Abstract
This present research work reports the possible effects and the underlying mechanism of atorvastatin on survival rate and cognitive disorders after sepsis. Sepsis is a life-threatening dysfunction that arises when the body’s response to infection causes injury to its own tissues and organs. Diffuse sepsis was induced by cecal ligation and puncture surgery (CLP) in ICR mice. 0.2 mg/kg body weight of atorvastatin was administrated intraperitoneally at 12 h before surgery. The survival of mice was calculated 24 h, 48 h, 72 h, and 96 h after CLP surgery. Two weeks later, open-field test and Morris water maze test were conducted to evaluate the protective effect of atorvastatin. Inflammatory cytokines in plasma, oxidative stress parameters, number of astrocytes, and neuronal cell deaths in the CA3 region of the hippocampus were examined using enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry. The results indicate that pretreatment with atorvastatin can increase survival percentage and improve cognitive function. Atorvastatin reversed all these alterations in parallel with a decrease in circulating levels of cytokines (IL-1β, IL-4, IL-6, and TNF-α) in plasma, inhibited the activities of oxidative stress parameters (lower TBARS levels, ratio of GSH/GSSH, and activities of SOD and CAT), enhanced the activity of citrate synthase in brain, and reduced the number of astrocytes and neuronal cell deaths in CA3 region of hippocampus. Overall, our results indicated that atorvastatin exhibited protective effects on survival rate and cognitive disorders after sepsis by inhibiting the release of inflammatory cytokines, oxidative stress, and neuronal apoptosis in brain tissue.
Keywords: Atorvastatin, Sepsis, Inflammation, Oxidative stress, Apoptosis
Introduction
Sepsis is a potentially fatal systemic inflammatory response to severe infection. As a common and frequent fatal clinical problem in intensive care unit (ICU), sepsis consumes vast healthcare resources worldwide. The mortality continues to remain high despite the multitude of therapeutic approaches targeting cardiovascular resuscitation and source control measures (Dellinger et al. 2012). Sepsis and its complications are the main causes of death in ICU patients, with a mortality rate as high as 30–70% (Jawad et al. 2012). Furthermore, it is important to note that patients who survived sepsis are prone to long-term physical, psychological, and cognitive disorders with health care and social implications (Singer et al. 2016; Iwashyna et al. 2010). Severe sepsis is characterized by a pronounced inflammatory response to infection (Tsiotou et al. 2005), imbalance of oxidants and antioxidants (Mantzarlis et al. 2017), as well as cell death in the hippocampus (Li et al. 2017). Sepsis-associated encephalopathy is a diffuse encephalopathy disorder caused by sepsis, with a reported incidence of 8–70% (Angus et al. 2006). It’s shown that SAE may cause long-term cognitive dysfunction in a large number of animal and clinical trials. Recent studies have revealed that 70% of sepsis survivors have cognitive dysfunction when they leave the hospital, and 45% of patients still have cognitive dysfunction one year after discharge (Hopkins et al. 2004). SAE patients have blood–brain barrier damage, neuronal dysfunction, persistent microglial cell activation, and even permanent brain damage (Sharshar et al. 2007). Sepsis may significantly increase the risk of neurodegenerative diseases and dementia. Therefore, it is imperative to explore new and effective therapeutic approaches to curb sepsis in patients.
HMG-CoA-reductase inhibitors (statins) such as atorvastatin have been shown to exhibit anti-inflammatory, antioxidant, and immune-modulating effects which may further relieve the process of sepsis (Blanco-Colio et al. 2003; Mekontso-Dessap and Brun-Buisson 2006). Traditionally, atorvastatin is prescribed for lowering the cholesterol levels in the blood of hyperlipidemia patients, thereby reducing the risk of ischemic heart disease and stroke (El-Nabarawi et al. 2017). Statins may be considered as novel therapeutic agents that may aid the treatment and prevention of sepsis by targeting several inflammatory and immune-modulating cascades involved in the development of sepsis in murine models (Ando et al. 2000; Ou et al. 2014). Many potential signaling pathways have been reported to participate in the protective effects of atorvastatin after septic injury. Statins reverse microvascular dysfunction during sepsis, reduce neuroinflammation, and prevent the development of long-term cognitive decline (Reis et al. 2016). But the potential mechanisms employed by atorvastatin to sepsis are still unknown. The animal model of sepsis is a necessary experimental platform to study the pathogenesis and clinical prevention and treatment of sepsis. This model simulates the perforation of human appendicitis or diverticulitis, resulting in gram-negative bacteria infection or gram-negative bacteria mainly mixed infection (Buras et al. 2005). The animal model of sepsis has sufficient morbidity and mortality. An imbalance in the activities of the two major brain antioxidant enzymes: superoxide dismutase (SOD) and catalase (CAT) is implicated in high mortality rate seen in experimental sepsis model and the incidence of septic encephalopathy. The levels of thiobarbituric acid-reactive species (TBARS), formed as a by-product of lipid peroxidation, are regarded as biomarkers of lipid peroxidation in the plasma and brain tissue. Its assay measures malondialdehyde (MDA) present in the sample, as well as malondialdehyde produced from lipid hydroperoxides by the hydrolytic conditions of the reaction. Citrate synthase is localized in the mitochondrial matrix and catalyzes the condensation of oxaloacetate and the acetyl group of acetyl coenzyme-A (acetyl CoA), the first step of the Krebs cycle (Scaini et al. 2011). Citrate synthase is also a quantitative enzyme marker for intact mitochondria (Marco et al. 1974). Similarly, the antioxidant biomarker ratio of glutathione (GSH)/glutathione disulfide (GSSG) is also associated with the mortality rate of septic shock (Kim et al. 2016). Statins reduce oxidative stress and inflammation, whether these oxidative stress parameters changes are also associated with atorvastatin needs further exploration. Astrocytes are closely related to nuclear neurons in the central nervous system, the synthesis of GFAP in the hippocampal area increases after severe brain function damage. Statin pretreatment can reduce the symptoms of neurological impairment in rats after cerebral ischemia and reperfusion and has a protective effect on neurons and oligodendrocytes in rats (Ma et al. 2013). But the effects of atorvastatin on the genesis and development of sepsis remain unknown.
