Abstract
MEG8 is involved in ischemia stroke, however, its role in ischemia stroke remains unknown. The current research aimed to investigate the effects and mechanisms of MEG8 in ischemic stroke. Mouse brain microvascular endothelial cells (BMECs) were treated by oxygen–glucose deprivation (OGD). Then, the expressions of MEG8 and miR-130a-5p were detected by quantitative reverse transcription-polymerase chain reaction (q-PCR). Cell counting kit-8 (CCK-8), wound-healing, tube formation, Western blot, and q-PCR assays were performed to detect the effects of MEG8 and miR-130a-5p on cell viability, migration, and angiogenesis and VEGFA expression. Bioinformatics, dual-luciferase reporter assay, and RNA immunoprecipitation analysis were carried out to investigate the targeting relationship between MEG8 and miR-130a-5p, and between miR-130a-5p and VEGFA. Then, rat middle cerebral artery occlusion (MCAO) model and MEG8 overexpression MCAO model were established, and neurological deficit and infarct volume of the model rats were evaluated. Finally, Western blot and q-PCR were carried out to detect the expressions of MEG8, miR-130a-5p, and VEGFA. MEG8 was upregulated and miR-130a-5p was downregulated in OGD-treated BMECs. MiR-130a-5p was found to be a target of MEG8, and VEGFA was predicted to be a potential target of miR-130a-5p. Downregulation of MEG8 inhibited the cell viability, migration, and angiogenesis and the expression of VEGFA via negatively regulating miR-130a-5p of BMECs treated by OGD/non-OGD. In addition, MEG8 reduced cerebral ischemia, neurological score and miR-130a-5p expression, and increased VEGFA expression of MCAO rat. Our findings proved that MEG8 regulates angiogenesis and attenuates cerebral ischemia after ischemic stroke via miR-130a-5p/VEGFA signaling.
Electronic supplementary material
The online version of this article (10.1007/s10571-020-00904-4) contains supplementary material, which is available to authorized users.
Keywords: Ischemic stroke, MEG8, miR-130a-5p, VEGFA, Angiogenesis
Introduction
Stroke, which is a disease with the second highest mortality all over the world, is characterized by high morbidity, disability, and mortality (Hankey 2017). Stroke can be divided into ischemic stroke and hemorrhagic stroke, the proportion of ischemic stroke is higher than 80% (Hankey 2017; Minnerup et al. 2012). Ischemic stroke is caused by the obstruction of blood into the cerebrum, leading to reduced intracranial blood flow, and cerebral ischemia and hypoxia that further causes damages to nervous system (Favate and Younger 2016; Meschia and Brott 2018). Under the condition of insufficient blood supply, angiogenesis of endothelial cells will be enhanced and new vessels will be generated as a natural process of restoring blood flow (Tsvetkov et al. 2016; Zhao and Li 2015). Previous study reported that angiogenesis is essential for cerebral ischemia repairing, thus, promoting angiogenesis is considered as a promising strategy for treating ischemic stroke (Hatakeyama et al. 2020).
Long non-coding RNAs (lncRNAs) are a class of non-coding RNAs that share many features with protein-coding RNAs (mRNAs), but they lack open-reading frame, have lower sequence conservation and lower expression levels (Jarroux et al. 2017). It has been increasingly found that abnormal expressions of lncRNAs are strongly related to multiple biological processes, such as epigenetic regulation, multifunction of stem cells, and immune surveillance (Ahmed and Liu 2018; Bernardes de Jesus et al. 2018; Zhou et al. 2018). In recent years, accumulating evidence showed that abnormal regulation of lncRNAs is closely related to the pathogenesis of ischemic stroke (Zhao et al. 2018). In addition, the effects of lncRNAs on angiogenesis after ischemic stroke are gradually uncovered (Heydari et al. 2019; Ruan et al. 2015; Yin et al. 2015). Intronic lncRNA MEG8, alternatively known as Rian, is identified to be up-regulated in many diseases and plays crucial role in cell proliferation and invasion (Sheng et al. 2019; Terashima et al. 2018). Researchers predicted that MEG8 is highly related to ischemic stroke by Subpathway-LNCE method (Xiao et al. 2019). However, the specific effect of MEG8 on ischemic stroke is still unknown.
It had been increasingly found that lncRNAs could competitively bind to certain miRNAs, and subsequently regulate miRNA-mediated downstream target genes (Khorkova et al. 2015). MiR-130a is a vertebrate-specific miRNA, and functions critically during cell cycle and angiogenesis (Xiao et al. 2014). A recent study has reported that miR-130a is upregulated in cerebral ischemia and has the ability to regulate the blood–brain barrier permeability (Wang et al. 2018). As a mature miRNA of miR-130a, miR-130a-5p also plays a critical role in many diseases and has multiple biological functions affecting cell behaviors such as cell growth, apoptosis, and metastasis (Liu et al. 2018; Xian et al. 2018; Xu et al. 2019). However, the role of miR-130a-5p in ischemic stroke still remains to be investigated.
The present study established cerebral ischemia models in vitro and in vivo to examine the effects of MEG8 and miR-130a-5p on angiogenesis of mouse brain microvascular endothelial cells (BMECs) and investigated their effects on ischemic stroke. The aim of this research was to investigate the specific effects and mechanisms of MEG8 in ischemic stroke.
Materials and Methods
Ethics Statement
All animal experiments were performed in accordance with the Guidelines of the China Council on Animal Care and Use. This study was approved by the Committee of Experimental Animals of People’s Hospital of Rizhao (Z201807321N). All possible efforts were made to minimize pain and discomfort caused to the animals. The animal experiments were performed in People’s Hospital of Rizhao.
Cell Culture and Oxygen–Glucose Deprivation (OGD) Treatment
Mouse BMECs (bEnd.3) (CRL-2299, American Type Culture Collection, ATCC, MD, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; C11995500BT, Gbico, MA, USA) containing 10% fetal bovine serum (FBS; 10437010, Gbico) in a humidified atmosphere with 5% CO2 at 37 °C.
OGD progression was developed on bEnd.3 cells to mimic ischemia in vitro. The reference of the OGD method was based on the method used in the research of Miao Zhao (Zhao et al. 2018). In brief, after cells had adhered to the wells, the medium was replaced by glucose-free DMEM, and the cells were transferred to an anaerobic environment containing 5% CO2 and 95% N2 at 37 °C and held for 2 h. Then, the cells were recovered under normal oxygen culture environment for later use, and the recovery time was different (12 h, 24 h and 48 h).
