ABSTRACT.
Free-living amoebae (FLA) are widely distributed in the environment. Among these, Acanthamoeba spp., Naegleria fowleri, Balamuthia mandrillaris, and Vermamoeba vermiformis have been reported as human pathogens with health effects ranging from lethal encephalitis to different epithelial disorders. Despite this, FLA still present many diagnostic challenges. The aim of this study was to develop a rapid and efficient multiplex real-time quantitative polymerase chain reaction (qPCR) to simultaneously detect Acanthamoeba spp., N. fowleri, B. mandrillaris, and V. vermiformis in different water sources. For the validation of the qPCR assay, 38 samples (19 tap water and 19 stagnant water sources) were analyzed. The qPCR assay accurately identified the four types of FLA with no cross-reactivity. Considering water samples with results subsequently confirmed by conventional PCR, the multiplex qPCR assay detected 18/38 (47.4%) positive samples (Acanthamoeba spp. in 44.7% and V. vermiformis in 31.6%) and growth in nonnutritive agar (NNA) cultures identified 7/38 (18.4%) positive samples. Of the tap water samples analyzed, 26.3% of samples positive for FLA were detected by growth in NNA culture whereas 31.6% were identified by qPCR. In addition, FLA were detected in 2/19 stagnant water samples (10.5%) by growth in NNA culture and in 12/19 stagnant water samples (63.2%) by qPCR. Neither N. fowleri nor B. mandrillaris was detected in the water samples analyzed. In conclusion, the qPCR developed showed its potential as a rapid tool for detection of Acanthamoeba spp., N. fowleri, B. mandrillaris, and V. vermiformis. Moreover, FLA species were detected in half of the water sources evaluated, suggesting the importance of the surveillance of these potential infectious agents.
INTRODUCTION
Amoebae are protists that move using pseudopods and feed by phagocytosis.1 Free-living amoebae (FLA) are widely distributed in the environment, in both water (freshwater and seawater) and soil, where they find favorable conditions for their growth. During recent decades, there have been an increasing number of reports relative to the presence of FLA in environmental freshwater sources. Typically, the diversity and the ecology of FLA in the environment have been tested mainly by culture. However, recent studies have added helpful information about the current diversity of FLA by the use of molecular techniques such as multiplex real-time quantitative polymerase chain reaction (qPCR).2 Infections caused by Acanthamoeba spp., Naegleria fowleri, and Balamuthia mandrillaris are reported around the world, presenting many diagnostic challenges.3
The most abundant FLA in the environment are those of the genus Acanthamoeba and the species Vermamoeba vermiformis, formerly classified as Hartmannella vermiformis.4 Acanthamoeba spp. are the most prevalent and widespread free-living heterotrophic protists in the world, have been widely studied, and are the cause of sight-threatening keratitis or encephalitis.5 On the other hand, V. vermiformis did not gain such a prominent health profile until recent years, when it attracted the attention of experts due to the discovery of its pathogenic capacity and its high prevalence in environments related to human activity. However, despite the health importance of these species, their detection and quantification in water are not regulated in Spain.
Because these infections are rare, it is difficult to diagnose them clinically, and if not suspected, they could be misdiagnosed or not diagnosed until an autopsy is carried out. Microscopy continues to be the main diagnostic method; however, experience with these pathogenic protozoa is not commonplace and requires expert counsel, which habitually delays the diagnosis.6 The detection and diagnosis methods used for these protozoa have varied, from culture and morphological characterization to classification by biochemical and immune tests to the most current molecular methods. Molecular methods have been developed to avoid the need for culture and are focused on nucleic acid fluorescence determination and in situ hybridization with oligonucleotide probes.7 Methods based on culture and morphological characterization are complex and slow and involve participation of qualified personnel to differentiate between FLA species3; for this reason, they are currently complemented with molecular tools. On the other hand, PCR-based methods offer a rapid and more sensitive alternative for the detection of FLA in samples where a low density of amoebae are expected.8 qPCR, according to the MIQE guidelines,9 has numerous advantages over traditional PCR, as shown by its high precision and accuracy in quantification, its high sensitivity, and its wide linear dynamic range.
The present research aims to develop a high-speed, simultaneous, and efficient multiplex qPCR capable of detecting the four FLA, specifically those of the Acanthamoeba genus and the species N. fowleri, B. mandrillaris, and V. vermiformis in different water sources.