The pleiotropic actions of atorvastatin provided the rationale for investigating their role in animal and clinical studies. In the present study, we explored whether administration of atorvastatin improved survival rate and cognitive dysfunction after sepsis. We also examined the possible underlying mechanism of atorvastatin which may be culpable in reducing the over-reaction of the inflammatory response as well as the decrease in the oxidative stress and apoptosis in the hippocampus. As one of the commonly used lipid-lowering agents in the clinical field, this study may provide new insights into the prescription of atorvastatin.
Methods
Animals and Drug Administration
80 male ICR mice (25 ± 3 g) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. Mice in experiments were all housed in the pathogen-free condition cage with free feed and water under independent ventilation system at 22 ± 1 °C and 12-h light: dark cycle with 50–55% relative humidity. Light was turned on at 7 am to 7 pm. Before surgery, all animals aged 3 months were bred in individual cages for 96 h. The ICR mice were randomly divided into four groups: 30 atorvastatin-treated sepsis, 30 saline-treated sepsis, 30 atorvastatin-treated Cons, and 30 saline-treated Cons. Atorvastatin (mother liquor) was at a concentration of 10 mg/ml, which was dissolved in ethanol, atorvastatin (work liquor) was at a concentration of 10 μg/ml, which lowish was diluted mother liquor with 0.9% NaCl at a ratio of 1:1000. The Con groups were treated with 0.9% NaCl containing ethanol only (without atorvastatin). Mice treated with atorvastatin were intraperitoneally administrated with atorvastatin (0.2 mg/kg body weight) at 12 h before surgery. (Merx et al. 2004; Kandasamy et al. 2011).
Induction of Sepsis by Cecal Ligation and Puncture (CLP)
CLP was performed as described by Wichterman et al. Chloral hydrate (10 ml/kg, 4%) was used to anesthetize the experimental mice. A 2-cm midline abdominal incision was performed after disinfecting with iodine volts. In order to avoid intestinal obstruction, the exposed cecum was ligated with 3–0 silk to the distal ileocecal valve. A 21-gauge needle was used to puncture the ligated cecum for once. Next, the punctured cecum was placed back to the abdomen and the incision was closed in layers. Con-operated mice suffered the same operation except for ligation and perforation of the cecum. All animals received saline solution (3 ml/100 g) subcutaneously for resuscitation immediately after the surgery. The study was conducted according to the procedures laid down by the Institutional Animal Ethics Committee.
Survival Rate
Mice in Con and CLP groups with or without atorvastatin administration were evaluated every 24 h consecutively for 4 days for survival analysis.
Open-Field Test
Related behavior tests were all performed in a sound-isolated room during 9:00 am–12:00 pm with XR-XZ301, a video-tracking system from Shanghai Soft Maze Information Technology Co, Ltd. Open-field test was performed to evaluate the cognitive function of exercise and anxiety mood of mice. On the 15th to 17th day after surgery, the mice were placed on the central open-field apparatus for 5 min in order to adapt to the new environment. On the 18th day after surgery, a single mouse was placed on the center area of apparatus for 5 min and the running traces were recorded. The total running distance (m), number of rearing and grooming events, the center occupancy (s), and mean speed (cm/s) were all recorded. The apparatus was thoroughly cleaned using 70% alcohol after each mouse testing period. Final results were computed for further statistical analysis.
Morris Water Maze Test
The Morris water maze test was widely used to examine the learning and spatial memory disorders among many neurological disease models (Hernandes et al. 2014). Following the open-field test, all mice were trained to find the platform for consecutive 4 days (19–22 days) with 60-s trial sessions and the time finding the platform was calculated. If the mice can not to find the platform within 60-s sessions, the experimenter would gently guide them to the platform. On the fifth day (23 days), the time spent in the target quadrant and the number of times the mice crossed the target quadrant were all recorded in the absence of the platform.
