Abstract
Spinal cord injury leads to loss of sensory motor functions below the damaged area, and can significantly affects physical and mental health. An effective spinal cord injury treatment is currently unavailable, in part, because of the intricacy of the brain, as well as the complex pathophysiological mechanism of the injury. Inflammation is an important biological process in multitudinous diseases, with no exception for spinal cord injury. Nuclear factor kappa beta (NF-κB) signaling pathway is a key inflammatory element, as it is involved in cell survival, apoptosis, proliferation, differentiation, and immune response. Activation of the NF-κB signaling pathway leads to the release of a large number of inflammatory factors that can affect tissue repair. Hence, the inhibition of inflammatory responses could improve the repair of injured spinal cord tissues. Secretory leukocyte protease inhibitor (SLPI) has anti-inflammatory and anti-bacterial properties, and promotes wound healing. SLPI can bind to the promoter region of tumor necrosis factor-αand interleukin-8 (IL-8) to inhibit the NF-κB signaling pathway. Additionally, SLPI can reduce secondary damages after spinal cord injury, and prevent further complications. In this report, we analyze the pathophysiological mechanism of spinal cord injury, the role of NF-κB signaling pathway following spinal cord injury, and how SLPI regulates the NF-κB signaling pathway to curtail inflammatory reaction.
Keywords: Ecretory leukocyte protease inhibitor, Spinal cord injury, Nuclear factor kappa beta signaling pathway, Inflammation, Oxidative stress
Introduction
Spinal cord injury (SCI) is a traumatic lesion of the central nervous system caused by multiple factors, including falling from high altitude, sport injury, and traffic accident. In the past decade, millions of people have suffered from SCI, with the incidence of SCI gradually increasing. The incidence of traumatic SCI (TSCI) was 10.6–22.6 per million per year in Québec, Canada (Thompson et al. 2015). On a global scale, the incidence of TSCI is approximately 700,000 individuals per year (James et al. 2019; Kumar et al. 2018). The associated pathophysiological processes of SCI can be categorized into two stages: primary and secondary injury. Primary SCI is a temporal and irreversible process, and can directly damage the spinal cord parenchyma owing to vertebral fracture and ligament avulsion (Pinchi et al. 2019). The resultant of this is dysfunction of blood supply to the spinal cord (Zhang et al. 2017). This, coupled with the destruction of the spinal cord tissue microenvironment, and aggregated immune cells to the spinal cord lesion culminate in the release of a large number of pro-inflammatory factors. Similarly, a large number of chemokines, oxidants, inducible nitric oxide synthase (iNOS) and matrix metalloproteinases (MMPs) are released. Upregulated MMPs expression is harmful, and can cause a variety of diseases (Lee et al. 2015). With the breakdown of blood spinal cord barrier (BSCB), the spinal cord undergoes ischemia–reperfusion injury and oxidative stress (Li et al. 2018; Lv et al. 2019).
