Abstract
Excessive mitochondrial fission has been implicated in the etiology of neuronal cell death in traumatic brain injury (TBI). In the present study, we examined the efficacy of melatonin (Mel) as a neuroprotective agent against TBI-induced oxidative damage and mitochondrial dysfunction. We assessed the impact of Mel post-treatment (10 mg/kg b.wt., i.p.) at different time intervals in TBI-subjected Wistar rats. We found that the Mel treatment significantly attenuated brain edema, oxidative damage, mitochondrial fission, and promoted mitochondrial fusion. Additionally, Mel-treated rats showed restoration of mitochondrial membrane potential and oxidative phosphorylation with a concomitant reduction in cytochrome-c release. Further, Mel treatment significantly inhibited the translocation of Bax and Drp1 proteins to mitochondria in TBI-subjected rats. The restorative role of Mel treatment in TBI rats was supported by the mitochondrial ultra-structural analysis, which showed activation of mitochondrial fusion mechanism. Mel enhanced mitochondrial biogenesis by upregulation of PGC-1α protein. Our results demonstrated the remedial role of Mel in ameliorating mitochondrial dysfunctions that are modulated in TBI-subjected rats and provided support for mitochondrial-mediated neuroprotection as a putative therapeutic agent in the brain trauma.
Keywords: Traumatic brain injury, Melatonin, Drp1, Mitochondria dysfunction, Neuroprotection, Repair
Introduction
Among the known neurological disorders, traumatic brain injury (TBI) is considered one of the major economic and medical burden in the world (Johnson and Griswold 2017). Contact injury in sports, road traffic accidents, and training or war time mishaps have been found to induce severe to mild TBI in civilians and veterans (Selassie et al. 2013). The mechanical damage in TBI is followed by multidimensional cascades of primary and secondary brain injuries that include a decrease in cerebral blood flow leading to oxygen deprivation, excessive reactive oxygen species (ROS) generation, inflammation, excitotoxicity, organelle dysfunction, and subsequently promotion of neuronal death (Talley Watts et al. 2014). Therefore, identification of the mitigatory strategies that target the mechanisms behind neuronal injury holds tremendous therapeutic potential for the possible treatment of TBI.
Mitochondria are highly dynamic ultra-structural and energy-producing sub-cellular organelles that have been implicated in numerous diseases and are considered excitotoxic in the brain damage (Zhao et al. 2015). Recent reports have suggested that mitochondrial perturbation contributes to secondary neuronal injury and death after brain trauma (Dobrachinski et al. 2017). Traumatic injury causes inflammation and compartment degeneration in neurons, eventually leading to mitochondrial dysfunction. All of these changes, in turn, cause excess mitochondrial fission and mitochondria-mediated neuronal death (Webster et al. 2015). In normal physiological conditions, dysfunctional mitochondria are salvaged and fused with other healthy mitochondria by highly selective mitofusin proteins (Mfn1 and Mfn2) or assiduously broken by the mitofission proteins (Drp1 and Fis1) if they are not deemed viable anymore (Meyer et al. 2017). In normal conditions, the total number of mitochondria in neurons are maintained through a dynamic equilibrium consisting of both fission and fusion mechanisms. A shift in the fission/fusion balance has been implicated in the etiology of various neurodegenerative disorders (Chen and Chan 2009). In acute TBI, oxidative stress has been found to increase, which is responsible for the imbalance in redox homeostasis (Ehsaei et al. 2015). This process results in the disruption of mitochondrial function and dynamics concomitantly leading to neuronal apoptosis (Sheridan and Martin 2010). In conclusion, the inhibition of mitochondrial fission and dysfunction could be a potential therapeutic strategy in TBI pathogenesis.
Melatonin (Mel) is a main secretory product of the pineal gland. It plays an important role in immune function, ROS foraging, autophagy, anti-inflammatory, and antioxidant activities in various models (Ding et al. 2015; Wu et al. 2016a). Recent reports have suggested that Mel provides mitochondrial-mediated neuroprotection in different toxic models. Mel is considered a broad spectrum protective neurohormone that shows neuroprotection via multiple cellular and molecular mechanisms in neuronal cells (Furio et al. 2007). However, the main causal mechanism that explains mitochondrial-mediated neuroprotection against TBI modulated mitochondrial dynamics remains poorly understood in the acute-brain injury.
Based on the existing literature, it is important to gain a better understanding of mitochondrial-mediated neuroprotection of Mel and its possible implications on mitochondrial dynamics and biogenesis in TBI-subjected rat brain. Thus, we examined the effect of Mel on TBI-induced behavioral impairments, oxidative damage, and mitochondria dysfunction after focal TBI in Wistar rats. Furthermore, we explored the efficacy of Mel administration on mitochondrial dynamics and its possible neuroprotective mechanisms. To the best of our knowledge, this is the first study of TBI where Mel treatment attenuated mitochondrial perturbation, promoted the mitochondrial fusion pathway, and provided new insights into the potential neurotherapeutic value of Mel for brain injury.
Materials and Methods
Chemicals
Bovine serum albumin (BSA), Butylated hydroxytoluene (BHT), Calcium chloride (CaCl2), Dihydroethidium (DHE), 2,7-dichlorodihydrofluorescein diacetate (DCDHF-DA), Ethanol, Ethylenediaminetetraacetic acid (EDTA), ethylene glycol-O,-O’-bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), Melatonin (M5250), Ortho-phosphoric acid (OPA), Sucrose, Tetramethylrhodamine ethyl ester (TMRE), Thiobarbituric acid (TBA), Trichloroacetic acid (TCA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Routine chemicals were purchased from Hi-Media labs Pvt. Ltd. (Mumbai, India).
Animals and Housing
Male Wistar rats (280–320 g, 12–14 weeks old) were obtained from the Central Animal House Facility (Registration No. 173/GO/ReBi/S/2000/CPCSEA) of Jamia Hamdard, New Delhi, India. Animals were kept in two per cage, an air-filtrated unit at temperature 23 ± 2 °C with a relative humidity of 65 ± 10% and 12 h light/dark cycle. Experimental animals were randomly divided into three groups as follows: Sham, TBI, and TBI + Mel. The Institutional Animal Ethics Committee approved the surgical and experimental procedures of Jamia Hamdard, New Delhi, India. Animals were allowed to have access to the standard rodent pellet diet and water ad libitum throughout the duration of the study.
TBI Model
Focal moderate TBI was induced by the Controlled Cortical Impact (CCI) injury method described earlier (Wu et al. 2016a). The experimental animals were anesthetized (75 mg/kg ketamine, 10 mg/kg xylazine b.wt., i.p.) and mounted on a stereotaxic frame (David Kopf Instrument, Tujunga, CA, USA) with ear bars and an incision bar. The scalp incision area was shaved and sterilized with 70% ethanol and Betadine. Body temperature was maintained with a heating blanket. A middle incision was made into the skull skin, and the skin was retracted to create a 5 mm diameter craniotomy by a trephine drill. Cotton swabs were used to halt and clean extra bleeding. The craniotomy was located on the bregma on the right side, with the medial edge of the craniotomy 1 mm lateral from midline and skull disk was removed without any disturbance. The injury was induced to the brain surface using a magnetic control pinpoint contusion impactor (4 mm diameter; PC1300; Hatteras Instrument, Cary, NC, USA) to a contusion depth of 2.5 mm, the velocity of 3 m/s and dwell time of 120 ms displacement of the brain. After contusion injury, the bone flap was put back and sealed using dental cement under sterile conditions. In the Sham group, animals received similar surgical procedures without contusion injury. After complete recovery from anesthesia, animals were returned to their home cages and kept at ambient temperature (23 ± 2 °C) and relative humidity (65 ± 10%) with a 12 h light/dark cycle and allowed free access to the pellet diet and purified drinking water. Injured brain tissue part was collected after 24 h for examining molecular and biochemical parameters following TBI.
