ABSTRACT
OBJECTIVE:
The objective of this review was to systematically analyze the trials on the effectiveness of perioperative warming in surgical patients.
METHODS:
A systematic review of the literature was undertaken. Clinical trials on perioperative warming were selected according to specific criteria and analyzed to generate summative data expressed as standardized mean difference (SMD).
RESULTS:
Twenty-five studies encompassing 3,599 patients in various surgical disciplines were retrieved from the electronic databases. Nineteen randomized trials on 1785 patients qualified for this review. The no-warming group developed statistically significant hypothermia. In the fixed effect model, the warming group had significantly less pain and lower incidence of wound infection, compared with the no-warming group. In the random effect model, the warming group was also associated with lower risk of post-anesthetic shivering. Both in the random and the fixed effect models, the warming group was associated with significantly less blood loss. However, there was significant heterogeneity among the trials.
CONCLUSION:
Perioperative warming of surgical patients is effective in reducing postoperative wound pain, wound infection and shivering. Systemic warming of the surgical patient is also associated with less perioperative blood loss through preventing hypothermia-induced coagulopathy. Perioperative warming may be given routinely to all patients of various surgical disciplines in order to counteract the consequences of hypothermia.
KEY WORDS: Body temperature regulation; Hypothermia; Blood loss, surgical; Shivering; Wound infection
RESUMO
OBJETIVO:
O objetivo desta revisão é analisar sistematicamente os ensaios sobre a eficácia do aquecimento perioperatório em pacientes cirúrgicos.
MÉTODOS:
Uma revisão sistemática da literatura foi realizada. Ensaios clínicos sobre aquecimento perioperatório foram selecionados segundo critérios específicos e analisados para gerar dados sumativo expresso na diferença média padronizada (standardized mean difference, SMD).
RESULTADOS:
Vinte e cinco estudos englobando 3.599 pacientes de várias disciplinas de cirurgia foram obtidos a partir de bases de dados eletrônicas. Dezenove ensaios aleatórios em 1.785 pacientes qualificados para esta revisão. Nenhum grupo de aquecimento desenvolveu estatisticamente significativa hipotermia. No modelo de efeito fixo, grupo de aquecimento tiveram significativamente menos dor e menor incidência de infecção na ferida quando comparado com o grupo de não-aquecimento. No modelo de efeito aleatório, grupo de aquecimento também foi associado a um menor risco de tremores pós-anestesia. Em ambos os modelos de efeitos fixos e aleatórios, o aquecimento foi significativamente associado com menor perda de sangue. No entanto, houve significativa heterogeneidade entre os ensaios.
CONCLUSÃO:
O aquecimento perioperatório de pacientes cirúrgicos é eficaz na redução da dor pós-operatória ferida, infecção ferida, e tremores. O aquecimento sistêmico do paciente cirúrgico também está associado com menor perda de sangue no perioperatório prevenindo hipotermia e induzindo coagulopatia. O aquecimento perioperatório pode ser administrado rotineiramente a todos os pacientes cirúrgicos de diversas disciplinas, a fim de neutralizar as consequências da hipotermia.
PALAVRAS-CHAVE: Regulação da temperatura corporal, Hipotermia, Perda sanguínea cirúrgica, Tremor por sensação de frio, Infecção dos ferimentos
Introduction
Hypothermia, defined as core temperature below 36 °C1,2,3 is common in operating theaters and has often been disregarded as an inevitable consequence of general anesthesia and surgery.2,4,5 The body’s core temperature is determined by the balance between heat loss and heat gain. Exposure to a cold operating theater environment and anesthetic-induced impairment of thermoregulatory control are two of the commonest contributing factors that tip the balance in favor of heat loss, thereby leading to hypothermia in surgical patients.1,6
Hypothermia confers distinct benefits as well as severe complications in surgical patients. The potential benefits include protection against the deleterious effects of cerebral ischemia and malignant hyperthermia.7 However, hypothermia may increase susceptibility to perioperative wound infection by causing vasoconstriction and impaired immunity. Vasoconstriction decreases the partial pressure of oxygen in tissue, which lowers the resistance to infection.8 The other commonly known adverse effects of hypothermia include shivering,9 prolonged duration of drug action,10 coagulopathy,11 myocardial ischemia and decreased resistance to surgical infections.12 Perioperative warming has been shown to reduce perioperative complications.13,14 Several prophylactic and therapeutic measures have been tried with the aim of reducing or abolishing the development of perioperative hypothermia. Various perioperative warming techniques like simple cotton blankets, carbon-fiber sheets, circulating hot water mattresses, forced air warming, warm fluid infusion and esophageal heat exchange systems9,15,16 are in use in all surgical disciplines. These perioperative warming systems are being used during the preoperative, intraoperative and postoperative phases with variable efficacy. The duration of perioperative warming is also under review and prolonged exposure of surgical patients to warming systems has proven to be quite effective in major elective abdominal surgery.17
The aim of this systematic review was to compare the efficacy of perioperative warming of surgical patients aimed at reducing the consequences of wound infection, coagulopathy, blood loss, postoperative pain and postoperative shivering, in relation to no warming.