Secondary SCI begins several minutes to weeks after primary SCI. The pathological mechanisms of secondary SCI include inflammatory response, vascular changes, apoptosis, free radical formation, and lipid peroxidation (Silva et al. 2014). Free radicals and lipid peroxidation cause destruction of Na+- K+-ATPase and oxidative death of spinal cord cells, which can lead to loss of neuronal function. During secondary SCI process, reactive oxygen species (ROS) activate the p38 mitogen-activated protein kinases (MAPK) signaling pathway, and induces cell apoptosis when the level of ROS is upregulated in spinal cord tissue (Gao et al. 2019). Interferon-gamma (IFN-γ) activates microglia cells to promote differentiation of neural precursor cells into neurons (Gaudet et al. 2018). Inflammatory cytokines, such as IFN-β, interleukin-4 (IL-4), IL-10 and IL-12 also participate in neural tissue repair (Ren et al. 2018). However, excessive inflammation can aggravate tissue damage. The key to SCI repair lies in balancing inflammation (Anwar et al. 2016). When ROS are released, polyunsaturated fatty acids in membrane lipids are destroyed. Cell membrane rupture results in the release of cellular contents (Fatima et al. 2015). Highly active substances, such as ROS, reactive nitrogen species (RNS), and free radicals can destroy cell membranes, protein structures and deoxyribonucleic acid (DNA) (Kertmen et al. 2018; Allison and Ditor 2015). With the occurrence of the above pathophysiological processes, spinal cord function is lost in varied degrees. Inflammation is the body’s adaptive, homeostatic response to localized-injury. Tight junction (TJ) and adheren junction (AJs) proteins of the BSCB restrict the movement of several molecules entering the spinal cord (Wang et al. 2018a). In the wake of SCI, the BSCB destroyed, and a series of inflammatory responses are activated. Destruction of the spinal cord produces cell fragments. These cellular fragments connect with pattern recognition receptors (PRRs) on inflammatory cells. Chemokines and cytokines are released by astrocytes, and microglia recruits neutrophils and macrophages (Orr and Gensel 2018). Neutrophils, macrophages, lymphocytes, and other leukocytes in the peripheral blood vessels enter the spinal cord tissue, and release inflammatory cytokines (O’Shea et al. 2017). Microglial cells are made up of M1 (classical activated) pro-inflammatory phenotype and M2 (alternatively activated) anti-inflammatory phenotype (Cherry et al. 2014). M1 cells secrete IL-1β, IL-6, IL-12, IFN-γ and tumor necrosis factor-α(TNF-α). M2 cells secrete IL-4, IL-10, IL-13 and transforming growth factor beta (TGF-β). The ratio of M1 to M2 influences the microenvironment of spinal cord tissue after injury. M1 type cells occupy a dominant position in SCI. This state is not conducive to SCI repair (Fan et al. 2018). After SCI, extravasated neutrophils secrete proteolytic enzymes. Proteolytic enzymes cause neuronal, glia, and endothelial cell injury (Anwar et al. 2016).
The Role of Nuclear Factor Kappa Beta (NF-κB) Signaling Pathway in SCI
NF-κB is a family of proteins consisting of complex polypeptide subunits. The NF-κB family includes RelA (also known as p65), p50, p52, RelB, and c-Rel. Inhibitors of NF-κB (IκBs) proteins include IκBα, IκBβ, IκBγ, and IκBε. Some IκBs that are located in the nucleus include Bcl-3, IκBL, Iκ BNS and IκB η. IκBL is a nuclear IκB (Chiba et al. 2013). In un-stimulated cells, NF-κB is located in the cytosol, and complexes with the inhibitory protein, IκBα. IκB kinase (IKK) complex degrades IκB by phosphorylating IkB proteins (Mettang et al. 2018). Intracellular IKK is activated when an external stimulator acts on the cell surface IL-1 receptor (IL-1R), toll-like receptor (TLR) family members (TLR3, TLR4, TLR7), and tumor necrosis factor receptor (TNFR) (Pires et al. 2018). Activated IKK leads to the inability of IκB to bind to NF-κB dimer. The NF-κB dimer can enter the nucleus, and regulate the transcription of target gene. NF-κB-inducing kinase (NIK) is then activated, which in turn, phosphorylates IKKα dimers (Boyce et al. 2015). IKKα converts p100 to p52. Finally, RelB/p52 complex enters the nucleus, and regulate transcription of the target gene.
Following SCI, there is a positive feedback mechanism between inflammation and NF-κB signaling pathway. The instigation of NF-κB signaling pathway leads to increased expressions of TNF-α, IL-1β, IL-6 and IL-8, and aggravated inflammatory response. The inflammatory response produces a cascade effect at the spinal cord lesion. Over-expression of pro-inflammatory factors trigger tissue damage, and activate the NF-κB signaling pathway (Karova et al. 2019; Chen et al. 2018).
