Abstract
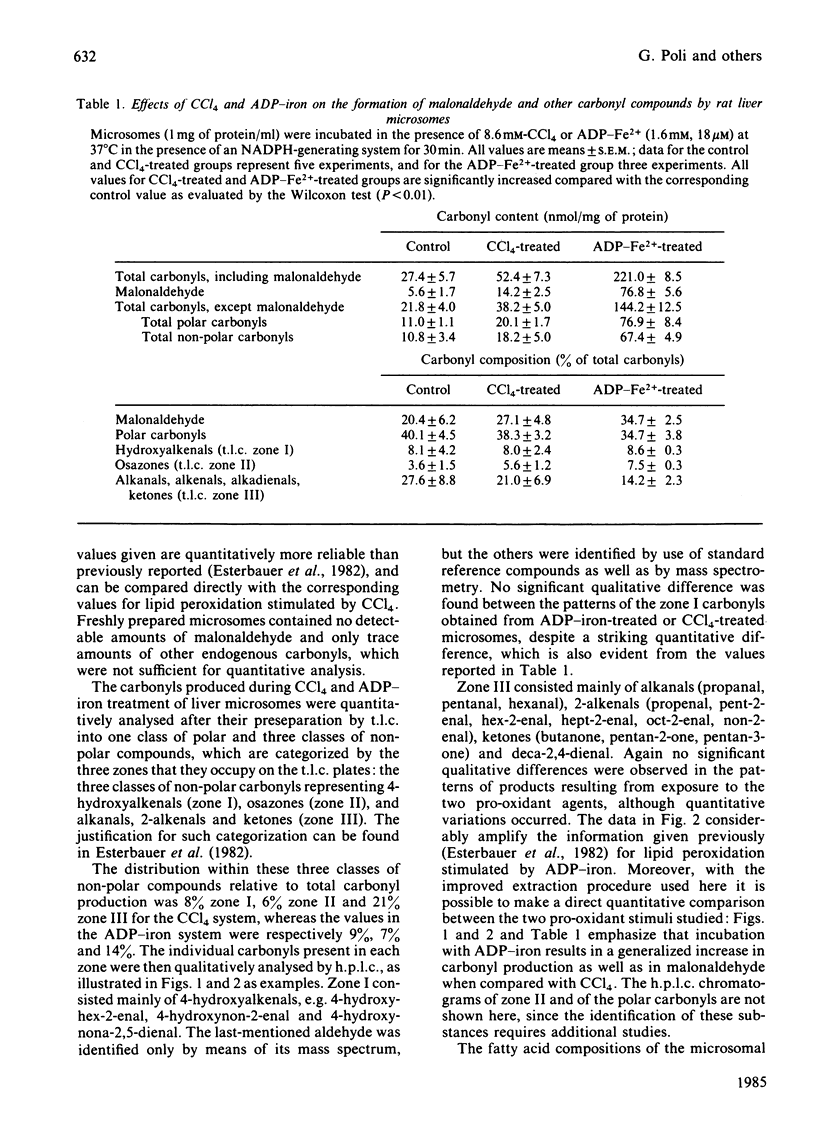
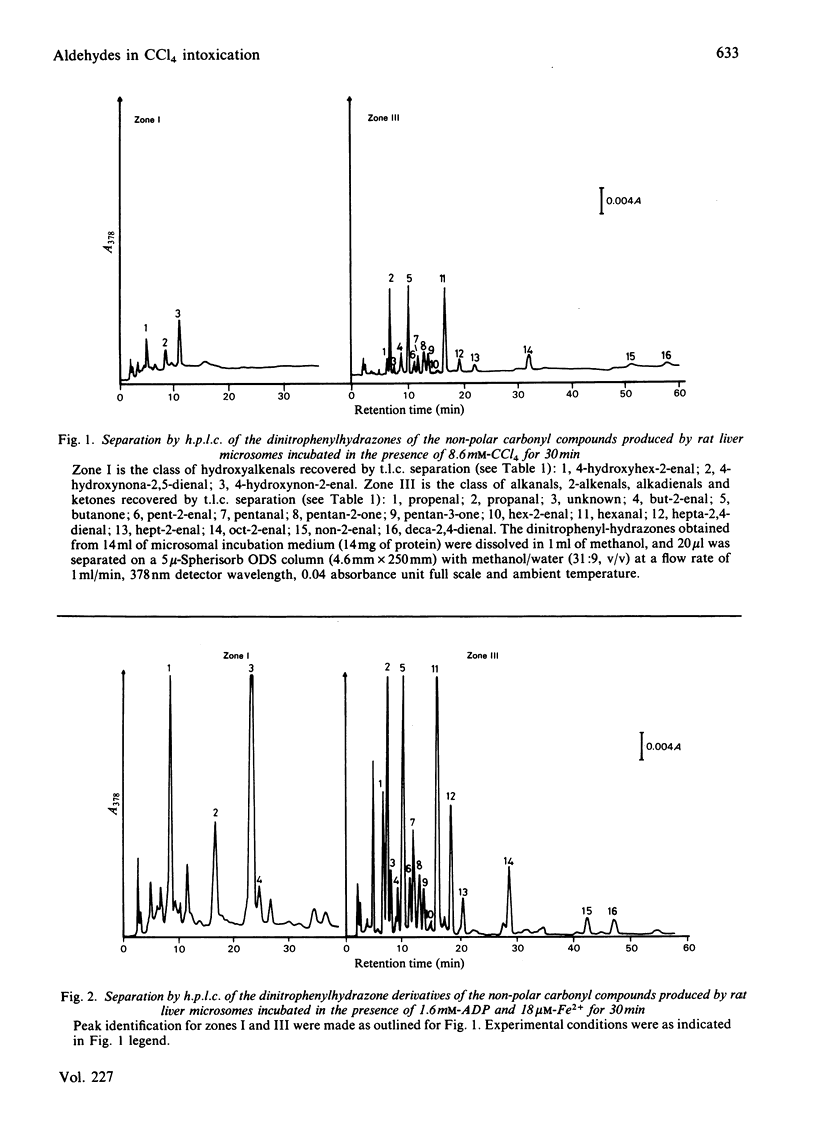
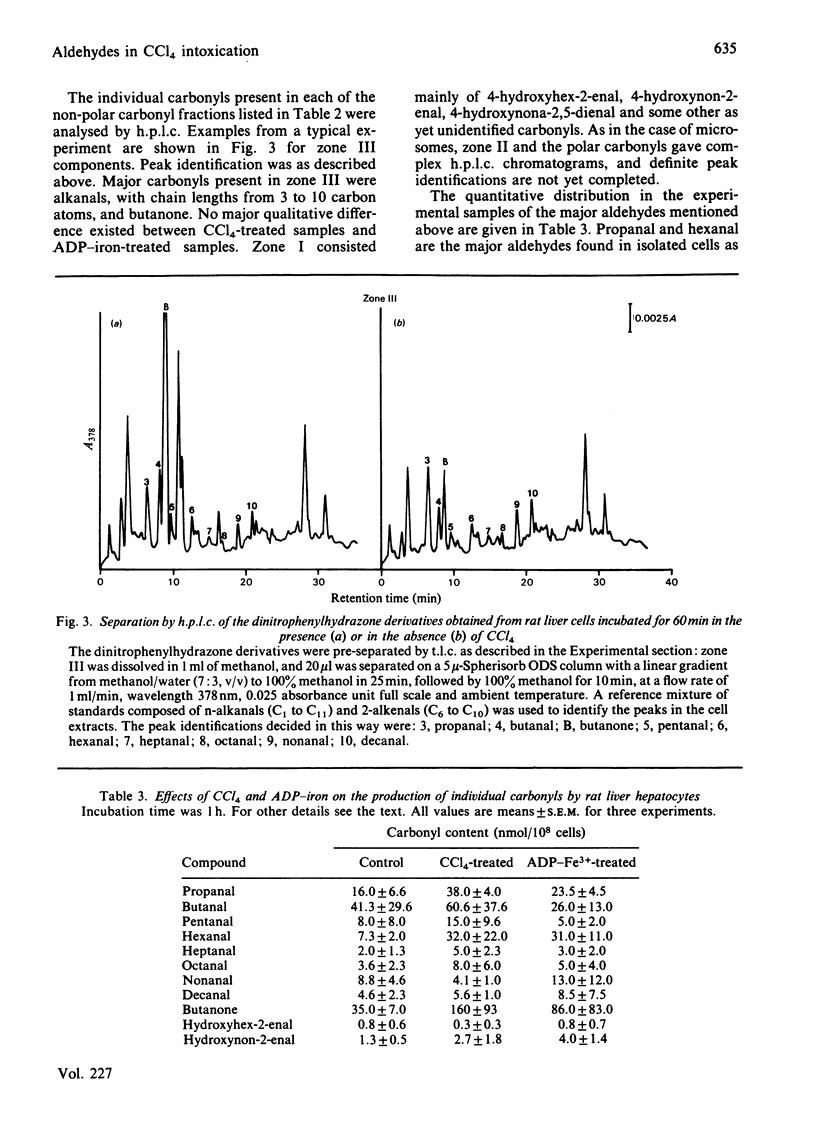
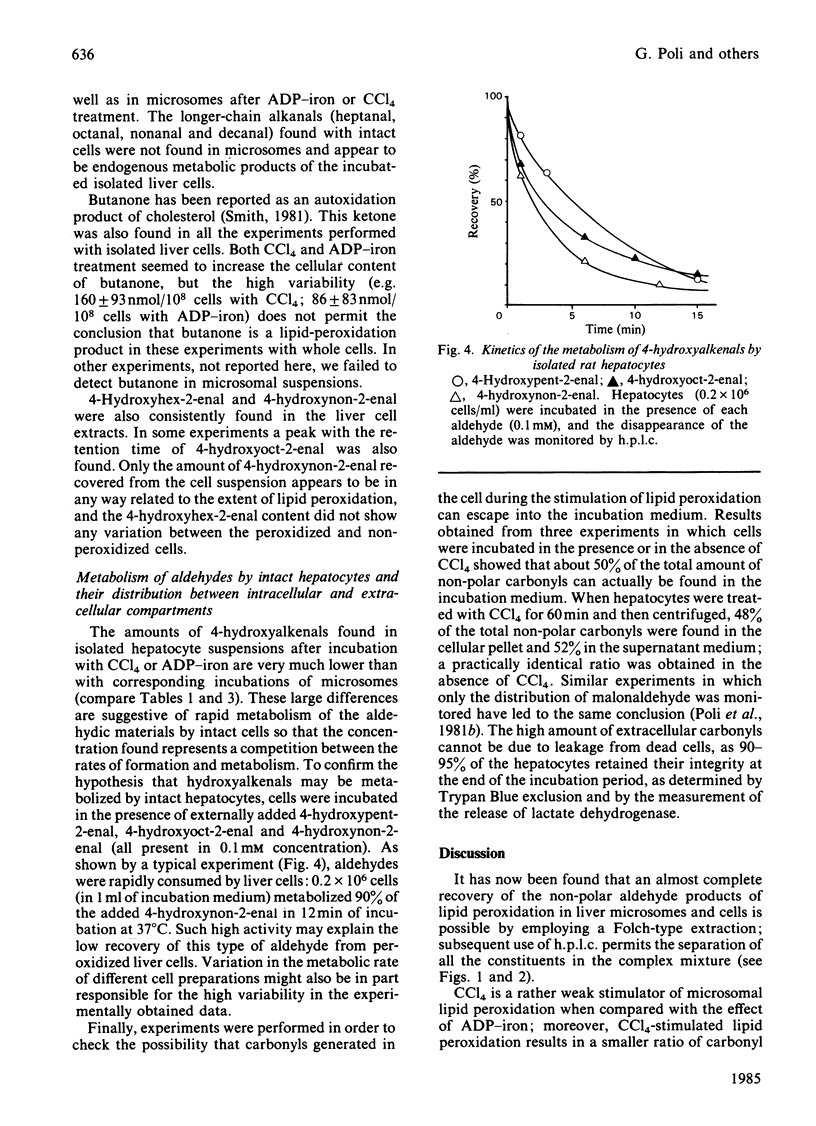
Carbonyl products were separated and identified in suspensions of rat liver microsomal fractions and in isolated hepatocytes, after stimulation of lipid peroxidation by incubation with the pro-oxidants CCl4 and ADP-iron. The carbonyl products were allowed to react with 2,4-dinitrophenylhydrazine, and the derivatives were extracted and separated by t.l.c. into three zones of non-polar materials, and one fraction of polar derivatives that remained at the origin. Separation of the individual non-polar hydrazones in each zone by h.p.l.c. demonstrated that zone I prepared from microsomal fraction or hepatocytes incubated with CCl4 or ADP-iron contained mainly 4-hydroxyhex-2-enal, 4-hydroxynon-2-enal and 4-hydroxynona-2,5-dienal. Zone III consisted mainly of the alkanals propanal, pentanal and hexanal, the 2-alkenals propenal, pent-2-enal, hex-2-enal, hept-2-enal, oct-2-enal and non-2-enal, the ketones butanone, pentan-2-one and pentan-3-one, and deca-2,4-dienal. Incubation of a microsomal fraction with ADP-iron was much more effective in producing malonaldehyde and other carbonyl products than an incubation with CCl4. Despite such quantitative differences, there were no obvious qualitative differences in the h.p.l.c. spectra obtained from zones I and III. However, the stoichiometric evaluation of fatty acid