Methods
Relevant prospective randomized controlled trials on perioperative warming among surgical patients published between January 1980 and June 2007 were identified through the Medical Literature Analysis and Retrieval System Online (Medline), Excerpta Medica (Embase), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane library and Pubmed databases. The search strategy for target articles was not limited by time, age or gender. However, through frequent and thorough searching, it was noticed that there were no published comparative, non-randomized or randomized trials in the literature before 1980. The terms “randomized trials on perioperative warming”, “trials on perioperative warming” and “warming in surgical patients” were used in combination with the headings “surgical patients”, “forced air warming”, “thermoregulation in anesthetized patients” and “warming blankets”. Relevant articles referenced in these publications were obtained. The “related article” function was also used to widen the search criteria. All abstracts, comparative studies, randomized trials, non-randomized trials and citations that were firstly scanned through were reviewed comprehensively in accordance with the Quality of Reporting of Meta-analyses (QUORUM) template for the literature search. Each article was critically reviewed to assess its eligibility for inclusion or exclusion in this review.
Statistical analysis was performed by a senior statistician, using the Statistics for Windows software in Microsoft Excel 2007®. The methods used were Hedges G statistic for the calculation of standardized mean difference (SMD), the inverse variance method for the fixed effect model and the DerSimonian/Laired method for the random effect model. The estimate of the difference between the two techniques was pooled depending on the effect weights in the results, which were determined by the variance in each trial estimate. Forest plots were used for graphical displays of results from the meta-analysis: the square around the estimate represents the accuracy of the estimation (sample size) and the line represents the 95% confidence interval.
Results
Twenty-five studies encompassing 3,599 patients in various surgical disciplines were retrieved from the electronic databases. Nineteen randomized controlled trials11,12,14,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 on 1,785 patients qualified for this review in accordance with the inclusion criteria (Figure 1). Six trials16,17,33,34,35,36 were excluded for the reasons mentioned in Figure 1. The characteristics of the trials included are given in Table 1. 11,12,14,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32
Figure 1. Quality of Reporting of Meta-Analyses (QUORUM) diagram template used in this review and results from the retrieval of randomized controlled trials (RCT).
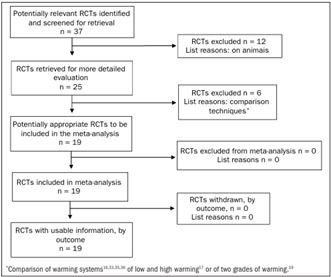
Table 1. Characteristics of included trials.
| Trial | Patients | Warming technique | Type of surgery | Outcome measurements |
|---|---|---|---|---|
| Melling and Leaper18 | 45 | Non-contact radiant heat system | General surgery | Pain and wound infection |
| Kim et al.19 | 40 | Forced warming blanket | Cardiothoracic | Temperature |
| Cavallini et al.20 | 76 | Forced warming blanket | Plastic surgery | Temperature, coagulation |
| Zhao et al.21 | 40 | Forced warming blanket | Abdominal surgery | Temperature, blood loss, shivering, extubation time |
| Scheck et al.22 | 30 | Carbon-fiber warming blanket | Trauma patients | Temperature |
| Xu et al.23 | 40 | Forced warming blanket and fluid warming device | Abdominal surgery | Temperature, blood loss, shivering, extubation time |
| Vanni et al.24 | 30 | NA | Abdominal surgery | Temperature |
| Persson and Lundberg25 | 59 | Forced warming blanket | Gynecological | Temperature, pain |
| Melling et al.14 | 421 | Forced warming blanket | General surgery | Wound infection |
| El-Rahmany et al.26 | 149 | Forced warming blanket | Cardiothoracic | Temperature, cardiovascular vital signs |
| Bock et al.27 | 40 | Forced warming blanket | Abdominal Surgery | Temperature, blood loss, stay, cost, transfusion. |
| Wongprasartsuk et al.28 | 26 | Forced warming blanket | Orthopedic | O2 consumption, CO2 production, pain, temperature |
| Frank et al.12 | 300 | Forced warming blanket | Vascular, thoracic and abdominal | Temperature, ischemic heart disease, cardiac arrest |
| Schmied et al.11 | 60 | Forced warming blanket | Orthopedics | Blood loss, transfusions |
| Kurz et al.29 | 200 | Forced warming blanket | Colorectal surgery | Wound infection, stay |
| Frank et al.30 | 74 | Forced warming blanket | Vascular, thoracic and abdominal | Neuroendocrine response, temperature, blood pressure, pulse rate |
| Frank et al.31 | 100 | NA | Vascular surgery | Temperature, cardiac stress |
| Camus et al.32* | 22 | Forced warming blanket | Abdominal Surgery | Temperature, shivering |
| Camus et al.32† | 33 | Forced warming blanket | Abdominal surgery | Temperature, shivering |
*limb a of trial; †limb b of trial. NA = not available. Heating technique was not mentioned in the trial but this group was definitely provided with perioperative warming.