SLPI Regulates NF-κB Signaling Pathway to Reduce Inflammatory Reaction
The Biological Characteristics of SLPI
SLPI, as a member of the whey acidic protein (WAP) family, is an 11.7-kDa inhibitor of cationic serine protease (Majchrzak-Gorecka et al. 2016). SLPI is produced by epithelial cells, and is found in mucosal fluids including digestive, lung and genital systems (Maffia et al. 2018). Some myeloid cells can produce SLPI (Zhong et al. 2017). Human SLPI has two homologous WAP domains. These are characterized by eight cysteine residues, and form four characteristic intramolecular disulfide bonds. SLPI WAP II domain is primarily responsible for the inhibition of proteases. The most significant role of SLPI is to inhibit neutrophil elastase (NE) (Choi et al. 2018). Regarding inflammation, SLPI is an anti-inflammatory mediator that protects the host tissues, and prevents proteolytic enzymes from over-damaging tissues (Prompunt et al. 2018). In recent years, SLPI has been reported to have beneficial effects in the treatment of medical conditions, such as ischemic heart disease (Prompunt et al. 2018; Wang et al. 2018b; Liu et al. 2014) (Table 1).
Table 1.
Beneficial effects of SLPI in diverse diseases
| Diseases | Species | Dose | Conclusions | References |
|---|---|---|---|---|
| Gonococcal infection | Human | 10 μg/ml | SLPI binds to gonococci and is bactericidal | Cooper et al. (2012) |
| Oral squamous cell carcinoma (OSCC) and oral premalignant lesion (OPML) | Human | 40 µg/ml | SLPI may promote OSCC and OPML apoptosis | Wang et al. (2018b) |
| Colorectal cancer | Human | – | SLPI can be used as a tumor marker in colorectal cancer | Liu et al. (2014) |
| Myocardial ischemia/reperfusion injury | Rats | 400 ng/ml | rhSLPI reduces ROS levels to protect cardiomyocytes | Prompunt et al. (2018) |
| Umbilical vein ischemia/reperfusion injury | Human | 1000 ng/ml | rhSLPI significantly increases the viability of human umbilical vein IRI | Nernpermpisooth et al. (2017) |
| Lung infection | Mice | – | SLPI-A16G inhibits lung infection in mice | Camper et al. (2016) |
| Papillary thyroid carcinoma | Human | – | Silenced secretory leukocyte protease inhibitor weaken papillary thyroid carcinoma cell migration | Xu et al. (2019) |
| Kidney injury | Human | – | A biomarker for acute kidney injury | Ambrosi et al. (2018) |
| CNS injury | Rats | 10 μg | SLPI can promote axonal regeneration | Hannila et al. (2013) |
| Head and neck cancer | Human | – | As a biomarker for head and neck cancer metastasis | Hoffmann et al. (2013) |
SLPI Inhibits Microbial Infection and Promote Wound Healing
SLPI inhibits viral infection, bacterial and fungal growth (Zhong et al. 2017), as well as downregulates mRNA expression and protein synthesis by inhibiting the growth of Escherichia coli (E. coli) (Matsuba et al. 2017). Further studies have shown SLPI to inhibit the growth of both Gram-positive and Gram-negative bacteria. Similarly, SLPI hinders human immunodeficiency virus (HIV) infection (Quabius et al. 2015). SLPI not only provides pathogen defense, but also plays an important role in inflammation, wound healing, and promotes cell proliferation in tissues and organs (Jeong et al. 2015; Munn and Garkavtsev 2018). SLPI-deficient mutational mice did aggravate inflammatory and elastase activity at injured site, resulting in slow healing of damaged wounds (Zhang et al. 2015).
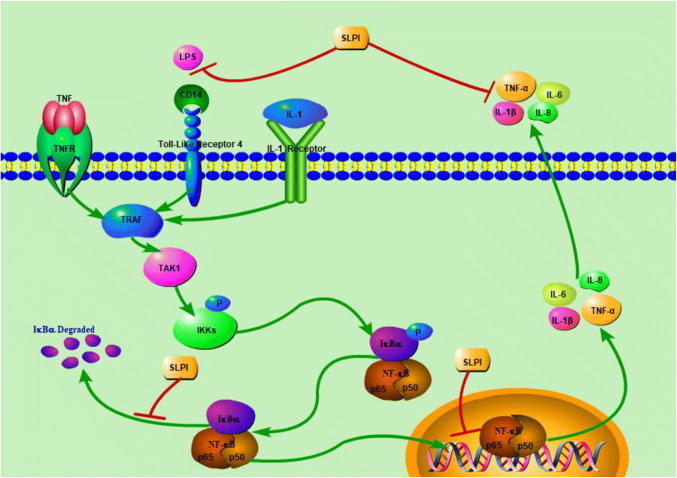
SLPI Reduces Inflammatory Reaction Via Inhibition of NF-κB Signaling Pathway (Fig. 1 )
Fig. 1.