loss and the production of malonaldehyde and other carbonyls suggests that the pathways of lipid peroxidation triggered by CCl4 and ADP-iron are different. The accumulation of carbonyl products of lipid peroxidation in isolated hepatocytes is strongly affected by their metabolism; in particular, 4-hydroxyalkenals were found to be metabolized very rapidly. Nonetheless, both CCl4 and ADP-iron produced stimulation in the production of malonaldehyde and non-polar carbonyl production. After incubation of rat hepatocytes with CCl4 or ADP-iron it was found that approx. 50% of the total amount of non-polar carbonyls produced during incubation escaped into the external medium. This was not leakage from dead cells, as 90-95% of the hepatocytes had retained their integrity at the end of the incubation. Release of carbonyl products from cells stimulated to undergo lipid peroxidation may be a mechanism for spreading an initial intracellular disturbance to affect critical targets outside the parent cell.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano E., Lott K. A., Slater T. F., Stier A., Symons M. C., Tomasi A. Spin-trapping studies on the free-radical products formed by metabolic activation of carbon tetrachloride in rat liver microsomal fractions isolated hepatocytes and in vivo in the rat. Biochem J. 1982 May 15;204(2):593–603. doi: 10.1042/bj2040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A., Fulceri R., Ferrali M., Ciccoli L., Esterbauer H., Comporti M. Detection of carbonyl functions in phospholipids of liver microsomes in CCl4- and BrCCl3-poisoned rats. Biochim Biophys Acta. 1982 Sep 14;712(3):628–638. doi: 10.1016/0005-2760(82)90292-2. [DOI] [PubMed] [Google Scholar]
