Abstract
Human immunodeficiency virus-type I (HIV-1) infection elicits antibodies (Abs) directed against several regions of the gp120 and gp41 envelope glycoproteins. Many of these Abs are able to neutralize T-cell-line-adapted strains (TCLA) of HIV-1, but only a few effectively neutralize primary HIV-1 isolates. The nature of HIV-1 neutralization has been carefully studied using human monoclonal Abs (MAbs), and the ability of such MAbs to act in synergy to neutralize HIV-1 has also been extensively studied. However, most synergy studies have been conducted using TCLA strains. To determine the nature of Ab interaction in HIV-1 primary isolate neutralization, a panel of 12 anti-HIV-1 human immunoglobulin G (IgG) MAbs, specific for epitopes in gp120 and gp41, were used. Initial tests showed that six of these MAbs, as well as sCD4, used individually, were able to neutralize the dualtropic primary isolate HIV-189.6; MAbs giving significant neutralization at 2 to 10 μg/ml included 2F5 (anti-gp41), 50-69 (anti-gp41), IgG1b12 (anti-gp120CD4bd), 447-52D (anti-gp120V3), 2G12 (anti-gp120), and 670-D (anti-gp120C5). For studies of reagent interaction, 16 binary combinations of reagents were tested for their ability to neutralize HIV-189.6. Reagent combinations tested included one neutralizing MAb with sCD4, six pairs consisting of two neutralizing MAbs, and nine pairs consisting of one neutralizing MAb with another non-neutralizing MAb. To assess the interaction of the latter type of combination, a new mathematical treatment of reagent interaction was developed since previously used methods could be used only when both reagents neutralize. Synergy was noted between sCD4 and a neutralizing anti-gp120V3 MAb. Antagonism was noted between two pairs of anti-gp41 MAbs (one neutralizing and one non-neutralizing). All of the other 13 pairs of MAbs tested displayed only additive effects. These studies suggest that Abs rarely act in synergy to neutralize primary isolate HIV-189.6; many anti-HIV-1 Abs act additively to mediate this biological function.
Passive immunization has established the role of antibodies (Abs) in the prevention and treatment of many viral infections, including polio, measles, rubella, mumps, varicella-zoster, rabies, and hepatitis A and B (6, 7, 33, 38, 39, 47, 53, 83, 88, 89). Similarly, administration of polyclonal or monoclonal Abs (MAbs) has been shown to prevent the infection of chimpanzees and SCID mice with human immunodeficiency virus type 1 (HIV-1) (27, 28, 32, 82, 85) and of macaques with SHIV (5, 61, 63, 86). Moreover, administration of immunoglobulin (Ig) preparations from the sera of HIV-infected individuals (HIVIG) into HIV-infected patients was associated with reduced p24 antigen (Ag) levels and/or increased CD4+ T-lymphocyte counts (46, 48, 56, 78, 96). Thus, Abs have been shown to have an important role in preventing HIV-1 infection and may participate in some aspects of controlling an established infection.
While the role of non-neutralizing Abs in preventing HIV-1 infection in in vivo models has not been confirmed as it has been in other virus systems (9, 11, 21, 37, 41, 45, 65, 67, 68), neutralizing MAbs (NMAbs) and HIVIG with neutralizing activity have been most frequently used and advocated for passive immunization against HIV-1. However, a major obstacle in developing effective prophylactic or therapeutic passive immunization strategies against HIV-1 is the paucity of Ab preparations that effectively neutralize primary HIV-1 isolates. Only a few MAbs have been shown capable of neutralizing a wide array of primary HIV-1 isolates (25, 35, 93). In addition, while most HIV+ sera have neutralizing activity against many primary isolates, and essentially all primary isolates can be neutralized by at least some sera, when panels of sera from HIV-1-infected individuals are tested against diverse primary isolates, only about 52 to 65% of the serum-virus combinations show neutralization, and both the Ab titers in sera and the levels of neutralization achieved are generally low (54, 70, 71, 76, 98). Furthermore, experiments of passive immunization in animal models suggest that a concentration of Ab inducing 99% neutralization in vitro may be necessary for significant protective effects in vivo (32, 79, 86), although it should be noted that the animal infectious doses used in these experiments far exceed the probable infectious dose to which humans are normally exposed.
These data suggest that, while Abs have potential promise as immunotherapeutic and prophylactic reagents, their use is also fraught with problems. Therefore, the finding that two or more Abs can act in synergy to neutralize virus infectivity suggests that appropriately selected combinations of Abs may be useful in protecting against infection in vivo, can help to delineate mechanisms that protect cells from infection, and may be useful in the design of passive and active immunization strategies. This phenomenon of Ab synergy has been described in the neutralization of many viruses; for example, enhanced neutralization by pairs of Abs has been described for the following viruses: vesicular stomatitis virus (97), West Nile virus (80), Sindbis virus (20), Japanese encephalitis virus (50), La Crosse virus (51), Newcastle disease virus (84), rubella virus (34), respiratory syncytial virus (2), and bovine herpesvirus type 4 (26). These studies suggested that one of the Abs in each pair could increase the binding or increase the avidity of the other Ab, resulting in a greater degree or greater breadth of neutralization.
The cooperativity of pairs of MAbs in neutralizing HIV-1 has been extensively studied over the past several years. Neutralization synergy was detected with a variety of anti-HIV-1 Abs to different gp120 or gp41 epitopes. Synergistic neutralization has been demonstrated most extensively between anti-gp120V3 Abs and anti-gp120CD4bd MAbs or sCD4 (1, 13, 17, 55, 60, 66, 69, 81, 91, 92, 95). In addition, synergy between two anti-gp120CD4bd MAbs or between anti-gp120CD4bd and anti-gp120C5 MAbs has been reported (55). The same effect has also been seen with double or triple combinations of an anti-gp120V2 MAb with an anti-gp120V3 and an anti-gp120CD4bd MAb (58, 95), with a combination of anti-gp120V3, anti-gp41 and anti-gp120 MAbs (58), and with combinations of anti-gp41, anti-gp120 MAbs, and HIVIG (58, 62). Moreover, in an HIV-1 env-mediated cell fusion assay which contrasts with the cell-free virus neutralization assays used by other investigators studying synergistic neutralization of HIV-1, Allaway et al. (1) observed synergistic interactions between the anti-gp41 NMAb 2F5 and anti-gp120CD4bd MAbs, and between anti-gp120V3 and anti-gp120CD4bd MAbs.
Primary isolates are clearly more relevant to human infection than are T-cell-line-adapted (TCLA) strains of HIV-1; however, most synergy studies have been conducted using TCLA strains of HIV-1. Only one group (62) has shown synergy against primary isolates by mixing human MAbs with a polyclonal preparation of IgG. Such an Ab cocktail consists of multiple Ab populations with diverse specificities against a variety of epitopes in gp120 and gp41, making it difficult to dissect the effect observed. The observed effects with such a preparation could derive from the sum of the synergistic, additive, and antagonistic effects of each Ab. To circumvent these ambiguities, combinations of MAbs could be studied, but there are few data concerning the ability of MAb combinations to neutralize primary isolates, a result, in part, of the paucity of human MAbs isolated from HIV-1-infected individuals which are able to neutralize primary isolates. However, it has been shown with other viruses that neutralizing activity can be conferred by combinations of two non-neutralizing MAbs or by one NMAb and one non-NMAb (2, 26, 34, 59). Consequently, a study of HIV-1 neutralization was undertaken using pairs of human MAbs directed against gp120 or gp41, whether of two NMAbs or of one NMAb and one non-NMAb. For this, the dualtropic primary isolate HIV-189.6 was tested with single reagents or binary combinations of 12 human anti-HIV-1 IgG MAbs specific for epitopes in gp120 and gp41.
MATERIALS AND METHODS
Antibodies.
Humans MAbs were used in this study. They included MAbs directed against five regions of gp120: V3 (MAb 447-52D [35]), CD4bd (MAbs IgG1b12 [15, 16] and 654-D [55]), CD4i (MAb 4.8D [72, 90]), C5 (MAbs 450-D [49] and 670-D), and MAb 2G12, directed against a complex gp120 epitope (93). MAbs to three regions of gp41 were also used: MAbs 50-69 and 246-D to cluster I (99), MAbs 98-6 and 1281 to cluster II (99), and MAb 2F5 to an epitope adjacent to cluster II at amino acids 662 to 667 (12). MAbs 2F5 and 2G12 were provided by J. Mascola (Henry M. Jackson Foundation); MAb IgG1b12 was provided by D. Burton. Recombinant sCD4 and MAb 4.8D were obtained from the NIH AIDS Research and Reference Reagent Program. All other MAbs were produced in our laboratory.
Neutralization assay.
