Abstract
Traditionally, hydrogen peroxide (H2O2) was formed from cellular oxidative metabolism and often viewed as toxic waste. In fact, H2O2 was a benefit messenger for neuron-glia signaling and synaptic transmission. Thus, H2O2 was a double-edged sword and neuroprotection vs. neurotoxicity produced by H2O2 was difficult to define. Nuclear factor erythroid 2-related factor 2 (Nrf2) has been implicated as an intracellular regulator of neuronal growth. Inactivation of Nrf2 participated in the development of Parkinson's disease (PD). Thus, suitable activation of Nrf2 was essential for the prevention and treatment of PD. This study aimed to explore whether H2O2-conferred neuroprotective effects to support neuronal survival. H2O2 were added into primary neuron-glia, neuron-astroglia and neuron-microglia co-cultures in concentration- and time-dependent manners. H2O2 increased dopamine (DA) neuronal survival in concentration- and time-dependent manners. In addition, glial cells Nrf2 activation involved in H2O2-supported DA neuronal survival with the following phenomenons. First, H2O2 activated Nrf2 signaling pathway. Second, H2O2 generated beneficial neuroprotection in neuron-glia, neuron-astroglia and neuron-microglia co-cultures but not in neuron-enriched cultures. Third, silence of Nrf2 in glial cells abolished H2O2-conferred DA neuronal survival. This study demonstrated that physiological concentration of H2O2-supported DA neuronal survival via activation of Nrf2 signaling in glial cells. Our data permit to re-evaluate the role of H2O2 in the pathogenesis and therapeutic strategies for PD.
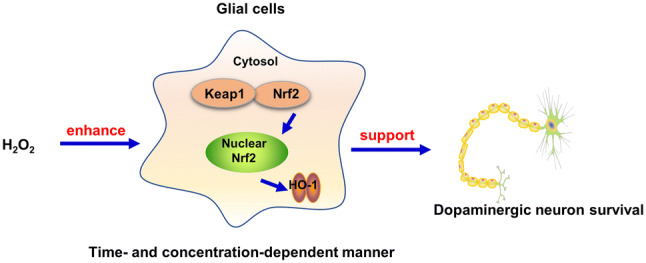
Graphic Abstract
Keywords: H2O2, Dopamine neurons, Glial cells, Nrf2
Introduction
The gradual, irreversible loss of dopamine (DA) neurons in the midbrain is a characteristic of Parkinson's disease (PD) (Magalingam et al. 2015). Unfortunately, little is known about the reasons why PD is unstoppable (Zheng et al. 2017). Therefore, there is no reliable clinical treatment to delay PD progression (Machado et al. 2016).
Hydrogen peroxide (H2O2) is formed from cellular oxidative metabolism and often considered as toxic waste (Lee et al. 2015). Excessive H2O2 is a key factor for many central nervous system (CNS) disorders, such as trauma and neurodegenerative diseases (Ohashi et al. 2016; Beltrán González et al. 2019). However, H2O2 is one unconventional and under-understood neuromodulator. In brain cells, H2O2 has been implicated as an intracellular regulator of neuronal activity, as well as a diffusible messenger for neuron-glia signaling and synaptic transmission (Ohashi et al. 2016; Kamsler and Segal 2003). In fact, the balance of neuroprotection vs. neurotoxicity produced in a given neuronal cell by H2O2 is difficult to define. However, the pathophysiological role of H2O2 in DA neuronal survival remains poorly understood.
Nuclear factor erythroid 2-related factor 2 (Nrf2) has been recognized as an intracellular regulator of neuronal growth (Bell et al. 2015). Once Nrf2 activated, Nrf2 transferred into nucleus from cytoplasm, initiating the expression of anti-oxidant proteases. Anti-oxidant proteases mainly include heme oxygenase-1 (HO-1) and NADPH quinone oxidoreductase 1 (NQO1). The high expression of HO-1 and NQO1 can delay the development of neurodegenerative diseases (Jeong et al. 2016; Huang et al. 2016). Aging is closely related to the loss of Nrf2 activity, inactivation of Nrf2 participated in the progress of PD (Johnson and Johnson 2015; Lastres-Becker et al. 2016). Thus, suitable activation of Nrf2 is essential for the prevention and treatment of PD.
H2O2 is well-suited as an intracellular signal transduction agent due to its low reactivity and high permeability. Currently, H2O2 is mainly focused on its neurotoxicity rather than neuroprotection. In the present study, primary rat midbrain neuron-glia co-cultures were applied to investigate different concentration of H2O2 on DA neuronal survival and further explore the role of Nrf2 signaling pathway on H2O2-mediated DA neuronal survival. These findings might provide a new understanding of H2O2 physiology.
