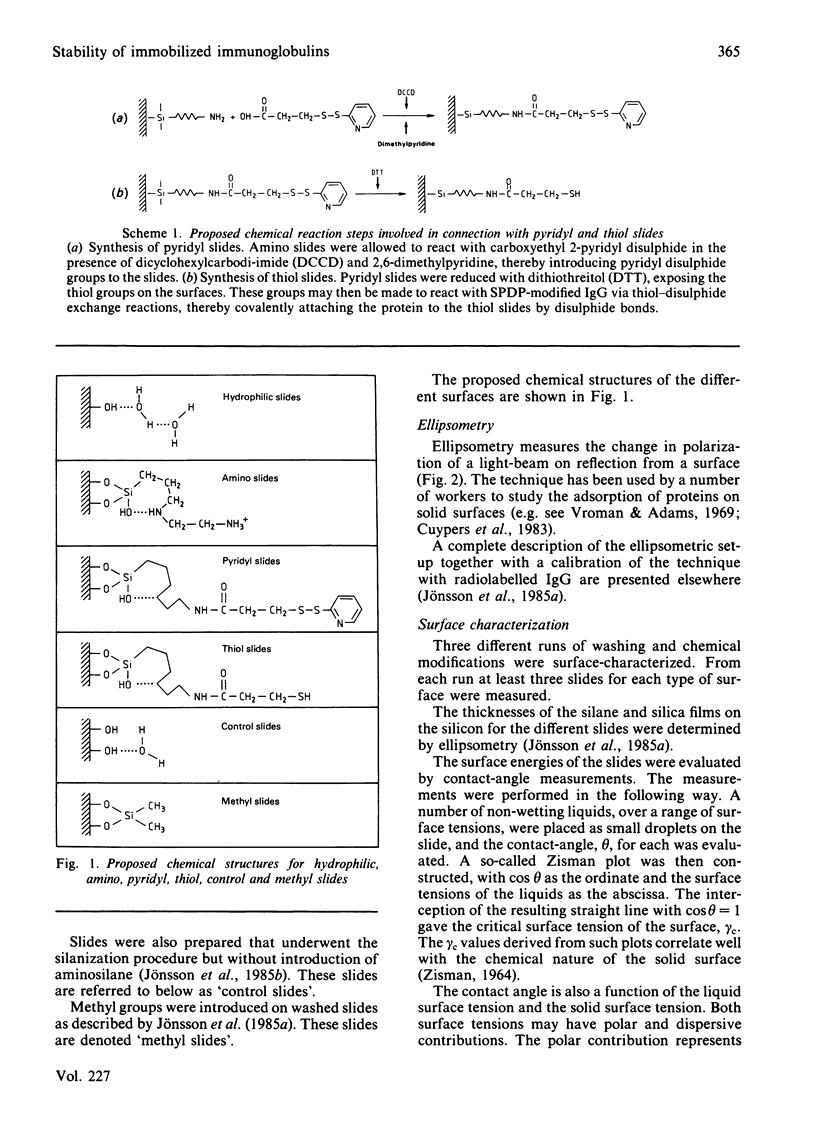
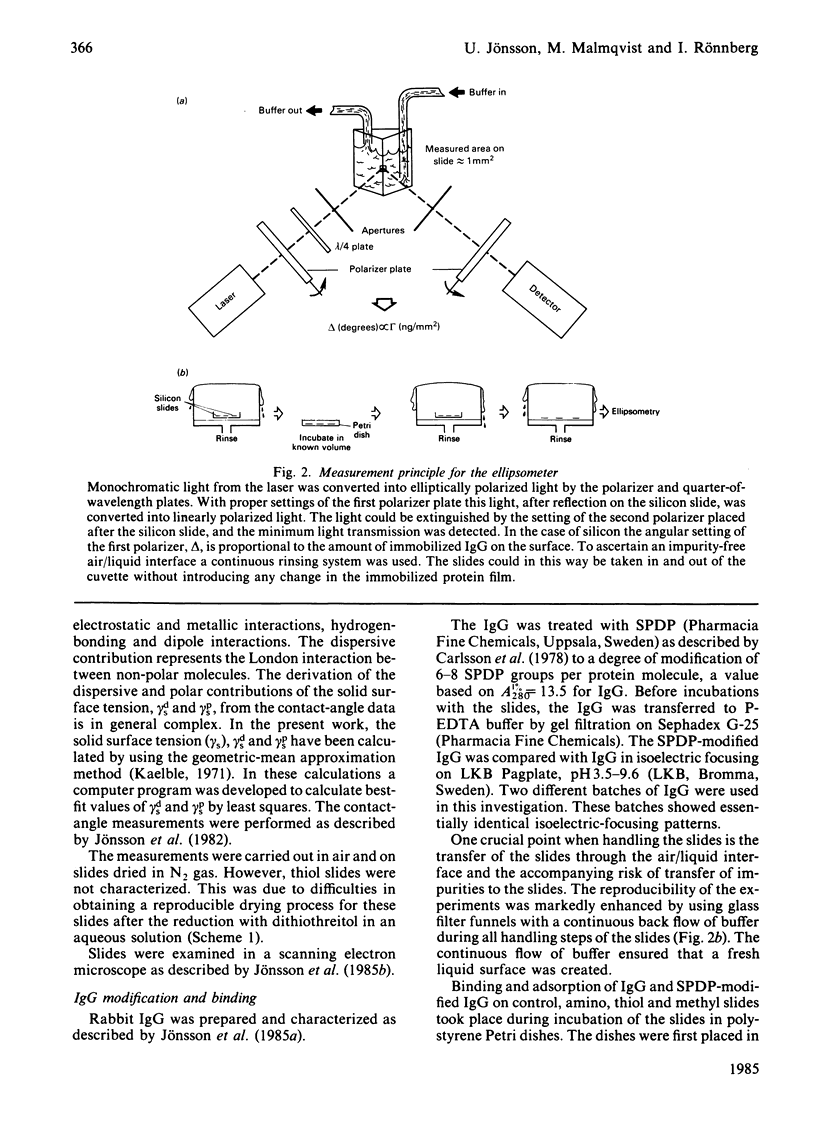
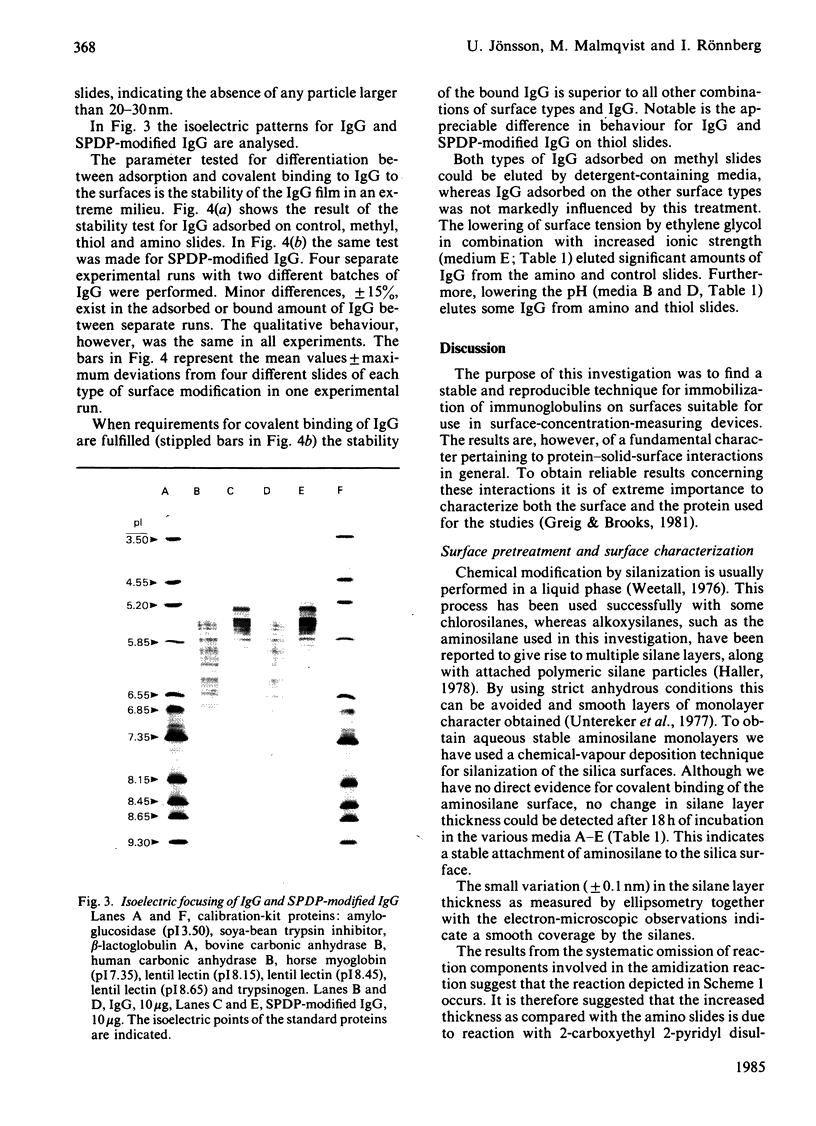
Abstract
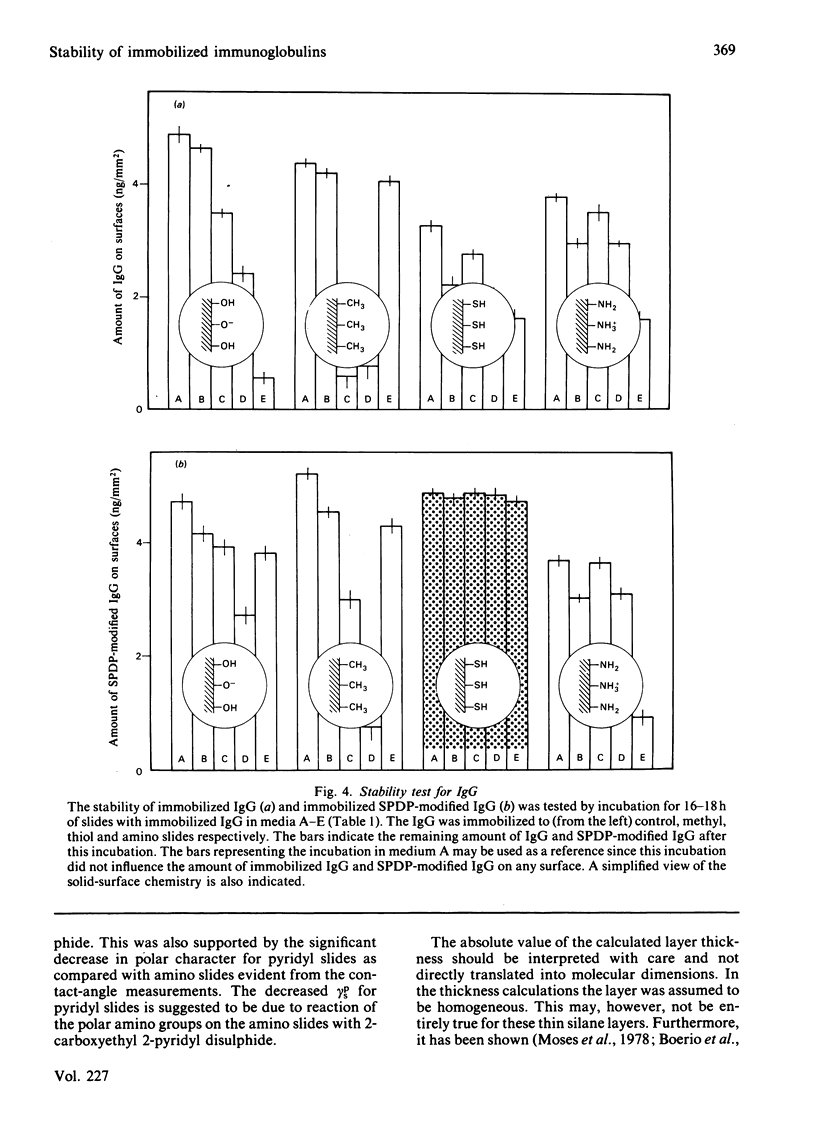
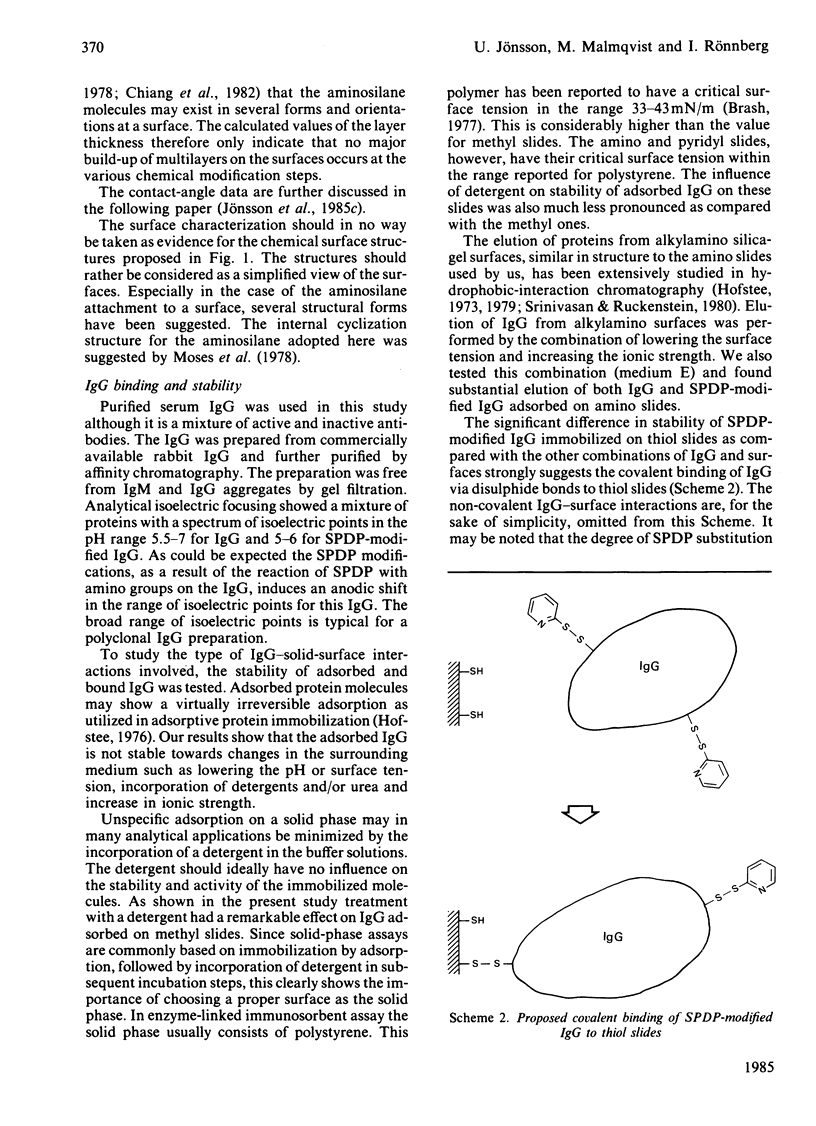
The development of new immunosensors based on surface-concentration-measuring devices requires a stable and reproducible immobilization of antibodies on well-characterized solid surfaces. We here report on the immobilization of immunoglobulin G (IgG) on chemically modified silica surfaces. Such surfaces may be used in various surface-oriented analytical methods. Reactive groups were introduced to the silica surfaces by chemical-vapour deposition of silane. The surfaces were characterized by ellipsometry, contact-angle measurements and scanning electron microscopy. IgG covalently bound by the use of thiol-disulphide exchange reactions, thereby controlling the maximum number of covalent bonds to the surface, was compared with IgG adsorbed on various silica surfaces. This comparison showed that the covalently bound IgG has a superior stability when the pH was lowered or incubation with detergents, urea or ethylene glycol was carried out. The result was evaluated by ellipsometry, an optical technique that renders possible the quantification of amounts of immobilized IgG. The results outline the possibilities of obtaining a controlled covalent binding of biomolecules to solid surfaces with an optimal stability and biological activity of the immobilized molecules.