Transfection
Small interfering RNAs for lncRNA MEG8 (siMEG8) (siB170103072135-1-5), and negative control (siNC) (siN0000002-1-5), miR-130a-5p mimic (miR10004593-1-5), miR-130a-5p inhibitor (miR20004593-1-5), mimic control (MC) (miR1N0000002-1-5), and inhibitor control (IC) (miR2N0000002-1-5) were purchased from RIBOBIO (Guangzhou, China). RNase-free H2O (ST876, Beyotime, Shanghai, China) was used to dilute these products to 20 μM and maintained at − 20 °C for later use. Before cell transfection, the cells were seeded into 6-well plates containing 2 ml complete medium at 1.0 × 106 cells. After cell grew to the confluence of 60–70%, 100 μl medium without FBS was used to dilute siMEG8, mimic, inhibitor, or control to a using concentration of 20 nM, then added with 3 μl lipo 2000 (11668-019, Invitrogen, MA, USA), mixed thoroughly and rested for 15 min at normal room temperature. Finally, the mixture was added into the cells, which were then added with 1.8 ml medium to allow the cells to grow for an additional 48 h. Experiments of control groups were conducted in parallel.
RNA Extraction and q-PCR
TRIzol reagent (15596, Invitrogen, MA, USA) was used to extract mRNAs and lncRNAs. In brief, the cells and cerebral samples were lysed by TRIzol and collected into a new 1.5 ml centrifugal tube (615001, Nest, Wuxi, China), which was then added with chloroform (C805334, Macklin, shanghai, China) and centrifuged for 20 min (14,000 × g). The supernatant was collected and mixed with an equal volume of isopropanol (H822173, Macklin). Then, the samples were centrifuged for 5 min (14,000 × g). RNA sediments were diluted using RNase-free H2O. MiRNAs were extracted using a MiRcute miRNA Isolation Kit (FP401, TianGEN, Beijing, China). In brief, the cells and cerebral samples were collected into 1.5 ml centrifugal tube and mixed with lysis buffer. 200 μl chloroform was added into the tube and shaken for 1 min. After resting the cells for 5 min at room temperature, the cell was centrifuged for 20 min (13,400×g) and miRNA solution was collected into a new 1.5 ml tube, into which ethanol (E801077, Macklin) was then added, and centrifuged for 15 min (13,400×g). The sediments were miRNAs, and RNase-free H2O was used to dilute the miRNA sediments.
Then, RNAs were reverse-transcribed into cDNAs using PrimeScript RT kit (RR037A, Takara, Dalian, China) according to the reference instructions. Finally, gene expression was detected by q-PCR assays using Verso 1-step RT-qPCR Kit (A15300, Thermo Scientific, MA, USA) in ABI 7500 Fast Real-Time PCR System (Applied Biosystems, CA, USA). The condition of q-PCR was set at 95 °C for 30 s, at 60 °C for 30 s, 45 cycles at 60 °C for 30 s. RNAs was quantified by 2−ΔΔCT method (Schmittgen and Livak 2008). All primer sequences used were as follows: MEG8 upstream: 5′-GCGTAGAGTCCTCGGGATGGATCTAAC-3′, MEG8 downstream: 5′-CCAGGGTAATTCCATGTGAAGATGCTC-3′; miR-130a-5p upstream: 5′-GCGCGGATCCAGGCGGCAAAAGGAAGAGTGGTG-3′, miR-130a-5p downstream: 5′-CGGCGAATTCCACAAGCACTGCATACAGAAGTAG-3′; VEGFA upstream: 5′-CTGCCGTCCGATTGAGACC-3′, VEGFA downstream: 5′-CCCCTCCTTGTACCACTGTC-3′; GAPDH upstream: 5′-GAAGGTGAAGGTCGGAGTC-3′, GAPDH downstream: 5′-GAAGATGGTGATGGATTTC-3′. U6 upstream: 5′-CTCGCTTCGGCAGCACA-3′, U6 downstream: 5′-ACGCTTCACGAATTTGCGT-3′.
CCK-8 Assays
CCK-8 (PA137267, Pierce, MA, USA) was performed to detect cell viability. After the transfection, the cells (at 1.0 × 104 cells) were laid into 96-well plates containing 100 μl complete medium. After let grow for 24 h, the cells were incubated by CCK-8 reagent (0.5 mg/ml) for 15 min. Finally, the absorbance of each well was detected at 450 nm using a microplate reader (Infinite M200 PRO, Tecan Austria GmbH, Austria).
Wound-Healing Assay
After the transfection, the cells (at 3.5 × 105) were placed into 6-well plates containing 2 ml of complete medium, and cultured until the cell confluence reached 95%. Then, a vertical wound in each well was created using a 20 μl pipette tip, and medium without FBS was added into each well. Images were collected at 0 and 24 h and observed under a phase-contrast optical microscope (Axio Lab.A1 pol; Leica, Solms, Germany). Image J software (Version 1.8.0) was used to analyze the images in this assay.
Tube Formation Assays
After the transfection, the cells in 200 µl medium were diluted to a density of 2 × 105 cells and seeded into a 48 well plate coated with 200 µl ECMatix gel (ECM625, Millipore, Billerica, MA, USA). The cells were then incubated for 4 h, and photographed under a phase-contrast optical microscope (Axio Lab.A1 pol; Leica, Solms, Germany) to observe and examine their tube‑like structures.
Luciferase Reporter Assays
Putative miR-130a-5p binding sites in MEG8 and VEGFA were respectively predicted using StarBase (https://starbase.sysu.edu.cn/mirMrna.php) and TargetScan (https://www.targetscan.org). The fragments of MEG8-3′-UTR with wide-type (MEG8-WT) and mutant (MEG8-MUT) binding sites for miR-130a-5p were inserted into pmirGLO luciferase Vectors (E1330, Promega, CA, USA). Moreover, the fragments of VEGFA-3′-UTR with wide-type (VEGFA-WT) and mutant (VEGFA -MUT) binding sites for miR-130a-5p were inserted into pmirGLO luciferase Vectors. MEG8-WT/MEG8-MUT and VEGFA-WT/ VEGFA-MUT were co-transfected with miR-130a-5p inhibitor using Lipo 2000 and continued to grow for 48 h. Next, the cells were collected to perform Dual-Luciferase Reporter Assay (Promega). Luciferase activity of the cells was determined by SpectraMax L fluorescence reader (Molecular Devices, CA, USA).