Role of SLPI in SCI. In the wake of SCI, SLPI directly suppresses the NF-κB signaling pathway, thus, exhibiting its anti-inflammatory effects. Sect. “SLPI reduces inflammatory reaction via inhibition of NF-κB signaling pathway (Fig. 1)” of the manuscript has the comprehensive elocution of this mechanism. “⊥” for inhibition and “ → ” for activation
SLPI has an anti-inflammatory effect, and is significant in the balancing of both inflammatory and anti-inflammatory damages. SLPI significantly hinders lipopolysaccharide (LPS), and regulates LPS-receptor connection to inhibit the activation of NF-κB signaling pathway (De Santis et al. 2016). Previous reports have shown that high expression of SLPI leads to LPS hyporesponsiveness (Jin et al. 1997). SLPI prevents LPS-induced degradation of IκBα in U937 cells (Taggart et al. 2002; Minari et al. 2017). IκB sequesters inactive NF-κB in the cytoplasm. When NF-κB signaling pathway is activated, IκB is rapidly degraded. NF-κB enters the nucleus, and regulates gene transcription (Majchrzak-Gorecka et al. 2016). This might be related to SLPI features against proteases. On the other hand, SLPI can bind and block NF-κB DNA binding sites. SLPI can enter the cytoplasm and nucleus, and bind to the promoter region of the IL-8 and TNF-α in the NF-κB regions to inhibit NF-κB signaling pathway (Taggart et al. 2005). This is also one of the mechanisms by which SLPI exerts its anti-inflammatory effects (Camper et al. 2016). SLPI reduces the release of inflammatory mediators by affecting LPS interaction with CD14 (Vandooren et al. 2018). Also, SLPI exerts anti-inflammatory functions through the downregulation of TNF-α (Klimenkova et al. 2014; Svensson et al. 2017). TNF-α is one of the activating factors of the NF-κB signaling pathway. SLPI indirectly inhibits the activation of NF-κB by this route. Moreover, the effect of SLPI on cytokines, such as IL-6, IL-8, IL-10 and IL-1β can effectively limit the NF-κB signaling pathway (Liu et al. 2018).
SLPI Improves SCI by Inhibiting NF-κB Signaling Pathway
Inflammation is an important pathophysiological process of secondary SCI. SLPI hinders the inflammatory responses in secondary injury by inhibiting the NF-κB signaling pathway, and reducing the release of pro-inflammatory cytokines. SLPI did inhibit the expression of polymeric immunoglobulin receptors (pIgR), and upregulated IκBβ by inhibiting the NF-κB signaling pathway (Mikami et al. 2015). Also, IκB was significantly increased in spinal cord tissue of SCI mice after treatment with recombinant SLPI (Ghasemlou et al. 2010). IL-1β impairs motor function recovery (Boato et al. 2013). Although SLPI did suppress the ATP-dependent secretion of IL-1β, it did not impair the release of ATP-independent secretion of IL-1β (Zakrzewicz et al. 2019). Thus, by inhibiting the release of IL-1β, the activation of the NF-κB signaling pathway is also hindered.