Determination of Plasma Cytokines by ELISA and Western Blotting
At the end of the behavior test, twelve (12) of the number of survival mice in each group were euthanized with carbon dioxide and cervical dislocation. Blood samples were collected by cardiac puncture, after using heparin to prevent coagulation, the blood samples were centrifuged at 3000 rpm, 4 °C, 10 min. The 200-µl separated plasma was immediately added with 800 µl 5% trichloroacetic acid for acidification and then frozen for subsequent measurement of IL-1β, IL-4, IL-6, and TNF-α. The inflammatory response induced after CLP surgery was measured by evaluating the levels of IL-1β, IL-4, IL-6, and TNF-α in the plasma area using an ELISA kit (Minneapolis, MN, USA). Samples and standards were prepared to be determined quantificationally by a microplate reader (Bio-Rad), and the results (pg/mg) were used to make the standard curve.
Determination of Oxidative Stress Parameters
The brain tissue in the four groups were defrosted and homogenized in buffers specific for each assay for the determination of the levels of thiobarbituric acid-reactive substances (TBARS), measurement of citrate synthase activity, superoxide dismutase (SOD) activity, catalase (CAT) activity, and the ratio of reduced to oxidized glutathione (GSH/GSSG). The protocols of oxidative stress parameters were referred to the previous studies published on 2016 (Catalao et al. 2016).
Immunohistochemistry (IHC) Staining
Another twelve (12) number of survival mice were killed by an overdose of thiopentone (50 mg/kg), transcardiac perfused with 30 mL heparinized sodium chloride (0.9%), and then fixed in 30 mL 4% paraformaldehyde. And the brains of the mice were immersed in 4% paraformaldehyde (PFA) overnight for dehydration with 10%, 20%, or 30% sucrose solution for dehydration. Coronal sections of paraffin coronal section (20 μm) were obtained using a microtome model 2155 and mounted on the slides coated with poly-l-lysine.
GFAP and NeuN were co-stained by immunohistochemistry method. The slices were washed 5 min × 3 times by PBS. After blocking with 10% donkey serum, primary antibodies against GFAP (rabbit, GAKO, 1:500) and NeuN (mouse, Millipore, 1:500) were applied overnight at 4 °C. Then rinsing in 5 min × 3 times with PBS, and incubating the slices with secondary Alexa Fluor 594 and 488 (Invitrogen, 1:500) antibodies for 1 h at room temperature. Finally, the slices were rinsed with PBS and cover slipped with DAPI (1:500, Invitrogen) for 5 min. After rinsed with PBS, the sections were fluorescence scanned with virtual microscopy scanner (Axio Scan, Carl Zeiss, Germany). The picture was obtained and analyzed by ImageJ software. Counting the number of GFAP-positive cells in the CA3 in the hippocampus, the total number of GFAP-positive cells in each hippocampus was summed and compared them between groups.
Histologic Evaluation
A parallel experiment was conducted as follows for histological assessment. Sections were stained with hematoxylin–eosin after these sections were cut and embedded in paraffin. The pathologic changes of brain tissue under an optical microscope (DP73, Olympus, Tokyo, Japan) were observed. The cell morphology and dead neurons were observed and calculated in the final results.
Statistical Analysis
GraphPad Prism 6.0 software was used for statistical analyses, and the experimental data were expressed as the mean ± SEM. Mann–Whitney U test was used to compare the 2 groups, and the exact 2 tails were obtained. The value of p < 0.05 was considered as statistically significant difference.
Results
Sepsis Survival is Extended by Atorvastatin Treatment
To evaluate whether pretreatment with atorvastatin in the acute phase of sepsis would exert a positive effect on overall survival on sepsis mice, we measured the survival percentage. As shown in Fig. 1, the survival rate among mice treated with saline (less alcohol) was 100% at 96 h. conversely, after CLP surgery; the survival percentage decreased drastically at 72–96 h, when saline (less alcohol) treatment was combined with CLP; and the survival percentage of saline and CLP group dropped to 30% compared with saline treatment group (p < 0.05). With the administration of atorvastatin treatment, the survival percentage increased significantly to 65% at 48 to 96 h compared with saline and CLP group (p < 0.05). This gain in survival indicates that pretreatment with atorvastatin before CLP surgery significant prolongs living time after sepsis.
Fig. 1.
