Abstract
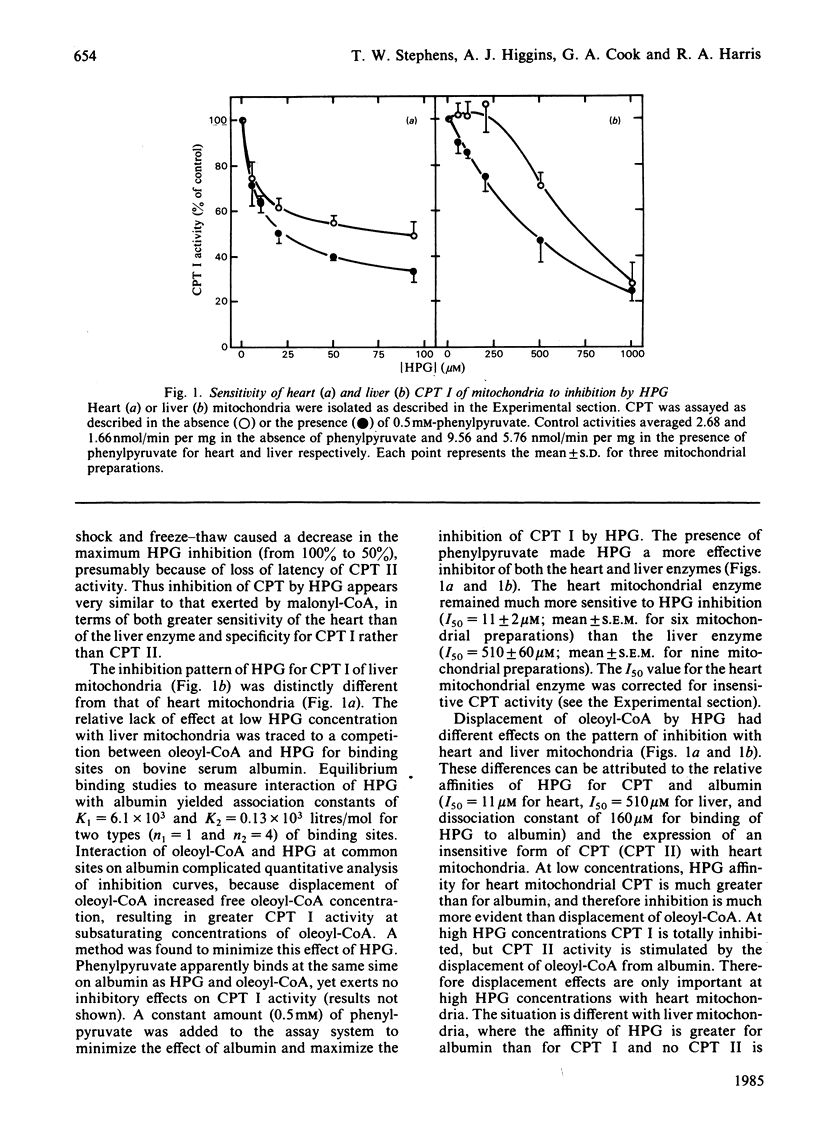
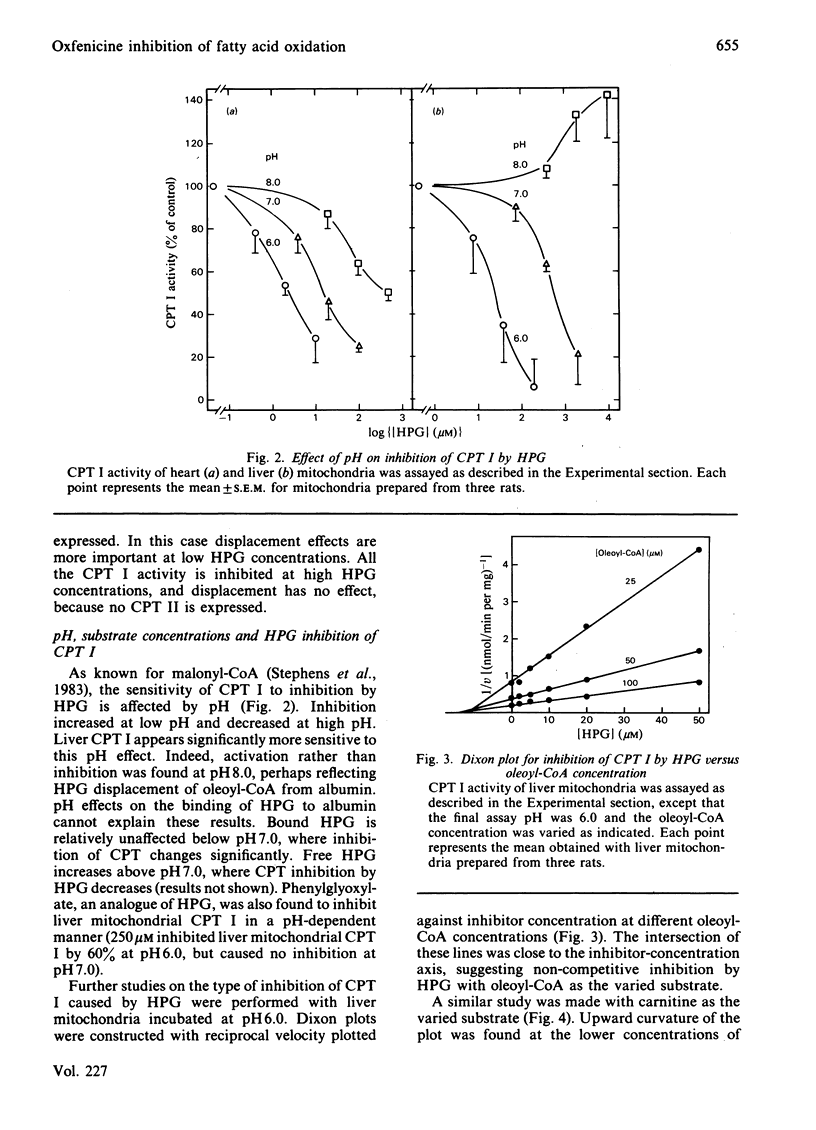
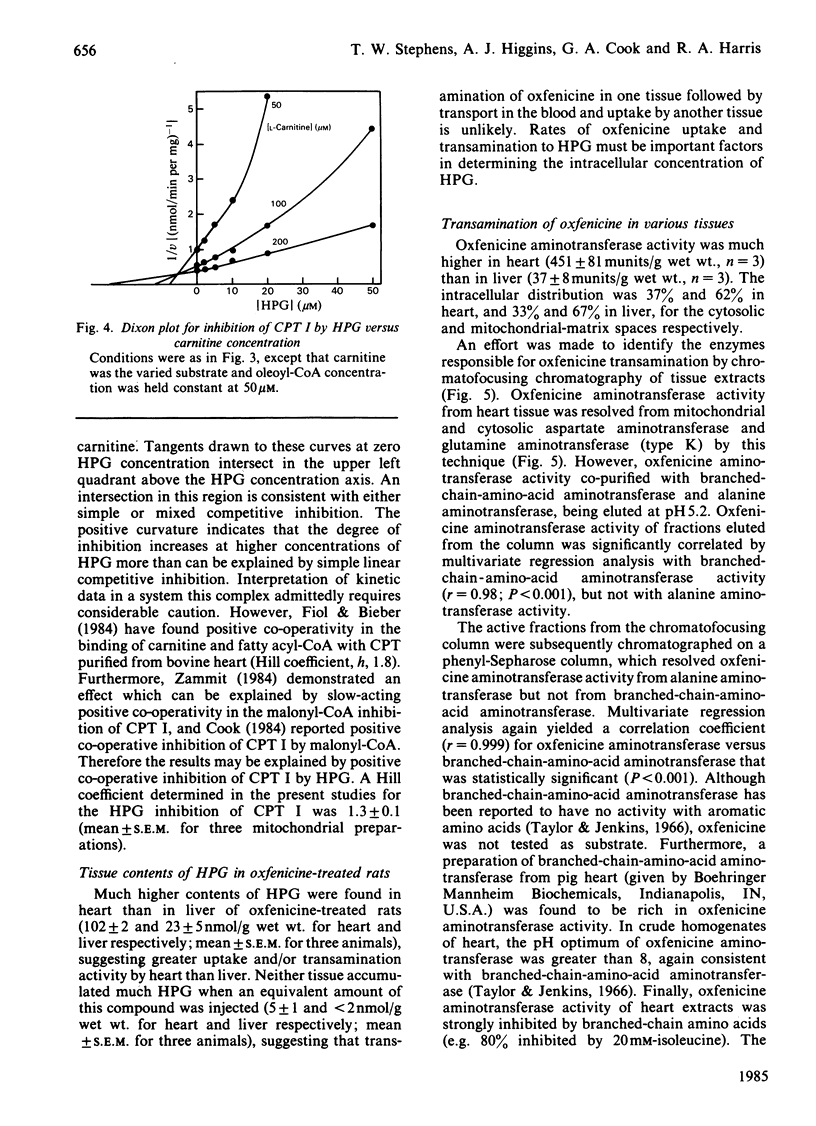
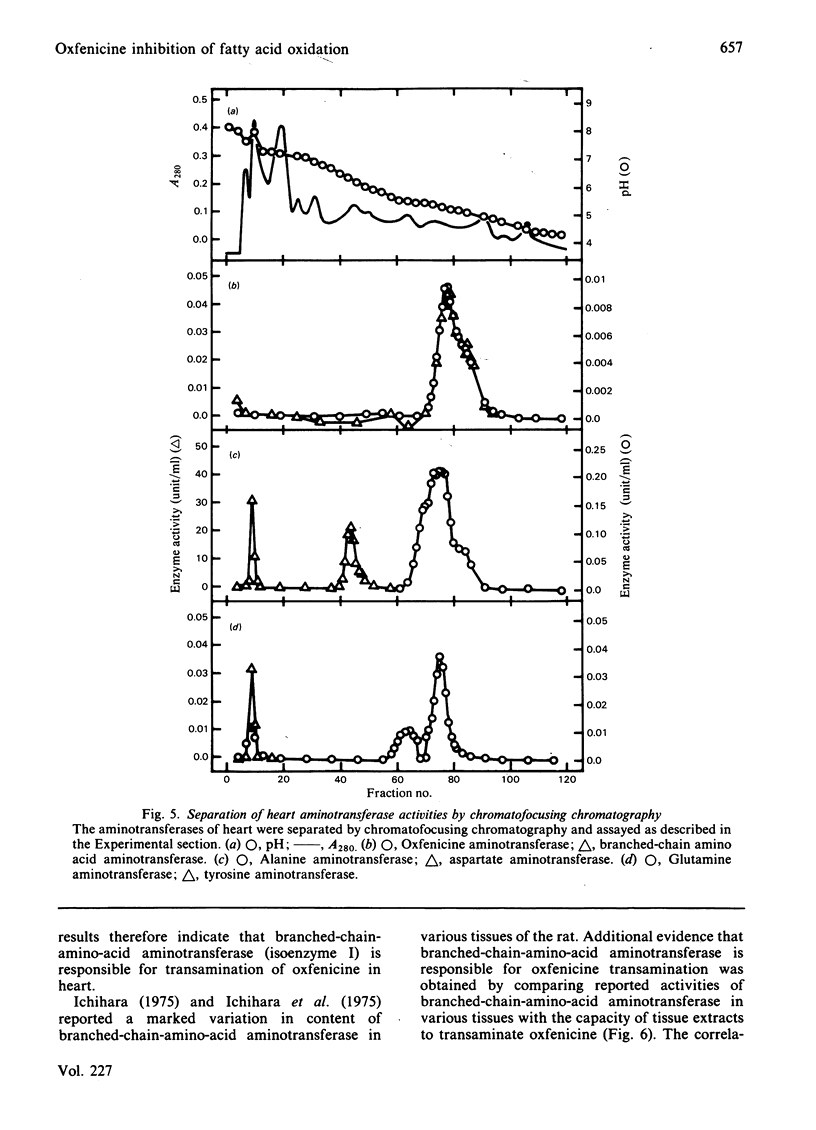
Oxfenicine [S-2-(4-hydroxyphenyl)glycine] is transaminated in heart and liver to 4-hydroxyphenylglyoxylate, an inhibitor of fatty acid oxidation shown in this study to act at the level of carnitine palmitoyltransferase I (EC 2.3.1.21). Oxfenicine was an effective inhibitor of fatty acid oxidation in heart, but not in liver. Tissue specificity of oxfenicine inhibition of fatty acid oxidation was due to greater oxfenicine transaminase activity in heart and to greater sensitivity of heart carnitine palmitoyltransferase I to inhibition by 4-hydroxyphenylglyoxylate [I50 (concentration giving 50% inhibition) of 11 and 510 microM for the enzymes of heart and liver mitochondria, respectively]. Branched-chain-amino-acid aminotransferase (isoenzyme I, EC 2.6.1.42) was responsible for the transamination of oxfenicine in heart. A positive correlation was found between the capacity of various tissues to transaminate oxfenicine and the known content of branched-chain-amino-acid aminotransferase in these tissues. Out of three observed liver oxfenicine aminotransferase activities, one may correspond to asparagine aminotransferase, but the major activity could not be identified by partial purification and characterization. As reported previously for malonyl-CoA inhibition of carnitine palmitoyltransferase I, 4-hydroxyphenylglyoxylate inhibition of this enzyme was found to be very pH-dependent. In striking contrast with