- Comporti M., Saccocci C., Dianzani M. U. Effect of CCl-4 in vitro and in vivo on lipid peroxidation of rat liver homogenates and subcellular fractions. Enzymologia. 1965 Nov 6;29(3):185–204. [PubMed] [Google Scholar]
- Esterbauer H., Cheeseman K. H., Dianzani M. U., Poli G., Slater T. F. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem J. 1982 Oct 15;208(1):129–140. doi: 10.1042/bj2080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Lang J., Zadravec S., Slater T. F. Detection of malonaldehyde by high-performance liquid chromatography. Methods Enzymol. 1984;105:319–328. doi: 10.1016/s0076-6879(84)05041-2. [DOI] [PubMed] [Google Scholar]
- Forni L. G., Packer J. E., Slater T. F., Willson R. L. Reaction of the trichloromethyl and halothane-derived peroxy radicals with unsaturated fatty acids: a pulse radiolysis study. Chem Biol Interact. 1983 Jul 15;45(2):171–177. doi: 10.1016/0009-2797(83)90066-2. [DOI] [PubMed] [Google Scholar]
- Frank H., Link B. Anaerobic metabolism of carbon tetrachloride and formation of catabolically resistant phospholipids. Biochem Pharmacol. 1984 Apr 1;33(7):1127–1130. doi: 10.1016/0006-2952(84)90524-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Packer J. E., Slater T. F., Willson R. L. Reactions of the carbon tetrachloride-related peroxy free radical (CC13O.2) with amino acids: pulse radiolysis evidence. Life Sci. 1978 Dec 25;23(26):2617–2620. doi: 10.1016/0024-3205(78)90378-8. [DOI] [PubMed] [Google Scholar]