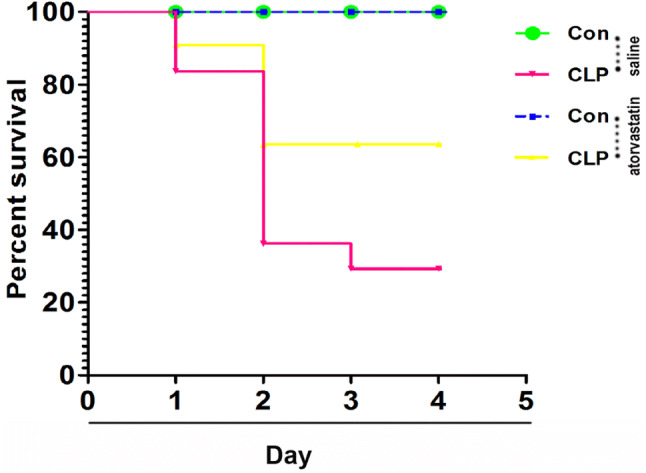
Pretreatment with atorvastatin increased the survival in CLP mice, (p < 0.05)
Atorvastatin Partially Reversed the Decreased Locomotor Activity and Anxiety-Like Behavior Induced by CLP
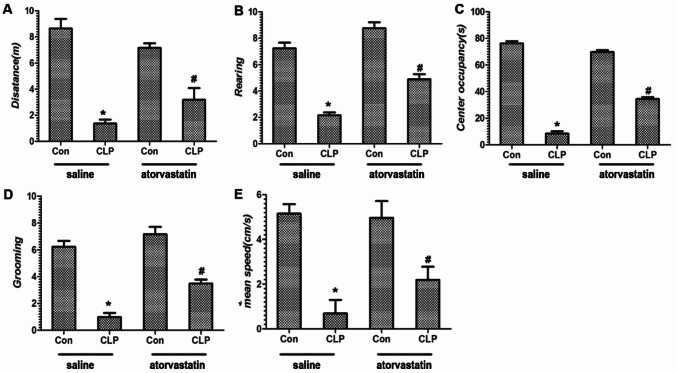
As represented in Fig. 2a–e, the behavior of the mice after CLP surgery exhibited a significant decreased in total distance (m) traveled, number of rearing or grooming, center occupancy (s), and mean speed (cm/s) in the open-field test, compared with the Con groups. However, pretreatment with atorvastatin markedly improved the behaviors loss of the mice after CLP surgery compared with saline + CLP group (p < 0.05). This result implied that pretreatment with atorvastatin partially reversed the decreased locomotor activity and anxiety-like behavior induced by CLP surgery; therefore, we conclude that atorvastatin improves the locomotor deficits and anxiety-like behavior induced by CLP procedure.
Fig. 2.
The behavior of mice in the open-field test. Parameters including total distance (a, m), rearing (b), center occupancy (c, s), number of grooming (d), and mean speed (e, cm/s) were measured to explore locomotor activities and anxiety-like behaviors in the experimental rats. Data are presented as mean ± SEM. *p < 0.05 versus saline + Con group, #p < 0.05 versus saline + CLP group
Application of Atorvastatin Improves Learning and Memory Disorders After CLP Surgery
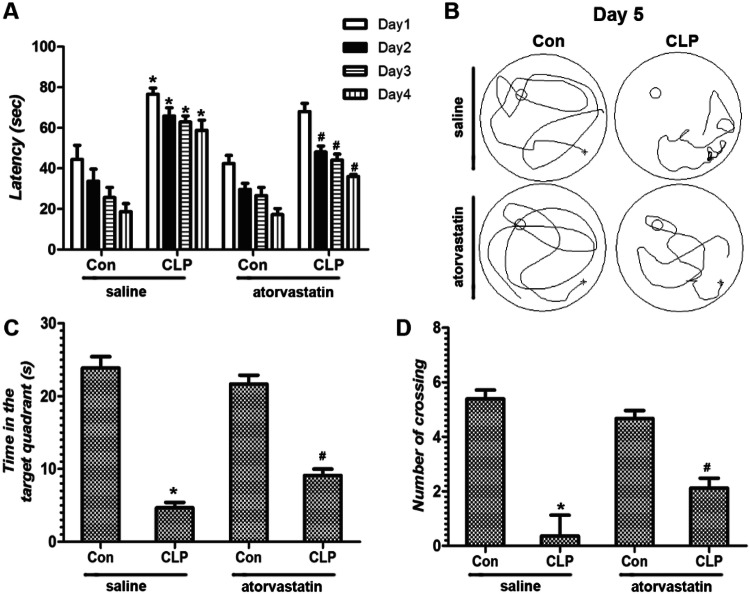
The testing trial was conducted followed the open-field test in the survival mice (n = 20, 20, 6, and 13 in saline + Con, atorvastatin + Con, saline + CLP, atorvastatin + CLP group). In the four probe test days, the swimming latency in saline + Con group to the platform was significantly increased in the saline + CLP group (p < 0.05, Fig. 3a). Pretreatment with atorvastatin before CLP surgery decreased the swimming latency compared with saline + CLP group (p < 0.05, Fig. 3a). In the trials of Morris water maze test, we detected that time in the target quadrant (s) and number of crossing the area once platform located in saline + Con group all decreased significantly compared with saline + CLP group (p < 0.05, Fig. 3b, c). Pretreatment with atorvastatin increased the time in the target quadrant (s) and number of crossing the area once platform located in atorvastatin + CLP group compared with saline + CLP group (p < 0.05, Fig. 3b, c). Overall, this data indicate that atorvastatin pre-administration rescued the early learning and memory deficits in the septic mice.
Fig. 3.
