Abstract
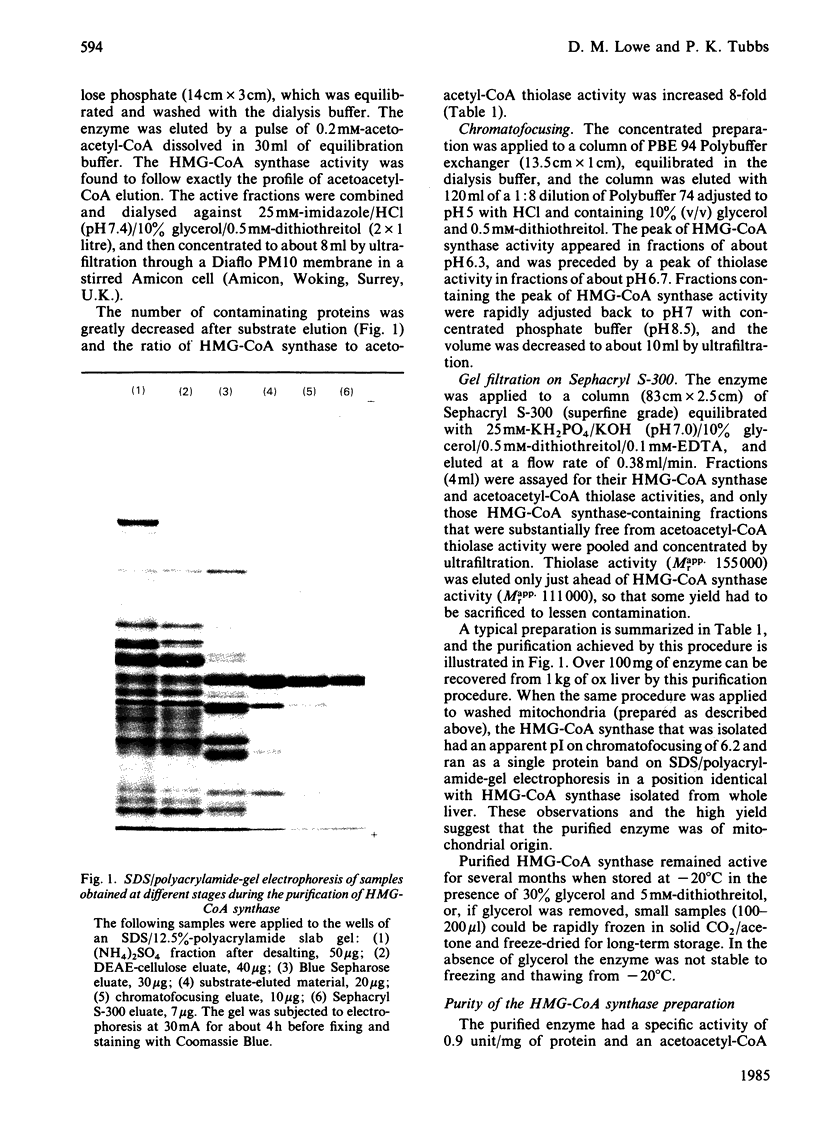
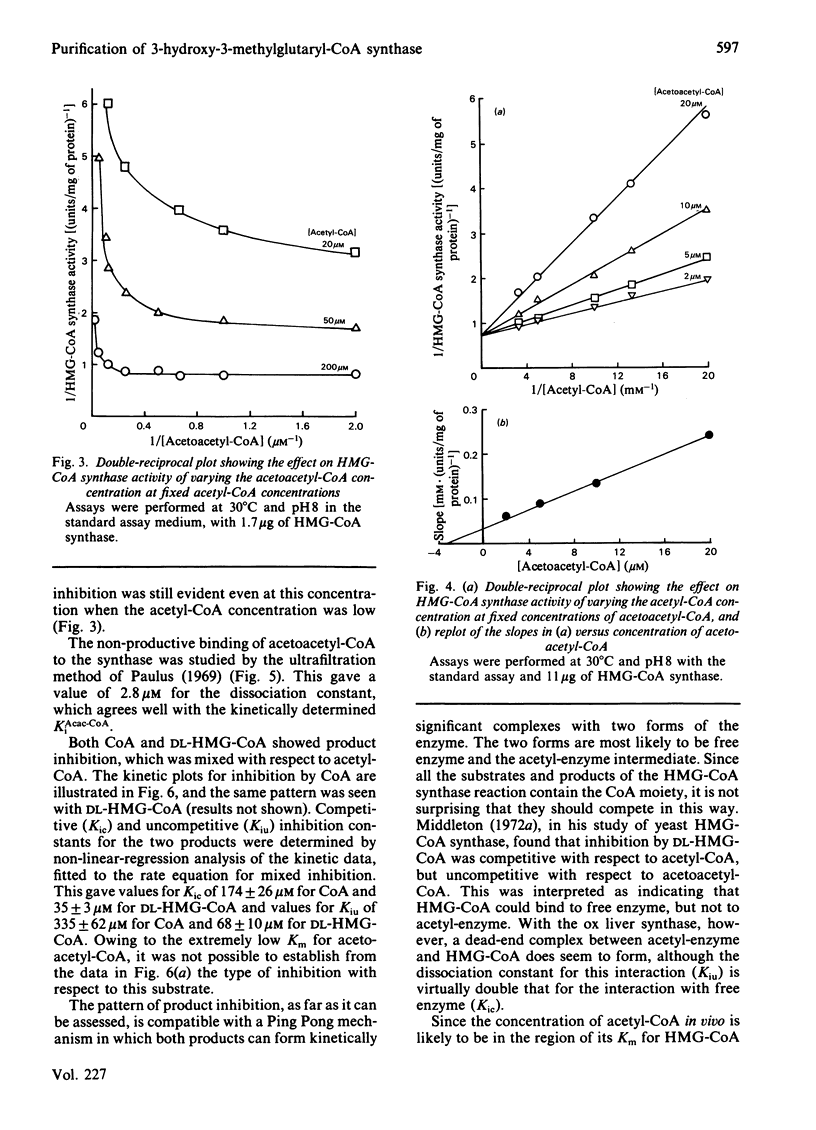
Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase (EC 4.1.3.5) was purified to homogeneity from ox liver and obtained essentially free from acetoacetyl-CoA thiolase activity. The purification procedure included substrate elution from cellulose phosphate and chromatofocusing. The relative molecular mas was about 100 000 and S20,w0 was 6.36S. The enzyme appears to be a dimer of identical subunits (Mr 47 900). The Km for acetoacetyl-CoA is extremely low (less than 0.5 microM), and acetoacetyl-CoA (Acac-CoA) gives marked substrate inhibition (KiAcac-CoA = 3.5 microM) that is competitive with respect to acetyl-CoA. Both CoA and DL-3-hydroxy-3-methylglutaryl-CoA give mixed product inhibition with respect to acetyl-CoA, which is compatible with a Ping Pong mechanism in which both products can form kinetically significant complexes with two forms of the enzyme. The two forms are most likely to be free enzyme and an acetyl-enzyme intermediate.