RNA Immunoprecipitation (RIP) Assay
The cells transfected with miR-130a-5p mimic or MC for 48 h were washed by cold PBS and lysed by RIP lysis buffer (RG129S, Beyotime). The cell lysates were incubated with magnetic beads conjugated with anti-Argonaute2 antibody (MA514861, Thermo Scientific) or negative control mouse IgG (31,203, Thermo Scientific). After the incubation, the beads were collected and washed, and RNAs were extracted using proteinase K. MEG8 was detected by performing reverse transcription PCR. The cell lysates served as the input.
Animal and Middle Cerebral Artery Occlusion (MCAO) Model Establishment
Forty adult male Sprague–Dawley rats (weighting 300–340 g, aged 9 to 10 weeks old) were purchased from SLAC Laboratory Animal Technology Co. (Shanghai, China). All experimental animals were fed in the same animal feeding unit and housed under 12-h dark/12 light cycle, assign 10 rats to each unit. The animals were randomly divided into four groups (n = 10), namely, Sham, MCAO, MCAO + NC, and MCAO + MEG8 groups. Before the operation, all the rats were fasted for 12 h and fed with drinking water. The rats were intraperitoneally injected with 2% of sodium pentobarbital (50 mg/kg) (B5646, APExBIO, Houston, USA). After the anesthesia, the median neck was cut to separate left common carotid artery, external carotid artery, and internal carotid artery. Then the common carotid artery and external carotid artery were ligatured, and a small area under the bifurcation of the common carotid artery was cut slowly. Next, MCAO bolt (1623, Cinontech, Beijing, China) was inserted 18–20 mm under the internal carotid artery until there was no resistance. After blocking for 2 h, the bolt was pulled out and the wound was stitched. During the operation, a temperature changing blanket was used to maintain the rectal temperature of the mouse at (37 ± 0.2) °C. The rats in the sham group were subjected to all surgical procedures except the creation of MCAO. The rats in MCAO + MEG8 were treated by lentivirus vector-mediated transformation of MEG8 for 14 days before MCAO. The rats in MCAO + NC group were treated by lentivirus vector-mediated transformation of negative control for 14 days before MCAO.
Neurological Scores Evaluation
Seven days after MCAO, we evaluated the neurological scores by the modified 5-score system. The scores are defined as follows: 0 (no observable deficits); 1 (difficult to fully extend the contralateral forelimb); 2 (unable to extend the contralateral forelimb); 3 (mild circling to the contralateral side); 4 (severe circling to the contralateral side); 5 (falling to the contralateral side). The neurological conditions of the animals were evaluated and scored by a pathologist blinded to treatment conditions.
Cerebral Infarct Volume Detection
Seven days after MCAO, the rats were anesthetized by intraperitoneal injection of 2% of sodium pentobarbital (50 mg/kg). Then, the rats with 350 g weight were sacrificed by cervical dislocation, and their brain tissues were collected. The coronal brain tissues were sectioned into 2-mm-thick slices, treated by TTC (2′3′5′-triphenyl tetrazolium chloride) (S19026, Yuanye, Shanghai, China) at 37 °C for 20 min, and then fixed by 10% formalin solution (P804537, Macklin). Finally, infarct volume was calculated using Image Proplus 6.0 (Media Cybernetics, MA, USA).
Western Blot Assay
Total proteins from the cells and cerebral samples were isolated by RIPA lysis buffer (P0013B, Beyotime), and a BCA assay kit (23250, Pierce, MA, USA) was used to detect the total protein concentrations. Finally, total proteins (30 µg) were separated in each lane on 10% SDS-PAGE gels (P0052A, Beyotime), electro-blotted and transferred to negative control (NC) membranes (HTS112M, Millipore). Next, the membranes were incubated by 5% non-fat for 2 h at normal temperature, and then incubated with relative first antibodies (VEGFA (1:1000, ab52917, 23kD, Abcam, CA, USA) and GAPDH (1:1000, 36kD, ab8245, Abcam)) overnight. The next day, HRP-conjugated secondary antibodies (goat anti-mouse IgG secondary antibody (1:5000, ab205719, Abcam) and goat anti-rabbit IgG secondary antibody (1:5000, ab205718, Abcam)) were used to incubate the membranes for 1 h at room temperature. Finally, SuperSignal West Pico Chemiluminescent Substrate (34078; Thermo Scientific, MA, USA) was used to incubate the membranes for detecting the signals. Image Lab™ Software (version 3.0) was used for densitometric analysis and quantification of the data (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Statistical Analysis
Student’s t-test and one-way ANOVA were performed to analyze the data in SPSS software (version 18.0). LSD and Dunnet's were used as post hoc tests (After analysis of variance, make a pairwise comparison of each population in the experiment). The comparison between the two groups was tested using LSD post-hoc analysis. The statistical data were shown as Mean ± standard deviation. All experiments were conducted in triplicate. P < 0.05 was considered as a statistically significant.
Results
MEG8 was High-Expressed and miR-130a-5p was Low-Expressed in BMECs Exposed to OGD
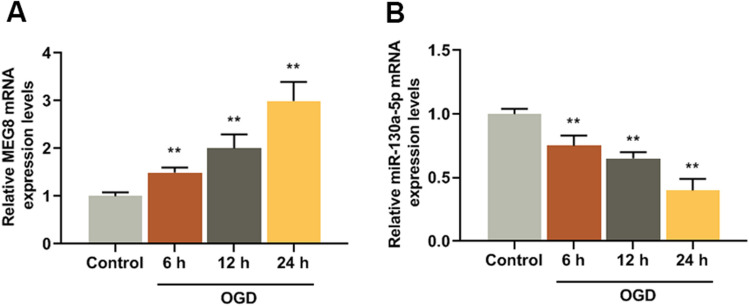
MEG8 and miR-130a-5p expression levels of the ischemic stroke model in vitro were detected, and we found that MEG8 expression was increased in the OGD group after reperfusion for 6, 12, and 24 h as compared with control group (F/q = 109.5, P = 0.000) (Fig. 1a). The expression of miR-130a-5p was reduced in OGD group after reperfusion for 6, 12, and 24 h as compared with control group (F/q = 132.6, P = 0.000) (Fig. 1b).
Fig. 1.