MATERIALS AND METHODS
Multiplex qPCR assay.
The multiplex qPCR assay was performed for the simultaneous molecular detection of Acanthamoeba spp., N. fowleri, B. mandrillaris, and V. vermiformis.
The primers used for the detection of Acanthamoeba spp., N. fowleri, and B. mandrillaris, reported by Qvarnstrom et al.,3 were specific to the region of the !8s ribosomal RNA gene and are detailed in Table 1.
Table 1.
Primers used in the qPCR for the detection of free-living amoebae under study
| Parasite Species | Primer (sequence) | DNA Fragment (bp) |
|---|---|---|
| Acanthamoeba spp.* | AcantF900 (5′-CCC AGA TCG TTT ACC GTG AA-3′) | 180 |
| AcantR1100 (5′-TAA ATA TTA ATG CCC CCA ACT ATC C-3′) | ||
| Naegleria fowleri * | NaeglF192 (3′-GTG CTG AAA CCT AGC TAT TGT AAC TCA GT-5′) | 153 |
| NaeglR344 (5′-CAC TAG AAA AAG CAA ACC TGA AAG G-3′) | ||
| Balamuthia mandrillaris * | BalaF1451 (5′-TAA CCT GCT AAA TAG TCA TGC CAA T-3′) | 171 |
| BalaR1621 (5′-CAA ACT TCC CTC GGC TAA TCA-3′) | ||
| Vermamoeba vermiformis | Hv1227F (5′-TTA CGA GGT CAG GAC ACT GT- 3′)† | 235 |
| VermRv (5′ TGCCTCAAACTTCCATTCGC-3′)‡ |
For V. vermiformis detection, we used the specific forward primer reported by Kuiper et al.,7 while the reverse primer was especially designed for this study by using the Primer3Plus software (https://www.primer3plus.com/). This pair of oligonucleotides amplifies a fragment of 235 bp, which also corresponds to a specific region of the 18S ribosomal RNA gene, as shown in Table 1.
The qPCR was performed using the following TaqMan probes adapted from a report by Qvarnstrom et al.3 to set up in our laboratory the technology for the detection of Acanthamoeba spp., N. fowleri, and B. mandrillaris, respectively: AcantProb (5′-JUN-CT GCC ACC GAA TAC ATT AGC ATG G-QSY-3′), NeglProb (5′-VIC-AT AGC AAT ATA TTC AGG GGA GCT GGG C-QSY-3′), and BalaProb (5′-6FAM-AG TAC TTC TAC CAA TCC AAC CGC CA-QSY-3′). For the detection of V. vermiformis, we specially designed a TaqMan probe for this study, VermProb (5′-ABI-TTG ATT CAG TGG GTG GTG GT-QSY-3′), by using Primer3Plus software (https://www.primer3plus.com/).
The qPCR mixture consisted of a 10-µL final volume using 10× TaqMan® multiplex master mix (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA), a 0.5 µM concentration of each primer, a 0.25 µM concentration of the probe, and 2 µL of the DNA sample (water or cultured FLA strains). This was set up in a QuantStudio 5 real-time PCR machine (Thermo Fisher Scientific, Madrid, Spain) under the following conditions: a first step of 95°C for 3 minutes, followed by 35 cycles consisting of two steps of 95°C for 15 s and 60°C for 1 minute.
Samples of cultured FLA strains of Acanthamoeba castellanii Neff (ATCC® 30011TM), N. fowleri (ATCC® 30808), and V. vermiformis (NCBI MT320010) cells were used to create a standard curve with serially diluted known concentrations (1, 0.1, 0.01, 0.001, and 0.0001 ng). For the detection of B. mandrillaris, we used as a positive control a DNA sample from our laboratory collection (GC-13 code) at a concentration of 1 ng.10 To test the specificity of qPCR detection of N. fowleri, DNA samples from the species Naegleria canariensis (code SP16H),11 Naegleria gruberi (ATCC® 30224), and Naegleria clarki (code CRET1),12 present in the laboratory collection, were used. A spiked sample was prepared using DNA of the four amoeba species, which was set up in every experiment.
