Abstract
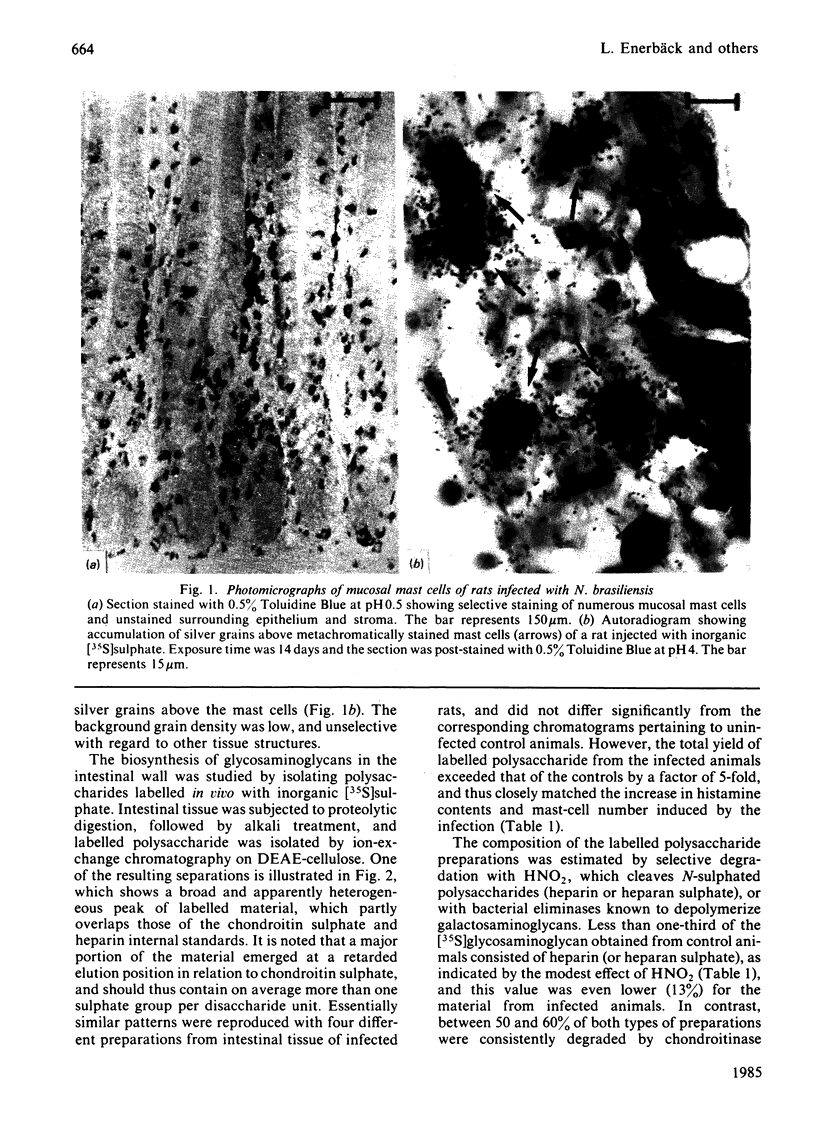
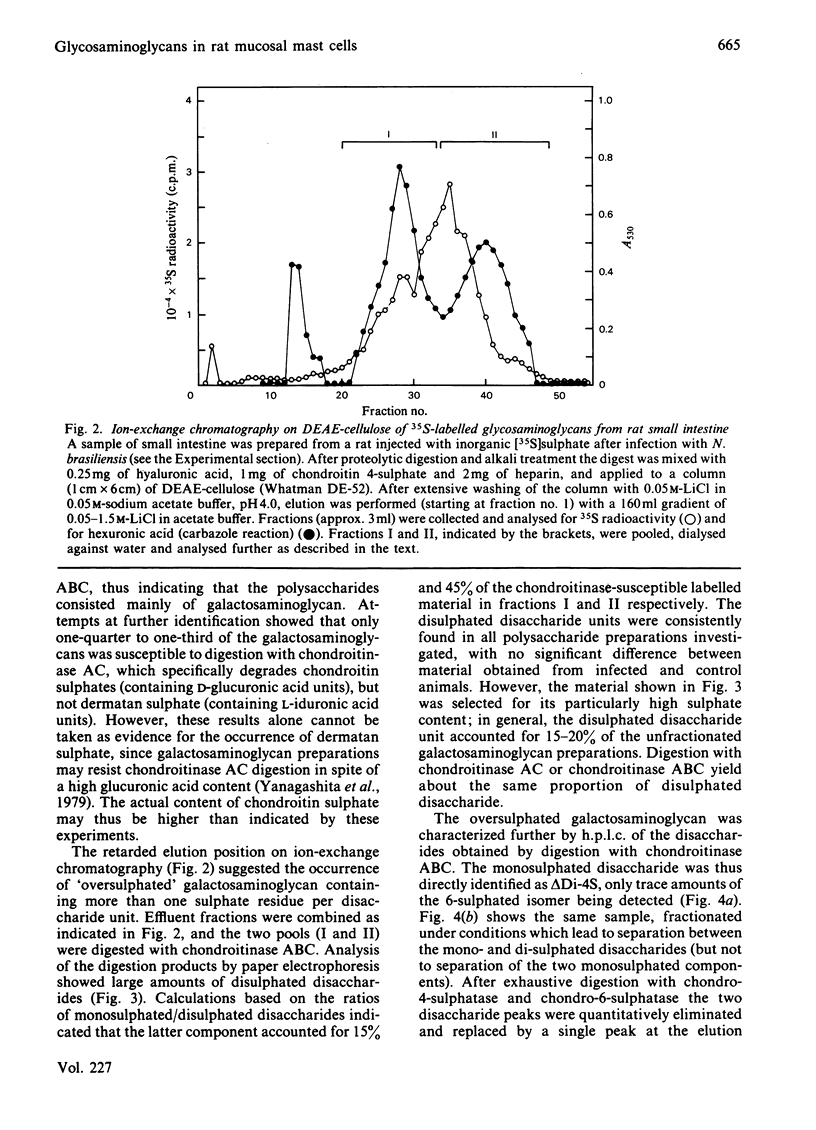
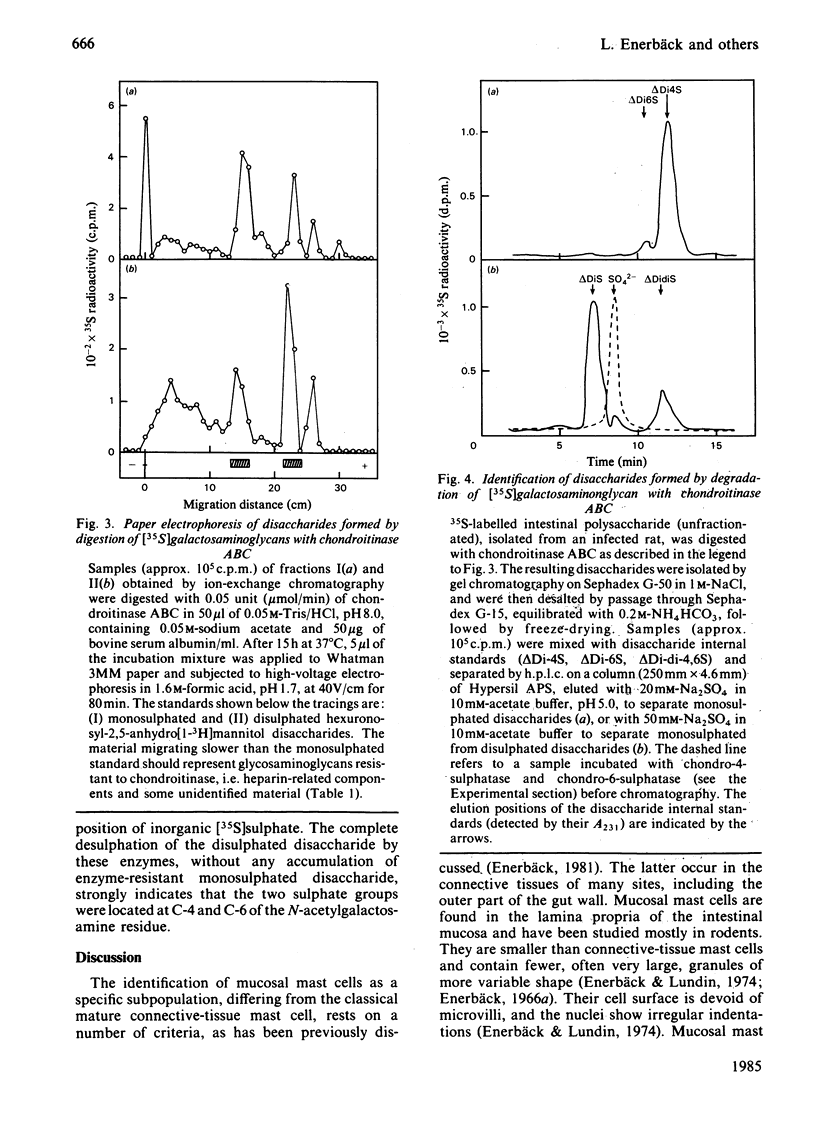
Rats were infected with the nematode Nippostrongylus brasiliensis, resulting in an approx. 5-fold increase in the number of mucosal mast cells and the histamine content of the intestinal (jejunum) wall. After injection of the infected animals with inorganic [35S]sulphate, a similar increase in the yield of labelled intestinal glycosaminoglycans was observed, compared with uninfected control rats. Autoradiography showed a highly selective labelling of the numerous mucosal mast cells and of the few connective-tissue mast cells in the subserosal region of the bowel. Analysis of the labelled polysaccharide from the infected animals showed that almost 60% of this material consisted of oversulphated galactosaminoglycan, whereas heparin-related polysaccharides accounted for only 13%. The galactosaminoglycan contained 4-monosulphated and 4,6-disulphated N-acetylgalactosamine residues in approx. 5:1 molar ratio, both being linked to D-glucuronic acid residues; the occurrence of L-iduronic acid units could not be excluded. No significant difference in structure was found between this polysaccharide and the corresponding component isolated from uninfected rats. It is concluded that the major polysaccharide produced by rat mucosal mast cells in vivo is an oversulphated galactosaminoglycan rather than heparin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allenmark S., Bergström S., Enerbäck L. A selective postcolumn o-phthalaldehyde-derivatization system for the determination of histamine in biological material by high-performance liquid chromatography. Anal Biochem. 1985 Jan;144(1):98–103. doi: 10.1016/0003-2697(85)90089-2. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Befus A. D., Pearce F. L., Gauldie J., Horsewood P., Bienenstock J. Mucosal mast cells. I. Isolation and functional characteristics of rat intestinal mast cells. J Immunol. 1982 Jun;128(6):2475–2480. [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D., Pearce F., Denburg J., Goodacre R. Mast cell heterogeneity: derivation and function, with emphasis on the intestine. J Allergy Clin Immunol. 1982 Dec;70(6):407–412. doi: 10.1016/0091-6749(82)90001-x. [DOI] [PubMed] [Google Scholar]
