Abstract
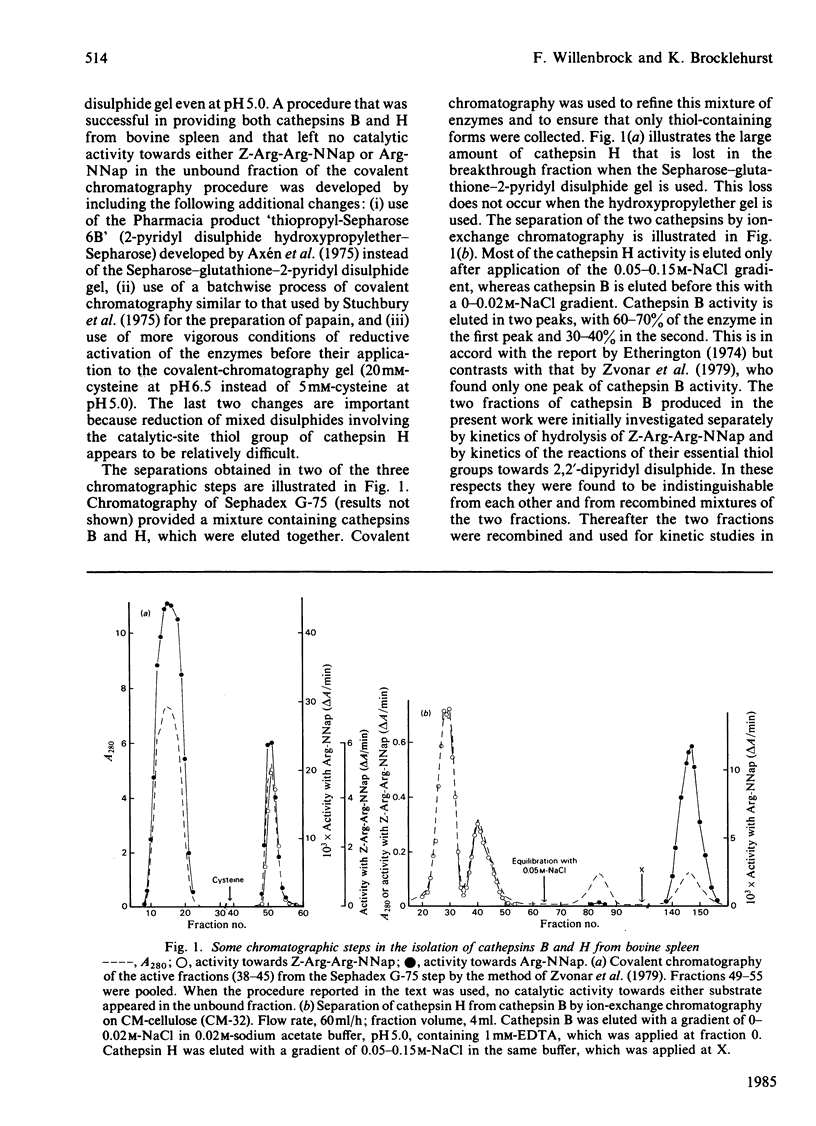
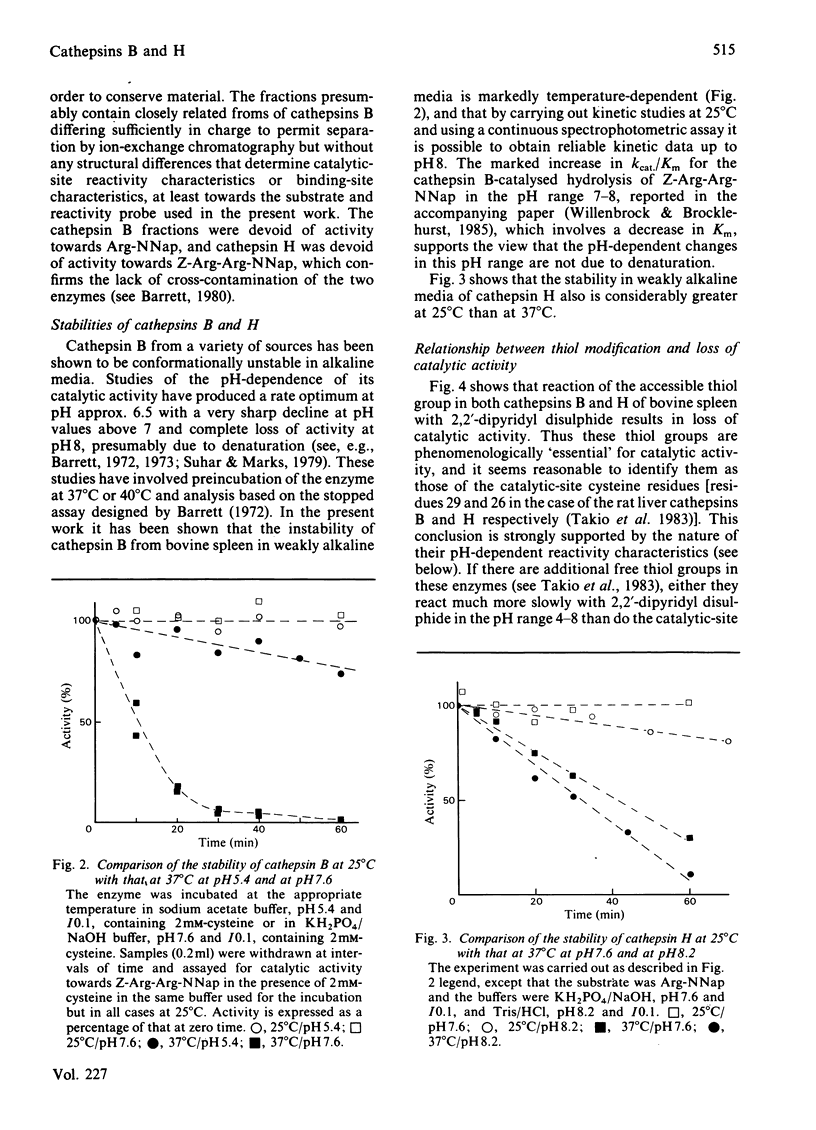
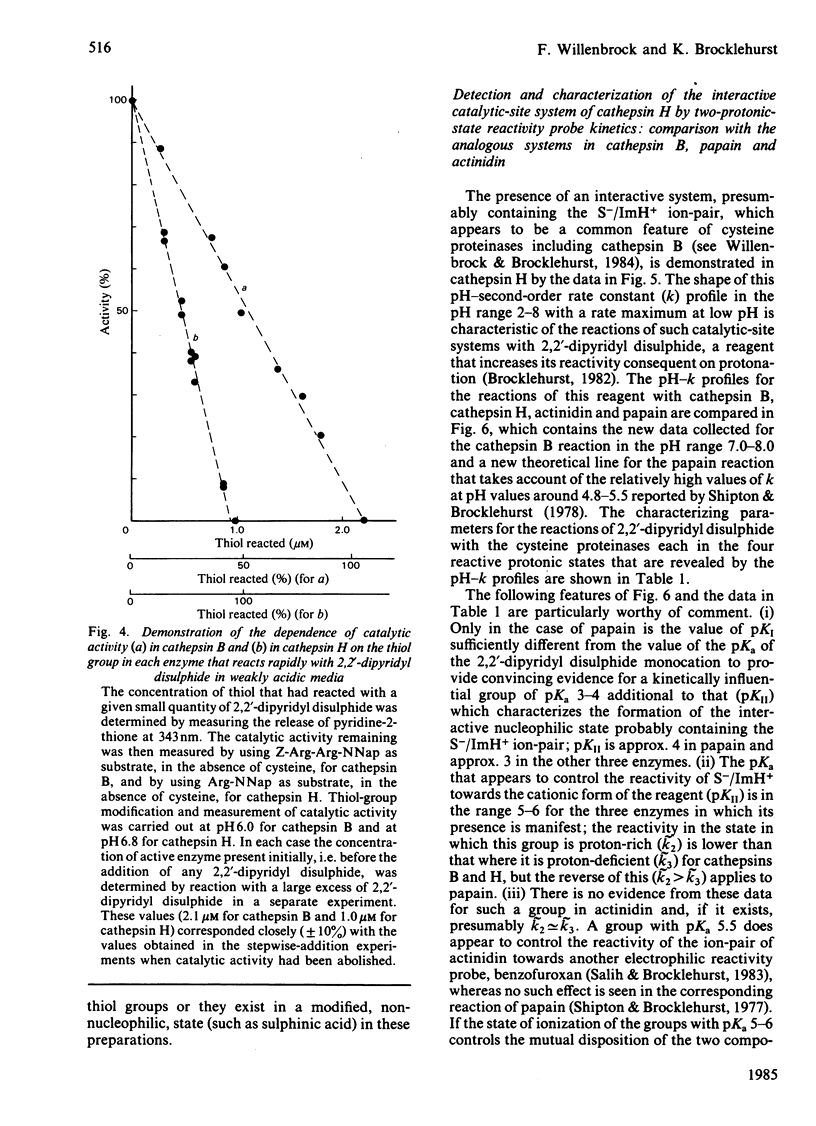
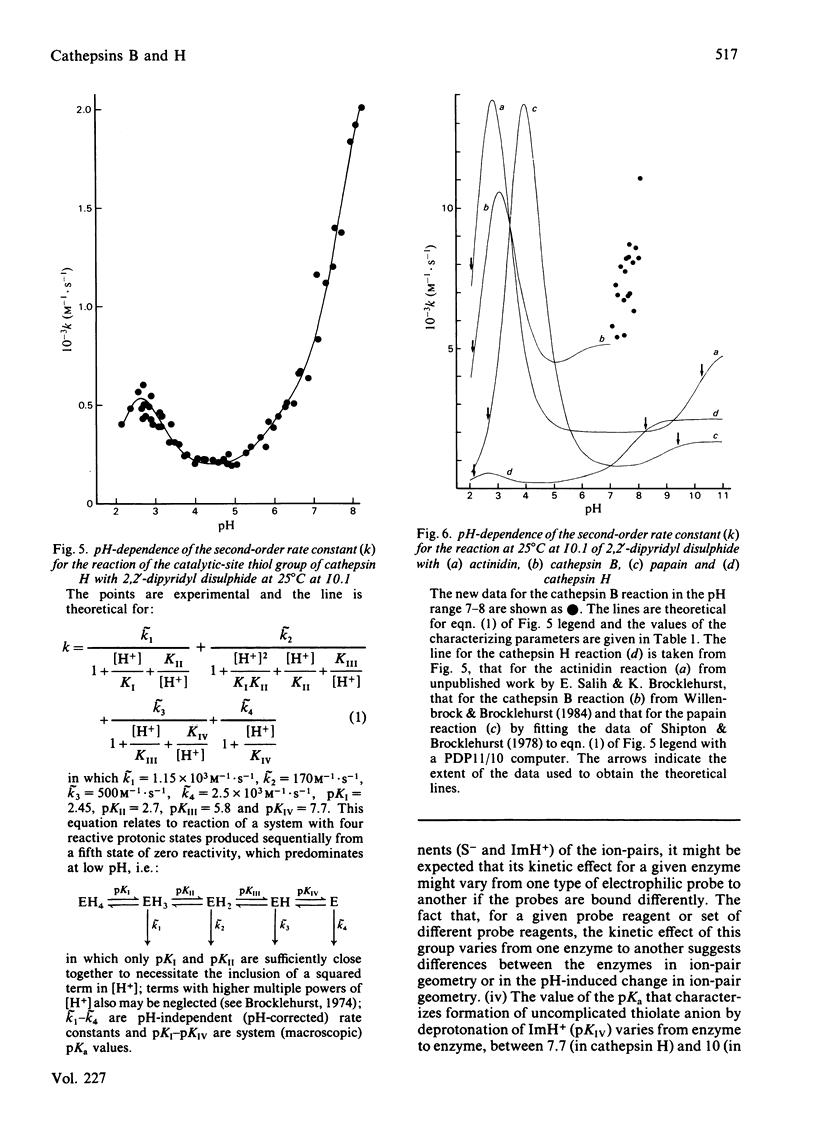
A procedure for the isolation of cathepsin B (EC 3.4.22.1) and of cathepsin H from bovine spleen involving covalent chromatography by thiol-disulphide interchange and ion-exchange chromatography was devised. The stabilities of both cathepsins in alkaline media are markedly temperature-dependent, and reliable kinetic data can be obtained at pH values up to 8 by working at 25 degrees C with a continuous spectrophotometric assay. Both enzyme preparations contain only one type of thiol group as judged by reactivity characteristics towards 2,2'-dipyridyl disulphide at pH values up to 8; in each case this thiol group is essential for catalytic activity. Cathepsin H was characterized by kinetic analysis of the reactions of its thiol group with 2,2'-dipyridyl disulphide in the pH range approx. 2-8 and the analogous study on cathepsin B [Willenbrock & Brocklehurst (1984) Biochem. J. 222, 805-814] was extended to include reaction at pH values up to approx. 8. Cathepsin H, like the other cysteine proteinases, was shown to contain an interactive catalytic-site system in which the nucleophilic character of the sulphur atom is maintained in acidic media. The considerable differences in catalytic site characteristics detected by this two-protonic-state reactivity probe between cathepsin B, cathepsin H, papain (EC 3.4.22.2) and actinidin (EC 