MEG8 was high-expressed and miR-130a-5p was low-expressed in BMECs cells exposed to OGD. a MEG8 expression in BMECs exposed to OGD was detected by q-PCR, GAPDH served as an internal control. b MiR-130a-5p expression in BMECs exposed to OGD were detected by q-PCR, and U6 served as an internal control. All experiments were conducted in triplicate. **P < 0.001, vs. control. OGD oxygen–glucose deprivation
Knocking Down MEG8 Inhibited the Viability, Migration, and Angiogenesis of BMECs
The role of MEG8 in BMECs was investigated. SiMEG8 was transfected into BMECs for the detection of cell viability, migration, and angiogenesis. As shown in Fig. 2a, b, the expressions of MEG8 were reduced as compared with control groups (Fig. 2a: P = 0.000; Fig. 2b: P = 0.000). In Fig. 2c, d, it could be observed that siMEG8 reduced the viability of BMECs with OGD treatment or without as compared with control groups (Fig. 2c: P = 0.000; Fig. 2d: P = 0.000). As for cell migration (Fig. 2e, f), siMEG8 also decreased the migration ability of BMECs exposed to OGD or not as compared with control groups (Fig. 2e: P = 0.000; Fig. 2f: P = 0.000). In addition, the tube formation assay (Fig. 2g, h) revealed that after knocking down MEG8, angiogenesis of BMECs to ODG or not were less than that in control groups (Fig. 2g: P = 0.002; Fig. 2f: P = 0.000). These results indicated that knocking down MEG8 inhibited the viability, migration, and angiogenesis of BMECs.
Fig. 2.
Knockdown of MEG8 inhibited the viability, migration, and angiogenesis of BMECs. a, b The transfection efficiency of MEG8 in BMECs exposed to OGD or not was detected by q-PCR, GAPDH served as an internal control. c, d Cell viabilities of BMECs exposed to OGD or not were analyzed by CCK-8 assays after transfection. e, f Cell migration of BMECs exposed to OGD or not was analyzed by wound-healing after transfection. g, h Cell angiogenesis of BMECs exposed to OGD or not was analyzed by tube formation assays after transfection. All experiments were conducted in triplicate. *P < 0.05, **P < 0.001, vs. control. OGD oxygen–glucose deprivation
Knocking Down MEG8 Inhibited the Expression of VEGFA of BMECs
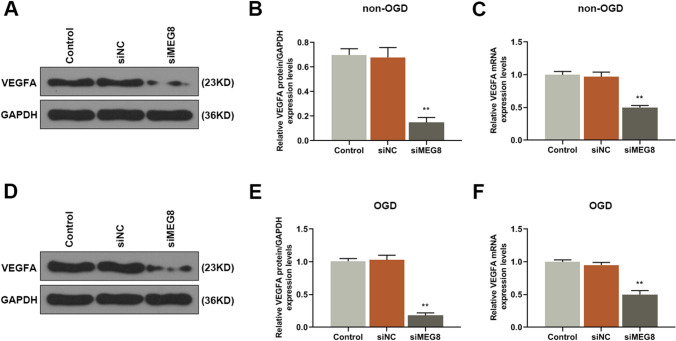
VEGFA is a key regulator of angiogenesis (Shi et al. 2018), therefore, its expression level of BMECs transfected with siMEG8 was detected. As shown in Fig. 3a–c, the results showed that siMEG8 down-regulated the expression of VEGFA both at protein and gene levels in BMECs without exposure to OGD as compared with control groups (Fig. 3b: P = 0.000; Fig. 3c: P = 0.000). Moreover, siMEG8 downregulated the protein and gene expressions of VEGFA in BMECs exposed to OGD as compared with control groups (Fig. 3e: P = 0.000; Fig. 3c: P = 0.000). These results further confirmed that knocking down MEG8 inhibited the angiogenesis of BMECs through down-regulating the expression of VEGFA.
Fig. 3.
Knocking down MEG8 inhibited the expression of VEGFA in BMECs. a, b, d, e The expression levels of VEGFA in BMECs exposed to OGD or not were detected by Western blot, GAPDH served as an internal control. c, f The expression levels of VEGFA of BMECs exposed to OGD or not were detected by q-PCR, GAPDH served as an internal control. All experiments were conducted in triplicate. **P < 0.001, vs. control. OGD oxygen–glucose deprivation
MEG8 Targeted miR-130a-5p and was Negatively Regulated its Expression
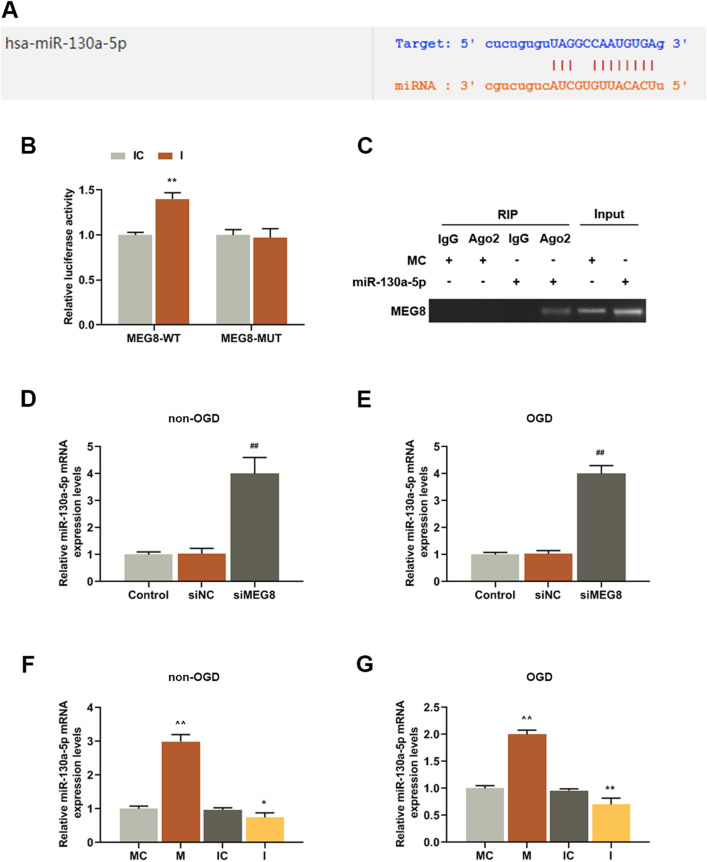
StarBase predicted that miR-130a-5p was a potential target of MEG8 (Fig. 4a). To further confirm the prediction, dual-luciferase reporter and RIP assays were conducted. As shown in Fig. 4b, luciferase activities of the cells co-transfected with miR-130a-5p inhibitor and MEG8-WT were increased as compared with IC group (P = 0.000). However, after co-transfection with miR-130a-5p inhibitor and MEG8-MUT, there was no difference in the luciferase activities compared with IC group. Furthermore, the result of RIP assay also confirmed the target relationship between MEG8 and miR-130a-5p (Fig. 4c). After transfecting siMEG8 into BMECs (Fig. 4d, e), a comparative analysis of miR-130a-5p expression level in the cells was conducted, and the results revealed that knocking down MEG8 significantly upregulated miR-130a-5p expression in BMECs exposed to OGD or not, as compared with control groups (Fig. 4d: P = 0.000; Fig. 4e: P = 0.000). This demonstrated that MEG8 negatively regulated miR-130a-5p expression. MiR-130a-5p mimic and inhibitor was then transfected into BMECs (Fig. 4f, g), and we observed that miR-130a-5p expression were up-regulated by miR-130a-5p mimic in BMECs exposed to OGD (Fig. 4f: P = 0.000) or not (Fig. 4g: P = 0.000) as compared with MC group. Moreover, miR-130a-5p expression were down-regulated by miR-130a-5p inhibitor in BMECs exposed to OGD (Fig. 4f: P = 0.0029) or not (Fig. 4g: P = 0.000) as compared with IC group.