Postoperative Care
Recovery of anesthesia took approximately 1–2 h after the surgery. The experimental animals were kept in a well-ventilated room at 22 ± 3 °C in individual cages (one animal per cage). All experimental rats received an intravenous injection of 0.9% sterile normal saline to prevent dehydration. Food and filtered water were kept in the cages for the rest of 24 h so that experimental animals could easily access these facilities without any physical disturbance due to the surgery.
Drug Administration
Mel was dissolved in 1.0% ethanol in normal saline (0.9%). The dose was freshly prepared before each injection. The half-life of Mel is about 40 min, so we chose to give the dose of 10 mg/kg b.wt. by intraperitoneally at different time intervals (0.5 h, 1 h, 2 h, 3 h, and 4 h) post-TBI as reported by Wu et al. (2016a).
Neurobehavioral Experiments
Behavioral experiments were performed to examine the effect of Mel on the behavioral alterations following the TBI. Animals were trained for three consecutive days before the TBI and final experiments were performed at 24 h after post-injury.
Motor Coordination Test
The motor coordination performance was evaluated by the rota-rod apparatus (Omnitech, Columbus, OH, USA) as previously described by Rasheed et al. (2018). Experimental animals were kept on a rotating rod at a speed of 10 rpm. The performance was evaluated as the ability of the animals to spend maximum time on the rotating rod. Results were expressed in seconds, and the maximum time was 180 s.
Grip Test
A Grip test was performed as previously described by Rasheed et al. (2018). A string wire 75 cm in length was pulled out between two solid subjects at 50 cm height. The results were expressed as scores and evaluated according to the following score; 0 = fall off, 1 = hangs onto string by two forepaws, 2 = hangs onto string by two forepaws but trying to climb on string, 3 = hangs onto string by forepaws, 4 = hangs onto string by forepaws plus tail wrapped around the string, and 5 = animals escaped.
Narrow Beam walk Test
Narrow Beam walk test was performed as described by Rasheed et al. (2018). Briefly, a wooden narrow beam consisted of recording the elapsed time (180 s) to traverse the beam from one side to another side. The final test consisted of three trials in each task. The average for each task was analyzed, and results were shown in seconds.
Gait Analysis
Gait patterns of animals was analyzed after focal trauma as previously described by Andrabi et al. (2017). In this experiment, we performed the stride length and stride width test. The results of stride length and width were analyzed in centimeters.
Brain Water Content
Brain water content was determined by the weight/dry method, as previously described by Chen et al. (2014). Briefly, the brain was isolated and immediately weighed to obtain the wet weight and then kept in hot airflow laminar at 105 °C for one day to obtain the dry weight. The brain water content was calculated as [(wet weight–dry weight)/wet weight] × 100%.
Nissl Staining
The animals were anesthetized with an overdose of ketamine and xylazine and perfused with 4% paraformaldehyde in phosphate buffer saline. After perfusion, the brain was removed and fixed in 10% formalin. Thereafter, 5-μm-thick coronal tissue sections were cut from paraffin-embedded brains and deparaffinized sections stained with 0.1% cresyl violet dye for 30 min using a standard protocol as described by Feng et al. (2017). The sections were examined using an inverted bright field microscope (Zeiss, Germany).
Transmission Electron Microscopy
Perfused brain tissue samples were fixed with 2.5% glutaraldehyde and 2.0% paraformaldehyde in phosphate buffer at 4 °C for 24 h. After fixation, the brain slices were cut using an ultra-microtome and embedded in 1% osmium tetroxide, dehydrated, and fixed in araldite CY 212. Ultra-thin slices were stained with uranyl acetate (for 25 min) and lead citrate (for 20 min) and examined under a TECNAI G20 transmission electron microscope (FEI, Eindhoven, the Netherlands) (Dongare et al. 2016).
Mitochondria Isolation
Mitochondria were isolated from injured brain tissue parts with different centrifugation speeds as described by Andrabi et al. (2017) and Waseem et al. (2016). Experimental animals were decapitated after being deeply anesthetized with an overdose of ketamine and xylazine, and dissected brain tissue samples were homogenized in 10% volumes (1:10 w/v) of ice-cold buffer A (10 mmol/L HEPES, 250 mmol/L sucrose, 1 mmol/L EGTA and 0.1% fat-free BSA adjusted pH 7.4 with Tris-buffer) using a mechanically driven Teflon-fitted Potter–Elvehjem type homogenizer. The homogenate sample was centrifuged at 1000×g for 8 min at 4 °C. The supernatant was collected and centrifuged again at 10,000×g for 10 min at 4 °C. Thereafter, the pellet was resuspended and washed twice with buffer B (250 mmol/L sucrose, 10 mmol/L HEPES, and 0.1 mmol/L EGTA) and centrifuged at 12,300×g, 4 °C for 10 min. The pellet was resuspended in 2 ml buffer C (250 mmol/L sucrose, 10 mmol/L HEPES, and 0.1% fat-free BSA) and centrifuged at 12,300×g, 4 °C for 10 min. Finally, the pellet was resuspended in buffer C. Analysis of mitochondrial oxidative stress parameters was performed on the same day.
Oxidative Stress Biomarkers
Oxidative stress parameters, including lipid peroxidation (LPO), glutathione (GSH), and mitochondrial ROS level were determined from isolated mitochondria. LPO rate was measured using a method as described earlier (Chaudhary and Parvez 2012) with some modifications. Briefly, the mitochondrial samples were incubated with 10 mmol/L BHT, 0.67% TBA, and 1.0% OPA at 90 °C for 45 min. After incubation, the reaction mixture was centrifuged at 1000×g and measured photometrically at 535 nm. The results were expressed as nmoles of TBARS formed/h/mg of protein using a molar extinction coefficient (MEC) 1.56 × 105 M−1 cm−1. GSH was performed as described by Andrabi et al. (2017). The result was expressed as nmoles of GSH/mg protein using a MEC 1.36 × 104 M−1 cm−1. Mitochondrial ROS was measured by flow cytometry using DCFDA dye. The flow cytometric acquisition of fluorescent intensities from 10,000 events was done using BD-LSR II. Histograms were generated, and statistical analysis was performed with FACS-DIVA software.
Measurement of Mitochondrial Membrane Potential
Mitochondrial membrane potential was analyzed by flow cytometry using TMRE dye, as described by Andrabi et al. (2017). Briefly, isolated mitochondrial samples were incubated in an analysis buffer with 100 nmol/L TMRE dye under the dark condition for 30 min at 25 °C. After incubation, flow cytometric acquisition of fluorescent intensities from 10,000 events was done using BD-LSR II. Histograms were generated, and statistical analysis was performed with FACS-DIVA software.
F1-F0 Synthase Activity
The activity of F1-F0 synthase was measured photometrically at 660 nm, as described by Andrabi et al. (2017). The enzyme activity was assayed in terms of hydrolysis of ATP to ADP and the generation of inorganic phosphate (Pi). The results were expressed as μg of Pi liberated/min/mg protein using a phosphate standard curve.