The GHOST cell neutralization assay was used throughout (18). GHOST-R5 or GHOST-X4 cells were used as target cells with the dualtropic primary isolate HIV-189.6 or the X4 primary isolate HIV-1MNp (John Sullivan, University of Massachusetts Medical School, Worcester), respectively. The latter isolate was derived from the spleen cells of patient MN and was never cultured in cell lines. The GHOST cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, 1% glutamine, 2% penicillin and streptomycin, and 500 μg of Geneticin, 50 μg of hygromycin, and 1 μg of puromycin/ml. Cell monolayers, when confluent, were resuspended using 0.25% trypsin. The cells were carried for 15 passages and then replaced with fresh cells from stocks frozen at the second or third passage. For the GHOST cell neutralization assay, 6 × 104 GHOST cells/well/0.5 ml were seeded into wells of 24-well tissue culture plates and allowed to grow for 24 h. HIV-1 virus stock was diluted to a predetermined concentration which had been found to result in ∼1,000 infected cells per 15,000 total cells measured cytofluorometrically at the end of the assay. Serial dilutions of sCD4, MAbs, or mixtures (present at a constant ratio in each experiment) were prepared in culture medium, and then equal volumes of appropriately diluted virus and MAbs were mixed and incubated at 37°C for 1 h before being applied to the GHOST cells in the presence of DEAE-dextran at 8 μg/ml. After overnight incubation, the virus-Ab-containing medium was removed, the cell monolayers were washed, and the cells incubated for 3 to 4 days. For harvest, cells were resuspended using 1 mM EDTA, fixed in 2% formaldehyde, and then analyzed using a FACScan flow cytometer (Becton Dickinson). The percent neutralization was calculated using the number of infected cells observed in the absence of Ab as the denominator. The irrelevant human MAb 860-55D specific for parvovirus B19 (3) was used at 10 μg/ml as a negative control in each experiment.
Calculation of neutralization and synergy.
Most of the methods used to analyze and define synergy are based on the analytical method of Chou and Talalay (19). Briefly, this method yields a parameter (the combination index) that describes the interactions among the Abs in a given combination. This method takes into account the potency (median effect dose values or antibody concentration at 50% neutralization [EC50]) and the shape (sigmoidicity) of the dose effect curve based on the median effect equation of Chou and Talalay. This requires that both Abs used in a combination be capable of neutralizing the virus used in the experiment. Because the majority of MAbs used in this study were not able to neutralize primary isolate HIV-189.6 by themselves, the Chou and Talalay method could not be used to analyze the effect of most of the Ab combinations tested. Therefore, as described in the Appendix, we developed a mathematical method to analyze the combination effect of two MAbs based on the comparison of the experimental effect of the combination of two MAbs to the effect predicted under the hypothesis that the two MAbs act neither in synergy nor in antagonism but rather in a statistically independent manner. (See the Appendix for a comparison of our method and other previously used methods.)
We employ the following model for independently acting Abs: a combination (mixture) of two MAbs neutralizes an HIV-1 isolate if at least one of the two does so. However, this need not be the case if the two MAbs act synergistically or if they are antagonistic, e.g., both MAbs may be required to act in some interactive manner to achieve neutralization. It is also assumed, in the experiments described below, that the two MAbs target distinct epitopes on the virus and therefore they are not mutually exclusive in their ability to bind; in particular, their actions involved in neutralization may be, but need not be, statistically independent of each other.
Denoted by p1 is the probability that one of the MAbs, MAb number 1 at dose (concentration) D1, neutralizes HIV-1. This probability is estimated by the proportion (fraction) of cells protected from the HIV-1 infection by this single MAb, i.e., in an experiment using MAb number 1 at dose D1 only. Similarly, let p2 be the probability that MAb number 2 at dose D2 neutralizes. Based on these two probabilities and the assumption of statistical independence, the probability, denoted by p12, that a combination will neutralize, can be calculated as follows: p12 = p1 + p2 − p1p2. In the absence of both synergy and antagonism, the predicted percent neutralization under these conditions is 100 × p12. Note that, for a meaningful comparison, the combination of the two MAbs must consist of a mixture made according to the doses D1 and D2. In most of our experiments D1 = D2; in these cases, the mixture has equal concentrations of each MAb. If there is neither synergy nor antagonism then, except for statistical variation (see details in the Appendix), the difference between the observed and the predicted percent neutralizations should be small. Significant differences between the observed experimental percentage neutralization and the predicted percentage neutralization (for each combination of reagents) were defined by 90% confidence intervals for p12, where the width of these intervals were based on the variation within and between experiments with each MAb.
RESULTS
Neutralization of HIV-189.6 by individual MAbs specific for gp120 or gp41, and by sCD4.
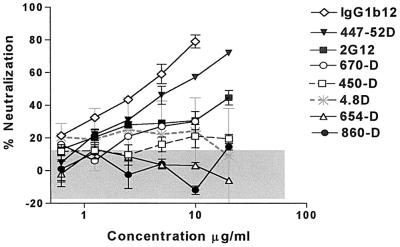
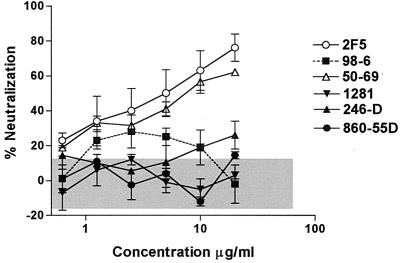
The capacity of a single MAb to neutralize HIV-189.6 is represented in Fig. 1 and 2. Two groups of MAbs were tested: MAbs directed against gp120 (Fig. 1) and MAbs directed against gp41 (Fig. 2). MAb 860-55D, specific for human parvovirus B19, was used as a negative control. This latter Ab was tested in each of 24 experiments at 10 μg/ml, and the percent neutralization it produced was used to calculate the 95% confidence limit of the assay. This value, 14%, was used as the cutoff point below which neutralization was considered nonsignificant.
FIG. 1.
Neutralization of HIV-189.6 by anti-gp120 MAbs. The percent neutralization of HIV-189.6, shown on the y axis, was determined with increasing amounts of anti-gp120 MAbs (x axis) or with an anti-parvovirus MAb, 860-55D, used as a negative control. The GHOST cell neutralization assay was used (18), and the results are represented as the mean of two or three separate experiments for each MAb. Significant neutralization was defined by the 95% confidence limit (shown as the shaded area in gray), established on the basis of 24 assays run with MAb 860-55D and defined in Materials and Methods.
FIG. 2.
Neutralization of HIV-189.6 by anti-gp41 MAbs. The percent neutralization of HIV-189.6, shown on the y axis, was determined with increasing amounts of anti-gp41 MAbs (x axis) or with an anti-parvovirus MAb, 860-55D, used as a negative control. The GHOST cell neutralization assay was used (18), and the results are represented as the mean of two or three separate experiments for each MAb. Significant neutralization was defined by the 95% confidence limit (shown as the shaded area in gray), as described in the legend of Fig. 1.
Among human anti-gp120 MAbs tested, the anti-gp120CD4bd MAb IgG1b12 was the most potent against HIV-189.6, with a 50% effective concentration (EC50) of 3 μg/ml (Fig. 1). The anti-gp120V3 loop MAb 447-52D was also potent, with an EC50 of 6 μg/ml. While MAbs 2G12 and 670-D did not achieve 50% neutralization, they displayed significant neutralizing activity (>14%) against HIV-189.6 at all concentrations tested of >2 μg/ml. The capacity of anti-gp41 MAbs to neutralize the same virus demonstrated that MAbs 2F5 and 50-69 were comparable to MAbs IgG1b12 and 447-52D, with EC50s of 4 and 6 μg/ml, respectively (Fig. 2). The other anti-gp41 MAbs tested in this study did not display significant neutralizing activity against primary isolate HIV-189.6.
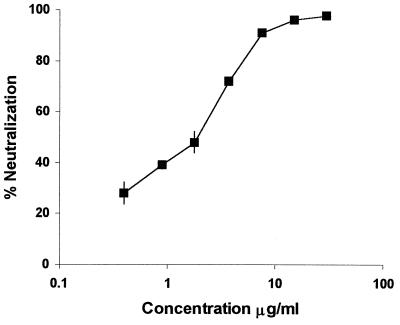
Soluble forms of the HIV-1 receptor CD4 (sCD4) have been shown to block viral infection of TCLA strains (24, 29, 44, 52, 73, 87). However, it was later shown that primary isolates of HIV-1 are more resistant to neutralization by sCD4 (4, 23, 73). When sCD4 was tested for HIV-189.6 neutralization, the EC50 was ∼5 μg/ml (Fig. 3). This result was consistent with previous studies showing an EC50 range of 1 to 10 μg/ml for neutralization of other primary isolates by sCD4 (31).