To test the detection limit of V. vermiformis by qPCR, the isolated strain V. vermiformis NCBI MT320010 on nonnutritive agar (NNA) from a previous work11 was used. As a negative control, an axenic culture of A. castellanii Neff (ATCC® 30011) in peptone yeast glucose (ATCC® 712) liquid culture medium, supplemented with 10 mg/mL of gentamicin (Sigma Aldrich, Madrid, Spain) was used. In addition, to evaluate the specific detection of V. vermiformis simultaneously with the other FLA detected in the previously reported multiplex qPCR, sterile water was coinfected with cultures of V. vermiformis and Acanthamoeba spp. Cultures presented an initial concentration of 106 cells/mL, and serial dilutions of 1:10 were prepared (range, 50,000 to 0.5 cells/mL).
The detection limit for the assay was set up considering one amoeba present per sample analyzed by qPCR, resulting in cycle threshold (Ct) values ranging from 34 to 35. However, the assay can detect lower concentrations that correspond to samples containing fewer than 1 amoeba per total amount of sample, which may be due to the presence of multiple copies of the PCR target gene. Samples with Ct values of >36 were considered negative.
Sampling and sample processing.
To evaluate the effectiveness of the developed technique, a total of 38 water samples were tested (Supplemental Table S1). Of the 38 samples, 19 corresponded to tapwater sample points that were selected through different municipalities of Tenerife (Canary Islands, Spain), from public and private sources. For each sampling point, 5 L of water was collected and then kept at 2–4°C until the samples were processed in the laboratory. The other 19 samples corresponded to different stagnant water sources from an animal reserve (such as ponds), where 1 L was collected and maintained as described above.
The collected samples were filtered with the aid of a vacuum pump and a filtration ramp using sterile 250-mL funnels and nitrocellulose filters with a 0.45-µm pore diameter (Pall Corporation, Madrid, Spain). The procedure was carried out under the influence of an alcohol burner to maintain sterile conditions. The filter was then cut with sterile scissors into two equal halves. One half was destined for the detection of FLA in 2% NNA plates, whereas the other half was intended for DNA extraction and subsequent FLA molecular detection by qPCR.
To obtain the total DNA, the half of the filter that was not seeded in NNA was resuspended into 15 mL of Page´s amoeba solution13 and subjected to agitation in a vortex (1 minute, 2,000 rpm). The filter was then removed, and the solution was centrifuged (10 minutes, 1,500 rpm). The supernatant was discarded until 1 mL was obtained, which was inoculated into the first well of a cartridge for DNA extraction by using a Maxwell® RSC cell DNA purification kit (AS1370) (Promega, Madrid, Spain) in a Maxwell RSC automated DNA system (Promega), in accordance with the manufacturer’s instructions. The obtained genomic DNA was used to perform the qPCR analysis.
The half of the filter intended for FLA isolation was seeded upside down in Petri dishes to allow amoebae to come into direct contact with the NNA medium. They were then sealed with Parafilm® and incubated at room temperature. Between 5 and 7 days later, the filters were removed, and the NNA plates were subjected to daily microscopic monitoring to detect the possible presence of amoebae, by following the morphological features of the Page key,14 and cloned by dilution in NNA until a monoxenic culture was obtained.15 Those monoxenic amoeba culture plates were subjected to DNA extraction using the Maxwell RSC, as described above.
Monoxenic FLA molecular characterization.
To perform molecular identification of the FLA isolated in the NNA plates, two different PCR assays were carried out. For those samples in which the trophozoite/cyst morphology matched the Acanthamoeba morphological characteristics,14 the Acanthamoeba-specific primers JDP1 5′- GGCCCAGATCGTTTACCGTGAA-3″and JDP2 5′-TCTCACAAGCTGCTAGGGAGTCA-3″16 were used. For the rest of the isolates, the following 18S rRNA universal FLA primers were used: P-FLAfw 5′-CGCGGTAATTCCAGCTCCAATAGC-3′/P-FLArv 5′-CAGGTTAAGGTCTCGTTCGTTAAC-3′.17 Both PCR amplifications were performed in a final 50-µL mixture containing 40 ng of DNA and 80 ng of DNA and in 35 and 40 cycles, respectively, with denaturation (95°C, 30 s), annealing (60°C, 30 s), and primer extension (72°C, 30 s). After the last cycle, a primer extension was maintained for 7 minutes at 72°C. PCR products were analyzed by electrophoresis through a 2% agarose gel and subsequently sequenced using the Macrogen Spain service (Madrid, Spain). The positive isolates were identified based on homology comparison with DNA sequences available in the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/).