Materials and Methods
Reagents
H2O2, leu-leu methyl ester (LME) and cytosine β-D-arabinofuranoside (ara-C) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Anti-kelch-like ECH-associated protein 1(Keap1) (Catalog No. ab119403), anti-Nrf2 (Catalog No. ab137550), anti-heme oxygenase (HO-1) (Catalog No.ab13248), anti-tyrosine hydroxylase (TH) (Catalog No. ab41528), anti-β-actin (Catalog No.ab179467), anti-Hsp90 (Catalog No. ab203126) and anti-Histone H (Catalog No.ab1791) antibodies were bought from Abcam (Cambridge, MA, USA). SYBR green polymerase chain reaction (PCR) master mix was purchased from Bio-Rad (CA, USA). Nrf2-siRNA and control-siRNA were obtained from Thermo Fisher Scientific (Waltham, MA, USA).
Primary Neuron-Glia Co-Cultures
Primary neuron-glia co-cultures were prepared from the ventral midbrain tissues of embryonic day 14 ± 0.5 Fischer 334 rats. After removing blood vessels and meninges, tissues were isolated and dissociated with gentle mechanical trituration. Dissociated cells were seeded at 2 × 106/well in 6-well plates. Cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air in DMEM/F12 containing 10% heat-inactivated fetal bovine serum (FBS), 50 U/ml penicillin and 50 mg/ml streptomycin. Changing the medium every three days. Seven days later, co-cultures were made up of 10% microglia, 50% astroglia and 40% neurons of which 2.5–3.5% were tyrosine hydroxylase (TH)-immunoreactive (IR) neurons (Chen et al. 2015).
Primary Mixed-Glia, Microglia-Enriched Cultures
Primary mixed-glia cultures were prepared from the whole brains of postnatal day one rats. After removing meninges and blood vessels, tissues were isolated and dissociated with gentle mechanical trituration. Cells were seeded in 150 cm3 cultures flask at the cell density of 5 × 107. Cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air in DMEM/F12 containing 10% heat-inactivated fetal bovine serum (FBS), 50 U/ml penicillin and 50 mg/ml streptomycin. For microglia-enriched cultures, after obtaining a confluent monolayer of glial cells (10–12 days after initial seeding), microglia were shaken off, collected and seeded.
Primary Neuron-Enriched, Neuron-Astroglia and Neuron-Microglia Cultures
For neuron-enriched cultures, two days after neuron-glia co-cultures seeding, ara-C (8 μM) was added into the cultures to suppress the glia cells proliferation. For neuron-astroglia cultures, one day after neuron-glia co-cultures seeding, LME (1 mM) was added into cultures to suppress microglia proliferation. Seven-day-old cultures were used for treatments (Gao et al. 2011). For neuron-microglia re-constituted cultures, the collected microglia-enriched culture (1 × 105/well) were seeded to 7-day-old neuron-enriched cultures. Then, the re-constituted cultures were used for treatments (Hu et al. 2012). Three types of co-cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air in DMEM/F12 containing 10% heat-inactivated fetal bovine serum (FBS), 50 U/ml penicillin and 50 mg/ml streptomycin.
Silence of Nrf2
Nrf2-siRNA (40 nM) or control-siRNA (40 nM) were added into primary mixed-glia cultures to inhibit Nrf2 expression. Twenty-four hours later, silence efficiency was measured by western blot assay and real-time RT-PCR assay.
Neuron-Glia Re-Constituted Cultures by Transwells
Mixed-glia cultures were planted in transwells and transfected by Nrf2-siRNA. Twenty-four hours later, Nrf2 gene silence in glial cells was determined. Then, transwells containing mixed-glia cell were washed by PBS and moved into 7-day-old neuron-enriched cultures within fresh medium. Accordingly, the re-constituted neuron-glia cultures were treated with H2O2 (80 μM) for 5 days. DA neuronal survival was assessed by immunocytochemical staining and western blotting.
[3H]DA Uptake Assays
Cultures were incubated for 20 min at 37 °C with 1 mM [3H]DA in Krebs–Ringer buffer (16 mM sodium phosphate, 119mMNaCl, 4.7 mM KCl, 1.8 mM CaCl2, 1.2 mM MgSO4, 1.3 mM EDTA, and 5.6 mM glucose; pH7.4). Cells were washed with ice-cold Krebs–Ringer buffer, and then collected in 1 N NaOH. Radioactivity was determined by liquid scintillation counting. Nonspecific DA uptake observed in the presence of mazindol (10 μM) was subtracted (Zhang et al. 2010; Hu et al. 2012).
Immunocytochemical Staining
The identification of DA neurons was reflected by the detection of TH. Cells were permeabilizated by 0.03% Triton X-100, fixed with 4% paraformaldehyde and closed with 10% goat serum. DA neurons were stained with the antibody against TH (1:500). Images were recorded with an inverted microscope (Nikon, Tokyo, Japan). Nine representative areas per well of a 6-well plate were counted under the microscope at 100 × magnification.