FIG. 3.
Neutralization of HIV-189.6 by sCD4. The percentage of neutralization of HIV-189.6, shown on the y axis, was determined with increasing amounts of sCD4 (x axis). The GHOST cell neutralization assay was used (18), and the results are represented as the mean of two separate experiments.
HIV-189.6 neutralization by MAb combinations.
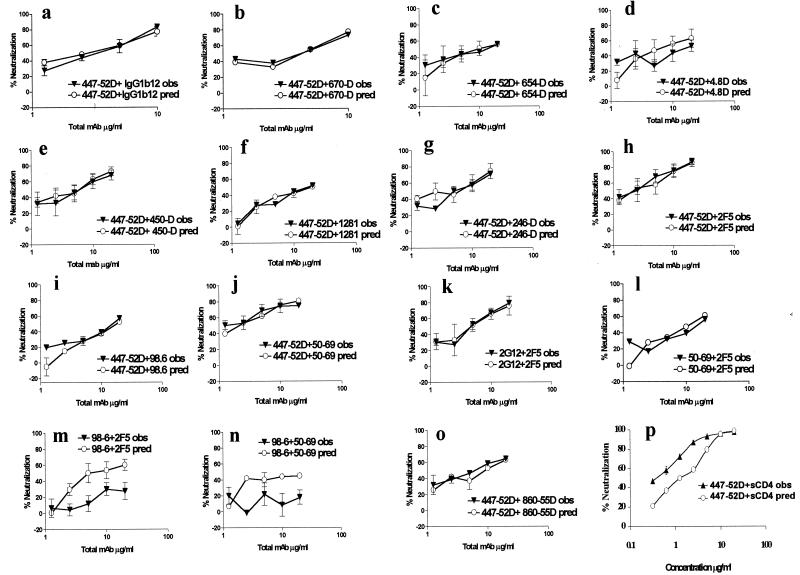
As shown above, only four of the 12 anti-HIV-1 MAbs were able to effect strong neutralization of HIV-189.6. In order to assess the interaction between two non-NMAbs or between one NMAb and one non-NMAb, a new mathematical treatment of reagent interaction was developed, since previous methods could not be used unless both reagents neutralize. Accordingly, the observed percentages of neutralization achieved when a fixed ratio of two MAbs was tested at several concentrations were compared to the percentages of neutralization predicted based on calculations of the level of neutralization that would be achieved if the two MAbs were acting in an additive manner. Significant differences between observed experimental values, and predicted additive effects were defined by the 90% confidence limits based on the variation within and between experiments. Figure 4 shows the observed dose-response neutralization curve (effect “obs”) for each tested combination of MAbs against HIV-189.6 and the predicted additive effect (effect “pred”) for that pair of MAbs.
FIG. 4.
Experimental effects of pairs of MAbs against gp120 and/or gp41 on neutralization of HIV-189.6 compared to the predicted additive effects. The percent neutralization of HIV-189.6 observed (obs, ▾), as well as the predicted additive effects (pred, ○) are shown on the y axis for 16 binary combinations of reagents, as the means of 2 or 4 independent experiments. The total amount of the combined MAbs used in the virus-MAb mixture for the neutralization assay is shown on the x axes.
In order to establish our mathematical model, we introduced in this series of experiments two controls: (i) a negative control, combining the NMAb 447-52D and the irrelevant anti-parvovirus MAb 860-55D, and (ii) a positive control for synergy, combining MAb 447-52D and sCD4. MAbs 447-52D and 860-55D were tested at increasing concentrations with the ratio of MAbs held constant at 1:1 (by weight), while MAb 447-52D and sCD4 were tested at a ratio of 1:3 (by weight), which gave us the best and clearest indication of synergy. As shown in Fig. 4o, at each concentration tested, the percentage of neutralization observed with the combination of MAbs 447-52D and 860-55D was comparable to the percentage of neutralization that would be predicted by calculations based on an additive interaction of these two MAbs. Given that MAb 860-55D has no neutralizing effect on HIV-189.6, the predicted curve for 447-52D and 860-55D is essentially the same as the curve for 447-52D alone. In the positive control experiment, synergy was observed between sCD4 and MAb 447-52D (Fig. 4p), as shown by the degree of neutralization obtained by the combination that was observed, which was significantly higher than the degree of neutralization that would have been predicted based on additive effects of these two reagents.
Neutralization of HIV-189.6 was then evaluated in several experiments in which equimolar amounts of different pairs of anti-HIV-1 MAbs were tested. Thus, 14 different combinations of MAbs, chosen from a panel of 12 human MAbs specific for regions of gp120 and gp41, were assessed. Three types of combinations were tested: those in which the two MAbs were directed against gp120, those in which one MAb was directed against gp120 and one was directed against gp41, and those in which the two MAbs were directed against gp41. These various combinations also allowed the testing of the effect of pairs of NMAbs or of pairs of MAbs in which one was non-neutralizing and one was neutralizing.
Combinations of two anti-gp120 MAbs.
The anti-gp120V3 loop MAb (447–52D) was combined with MAbs directed against gp120 epitopes CD4bd (MAbs IgG1b12 or 654-D), CD4i (MAb 4.8D), or C5 (MAbs 450-D or 670-D). As shown in Fig. 4a to e, the effect observed with all of these combinations was, in each case, similar to the predicted additive effect, whether the combination represented two neutralizing antibodies (e.g., 447-52D and IgG1b12) or one NMAb and one non-NMAb (e.g., 447-52D and 670-D). The difference between the observed effect and the predicted additivity was not significant based on the 90% confidence limit. The same additive effect was observed when 447-52D and IgG1b12 were tested against another primary isolate, HIV-1MNp. Furthermore, the results with HIV-189.6 were similar whether GHOST-R5 or GHOST-X4 cells were used (data not shown).
Combinations of one anti-gp120 MAb and one anti-gp41 MAb.
The envelope of HIV-1 is composed by glycoprotein gp120 noncovalently associated with glycoprotein gp41. In the native envelope of HIV-1, epitopes in gp41 are partially or completely hidden by their association with gp120 or by the structure of these molecules assembled into trimers (8, 75, 77). However, some anti-gp41 MAbs have been shown to neutralize HIV-1, including MAbs 2F5 (25, 94), 98-6 (43), clone 3 (22), and 50-69 (see Fig. 2). Therefore, we examined whether anti-gp120 MAbs could increase the neutralizing activity of anti-gp41 MAbs. As shown in Fig. 4f to k, all six combinations of anti-gp120 and anti-gp41 MAbs tested, whether consisting of two NMAbs (2F5 with 447-52D or 2G12) or of one neutralizing and one non-NMAb (447–52D with 246-D), displayed an observed effect comparable to the predicted additive effect.
Combinations of two anti-gp41 MAbs.
Finally, we examined the effect of combinations of two anti-gp41 MAbs on HIV-1 neutralization. This type of combination has not been examined to date on either TCLA or primary isolates of HIV-1. Three combinations of anti-gp41 MAbs were tested for their ability to neutralize HIV-189.6: the effect observed with the two neutralizing anti-gp41 MAbs, 50-69 and 2F5, was similar to the predicted additive effect (Fig. 4l). These two MAbs recognize distinct epitopes in gp41. Two other anti-gp41 combinations (MAb 98.6 with either 2F5 or 50-69 [Fig. 4m and n]) displayed a significant difference between the observed and the predicted additive effects. For these latter two combinations, the observed effect of each mixture was significantly less than that of the predictive additive effect. Interestingly, antagonism occurred between two MAbs (50-69 and 98-6), which recognize different gp41 epitopes (cluster I and cluster II, respectively [99]) and between two MAbs (98-6 and 2F5) which recognize different epitopes in a contiguous portion of the cluster II region of gp41.
Additivity characterizes the interaction of most MAbs tested.
Table 1 summarizes the results of the 16 combinations of reagents tested for neutralization of HIV-189.6. Of the 16 combinations, 13 demonstrated additivity. Synergy was observed only between sCD4 and an anti-gp120V3 MAb, while antagonism was demonstrated between two combinations of anti-gp41 MAbs, one of which was non-neutralizing for HIV-189.6 (MAb 98-6) while the other member of the pair (either MAb 50-69 or MAb 2F5) could neutralize this virus.
TABLE 1.