The behavior of mice on learning and spatial memory functions was evaluated by the MWM test. a The swimming latency (s) showed in the 4 probe test days. b Traces of swimming track on day 5. c Time in the target quadrant (s). d The number of crossing the area once platform once located. Data are presented as mean ± SEM. *p < 0.05 versus saline + Con group, #p < 0.05 versus saline + CLP group
Atorvastatin Negatively Regulates the Levels of Inflammatory Cytokines in Brain Tissue
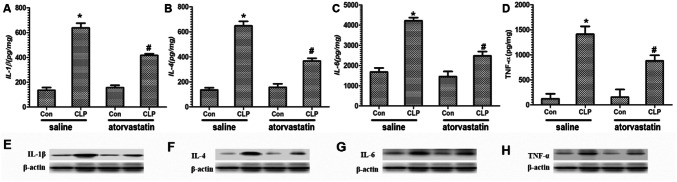
Plasma inflammatory cytokines levels were measured on septic mice by ELISA and western blotting analysis at 2 h after the CLP model. As expected, our results revealed that sepsis led to increased levels of IL-1β, IL-4, IL-6, and TNF-α in the saline group compared with atorvastatin treatment (p < 0.05, Fig. 4). With atorvastatin treatment, the concentration of IL-1β, IL-4, IL-6, and TNF-α levels was markedly reduced compared with the saline group (p < 0.05). Our results suggest atorvastatin exhibits a protective effect, as evidenced by a reduction in inflammatory.
Fig. 4.
Effect of atorvastatin on the levels of inflammatory cytokines in brain tissue. a–d was measured by ELISA assay and e, h was measured by western blotting. a, e Levels of IL-1. b, f Levels of IL-4. c, g Levels of IL-6. d, h Levels of TNF-α. Data are presented as mean ± SEM. *p < 0.05 versus saline + Con group, #p < 0.05 versus saline + CLP group
Atorvastatin Inhibited Oxidative Stress in Brain Tissue
TBARSs were evaluated via the reaction of cellular peroxidation products with thiobarbituric acid, where 80% of these substances are malondialdehyde (dos Santos et al. 2014). Figure 5a shows that levels of oxidative products TBARS increased in CLP versus Con group (p < 0.05). Atorvastatin treatment significantly reversed the increasing effect of TBARS (p < 0.05).
Fig. 5.
Effects of atorvastatin on changes in oxidative stress parameters in four groups of mice. a The levels of TBARS. b The activity of citrate synthase. c The activity of SOD. d The activity of CAT. E: the ratio of GSH/GSSG. Data are presented as mean ± SEM. *p < 0.05 versus saline + Con group, #p < 0.05 versus saline + CLP group
As one of the important mitochondrial enzymes, citrate synthase activity was evaluated in the brain tissues. Citrate synthase activity was markedly decreased in the CLP both in saline and in atorvastatin treatment compared with Con group (p < 0.05). We further estimate the effect of atorvastatin on several biomarkers of antioxidative defense system, including the ratio of GSH/GSSG and the activities of two important antioxidant enzymes SOD and CAT. As shown in the Fig. 5c, e, the levels of oxidative products SOD and GSH/GSSG increased robustly in CLP group versus Con group (p < 0.05). Atorvastatin treatment significantly decreases the increasing effect of SOD and GSH/GSSG. (p < 0.05). Figure 5d shows that pretreatment of atorvastatin significantly increased the level of oxidative product of CAT(p < 0.05).
Atorvastatin Partially Attenuated the Astrocytes in Hippocampus CA3 Region
Astrocytes, one of the important morphological indices of inflammation, would be induced into proliferation after CLP surgery. GFAP-positive cells were observed in brains of saline + Con, saline + CLP, atorvastatin + Con, and atorvastatin +CLP mice. The number of astrocytes per mm2 increased significantly after CLP surgery (p < 0.05, Fig. 6). Pre-administration with atorvastatin effectively inhibited the increasing effect of GFAP-positive cell number after CLP surgery versus saline + CLP group (p < 0.05, Fig. 6). This result shows that atorvastatin prevented apoptosis rate in the cerebral cortex region of CLP-induced septic rats.
Fig. 6.

The GFAP-positive (red) and NeuN-positive cells (green, a–d) and number of astrocytes per mm2 (e) in the hippocampal CA3 region among four groups. Data are presented as mean ± SEM. *p < 0.05 versus saline + Con group, #p < 0.05 versus saline + CLP group
Histological Changes in Hippocampus CA3 Region
HE staining was used to detect the histological changes induced by CLP procedure in the hippocampus CA3 region. CLP surgery produced a significant reduction in the number of CA3 neurons in the hippocampus when compared with saline + CLP group (p < 0.05, Fig. 7). Pretreatment with atorvastatin remarkable rescued the neuronal loss in hippocampus CA3 region compared with saline + CLP group (p < 0.05). This revealed that administration of atorvastatin significantly rescued the neuronal loss after CLP surgery.
Fig. 7.