3.4.22.14) are discussed. Reaction with 2,2'-dipyridyl disulphide in acidic media, which is known to provide a rapid spectrophotometric active centre titration for many cysteine proteinases, is applicable to cathepsin H. This is useful because other active-centre titrations have proved unsuitable in view of the relatively low reactivity of the thiol group in cathepsin H.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajkowski A. S., Frankfater A. Steady state kinetic evidence for an acyl-enzyme intermediate in reactions catalyzed by bovine spleen cathepsin B. J Biol Chem. 1983 Feb 10;258(3):1645–1649. [PubMed] [Google Scholar]
- Barrett A. J. A new assay for cathepsin B1 and other thiol proteinases. Anal Biochem. 1972 May;47(1):280–293. doi: 10.1016/0003-2697(72)90302-8. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Hanada K. E-64 [L-trans-epoxysuccinyl-leucyl-amido(4-guanidino)butane] and related epoxides as inhibitors of cysteine proteinases. Acta Biol Med Ger. 1981;40(10-11):1513–1517. [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Baines B. S., Malthouse J. P. Differences in the interaction of the catalytic groups of the active centres of actinidin and papain. Rapid purification of fully active actinidin by covalent chromatography and characterization of its active centre by use of two-protonic-state reactivity probes. Biochem J. 1981 Sep 1;197(3):739–746. doi: 10.1042/bj1970739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography. Preparation of fully active papain from dried