Western Blot
Injured brain tissue was collected and homogenized in ice-cold RIPA lysis buffer containing a protease inhibitor cocktail (Sigma, USA) using a hand homogenizer. Samples were sonicated at 40% energy for 2 min with 20 s on and 10 s off cycles and cleared by centrifugation at 20,000×g for 20 min at 4 °C. The protein was quantified from the supernatant with Bradford reagents, and the samples were stored at −80 °C. Isolation of sub-cellular fractions was done using fractionation kits (BioVision, Inc. USA) according to the manufacturer guidelines. An equal amount of protein (50–70 μg/well) was loaded in 10–15% sodium dodecyl sulfate–polyacrylamide gel (SDS-PAGE) followed by electrophoresis at 20 mA for each gel. After resolving, proteins were transferred from gels to PVDF membranes (Merck Millipore) for 90 min at 75 mA using the Mini Trans-Blot Cell apparatus (Bio-Rad, Hercules, CA, USA). In addition, membranes were blocked with 5% non-fat dry milk for 60 min at room temperature. Membranes were washed 3 times for 15 min with phosphate buffer saline and 0.1% tween-20 (PBST) and incubated overnight at 4 °C with appropriate primary antibodies diluted as desired Drp1 (1:1000, #ab56788; Abcam, Cambridge, UK); OXPHOS (1:1000, #45–8099; Thermo Scientific, USA); OPA1 (1:1000, #E-AB-63977), Fis1 (1:1000, #E-AB-61132), and Mfn2 (1:1000, #E-AB-64745) all from Elabscience Biotechnology Inc; Bcl-2 (1:500, #sc-7382), β-Actin (1:500, #sc-81178), Cox-IV (1:500, #sc-292652) and BAX (1:500, #sc-439) all from Santa Cruz Biotechnology, CA USA; cleaved caspase-3 (1:1000, #D175), histone H3 (1:2000, #D1H2) and cytochrome-c (1:1000, #136F3) all from Cell Signaling, MA, USA. Next day, membranes were washed thrice with PBST and incubated with horseradish peroxidase-conjugated secondary antibodies (diluted 1:5000, anti-mouse IgG, and anti-rabbit IgG antibody) for 90 min. at room temperature. The bands were detected by enhanced chemiluminescence reaction plus (Thermo Scientific, USA), and densitometric analysis was performed by ImageJ software (1.50 Version, NIH, USA).
Statistical Analysis
All experimental results were expressed as mean ± standard error (SD) and analyzed using analysis of variance (ANOVA) followed by Tukey’s post hoc test. A value of p < 0.05 was considered significant. All results were analyzed by Graph Prism 6 Software (GraphPad Software Inc., San Diego, CA, USA).
Results
Effect of Mel on Neuronal and Mitochondrial Damage After TBI
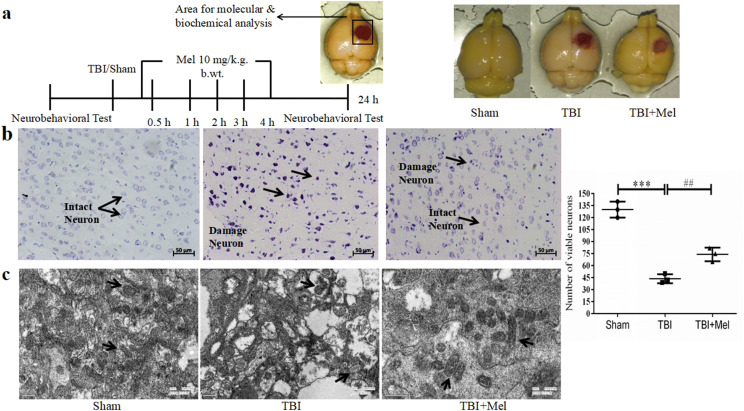
Histological analysis of coronal brain sections of experimental rats after subjecting them to TBI and Mel treatment at 24 h (Fig. 1b). The sections from the Sham group showed normal neuronal structure with no morphological changes full of the clear and intact neuron, as indicated by lightly stained cells. The sections from the TBI group, on the other hand, showed degenerative changes or neuronal death with shrunken neuronal bodies, condensed nuclei, as indicated by dark cytoplasm staining. Administration of Mel to TBI animals ameliorated neuronal damage and alleviated the neuronal loss in brain sections. Additionally, we used Electron microscopy to examine the structural changes for mitochondrial fragmentation and fusion. In the TBI group, electron microscopy images showed that TBI increased the number of smaller mitochondria with fragmented and spotted structures as compared to the Sham group. However, Mel treatment increased the mitochondrial size and reduced the mitochondrial fragmentation when compared to the only TBI group (Fig. 1c).
Fig. 1.
a A timeline of the experimental paradigm and images of the brain after CCI injury. b Representative brain images of Nissl staining of Sham, TBI, and TBI + Mel groups. The cortical neuronal area of the Sham group shows intact neurons. The coronal tissue section area of the TBI group shows damaged neurons. Mel treatment shows partial rescue of neuronal loss in the TBI + Mel group. c The image of the TBI group shows the fragmented and smaller size of mitochondria. Treatment of Mel reduced the mitochondrial fragmentation via the activation of the mitochondria fusion pathway. Data are presented as mean ± SD. ***P < 0.001, TBI vs. the Sham group; ##P < 0.01 TBI + Mel vs. the TBI group (n = 3 in each group)
Effect of Mel on Behavioral Alterations After TBI
In behavioral experiments, we confirmed the neuroprotective properties of Mel against TBI-induced behavioral impairments. We studied various behavioral parameters at 24 h post-injury. We performed a grip test wherein TBI group animals failed to achieve the high mean scores [F (2, 15) = 27.43, P < 0.0001] as compared to the Sham group animals after subjecting to trauma. Mel-treated animals achieved a high mean score (#P < 0.05) against the TBI group animals (Fig. 2a). Secondly, we performed the rota-rod test for muscular coordination performance. We found a significant decrease [F (2, 15) = 41.8, P < 0.0001] in the muscular coordination test in the TBI group as compared to the Sham group. However, Mel treatment showed a significant improvement in muscular coordination performance (#P < 0.05) as compared to the TBI group animals (Fig. 2b). Third, we performed a narrow beam walk test, and we found that the TBI group animals were unable to walk on the narrow beam and spent more time to transverse the beam [F (2, 15) = 21.65, P < 0.0001] when compared to the Sham group. We also noted that the Mel treatment group demonstrated improved performance on the beam walk test (##P < 0.01) when compared with the TBI group (Fig. 2c). Focal traumatic brain injury induced gait disabilities in TBI-subjected rats. We observed significant reduction in stride length [F (2, 15) = 13.0, P = 0.0005] and stride width [F (2, 15) = 10.21, P = 0.0016] in the TBI group as compared to the Sham group. However, the stride length was significantly improved (#P < 0.05) after Mel treatment. But Mel treatment did not show any significant changes in stride width against the TBI group (Fig. 2d, e).
Fig. 2.
Effect of Mel on behavioral alterations after TBI. a Grip test. b Motor coordination test. c Beam walk test. d Stride length. e Stride width. Data are presented as mean ± SD. ***P < 0.001 and **P < 0.01, TBI vs. the Sham group; ##P < 0.01 and #P < 0.05, TBI + Mel vs. the TBI group (n = 6 in each group)
Mel Treatment Reduces Brain Edema After TBI
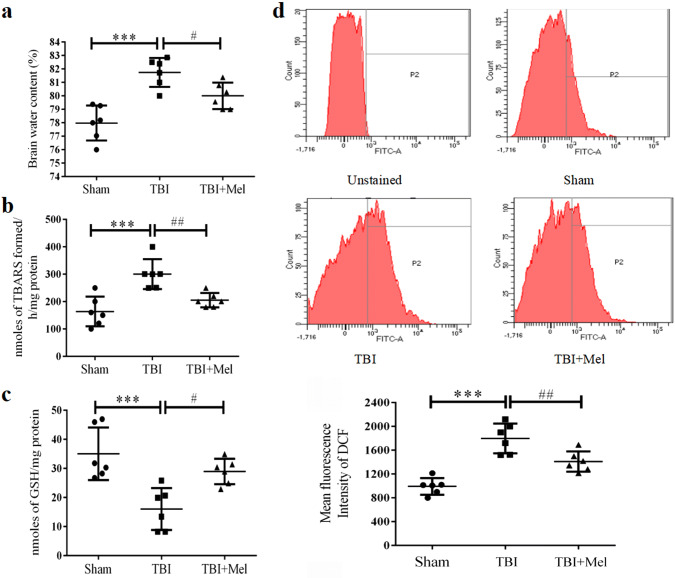
The brain water content was determined to confirm the effect of focal injury and Mel treatment at the macroscopic level following a TBI. As shown in Fig. 3a, the TBI group rats showed a significant increase in brain water content [F (2, 15) = 16.7, P = 0.0002] as compared to the Sham group at 24 h after post-injury. However, the experimental animals treated with Mel had less water content (#P < 0.05) as compared to the TBI group.