Methodological quality of studies included
The characteristics of the trials included are explained comprehensively in Table 2 for methodological quality analysis. 11,12,14,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 The Mantel-Haenszel fixed effect model was used to compute robustness and susceptibility to any outlier among these trials. The allocation, concealment and blinding of the investigator or assessor were not clearly reported, and consequently the methodological quality of the trials was considered inadequate and the results from our review may be considered biased. Heterogeneity (clinical and methodological diversity) was seen among all these trials (Chart 1). Limited availability of data on various outcome variables and lack of a major multicenter double blind randomized controlled trial restricted this review with regard to detailed sub-group analysis. However, a subgroup analysis of trials with clearly reported allocation concealment was performed. We felt that performing sensitivity analysis was not relevant due to limited numbers of studies. We attempted to assess publication bias by using funnel plots, but this was difficult to compute due to the small numbers of patients.
Table 2. The randomized controlled trials included, all of them with stated inclusion and exclusion criteria.
| Trial | Baseline comparables | Blinding | Technique of randomization | Allocation concealment | Intention to treat analysis |
|---|---|---|---|---|---|
| Melling and Leaper18 | Stated | Yes | Random number technique | Yes | No |
| Kim et al.19 | Stated | No | Sealed envelopes | Yes | No |
| Cavallini et al.20 | Stated | No | Random assigning | No | No |
| Zhao et al.21 | Stated | No | Not given | No | No |
| Scheck et al.22 | Stated | No | Not given | No | No |
| Xu et al.23 | Stated | No | Not given | No | No |
| Vanni et al.24 | Stated | Yes | Sealed envelopes | Yes | No |
| Melling et al.14 | Stated | No | Sealed envelopes | No | Yes |
| Persson and Lundberg25 | Stated | No | Not given | No | No |
| El-Rahmany et al.26 | Stated | No | Computerized | No | Yes |
| Bock et al.27 | Stated | No | Random assigning | No | Yes |
| Wongprasartsuk et al.28 | Stated | No | Random assigning | Yes | Yes |
| Frank et al.12 | Stated | No | Computerized | No | No |
| Schmied et al.11 | Stated | No | Random assigning | Yes | Yes |
| Kurz et al.29 | Stated | Yes | Not given | No | No |
| Frank et al.30 | Stated | Yes | Computerized | No | No |
| Frank et al.31 | Stated | No | Computerized | No | No |
| Camus et al.32 | Not stated | No | Not given | No | No |
RCT = randomized controlled trial.
Chart 1. Causes of heterogeneity.

Hypothermia
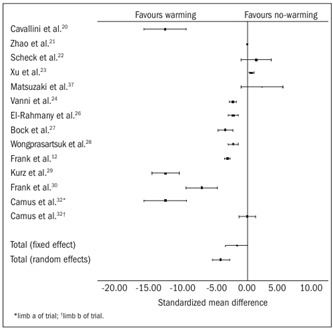
Fourteen trials12,20,21,22,23,24,26,27,28,29,30,31,32,37 contributed towards the combined analysis on the development of hypothermia in the no-warming group. In both the fixed and the random effect models, the no-warming group developed statistically significant hypothermia [fixed effect SMD -1.78, 95% confidence interval, CI (-1.96, -1.61), P = 0.0000, degrees of freedom, df = 13, z = -20.25; and random effect SMD -4.44, 95% CI (-5.92, -2.95), P = 0.0000, df = 13, z = -5.92; Table 3 12,20,21,22,23,24,26,27,28,29,30,32,37 and Figure 2].