Western Blotting
Nuclear and cytosol protein were extracted with Nuclear-Cytosol Extraction Kit. Equal amounts of protein (10 μg/lane) were separated on 4–12% Nu-PAGE gel. The membranes were blocked with 5% nonfat milk and incubated with primary antibody (rabbit anti-TH antibody (1: 500), rabbit anti- Nrf2 antibody (1: 1000), rabbit anti- Keap1 (1:1000), rabbit anti- HO-1antibody (1: 1000), rabbit anti- Histone H antibody (1: 1000), mouse anti-β-actin (1:3000) overnight at 4 °C. After washing by PBS, membranes were incubated with anti-rabbit or mouse IgG (1:2000) for 1.5 h at 25 °C. ECL substrate was used as a detection system. β-actin was included as an internal standard to monitor loading errors. Quantity One software system (Bio-Rad, Hercules, CA, USA) were used for densitometric analysis.
Real-Time RT-PCR Assay
The total RNA isolated from whole cell was prepared using RNeasy kit according to the manufacturer’s instructions. The primer sequences were as follows were: Nrf2, CAGTCTTCACCACCCCTGAT (F), CAGTGAGGGGATCGATGAGT(R); Keap1, AGGAATGAGTGGCGGATGAT (F), GCGCTCCACACTGTTCAACT(R); HO-1, AGAGGCTAAGACCGCCTTCC (F), TCTGACGAAGTGACGCCATC(R); β-actin, GTATGACTCCACTCACGGCAAA (F), GGTCTCGCTCCTGGAAGATG(R). Accordingly, β-actin was included as an internal standard to monitor loading errors.
Statistical Analysis
Results were expressed as mean ± standard error of the mean (SEM). Statistical comparisons were analyzed using GraphPad Prism 5 software and SPSS 18.0 software. After one-way analysis of variance (ANOVA) expressed significant differences, post-hoc Fisher’s least significant difference (LSD) were used for all pairwise comparisons among means. A value of P < 0.05 was considered statistically significant.
Results
H2O2 Increased DA Neuronal Survival in Concentration- and Time-Dependent Manners
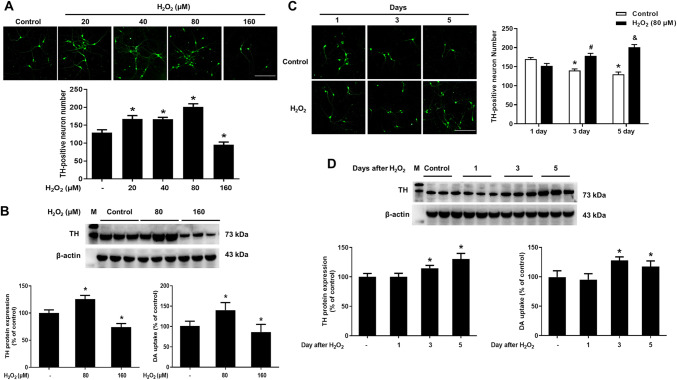
Seven-day-old primary neuron-glia co-cultures were performed to detect the effects of H2O2 on DA neuronal survival. Five days later, DA neurons were determined by immunocytochemical staining, western blot and [3H]DA uptake assay. As shown in Fig. 1a, H2O2 (20, 40, 80 and 160 μM) were added into neuron-glia co-cultures for 5 days, immunostaining of DA neurons indicated that H2O2 (20–80 μM) increased DA neuronal survival, displaying the enhanced TH immunoreactivity with H2O2 (80 μM) almost. Furthermore, H2O2 had neurotoxicity up to the concentration of 160 μM, exhibiting more interrupted immunoreactivity in neuronal processes. TH protein expressions and [3H]DA uptake assay were parallel with immunostaining results (Fig. 1b). Thus, H2O2-conferred DA neuronal survival in a concentration-dependent manner. In addition, as shown in Fig. 1c, after H2O2 (80 μM) treatment for 1, 3 and 5 days, DA neurons in control group decreased time-dependently. However, 1, 3 and 5 day after cultures exposure to H2O2 (80 μM), DA neuronal number had no significant difference at 1 day, increased slightly at 3 days and peaked at 5 days. Similar results were shown in TH protein quantification and [3H]DA uptake assay (Fig. 1d). Collectively, H2O2-elicited DA neuronal survival in concentration- and time-dependent manners.
Fig. 1.