- Enerbäck L. Berberine sulphate binding to mast cell polyanions: a cytofluorometric method for the quantitation of heparin. Histochemistry. 1974;42(4):301–313. doi: 10.1007/BF00492678. [DOI] [PubMed] [Google Scholar]
- Enerbäck L., Berlin G., Svensson I., Rundquist I. Quantitation of mast cell heparin by flow cytofluorometry. J Histochem Cytochem. 1976 Dec;24(12):1231–1238. doi: 10.1177/24.12.63510. [DOI] [PubMed] [Google Scholar]
- Enerbäck L., Lundin P. M. Ultrastructure of mucosal mast cells in normal and compound 48-80-treated rats. Cell Tissue Res. 1974 Jul 3;150(1):95–105. doi: 10.1007/BF00220383. [DOI] [PubMed] [Google Scholar]
- Enerbäck L., Löwhagen G. B. Long term increase of mucosal mast cells in the rat induced by administration of compound 48/80. Cell Tissue Res. 1979 May 18;198(2):209–215. doi: 10.1007/BF00232005. [DOI] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66(3):303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. I. Effects of fixation. Acta Pathol Microbiol Scand. 1966;66(3):289–302. doi: 10.1111/apm.1966.66.3.289. [DOI] [PubMed] [Google Scholar]
- Enerbäck L., Wingren U. Histamine content of peritoneal and tissue mast cells of growing rats. Histochemistry. 1980;66(2):113–124. doi: 10.1007/BF00494639. [DOI] [PubMed] [Google Scholar]
- Horner A. A. Macromolecular heparin from rat skin. Isolation, characterization, and depolymerization with ascorbate. J Biol Chem. 1971 Jan 10;246(1):231–239. [PubMed] [Google Scholar]
- Jansson L., Ogren S., Lindahl U. Macromolecular properties and end-group analysis of heparin isolated from bovine liver capsule. Biochem J. 1975 Jan;145(1):53–62. doi: 10.1042/bj1450053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset S. O., Kjellén L., Seljelid R., Lindahl U. Changes in glycosaminoglycan biosynthesis during differentiation in vitro of human monocytes. Biochem J. 1983 Mar 15;210(3):661–667. doi: 10.1042/bj2100661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset S. O., Seljelid R., Lindahl U. Modulation of the morphology and glycosaminoglycan biosynthesis of human monocytes, induced by culture substrates. Biochem J. 1984 May 1;219(3):793–799. doi: 10.1042/bj2190793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Metcalfe D. D., Lewis R. A., Silbert J. E., Rosenberg R. D., Wasserman S. I., Austen K. F. Isolation and characterization of heparin from human lung. J Clin Invest. 