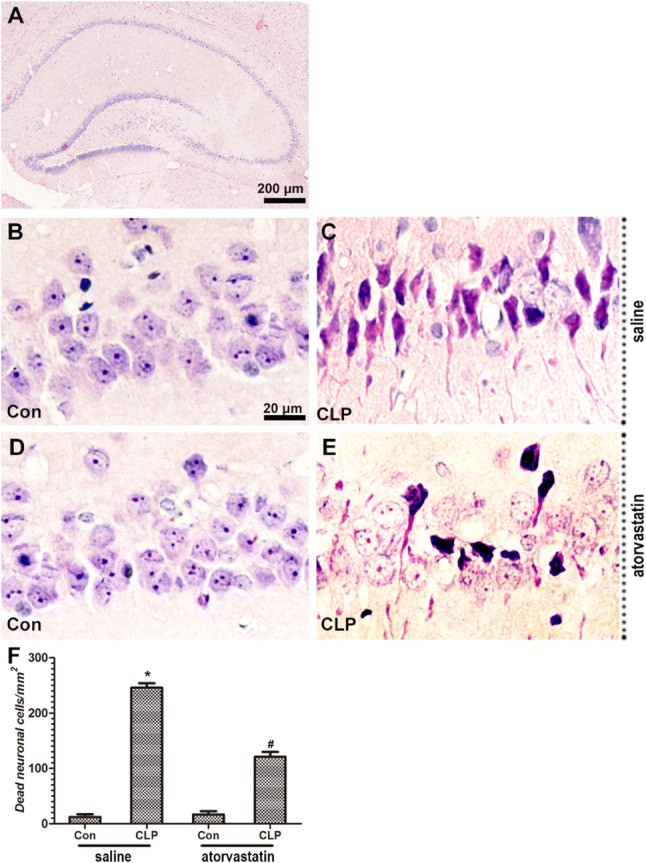
The morphological structures (a–e) and number of dead neuronal cells per mm2 (f) in the hippocampal CA3 region among four groups (HE, a × 40, b–e × 400). Data are presented as mean ± SEM. *p < 0.05 versus saline + Con group, #p < 0.05 versus saline + CLP group
Discussion
In this present study, we found that pretreatment with atorvastatin can protect mice brain from sepsis via anti-inflammatory and antioxidant effect after CLP surgery, characterized by a higher survival rate, lower impairment in locomotor and cognitive functions, and learning and memory deficits. We provided several lines of evidence that atorvastatin pretreatment significantly alleviates inflammatory cytokines in plasma, oxidative stress, proliferation of astrocytes, and neuronal death in the brain. On this basis, the use of atorvastatin had great potential to facilitate injury from acute sepsis.
Atorvastatin is usually absorbed through the small bowel mucosa and taken up by the hepatocytes, where it will be metabolized by the cytochrome P450 3A4 system (Kruger et al. 2009). A Previous study revealed that atorvastatin concentration in the plasma of critically ill patients with sepsis remained supra-therapeutic for up to 20 h after a single dose (Kruger et al. 2009). Early research also revealed that the permeability value of atorvastatin is close to zero and also the blood–brain barrier penetration is estimated to be < 5% due to its large molecular weight, which suggested a reduction in the use of atorvastatin as a therapeutic agent (Sierra et al. 2011). Despite of these prosperities, the neurocognitive protection of atorvastatin can’t be ignored. Excessive inflammation and oxidative stress were recognized as the underlying mechanism in cognitive impairments caused by sepsis. In our study, we examined the levels of inflammatory cytokines in plasma and found that with atorvastatin treatment, CLP group reduced the concentration of plasma IL-1β, IL-4, IL-6, and TNF-α compared with saline + Con group (p < 0.05). To investigate the effect of atorvastatin on cognitive function after sepsis, we performed the open-field test and MWM test. Our results indicate that atorvastatin pre-administration rescued early learning and memory deficits in the septic mice.
A series of studies have studied the relationship between stains and sepsis. Reverting neuroinflammation and microcirculatory/endothelial dysfunction are involved in the stains’ mechanism toward sepsis-induced cognitive dysfunction (Reis et al. 2017). Improved susceptibility to endothelial nitric oxide synthase stimulation and reduced endothelial adhesion of leukocytes has been proved to be relevant in the protective effect of statins (atorvastatin included) treatment after onset of sepsis (Merx et al. 2005). What’s more, as an important reason leading to poor outcomes of septic patients, myocardial dysfunctions are key targets through preserving β1-adrenoreceptor signaling and decreasing cAMP response by pretreatment of septic mice with atorvastatin (Coldewey and Thiemermann 2014; Thangamalai et al. 2014). Atorvastatin also attenuates LPS-induced TNF-α expression and production by activating HO-1 via the ERK and p38 MAPK pathways in models mimicked inflammatory diseases such as sepsis (Wang et al. 2014). Besides the inflammatory pathway, insulin signaling also plays important roles in the effects of atorvastatin in septic rats (Calisto et al. 2010). We further confirmed that inhibition effects on oxidative stress and proliferation of astrocyte, neuronal deaths in hippocampus all related with the protective effects of pretreatment with sepsis. Sepsis is a complex and fatal disease which many organs and signals involved in the genesis and development. However, although several studies have proved promising results regarding stains as an adjunctive treatment for sepsis in animals, a clinical trial named ASEPSIS reported that the administration of atorvastatin reduced clinical progression of sepsis but did not improve mortality (Rachoin et al. 2013). The precise mechanisms underlying the gaps between murine animals and human are still unknown yet. We believe that the atorvastatin will benefit more septic patients with further exploration and bridge the gaps in the future.