H2O2 increased DA neuronal survival in concentration and time-dependent manners. Seven-day-old neuron-glia co-cultures were treated with various concentrations of H2O2 for 5 days. DA neurons were measured by immunofluorescence staining, western blot assay and [3H]DA uptake assay (a and b). In addition, DA neuronal survival after H2O2 (80 μM) addition for 1, 3 and 5 days was detected via immunofluorescence staining, western blot assay and [3H]DA uptake assay (c and d). Results were mean ± SEM from 3 individual experiments in triplicate in each experiment. *p < 0.05, versus 1 day control cultures; #p < 0.05, versus 3 days control cultures; &p < 0.05, versus 5 days control cultures. Scale bar = 100 μm
H2O2 Activated Nrf2 Signaling Pathway in a Concentration-Dependent Manner
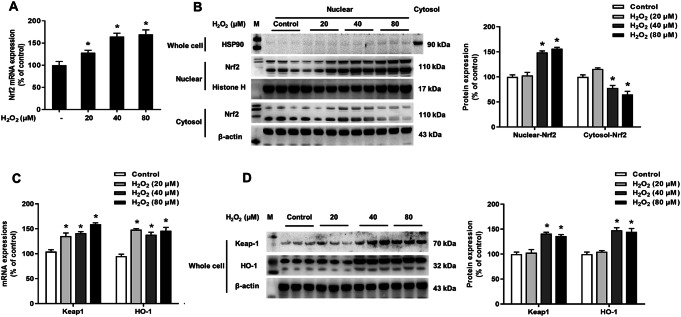
Primary neuron-glia co-cultures were exposure to H2O2 (20–80 μM) for 5 days. The effects of H2O2 on Nrf2 signaling pathway activation were detected. As indicated in Fig. 2a, the expression of Nrf2 mRNA were upregulated with H2O2 (20–80 μM) exposure. In addition, to further detect whether H2O2 affected Nrf2 distribution, the protein expressions of Nrf2 in cytosol and nuclear were measured. HSP90 expressed in cytosol but not in nuclear, cytosolic marker HSP90 were used to test the purity of nuclear protein. There is no cytosol protein involved in the nuclear protein from HSP90 protein expression. Furthermore, western blot data indicated that H2O2 (40 and 80 μM) concentration-dependently stimulated Nrf2 nuclear translocation (Fig. 2b). Moreover, H2O2 (20–80 μM) increased mRNA levels of Keap1 and HO-1 (Fig. 2c). In addition, H2O2 (40 and 80 μM) increased Keap1 and HO-1protein expression (Fig. 2d).
Fig. 2.
H2O2 activated Nrf2 signaling pathway in a concentration-dependent manner. H2O2 (20, 40 and 80 μM) were added into primary neuron-glia co-cultures for 5 days. The mRNA and protein expressions of Nrf2 (a and b), Keap1and HO-1 (c and d) in whole cells/nuclear/cytosol were determined by real-time RT-PCR and western blot assay, respectively. Results were mean ± SEM from 3 individual experiments in triplicate in each experiment. *p < 0.05, versus control cultures
H2O2-Induced Nrf2 Signaling Pathway Activation in a Time-Dependent Manner
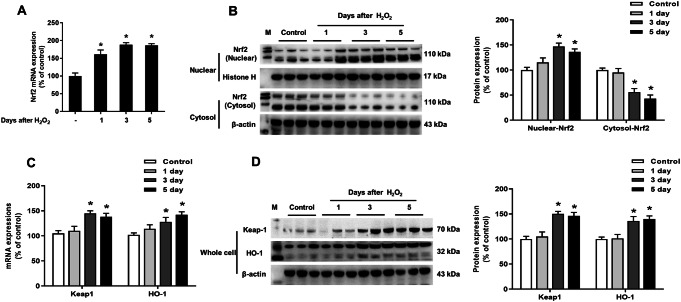
To further determine whether H2O2-induced Nrf2 signaling pathway activation was in a time-dependent manner, primary neuron-glia co-cultures were exposure to H2O2 (80 μM) for 1, 3 and 5 days, respectively. As indicated in Fig. 3a, b, time course study of mRNA and protein expressions analysis revealed that H2O2 increased Nrf2 mRNA expression from H2O2 stimulation for 1 day. H2O2 increased Nrf2 translocation from H2O2 stimulation for 3 day. Furthermore, H2O2-induced Nrf2 downstream signaling Keap1 and HO-1 activation were more pronounced after H2O2 treatment for 3 and 5 days (Fig. 3c).
Fig. 3.
H2O2-induced Nrf2 signaling pathway activation in a time-dependent manner. Midbrain neuron-glia co-cultures were treated with H2O2 (80 μM) for 1, 3 and 5 days. The mRNA and protein expressions of Nrf2 (a and b), Keap1 and HO-1 (c and d) in whole cells/nuclear/cytosol were detected by real-time RT-PCR and western blot assay. Results were mean ± SEM from 3 individual experiments in triplicate in each experiment. *p < 0.05, versus control cultures
Glial Cells were Indispensable for H2O2-Supported DA Neuronal Survival
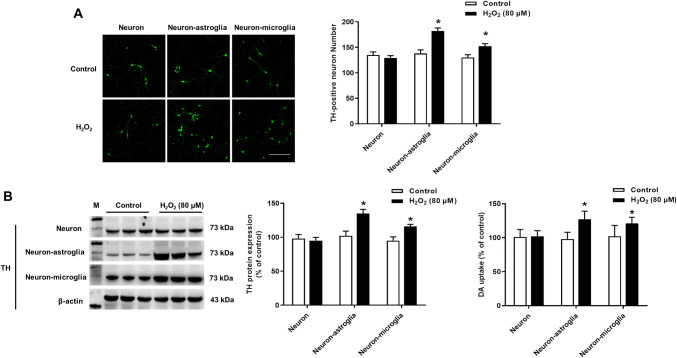
To explore which cell type H2O2 targeted to support DA neuronal survival, primary neuron-enriched, neuron-astroglia and neuron-microglia co-cultures were applied. Five days after H2O2 addition, DA neuronal survival was assessed by immunocytochemical staining (Fig. 4a), western blot assay and [3H]DA uptake assay (Fig. 4b). DA neuronal number, TH protein expression and the capacity to take up DA were increased in neuron-astroglia and neuron-microglia cultures after H2O2 treatment, but not in neuron-enriched cultures. This result suggested that glial cells were indispensable for H2O2-elicited DA neuronal survival.