SCI can cause ischemia and hypoxia in spinal cord tissues. In cerebral ischemia, serum levels of SLPI are increased. SLPI mRNA levels are also significantly increased after 12 h of cerebral ischemia, and even after 5 days (Hannila 2015). Human umbilical vein endothelial cells are used to make ischemia–reperfusion injury model. Recombinant human SLPI improved the survival rate of the human umbilical vein endothelial cells (Nernpermpisooth et al. 2017). In addition, after SCI, MMPs are involved in BSCB disruption and inflammatory reaction (Zhang et al. 2018). In bleomycin model of pulmonary fibrosis, the active-MMP-9 to pro-MMP-9 ratio was significantly increased (Habgood et al. 2016). In optic nerve crush model, SLPI administration culminated in significant regeneration of retinal ganglion cell axons (Hannila et al. 2013). Hence, SLPI can promote axonal regeneration, in addition to possessing anti-inflammatory and anti-bacterial effects. SLPI levels are considerably increased in serum levels of patients following ischemic stroke (Iłzecka and Stelmasiak 2002). In cerebral ischemic injury, SLPI is expressed in neurons and astrocytes between 24 to 72 h in the peri-infarct zone (Wang et al 2003). In experimented cultures, astrocytes did release a large number of SLPI following pro-inflammatory stimulation; however, microglial was not detected (Suh et al. 2012). Also, SLPI is highly expressed in amyotrophic lateral sclerosis (ALS)-human bone marrow mesenchymal stem cells (hMSC) (Lilo et al. 2013). In chronic demyelinating animal model of multiple sclerosis, SLPI promotes proliferation of adult neural stem cells, and oligodendroglial differentiation (Mueller et al. 2008). Smad2 expression, an intermediate in TGF-β signaling pathway, could be the underlying mechanism (Siddiq and Hannila 2015). In a SCI model of SLPI transgenic over-expressed mice, SLPI improved motor function, and reduced secondary damage. Furthermore, SLPI downregulated TNF-α expression in spinal cord tissue (Ghasemlou et al. 2010). This indicates that the high expression of SLPI has a recuperative effect in SCI. Previous studies have shown SLPI gene expression to be upregulated after SCI in humans, and that its effect might reduce skeletal muscle atrophy (Urso et al. 2007).
Summary and Perspective
NF-κB signaling pathway plays an important role in inflammatory response after SCI. It also participates in cell physiological processes, such as cell survival, apoptosis, proliferation, differentiation, and immune responses. As a protease inhibitor, SLPI has been widely studied owing to its anti-inflammatory and anti-bacterial effects. Its beneficial effects in ischemia and axonal regeneration, in the wake of SCI, have been reported. SLPI promotes proliferation of adult neural stem cells and oligodendroglial differentiation. Furthermore, following pro-inflammatory stimulation, SLPI can be abundantly expressed in neurons and astrocytes in the peri-infarct zone after ischemic injury of nerve tissue. Moreover, SLPI inhibits NF-κB signaling pathway. However, the specific molecular mechanism of NF-κB following SCI warrants further studies. Such studies could focus on the interactions between NF-κB, and TNF-a, Ikba, Hes1 and Smad2 molecules. Also, molecular pathways, such as cAMP-dependent and Cdh1-APC-SnoN, need further clarification in their role relating to nerve repair in spinal cord injury. Studies addressing these suggestions could facilitate the development of an effective pharmaceutical agent for SCI.
Author Contributions
XL designed the study. RT, BOAB, YM, YZ CZ, JJ and XL prepared and revised the manuscript. All authors approved the final paper.
Funding
This study was funded by the Natural Science Foundation of Zhejiang Province (Grant No. LY19H170001).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Renzhe Tang and Benson O. A. Botchway have contributed equally to this work.