Full text
PDF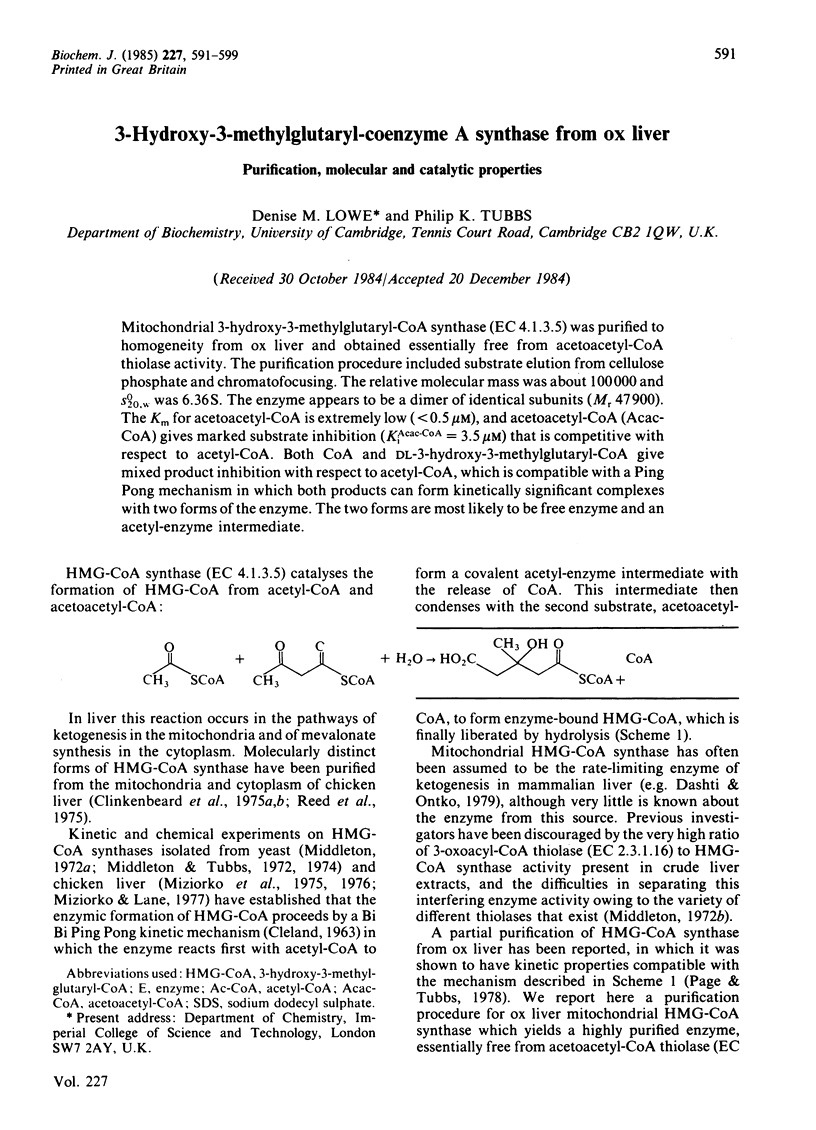






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aragón J. J., Lowenstein J. M. A survey of enzymes which generate or use acetoacetyl thioesters in rat liver. J Biol Chem. 1983 Apr 25;258(8):4725–4733. [PubMed] [Google Scholar]
- Baird G. D., Hibbitt K. G., Lee J. Enzymes involved in acetoacetate formation in various bovine tissues. Biochem J. 1970 May;117(4):703–709. doi: 10.1042/bj1170703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Clinkenbeard K. D., Reed W. D., Mooney R. A., Lane M. D. Intracellular localization of the 3-hydroxy-3-methylglutaryl coenzme A cycle enzymes in liver. Separate cytoplasmic and mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A generating systems for cholesterogenesis and ketogenesis. J Biol Chem. 1975 Apr 25;250(8):3108–3116. [PubMed] [Google Scholar]
- Corkey B. E., Brandt M., Williams R. J., Williamson J. R. Assay of short-chain acyl coenzyme A intermediates in tissue extracts by high-pressure liquid chromatography. Anal Biochem. 1981 Nov 15;118(1):30–41. doi: 10.1016/0003-2697(81)90152-4. [DOI] [PubMed] [Google Scholar]
- Dashti N., Ontko J. A. Rate-limiting function of 3-hydroxy-3-methylglutaryl-coenzyme A synthase in ketogenesis. Biochem Med. 1979 Dec;22(3):365–374. doi: 10.1016/0006-2944(79)90024-3. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Gehring U., Riepertinger C., Lynen F. Reinigung und Kristallisation der Thiolase, Untersuchungen zum Wirkungsmechanismus. Eur J Biochem. 1968 Nov;6(2):264–280. doi: 10.1111/j.1432-1033.1968.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Greville G. D., Tubbs P. K. The catabolism of long chain fatty acids in mammalian tissues. Essays Biochem. 1968;4:155–212. [PubMed] [Google Scholar]
- Ishitani K., Niitsu Y., Listowsky I. Characterization of the different polypeptide components and analysis of subunit assembly in ferritin. J Biol Chem. 1975 Apr 25;250(8):3124–3128. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowe D. M., Tubbs P. K. 3-Hydroxy-3-methylglutaryl-coenzyme A synthase from ox liver. Properties of its acetyl derivative. Biochem J. 1985 Apr 15;227(2):601–607. doi: 10.1042/bj2270601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menahan L. A., Hron W. T., Hinkelman D. G., Miziorko H. M. Interrelationships between 3-hydroxy-3-methylglutaryl-CoA synthase, acetoacetyl-CoA and ketogenesis. Eur J Biochem. 1981 Oct;119(2):287–294. doi: 10.1111/j.1432-1033.1981.tb05606.x. [DOI] [PubMed] [Google Scholar]