Fig. 4.
Glial cells were indispensable for H2O2-supported DA neuronal survival. Three types of cultures including neuron-enriched, neuron-astroglia and neuron-microglia re-constituted cultures were treated with H2O2 (80 μM). Five days later, DA neuronal survival was detected by TH staining, TH protein expression and [3H]DA uptake assay (a and b). Results were mean ± SEM from 3 individual experiments in triplicate in each experiment. *p < 0.05, versus control cultures. Scale bar = 100 μm
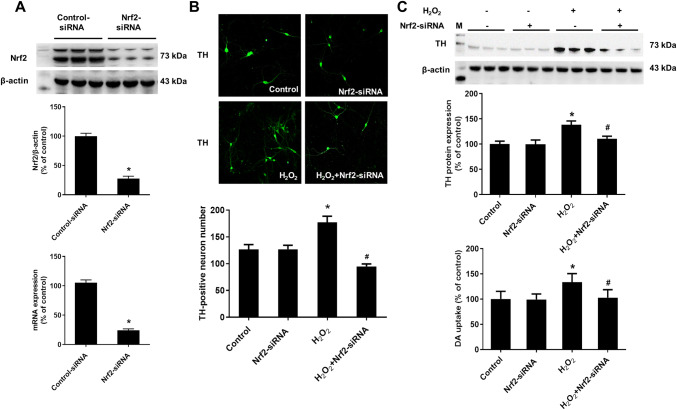
Silence of Nrf2 in Glial Cells Abolished H2O2-Supported DA Neuronal Survival
Nrf2-siRNA was applied to determine whether H2O2-induced activation of Nrf2 in glial cells contributed to DA neuronal survival. First, primary mixed-glial cells were planted in transwells. Twenty-four hours later, Nrf2-siRNA (40 nM) were added into transwells for another 24 h. We first detected the silence rate. Nrf2-siRNA markedly suppressed Nrf2 expressions both in mRNA and protein (Fig. 5a). Thus, Nrf2 in mixed-glia cell were knockout selectively. Then, transwells were washed by PBS and moved into 7-day-old neuron-enriched cultures within fresh medium, the new neuron-glia cultures were created and treated with H2O2 for 5 days. DA neuronal survival was assessed by immunocytochemical staining, western blot assay and [3H]DA uptake assay. As indicated in Fig. 5b, c, DA neuronal survival has no significant difference in control and Nrf2-siRNA groups. In addition, H2O2-induced increase in DA neuronal staining, TH protein level and increased capacity to take up DA were abolished by Nrf2-siRNA treatment. Together, glial cells Nrf2 was the target of H2O2 to support DA neuronal survival.
Fig. 5.
Silence of Nrf2 in glial cells abolished H2O2-supported DA neuronal survival. Primary mixed-glial cells were planted in transwells. Twenty-four hours later, Nrf2-siRNA (40 nM) were added into transwells for another 24 h. We first detected the silence rate using real-time RT-PCR and Western blotting (a). After washing the mixed-glial cells in the upper chamber with PBS, cells were transferred into lower chamber containing neuron-enriched cultures. Thus, the new neuron-glia cultures were created and treated with H2O2 for 5 days. DA neurons were detected by immunofluorescence staining, western blot assay and [3H]DA uptake assay (b and c). Results were mean ± SEM from 3 individual experiments in triplicate in each experiment. *p < 0.05, versus control cultures; #p < 0.05, versus H2O2-treated cultures. Scale bar = 100 μm
Discussion
This study provided new insights into H2O2 with the finding that physiological concentration of H2O2 increased DA neuronal survival in concentration- and time-dependent manners. In addition, glial cells Nrf2 activation involved in H2O2-supported DA neuronal survival with the following phenomenons. First, H2O2 activated Nrf2 signaling pathway in concentration- and time-dependent manners. Second, H2O2 generated beneficial neuroprotection in neuron-glia, neuron-astroglia and neuron-microglia co-cultures but not in neuron-enriched cultures. Third, silence of Nrf2 in glial cells abolished H2O2-conferred DA neuronal survival.