Effect of reagent combinations
| Domain | Epitopea | MAb 1 | MAb 2 | Effectb |
|---|---|---|---|---|
| gp120+gp120 | CD4bd + V3 loop | IgG1b12 | 447-52D | ADD |
| CD4bd + V3 loop | 654-D | 447-52D | ADD | |
| CD4i + V3 loop | 4.8D | 447-52D | ADD | |
| C-term + V3 loop | 450-D | 447-52D | ADD | |
| C-term + V3 loop | 670-D | 447-52D | ADD | |
| gp41+gp120 | Cluster I + V3 loop | 50-69 | 447-52D | ADD |
| Cluster I + V3 loop | 246-D | 447-52D | ADD | |
| Cluster II + V3 loop | 2F5 | 447-52D | ADD | |
| Cluster II + V3 loop | 98-6 | 447-52D | ADD | |
| Cluster II + V3 loop | 1281 | 447-52D | ADD | |
| Cluster II + Conf | 2F5 | 2G12 | ADD | |
| gp41+gp41 | Cluster I + II | 50-69 | 2F5 | ADD |
| Cluster I + II | 50-69 | 98-6 | ANT | |
| Cluster II+ II | 98-6 | 2F5 | ANT | |
| Others | Parvovirus + V3 loop | 860 | 447-52D | ADD |
| sCD4 + V3 loop | sCD4 | 447-52D | SYN |
Conf, conformational epitope in gp120; CD4bd, CD4 binding domain.
ADD, additive; ANT, antagonistic; SYN, synergistic.
DISCUSSION
Various combinations of human MAbs have been studied over the past several years which have shown additive, synergistic, or antagonistic effects on the neutralization of HIV-1 (13, 14, 40, 55, 57, 58, 61, 62, 81, 91, 92). In most of these studies, TCLA strains of HIV-1 were used, and most frequently the authors of these reports have used a computer model developed by Chou and Talalay (19) to analyze and define synergy. This method was originally developed to define the interaction of drug combinations and requires that each reagent in the combination display an effect which can be represented by a dose-response curve (see Appendix). This requirement excludes the possibility of analyzing combinations of reagents such as Abs where at least one member of the pair is non-neutralizing, i.e., displays no dose-response curve in the neutralization of the test virus strain. Moreover, this computer model can define an interaction as synergistic when the increase in neutralization observed is due to intra-experimental variation (81).
Since most Abs to the envelope glycoproteins do not neutralize HIV-1 primary isolates, yet are present as a complex polyclonal mixture in HIV+ sera, defining the functional interaction of non-neutralizing Abs and of neutralizing and non-neutralizing Abs could reveal important interactions useful for designing active and passive immunization regimens against HIV-1. To conduct such studies, it was necessary to develop a mathematical method that would be generally applicable for the analysis of the effects of Ab combinations, be they representatives of neutralizing or non-neutralizing Abs. Such a method was developed (see Appendix) and applied here. We demonstrated a synergistic interaction between sCD4 and an anti-gp120V3 loop MAb, 447-52D, confirming other studies showing synergistic effects between sCD4 and anti-gp120V3 MAbs (1, 81). However, additivity characterized the vast majority of MAb interactions, while antagonism was noted in two cases.
As noted above, some combinations of Abs against gp120 have previously been found to have a positive cooperative effect in neutralizing HIV-1; however, our results indicate that synergy appears to be the exception rather than the rule. Thus, all five combinations of anti-gp120 MAbs tested (Fig. 4a to e), whether of two NMAbs or of one NMAb and one non-NMAb, displayed only additive effects in neutralizing the dualtropic primary isolate HIV-189.6. Interestingly, one combination, MAbs 447-52D (anti-gp120V3) and 654-D (anti-gp120CD4bd), had previously been shown to synergize in neutralizing the TCLA HIV-1IIIB strain (55); however, we show here that this combination has only an additive effect against primary isolate HIV-189.6. This result supports the hypothesis that epitope presentation, conformation and/or flexibility of the HIV-1 envelope appear to differ between TCLA and primary HIV-1 isolates (10, 30, 74, 100).
While several anti-gp41 MAbs have displayed neutralizing activity against HIV-1, only one, MAb 2F5, has shown broad activity against primary isolates (22, 25, 43, 94). In this study we report that another anti-gp41 (50–69), specific for cluster I, is able to neutralize primary isolate HIV-189.6. The analysis of the ability of various anti-gp41 MAbs to neutralize this primary isolate in combination with anti-gp120 MAbs (447-52D or 2G12) showed only additive effects (Fig. 4f to k). Similar results were obtained with the TCLA strain of HIV-1IIIB (55). However, Li et al. showed that the combination of an anti-gp120V3 MAb (694/98D) and anti-gp41 MAb (2F5) could display synergy against SHIV-1vpu (expressing the envelope of HIV-1IIIB) (57).
Among the three pairs of anti-gp41 MAbs tested, two displayed antagonism in the neutralization of HIV-189.6. Thus, when MAb 98.6 (which does not neutralize HIV-189.6) was used in combination with MAb 2F5 (Fig. 4m) or with MAb 50-69 (Fig. 4n) (both of which neutralize HIV-189.6 [Fig. 2]), the potency of neutralization was reduced. This is the first time that antagonism has been shown between two anti-gp41 MAbs, although a similar antagonistic effect in HIV-1 neutralization has been previously reported with a pair of anti-gp120 MAbs directed against gp120 (with Abs to gp120V3 and anti-gp120CD4bd [40]). Antagonism between Abs has also been demonstrated in other virus systems (42, 64). Several explanations could explain this phenomenon of antagonism: negative cooperativity between two MAbs could reflect conformational rearrangements induced by one of the Abs, inhibiting subsequent interactions with the neutralizing Ab of the pair. Abs might also interfere with each other's binding by steric hindrance (26); the antagonism between MAbs 98-6 and 2F5 might be an example of this. Thus, Gorny and Zolla-Pazner (36) recently showed that MAbs 98-6 and 2F5 could bind to peptides and peptide complexes representing the prefusogenic and fusogenic forms of gp41. In that study, 98-6 had a higher affinity for the peptide complexes representing the fusogenic form, than did 2F5. Thus, the binding of 98-6, which fails to neutralize HIV-189.6, could interfere with the binding of 2F5, leading to the antagonism noted (Fig. 4m). In contrast, MAbs 50-69 and 2F5 recognize distinct epitopes in gp41 and display independent (additive) reactivity against HIV-189.6.
The data presented in this study suggest that many anti-HIV-1 Abs act additively to neutralize HIV-189.6. While synergy and antagonism have been documented here and elsewhere, it would appear that the predominant phenomenon characterizing Ab interaction is additivity. Given that relatively few anti-HIV-1 Abs characterized to date have broad neutralizing activity and given the extreme polyclonality of the human Ab response to HIV-1, it seems plausible that the additive interactions between Abs may contribute whatever neutralizing activity is observed in HIV+ sera and that antagonism probably does not account for the modest neutralizing activity in these sera. Continued studies of MAb combinations and of MAbs mixed with HIVIG preparations should illuminate the search for reagents useful in passive immunization regimens and in the design of antigens that will elicit the most effective protective Ab responses.
ACKNOWLEDGMENTS
This study was supported in part by grants from the National Institutes of Health (AI 32424, AI 36085, HL 59725, and AI 27742) and by the Research Center for AIDS and HIV Infection.
We acknowledge the provision of antibodies, either directly or indirectly, from Denis Burton, Hermann Katinger, James Robinson, and John Mascola.
Appendix
There are a number of definitions of synergy in the literature. The purpose of this appendix is to give a general but mathematically precise definition that is nevertheless intuitive and comprehensive for nonmathematicians. Synergy is defined by a mathematical definition and specifically in terms of the problem which motivated this study, i.e., the definition of synergy among MAbs in neutralizing the HIV-1 virus. The present approach is contrasted with those of Mascola et al. (62) and Chou and Talalay (19).
Mathematical definition. Let X and Y be random objects and let f be a function with value Z = f(X,Y). Z may be viewed as the response of a system to the simultaneous stimuli X and Y. Let Eind denote the expected value of Z under the hypothesis that X and Y are statistically independent random objects. Then (using discrete variables for illustration), Eind = Σ f(x,y) pX(x) pY(y), where the sum is over all possible values (x,y) and pX(x) is the probability of the event that X = x. Similarly, pY(y) is the probability of the event that Y = y.
pX,Y(x,y) is the probability of the event that simultaneously X = x and Y = y. Let E denote the expected value of Z with no assumptions about the dependence or independence of X and Y: E = Σ f(x,y) pX,Y(x,y), where the sum is again over all possible values of the pair (x,y).
We say that (i) there is synergy between X and Y if E > Eind, (ii) there is independence between X and Y if E = Eind, and (iii) there is antagonism between X and Y if E < Eind.
A “synergy index” (SI) may be defined as the percent excess of E over Eind, i.e., SI = 100(E − Eind)/Eind, whose negative values indicate antagonism.