- Middleton B. The existence of ketoacyl-CoA thiolases of differing properties and intracellular localization in ox liver. Biochem Biophys Res Commun. 1972 Jan 31;46(2):508–515. doi: 10.1016/s0006-291x(72)80168-2. [DOI] [PubMed] [Google Scholar]
- Middleton B. The kinetic mechanism of 3-hydroxy-3-methylglutaryl-coenzyme A synthase from baker's yeast. Biochem J. 1972 Jan;126(1):35–47. doi: 10.1042/bj1260035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton B., Tubbs P. K. The purification and some properties of 3-hydroxy-3-methylglutaryl-coenzyme A synthase from Baker's yeast. Biochem J. 1972 Jan;126(1):27–34. doi: 10.1042/bj1260027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miziorko H. M., Clinkenbeard K. D., Reed W. D., Lane M. D. 3-Hydroxy-3-methylglutaryl coenzyme A synthase. Evidence for an acetyl-S-enzyme intermediate and identification of a cysteinyl sulfhydryl as the site of acetylation. J Biol Chem. 1975 Aug 10;250(15):5768–5773. [PubMed] [Google Scholar]
- Miziorko H. M., Lane M. D. 3-Hydroxy-3-methylgutaryl-CoA synthase. Participation of acetyl-S-enzyme and enzyme-S-hydroxymethylgutaryl-SCoA intermediates in the reaction. J Biol Chem. 1977 Feb 25;252(4):1414–1420. [PubMed] [Google Scholar]
- Miziorko H. M., Shortle D., Lane M. D. Trapping of a novel coenzyme A containing intermediate of 3-hydroxy-3-methylglutaryl-CoA synthase. Biochem Biophys Res Commun. 1976 Mar 8;69(1):92–98. doi: 10.1016/s0006-291x(76)80277-x. [DOI] [PubMed] [Google Scholar]
- Page M. A., Tubbs P. K. Some properties of 3-hydroxy-3-methylglutaryl-coenzyme A synthase from ox liver. Biochem J. 1978 Sep 1;173(3):925–928. doi: 10.1042/bj1730925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus H. A rapid and sensitive method for measuring the binding of radioactive ligands to proteins. Anal Biochem. 1969 Oct 15;32(1):91–100. doi: 10.1016/0003-2697(69)90107-9. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Reed W. D., Clinkenbeard D., Lane M. D. Molecular and catalytic properties of mitochondrial (ketogenic) 3-hydroxy-3-methylglutaryl coenzyme A synthase of liver. J Biol Chem. 1975 Apr 25;250(8):3117–3123. [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Wieland O. H. Distribution of metabolites between the cytosolic and mitochondrial compartments of hepatocytes isolated from fed rats. Hoppe Seylers Z Physiol Chem. 1978 Jul;359(7):785–798. doi: 10.1515/bchm2.1978.359.2.785. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]