the kinetics of malonyl-CoA inhibition, 4-hydroxyphenylglyoxylate inhibition was not affected by oleoyl-CoA concentration, but was partially reversed by increasing carnitine concentrations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman G., Atkinson L., Metcalfe J., Jackson N., Jewitt D. E. Beneficial effect of enhanced myocardial carbohydrate utilisation after oxfenicine (L-hydroxyphenylglycine) in angina pectoris. Eur Heart J. 1980 Aug;1(4):247–253. doi: 10.1093/oxfordjournals.eurheartj.a061126. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. A., Howlett G. J., Schachman H. K. A sedimentation equilibrium method for determining molecular weights of proteins with a tabletop high speed air turbine centrifuge. J Biol Chem. 1978 Apr 10;253(7):2073–2077. [PubMed] [Google Scholar]
- Cappuccino C. C., Kadowaki H., Knox W. E. Assay of leucine aminotransferase in rat tissues and tumors. Enzyme. 1978;23(5):328–338. doi: 10.1159/000458597. [DOI] [PubMed] [Google Scholar]
- Chao D. L., Davis E. J. Studies on the role of Mg 2+ and the Mg 2+ -stimulated adenosine triphosphatase in oxidative phosphorylation. Biochemistry. 1972 May 9;11(10):1943–1952. doi: 10.1021/bi00760a032. [DOI] [PubMed] [Google Scholar]
- Cook G. A. Differences in the sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA are due to differences in Ki values. J Biol Chem. 1984 Oct 10;259(19):12030–12033. [PubMed] [Google Scholar]
- Cooper A. J. Asparagine transaminase from rat liver. J Biol Chem. 1977 Mar 25;252(6):2032–2038. [PubMed] [Google Scholar]