RESULTS
The qPCR assay setup accurately identified the four types of FLA with no cross-reactivity. The present qPCRs adapted from the report of Qvarnstrom et al.3 demonstrated the expected performance. Samples of cultured FLA strains used as positive controls with known concentrations were amplified under optimal conditions for Acanthamoeba spp., N. fowleri, and B. mandrillaris, as was the spiked sample (Supplemental Figure S1). The qPCR was able to identify these FLA at a parasite count as low as 0.3 and 0.9 for Acanthamoeba spp. and N. fowleri, respectively. The qPCR performed for the detection of N. fowleri showed negative results for the samples corresponding to the species N. clarki, N. canariensis, and N. gruberi.
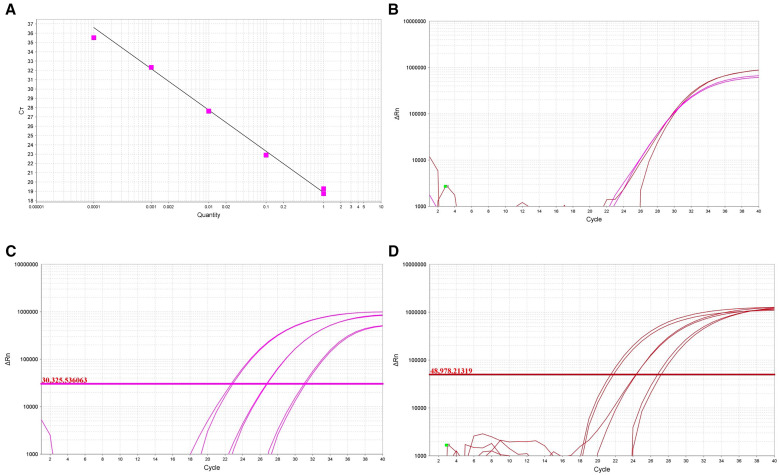
The qPCR assay designed for V. vermiformis had a high sensitivity, with a detection limit of 0.0001 ng of genomic material, as shown in Figure 1A. This qPCR showed an efficiency (E) of 100.3% and a correlation (R2) of 0.96. The results of the analyzed samples corresponding to infection of sterile water with Acanthamoeba spp. at concentrations of 50,000 cells/mL, 5,000 cells/mL, and 500 cells/mL are shown in Figure 1C. The results of the qPCR analysis obtained for infection with V. vermiformis at the same concentrations are shown in Figure 1D. In the same way, the qPCR setup in our laboratory for the four FLA of interest was positively validated by testing a sample of environmental water previously treated and analyzed that was positive for Acanthamoeba spp. and V. vermiformis (Figure 1B).
Figure 1.
(A) Standard curve with serially diluted concentrations (1, 0.1, 0.01, 0.001, and 0.0001 ng) of cultured Vermamoeba vermiformis (slope = –3.314; R2 = 0.96; efficiency = 100.3%). (B) Simultaneous detection of V. vermiformis (pink line) and Acanthamoeba spp. (red line) in a recreational water sample. (C and D) Quantitative polymerase chain reaction plot showing coinfection of sterile water with cultures of Acanthamoeba spp. (C) and V. vermiformis (D) at concentrations of 50,000, 5,000, and 500 cells/mL.
Validation: Detection of FLA by the multiplex qPCR assay in recreational water samples.
For the validation of the multiplex qPCR assay for detection of the four FLA, 38 samples from different recreational sources of water were analyzed. As shown in Table 2, in 18.4% (7/38) of the total samples studied, FLA growth was observed in NNA cultures (Acanthamoeba spp. in 31.6% and V. vermiformis in 10.5% of the analyzed samples). Among these positive cases, six samples, i.e., FW5, FW8, FW10, FW17, AR18, and AR19 (75%, 6/8), were identified by the traditional PCR assay as strains belonging to the Acanthamoeba genus (Table 2; Supplemental Table S1). In addition, samples FW2, FW10, AR18, and AR19 were found to be positive for V. vermiformis growth on NNA (50%, 4/8) (Supplemental Table S1), and these results were confirmed by traditional PCR. The sequences of the positive isolates have been submitted to GenBank under accession numbers OR940142 to OR940151 (Supplemental Table S1). It is important to note that samples FW10, AR18, and AR19 were positive for the NNA culture of both types of FLA.