papaya latex. Biochem J. 1973 Jul;133(3):573–584. doi: 10.1042/bj1330573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. A novel reactivity of papain and a convenient active site titration in the presence of other thiols. FEBS Lett. 1970 Jul 29;9(2):113–116. doi: 10.1016/0014-5793(70)80327-1. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Mushiri S. M., Patel G., Willenbrock F. A marked gradation in active-centre properties in the cysteine proteinases revealed by neutral and anionic reactivity probes. Reactivity characteristics of the thiol groups of actinidin, ficin, papain and papaya peptidase A towards 4,4'-dipyridyl disulphide and 5,5'-dithiobis-(2-nitrobenzoate) dianion. Biochem J. 1983 Mar 1;209(3):873–879. doi: 10.1042/bj2090873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Salih E., Lodwig T. S. Differences between the electric fields of the catalytic sites of papain and actinidin detected by using the thiol-located nitrobenzofurazan label as a spectroscopic reporter group. Biochem J. 1984 Jun 1;220(2):609–612. doi: 10.1042/bj2200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. Two-protonic-state electrophiles as probes of enzyme mechanisms. Methods Enzymol. 1982;87:427–469. doi: 10.1016/s0076-6879(82)87026-2. [DOI] [PubMed] [Google Scholar]
- Etherington D. J. The purification of bovine cathepsin B1 and its mode of action on bovine collagens. Biochem J. 1974 Mar;137(3):547–557. doi: 10.1042/bj1370547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans B., Shaw E. Inactivation of cathepsin B by active site-directed disulfide exchange. Application in covalent affinity chromatography. J Biol Chem. 1983 Sep 10;258(17):10227–10232. [PubMed] [Google Scholar]