- Poli G., Cheeseman K., Slater T. F., Dianzani M. U. The role of lipid peroxidation in CCl4-induced damage to liver microsomal enzymes: comparative studies in vitro using microsomes and isolated liver cells. Chem Biol Interact. 1981 Oct;37(1-2):13–24. doi: 10.1016/0009-2797(81)90162-9. [DOI] [PubMed] [Google Scholar]
- Poli G., Chiarpotto E., Albano E., Biasi F., Cecchini G., Gravela E., Dianzani M. U. Biochemical evidence for chemical and/or topographic differences in the lipoperoxidative processes induced by CCl4 and iron. Chem Biol Interact. 1983 Mar;43(3):253–261. doi: 10.1016/0009-2797(83)90109-6. [DOI] [PubMed] [Google Scholar]
- Poli G., Gravela E., Albano E., Dianzani M. U. Studies on fatty liver with isolated hepatocytes. II. The action of carbon tetrachloride on lipid peroxidation, protein, and triglyceride synthesis and secretion. Exp Mol Pathol. 1979 Feb;30(1):116–127. doi: 10.1016/0014-4800(79)90086-8. [DOI] [PubMed] [Google Scholar]
- Poyer J. L., McCay P. B., Lai E. K., Janzen E. G., Davis E. R. Confirmation of assignment of the trichloromethyl radical spin adduct detected by spin trapping during 13C-carbon tetrachloride metabolism in vitro and in vivo. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1154–1160. doi: 10.1016/0006-291x(80)90540-9. [DOI] [PubMed] [Google Scholar]