Fig. 3.
Effect of Mel on brain edema and oxidative stress parameters. a Brain edema. b LPO. c GSH. d ROS. Data are presented as mean ± SD. ***P < 0.001, TBI vs. the Sham group; ##P < 0.01 and #P < 0.05, TBI + Mel vs. the TBI group (n = 6 in each group)
Mel Treatment Attenuates Oxidative Stress in the Injured Brain After TBI
We also determined the antioxidant property of Mel at organelle level such as mitochondria against TBI-induced oxidative damage. We performed biochemical parameters such as LPO, GSH, and ROS in isolated mitochondria samples. As shown in Fig. 3b and c, our experimental outcomes showed that a significant decrease in mitochondrial GSH level [F (2, 15) = 11.0, P = 0.0011] and increase in LPO rate [F (2, 15) = 13.2, P = 0.0005] were observed in the TBI group as compared to the Sham group. We also observed that the Mel treatment significantly enhanced the GSH level (#P < 0.05) and inhibited the LPO rate (##P < 0.01) when compared to the TBI group. Also, we measured mitochondrial ROS by changes in the mean fluorescence intensity of ROS-sensitive DCFDA dye. As shown in Fig. 3d, the level of ROS significantly increased [F (2, 15) = 26.18, P < 0.0001] in the TBI group as compared to the Sham group. Further, the Mel administration was able to scavenge the ROS (##P < 0.01) when compared to the TBI group.
Mel Treatment Reduces the Expression of Cleaved Caspase-3 and Increased the Expression Level of Bcl-2 After TBI
To explore the anti-apoptotic property of Mel, we evaluated the protein expression level of Bcl-2 and cleaved caspase-3 by western blot analysis. As shown in Fig. 4, the expression level of cleaved caspase-3 protein was substantially upregulated (***P < 0.001) after TBI when compared to the Sham group. Mel administration significantly inhibited (#P < 0.05) the expression of cleaved caspase-3 when compared to the TBI group. However, the expression of anti-apoptotic protein Bcl-2 was downregulated (**P < 0.01) in the TBI group when compared to the Sham group. Finally, Mel treatment was able to upregulate the level of Bcl-2 protein (#P < 0.05) against the TBI group.
Fig. 4.
Effects of Mel on the expression level of Bcl-2 and cleaved caspase-3 following TBI. Representative immunoblots are showing the expression of Bcl-2 and cleaved caspase-3 in the penumbra tissue lysate after injury. The relative optical density of protein bands was analyzed and normalized to β-Actin. Data are presented as mean ± SD. ***P < 0.001 and **P < 0.01, TBI vs. the Sham group; # P < 0.05, TBI + Mel vs. the TBI group (n = 5 in each group)
Mel Treatment Reinstates Mitochondrial Membrane Potential and Inhibits Cytochrome-c Release After TBI
Disturbance in mitochondrial membrane potential (Δψm) is an early and key indicator of mitochondrial dysfunction and mitochondrial-mediated cell death following a brain injury. The Δψm was evaluated by changes in the mean fluorescence intensity of TMRE dye. TMRE fluorescence intensity showed a significant decrease in the TBI group [F (2, 15) = 29.8, P < 0.0001] as compared to the Sham group. However, the treatment of Mel retained Δψm (##P < 0.01) against the TBI group. Disruption in Δψm led to loss of mitochondrial integrity and release of several apoptogenic proteins, mostly cytochrome-c into the cytoplasm. Immunoblotting analysis of cytosolic and mitochondrial fractions showed that TBI disturbed Δψm and significantly increased the leakage of cytochrome-c into the cytoplasm. Further, Mel treatment significantly inhibited cytochrome-c release (#P < 0.05) as compared to the TBI group and reduced mitochondrial-mediated neuronal cell death (Fig. 5).
Fig. 5.
Effects of Mel on mitochondrial membrane potential and cytochrome-c release: Mel improves mitochondrial membrane integrity and inhibits cytochrome-c release after TBI. The relative optical density of protein bands was analyzed and normalized to β-Actin in the cytosolic fraction and COX-IV in the mitochondrial fraction. Data are presented as mean ± SD. ***P < 0.001 and **P < 0.01, TBI vs. the Sham group; ##P < 0.01 and #P < 0.05, TBI + Mel vs. the TBI group (n = 6 for ΔΨm and n = 5 for Immunoblot)
Effect of Mel Treatment on PGC1-α and OXPHOS Expression Level After TBI
It has been previously reported that mitochondrial dysfunction, such as perturbation in biogenesis, is associated with brain injury (Chen et al. 2011). Therefore, we analyzed the expression level of PGC1-α and OXPHOS proteins by immunoblotting technique (Fig. 6a, b). We examined the expression of PGC1-α protein in the nuclear fraction in the TBI and TBI + Mel group against the Sham group. We found that the expression level of PGC1-α increased from Sham through TBI to TBI + Mel group, and significantly increased (*P < 0.05) in the TBI + Mel group only when compared to the Sham group. Additionally, we investigated the expression level of OXPHOS proteins, which showed a significant downregulation of C-I, C-II, C-III, and C-V (**P < 0.01 and *P < 0.05) proteins in TBI group as compared to the Sham group. However, Mel treatment to TBI group ameliorated mitochondrial dysfunction and significantly increased the expression level of C-I, and C-V complex proteins (#P < 0.05) against the TBI group. Finally, the results indicated that Mel significantly enhanced mitochondrial biogenesis and improved mitochondrial function following TBI.
Fig. 6.
Effect of Mel on the expression level of PGC1-α and OXPHOS proteins after TBI. Mel accelerated the translocation of PGC1-α from the cytosol to the nucleus and significantly increased the expression level of C-I and C-IV of OXPHOS proteins. The relative optical density of protein bands was analyzed and normalized to β-Actin in cytoplasm fraction and histone in the nuclear fraction after TBI. Data are presented as mean ± SD. **P < 0.01 and * P < 0.05, TBI vs. the Sham group; #P < 0.05, TBI + Mel vs. the TBI group (n = 5 in each group)
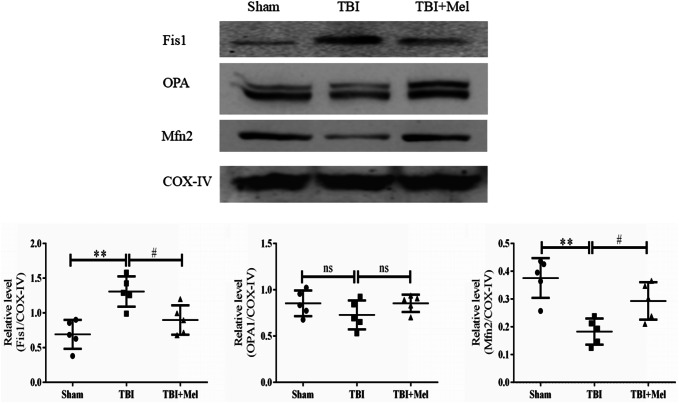
Mel Treatment Inhibits Drp1-Mediated Mitochondrial Fission and Enhanced Mitochondrial Fusion Protein Expression After TBI
To assess the role of Mel in mitochondrial dynamics against TBI, we investigated the expression of mitochondrial fission/fusion proteins in cytosolic and mitochondrial fractions. As shown in Fig. 7, western blot analysis showed that the expression level of Drp1 was significantly decreased in the cytoplasm (**P < 0.01) and increased in the mitochondrial fraction (**P < 0.01) in the TBI group as compared to the Sham group. Since the increased level of Drp1 protein is known to cause mitochondrial fission (Filippi et al. 2017), this indicates that Mel treatment significantly inhibited fission by regulating the level of Drp1. We also analyzed the expression level of Bax in cytosol and mitochondria upon Mel treatment. The level of Bax expression significantly decreased in the cytosol (**P < 0.01) but increased in the mitochondria in the TBI group (**P < 0.01) as compared to the Sham group. However, there was an opposite trend in the Mel-treated group (#P < 0.05) as compared to the TBI group. Furthermore, we determined the expression of mitochondrial fission promoting protein Fis1 in the mitochondrial fraction. The expression level of Fis1 protein was significantly upregulated in the TBI group (**P < 0.01) as compared to the Sham group. While Mel treatment significantly downregulated the Fis1 protein level (*P < 0.05) as compared to the TBI group, the expression level of mitochondrial fusion protein Mfn2 was significantly downregulated (**P < 0.01) in the TBI group animals as compared to the Sham group. Although, Mel treatment led to a significant upregulation of Mfn2 (#P < 0.05) in the TBI + Mel group, the expression of the OPA1 protein level did not change, as shown in Fig. 8.