H2O2 is a stable reactive oxygen species and important neuromodulator in synaptic and neuronal activity (Gerich et al. 2009; Groeger et al. 2009). Centrally, endogenous H2O2 is elevated during bouts of hypoxia-reoxygenation and aging, this upregulation may be a spontaneous compensation mechanism trying to resist harmful factors (Ostrowski et al. 2014). Specifically, exogenous H2O2 (0.01–1 mM) in the central nervous system has been shown to mimic the concentration of H2O2 released endogenously by cellular effectors (Schroder and Eaton, 2008). This study indicated that the effective concentrations of H2O2 (20–80 μM) increased DA neuronal survival, while H2O2 (160 μM) showed neurotoxicity in primary neuron-glia co-cultures. Several lines of evidence indicated that insulin receptors played an important role in synaptic function, memory formation and neuroprotection (Chiu and Cline, 2010). Additionally, endogenous H2O2 signal surpassed a certain threshold, facilitating insulin receptor autophosphorylation and prolonging the duration of time for which the insulin receptor remained active (Persiyantseva et al. 2013). Likewise, H2O2 also modulated DA neuron activity and somatodendritic DA release in the substantia nigra pars compacta (SNc). Regulation of the nigrostriatal DA system is critical as the central role this pathway played in the control of movement by the basal ganglia (Patel and Rice, 2012; Rice 2011). Interestingly, H2O2 at effective concentration (80 μM) supported neuronal survival in neuron-astroglia, neuron-microglia and neuron-glia cultures but not in neuron-enriched cultures. Therefore, it was likely that H2O2-elicited neuroprotection was mediated through an indirect mechanism. This study confirmed this possibility that H2O2-conferred neuroprotection was glial cells-dependent.
Furthermore, this study indicated that H2O2 stimulated Nrf2 signaling pathway activation. The Nrf2 pathway is the predominant signaling pathway used for oxidative stress stimuli and initiating innate immunity (Liddell et al. 2016; Helou et al. 2019).
Increasing evidence implicated a role for H2O2 as a neuromodulator requires more subtle regulation by the anti-oxidant network. This network allowed H2O2 to reach functional concentrations intracellularly to prevent oxidative stress (Rice 2011). In addition, Nrf2 inactivation in brain accompanied with aging participated in the progress of neurodegenerative disease (Gureev and Popov 2019). Dimethylfumarate (DMF) is a putative Nrf2 activator, DMF exerted neuroprotective effects and protected against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced DA neurotoxicity in wild-type but not in Nrf2−/− mice (Ahuja et al. 2016). Furthermore, Nrf2 activity in astrocytes and meningeal cells link the neurotoxic actions of dopamine to neuroprotective pathways that may potentially modulate ischemic injury and neurodegeneration (Shih et al. 2007). Moreover, the protective features of astrocytes against oxidative stress are more prominent in striatal astrocytes, possibly by secreting humoral factors in striatal astrocytes (Asanuma et al. 2019). Additionally, AngII acts as a signaling molecule to activate NRF2 and KLF9 neuroprotective pathways in cellular and animal models of PD (Parga et al. 2018). Therefore, appropriate Nrf2 signaling pathway activation was a vital neuroprotective regulator of PD (de Vries et al. 2008; Zhang et al. 2019). This study demonstrated that H2O2 concentration- and time-dependently stimulated Nrf2 signaling activation. Although the critical role of Nrf2 pathway in DA neuronal protection was well documented, it was still unclear whether glial cells Nrf2 pathway was involved in H2O2-supported DA neuronal survival. Thus, we assumed that physiological concentration of H2O2 might trigger spontaneous anti-oxidant stress by glial cells Nrf2 activation to protect DA neurons. Given these assumptions, Nrf2-siRNA was performed in primary mixed-glia cultures. As a result, silence of glial cells Nrf2 abolished H2O2-induced DA neuronal survival, which may be related to glial cell function. Microglia not only constitute the first line of defense against pathogens by regulating components of innate immunity, but also regulate the adaptive arms of immune responses (Yin et al. 2017).Astroglia are the most abundant cells in the brain, which provide for nutritional support and control CNS homeostasis (Verkhratsky and Nedergaard 2018). Absence of microglia and astroglia lead to immunity weakness, neurotrophic factors decrease and homeostasis imbalance. Thus, DA neurons are more vulnerable without glia cell. In addition, one way to support DA neurons is to transfer Nrf2-regulated gene products from astrocytes to neurons via microvesicles or exsosomes. Nerve growth factor (NGF) and glial cell-derived neurotrophic factor (GDNF) produced by either astrocytes or neurons enhances Nrf2 gene expression via an autocrine or paracrine manner (Wang et al. 2019; Valdovinos-Flores et al. 2019). One of the possibilities is the formation of positive feedback loop between NGF and Nrf2 (Naveilhan et al. 1994; Mimura et al. 2011). GDNF is a potent neuroprotective molecule for DA neurons and can be secreted from hydrogen peroxide-challenged astrocytes (Fonseca et al. 2014). These results suggested that H2O2 regulated DA neuronal survival through a Nrf2-dependent manner in glial cells.
Together, this study indicated that H2O2 played an important role in DA neuronal survival through a Nrf2-dependent manner. This study permits to re-evaluate the role of H2O2 in the pathogenesis and therapeutic studies of PD. Moreover, given the potential therapeutic use of anti-oxidants, these results might provide new avenues into the mechanisms and their site of actions.