- Cooper A. J., Meister A. Action of liver glutamine transaminase and L-amino acid oxidase on several glutamine analogs. Preparation and properties of the 4-S, O, and NH analogs of alpha-ketoglutaramic acid. J Biol Chem. 1973 Dec 25;248(24):8499–8505. [PubMed] [Google Scholar]
- Cooper A. J., Meister A. Isolation and properties of a new glutamine transaminase from rat kidney. J Biol Chem. 1974 Apr 25;249(8):2554–2561. [PubMed] [Google Scholar]
- Cooper A. J. Purification of soluble and mitochondrial glutamine transaminase K from rat kidney. Use of a sensitive assay involving transamination between L-phenylalanine and alpha-keto-gamma-methiolbutyrate. Anal Biochem. 1978 Sep;89(2):451–460. doi: 10.1016/0003-2697(78)90374-3. [DOI] [PubMed] [Google Scholar]
- Farmer B. B., Mancina M., Williams E. S., Watanabe A. M. Isolation of calcium tolerant myocytes from adult rat hearts: review of the literature and description of a method. Life Sci. 1983 Jul 4;33(1):1–18. doi: 10.1016/0024-3205(83)90706-3. [DOI] [PubMed] [Google Scholar]
- Fiol C. J., Bieber L. L. Sigmoid kinetics of purified beef heart mitochondrial carnitine palmitoyltransferase. Effect of pH and malonyl-CoA. J Biol Chem. 1984 Nov 10;259(21):13084–13088. [PubMed] [Google Scholar]
- Harris R. A. Studies on the inhibition of hepatic lipogenesis by N-6,O-2'-dibutyryl adenosine 3',5'-monophosphate. Arch Biochem Biophys. 1975 Jul;169(1):168–180. doi: 10.1016/0003-9861(75)90330-6. [DOI] [PubMed] [Google Scholar]
- Higgins A. J., Morville M., Burges R. A., Blackburn K. J. Mechanism of action of oxfenicine on muscle metabolism. Biochem Biophys Res Commun. 1981 May 15;100(1):291–296. doi: 10.1016/s0006-291x(81)80095-2. [DOI] [PubMed] [Google Scholar]