Fig. 4.
MEG8 targeted miR-130a-5p and negatively regulated its expression. a The predictive binding sites of miR-130a-5p in MEG8 sequences. b Dual-luciferase assay validated that MEG8 targeted miR-130a-5p in BMECs, luciferase from firefly was used as a reporter gene and luciferase from sea kidney served as internal reference gene. **P < 0.001, vs. IC. c BMECs were transfected with miR-130a-5p mimic or MC for 48 h. The association between MEG8 and miR-130a-5p was detected by RIP assay. d, e The expression levels of miR-130a-5p of BMECs exposed to OGD or not were detected by q-PCR, U6 served as an internal control. ##P < 0.001, vs. siNC. f, g The transfection efficiencies of miR-130a-5p mimic and inhibitor in bEnd.3 cells exposed to OGD or not were detected by q-PCR, U6 served as an internal control. ^^P < 0.001, vs. MC; **P < 0.001, vs. IC. All experiments were conducted three times. MC miR-130a-5p mimic control, M miR-130a-5p mimic, IC miR-130a-5p inhibitor control, I miR-130a-5p inhibitor, OGD oxygen–glucose deprivation, RIP RNA immunoprecipitation
MEG8 Regulated the Viability, Migration, and Angiogenesis of BMECs via Regulating miR-130a-5p
As shown in Fig. 5a and b, both in the presence of OGD or not, viability of BMECs was reduced by miR-130a-5p mimic as compared with MC group (Fig. 5a: P = 0.000; Fig. 5b: P = 0.000), and increased by miR-130a-5p inhibitor as compared with IC group (Fig. 5a: P = 0.000; Fig. 5b: P = 0.000), however, the promoting effect of miR-130a-5p inhibitor was partly reversed by siMEG8 as compared with siMEG8 + I group (Fig. 5a: P = 0.000; Fig. 5b: P = 0.000). As for cell migration (Fig. 5c, d), in the presence of OGD or not, the relative rate of BMECs migration was reduced by miR-130a-5p mimic as compared with MC group (Fig. 5c: P = 0.000; Fig. 5d: P = 0.000) but increased by miR-130a-5p inhibitor as compared with IC group (Fig. 5c: P = 0.000; Fig. 5d: P = 0.000), however, the promoting effect of miR-130a-5p inhibitor was partly reversed by siMEG8 as compared with siMEG8 + I group (Fig. 5c: P = 0.000; Fig. 5d: P = 0.000). Moreover, the tube formation assays revealed that in the presence of OGD or not, the angiogenesis of BMECs was weakened by miR-130a-5p mimic as compared with MC group (Fig. 5e: P = 0.000; Fig. 5f: P = 0.000), but increased by miR-130a-5p inhibitor as compared with IC group (Fig. 5e: P = 0.000; Fig. 5f: P = 0.000), while siMEG8 also inhibited the promoting effect of miR-130a-5p inhibitor on cell angiogenesis as compared with siMEG8 + I group (Fig. 5e: P = 0.000; Fig. 5f: P = 0.000). These results indicated that MEG8 regulated viability, migration, and angiogenesis of BMECs via regulating miR-130a-5p.
Fig. 5.
MEG8 regulated the viability, migration, and angiogenesis of BMECs via regulating miR-130a-5p. a, b Cell viabilities of BMECs exposed to OGD or not were analyzed by CCK-8 assays after transfection. c, d Cell migration of bEnd.3 cells exposed to OGD or not was analyzed by wound-healing assays after transfection. e, f Cell angiogenesis of in bEnd.3 cells exposed to OGD or not was analyzed by tube formation assays after transfection. All experiments were conducted in triplicate. (^^P < 0.001, vs. MC; **P < 0.001, vs. IC; ΔΔP < 0.001, vs. I). MC miR-130a-5p mimic control, M miR-130a-5p mimic, IC miR-130a-5p inhibitor control, I miR-130a-5p inhibitor, OGD oxygen–glucose deprivation
MEG8 Regulated the Expression of VEGFA via Regulating miR-130a-5p and miR-130a-5p Targeted VEGFA
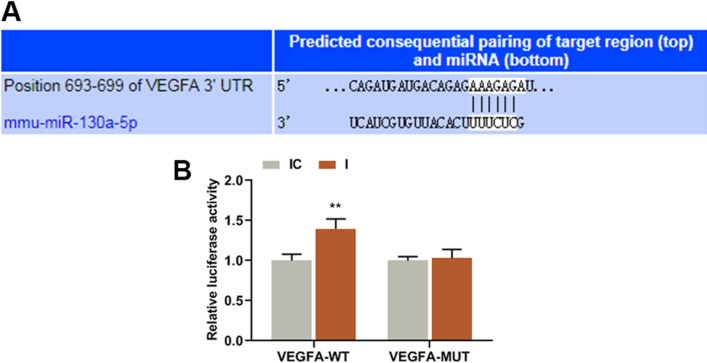
As shown in Fig. 6a–c, the results showed that in the presence of OGD, the protein and gene expression levels of VEGFA were reduced by miR-130a-5p mimic as compared with MC groups (Fig. 6b: P = 0.000; Fig. 6c: P = 0.000), but were promoted by miR-130a-5p inhibitor as compared with IC groups (Fig. 6b: P = 0.000; Fig. 6c: P = 0.000), while the promoting effect of miR-130a-5p inhibitor on VEGFA expression was partly reversed by siMEG8 as compared with siMEG8 + I groups (Fig. 6b: P = 0.000; Fig. 6c: P = 0.000). At the same time, in OGD condition (Fig. 6D-E), the protein and gene expression levels of VEGFA were reduced by miR-130a-5p mimic as compared with MC groups (Fig. 6e: P = 0.000; Fig. 6f: P = 0.000), but were promoted by miR-130a-5p inhibitor as compared with IC group (Fig. 6e: P = 0.000; Fig. 6f: P = 0.000), while the promoting effect of miR-130a-5p inhibitor on VEGFA expression was partly reversed by siMEG8 as compared with siMEG8 + I group (Fig. 6e: P = 0.000; Fig. 6f: P = 0.000). These results indicated that MEG8 regulated the expression of VEGFA via regulating miR-130a-5p. Therefore, we further investigated whether miR-130a-5p was correlated with VEGFA. As shown in Fig. 7a, TargetScan predicted that VEGFA was a possible target of miR-130a-5p, as VEGFA-3′-UTR contained a target sequence base pair for miR-130a-5p. To further confirm the prediction, dual-luciferase reporter assay was conducted. In Fig. 7b, it can be observed that luciferase activities of the cells co-transfected with miR-130a-5p inhibitor and VEGFA-WT were increased as compared with IC group (Fig. 7b: P = 0.000), while there was no difference in the luciferase activities of the cells co-transfected with miR-130a-5p inhibitor and VEGFA-MUT compared with IC group.