Synergy between neutralizing MAbs. The intuitive definition of synergy is sometimes stated as the event when the combined action of two agents exceeds the sum of their actions alone. Note that if the “action,” i.e., the desired effect, is measured by the percent or the fraction of the virus that was neutralized, then this intuitive definition is meaningless when both agents neutralize more than half of the viruses. Thus, if we are to calculate the sum of anything, it has to be the amounts of agents used and not their effect. Also, presumably, “more is better,” so any comparisons between combined and separate effects must be such that the individual dosages of two MAbs in the mixture are exactly the same dosages at which they were separately measured for percent neutralization.
Consider the special case of the mathematical definition where X is the characteristic function or indicator (1 or 0, according to occurrence or nonoccurrence) of the event when a virus particle is neutralized by MAb1 and Y is the indicator of the event when a virus is neutralized by MAb2. Let f(x,y) = 1 − (1 − x)(1 − y); then Z = f(X,Y) is the indicator of the event that the virus is neutralized by at least one of the two MAbs.
It follows that Eind = 1 − q1q2, where now Eind becomes the probability that the virus particle is neutralized by at least one of the two antibodies, whereas q1 is the probability that MAb1 alone fails to neutralize and q2 is the probability that MAb2 alone fails to neutralize the virus particle.
In the above, synergy was defined between two random objects, but the definition easily generalizes to any number of objects. For example, if there are four antibodies in a neutralizing experiment, then the probability that at least one of the four succeeds is 1 − q1q2q3q4, provided that we assume mutual independence among the antibodies.
Experimental error. In practice, E is estimated by Z* which is an average of some replications of Z, and Eind is estimated by Eind* = 1 − q1*q2*, where 1 − q1* and 1 − q2* are the fractions of neutralization achieved by each antibody. Independence is rejected in favor of synergy or antagonism if |E* − Eind*|/S exceeds a fractile of a Student t test distribution. Equivalently, independence is rejected if E* is not inside the corresponding confidence interval for Eind. The standard deviation S may be obtained from the relation S2 = S2(E*) + S2(Eind*) because E* and Eind* are based on different experiments that are, of necessity, independent so that the variance of E* − Eind* is the sum of their variances.
Comparison with other methods. We now reintroduce the neutralization model in a more intuitive way and compare it to other methods found in the literature. The probability p of neutralization is defined this way: starting with one or more MAbs at some total concentration (dose) D, a virus particle is introduced, which is then either neutralized or not. Let p = p(D) denote the probability that the virus is neutralized at dose D, so that q = 1 − p is the probability it is not neutralized at that dose. In an actual experiment, a large number (N) of virus particles will be introduced. Then, the percent neutralization achieved will be ca. 100p; the expected number neutralized will be Np and the variance of the number neutralized will be Npq.
The dose is varied as D1,D2, … , Dk, resulting in the dose responses p(D1), … ,p(Dk). One may plot p against D and get a dose-response curve by joining these data points with straight lines. This is repeated for each of two MAbs as well as for a combination, i.e., a mixture of the two.
Both synergy and antagonism and their absence are defined as described above: there is neither synergy nor antagonism in the combination of MAbs if the two events (“MAb1 neutralizes” and “MAb2 neutralizes”) are statistically independent. In this case, the probability Eind that the combination neutralizes is Eind = Prob (at least one of the two MAbs neutralizes) = p1 + p2 − p1p2 = 1 − q1q2, where p1 is the probability that the MAb1 portion of the mix neutralizes and p2 is the probability for MAb2. According to our definition, there is synergy between the MAbs at a fixed concentration D whenever the neutralization probability E of the mixture is greater than what it would be for independently acting MAbs, i.e., if E > Eind. This is not the definition used in the literature of virology, but we will show that this definition nevertheless implies some definitions suggested by both Mascola et al. (62) and Chou and Talalay (19).
We prefer the definition developed here because it does not require the additional assumptions made by these previously cited authors in their description of the dose-response curves associated with the individual and with the combined MAbs. In order to answer our question, whether the two MAbs act synergistically or not, it is not necessary to model the dose-response relationships, which is what the authors in the other studies have done. Rather, we simply measure E and compare it to Eind, making the necessary allowances for statistical error in the measurements.
We now compare and relate our model-free definition of synergy and antagonism to the definitions used by Mascola et al. and by Chou and Talalay. At the risk of excess, we repeat that none of the following dose-response modeling is necessary for our problem. Nevertheless, these models must be examined because of their pervasive use in the literature.
Mascola et al. model the dose-response curve for a single MAb as follows: q(D) = 1/[1 + (D/k)] in the “first order” case. Here, k is a constant obtained by fitting this curve to dose-response data, obviously, q(k) = p(k) = 1/2. These authors mention the general case wherein D/k is replaced by D/k to some power B, and this second constant B is also to be learned from the data. However, these authors seem not to employ the general case because they model the dose-response for a mixture of two MAbs as follows: q12(D1,D2) = 1/[1 + (D1/k1) + (D2/k2)]. Mascola et al. refer to this as the “predicted effect” and assert synergy between the MAbs if the measured effect of the mixture exceeds this predicted effect. Note that this expression contains no product term. In fact, the corresponding odds ratio is as follows: p12/q12 = (D1/k1) + (D2/k2), which is exactly the Chou and Talalay index for the case of mutually exclusive drugs, e.g., for two MAbs having the same target epitope. This is puzzling because Mascola et al. do not suggest that 2F5 and 2G12 (let alone the polyclonal HIVIG in their triple mixture) target the same epitope or are for some other reasons mutually exclusive in their action. We do not need to sort out this complication, because it is not necessary to model the dose-response curve to detect synergy or antagonism.
In the case of distinct target epitopes (such as those recognized by 2F5 and 2G12), our simple setup, together with the model of Mascola et al. for single MAbs, yields by statistical independence of the actions of the two MAbs the following: q12(D1,D2) = 1/[1 + (D1/k1) + (D2/k2) + (D1/k1)(D2/k2)].
The corresponding odds ratio is p12/q12 = (D1/k1) + (D2/k2) + (D1/k1)(D2/k2), which is exactly the Chou and Talalay index for the case of drugs that are not mutually exclusive, e.g., for two MAbs having distinct target epitopes. Thus, we see that there is no contradiction, at least in the “first-order” case, between our statistical but model-free definition of synergy and the more complicated model-based definitions of Mascola et al. and Chou and Talalay.
The theory of Chou and Talalay is based on enzyme kinetics. Its central tenet, the “mass action law” (based on Michaelis-Menten theory) is that the odds ratio curve corresponding to any dose-response curve has the form p12/q12 = [(D1/k1) + (D2/k2) + (D1/k1)(D2/k2)]B; however, the product term is dropped in the case of mutually exclusive drugs. (We ignore this case.)
We now assert that if B is different from 1, then such a dose-response curve cannot describe the statistically independent actions (as in our model-free setup) of two MAbs. This is because the dose-response and odds ratio curves of the individual MAbs also must follow this form, and we know that, as in Mascola et al., q1 = 1/[1 + (D1/k1)B1] and q2 = 1/[1 + (D2/k2)B2]. Thus, q12 = q1q2 = {1/[1 + (D1/k1)B1]}{1/[1 + (D2/k2)B2]} and then the corresponding odds ratio function has the form p12/q12 = (D1/k1)B1 + (D2/k2)B2 + (D1/k1)B1(D2/k2)B2. This form does not agree with that of Chou and Talalay, not even if B1 = B2 = B, unless B = 1.