Acknowledgements
All experiments were supported by National Natural Science Foundation of China (No. 81760658), High-level Innovative Talents of Guizhou Province (No. 20164027), Governor Talent Foundation of Guizhou Province (No. 201288), Innovation Research Group Project of Education Department of Guizhou Province (no. 2016038), and Excellent Young Talents of Zunyi Medical University.
Abbreviations
- ara-C
Cytosine β-D-arabinofuranoside
- CNS
Central nervous system
- DA
Dopamine
- DMF
Dimethylfumarate
- GDNF
Glial cell-derived neurotrophic factor
- HO-1
Heme oxygenase-1
- H2O2
Hydrogen peroxide
- Keap1
Kelch-like ECH-associated protein 1
- LME
Leu-leu methyl ester
- NGF
Nerve growth factor
- Nrf2
Nuclear factor erythroid 2-related factor 2
- NQO1
NADPH quinone oxidoreductase 1
- PD
Parkinson's disease
- SNc
Substantia nigra pars compacta
- TH
Tyrosine hydroxylase
Author Contribution
FZ designed all the experiments. GQW performed the experiments and wrote the manuscript. QYY, CQZ, DDL, and JJL participated in the data analysis and approved the submitted manuscript.
Compliance with Ethical Standards
Conflicts of interest
The authors declared no conflicts of interest.
Ethics Approval
All animal experiments were performed in accordance with National Institute of Health Guideline for the Animal Care and Use of Laboratory Animal and approved protocol by the institutional Animal Care and Use Committee at Zunyi Medical University (Zunyi, China).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahuja M, Ammal Kaidery N, Yang L, Calingasan N, Smirnova N, Gaisin A (2016) Distinct Nrf2 signaling mechanisms of fumaric acid esters and their role in neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced experimental Parkinson's-like disease. J Neurosci 36:6332–6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma M, Okumura-Torigoe N, Miyazaki I, Murakami S, Kitamura Y, Sendo T (2019) Region-specific neuroprotective features of astrocytes against oxidative stress induced by 6-hydroxydopamine. Int J Mol Sci 20:pii.E598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KF, Al-Mubarak B, Martel MA, McKay S, Wheelan N, Hasel P (2015) Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat Commun 6:7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán González AN, López Pazos MI, Calvo DJ (2019) Reactive oxygen species in the regulation of the GABA mediated inhibitory neurotransmission. Neuroscience pii S0306–4522(19):30395–30401 [DOI] [PubMed] [Google Scholar]
- Chen SH, Oyarzabal EA, Sung YF, Chu CH, Wang Q, Chen SL (2015) Microglial regulation of immunological and neuroprotective functions of astroglia. Glia 63:118–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SL, Cline HT (2010) Insulin receptor signaling in the development of neuronal structure and function. Neural Dev 5:7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J (2008) Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med 45:1375–1383 [DOI] [PubMed] [Google Scholar]
- Fonseca CP, Gama S, Saavedra A, Baltazar G (2014) H2O2- or I-DOPA-injured dopaminergic neurons trigger the release of soluble mediators that up-regulate striatal GDNF through different signalling pathways. Biochim Biophys Acta 1842:927–234 [DOI] [PubMed] [Google Scholar]
- Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS (2011) HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci 31:1081–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich FJ, Funke F, Hildebrandt B, Fasshauer M, Müller M (2009) H2O2-mediated modulation of cytosolic signaling and organelle function in rat hippocampus. Pflugers Arch 458:937–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger G, Quiney C, Cotter TG (2009) Hydrogen peroxide as a cell-survival signaling molecule. Antioxid Redox Signal 11:2655–2671 [DOI] [PubMed] [Google Scholar]
- Gureev AP, Popov VN (2019) Nrf2/ARE pathway as a therapeutic target for the treatment of Parkinson diseases. Neurochem Res 44:2273–2279 [DOI] [PubMed] [Google Scholar]
- Helou DG, Martin SF, Pallardy M, Chollet-Martin S, Kerdine-Römer S (2019) Nrf2 involvement in chemical-induced skin innate immunity. Front Immunol 10:1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JY, Yuan YH, Yan JQ, Wang YN, Chu SF, Zhu CG (2016) 20C, a bibenzyl compound isolated from Gastrodia elata, protects PC12 cells against rotenone-induced apoptosis via activation of the Nrf2/ARE/HO-1 signaling pathway. Acta Pharmacol Sin 37:731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XM, Zhou H, Zhang D, Yang SF, Qian L, Wu HM (2012) Clozapine protects dopaminergic neurons from inflammation-induced damage by inhibiting microglial overactivation. J Neuroimmune Pharmacol 7:187–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong YH, Park JS, Kim DH, Kim HS (2016) Lonchocarpine increases Nrf2/ARE-mediated antioxidant enzyme expression by modulating AMPK and MAPK signaling in brain astrocytes. Biomol Ther 24:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DA, Johnson JA (2015) Nrf2-a therapeutic target for the treatment of