Fig. 6.
MEG8 regulated the expression of VEGFA via regulating miR-130a-5p. a, b, d, e The expression levels of VEGFA in bEnd.3 cells exposed to OGD or not was analyzed by tube formation assays were detected by Western blot, GAPDH served as an internal control. c, f The expression levels of VEGFA in bEnd.3 cells exposed to OGD or not was analyzed by tube formation assays were detected by q-PCR, GAPDH served as an internal control. All experiments were conducted in triplicate. ^^P < 0.001, vs. MC; **P < 0.001, vs. IC; ΔΔP < 0.001, vs. I. MC miR-130a-5p mimic control, M miR-130a-5p mimic, IC miR-130a-5p inhibitor control, I miR-130a-5p inhibitor, OGD oxygen–glucose deprivation
Fig. 7.
miR-130a-5p targeted VEGFA. a The predictive binding sites of VEGFA-3′-UTR contained a binding site of miR-130a-5p. b Luciferase assay validated that miR-130a-5p targeted VEGFA in bEnd.3 cells, luciferase from firefly was used as a reporter gene and luciferase from sea kidney as internal reference gene. **P < 0.001, vs. IC
Overexpression of MEG8 Attenuated Cerebral Ischemia in MCAO Rats
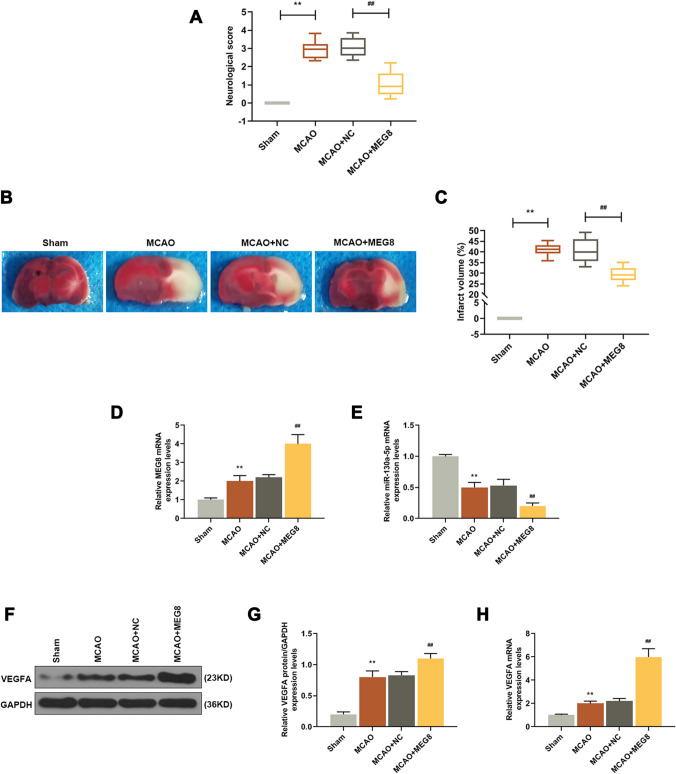
Additionally, the effect of MEG8 on ischemic stroke was further explored using MCAO rat models with MEG8 overexpressed by intracerebroventricular injection of MEG8 lentivirus. After the model had been established successfully, the neurological score (Fig. 8a) and relative infarct volume (Fig. 8b, c) in MCAO groups were increased when compared with sham groups (Fig. 8a: P = 0.000; Fig. 6e: P = 0.000), while in MCAO + MEG8 group, the score was significantly reduced than that in MCAO + NC group (Fig. 8a: P = 0.000; Fig. 6e: P = 0.000). The results indicated that overexpression of MEG8 could inhibit cerebral ischemia in ischemic stroke.
Fig. 8.
Overexpression of MEG8 attenuated cerebral ischemia in MCAO rats and the attenuating effect of MEG8 overexpression on cerebral ischemia was related to the miR-130a-5p/VEGFA signaling. a A neurological score of MCAO rats was evaluated by the modified 5-score system. b, c The cerebral infarct volume of MCAO rats was detected by TTC histology. (**P < 0.001, vs. Sham; ##P < 0.001, vs. MCAO + MEG8). d The expression level of MEG8 in MCAO rat samples was detected by q-PCR, GAPDH served as an internal control. e The expression level of miR-130a-5p in MCAO rat samples was detected by q-PCR, U6 served as an internal control. f, g The expression level of VEGFA in MCAO rat samples was detected by Western blot, GAPDH served as an internal control. h The expression level of VEGFA in MCAO rat samples was detected by q-PCR, GAPDH served as an internal control. All experiments were conducted in triplicate. (**P < 0.001, vs. Sham; ##P < 0.001, vs. MCAO + MEG8). MCAO middle cerebral artery occlusion, TTC 2′3′5′-triphenyl tetrazolium chloride
The Attenuation of MEG8 Overexpression on Cerebral Ischemia was Related to the miR-130a-5p/VEGFA Signaling
Based on current findings, we further detected the expressions of MEG8, miR-130a-5p, and VEGFA in rats’ cerebral samples. As shown in Fig. 8d, the expressions of MEG8 in MCAO group were higher than those in Sham group (P = 0.000), and the expression of MEG8 in MCAO + MEG8 group was also higher than that in MCAO + NC group (P = 0.000). As demonstrated in Fig. 8e, the expression of miR-130a-5p in MCAO group was reduced than that in Sham group (P = 0.000), and it was further reduced in MCAO + MEG8 group than that in MCAO + NC group (P = 0.000). As for the protein expression of VEGFA (Fig. 8f, g), it was up-regulated in MCAO group than that in Sham group (P = 0.000), and was increased more in MCAO + MEG8 group than that in MCAO + NC group (P = 0.000). As for gene expression level (Fig. 8h), it was upregulated in MCAO group than that in Sham group (P = 0.000), and was more up-regulated in MCAO + MEG8 group than that in MCAO + NC group (P = 0.000). Thus, these results showed that the effects of MEG8 on ischemic stroke were related to the miR-130a-5p/VEGFA signaling.
