Abstract
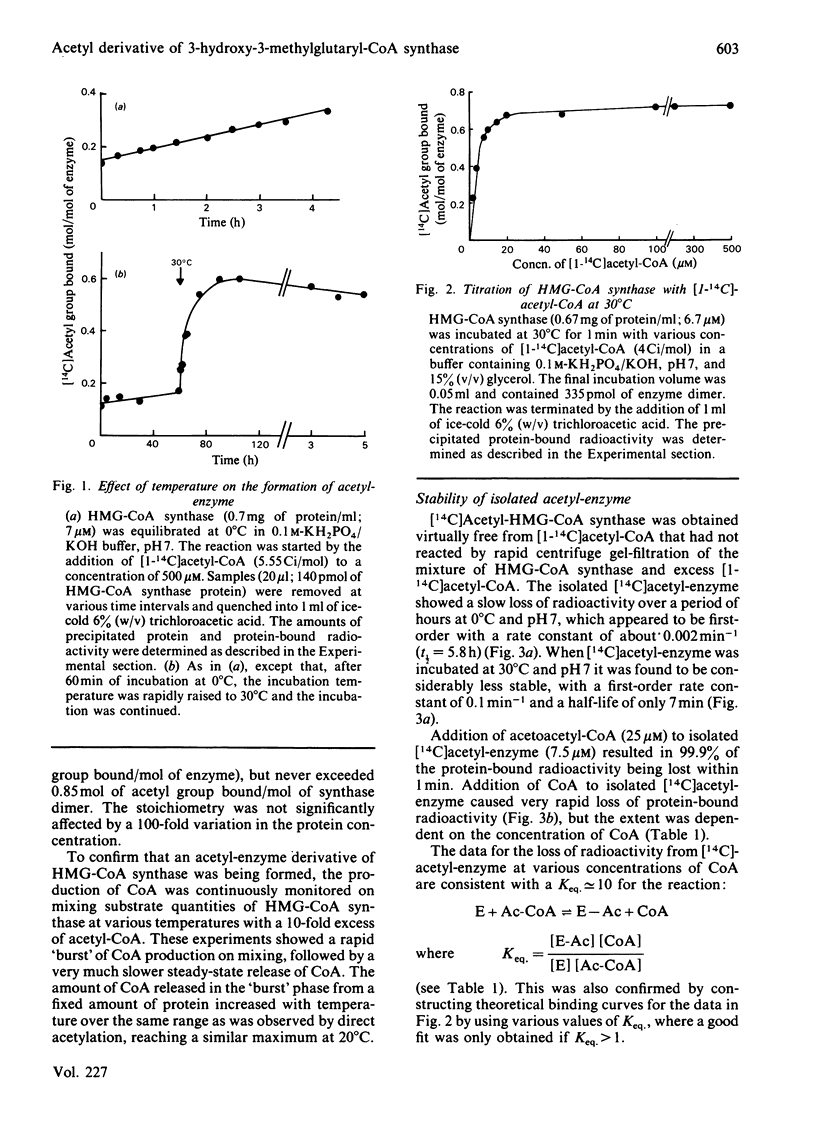
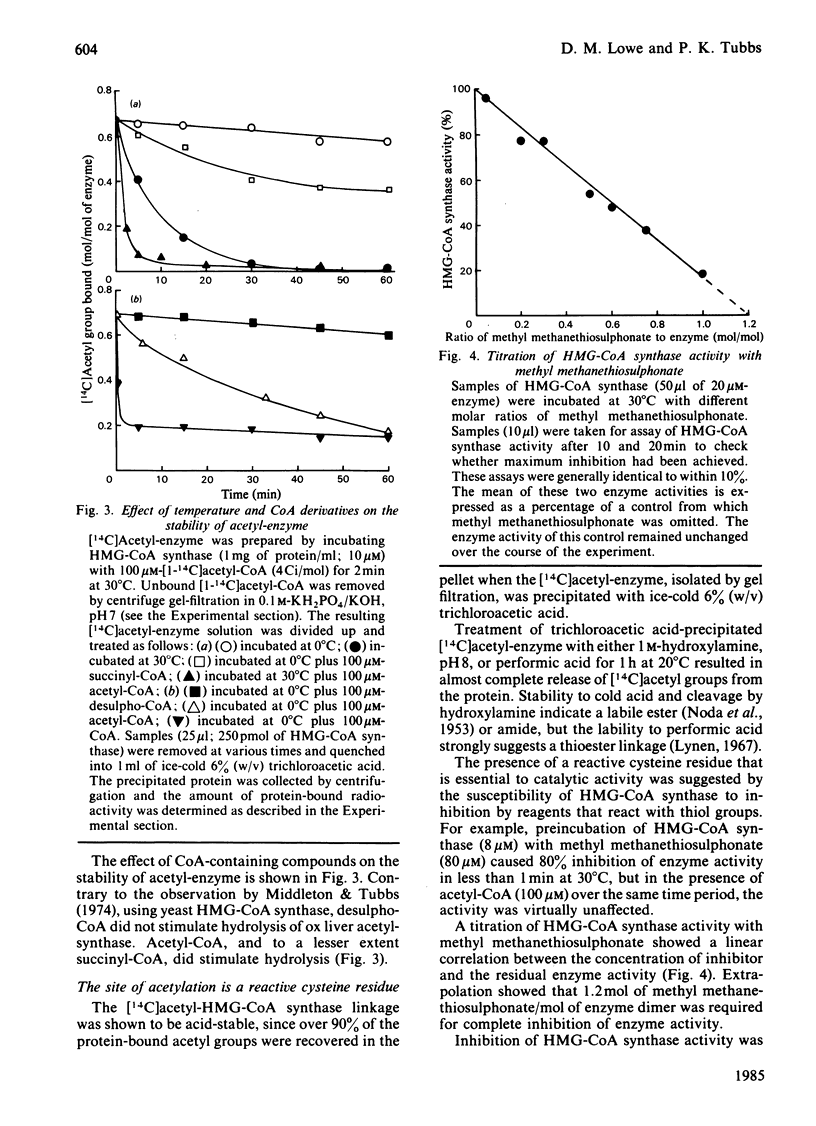
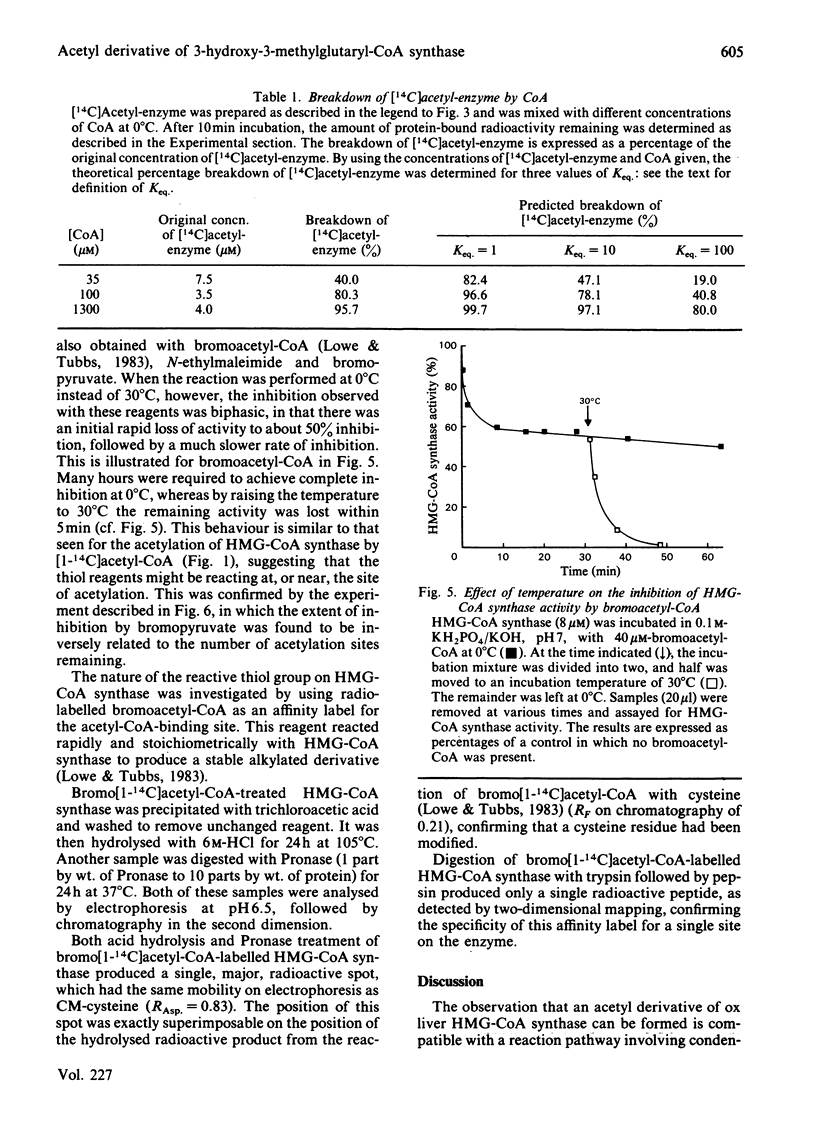
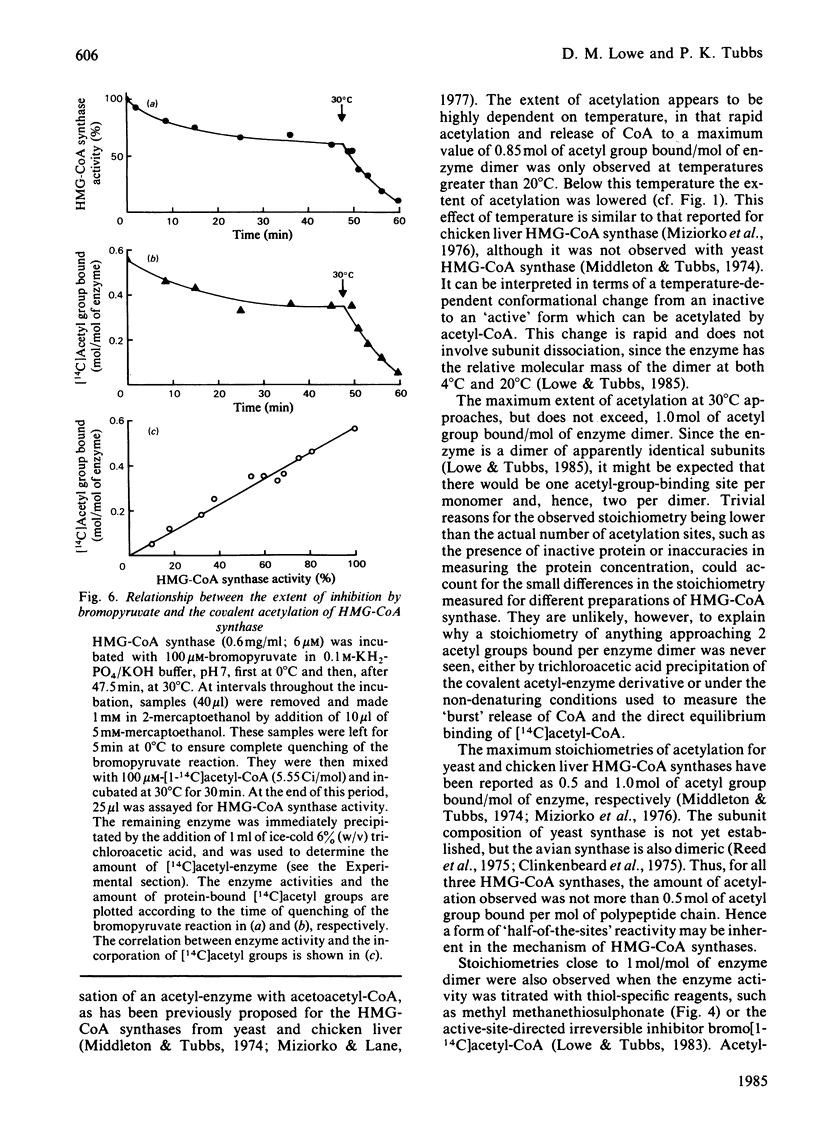
Ox liver mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase (EC 4.1.3.5) reacts with acetyl-CoA to form a complex in which the acetyl group is covalently bound to the enzyme. This acetyl group can be removed by addition of acetoacetyl-CoA or CoA. The extent of acetylation and release of CoA were found to be highly temperature-dependent. At temperatures above 20 degrees C, a maximum value of 0.85 mol of acetyl group bound/mol of enzyme dimer was observed. Below this temperature the extent of rapid acetylation was significantly lowered. Binding stoichiometries close to 1 mol/mol of enzyme dimer were also observed when the 3-hydroxy-3-methylglutaryl-CoA synthase activity was titrated with methyl methanethiosulphonate or bromoacetyl-CoA. This is taken as evidence for a 'half-of-the-sites' reaction mechanism for the formation of 3-hydroxy-3-methylglutaryl-CoA by 3-hydroxy-3-methylglutaryl-CoA synthase. The Keq. for the acetylation was about 10. Isolated acetyl-enzyme