Table 2.
FLA detected by NNA culture and multiplex qPCR methods in water samples from Tenerife Island
| Water Sample | NNA Culture + PCR | qPCR | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ac | Nf | Bm | Vv | Ac | Nf | Bm | Vv | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Tap water (n = 19) | 4 | 21.0 | 0 | 0 | 0 | 0 | 2 | 10.5 | 5 | 26.3 | 0 | 0 | 0 | 0 | 3 | 15.8 |
| Stagnant water (n = 19) | 2 | 10.5 | 0 | 0 | 0 | 0 | 2 | 10.5 | 12 | 63.2 | 0 | 0 | 0 | 0 | 8 | 42.1 |
| Total (N = 38) | 6 | 15.8 | 0 | 0 | 0 | 0 | 4 | 10.3 | 17 | 44.7 | 0 | 0 | 0 | 0 | 11 | 28.9 |
| 7/38 (18.4%) | 18/38 (47.4%) | |||||||||||||||
Ac = Acanthamoeba spp.; Bm = B. mandrillaris; FLA = free-living amoebae; Nf = N. fowleri; NNA = nonnutritive agar; qPCR = real-time quantitative polymerase chain reaction; Vv = V. vermiformis.
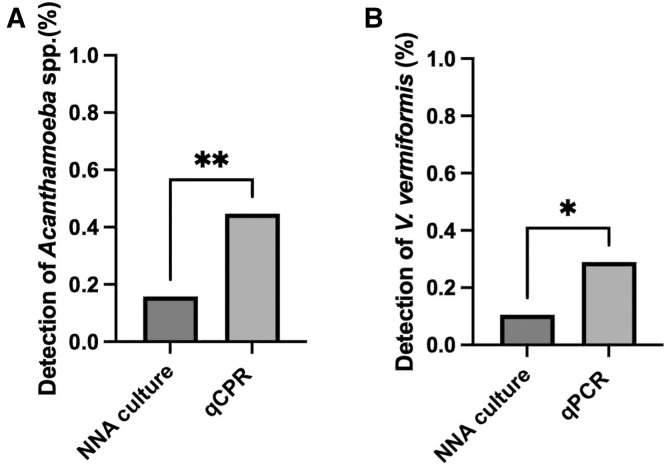
On the other hand, the multiplex qPCR assay identified 47.4% of positive samples (18/38), with detection of Acanthamoeba spp. in 44.7% (17/38) of the analyzed samples and detection of V. vermiformis in 31.6% (12/38) (Figure 2). Neither N. fowleri nor B. mandrillaris was detected in the water samples submitted for analysis. As shown in Supplemental Table S1, the qPCR assay detected FLA in 13 samples for which the NNA cultures were negative but traditional PCR results were positive and in 2 samples that were culture positive but negative for traditional PCR.
Figure 2.
Detection of amoebae was performed by culture cell growth in nonnutritive agar (NNA) and by the developed multiplex quantitative polymerase chain reaction (qPCR) using the 38 water samples analyzed. Comparisons were tested by an unpaired t test. (A) Acanthamoeba spp. were detected by qPCR in 44.7% of the analyzed samples, in contrast to the 18.4% positive samples by culture growth (**, P = 0.005). (B) Vermamoeba vermiformis was detected in 31.6% of the analyzed samples by qPCR, whereas NNA culture growth identified 10.5% of positives samples (*, P = 0.04).
DISCUSSION
In this study, we demonstrated the utility of a qPCR-based technique in a multiplex variant that allows the simultaneous detection of four FLA, Acanthamoeba spp., N. fowleri, B. mandrillaris, and V. vermiformis, in the same sample. The proposed assay showed high specificity and sensitivity, allowing the detection and processing of a high number of samples.