Fig. 7.
Effect of Mel on the Drp1 and Bax protein expression in the cytosol as well as mitochondria. The relative optical density of protein bands were analyzed and normalized to β-Actin for cytosol and COX-IV for mitochondria. Data are presented as mean ± SD. **P < 0.01, TBI vs. the Sham group; ## P < 0.01 and #P < 0.05, TBI + Mel vs. the TBI group (n = 5 in each group)
Fig. 8.
Effect of Mel on the expression level of Fis1, OPA1, and Mfn2 proteins. The relative optical density of protein bands was analyzed and normalized to COX-IV. Data are presented as mean ± SD. **P < 0.01, TBI vs. the Sham group; #P < 0.05, TBI + Mel vs. the TBI group (n = 5 in each group)
Effect of Mel Treatment on F1-F0 Synthase Activity After TBI
F1-F0 synthase, also known as ATP synthase, is a component of the electron transport chain and plays an important role in the production of cellular energy. The activity of ATP synthase significantly decreased in mitochondrial sample of TBI group [F (2, 15) = 44.5, P < 0.0001] as compared to the Sham group. In addition, Mel treatment significantly elevated the ATP synthase activity (#P < 0.05) as compared to the TBI group, as shown in Fig. 9.
Fig. 9.

Mel significantly promote the activity of ATP synthase. Data are presented as mean ± SD. ***P < 0.001, TBI vs. the Sham group; #P < 0.05, TBI + Mel vs. the TBI group (n = 6 in each group)
Discussion
In the present study, we examined the neuroprotective effects of exogenous administration of Mel on mitochondrial-mediated neuronal cell death and neurobehavioral changes induced by traumatic injury in Wistar rats. The CCI model of TBI in rodents is widely accepted to mimic features of accidental brain injury in humans (Dixon et al. 1991; Morales et al. 2005). Primary and secondary injuries are associated with TBI have been found to cause neurochemical and behavioral implications (Ates et al. 2006). Notably, Wu et al. (2018) have reported an association link between TBI and mitochondrial perturbations. Multiple studies have demonstrated that TBI is associated with behavioral abnormalities, and Mel treatment has been shown to exert neuroprotective effects in various models of TBI (Ismailoglu et al. 2012). However, its role as exogenous mito-protectant has not been elucidated so far. Therefore, we sought to investigate the protective effects of Mel on mitochondrial dysfunction, dynamics, and biogenesis at 24 h following TBI. Our results showed that post-injury treatment with repeated doses of Mel activates mitochondrial fusion pathway through the upregulation of mitofusin protein and maintain a balance in mitochondrial dynamics equilibrium by reducing the availability of Drp1 protein and expression level of Fis1 protein on mitochondrial site. We also found a restorative effect of Mel on Δψm disruption changes in the level of oxidative phosphorylation (OXPHOS) proteins and bioenergetics loss after TBI. To our knowledge, this is the first study evaluating the modulation of mitochondrial dynamics and mitochondria-mediated neuroprotection in a rat CCI model of TBI.
Similar to a prior study, we found that TBI-subjected rats showed behavioral dysfunction as evidenced by performance on rota-rod, grip test, beam balance test and stride length. The current study showed that Mel administration improved the behavioral test after trauma. Our data are thus in agreement with the earlier reports by other research group where behavioral impairments have been improved following administration of Mel (Wu et al. 2016a). In the current study, we used the dose of Mel 10 mg/kg b.wt. based on previous studies (Ding et al. 2015; Wu et al. 2016a). Mel is highly lipophilic and easily passes through the blood–brain barrier. The multiple doses of Mel were injected at 0.5, 1, 2, 3, and 4 h post-TBI due to its short half-life. Previously published research articles have exhibited the critical estimation of the restorative time window after the initial insult of TBI. It is essential to begin intercessions at the earliest opportunity after TBI, ideally within 4 h after injury, to accomplish the most extreme protective effects (Ding et al. 2015).
Exacerbation of free radical production is the initial manifestation of brain injuries. Increased levels of superoxide ion and hydroxyl radicals result in a weak antioxidant defense system in neuropathological conditions (Ozdemir et al. 2005; Ashafaq et al. 2017; Wang et al. 2014). Oxidative stress disturbs the biological macromolecules, which can lead to perturbation in sub-cellular organelle function such as mitochondrial function and implicated in pathological brain insult (Watts 2016; Cui et al. 2012). Furthermore, TBI significantly increased brain water content which is the most common reason for high mortality and morbidity in traumatic circumstances (Yuruker et al. 2015; Ozdemir et al. 2005). In this study, we investigated oxidative stress biomarkers in mitochondria and brain edema in the brain tissue after a CCI injury. When TBI ensues, oxidative stress and brain edema are significantly increased due to tissue damage and concomitant loss of sub-cellular and neuronal integrity. As a result, there is direct impact of mitochondrial function and cellular energy-producing capacity after neuronal dysfunction (Wang et al. 2014; Areti et al. 2017). Alterations in specific biomarkers of oxidative stress such as LPO and GSH were analyzed spectrophotometrically after TBI. Additionally, ROS and Δψm were measured by flow cytometry. Our data showed that Mel treatment maintained the mitochondrial integrity by decreasing the oxidation of lipids of mitochondrial membranes, brain water content and increased the GSH level, thereby indicating a balance between oxidant and antioxidant equilibrium and anti-inflammatory effects of Mel. Previously, it has been shown that Δψm disruption accompanies mitochondrial dysfunction that may initiate activation of cascades leading to mitochondrial-mediated neuronal death (Waseem et al. 2016). Also, loss of mitochondrial integrity has been reported to disturb the function of mitochondrial permeability transition pore leading to cytochrome-c release from mitochondria into the cytosol following traumatic injury (Cheng et al. 2012). The presence of cytochrome-c in cytosol triggers apoptosis-associated pathways (Webster, 2012). Our results support the earlier findings (Waseem et al. 2017) that Mel treatment mitigates cytochrome-c leakage by promoting Δψm integrity and mitochondrial function through inhibition of lipid peroxidation and showed anti-inflammatory, antioxidant, anti-apoptotic, and anxiolytic effects (Ismailoglu et al. 2012; Tabassum et al. 2017; Yuruker et al. 2015).