Full text
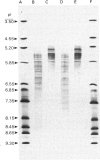
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baszkin A., Lyman D. J. The interaction of plasma proteins with polymers. I. Relationship between polymer surface energy and protein adsorption/desorption. J Biomed Mater Res. 1980 Jul;14(4):393–403. doi: 10.1002/jbm.820140406. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradie J. D., Govender M., Visser L. ELISA solid phase: partial denaturation of coating antibody yields a more efficient solid phase. J Immunol Methods. 1983 May 13;59(3):289–299. doi: 10.1016/0022-1759(83)90190-4. [DOI] [PubMed] [Google Scholar]
- Cuypers P. A., Corsel J. W., Janssen M. P., Kop J. M., Hermens W. T., Hemker H. C. The adsorption of prothrombin to phosphatidylserine multilayers quantitated by ellipsometry. J Biol Chem. 1983 Feb 25;258(4):2426–2431. [PubMed] [Google Scholar]
- Giaever I. Visual detection of carcinoembryonic antigen on surfaces. J Immunol. 1976 Mar;116(3):766–771. [PubMed] [Google Scholar]
- Hofstee B. H. Hydrophobic affinity chromatography of proteins. Anal Biochem. 1973 Apr;52(2):430–448. doi: 10.1016/0003-2697(73)90046-8. [DOI] [PubMed] [Google Scholar]
- Jönsson U., Malmqvist M., Rönnberg I. Immobilization of immunoglobulins on silica surfaces. Kinetics of immobilization and influence of ionic strength. Biochem J. 1985 Apr 15;227(2):373–378. doi: 10.1042/bj2270373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. R. The affinity electrode. Application to the assay of human serum albumin. FEBS Lett. 1979 Oct 15;106(2):405–408. doi: 10.1016/0014-5793(79)80542-6. [DOI] [PubMed] [Google Scholar]
- Nygren H., Sandström T., Stenberg M. Direct visual detection of protein antigen: importance of surface concentration. J Immunol Methods. 1983 Apr 29;59(2):145–149. doi: 10.1016/0022-1759(83)90025-x. [DOI] [PubMed] [Google Scholar]
- Watkins R. W., Robertson C. R. A total internal-reflection technique for the examination of protein adsorption. J Biomed Mater Res. 1977 Nov;11(6):915–938. doi: 10.1002/jbm.820110611. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Nagasawa Y., Shuto S., Tsubomura H., Sawai M., Okumura H. Antigen--antibody reaction investiaged with use of a chemically modified electrode. Clin Chem. 1980 Oct;26(11):1569–1572. [PubMed] [Google Scholar]
- van Oss C. J., Absolom D. R., Grossberg A. L., Neumann A. W. Repulsive van der Waals forces. I. Complete dissociation of antigen-antibody complexes by means of negative van der Waals forces. Immunol Commun. 1979;8(1):11–29. doi: 10.3109/08820137909044703. [DOI] [PubMed] [Google Scholar]