References
- Allison DJ, Ditor DS (2015) Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord 53:14–18 [DOI] [PubMed] [Google Scholar]
- Ambrosi N, Caro F, Guerrieri D et al (2018) SLPI expression on kidney biopsies correlates with better clinical outcomes. Transplantation 102:S326–S327 [Google Scholar]
- Anwar MA, Al Shehabi TS, Eid AH (2016) Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci 10:98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boato F, Rosenberger K, Nelissen S et al (2013) Absence of IL-1beta positively affects neurological outcome, lesion development and axonal plasticity after spinal cord injury. J Neuroinflamm 10:6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce BF, Xiu Y, Li J et al (2015) NF-kappaB-mediated regulation of osteoclastogenesis. Endocrinol Metab (Seoul) 30:35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper N, Glasgow AM, Osbourn M et al (2016) A secretory leukocyte protease inhibitor variant with improved activity against lung infection. Mucosal Immunol 9:669–676 [DOI] [PubMed] [Google Scholar]
- Chen S, Ye J, Chen X et al (2018) Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. J Neuroinflamm 15:150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O'Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflamm 11:98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Inoko H, Kimura M (2013) Role of nuclear IkappaBs in inflammation regulation. Biomol Concepts 4:187–196 [DOI] [PubMed] [Google Scholar]
- Choi SR, Hong SS, Kim J et al (2018) Neutrophil elastase in cervical fluid in women with short cervical length. Taiwan J Obstet Gynecol 57:407–410 [DOI] [PubMed] [Google Scholar]
- Cooper MD, Roberts MH, Barauskas OL et al (2012) Secretory leukocyte protease inhibitor binds to Neisseria gonorrhoeae outer membrane opacity protein and is bactericidal. Am J Reprod Immunol 68:116–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis S, Kunde D, Serino G et al (2016) Secretory leukoprotease inhibitor is required for efficient quercetin-mediated suppression of TNF beta secretion. Oncotarget 7:75800–75809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B, Wei Z, Yao X et al (2018) Microenvironment imbalance of spinal cord injury. Cell Transplant 27:853–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima G, Sharma VP, Das SK et al (2015) Oxidative stress and antioxidative parameters in patients with spinal cord injury: implications in the pathogenesis of disease. Spinal Cord 53:3–6 [DOI] [PubMed] [Google Scholar]
- Gao L, Zhang Z, Xu W et al (2019) Natrium benzoate alleviates neuronal apoptosis via the DJ-1-related anti-oxidative stress pathway involving Akt phosphorylation in a rat model of traumatic spinal cord injury. Front Mol Neurosci 12:42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet AD, Fonken LK, Watkins LR et al (2018) MicroRNAs: roles in regulating neuroinflammation. Neuroscientist 24:221–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemlou N, Bouhy D, Yang J et al (2010) Beneficial effects of secretory leukocyte protease inhibitor after spinal cord injury. Brain 133:126–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habgood AN, Tatler AL, Porte J et al (2016) Secretory leukocyte protease inhibitor gene deletion alters bleomycin-induced lung injury, but not development of pulmonary fibrosis. Lab Invest 96:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannila SS (2015) Secretory leukocyte protease inhibitor (SLPI): emerging roles in CNS trauma and repair. Neuroscientist 21(6):630–636 [DOI] [PubMed] [Google Scholar]
- Hannila SS, Siddiq MM, Carmel JB et al (2013) Secretory leukocyte protease inhibitor reverses inhibition by CNS myelin, promotes regeneration in the optic nerve, and suppresses expression of the transforming growth factor-beta signaling protein Smad2. J Neurosci 33:5138–5151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Quabius ES, Tribius S et al (2013) Human papillomavirus infection in head and neck cancer: the role of the secretory leukocyte protease inhibitor. Oncol Rep 29:1962–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iłzecka J, Stelmasiak Z (2002) Increased serum levels of endogenous protectant secretory leukocyte protease inhibitor in acute ischemic stroke patients. Cerebrovasc Dis 13(1):38–42 [DOI] [PubMed] [Google Scholar]
- James SL, Theadom A, Ellenbogen RG et al (2019) Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18:56–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J-O, Wang G, Jeong S-J et al (2015) Function of secretory leukocyte protease inhibitor (SLPI) in odontoblast during Mouse tooth development. J Nanosci Nanotechnol 15:120–124 [DOI] [PubMed] [Google Scholar]