Table 3. Temperature changes: combined analysis.
| Warming group | Control group | |
|---|---|---|
| Cavallini et al.20 | 36 ± 0.6 C | 34 ± 1.0 |
| Zhao et al.21 | 36.4 ± 0.4 C | 35.3 ± 0.5 C |
| Scheck et al.22 | 36.4 ± 0.2 C | 34.7 ± 0.6 C |
| Xu et al.23 | 36.4 ± 0.4 C | 35.3 ± 0.5 C |
| Vanni et al.24 | 34.2 ± 1.1 C | 34.1 ± 0.9 C |
| El-Rahmany et al.26 | 34.5 ± 0.1 C | 34.5 ± 0.1 C |
| Bock et al.27 | 36.5 C | 35.5 C |
| Wongprasartsuk et al.28 | 36.9 ± 0.55 C | 36.2 ± 0.87 C |
| Frank et al.12 | 36.7 ± 0.1 C | 35.4 ± 0.1 C |
| Kurz et al.29 | 36.6 ± 0.5 C | 34.7 ± 0.6 C |
| Frank et al.30 | 36.7 ± 0.1 C | 35.3 ± 0.1 C |
| Camus et al.32* | 36.4 ± 0.1 C | 34.6 ± 0.3 C |
| Camus et al.32† | 37.1 ± 0.1 C | 35.1 ± 0.2 C |
| Matsuzaki et al.37 | 36.9 ± 0.3 C | 36.6 ± 0.5 C |
*limb a of trial; †limb b of trial.
Figure 2. Hypothermia: combined analysis of the randomized controlled trials in the review.
Postoperative pain
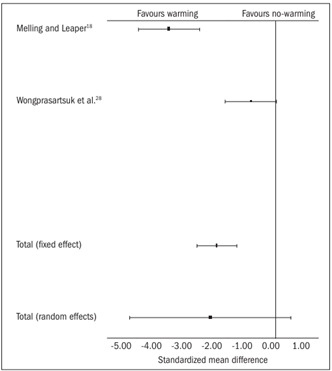
Two trials18,28 contributed towards the combined analysis on postoperative pain. In the fixed effect model, the warming group had significantly less pain [SMD -1.84, 95% CI (-2.45, -1.22), P = 0.0000, df = 1, z = -5.8]. In the random effect model, this difference was not statistically significant between the two groups [SMD -2.0, 95% CI (-4.5, 0.46), P = 0.11, df = 1, z = -1.59; Table 4 18,28 and Figure 3]. However, there was significant heterogeneity among the trials (Q = 16.28, P = 0.001).
Table 4. Postoperative pain: combined analysis.
Figure 3. Postoperative pain: combined analysis of the randomized controlled trials in this review.
Wound infection
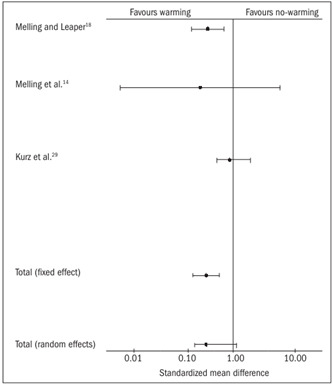
Three trials14,18,29 contributed towards the combined analysis on the postoperative wound infection rate. In the fixed effect model, the warming group was associated with lower risk of developing postoperative wound infection [SMD 0.32, 95% CI (0.18-0.56), P = 0.0001, df = 2, z = -3.99; Table 5 14,18,29 and Figure 4], compared with the no-warming group. There was no heterogeneity among the trials (Q = 0.06, P = 0.96).
Table 5. Wound infection: combined analysis.
Figure 4. Wound infection: combined analysis of the randomized controlled trials in this review.
Shivering
Five trials21,23,24,32 contributed towards the combined analysis on postoperative shivering. In the random effect model, the warming group was associated with lower risk of post-anesthetic shivering [SMD 0.01, 95% CI (0.001-0.08), P = 0.0000, df = 4, z = -4.43; Table 6 21,23,24,32 and Figure 5], compared with the no-warming group. There was no heterogeneity among the trials (Q = 0.082, P = 0.9980).