REFERENCES
- 1.Allaway G P, Ryder A M, Beaudry G A, Maddon P J. Synergistic inhibition of HIV-1 envelope-mediated cell fusion by CD4-based molecules in combination with antibodies to gp120 or gp41. AIDS Res Hum Retrovir. 1993;9:581–587. doi: 10.1089/aid.1993.9.581. [DOI] [PubMed] [Google Scholar]
- 2.Anderson L J, Bingham P, Hierholzer J C. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J Virol. 1988;62:4232–4238. doi: 10.1128/jvi.62.11.4232-4238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakelov S, Gorny M K, Williams C, Riggin C H, Brady F, Collett M S, Zolla-Pazner S. Generation of neutralizing anti-B19 parvovirus human monoclonal antibodies from patients infected with human immunodeficiency virus. J Infect Dis. 1993;168:580–585. doi: 10.1093/infdis/168.3.580. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Smith D H, Marsters S A, Riddle L, Gregory T J, Ho D D, Capon D J. Resistance of primary isolates of human immunodeficiency virus type 1 to soluble CD4 is independent of CD4-rgp120 binding affinity. Proc Natl Acad Sci USA. 1991;88:7056–7060. doi: 10.1073/pnas.88.16.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba T W, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini L A, Posner M R, Katinger H, Stiegler G, Bernacky B J, Rizvi T A, Schmidt R, Hill L R, Keeling M E, Lu Y, Wright J E, Chou T C, Ruprecht R M. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 6.Bahmanyar M, Fayaz A, Nour-Salehi S, Mohammadi M, Koprowski H. Successful protection of humans exposed to rabies infection. Postexposure treatment with the new human diploid cell rabies vaccine and antirabies serum. JAMA. 1976;236:2751–2754. [PubMed] [Google Scholar]
- 7.Beasley R P, Hwang L Y, Stevens C E, Lin C C, Hsieh F J, Wang K Y, Sun T S, Szmuness W. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology. 1983;3:135–141. doi: 10.1002/hep.1840030201. [DOI] [PubMed] [Google Scholar]
- 8.Blacklow S C, Lu M, Kim P S. A trimeric subdomain of the simian immunodeficiency virus envelope glycoprotein. Biochemistry. 1995;34:14955–14962. doi: 10.1021/bi00046a001. [DOI] [PubMed] [Google Scholar]
- 9.Boere W A, Benaissa-Trouw B J, Harmsen M, Kraaijeveld C A, Snippe H. Neutralizing and non-neutralizing monoclonal antibodies to the E2 glycoprotein of Semliki Forest virus can protect mice from lethal encephalitis. J Gen Virol. 1983;64:1405–1408. doi: 10.1099/0022-1317-64-6-1405. [DOI] [PubMed] [Google Scholar]
- 10.Bou-Habib D C, Roderiquez G, Oravecz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandriss M W, Schlesinger J J, Walsh E E, Briselli M. Lethal 17D yellow fever encephalitis in mice. I. Passive protection by monoclonal antibodies to the envelope proteins of 17D yellow fever and dengue 2 viruses. J Gen Virol. 1986;67:229–234. doi: 10.1099/0022-1317-67-2-229. [DOI] [PubMed] [Google Scholar]
- 12.Buchacher A, Predl R, Tauer C, Purtscher M, Gruber G, Heider R, Steindl F, Trkola A, Jungbauer A, Katinger H. Human monoclonal antibodies against gp41 and gp120 as potential agents for passive immunization. Vaccines. 1992;92:191–194. [Google Scholar]
- 13.Buchbinder A, Karwowska S, Gorny M K, Burda S T, Zolla-Pazner S. Synergy between human monoclonal antibodies to HIV extends their effective biologic activity against homologous and divergent strains. AIDS Res Hum Retrovir. 1992;8:425–427. doi: 10.1089/aid.1992.8.425. [DOI] [PubMed] [Google Scholar]
- 14.Burkly L, Mulrey N, Blumenthal R, Dimitrov D S. Synergistic inhibition of human immunodeficiency virus type 1 envelope glycoprotein-mediated cell fusion and infection by an antibody to CD4 domain 2 in combination with anti-gp120 antibodies. J Virol. 1995;69:4267–4273. doi: 10.1128/jvi.69.7.4267-4273.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton D R, Barbas C F, Persson M A, Koenig S, Chanock R M, Lerner R A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci USA. 1991;88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, III Barbas C F. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 17.Cavacini L A, Emes C L, Power J, Buchbinder A, Zolla-Pazner S, Posner M R. Human monoclonal antibodies to the V3 loop of HIV-1/gp120 mediate variable and distinct effects on binding and viral neutralization by a human monoclonal antibody to the CD4 binding site. J AIDS. 1993;6:353–358. [PubMed] [Google Scholar]
- 18.Cecilia D, KewalRamani V N, O'Leary J, Volsky B, Nyambi P N, Burda S, Xu S, Littman D R, Zolla-Pazner S. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J Virol. 1998;72:6988–6996. doi: 10.1128/jvi.72.9.6988-6996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou T C, Talalay T P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:22–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 20.Clegg J C, Chanas A C, Gould E A. Conformational changes in Sindbis virus E1 glycoprotein induced by monoclonal antibody binding. J Gen Virol. 1983;64:1121–1126. doi: 10.1099/0022-1317-64-5-1121. [DOI] [PubMed] [Google Scholar]
- 21.Corbeil S, Seguin C, Trudel M. Involvement of the complement system in the protection of mice from challenge with respiratory syncytial virus Long strain following passive immunization with monoclonal antibody 18A2B2. Vaccine. 1996;14:521–525. doi: 10.1016/0264-410X(95)00222-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotropia J, Ugen K E, Kliks S, Broliden K, Broliden P-A, Hoxie J A, Srikantan V, Williams W V, Weiner D B. A human monoclonal antibody to HIV-1 gp41 with neutralizing activity against diverse laboratory isolates. J AIDS Hum Retrovir. 1996;12:221–232. doi: 10.1097/00042560-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Daar E S, Li X L, Moudgil T, Ho D D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci USA. 1990;87:6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deen K C, Mc Dougal J S, Inacker R, Folena-Wasserman G, Arthos J, Rosenberg J, Maddon P J, Axel R, Sweet R W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988;331:82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- 25.D'Souza M P, Livnat D, Bradac J A, Bridges S H. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. AIDS Clinical Trials Group Antibody Selection Working Group. J Infect Dis. 1997;175:1056–1062. doi: 10.1086/516443. [DOI] [PubMed] [Google Scholar]
- 26.Dubuisson J, Guillaume J, Boulanger D, Thiry E, Bublot M, Pastoret P P. Neutralization of bovine herpesvirus type 4 by pairs of monoclonal antibodies raised against two glycoproteins and identification of antigenic determinants involved in neutralization. J Gen Virol. 1990;71:647–653. doi: 10.1099/0022-1317-71-3-647. [DOI] [PubMed] [Google Scholar]
- 27.Emini E A, Nara P L, Schleif W A, Lewis J A, Davide J P, Lee D R, Kessler J, Conley S, Matsushita S, Putney S D, et al. Antibody-mediated in vitro neutralization of human immunodeficiency virus type 1 abolishes infectivity for chimpanzees. J Virol. 1990;64:3674–3678. doi: 10.1128/jvi.64.8.3674-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D, Matsushita S, Cobb. Jett K E C M, et al. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 29.Fisher R A, Bertonis J M, Meier W, Johnson V A, Costopoulos D S, Liu T, Tizard R, Walker B D, Hirsch M S, Schooley R T. HIV infection is blocked in vitro by recombinant soluble CD4. Nature. 1988;331:76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- 30.Fouts T R, Binley J M, Trkola A, Robinson J E, Moore J P. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J Virol. 1997;71:2779–2785. doi: 10.1128/jvi.71.4.2779-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gauduin M C, Allaway G P, Maddon P J, Barbas C F R, Burton D R, Koup R A. Effective ex vivo neutralization of human immunodefiency virus type 1 in plasma by recombinant immunoglobulin molecules. J Virol. 1996;70:2586–2592. doi: 10.1128/jvi.70.4.2586-2592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauduin M C, Parren P W, Weir R, Barbas C F, Burton D R, Koup R A. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 33.Gellis S S, Mc Guinnes A C, Peters M. Study of prevention of mumps orchitis by gamma globulin. Am J Med Sci. 1945;210:661–664. [Google Scholar]
- 34.Gerna G, Revello M G, Dovis M, Petruzzelli E, Achilli G, Percivalle E, Torsellini M. Synergistic neutralization of rubella virus by monoclonal antibodies to viral haemagglutinin. J Gen Virol. 1987;68:2007–2012. doi: 10.1099/0022-1317-68-7-2007. [DOI] [PubMed] [Google Scholar]