is stable for many hours at 0 degrees C and pH 7, but is hydrolysed at 30 degrees C with a half-life of 7 min. This hydrolysis is stimulated by acetyl-CoA and slightly by succinyl-CoA, but not by desulpho-CoA. The site of acetylation has been identified as the thiol group of a reactive cysteine residue by affinity-labelling with the substrate analogue bromo[1-14C]acetyl-CoA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chase J. F., Middleton B., Tubbs P. K. A coenzyme A analogue, desulpho-coA; preparation and effects on various enzymes. Biochem Biophys Res Commun. 1966 Apr 19;23(2):208–213. doi: 10.1016/0006-291x(66)90529-8. [DOI] [PubMed] [Google Scholar]
- Ishitani K., Niitsu Y., Listowsky I. Characterization of the different polypeptide components and analysis of subunit assembly in ferritin. J Biol Chem. 1975 Apr 25;250(8):3124–3128. [PubMed] [Google Scholar]
- Lowe D. M., Tubbs P. K. 3-Hydroxy-3-methylglutaryl-coenzyme A synthase from ox liver. Purification, molecular and catalytic properties. Biochem J. 1985 Apr 15;227(2):591–599. doi: 10.1042/bj2270591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. M., Tubbs P. K. Preparation of bromo[1-14C]acetyl-coenzyme A as an affinity label for acetyl-coenzyme A binding sites. Anal Biochem. 