Mitochondria produce cellular fuel in the form of adenosine triphosphate (ATP) by OXPHOS (Chen et al. 2016). Decreased OXPHOS may cause energy depletion and impairment in the brain function, which is frequently observed in various neurodegenerative disorders (Hroudová et al. 2014). In TBI, injured neurons require high levels of oxygen and glucose because of interruption in cerebral blood flow due to the blood–brain barrier leakage (Chen et al. 2014). These pathological consequences may create an ischemic condition, cause oxidative stress, and mitochondrial dysfunction in the brain resulting in truncated ATP production (Alluri et al. 2016; Chen et al. 2016; Kim et al. 2016). In the present study, we examined the expression level of mitochondrial complexes (C-I and C-V) proteins. A downregulation in the expression of OXPHOS associated proteins suggests low energy production, which affects neuronal repair mechanism and neuronal function (Chen et al. 2014). Our data showed that the expression of OXPHOS proteins is significantly downregulated in the TBI-subjected rat, which is a signature of mitochondrial dysfunction and low energy production. However, Mel treatment significantly upregulated the expression of OXPHOS proteins and augmented ATP synthase activity, which is an indication of improved mitochondria function following TBI. Neuronal cells use ATP as cellular fuel. Therefore, to analyze the ATP level, we examined the ATP synthase activity in the isolated mitochondrial sample. Mel treatment significantly increased the activity of ATP synthase after TBI and also increased the expression level of C-V-ATP5A protein.
Mitochondrial biogenesis is a key indicator of mitochondrial quality control, which activates through the peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) protein. The upregulation of PGC-1α also co-activates transcription factors such as Nrf1, Nrf2. In addition, it promotes antioxidant genes, mitochondrial function, and cellular energy generation in neurodegenerative disorders (Min et al. 2015). It has been demonstrated that PGC-1α is a key regulator of mitochondrial biogenesis and mitochondrial fusion under a range of pathological conditions of neuronal injury (Xi et al. 2018; Ding et al. 2018). Our results showed that Mel treatment accelerates the translocation of PGC-1α protein to the nucleus where it activates the various antioxidant genes and helps to attenuate oxidative damage and improve mitochondrial biogenesis, which finally restores the mitochondrial quality control.
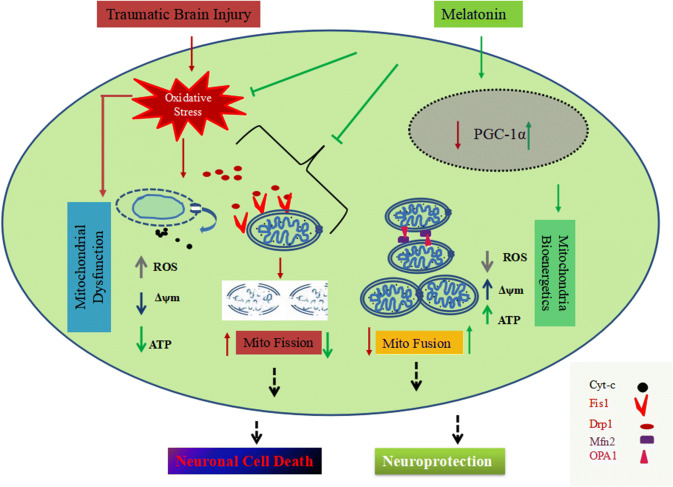
Mitochondria are highly dynamic organelle that maintain their size, position, and function via mitochondrial fission and fusion mechanism (Peng et al. 2016). It has been demonstrated that mitochondria are the primary source of free radical generation, and increased reactive oxygen and nitrogen species production can lead to an imbalance between fission and fusion in mitochondria, which could damage mitochondria (Hung et al. 2018; Cid-Castro et al. 2018). The previously published data have indicated that mitochondrial fission stimulates apoptotic-associated pathways in mitochondria due to cytochrome-c release, which plays an important role in the pathogenesis of neurodegenerative diseases (Bordt et al. 2017). Drp1 and Fis1 are essential proteins whose upregulation are involved in the mitochondria fission mechanism (Xi et al. 2018; Weil et al. 2018; Fischer et al. 2016). Drp1 is a guanosine triphosphatase protein found in the cytosol. It plays a pivotal role in mitochondrial fragmentation and mitochondria-dependent neuronal death by forming clusters of spirals, e.g., clusters with Fis1 protein found at the outer membrane of mitochondria with Fis1 protein. Many studies have shown upregulation of Drp1 leads to Bax-dependent cytochrome-c release and re-distribution due to migration from mitochondria to cytosol in traumatic conditions. This, in turn, results in the activation of caspase protease executioners (Li et al. 2015; Wu et al. 2016b; Bordt et al. 2017). Drp1 and Fis1 inhibition prevents mitochondrial fission and cell death and stabilizes mitochondrial dynamics in different models (Xie et al. 2018; Qi et al. 2013). Our results showed that TBI activates the mitochondrial fission pathway by promoting the upregulation of mitofission proteins. For the mitochondrial fusion mechanism, we checked the expression level of Mfn2 protein, which is an outer membrane fusion protein, responsible for mitochondrial fusion (Chen et al. 2003). The fusion of two mitochondria results inhibited cytochrome-c release, increased Δψm, and consequently increased more production of cellular energy. Excessive rate of fission is one of the major causes of mitochondrial perturbation and neuronal cell death (Bordt et al. 2017; Flippo and Strack 2017; Li et al. 2017). We presume that the post-injury treatment of Mel improved the mitochondrial function via inhibition of mitochondrial fission and modulation of mitochondrial fusion pathway (Fig. 10). However, additional molecular studies need to be done to understand the action of Mel against Drp1-mediated mitochondrial fission in the present outcomes. Our findings constitute strong evidence that Mel has an immediate protective action on mitochondrial function and mitochondrial dynamics, which might contribute to its neuronal cell protection against TBI.
Fig. 10.
Schematic diagram showing mitochondrial-mediated neuroprotection against TBI-induced mitochondrial fission and mitochondrial dysfunction through Drp1 and PGC-1α pathway. Mel positively regulates the expression of PGC-1α in the nucleus and improves mitochondrial function, thereby inhibiting the translocation of Bax, Drp1, and cytochrome-c release. TBI significantly increases oxidative damage and mitochondrial dysfunction, which directly affects mitochondrial dynamics. Upregulation of fission proteins, triggers mitochondrial fission and, subsequently, leads to low mitochondrial ATP production. Cytochrome-c migrates to the cytosol, which activates cascades and results in mitochondrial-mediated neuronal cell death. Treatment with melatonin increases the expression of PGC-1α, and Mfn2, and thus prevents oxidative damage and Drp1-mediated mitochondrial fragmentation. In summary, melatonin provides mitochondrial-mediated neuroprotection and promotes mitochondrial fusion following TBI-induced mitochondrial perturbation
It is important to acknowledge some of the limitations of the current study. First, this study only demonstrates that Mel provides neuroprotective effects early point, and further investigation needs to be performed to investigate the long-term neuromodulatory effects of Mel after TBI. However, the remedial time point, the optimal dosage, and other drug administration routes of Mel treatment in acute TBI need to be addressed. Further, the long-term effects of mitochondrial fission/fusion following TBI should be evaluated as well.
Conclusion
To the best of our knowledge, this is the first study to evaluate the effect of Mel on mitochondrial-mediated neuroprotection in the CCI model of TBI. Mitochondria fission is one of the hallmarks of TBI-induced neuronal death and dysfunction. TBI upregulates the expression of mitochondrial fission proteins and downregulates mitochondrial fusion proteins. It also activates neuronal apoptotic pathways and impairs biogenesis in the injured brain. All of these effects are attenuated by Mel treatment, as shown in the current study. Our data strongly suggest that TBI activates mitochondrial fission, which might play a central role in the mitochondrial perturbation that, in turn, contributes to neuronal dysfunction and death after a TBI. Findings from the present study indicate that the therapeutic benefits of Mel treatment might be due to its inhibitory effect on mitochondrial fission and augmentation of mitochondrial function via mitochondrial dynamics pathway.