- 35.Gorny M K, Conley A J, Karwowska S, Buchbinder A, Xu J-Y, Emini E A, Koenig S, Zolla-Pazner S. Neutralization of diverse HIV-1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992;66:7538–7542. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorny M K, Zolla-Pazner S. Recognition of free and complexed peptides representing the prefusogenic and fusogenic forms of HIV-1 gp41 by human monoclonal antibodies. J Virol. 2000;74:6186–6192. doi: 10.1128/jvi.74.13.6186-6192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gould E A, Buckley A, Barrett A D, Cammack N. Neutralizing (54K) and non-neutralizing (54K and 48K) monoclonal antibodies against structural and non-structural yellow fever virus proteins confer immunity in mice. J Gen Virol. 1986;67:591–595. doi: 10.1099/0022-1317-67-3-591. [DOI] [PubMed] [Google Scholar]
- 38.Groothius J R, Simoes E A F, Levin M J, Hall C B, Long C E, Rodriguez W J, Arrobio J, Meissner C H, Fulton D R, Welliver R C, Tristram D A, Siber G R, Prince G A, Van Raden M, Hemming V G. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 39.Hammon W M, Coriel L L, Wehrle P F. Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. JAMA. 1953;151:1272–1285. [PubMed] [Google Scholar]
- 40.Hansen J E, Sorensen A M, Olofsson S, Osinaga E, Roseto A. Combination effect on HIV infection in vitro of soluble CD4 and HIV-neutralizing antibodies. Arch Virol. 1994;134:179–184. doi: 10.1007/BF01379116. [DOI] [PubMed] [Google Scholar]
- 41.Harty J T, Chan S P, Contag C H, Plagemann P G. Protection of C58 mice from lactate dehydrogenase-elevating virus-induced motor neuron disease by non-neutralizing antiviral antibodies without interference with virus replication. J Neuroimmunol. 1987;15:195–206. doi: 10.1016/0165-5728(87)90093-2. [DOI] [PubMed] [Google Scholar]
- 42.Heinz F X, Berger R, Tuma W, Kunz C. A topological and functional model of epitopes on the structural glycoprotein of tick-borne encephalitis virus defined by monoclonal antibodies. Virology. 1983;126:525–537. doi: 10.1016/s0042-6822(83)80010-5. [DOI] [PubMed] [Google Scholar]
- 43.Hioe C E, Xu S, Chigurupati P, Burda S, Williams C, Gorny M K, Zolla-Pazner S. Neutralization of HIV-1 primary isolates by polyclonal and monoclonal human antibodies. Int Immunol. 1997;9:1281–1290. doi: 10.1093/intimm/9.9.1281. [DOI] [PubMed] [Google Scholar]
- 44.Hussey R E, Richardson N E, Kowalski M, Brown N R, Chang H C, Siliciano R F, Dorfman T, Walker B, Sodroski J, Reinherz E L. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature. 1988;331:78–81. doi: 10.1038/331078a0. [DOI] [PubMed] [Google Scholar]
- 45.Iacono-Connors L C, Smith J F, Ksiazek T G, Kelley C L, Schmaljohn C S. Characterization of Langat virus antigenic determinants defined by monoclonal antibodies to E, NS1 and preM and identification of a protective, non-neutralizing preM-specific monoclonal antibody. Virus Res. 1996;43:125–136. doi: 10.1016/0168-1702(96)01325-1. [DOI] [PubMed] [Google Scholar]
- 46.Jackson G G, Perkins J T, Rubenis M, Paul D A, Knigge M, Despotes J C, Spencer P. Passive immunoneutralization of human immunodeficiency virus in patients with advanced AIDS. Lancet. 1988;ii:647–652. doi: 10.1016/s0140-6736(88)90468-0. [DOI] [PubMed] [Google Scholar]
- 47.Janeway C A. Use of concentrated human serum gamma-globulin in the prevention and treatment of measles. Bull N Y Acad Med. 1945;21:202–220. [PMC free article] [PubMed] [Google Scholar]
- 48.Karpas A, Hill F, Youle M, Cullen V, Gray J, Byron N, Hayhoe F, Tenant-Flowers M, Howard L, Gilgen D, Oates J K, Hawkins D, Gazzard B. Effects of passive immunization in patients with the acquired immunodeficiency syndrome-related complex and acquired immunodeficiency syndrome. Proc Natl Acad Sci USA. 1988;85:9234–9237. doi: 10.1073/pnas.85.23.9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karwowska S, Gorny M K, Buchbinder A, Gianakakos V, Williams C, Fuerst T, Zolla-Pazner S. Production of human monoclonal antibodies specific for conformational and linear non-V3 epitopes of gp120. AIDS Res Hum Retrovir. 1992;8:1099–1106. doi: 10.1089/aid.1992.8.1099. [DOI] [PubMed] [Google Scholar]
- 50.Kimura-Kuroda J, Yasui K. Topographical analysis of antigenic determinants on envelope glycoprotein V3 (E) of Japanese encephalitis virus, using monoclonal antibodies. J Virol. 1983;45:124–132. doi: 10.1128/jvi.45.1.124-132.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kingsford L. Enhanced neutralization of La Crosse virus by the binding of specific pairs of monoclonal antibodies to the G1 glycoprotein. Virology. 1984;136:265–273. doi: 10.1016/0042-6822(84)90163-6. [DOI] [PubMed] [Google Scholar]
- 52.Klasse P J, McKeating J A. Soluble CD4 and CD4 immunoglobulin-selected HIV-1 variants: a phenotypic characterization. AIDS Res Hum Retrovir. 1993;9:595–604. doi: 10.1089/aid.1993.9.595. [DOI] [PubMed] [Google Scholar]
- 53.Korns R F. Prevention of german measles with immune serum globulin. J Infect Dis. 1952;90:183–189. doi: 10.1093/infdis/90.2.183. [DOI] [PubMed] [Google Scholar]
- 54.Kostrikis L G, Cao Y, Ngai H, Moore J P, Ho D D. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J Virol. 1996;70:445–458. doi: 10.1128/jvi.70.1.445-458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laal S, Burda S, Gorny M K, Karwowska S, Buchbinder A, Zolla-Pazner S. Synergistic neutralization of human immunodeficiency virus type 1 by combinations of human monoclonal antibodies. J Virol. 1994;68:4001–4008. doi: 10.1128/jvi.68.6.4001-4008.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levy J A, Youvan T, Lee M L. Passive hyperimmune plasma therapy in the treatment of acquired immunodeficiency symdrome: results of a 12-month multicenter double-blind controlled trial. Blood. 1994;84:2130–2135. [PubMed] [Google Scholar]
- 57.Li A, Baba T W, Sodroski J, Zolla-Pazner S, Gorny M K, Robinson J, Posner M R, Katinger H, Barbas III C F, Burton D R, Chou T C, Ruprecht R M. Synergistic neutralization of a chimeric SIV/HIV type 1 virus with combinations of human anti-HIV type 1 envelope monoclonal antibodies or hyperimmune globulins. AIDS Res Hum Retrovir. 1997;13:647–656. doi: 10.1089/aid.1997.13.647. [DOI] [PubMed] [Google Scholar]
- 58.Li A, Katinger H, Posner M R, Cavacini L, Zolla-Pazner S, Gorny M K, Sodroski J, Chou T C, Baba T W, Ruprecht R M. Synergistic neutralization of simian-human immunodeficiency virus SHIV-vpu+ by triple and quadruple combinations of human monoclonal antibodies and high-titer anti-human immunodeficiency virus type 1 immunoglobulins. J Virol. 1998;72:3235–3240. doi: 10.1128/jvi.72.4.3235-3240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lussenhop N O, Goertz R, Wabuke-Bunoti M, Gehrz R, Kari B. Epitope analysis of human cytomegalovirus glycoprotein complexes using murine monoclonal antibodies. Virology. 1988;164:362–372. doi: 10.1016/0042-6822(88)90549-1. [DOI] [PubMed] [Google Scholar]
- 60.Maeda Y, Matsushita S, Hattori T, Murakami T, Takatsuki K. Changes in the reactivity and neutralizing activity of a type-specific neutralizing monoclonal antibody induced by interaction of soluble CD4 with gp120. AIDS Res Hum Retrovir. 1992;8:2049–2054. doi: 10.1089/aid.1992.8.2049. [DOI] [PubMed] [Google Scholar]
- 61.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mascola J R, Louder M K, VanCott T C, Sapan C V, Lambert J S, Muenz L R, Bunow B, Birx D L, Robb M L. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J Virol. 1997;71:7198–7206. doi: 10.1128/jvi.71.10.7198-7206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mascola J R, Stiegler G, VanCott T C, Katinger H, Carpenter C B, Hanson C E, Beary H, Hayes D, Frankel S S, Birx D L, Lewis M G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 64.Massey R J, Schochetman G. Viral epitopes and monoclonal antibodies: isolation of blocking antibodies that inhibit virus neutralization. Science. 1981;213:447–449. doi: 10.1126/science.6264601. [DOI] [PubMed] [Google Scholar]
- 65.McCullough K C, Crowther J R, Butcher R N, Carpenter W C, Brocchi E, Capucci L, De Simone F. Immune protection against foot-and-mouth disease virus studied using virus-neutralizing and non-neutralizing concentrations of monoclonal antibodies. Immunology. 1986;58:421–428. [PMC free article] [PubMed] [Google Scholar]
- 66.McKeating J A, Cordell J, Dean C J, Balfe P. Synergistic interaction between ligands binding to the CD4 binding site and V3 domain of human immunodeficiency virus type 1 gp120. Virology. 1992;191:732–742. doi: 10.1016/0042-6822(92)90249-o. [DOI] [PubMed] [Google Scholar]