1983 Jul 15;132(2):276–284. doi: 10.1016/0003-2697(83)90008-8. [DOI] [PubMed] [Google Scholar]
- Lynen F. The role of biotin-dependent carboxylations in biosynthetic reactions. Biochem J. 1967 Feb;102(2):381–400. doi: 10.1042/bj1020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton B. The kinetic mechanism of 3-hydroxy-3-methylglutaryl-coenzyme A synthase from baker's yeast. Biochem J. 1972 Jan;126(1):35–47. doi: 10.1042/bj1260035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton B., Tubbs P. K. The purification and some properties of 3-hydroxy-3-methylglutaryl-coenzyme A synthase from Baker's yeast. Biochem J. 1972 Jan;126(1):27–34. doi: 10.1042/bj1260027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miziorko H. M., Clinkenbeard K. D., Reed W. D., Lane M. D. 3-Hydroxy-3-methylglutaryl coenzyme A synthase. Evidence for an acetyl-S-enzyme intermediate and identification of a cysteinyl sulfhydryl as the site of acetylation. J Biol Chem. 1975 Aug 10;250(15):5768–5773. [PubMed] [Google Scholar]
- Miziorko H. M., Lane M. D. 3-Hydroxy-3-methylgutaryl-CoA synthase. Participation of acetyl-S-enzyme and enzyme-S-hydroxymethylgutaryl-SCoA intermediates in the reaction. J Biol Chem. 1977 Feb 25;252(4):1414–1420. [PubMed] [Google Scholar]
- Miziorko H. M., Shortle D., Lane M. D. Trapping of a novel coenzyme A containing intermediate of 3-hydroxy-3-methylglutaryl-CoA synthase. Biochem Biophys Res Commun. 1976 Mar 8;69(1):92–98. doi: 10.1016/s0006-291x(76)80277-x. [DOI] [PubMed] [Google Scholar]
- Page M. A., Tubbs P. K. Some properties of 3-hydroxy-3-methylglutaryl-coenzyme A synthase from ox liver. Biochem J. 1978 Sep 1;173(3):925–928. doi: 10.1042/bj1730925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus H. A rapid and sensitive method for measuring the binding of radioactive ligands to proteins. Anal Biochem. 1969 Oct 15;32(1):91–100. doi: 10.1016/0003-2697(69)90107-9. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Reed W. D., Clinkenbeard D., Lane M. D. Molecular and catalytic properties of mitochondrial (ketogenic) 3-hydroxy-3-methylglutaryl coenzyme A synthase of liver. J Biol Chem. 1975 Apr 25;250(8):3117–3123. [PubMed] [Google Scholar]