Monitoring and detection of FLA in water systems are crucial, as these amoebae pose a potential risk to public health.18 Culture and traditional PCR-based approaches have been the diagnostic methods of choice to date, but they are lengthy procedures that require precision and training.3 Quantitative PCR is a method of high specificity and sensitivity, with many advantages in comparison to conventional PCR.7 The qPCR developed in this study has been shown to be useful for detecting the presence of these four types of amoebae in aquatic environments, requiring less time for genetic detection than that of conventional techniques and reducing the high cost because of its multiplex format.
Our findings are in accordance with results obtained by other researchers on the presence of FLA of the genus Acanthamoeba in different water sources.19,20 According to Loret and Greub, the observed prevalence is related to the amount of organic matter present in the water, with a high frequency in sediments and biofilms, where ecological niches in which the amoebae can feed on bacteria are constituted.18 Despite their ubiquitous nature and the fact that not all species are pathogenic, members of the genus Acanthamoeba are considered a biosafety level 2 pathogen since they can cause amoebic granulomatous encephalitis, amoebic keratitis, and corneal infections. The genus is classified into 23 genotypes (T1 to T23),21,22 with T4 as the most common associated with these diseases.23 Although the trophozoites are the cause of the infection, the cysts ensure its persistence in the environment.24
Together with amoebae of the Acanthamoeba genus, V. vermiformis is one of the most environmentally widespread FLA.4 Moreover, V. vermiformis has been reported as a thermotolerant protozoan capable of colonizing heated bodies of water.25,26 In addition, there are several studies which report the capacity of V. vermiformis to act as a vehicle for pathogenic bacteria that have acquired resistance to the protozoan digestive enzymes.4 The ability of V. vermiformis to be present everywhere and its thermotolerance make it a possible source of pathogens and enable this protozoan and its guests to proliferate in harsh environments.
It is important to highlight that the tapwater samples as well as the stagnant water samples used for the validation of our technique are not environmental freshwater but come from water treatment systems. These systems are regulated by Spanish legislation (RD 3/2023),27 and the presence of parasites is evaluated only when the turbidity values are higher than 4 UTN (nephelometric turbidity units) and only if the sanitary authorities consider it necessary. Therefore, the presence of these pathogenic FLA is not regulated. Among the most common protozoa found in water resources, FLA are able to accomplish their life cycle without a host organism.28 These organisms have been isolated from freshwater, tapwater, brackish water, seawater, water treatment plants, and sanitary water, among others.29 Considering the environmental ubiquity of these protozoa and their capacity to cause several human diseases, the CDC has considered them emerging pathogens.30
The present work has confirmed the presence of Acanthamoeba spp. and V. vermiformis strains, detected by different methods, in water samples from Tenerife. Importantly, the proposed multiplex qPCR set up in this study was able to detect Acanthamoeba spp. and V. vermiformis species of FLA in cases where culture and traditional PCR results were negative, demonstrating greater sensitivity in detection. The multiplex qPCR developed was able to detect 2.4 and 3 times more parasites (Acanthamoeba spp. and V. vermiformis, respectively) in the water samples analyzed. The sensitivity of the assay allowed us to detect a very small amount of template DNA corresponding to a parasite count as low as 0.3 to 1 amoeba for the four species evaluated. It is important to note that in two of the samples analyzed (FW8 and FW17, corresponding to Acanthamoeba isolates OR940145 and OR940144, respectively), the presence of FLA was evidenced by culture and traditional PCR but not by the qPCR assay. To explain this fact, it is important to highlight that both samples consisted of 5 L of filtered water and that the nitrocellulose membrane used was cut and divided into two halves. The presence of Acanthamoeba could have gone undetected because this protozoan was adhered to the half filter used for culturing. Considering the demonstrated sensitivity of the qPCR technique and the large water volume processed, the absence of detection of the evaluated FLA in the present study may be due to a very low quantity of FLA in the water sample. However, as there is no current Spanish legislation related to FLA in environmental resources, report of its presence is based on detection or no detection. Therefore, to avoid an information loss and to evaluate the presence of FLA from natural resources, the present work promotes a combination of methods based on culture and qPCR. Nevertheless, in accordance with the report of Aykur and Dagci,31 qPCR seems to be the best strategy for clinical diagnosis because of its promptness and sensitivity.