- 67.McLain L, Dimmock N J. Protection of mice from lethal influenza by adoptive transfer of non-neutralizing haemagglutination-inhibiting IgG obtained from the lungs of infected animals treated with defective interfering virus. J Gen Virol. 1989;70:2615–2624. doi: 10.1099/0022-1317-70-10-2615. [DOI] [PubMed] [Google Scholar]
- 68.Mendoza Q P, Stanley J, Griffin D E. Monoclonal antibodies to the E1 and E2 glycoproteins of Sindbis virus: definition of the epitopes and efficiency of protection from fatal encephalitis. J Gen Virol. 1988;69:3015–3022. doi: 10.1099/0022-1317-69-12-3015. [DOI] [PubMed] [Google Scholar]
- 69.Montefiori D C, S. G B, Zhou J, Bucco R A, Schwartz D H, Cavacini L A, Posner M R. V3-specific neutralizing antibodies in sera from HIV-1 gp160-immunized volunteers block virus fusion and act synergistically with human monoclonal antibody to the conformation-dependent CD4 binding site of gp120. NIH-NIAID AIDS Vaccine Clinical Trials Network. J Clin Investig. 1993;92:840–847. doi: 10.1172/JCI116658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moog C, Fleury H J A, Pellegrin I, Kirn A, Aubertin A M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moore J P, Cao Y, Leu J, Qin L, Korber B, Ho D D. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J Virol. 1996;70:427–444. doi: 10.1128/jvi.70.1.427-444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moore J P, Yoshiyama H, Ho D D, Robinson J E, Sodroski J. Antigenic variation in gp120s from molecular clones of HIV-1 LAI. AIDS Res Hum Retrovir. 1993;9:1185–1193. doi: 10.1089/aid.1993.9.1185. [DOI] [PubMed] [Google Scholar]
- 73.Moore J P, Burkly L C, Connor R I, Cao Y, Tizard R, Ho D D, Fisher R A. Adaptation of two primary human immunodefiency virus type 1 isolates to growth in transformed T cell lines correlates with alterations in the responses of their envelope glycoproteins to soluble CD4. AIDS Res Hum Retrovir. 1993;9:529–539. doi: 10.1089/aid.1993.9.529. [DOI] [PubMed] [Google Scholar]
- 74.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas III C F, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore J P, Sattentau Q, Wyatt R, Sodroski J. Probing the structure of the HIV surface glycoprotein gp120 with a panel of MAbs. J Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nyambi P N, Nkengasong J, Lewi P, Andries K, Janssens W, Fransen K, Heyndrickx L, Piot P, van der Groen G. Multivariate analysis of human immunodeficiency virus type 1 neutralization data. J Virol. 1996;70:6235–6243. doi: 10.1128/jvi.70.9.6235-6243.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nyambi P N, Mbah H A, Burda S, Williams C, Gorny M K, Nadas A, Zolla-Pazner S. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus-type 1 virions of group M. J Virol. 2000;74:7096–7107. doi: 10.1128/jvi.74.15.7096-7107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Osther K, Wiik A, Black F, Skinhoj P, Kellerman M G, Ugen K, Williams W V, Weiner D B. PASSHIV-1 treatment of patients with HIV-1 infection. A preliminary report of a Phase I trial of hyperimmune porcine immunoglobulin to HIV-1. AIDS. 1992;6:1457–1464. doi: 10.1097/00002030-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 79.Parren P W, Gauduin M C, Koup R A, Poignard P, Fisicaro P, Burton D R, Sattentau Q J. Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design. Immunol Lett. 1997;57:105–112. doi: 10.1016/s0165-2478(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 80.Peiris J S, Porterfield J S, Roehrig J T. Monoclonal antibodies against the flavivirus West Nile. J Gen Virol. 1982;58:283–289. doi: 10.1099/0022-1317-58-2-283. [DOI] [PubMed] [Google Scholar]
- 81.Potts B J, Field K G, Wu Y, Posner M, Cavacini L, White-Scharf M. Synergistic inhibition of HIV-1 by CD4 binding domain reagents and V3-directed monoclonal antibodies. Virology. 1993;197:415–419. doi: 10.1006/viro.1993.1604. [DOI] [PubMed] [Google Scholar]
- 82.Prince A M, Reesink H, Pascual D, Horowitz B, Hewlett I, Murthy K K, Cobb K E, Eichberg J W. Prevention of HIV infection by passive immunization with HIV immunoglobulin. AIDS Res Hum Retrovir. 1991;7:971–973. doi: 10.1089/aid.1991.7.971. [DOI] [PubMed] [Google Scholar]
- 83.Ross A H. Modification of chicken pox in family contacts by administration of gamma globulin. N Engl J Med. 1962;267:369–376. doi: 10.1056/NEJM196208232670801. [DOI] [PubMed] [Google Scholar]
- 84.Russel, P. H. 1986. The synergistic neutralization of Newcastle disease virus by two monoclonal antibodies to its haemagglutinin-neuramidase protein. Arch. Virol. 135–144. [DOI] [PubMed]
- 85.Safrit J T, Fung M S, Andrews C A, Braun D G, Sun W N, Chang T W, Koup R A. hu-PBL-SCID mice can be protected from HIV-1 infection by passive transfer of monoclonal antibody to the principal neutralizing determinant of envelope gp120. AIDS. 1993;7:15–21. doi: 10.1097/00002030-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 86.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 87.Smith D H, Byrn R A, Marsters S A, Gregory T, Groopman J E, Capon D J. Blocking of HIV-1 infectivity by soluble, secreted form of the CD4 antigen. Science. 1987;238:1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- 88.Snydman D R, Werner B G, Heinze-Lacey B, Berardi V P, Tilney N L, Kirkman R L, Milford E L, Cho S I, Bush H L, Jr, Levey A S. Use of cytomegalovirus immune globin to prevent cytomegalovirus disease in renal-transplant recipients. N Engl J Med. 1987;317:1049–1054. doi: 10.1056/NEJM198710223171703. [DOI] [PubMed] [Google Scholar]
- 89.Stokes J, Jr, Neefe J R. The prevention and attenuation of infectious hepatitis by gamma globulin. JAMA. 1945;127:144–145. [Google Scholar]
- 90.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved HIV-type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thali M, Furman C, Wahren B, Posner M, Ho D D, Robinson J, Sodroski J. Cooperativity of neutralizing antibodies directed against the V3 and CD4 binding regions of the human immunodeficiency virus gp120 envelope glycoprotein. J Acquir Immune Defic Syndr. 1992;5:591–599. [PubMed] [Google Scholar]
- 92.Tilley S A, Honnen W J, Racho M E, Chou T C, Pinter A. Synergistic neutralization of HIV-1 by human monoclonal antibodies against the V3 loop and the CD4-binding site of gp120. AIDS Res Hum Retrovir. 1992;8:461–467. doi: 10.1089/aid.1992.8.461. [DOI] [PubMed] [Google Scholar]
- 93.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vijh-Warrier S, Pinter A, Honnen W J, Tilley S A. Synergistic neutralization of human immunodeficiency virus type 1 by a chimpanzee monoclonal antibody against the V2 domain of gp120 in combination with monoclonal antibodies against the V3 loop and the CD4-binding site. J Virol. 1996;70:4466–4473. doi: 10.1128/jvi.70.7.4466-4473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vittecoq D, Mattlinger B, Barre-Sinoussi F, Courouce A M, Rouzioux C, Doinel C, Bary M, Viard J P, Bach J F, Rouger P, Lefrere J J. Passive immunotherapy in AIDS: a randomized trial of serial human immunodeficiency virus-positive transfusions of plasma rich in p24 antibodies versus transfusions of seronegative plasma. J Infect Dis. 1992;165:364–368. doi: 10.1093/infdis/165.2.364. [DOI] [PubMed] [Google Scholar]
- 97.Volk W A, Synder R M, Benjamin D C, Wagner R R. Monoclonal antibodies to the glycoprotein of vesicular stomatitis virus: comparative neutralizing activity. J Virol. 1982;42:220–227. doi: 10.1128/jvi.42.1.220-227.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weber J, Fenyo E-M, Beddows S, Kaleebu P, Bjorndal A. Neutralization serotypes of HIV-1 field isolates are not predicted by genetic subtype. The WHO Network for HIV Isolation and Characterization. J Virol. 1996;70:7827–7832. doi: 10.1128/jvi.70.11.7827-7832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu J-Y, Gorny M K, Palker T, Karwowska S, Zolla-Pazner S. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J Virol. 1991;65:4832–4838. doi: 10.1128/jvi.65.9.4832-4838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.York J, Follis K E, Trahey M, Nyambi P N, Zolla-Pazner S, Nunberg J H. Antibody binding and neutralization of primary and T-cell line-adapted isolates of human immunodeficiency virus type1. J Virol. 2001;75:2741–2752. doi: 10.1128/JVI.75.6.2741-2752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]