Acknowledgements
Mohd Salman is a recipient of the Senior Research Fellowship from ICMR [F.No.3/1/2/4/Trauma/2009-NCD-1], and Pooja Kaushik is a recipient of the Senior Research Fellowship from University Grant Commission [F.No.-25-1/2014-15(BSR)/7-91/2017/(BSR)]. Dr. Heena Tabassum is grateful to the Department of Science and Technology, Government of India, for financial grant (DST Cognitive Science Initiative Program, sanction no. SR/CSI/PDF-76/2012). The Grant no.[2016/001070/HS)], received as Extramural Research Grant from the Science and Engineering Research Board, New Delhi, Government of India, to Prof. Suhel Parvez is also thankfully acknowledged. The Grant no.[SR/FST/LS-I/2017/05(C)] and [SR/PURSE Phase2/39 (C)] received from the Ministry of Science & Technology, Government of India, to Jamia Hamdard under DST-FIST and DST-PURSE programs are also thankfully acknowledged. The authors would like to thank Dr. Pradeep K. Rai, BD-JH FACS Academy, BD Biosciences, India for his help in flow cytometry analysis.
Author Contributions
MS, HT and SP designed the study. MS, PK, and HT conducted the experiments and analyzed the data. MS, HT and SP wrote the manuscript. SP and HT critically revised the manuscript. All authors have read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no potential conflict of interest.
Ethical Approval
All Surgical and experimental procedures were performed in accordance with the Institutional Animal Ethics Committee of Jamia Hamdard, New Delhi, India.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alluri H, Wilson RL, Anasooya Shaji C et al (2016) Melatonin preserves blood-brain barrier integrity and permeability via matrix metalloproteinase-9 inhibition. PLoS ONE 11:e0154427. 10.1371/journal.pone.0154427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi SS, Parvez S, Tabassum H (2017) Progesterone induces neuroprotection following reperfusion-promoted mitochondrial dysfunction after focal cerebral ischemia in rats. Dis Model Mech 10:787–796. 10.1242/dmm.025692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Areti A, Komirishetty P, Akuthota M et al (2017) Melatonin prevents mitochondrial dysfunction and promotes neuroprotection by inducing autophagy during oxaliplatin-evoked peripheral neuropathy. J Pineal Res 62:e12393. 10.1111/jpi.12393 [DOI] [PubMed] [Google Scholar]
- Ashafaq M, Tabassum H, Parvez S (2017) Modulation of behavioral deficits and neurodegeneration by tannic acid in experimental stroke challenged wistar rats. Mol Neurobiol 54:5941–5951. 10.1007/s12035-016-0096-8 [DOI] [PubMed] [Google Scholar]
- Ates O, Cayli S, Gurses I et al (2006) Effect of pinealectomy and melatonin replacement on morphological and biochemical recovery after traumatic brain injury. Int J Dev Neurosci 24:357–363. 10.1016/j.ijdevneu.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Bordt EA, Clerc P, Roelofs BA et al (2017) The putative drp1 inhibitor mdivi-1 is a reversible mitochondrial complex I inhibitor that modulates reactive oxygen species. Dev Cell 40:583–594.e6. 10.1016/j.devcel.2017.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary S, Parvez S (2012) An in vitro approach to assess the neurotoxicity of valproic acid-induced oxidative stress in cerebellum and cerebral cortex of young rats. Neuroscience 225:258–268. 10.1016/j.neuroscience.2012.08.060 [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC (2009) Mitochondrial dynamics-fusion, fission, movement, and mitophagy-in neurodegenerative diseases. Hum Mol Genet 18:R169–R176. 10.1093/hmg/ddp326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chan YL, Nguyen LT et al (2016) Moderate traumatic brain injury is linked to acute behaviour deficits and long term mitochondrial alterations. Clin Exp Pharmacol Physiol 43:1107–1114. 10.1111/1440-1681.12650 [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ et al (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160:189–200. 10.1083/jcb.200211046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang L, Wu C et al (2014) Melatonin-enhanced autophagy protects against neural apoptosis via a mitochondrial pathway in early brain injury following a subarachnoid hemorrhage. J Pineal Res 56:12–19. 10.1111/jpi.12086 [DOI] [PubMed] [Google Scholar]
- Chen S-D, Yang D-I, Lin T-K et al (2011) Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci 12:7199–7215. 10.3390/ijms12107199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Kong R, Zhang L, Zhang J (2012) Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br J Pharmacol 167:699–719. 10.1111/j.1476-5381.2012.02025.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid-Castro C, Hernández-Espinosa DR, Morán J (2018) ROS as regulators of mitochondrial dynamics in neurons. Cell Mol Neurobiol 38:995–1007. 10.1007/s10571-018-0584-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Kong Y, Zhang H (2012) Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct 2012:1–13. 10.1155/2012/646354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K, Xu J, Wang H et al (2015) Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochem Int 91:46–54. 10.1016/j.neuint.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Ding M, Feng N, Tang D et al (2018) Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1α pathway. J Pineal Res 65:e12491. 10.1111/jpi.12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrachinski F, da Rosa GR, Sartori G et al (2017) Regulation of mitochondrial function and glutamatergic system are the target of guanosine effect in traumatic brain injury. J Neurotrauma 34:1318–1328. 10.1089/neu.2016.4563 [DOI] [PubMed] [Google Scholar]
- Dongare S, Gupta SK, Mathur R et al (2016) Zingiber officinale attenuates retinal microvascular changes in diabetic rats via anti-inflammatory and antiangiogenic mechanisms. Mol Vis 22:599–609 [PMC free article] [PubMed] [Google Scholar]
- Edward Dixon C, Clifton GL, Lighthall JW et al (1991) A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods 39:253–262. 10.1016/0165-0270(91)90104-8 [DOI] [PubMed] [Google Scholar]
- Ehsaei M, Khajavi M, Arjmand MH et al (2015) Prooxidant–antioxidant balance in patients with traumatic brain injury. Acta Neurol Belg 115:69–73. 10.1007/s13760-014-0300-4 [DOI] [PubMed] [Google Scholar]
- Feng Y, Gao J, Cui Y et al (2017) Neuroprotective Effects of Resatorvid Against Traumatic Brain Injury in Rat: Involvement of Neuronal Autophagy and TLR4 Signaling Pathway. Cell Mol Neurobiol 37:155–168. 10.1007/s10571-016-0356-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi BM, Abraham MA, Silva PN et al (2017) Dynamin-related protein 1-dependent mitochondrial fission changes in the dorsal vagal complex regulate insulin action. Cell Rep 18:2301–2309. 10.1016/j.celrep.2017.02.035 [DOI] [PubMed] [Google Scholar]
- Fischer TD, Hylin MJ, Zhao J et al (2016) Altered mitochondrial dynamics and TBI pathophysiology. Front Syst Neurosci. 10.3389/fnsys.2016.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flippo KH, Strack S (2017) Mitochondrial dynamics in neuronal injury, development and plasticity. J Cell Sci 130:671–681. 10.1242/jcs.171017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furio AM, Brusco LI, Cardinali DP (2007) Possible therapeutic value of melatonin in mild cognitive impairment: a retrospective study. J Pineal Res 43:404–409. 10.1111/j.1600-079X.2007.00491.x [DOI] [PubMed] [Google Scholar]