The qPCR designed to simultaneously detect Acanthamoeba spp., N. fowleri, B. mandrillaris, and V. vermiformis was developed with the aim of improving the efficient and fast detection of these parasites in water samples but also to make available FLA detection in different kinds of samples, such as clinical or environmental (tap water, stagnant water, and soil samples). The resulting qPCR is a robust assay that can be set up in most current molecular laboratories without the need of the expertise required for morphology-based tests. Nevertheless, ideally and in relation to our findings, this method should complement existing conventional laboratory methods for FLA diagnosis.
CONCLUSION
In conclusion, the multiplex qPCR for Acanthamoeba spp., N. fowleri, B. mandrillaris, and V. vermiformis has demonstrated its potential as a rapid and sensitive tool for the detection of these FLA in different water sources. The high performance of the qPCR technique can complement routine diagnosis. Moreover, by use of the developed tool, the presence of FLA species was detected in half of the water sources evaluated in the current study. Because FLA pose a direct threat as infectious agents and as transmitters of pathogenic bacteria, their presence should be considered by the authorities.
Supplemental Materials
ACKNOWLEDGMENT
We express our gratitude to the IUETSPC-University of La Laguna for its facilities.
Note: Supplemental material appears at www.ajtmh.org.
Contributor Information
Elizabeth Córdoba-Lanús, Email: acordoba@ull.edu.es.
José E. Piñero, Email: jpinero@ull.edu.es.
Data Availability
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as additional information.
REFERENCES
- 1. Shi Y, Queller DC, Tian Y, Zhang S, Yan Q, He Z, He Z, Wu C, Wang C, Shu L, 2021. The ecology and evolution of amoeba-bacterium interactions. Appl Environ Microbiol 87: e01866-e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Samba-Louaka A, Delafont V, Rodier MH, Cateau E, Héchard Y, 2019. Free-living amoebae and squatters in the wild: Ecological and molecular features. FEMS Microbiol Rev 43: 415–434. [DOI] [PubMed] [Google Scholar]
- 3. Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ, 2006. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol 44: 3589–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siddiqui R, Makhlouf Z, Khan NA, 2021. The increasing importance of Vermamoeba vermiformis. J Eukaryot Microbiol 8: e12857. [DOI] [PubMed] [Google Scholar]
- 5. Lorenzo-Morales J, Khan NA, Walochnik J, 2015. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 22: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kofman A, Guarner J, 2022. Infections caused by free-living amoebae. J Clin Microbiol 60: e0022821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuiper MW, Valster RM, Wullings BA, Boonstra H, Smidt H, van der Kooij D, 2006. Quantitative detection of the free-living amoeba Hartmannella vermiformis in surface water by using real-time PCR. Appl Environ Microbiol 72: 5750–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khairnar K, Tamber GS, Ralevski F, Pillai DR, 2011. Comparison of molecular diagnostic methods for the detection of Acanthamoeba spp. from clinical specimens submitted for keratitis. Diagn Microbiol Infect Dis 70: 499–506. [DOI] [PubMed] [Google Scholar]
- 9. Bustin SA. et al. , 2009. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622. [DOI] [PubMed] [Google Scholar]
- 10. Cabello-Vílchez AM. et al. , 2014. The isolation of Balamuthia mandrillaris from environmental sources from Peru. Parasitol Res 113: 2509–2513. [DOI] [PubMed] [Google Scholar]
- 11. Reyes-Batlle M, Gabriel MF, Rodríguez-Expósito R, Felgueiras F, Sifaoui I, Mourão Z, de Oliveira Fernandes E, Piñero JE, Lorenzo-Morales J, 2021. Evaluation of the occurrence of pathogenic free-living amoeba and bacteria in 20 public indoor swimming pool facilities. MicrobiologyOpen 10: e1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonilla-Lemus P, Rojas-Hernández S, Ramírez-Flores E, Castillo-Ramírez DA, Monsalvo-Reyes AC, Ramírez-Flores MA, Barrón-Graciano K, Reyes-Batlle M, Lorenzo-Morales J, Carrasco-Yépez MM, 2020. Isolation and identification of Naegleria species in irrigation channels for recreational use in Mexicali Valley, Mexico. Pathogens 9: 820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Page FC, 1967. Taxonomic criteria for limax amoebae, with descriptions of 3 new species of Hartmannella and 3 of Vahlkampfia. J Protozool 14: 499–521. [DOI] [PubMed] [Google Scholar]