Table 6. Trials on postoperative shivering: combined analysis.
| Warming group | Control group | |
|---|---|---|
| Zhao et al.21 | 0/20 | 6/20 |
| Xu et al.23 | 0/20 | 6/20 |
| Vanni et al.24 | 0/20 | 5/10 |
| Camus et al.32* | 1/11 | 9/11 |
| Camus et al.32† | 2/22 | 7/22 |
*limb a of trial; †limb b of trial.
Figure 5. Shivering: combined analysis of the randomized controlled trials in this review.

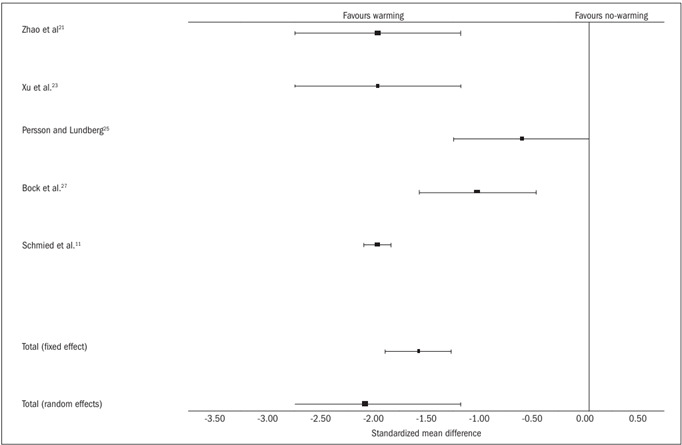
Blood loss
Five trials11,21,23,25,27 contributed towards the combined analysis on perioperative blood loss. Both in the random and in the fixed effect models, the warming group was associated with significantly less blood loss [random effect SMD -1.60, 95% CI (-1.92, -1.29), P = 0.0000, df = 4, z = -9.99; and fixed effect SMD -2.10, 95% CI (-3.31, -0.89), P = 0.0007, df = 4, z = -3.40; Table 7 11,21,23,25,27 and Figure 6]. However, there was significant heterogeneity among the trials (Q = 55.77, P = 0.0000).
Table 7. Trials on blood loss: combined analysis.
Figure 6. Blood loss: combined analysis of the randomized controlled trials in this review.
Myocardial dysfunction, coagulopathy and stress hormone imbalance
There was insufficient data in the trials available to assess hypothermia-induced myocardial dysfunction, coagulopathy and stress hormone imbalance.
Subgroup analysis
In the subgroup analysis, trials with allocation concealment2,11,19,24,28 were analyzed separately. Two trials24,28 contributed towards the calculation of hypothermia. The warming group was at less risk of developing hypothermia, compared with the no-warming group (P = 0.0163). Two trials18,28 contributed towards the calculation of postoperative pain. The warming group was associated with less postoperative wound pain, compared with the non-warming group (P = 0.0001). The combined calculation of perioperative blood loss, wound infection and postoperative shivering could not be performed because of insufficient data quoted in the trials.
Discussion
Patients in various surgical disciplines are exposed to numerous factors that may alter thermoregulatory mechanisms and result in postoperative hypothermia, including a cold operating theater, cold intravenous fluids, cold blood transfusions, cold antiseptic skin preparations and anesthesia.1,38,39 The latter obliterates behavioral responses and inhibits afferent input, thereby lowering the temperature threshold for thermoregulatory responses to hypothermia and preventing efferent responses.40 Some patients are particularly at higher risk of developing hypothermia: the factors involved include surgery lasting for more than two hours, extremes of age, trauma, abdominal surgery, thoracic surgery, massive transfusions of intravenous fluids or blood and massive blood or fluid loss.38,39 Inadvertent perioperative hypothermia prolongs the recovery time and also increases blood loss, surgical site infection and total hospital stay.8,39
Perioperative skin warming has been shown to reduce the initial postinduction hypothermia, intraoperative hypothermia and postoperative shivering, even for procedures lasting for more than three hours.9 Furthermore, a single hour of preoperative skin surface warming has been reported to reduce the rate at which core hypothermia developed during the first hour of anesthesia.33 Our analysis shows that the no-warming group is at significant risk of developing perioperative hypothermia, which in turn can give rise to significant perioperative morbidity.