- Higgins A. J., Morville M., Burges R. A., Gardiner D. G., Page M. G., Blackburn K. J. Oxfenicine diverts rat muscle metabolism from fatty acid to carbohydrate oxidation and protects the ischaemic rat heart. Life Sci. 1980 Sep 15;27(11):963–970. doi: 10.1016/0024-3205(80)90106-x. [DOI] [PubMed] [Google Scholar]
- Ichihara A. Isozyme patterns of branched-chain amino acid transaminase during cellular differentiation and carcinogenesis. Ann N Y Acad Sci. 1975 Aug 22;259:347–354. doi: 10.1111/j.1749-6632.1975.tb25431.x. [DOI] [PubMed] [Google Scholar]
- Ichihara A., Noda C., Goto M. Transaminase of brainched chain amino acids. X. High activity in stomach and pancreas. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1313–1318. doi: 10.1016/0006-291x(75)90170-9. [DOI] [PubMed] [Google Scholar]
- Livesey G., Lund P. Binding of branched-chain 2-oxo acids to bovine serum albumin. Biochem J. 1982 Apr 15;204(1):265–272. doi: 10.1042/bj2040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune S. A., Harris R. A. Mechanism responsible for 5-(tetradecyloxy)-2-furoic acid inhibition of hepatic lipogenesis. J Biol Chem. 1979 Oct 25;254(20):10095–10101. [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Mills S. E., Long C. S., Foster D. W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983 Jul 15;214(1):21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Effects of pH on the interaction of substrates and malonyl-CoA with mitochondrial carnitine palmitoyltransferase I. Biochem J. 1984 Apr 