- Green G. D., Shaw E. Peptidyl diazomethyl ketones are specific inactivators of thiol proteinases. J Biol Chem. 1981 Feb 25;256(4):1923–1928. [PubMed] [Google Scholar]
- Salih E., Brocklehurst K. Investigation of the catalytic site of actinidin by using benzofuroxan as a reactivity probe with selectivity for the thiolate-imidazolium ion-pair systems of cysteine proteinases. Evidence that the reaction of the ion-pair of actinidin (pKI 3.0, pKII 9.6) is modulated by the state of ionization of a group associated with a molecular pKa of 5.5. Biochem J. 1983 Sep 1;213(3):713–718. doi: 10.1042/bj2130713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz W. N., Barrett A. J. Human cathepsin H. Biochem J. 1980 Nov 1;191(2):487–497. doi: 10.1042/bj1910487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Brochlehurst K. Characterization of the papain active centre by using two-protonic-state electrophiles as reactivity probes. Evidence for nucleophilic reactivity in the un-interrupted cysteine-25-histidine-159 interactive system. Biochem J. 1978 May 1;171(2):385–401. doi: 10.1042/bj1710385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Brocklehurst K. Benzofuroxan as a thiol-specific reactivity probe. Kinetics of its reactions with papain, ficin, bromelain and low-molecular-weight thiols. Biochem J. 1977 Dec 1;167(3):799–810. doi: 10.1042/bj1670799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhar A., Marks N. Purification and properties of brain cathepsin B. Evidence for cleavage of pituitary lipotropins. Eur J Biochem. 1979 Nov 1;101(1):23–20. doi: 10.1111/j.1432-1033.1979.tb04211.x. [DOI] [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Teller D. C., Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. A general framework of cysteine-proteinase mechanism deduced from studies on enzymes with structurally different analogous catalytic-site residues Asp-158 and -161 (papain and actinidin), Gly-196 (cathepsin B) and Asn-165 (cathepsin H). Kinetic studies up to pH 8 of the hydrolysis of N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide catalysed by cathepsin B and of L-arginine 2-naphthylamide catalysed by cathepsin H. Biochem J. 1985 Apr 15;227(2):521–528. doi: 10.1042/bj2270521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Natural structural variation in enzymes as a tool in the study of mechanism exemplified by a comparison of the catalytic-site structure and characteristics of cathepsin B and papain. pH-dependent kinetics of the reactions of cathepsin B from bovine spleen and from rat liver with a thiol-specific two-protonic-state probe (2,2'-dipyridyl disulphide) and with a specific synthetic substrate (N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide). Biochem J. 1984 Sep 15;222(3):805–814. doi: 10.1042/bj2220805. [DOI] [PMC free article] [PubMed] [Google Scholar]


