, F. The associations of lipids and lipid ratios with stroke: a prospective cohort study. J. Clin. Hypertens. (Greenwich) 21, 127–135. 10.1111/jch.13441 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, J. T. et al. Admission LDL-cholesterol, statin pretreatment and early outcomes in acute ischemic stroke. J. Clin. Lipidol.10.1016/j.jacl.2023.08.002 (2023). [DOI] [PubMed]
- 9.Kim, J. T. et al. Statin treatment in patients with stroke with low-density lipoprotein cholesterol levels below 70 mg/dL. J. Am. Heart Assoc. 12, e030738. 10.1161/JAHA.123.030738 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, B. J. et al. Current status of acute stroke management in Korea: a report on a multicenter, comprehensive acute stroke registry. Int. J. Stroke 9, 514–518. 10.1111/ijs.12199 (2014). [DOI] [PubMed]
- 11.Kim, B. J. et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke-fifth division registry in South Korea. J. Stroke 17, 38–53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams, H. P. Jr. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24, 35–41 (1993). [DOI] [PubMed] [Google Scholar]
- 13.Ko, Y. et al. MRI-based Algorithm for Acute ischemic stroke subtype classification. J. Stroke 16, 161–172. 10.5853/jos.2014.16.3.161 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson, U. K., Fazio, S. & Linton, M. F. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr. Atheroscler. Rep. 14, 1. 10.2011/11/22 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arsenault, B. J. et al. Beyond low-density lipoprotein cholesterol: respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J. Am. Coll. Cardiol. 55, 35–41 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Wen, J. et al. Associations of non-high-density lipoprotein cholesterol, triglycerides and the total cholesterol/HDL-c ratio with arterial stiffness independent of low-density lipoprotein cholesterol in a Chinese population. Hypertens. Res. 42, 1223–1230. 10.1038/s41440-019-0251-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka, F. et al. Predictive value of lipoprotein indices for residual risk of acute myocardial infarction and sudden death in men with low-density lipoprotein cholesterol levels < 120 mg/dl. Am. J. Cardiol. 112, 1063–1068. 10.1016/j.amjcard.2013.05.049 (2013). [DOI] [PubMed]
- 18.Liu, J. et al. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am. J. Cardiol. 98, 1363–1368 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Authors/Task Force M Guidelines ESCCfP and Societies ESCNC. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis 290, 140–20520190831. 10.1016/j.atherosclerosis.2019.08.014 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Yang, T. et al. Correlation between the triglyceride-to-high-density lipoprotein cholesterol ratio and other unconventional lipid parameters with the risk of prediabetes and type 2 diabetes in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc. Diabetol. 21, 93 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun, T. et al. Predictive value of LDL/HDL ratio in coronary atherosclerotic heart disease. BMC Cardiovasc. Disord. 22, 273. 10.1186/s12872-022-02706-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okuzumi, A. et al. Impact of low-density lipoprotein to high-density lipoprotein ratio on aortic arch atherosclerosis in unexplained stroke. J. Neurol. Sci. 326, 83–88 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Liu, L. et al. Association of LDL-C/HDL-C ratio with stroke outcomes within 1 year after onset: a hospital-based follow-up study. Front. Neurol. 11, 408. 10.3389/fneur.2020.00408 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mora, S. et al. On-treatment non-high-density lipoprotein cholesterol, apolipoprotein B, triglycerides, and lipid ratios in relation to residual vascular risk after treatment with potent statin therapy: JUPITER (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin). J. Am. Coll. Cardiol. 59, 1521–1528. 10.1016/j.jacc.2011.12.035 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleindorfer, D. O. et al. 2021 Guideline for the prevention of stroke in patients With stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke 52, e364-e467. 10.1161/STR.0000000000000375 (2021). [DOI] [PubMed]
- 26.Liu, Y. et al. Non-traditional lipid profiles and the risk of stroke: a systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 33, 698–714. 10.1016/j.numecd.2023.01.003 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Deng, Q. W. et al. The short-term prognostic value of the triglyceride-to-high-density lipoprotein cholesterol ratio in acute ischemic stroke. Aging Dis. 9, 498–506. 10.14336/AD.2017.0629 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahabreh, I. J. & Kent, D. M. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA 305, 822–823. 10.1001/jama.2011.163 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study are available upon reasonable request following submission of a legitimate academic research proposal to be assessed by the CRCS-K steering committee. One may request to contact the corresponding author regarding data availability.