- 14. Page FC, 1988. A New Key to Freshwater and Soil Gymnamoebae: With Instructions for Culture. Cumbria, United Kingdom: Freshwater Biological Association. [Google Scholar]
- 15. Lorenzo-Morales J, Monteverde-Miranda CA, Jiménez C, Tejedor ML, Valladares B, Ortega-Rivas A, 2005. Evaluation of Acanthamoeba isolates from environmental sources in Tenerife, Canary Islands, Spain. Ann Agric Environ Med 12: 233–236. [PubMed] [Google Scholar]
- 16. Schroeder JM, Booton GC, Hay J, 2001. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoeba from humans with keratitis and from sewage sludge. J Clin Microbiol 39: 1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsvetkova N, Schild M, Panaiotov S, Kurdova-Mintcheva R, Gottstein B, Walochnik J, Aspöck H, Lucas MS, Müller N, 2004. The identification of free-living environmental isolates of amoebae from Bulgaria. Parasitol Res 92: 405–413. [DOI] [PubMed] [Google Scholar]
- 18. Loret JF, Greub G, 2010. Free-living amoebae: Biological by-passes in water treatment. Int J Hyg Environ Health 13: 167–175. [DOI] [PubMed] [Google Scholar]
- 19. Bagheri H, Shafiei R, Shafiei F, Sajjadi S, 2010. Isolation of Acanthamoeba spp. from drinking waters in several hospitals of Iran. Iran J Parasitol 5: 19–25. [PMC free article] [PubMed] [Google Scholar]
- 20. Milanez G. et al. , 2020. Detection of Acanthamoeba spp. in two major water reservoirs in the Philippines. J Water Health 18: 118–126. [DOI] [PubMed] [Google Scholar]
- 21. Fuerst PA, Booton GC, 2020. Species, sequence types and alleles: Dissecting genetic variation in Acanthamoeba. Pathogens 9: 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Putaporntip C, Kuamsab N, Nuprasert W, Rojrung R, Pattanawong U, Tia T, Yanmanee S, Jongwutiwes S, 2021. Analysis of Acanthamoeba genotypes from public freshwater sources in Thailand reveals a new genotype, T23 Acanthamoeba bangkokensis sp. nov. Sci Rep 11: 17290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siddiqui R, Khan NA, 2012. Biology and pathogenesis of Acanthamoeba. Parasit Vectors 5: 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chávez-Munguía B, Omaña-Molina M, González-Lázaro M, González-Robles A, Bonilla P, Martínez-Palomo A, 2005. Ultrastructural study of encystation and excystation in Acanthamoeba castellanii. J Eukaryot Microbiol 52: 153–158. [DOI] [PubMed] [Google Scholar]
- 25. Delafont V, Rodier MH, Maisonneuve E, Cateau E, 2018. Vermamoeba vermiformis: A free-living amoeba of interest. Microb Ecol 76: 991–1001. [DOI] [PubMed] [Google Scholar]
- 26. Fouque E, Héchard Y, Hartemann P, Humeau P, Trouilhé MC, 2015. Sensitivity of Vermamoeba (Hartmannella) vermiformis cysts to conventional disinfectants and protease. J Water Health 13: 302–310. [DOI] [PubMed] [Google Scholar]
- 27. Ministry of the Presidency , 2023. Royal Decree 3/2023. Spanish Royal Decree—Law establishing the technical-sanitary criteria for the quality of drinking water, its control and supply.
- 28. Cateau E, Delafont V, Hechard Y, Rodier MH, 2014. Free-living amoebae: What part do they play in healthcare-associated infections? J Hosp Infect 87: 131–140. [DOI] [PubMed] [Google Scholar]
- 29. Scheikl U, Sommer R, Kirschner A, Rameder A, Schrammel B, Zweimüller I, Wesner W, Hinker M, Walochnik J, 2014. Free-living amoebae (FLA) co-ocurring with legionellae in industrial waters. Eur J Protistol 50: 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Visvesvara GS, 2013. Infections with free-living amebae. Handb Clin Neurol 114: 153–168. [DOI] [PubMed] [Google Scholar]
- 31. Aykur M, Dagci H, 2021. Evaluation of molecular characterization and phylogeny for quantification of Acanthamoeba and Naegleria fowleri in various water sources, Turkey. PLoS One 16: e0256659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as additional information.