Discussion
The current study aimed to investigate the effects of MEG8 and miR-130a-5p on ischemic stroke and the potential mechanisms. After the establishment of the ischemic stroke model in vitro and in vivo, we found that knocking down MEG8 could inhibit the viability, migration, and angiogenesis of BMECs and the expression of VEGFA via negatively regulating miR-130a-5p. In addition, in ischemic stroke rat model in vivo, MEG8 had neuroprotective function and the ability to inhibit cerebral ischemic after ischemic stroke through mediating miR-130a-5p/VEGFA signaling.
Angiogenesis, which is a multi-step and multi-stage process, can induce the formation of new capillary and recovery of neurological functions (Tsvetkov et al. 2016; Zhao and Li 2015). Therefore, it is widely considered that enhancing angiogenesis is a promising strategy for treating ischemic stroke. Increasing number of reports reported that lncRNAs play important roles in angiogenesis of BMECs, for example, Zhao et al. demonstrated that lncRNA SNHG12 could enhance angiogenesis after ischemic stroke (Zhao et al. 2018); Li et al. found that lncRNA HIF1A-AS2 promotes angiogenesis in ischemic stroke (Li et al. 2017); knocking down MEG3 inhibits angiogenesis during ischemic brain injury (Liu et al. 2017). Recently, researchers predicted that MEG8 is closely related to ischemic stroke by subpathway-LNCE method (Xiao et al. 2019). However, the specific effect of MEG8 on ischemic stroke still remains unknown. In this study, we found that MEG8 was up-regulated in BMECs exposed to OGD, and that knocking down MEG8 inhibited the viability, migration, and angiogenesis of BMECs and the expression of VEGFA induced by OGD, moreover, MEG8 knockdown also had the same effect on BMECs without the induction of OGD. These results suggested that MEG8 plays important role in the ischemic stroke through distinct mechanisms.
We further explored the mechanism of MEG8 in affecting ischemic stroke. lncRNAs could competitively bind to certain miRNAs to further realize their functions (Khorkova et al. 2015). For example, SNHG12 promotes angiogenesis via targeting miR-130 in ischemic stroke (Zhao et al. 2018); HIF1A-AS2 promotes angiogenesis in ischemic stroke through competitively binding with miR-153-3p (Li et al. 2017); miR-377 could be targeted by NEAT1 and then enhance angiogenesis in OGD-induced BMECs (Zhou et al. 2019). Therefore as miR-130a-5p was downregulated in BMECs under OGD exposure in this study, we further explored whether there was a targeting relationship between MEG8 and miR-130a-5p. After using bioinformatics and performing dual-luciferase, and RIP analyses, we found that miR-130a-5p was a target of MEG8. Furthermore, MEG8 negatively regulated miR-130a-5p expression in the condition of OGD or not. Additionally, the effects of miR-130a-5p inhibitor on the viability, migration, and angiogenesis of BMECs and on the expression of VEGFA were partly reversed by siMEG8. These results suggested that the effects of MEG8 on BMECs were mediated through negatively regulating miR-130a-5p expression. Researchers reported that miRNAs regulate angiogenesis after stroke through targeting VEGFA (Shi et al. 2018; Sun et al. 2016; Zhao et al. 2018). Consistently, we demonstrated that VEGFA was a target of miR-130a-5p through bioinformatics and dual-luciferase analysis. The results indicated that MEG8 could function as a competing endogenous RNA (ceRNA). To make our present results more convincing, we established ab ischemic stroke model in rats, and further verified the effect of MEG8 on ischemic stroke. The in vivo study showed that MEG8 had neuroprotective functions and had the ability to inhibit cerebral ischemic after ischemic stroke. In addition, such effects of MEG8 on ischemic stroke were mediated via miR-130a-5p/VEGFA signaling.
In conclusion, the current study revealed that MEG8 promotes angiogenesis and alleviates cerebral ischemia. MEG8 functions as a ceRNA for miR-130a-5p in mediating VEGFA expression. The present findings provide better understandings of ischemic stroke and novel therapeutic target for the disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author Contributions
Substantial contributions to conception and design: SS, LS, WZ; data acquisition, data analysis and interpretation: JL, JH, JZ; drafting the article or critically revising it for important intellectual content: SS, LS, HX; final approval of the version to be published: all authors; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved: HX.
Funding
No funding was received.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving Human Participants and/or Animals
This study was approved by the Committee of Experimental Animals of People’s Hospital of Rizhao (Z201807321N). Every effort was made to minimize pain and discomfort to the animals. The animals’ experiments were performed in People’s Hospital of Rizhao. No human are involved in this research.