- Hroudová J, Singh N, Fišar Z (2014) Mitochondrial dysfunctions in neurodegenerative diseases: relevance to alzheimer’s disease. Biomed Res Int 2014:1–9. 10.1155/2014/175062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CH-L, Cheng SS-Y, Cheung Y-T et al (2018) A reciprocal relationship between reactive oxygen species and mitochondrial dynamics in neurodegeneration. Redox Biol 14:7–19. 10.1016/j.redox.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismailoglu O, Atilla P, Palaoglu S et al (2012) The therapeutic effects of melatonin and nimodipine in rats after cerebral cortical injury. Turk Neurosurg. 10.5137/1019-5149.JTN.6197-12.1 [DOI] [PubMed] [Google Scholar]
- Johnson WD, Griswold DP (2017) Traumatic brain injury: a global challenge. Lancet Neurol 16:949–950. 10.1016/S1474-4422(17)30362-9 [DOI] [PubMed] [Google Scholar]
- Kim DI, Lee KH, Gabr AA et al (2016) Aβ-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis. Biochim Biophys Acta - Mol Cell Res 1863:2820–2834. 10.1016/j.bbamcr.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Li H-N, Zimmerman M, Milledge GZ et al (2017) Water-soluble coenzyme Q10 reduces rotenone-induced mitochondrial fission. Neurochem Res 42:1096–1103. 10.1007/s11064-016-2143-2 [DOI] [PubMed] [Google Scholar]
- Li Y, Wang P, Wei J et al (2015) Inhibition of Drp1 by Mdivi-1 attenuates cerebral ischemic injury via inhibition of the mitochondria-dependent apoptotic pathway after cardiac arrest. Neuroscience 311:67–74. 10.1016/j.neuroscience.2015.10.020 [DOI] [PubMed] [Google Scholar]
- Meyer JN, Leuthner TC, Luz AL (2017) Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 391:42–53. 10.1016/j.tox.2017.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Huo X, Xiang L et al (2015) Protective effect of Dl-3n-butylphthalide on learning and memory impairment induced by chronic intermittent hypoxia-hypercapnia exposure. Sci Rep 4:5555. 10.1038/srep05555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales DM, Marklund N, Lebold D et al (2005) Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience 136:971–989. 10.1016/j.neuroscience.2005.08.030 [DOI] [PubMed] [Google Scholar]
- Ozdemir D, Uysal N, Gonenc S et al (2005) Effect of melatonin on brain oxidative damage induced by traumatic brain injury in immature rats. Physiol Res 54:631–637 [PubMed] [Google Scholar]
- Peng K, Tao Y, Zhang J et al (2016) Resveratrol regulates mitochondrial biogenesis and fission/fusion to attenuate rotenone-induced neurotoxicity. Oxid Med Cell Longev 2016:1–12. 10.1155/2016/6705621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Qvit N, Su Y-C, Mochly-Rosen D (2013) A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci 126:789–802. 10.1242/jcs.114439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed MZ, Andrabi SS, Salman M et al (2018) Melatonin improves behavioral and biochemical outcomes in a rotenone-induced rat model of parkinson’s disease. J Environ Pathol Toxicol Oncol 37:139–150. 10.1615/JEnvironPatholToxicolOncol.2018025666 [DOI] [PubMed] [Google Scholar]
- Selassie AW, Wilson DA, Pickelsimer EE et al (2013) Incidence of sport-related traumatic brain injury and risk factors of severity: a population-based epidemiologic study. Ann Epidemiol 23:750–756. 10.1016/j.annepidem.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C, Martin SJ (2010) Mitochondrial fission/fusion dynamics and apoptosis. Mitochondrion 10:640–648. 10.1016/j.mito.2010.08.005 [DOI] [PubMed] [Google Scholar]
- Tabassum H, Ashafaq M, Parvez S, Raisuddin S (2017) Role of melatonin in mitigating nonylphenol-induced toxicity in frontal cortex and hippocampus of rat brain. Neurochem Int 104:11–26. 10.1016/j.neuint.2016.12.010 [DOI] [PubMed] [Google Scholar]
- Talley Watts L, Long JA, Chemello J et al (2014) Methylene blue is neuroprotective against mild traumatic brain injury. J Neurotrauma 31:1063–1071. 10.1089/neu.2013.3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-W, Wang H-D, Cong Z-X et al (2014) Puerarin ameliorates oxidative stress in a rodent model of traumatic brain injury. J Surg Res 186:328–337. 10.1016/j.jss.2013.08.027 [DOI] [PubMed] [Google Scholar]
- Waseem M, Sahu U, Salman M et al (2017) Melatonin pre-treatment mitigates SHSY-5Y cells against oxaliplatin induced mitochondrial stress and apoptotic cell death. PLoS ONE 12:e0180953. 10.1371/journal.pone.0180953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waseem M, Tabassum H, Parvez S (2016) Neuroprotective effects of melatonin as evidenced by abrogation of oxaliplatin induced behavioral alterations, mitochondrial dysfunction and neurotoxicity in rat brain. Mitochondrion 30:168–176. 10.1016/j.mito.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Watts L (2016) Stimulating mitochondria to protect the brain following traumatic brain injury. Neural Regen Res. 10.4103/1673-5374.191205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KA (2012) Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Future Cardiol 8:863–884. 10.2217/fca.12.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KM, Wright DK, Sun M et al (2015) Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long-term outcomes in a rat model of repeated mild traumatic brain injury. J Neuroinflammation 12:238. 10.1186/s12974-015-0457-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil R, Laplantine E, Curic S, Génin P (2018) Role of optineurin in the mitochondrial dysfunction: potential implications in neurodegenerative diseases and cancer. Front Immunol. 10.3389/fimmu.2018.01243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Shao A, Zhao M et al (2016a) Melatonin attenuates neuronal apoptosis through up-regulation of K + -Cl − cotransporter KCC2 expression following traumatic brain injury in rats. J Pineal Res 61:241–250. 10.1111/jpi.12344 [DOI] [PubMed] [Google Scholar]
- Wu Q, Gao C, Wang H et al (2018) Mdivi-1 alleviates blood-brain barrier disruption and cell death in experimental traumatic brain injury by mitigating autophagy dysfunction and mitophagy activation. Int J Biochem Cell Biol 94:44–55. 10.1016/j.biocel.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Wu Q, Xia S-X, Li Q-Q et al (2016b) Mitochondrial division inhibitor 1 (Mdivi-1) offers neuroprotection through diminishing cell death and improving functional outcome in a mouse model of traumatic brain injury. Brain Res 1630:134–143. 10.1016/j.brainres.2015.11.016 [DOI] [PubMed] [Google Scholar]
- Xi Y, Feng D, Tao K et al (2018) MitoQ protects dopaminergic neurons in a 6-OHDA induced PD model by enhancing Mfn2-dependent mitochondrial fusion via activation of PGC-1α. Biochim Biophys Acta—Mol Basis Dis 1864:2859–2870. 10.1016/j.bbadis.2018.05.018 [DOI] [PubMed] [Google Scholar]
- Xie L, Shi F, Tan Z et al (2018) Mitochondrial network structure homeostasis and cell death. Cancer Sci 109:3686–3694. 10.1111/cas.13830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yürüker V, Nazıroğlu M, Şenol N (2015) Reduction in traumatic brain injury-induced oxidative stress, apoptosis, and calcium entry in rat hippocampus by melatonin: possible involvement of TRPM2 channels. Metab Brain Dis 30:223–231. 10.1007/s11011-014-9623-3 [DOI] [PubMed] [Google Scholar]
- Zhao L, Li S, Wang S et al (2015) The effect of mitochondrial calcium uniporter on mitochondrial fission in hippocampus cells ischemia/reperfusion injury. Biochem Biophys Res Commun 461:537–542. 10.1016/j.bbrc.2015.04.066 [DOI] [PubMed] [Google Scholar]