Perioperative systemic warming, in addition to standard forced warm air intraoperative warming, significantly reduces blood loss and complications in patients.17 These findings corroborate those from the independent studies of Schmied et al.11 and Winkler et al.34 In the latter study on blood loss following total hip arthroplasty, even a small difference in median core intraoperative temperature of 0.5 °C resulted in significantly less blood loss among the patients who were warmed. This excessive blood loss in hypothermic patients is due to hypothermia-induced coagulopathy41,42 that results from impaired platelet aggregation and prolonged bleeding time. Bleeding time depends on several variables, including the number and function of platelets, white and red cell counts, vascular factors, hormones and temperature. Although studies have been widely conducted, the bleeding time test does not strictly correlate with surgical bleeding.41,43 Nonetheless, with standardized techniques and knowledge of the merits and limitations of the bleeding time test, it is useful for diagnosing hemostasis disorders, guiding their therapy and warning of unexpected bleeding complications in surgical patients.44 Stensrud et al.45 evaluated the effects of intraoperative hypothermia on blood transfusion during cardiac surgery. They reported that even though no differences in total blood requirements were reported between patients receiving a normothermic cardiopulmonary bypass and those receiving a hypothermic bypass, the hypothermic patients showed an activated partial thromboplastin time that was prolonged by nearly 8%, compared with patients who were actively warmed. No differences were observed in prothrombin time and fibrinogen concentrations. Our study confirms that perioperative warming can significantly reduce bleeding following surgery and that it may be recommended for regular use.
The risk of wound infection in patients undergoing colonic surgery ranges from 9-27%46 and it may be reduced by two-thirds among patients who receive perioperative warming.8,46 By extending the warming period, to two hours before and after surgery, the incidence of wound infection can be further reduced from 27% to 13% and overall complications can be reduced from 54% to 32%.17 Our review concludes that perioperative warming can significantly reduce the incidence of wound infection.
There was significant heterogeneity among the trials (Chart 1). There may be many reasons for heterogeneity, including combined analysis on trials from various surgical disciplines, combined analysis on trials in which different types of anesthesia (general, spinal or combined epidural and spinal) are used in variable doses and inclusion of trials in which warming was given to different parts of the body. The results from the studies included in this review were also inconsistent. No major multicenter, randomized, controlled trial was reported in the literature. Thus, it was difficult to find high quality, unbiased data for analysis. Nonetheless, this is the only reported systematic review on the role of perioperative warming among surgical patients.
Conclusion
In conclusion, perioperative warming of surgical patients is effective for reducing postoperative wound pain, wound infection and shivering. Systemic warming of surgical patients is also associated with less perioperative blood loss, by preventing hypothermia-induced coagulopathy. Perioperative warming may be given routinely to all patients in various surgical disciplines in order to counteract the consequences of hypothermia.
Department of Colorectal Surgery, Worthing Hospital, Worthing, West Sussex, United Kingdom
Sources of funding: None
References
- 1.Buggy DJ, Crossley AW. Thermoregulation, mild perioperative hypothermia and postanaesthetic shivering. Br J Anaesth. 2000;84(5):615–628. doi: 10.1093/bja/84.5.615. [DOI] [PubMed] [Google Scholar]
- 2.Morley-Forster PK. Unintentional hypothermia in the operating room. Can Anaesth Soc J. 1986;33(4):515–528. doi: 10.1007/BF03010982. [DOI] [PubMed] [Google Scholar]
- 3.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 4.Slotman GJ, Jed EH, Burchard KW. Adverse effects of hypothermia in postoperative patients. Am J Surg. 1985;149(4):495–501. doi: 10.1016/s0002-9610(85)80046-5. [DOI] [PubMed] [Google Scholar]
- 5.Sessler DI. Mild perioperative hypothermia. N Engl J Med. 1997;336(24):1730–1737. doi: 10.1056/NEJM199706123362407. [DOI] [PubMed] [Google Scholar]
- 6.Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95(2):531–543. doi: 10.1097/00000542-200108000-00040. [DOI] [PubMed] [Google Scholar]