- Recknagel R. O. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967 Jun;19(2):145–208. [PubMed] [Google Scholar]
- Recknagel R. O., Ghoshal A. K. Lipoperoxidation as a vector in carbon tetrachloride hepatotoxicity. Lab Invest. 1966 Jan;15(1 Pt 1):132–148. [PubMed] [Google Scholar]
- Reynolds E. S. Liver parenchymal cell injury. IV. Pattern of incorporation of carbon and chlorine from carbon tetrachloride into chemical constituents of liver in vivo. J Pharmacol Exp Ther. 1967 Jan;155(1):117–126. [PubMed] [Google Scholar]
- Schauenstein E. Autoxidation of polyunsaturated esters in water: chemical structure and biological activity of the products. J Lipid Res. 1967 Sep;8(5):417–428. [PubMed] [Google Scholar]
- Slater T. F. Biochemical Pathology in microtime. Panminerva Med. 1976 Sep-Oct;18(9-10):381–390. [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J. 1971 Aug;123(5):805–814. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. T., Thor H., Hartizell P., Orrenius S. The measurement of lipid peroxidation in isolated hepatocytes. Biochem Pharmacol. 1982 Jan 1;31(1):19–26. doi: 10.1016/0006-2952(82)90230-1. [DOI] [PubMed] [Google Scholar]
- Tomasi A., Billing S., Garner A., Slater T. F., Albano E. The metabolism of halothane by hepatocytes: a comparison between free radical spin trapping and lipid peroxidation in relation to cell damage. Chem Biol Interact. 1983 Sep 15;46(3):353–368. doi: 10.1016/0009-2797(83)90019-4. [DOI] [PubMed] [Google Scholar]