15;219(2):601–608. doi: 10.1042/bj2190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and related compounds with mitochondria from different rat tissues. Relationship between ligand binding and inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 Jul 15;214(1):83–91. doi: 10.1042/bj2140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Takada Y., Oota Y. Intraperoxisomal and intramitochondrial localization, and assay of pyruvate (glyoxylate) aminotransferase from rat liver. Hoppe Seylers Z Physiol Chem. 1979 Jul;360(7):919–927. doi: 10.1515/bchm2.1979.360.2.919. [DOI] [PubMed] [Google Scholar]
- Stephens T. W., Cook G. A., Harris R. A. Effect of pH on malonyl-CoA inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 May 15;212(2):521–524. doi: 10.1042/bj2120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. T., Jenkins W. T. Leucine aminotransferase. II. Purification and characterization. J Biol Chem. 1966 Oct 10;241(19):4396–4405. [PubMed] [Google Scholar]
- Vercesi A., Reynafarje B., Lehninger A. L. Stoichiometry of H+ ejection and Ca2+ uptake coupled to electron transport in rat heart mitochondria. J Biol Chem. 1978 Sep 25;253(18):6379–6385. [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Zammit V. A. Time-dependence of inhibition of carnitine palmitoyltransferase I by malonyl-CoA in mitochondria isolated from livers of fed or starved rats. Evidence for transition of the enzyme between states of low and high affinity for malonyl-CoA. Biochem J. 1984 Mar 1;218(2):379–386. doi: 10.1042/bj2180379. [DOI] [PMC free article] [PubMed] [Google Scholar]