- 7.Sessler DI. Consecuencias y prevención de la hipotermia intraoperatoria moderada [Consequences and prevention of mild intraoperative hypothermia] Rev Esp Anestesiol Reanim. 1997;44(2):45–46. [PubMed] [Google Scholar]
- 8.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 9.Just B, Trévien V, Delva E, Lienhart A. Prevention of intraoperative hypothermia by preoperative skin-surface warming. Anesthesiology. 1993;79(2):214–218. doi: 10.1097/00000542-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Heier T, Caldwell JE, Sessler DI, Miller RD. Mild intraoperative hypothermia increase duration of action and spontaneous recovery of vecuronium blockade during nitrous oxide-isoflurane anesthesia in humans. Anesthesiology. 1991;74(5):815–819. doi: 10.1097/00000542-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347(8997):289–292. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- 12.Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997;277(14):1127–1134. [PubMed] [Google Scholar]
- 13.Kurz A, Sessler DI, Narzt E, et al. Postoperative hemodynamic and thermoregulatory consequences of intraoperative core hypothermia. J Clin Anesth. 1995;7(5):359–366. doi: 10.1016/0952-8180(95)00028-g. [DOI] [PubMed] [Google Scholar]
- 14.Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358(9285):876–880. doi: 10.1016/S0140-6736(01)06071-8. [DOI] [PubMed] [Google Scholar]
- 15.Hynson JM, Sessler DI, Moayeri A, McGuire J, Schroeder M. The effects of preinduction warming on temperature and blood pressure during propofol/nitrous oxide anesthesia. Anesthesiology. 1993;79(2):219–228. doi: 10.1097/00000542-199308000-00005. discussion 21A-22A. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen YH, Leikersfeldt G, Drenck NE. Forced-air surface warming versus oesophageal heat exchanger in the prevention of peroperative hypothermia. Acta Anaesthesiol Scand. 1998;42(3):348–352. doi: 10.1111/j.1399-6576.1998.tb04928.x. [DOI] [PubMed] [Google Scholar]
- 17.Wong PF, Kumar S, Bohra A, Whetter D, Leaper DJ. Randomized clinical trial of perioperative systemic warming in major elective abdominal surgery. Br J Surg. 2007;94(4):421–426. doi: 10.1002/bjs.5631. [DOI] [PubMed] [Google Scholar]
- 18.Melling AC, Leaper DJ. The impact of warming on pain and wound healing after hernia surgery: a preliminary study. J Wound Care. 2006;15(3):104–108. doi: 10.12968/jowc.2006.15.3.26879. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Shinn H, Oh YJ, Hong YW, Kwak HJ, Kwak YL. The effect of skin surface warming during anesthesia preparation on preventing redistribution hypothermia in the early operative period of off-pump coronary artery bypass surgery. Eur J Cardiothorac Surg. 2006;29(3):343–347. doi: 10.1016/j.ejcts.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Cavallini M, Baruffaldi Preis FW, Casati A. Effects of mild hypothermia on blood coagulation in patients undergoing elective plastic surgery. Plast Reconstr Surg. 2005;116(1):316–321. doi: 10.1097/01.prs.0000170798.45679.7a. discussion 322-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Luo AL, Xu L, Huang YG. Forced-air warming and fluid warming minimize core hypothermia during abdominal surgery. Chin Med Sci J. 2005;20(4):261–264. [PubMed] [Google Scholar]
- 22.Scheck T, Kober A, Bertalanffy P, et al. Active warming of critically ill trauma patients during intrahospital transfer: a prospective, randomized trial. Wien Klin Wochenschr. 2004;116(3):94–97. doi: 10.1007/BF03040703. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, Zhao J, Huang YG, Luo AL. [The effect of intraoperative warming on patient core temperature] Zhonghua Wai Ke Za Zhi. 2004;42(16):1010–1013. [PubMed] [Google Scholar]
- 24.Vanni SM, Braz JR, Módolo NS, Amorim RB, Rodrigues GR., Jr Preoperative combined with intraoperative skin-surface warming avoids hypothermia caused by general anaesthesia and surgery. J Clin Anesth. 2003;15(2):119–125. doi: 10.1016/s0952-8180(02)00512-3. [DOI] [PubMed] [Google Scholar]
- 25.Persson K, Lundberg J. Perioperative hypothermia and postoperative opioid requirements. Eur J Anaesthesiol. 2001;18(10):679–686. doi: 10.1046/j.1365-2346.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 26.El-Rahmany HK, Frank SM, Schneider GM, et al. Forced-air warming decreases vasodilator requirement after coronary artery bypass surgery. Anesth Analg. 2000;90(2):286–291. doi: 10.1097/00000539-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Bock M, Müller J, Bach A, Böhrer H, Martin E, Motsch J. Effects of preinduction and intraoperative warming during major laparotomy. Br J Anaesth. 1998;80(2):159–163. doi: 10.1093/bja/80.2.159. [DOI] [PubMed] [Google Scholar]