1979 Dec;64(6):1537–1543. doi: 10.1172/JCI109613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe D. D., Soter N. A., Wasserman S. I., Austen K. F. Identification of sulfated mucopolysaccharides including heparin in the lesional skin of a patient with mastocytosis. J Invest Dermatol. 1980 Apr;74(4):210–215. doi: 10.1111/1523-1747.ep12541737. [DOI] [PubMed] [Google Scholar]
- Metcalfe D. D., Wasserman S. I., Austen K. F. Isolation and characterization of sulphated mucopolysaccharides from rat leukaemic (RBL-1) basophils. Biochem J. 1980 Feb 1;185(2):367–372. doi: 10.1042/bj1850367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. R., Walshaw R. Immune reactions in mucous membranes. IV. Histochemistry of intestinal mast cells during helminth expulsion in the rat. Am J Pathol. 1972 Oct;69(1):195–208. [PMC free article] [PubMed] [Google Scholar]
- Nader H. B., Takahashi H. K., Straus A. H., Dietrich C. P. Selective distribution of the heparin in mammals: conspicuous presence of heparin in lymphoid tissues. Biochim Biophys Acta. 1980 Jan 3;627(1):40–48. doi: 10.1016/0304-4165(80)90121-x. [DOI] [PubMed] [Google Scholar]
- Nawa Y., Miller H. R. Protection against Nippostrongylus brasiliensis by adoptive immunization with immune thoracic duct lymphocytes. Cell Immunol. 1978 Apr;37(1):51–60. doi: 10.1016/0008-8749(78)90173-9. [DOI] [PubMed] [Google Scholar]
- Ogren S., Lindahl U. Degradation of heparin in mouse mastocytoma tissue. Biochem J. 1971 Dec;125(4):1119–1129. doi: 10.1042/bj1251119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Berg B., Fransson L. A., Nordén A. The identity of the metachromatic substance of basophilic leucocytes. Scand J Haematol. 1970;7(6):440–444. doi: 10.1111/j.1600-0609.1970.tb01929.x. [DOI] [PubMed] [Google Scholar]
- Orenstein N. S., Galli S. J., Dvorak A. M., Silbert J. E., Dvorak H. F. Sulfated glycosaminoglycans of guinea pig basophilic leukocytes. J Immunol. 1978 Aug;121(2):586–592. [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Akiyama F., Schmid K., Austen K. F. Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J Biol Chem. 1982 Jun 25;257(12):7229–7236. [PubMed] [Google Scholar]
- Scott J. E., Dorling J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie. 1965 Oct 1;5(3):221–233. doi: 10.1007/BF00306130. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Austen K. F. Effect of p-nitrophenyl-beta-D-xyloside on proteoglycan and glycosaminoglycan biosynthesis in rat serosal mast cell cultures. J Biol Chem. 1982 Jan 10;257(1):253–259. [PubMed] [Google Scholar]