neurodegenerative diseases. Free Radic Biol Med 88:253–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamsler A, Segal M (2003) Hydrogen peroxide modulation of synaptic plasticity. J Neurosci 23:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastres-Becker I, García-Yagüe AJ, Scannevin RH, Casarejos MJ, Kügler S, Rábano A (2016) Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson's disease. Antioxid Redox Signal 25:61–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Patel JC, O'Neill B, Rice ME (2015) Inhibitory and excitatory neuromodulation by hydrogen peroxide: translating energetics to information. J Physiol 593:3431–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell JR, Lehtonen S, Duncan C, Keksa-Goldsteine V, Levonen AL, Goldsteins G (2016) Pyrrolidine dithiocarbamate activates the Nrf2 pathway in astrocytes. J Neuroinflammation 13:49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalingam KB, Radhakrishnan AK, Haleagrahara N (2015) Protective mechanisms of flavonoids in Parkinson's disease. Oxid Med Cell Longev 2015:314560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado V, Zöller T, Attaai A, Spittau B (2016) Microglia-mediated neuroinflammation and neurotrophic factor-induced protection in the MPTP mouse model of parkinson’s disease-lessons from transgenic mice. Int J Mol Sci 17:pii:E151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura J, Kosaka K, Maruyama A, Satoh T, Harada N, Yoshida H (2011) Nrf2 regulates NGF mRNA induction by carnosic acid in T98G glioblastoma cells and normal human astrocytes. J Biochem 150:209–217 [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Jehan F, Baudet C, Wion D, Brachet P (1994) Reactive oxygen species influence nerve growth factor synthesis in primary rat astrocytes. J Neurochem 62:2178–2186 [DOI] [PubMed] [Google Scholar]
- Ohashi M, Hirano T, Watanabe K, Katsumi K, Ohashi N, Baba H (2016) Hydrogen peroxide modulates synaptic transmission in ventral horn neurons of the rat spinal cord. J Physiol 594:115–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski TD, Hasser EM, Heesch CM, Kline DD (2014) H2O2 induces delayed hyperexcitability in nucleus tractus solitarii neurons. Neuroscience 262:53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JC, Rice ME (2012) Classification of H2O2 as a neuromodulator that regulates striatal dopamine release on a subsecond time scale. ACS Chem Neurosci 3:991–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parga JA, Rodriguez-Perez AI, Garcia-Garrote M, Rodriguez-Pallares J, Labandeira-Garcia JL (2018) Angiotensin II induces oxidative stress and upregulates neuroprotective signaling from the NRF2 and KLF9 pathway in dopaminergic cells. Free Radic Biol Med 129:394–406 [DOI] [PubMed] [Google Scholar]
- Persiyantseva NA, Storozhevykh TP, Senilova YE, Gorbacheva LR, Pinelis VG, Pomytkin IA (2013) Mitochondrial H2O2 as an enable signal for triggering autophosphorylation of insulin receptor in neurons. J Mol Signal 8:11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME (2011) H2O2: a dynamic neuromodulator. Neuroscientist 17:389–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Erb H, Murphy TH (2007) Dopamine activates Nrf2-regulated neuroprotective pathways in astrocytes and meningeal cells. J Neurochem 101:109–119 [DOI] [PubMed] [Google Scholar]
- Schroder E, Eaton P (2008) Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: issues and considerations. Curr Opin Pharmacol 8:153–159 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M (2018) Physiology of astroglia. Physiol Rev 98:239–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdovinos-Flores C, Limón-Pacheco JH, León-Rodríguez R, Petrosyan P, Garza-Lombó C (2019) Systemic L-buthionine -S-R-sulfoximine treatment increases plasma NGF and upregulates L-cys/L-cys2 transporter and γ-glutamylcysteine ligase mRNAs through the NGF/TrkA/Akt/Nrf2 pathway in the striatum. Front Cell Neurosci 13:325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GQ, Zhang B, He XM, Li DD, Shi JS, Zhang F (2019) Naringenin targets on astroglial Nrf2 to support dopaminergic neurons. Pharmacol Res 139:452–459 [DOI] [PubMed] [Google Scholar]
- Yin J, Valin KL, Dixon ML, Leavenworth JW (2017) The role of microglia and macrophages in cns homeostasis, autoimmunity, and cancer. J Immunol Res 2017:5150678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wang GQ, He JY, Yang QY, Li DD, Li JJ (2019) Icariin attenuates neuroinflammation and exerts dopamine neuroprotection via an Nrf2-dependent manner. J Neuroinflammation 16:92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Qian L, Flood PM, Shi JS, Hong JS, Gao HM (2010) Inhibition of IκB kinase-β protects dopamine neurons against lipopolysaccharide-induced neurotoxicity. J. Pharmacol. Exp. Ther. 333(3):822–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Liu C, Fan Y, Yan P, Shi D, Zhang Y (2017) Neuroprotection by paeoniflorin in the MPTP mouse model of Parkinson's disease. Neuropharmacology 116:412–420 [DOI] [PubMed] [Google Scholar]