- 28.Wongprasartsuk P, Konstantatos A, McRae R. The effect of forced air warming on postoperative oxygen consumption and temperature in elective orthopaedic surgery. Anaesth Intensive Care. 1998;26(3):267–271. doi: 10.1177/0310057X9802600306. [DOI] [PubMed] [Google Scholar]
- 29.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 30.Frank SM, Higgins MS, Breslow MJ, et al. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. A randomized clinical trial. Anesthesiology. 1995;82(1):83–93. doi: 10.1097/00000542-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Frank SM, Beattie C, Christopherson R, et al. Unintentional hypothermia is associated with postoperative myocardial ischemia. The Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology. 1993;78(3):468–476. doi: 10.1097/00000542-199303000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Camus Y, Delva E, Just B, Lienhart A. Leg warming minimizes core hypothermia during abdominal surgery. Anesth Analg. 1993;77(5):995–999. doi: 10.1213/00000539-199311000-00021. [DOI] [PubMed] [Google Scholar]
- 33.Grocott HP, Mathew JP, Carver EH, et al. A randomized controlled trial of Arctic Sun Temperature Management System versus conventional methods for preventing hypothermia during off-pump cardiac surgery. Anesth Analg. 2004;98(2):298–302. doi: 10.1213/01.ANE.0000096242.06561.C0. [DOI] [PubMed] [Google Scholar]
- 34.Winkler M, Akça O, Birkenberg B, et al. Aggressive warming reduces blood loss during hip arthroplasty. Anesth Analg. 2000;91(4):978–984. doi: 10.1097/00000539-200010000-00039. [DOI] [PubMed] [Google Scholar]
- 35.Leben J, Tryba M, Kurz-Müller K, Schregel W. Prävention intraoperativer Hypothermie bei Kindern [Prevention of intraoperative hypothermia in children] Anaesthesist. 1998;47(6):475–478. doi: 10.1007/s001010050585. [DOI] [PubMed] [Google Scholar]
- 36.Hofer CK, Worn M, Tavakoli R, et al. Influence of body core temperature on blood loss and transfusion requirements during off-pump coronary artery bypass grafting: a comparison of 3 warming systems. J Thorac Cardiovasc Surg. 2005;129(4):838–843. doi: 10.1016/j.jtcvs.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Matsuzaki Y, Matsukawa T, Ohki K, Yamamoto Y, Nakamura M, Oshibuchi T. Warming by resistive heating maintains perioperative normothermia as well as forced air heating. Br J Anaesth. 2003;90(5):689–691. doi: 10.1093/bja/aeg106. [DOI] [PubMed] [Google Scholar]
- 38.Macario A, Dexter F. What are the most important risk factors for a patient’s developing intraoperative hypothermia? Anesth Analg. 2002;94(1):215–220. doi: 10.1097/00000539-200201000-00042. table of contents. [DOI] [PubMed] [Google Scholar]
- 39.Hildebrand F, Giannoudis PV, van Griensven M, Chawda M, Pape HC. Pathophysiologic changes and effects of hypothermia on outcome in elective surgery and trauma patients. Am J Surg. 2004;187(3):363–371. doi: 10.1016/j.amjsurg.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Sessler DI. Perioperative heat balance. Anesthesiology. 2000;92(2):578–596. doi: 10.1097/00000542-200002000-00042. [DOI] [PubMed] [Google Scholar]
- 41.Krause KR, Howells GA, Buhs CL, et al. Hypothermia-induced coagulopathy during hemorrhagic shock. Am Surg. 2000;66(4):348–354. [PubMed] [Google Scholar]
- 42.Lynn M, Jeroukhimov I, Klein Y, Martinowitz U. Updates in the management of severe coagulopathy in trauma patients. Intensive Care Med. 2002;28(Suppl 2):S241–S247. doi: 10.1007/s00134-002-1471-7. [DOI] [PubMed] [Google Scholar]
- 43.Valeri CR, MacGregor H, Cassidy G, Tinney R, Pompei F. Effects of temperature on bleeding time and clotting time in normal male and female volunteers. Crit Care Med. 1995;23(4):698–704. doi: 10.1097/00003246-199504000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Wolberg AS, Meng ZH, Monroe DM 3rd, Hoffman M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004;56(6):1221–1228. doi: 10.1097/01.ta.0000064328.97941.fc. [DOI] [PubMed] [Google Scholar]
- 45.Stensrud PE, Nuttall GA, de Castro MA, et al. A prospective, randomized study of cardiopulmonary bypass temperature and blood transfusion. Ann Thorac Surg. 1999;67(3):711–715. doi: 10.1016/s0003-4975(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 46.Greif R, Akça O, Horn EP, Kurz A, Sessler DI. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. Outcomes Research Group. N Engl J Med. 2000;342(3):161–167. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]