Informed Consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed W, Liu ZF (2018) Long non-coding RNAs: novel players in regulation of immune response upon herpesvirus infection. Front Immunol 9:761. 10.3389/fimmu.2018.00761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes de Jesus B, Marinho SP, Barros S, Sousa-Franco A, Alves-Vale C, Carvalho T, Carmo-Fonseca M (2018) Silencing of the lncRNA Zeb2-NAT facilitates reprogramming of aged fibroblasts and safeguards stem cell pluripotency. Nat Commun 9:94. 10.1038/s41467-017-01921-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favate AS, Younger DS (2016) Epidemiology of ischemic stroke. Neurol Clin 34:967–980. 10.1016/j.ncl.2016.06.013 [DOI] [PubMed] [Google Scholar]
- Hankey GJ (2017) Stroke. Lancet 389:641–654. 10.1016/S0140-6736(16)30962-X [DOI] [PubMed] [Google Scholar]
- Hatakeyama M, Ninomiya I, Kanazawa M (2020) Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen Res 15:16–19. 10.4103/1673-5374.264442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari E, Alishahi M, Ghaedrahmati F, Winlow W, Khoshnam SE, Anbiyaiee A (2019) The role of non-coding RNAs in neuroprotection and angiogenesis following ischemic stroke. Metab Brain Dis. 10.1007/s11011-019-00485-2 [DOI] [PubMed] [Google Scholar]
- Jarroux J, Morillon A, Pinskaya M (2017) History, discovery, and classification of lncRNAs. Adv Exp Med Biol 1008:1–46. 10.1007/978-981-10-5203-3_1 [DOI] [PubMed] [Google Scholar]
- Khorkova O, Hsiao J, Wahlestedt C (2015) Basic biology and therapeutic implications of lncRNA. Adv Drug Deliv Rev 87:15–24. 10.1016/j.addr.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang M, Mei Z, Cao W, Yang Y, Wang Y, Wen A (2017) lncRNAs HIF1A-AS2 facilitates the up-regulation of HIF-1alpha by sponging to miR-153-3p, whereby promoting angiogenesis in HUVECs in hypoxia. Biomed Pharm 96:165–172. 10.1016/j.biopha.2017.09.113 [DOI] [PubMed] [Google Scholar]
- Liu D et al (2018) MiR-130a-5p prevents angiotensin II-induced podocyte apoptosis by modulating M-type phospholipase A2 receptor. Cell Cycle 17:2484–2495. 10.1080/15384101.2018.1542901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J et al (2017) Downregulation of the long non-coding RNA Meg3 promotes angiogenesis after ischemic brain injury by activating notch signaling. Mol Neurobiol 54:8179–8190. 10.1007/s12035-016-0270-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschia JF, Brott T (2018) Ischaemic stroke. Eur J Neurol 25:35–40. 10.1111/ene.13409 [DOI] [PubMed] [Google Scholar]
- Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C (2012) Neuroprotection for stroke: current status and future perspectives. Int J Mol Sci 13:11753–11772. 10.3390/ijms130911753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan L, Wang B, ZhuGe Q, Jin K (2015) Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res 1623:166–173. 10.1016/j.brainres.2015.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108 [DOI] [PubMed] [Google Scholar]
- Sheng F et al (2019) Aberrant expression of imprinted lncRNA MEG8 causes trophoblast dysfunction and abortion. J Cell Biochem 120:17378–17390. 10.1002/jcb.29002 [DOI] [PubMed] [Google Scholar]
- Shi FP, Wang XH, Zhang HX, Shang MM, Liu XX, Sun HM, Song YP (2018) MiR-103 regulates the angiogenesis of ischemic stroke rats by targeting vascular endothelial growth factor (VEGF). Iran J Basic Med Sci 21:318–324. 10.22038/IJBMS.2018.27267.6657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Tao S, Liu L, Guo D, Xia Z, Huang M (2016) miR1405p regulates angiogenesis following ischemic stroke by targeting VEGFA. Mol Med Rep 13:4499–4505. 10.3892/mmr.2016.5066 [DOI] [PubMed] [Google Scholar]
- Terashima M, Ishimura A, Wanna-Udom S, Suzuki T (2018) MEG8 long noncoding RNA contributes to epigenetic progression of the epithelial-mesenchymal transition of lung and pancreatic cancer cells. J Biol Chem 293:18016–18030. 10.1074/jbc.RA118.004006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov D et al (2016) Better understanding of phosphoinositide 3-kinase (PI3K) pathways in vasculature: towards precision therapy targeting angiogenesis and tumor blood supply. Biochemistry (Mosc) 81:691–699. 10.1134/S0006297916070051 [DOI] [PubMed] [Google Scholar]
- Wang Y et al (2018) MicroRNA-130a regulates cerebral ischemia-induced blood-brain barrier permeability by targeting Homeobox A5. FASEB J 32:935–944. 10.1096/fj.201700139RRR [DOI] [PubMed] [Google Scholar]
- Xian X, Tang L, Wu C, Huang L (2018) miR-23b-3p and miR-130a-5p affect cell growth, migration and invasion by targeting CB1R via the Wnt/beta-catenin signaling pathway in gastric carcinoma. Onco Targets Ther 11:7503–7512. 10.2147/OTT.S181706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F et al (2014) A novel function of microRNA 130a–3p in hepatic insulin sensitivity and liver steatosis. Diabetes 63:2631–2642. 10.2337/db13-1689 [DOI] [PubMed] [Google Scholar]
- Xiao ZX, Olsen N, Zheng SG (2019) The essential role of costimulatory molecules in systemic lupus erythematosus. Lupus 28:575–582. 10.1177/0961203319829818 [DOI] [PubMed] [Google Scholar]
- Xu CH, Xiao LM, Liu Y, Chen LK, Zheng SY, Zeng EM, Li DH (2019) The lncRNA HOXA11-AS promotes glioma cell growth and metastasis by targeting miR-130a-5p/HMGB2. Eur Rev Med Pharmacol Sci 23:241–252. 10.26355/eurrev_201901_16770BH [DOI] [PubMed] [Google Scholar]
- Yin KJ, Hamblin M, Chen YE (2015) Angiogenesis-regulating microRNAs and ischemic stroke. Curr Vasc Pharmacol 13:352–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Wang J, Xi X, Tan N, Zhang L (2018) SNHG12 promotes angiogenesis following ischemic stroke via regulating miR-150/VEGF pathway. Neuroscience 390:231–240. 10.1016/j.neuroscience.2018.08.029 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Li Z (2015) Angiogenesis. BioMed Res Int 2015:135861. 10.1155/2015/135861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Lin Z, Pang X, Tariq MA, Ao X, Li P, Wang J (2018) Epigenetic regulation of long non-coding RNAs in gastric cancer. Oncotarget 9:19443–19458. 10.18632/oncotarget.23821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZW, Zheng LJ, Ren X, Li AP, Zhou WS (2019) LncRNA NEAT1 facilitates survival and angiogenesis in oxygen-glucose deprivation (OGD)-induced brain microvascular endothelial cells (BMECs) via targeting miR-377 and upregulating SIRT1 VEGFA, and BCL-XL. Brain Res 1707:90–98. 10.1016/j.brainres.2018.10.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.