Funding
This work was supported by the Jiangsu province natural science foundation of China (BK20151204).
Data Availability
Data used in your manuscript are all placed in our manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xuhua Ge, Email: gexuhuaxzyy@aliyun.com.
Guoji Zhu, Email: biozhuoguoji@163.com.
References
- Ando H, Takamura T, Ota T, Nagai Y, Kobayashi K (2000) Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther 294(3):1043–1046 [PubMed] [Google Scholar]
- Angus DC, Pires Pereira CA, Silva E (2006) Epidemiology of severe sepsis around the world. Endocr Metab Immune Disord. 10.2174/187153006777442332 [DOI] [PubMed] [Google Scholar]
- Blanco-Colio LM, Tunon J, Martin-Ventura JL, Egido J (2003) Anti-inflammatory and immunomodulatory effects of statins. Kidney Int 63(1):12–23. 10.1046/j.1523-1755.2003.00744.x [DOI] [PubMed] [Google Scholar]
- Buras JA, Holzmann B, Sitkovsky M (2005) Model organisms: animal models of sepsis: setting the stage. Dress Nat Rev Drug Discov 4(10):854–865. 10.1038/nrd1854 [DOI] [PubMed] [Google Scholar]
- Calisto KL, Carvalho Bde M, Ropelle ER, Mittestainer FC, Camacho AC, Guadagnini D, Carvalheira JB, Saad MJ (2010) Atorvastatin improves survival in septic rats: effect on tissue inflammatory pathway and on insulin signaling. PLoS ONE 5(12):e14232. 10.1371/journal.pone.0014232 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Catalao CH, Santos-Junior NN, da Costa LH, Souza AO, Alberici LC, Rocha MJ (2016) Brain oxidative stress during experimental sepsis is attenuated by simvastatin administration. Mol Neurobiol. 10.1007/s12035-016-0218-3 [DOI] [PubMed] [Google Scholar]
- Coldewey SM, Thiemermann C (2014) Pleiotropic effects of atorvastatin in experimental sepsis: preservation of beta1-adrenoreceptor signaling in the heart. Shock 41(5):458–459. 10.1097/SHK.0000000000000154 [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R (2012) Surviving Sepsis Campaign Guidelines Committee including The Pediatric S (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med 39(2):165–228. 10.1007/s00134-012-2769-8 [Google Scholar]
- dos Santos RS, Donadio MV, da Silva GV, Blattner CN, Melo DA, Nunes FB, Dias FS, Squizani ED, Pedrazza L, Gadegast I, de Oliveira JR (2014) Immediate effects of chest physiotherapy on hemodynamic, metabolic, and oxidative stress parameters in subjects with septic shock. Respir Care 59(9):1398–1403. 10.4187/respcare.02859 [DOI] [PubMed] [Google Scholar]
- El-Nabarawi N, El-Wakd M, Salem M (2017) Atorvastatin, a double weapon in osteoporosis treatment: an experimental and clinical study. Drug Des Dev Ther 11:1383–1391. 10.2147/DDDT.S133020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandes MS, D’Avila JC, Trevelin SC, Reis PA, Kinjo ER, Lopes LR, Castro-Faria-Neto HC, Cunha FQ, Britto LR, Bozza FA (2014) The role of Nox2-derived ROS in the development of cognitive impairment after sepsis. J Neuroinflamm 11:36. 10.1186/1742-2094-11-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins RO, Weaver LK, Chan KJ, Orme JF (2004) Quality of life, emotional, and cognitive function following acute, respiratory distress syndrome. J Int Neuropsychol Soc 10(07):1005–1017. 10.1017/s135561770410711x [DOI] [PubMed] [Google Scholar]
- Iwashyna TJ, Ely EW, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304(16):1787–1794. 10.1001/jama.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad I, Lukši I, Rafnsson SB (2012) Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health 2(1):10404. 10.7189/jogh.02.010404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Prawez S, Choudhury S, More AS, Ahanger AA, Singh TU, Parida S, Mishra SK (2011) Atorvastatin prevents vascular hyporeactivity to norepinephrine in sepsis: role of nitric oxide and alpha(1)-adrenoceptor mRNA expression. Shock 36(1):76–82. 10.1097/SHK.0b013e31821a4002 [DOI] [PubMed] [Google Scholar]
- Kim JS, Kwon WY, Suh GJ, Kim KS, Jung YS, Kim SH, Lee SE (2016) Plasma glutathione reductase activity and prognosis of septic shock. J Surg Res 200(1):298–307. 10.1016/j.jss.2015.07.044 [DOI] [PubMed] [Google Scholar]
- Kruger PS, Freir NM, Venkatesh B, Robertson TA, Roberts MS, Jones M (2009) A preliminary study of atorvastatin plasma concentrations in critically ill patients with sepsis. Intensive Care Med 35(4):717–721. 10.1007/s00134-008-1358-3 [DOI] [PubMed] [Google Scholar]