- Stevens R. L., Razin E., Austen K. F., Hein A., Caulfield J. P., Seno N., Schmid K., Akiyama F. Synthesis of chondroitin sulfate E glycosaminoglycan onto p-nitrophenyl-beta-D-xyloside and its localization to the secretory granules of rat serosal mast cells and mouse bone marrow-derived mast cells. J Biol Chem. 1983 May 10;258(9):5977–5984. [PubMed] [Google Scholar]
- Tas J., Berndsen R. G. Does heparin occur in mucosal mast cells of the rat small intestine? J Histochem Cytochem. 1977 Sep;25(9):1058–1062. doi: 10.1177/25.9.71326. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Bäckström G., Lindahl U. Further characterization of the antithrombin-binding sequence in heparin. Carbohydr Res. 1982 Mar 1;100:393–410. doi: 10.1016/s0008-6215(00)81050-2. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Hök M., Lindahl U., Abildgaard U., Langholm R. Isolation and characterization of heparin from human mastocytoma tissue. Thromb Haemost. 1980 Dec 19;44(3):125–129. [PubMed] [Google Scholar]
- Wingren U., Enerbäck L., Ahlman H., Allenmark S., Dahlström A. Amines of the mucosal mast cell of the gut in normal and nematode infected rats. Histochemistry. 1983;77(2):145–158. doi: 10.1007/BF00506557. [DOI] [PubMed] [Google Scholar]
- Wingren U., Enerbäck L. Mucosal mast cells of the rat intestine: a re-evaluation of fixation and staining properties, with special reference to protein blocking and solubility of the granular glycosaminoglycan. Histochem J. 1983 Jun;15(6):571–582. doi: 10.1007/BF01954148. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Gruzenski G. M., Lagunoff D. Immunofluorescent localization of a serine protease in rat small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2785–2789. doi: 10.1073/pnas.75.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Miller H. R. Quantitative analysis of mucosal mast cell protease in the intestines of Nippostrongylus-infected rats. Immunology. 1982 Jul;46(3):487–495. [PMC free article] [PubMed] [Google Scholar]
- Yurt R. W., Leid R. W., Jr, Spragg J., Austen K. F. Immunologic release of heparin from purified rat peritoneal mast cells. J Immunol. 1977 Apr;118(4):1201–1207. [PubMed] [Google Scholar]