- Li Y, Wang F, Luo Y (2017) Ginsenoside Rg1 protects against sepsis-associated encephalopathy through beclin 1-independent autophagy in mice. J Surg Res 207:181–189. 10.1016/j.jss.2016.08.080 [DOI] [PubMed] [Google Scholar]
- Ma M, Uekawa K, Hasegawa Y, Nakagawa T, Katayama T, Sueta D et al (2013) Pretreatment with rosuvastatin protects against focal cerebral ischemia/reperfusion injury in rats through attenuation of oxidative stress and inflammation. Brain Res 1519:87–94. 10.1016/j.brainres.2013.04.040 [DOI] [PubMed] [Google Scholar]
- Mantzarlis K, Tsolaki V, Zakynthinos E (2017) Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid Med Cell Longev 2017:5985209. 10.1155/2017/5985209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco R, Pestana A, Sebastian J, Sols A (1974) Oxaloacetate metabolic crossroads in liver. Enzyme compartmentation and regulation of gluconeogenesis. Mol Cell Biochem 3(1):53–70 [DOI] [PubMed] [Google Scholar]
- Mekontso-Dessap A, Brun-Buisson C (2006) Statins: the next step in adjuvant therapy for sepsis? Intensive Care Med 32(1):11–14. 10.1007/s00134-005-2860-5 [DOI] [PubMed] [Google Scholar]
- Merx MW, Liehn EA, Janssens U, Lutticken R, Schrader J, Hanrath P, Weber C (2004) HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation 109(21):2560–2565. 10.1161/01.CIR.0000129774.09737.5B [DOI] [PubMed] [Google Scholar]
- Merx MW, Liehn EA, Graf J, van de Sandt A, Schaltenbrand M, Schrader J, Hanrath P, Weber C (2005) Statin treatment after onset of sepsis in a murine model improves survival. Circulation 112(1):117–124. 10.1161/CIRCULATIONAHA.104.502195 [DOI] [PubMed] [Google Scholar]
- Ou SY, Chu H, Chao PW, Ou SM, Lee YJ, Kuo SC, Li SY, Shih CJ, Chen YT (2014) Effect of the use of low and high potency statins and sepsis outcomes. Intensive Care Med 40(10):1509–1517. 10.1007/s00134-014-3418-1 [DOI] [PubMed] [Google Scholar]
- Rachoin JS, Cerceo E, Dellinger RP (2013) A new role for statins in sepsis. Crit Care 17(1):105. 10.1186/cc11907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis PA, Alexandre PCB, D’Avila Joana C, Siqueira LD, Bozza FA (2016) Statins prevent cognitive impairment after sepsis by reverting neuroinflammation, and microcirculatory/endothelial dysfunction. Brain Behav Immun 60:293. 10.1016/j.bbi.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Reis PA, Alexandre PC, D’Avila JC, Siqueira LD, Antunes B, Estato V, Tibirica EV, Verdonk F, Sharshar T, Chretien F, Castro-Faria-Neto HC, Bozza FA (2017) Statins prevent cognitive impairment after sepsis by reverting neuroinflammation, and microcirculatory/endothelial dysfunction. Brain Behav Immun 60:293–303. 10.1016/j.bbi.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Scaini G, Rochi N, Benedet J, Ferreira GK, Teodorak BP, Comim CM, Constantino Lde S, Vuolo F, Constantino LC, Quevedo J, Streck EL, Dal-Pizzol F (2011) Inhibition of brain citrate synthase activity in an animal model of sepsis. Rev Bras Ter Intensiva 23(2):158–163 [PubMed] [Google Scholar]
- Sharshar T, Carlier R, Bernard F, Guidoux Céline, Brouland JP, Nardi O et al (2007) Brain lesions in septic shock: a. Intensive Care Med 33(5):798–806. 10.1007/s00134-007-0598-y [DOI] [PubMed] [Google Scholar]
- Sierra S, Ramos MC, Molina P, Esteo C, Vazquez JA, Burgos JS (2011) Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis 23(2):307–318. 10.3233/JAD-2010-101179 [DOI] [PubMed] [Google Scholar]
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8):801–810. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangamalai R, Kandasamy K, Sukumarn SV, Reddy N, Singh V, Choudhury S, Parida S, Singh TU, Boobalan R, Mishra SK (2014) Atorvastatin prevents sepsis-induced downregulation of myocardial beta1-adrenoceptors and decreased cAMP response in mice. Shock 41(5):406–412. 10.1097/SHK.0000000000000138 [DOI] [PubMed] [Google Scholar]
- Tsiotou AG, Sakorafas GH, Anagnostopoulos G, Bramis J (2005) Septic shock; current pathogenetic concepts from a clinical perspective. Med Sci Monit 11(3):RA76–RA85 [PubMed] [Google Scholar]
- Wang XQ, Luo NS, Salah ZQ, Lin YQ, Gu MN, Chen YX (2014) Atorvastatin attenuates TNF-alpha production via heme oxygenase-1 pathway in LPS-stimulated RAW264.7 macrophages. Biomed Environ Sci 27(10):786–793. 10.3967/bes2014.114 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in your manuscript are all placed in our manuscript.