- Jin FY, Nathan C, Radzioch D et al (1997) Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell 88(3):417–426 [DOI] [PubMed] [Google Scholar]
- Karova K, Wainwright JV, Machova-Urdzikova L et al (2019) Transplantation of neural precursors generated from spinal progenitor cells reduces inflammation in spinal cord injury via NF-kappaB pathway inhibition. J Neuroinflamm 16(1):12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertmen H, Celikoglu E, Ozturk OC et al (2018) Comparative effects of methylprednisolone and tetracosactide (ACTH1-24) on ischemia/reperfusion injury of the rabbit spinal cord. Arch Med Sci 14:1459–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimenkova O, Ellerbeck W, Klimiankou M et al (2014) A lack of secretory leukocyte protease inhibitor (SLPI) causes defects in granulocytic differentiation. Blood 123:1239–1249 [DOI] [PubMed] [Google Scholar]
- Kumar R, Lim J, Mekary RA et al (2018) Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg 113:e345–e363 [DOI] [PubMed] [Google Scholar]
- Lee JY, Choi HY, Na WH et al (2015) 17beta-estradiol inhibits MMP-9 and SUR1/TrpM4 expression and activation and thereby attenuates BSCB disruption/hemorrhage after spinal cord injury in male rats. Endocrinology 156:1838–1850 [DOI] [PubMed] [Google Scholar]
- Li Q, Gao S, Kang Z, Zhang M et al (2018) Rapamycin enhances mitophagy and attenuates apoptosis after spinal ischemia-reperfusion injury. Front Neurosci 12:865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilo E, Wald-Altman S, Solmesky LJ et al (2013) Characterization of human sporadic ALS biomarkers in the familial ALS transgenic mSOD1(G93A) mouse model. Hum Mol Genet 22(23):4720–4725 [DOI] [PubMed] [Google Scholar]
- Liu G, Yang J, Zhao Y et al (2014) Expression of secretory leukocyte protease inhibitor detected by immunohistochemistry correlating with prognosis and metastasis in colorectal cancer. World J Surg Oncol 12:369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Ma Z, Sun WW et al (2018) Upregulated expression of secretory leukocyte protease inhibitor in lung by inhalation of high concentration of sulfur dioxide. Chin Med J (Engl) 131:2005–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv R, Du L, Zhang L et al (2019) Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci 217:119–127 [DOI] [PubMed] [Google Scholar]
- Maffia PC, Guerrieri D, Villalonga X et al (2018) Cementoin-SLPI fusion protein binds to human monocytes and epithelial cells and shows higher biological activity than SLPI. Sci Rep 8:5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrzak-Gorecka M, Majewski P, Grygier B et al (2016) Secretory leukocyte protease inhibitor (SLPI), a multifunctional protein in the host defense response. Cytokine Growth Factor Rev 28:79–93 [DOI] [PubMed] [Google Scholar]
- Matsuba S, Yabe-Wada T, Takeda K et al (2017) Identification of secretory leukoprotease inhibitor as an endogenous negative regulator in allergic effector cells. Front Immunol 8:1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettang M, Reichel SN, Lattke M et al (2018) IKK2/NF-kappaB signaling protects neurons after traumatic brain injury. FASEB J 32:1916–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami Y, Iwase T, Komiyama Y et al (2015) Secretory leukocyte protease inhibitor inhibits expression of polymeric immunoglobulin receptor via the NF-kappaB signaling pathway. Mol Immunol 67:568–574 [DOI] [PubMed] [Google Scholar]
- Minari ALA, Oyama LM, Dos Santos RVT (2017) The Secretory Leukocyte Protease Inhibitor mRNA expression is involved with inflammatory control after downhill exercise in the triceps brachii intermediary head in Wistar rats. J Muscle Res Cell Motil 38:231–239 [DOI] [PubMed] [Google Scholar]
- Mueller AM, Pedré X, Stempfl T et al (2008) Novel role for SLPI in MOG-induced EAE revealed by spinal cord expression analysis. J Neuroinflamm 5:20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn LL, Garkavtsev I (2018) SLPI: a new target for stopping metastasis. Aging (Albany NY) 10:13–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nernpermpisooth N, Prompunt E, Kumphune S (2017) An in vitro endothelial cell protective effect of secretory leukocyte protease inhibitor against simulated ischaemia/reperfusion injury. Exp Ther Med 14:5793–5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea TM, Burda JE, Sofroniew MV (2017) Cell biology of spinal cord injury and repair. J Clin Invest 127:3259–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr MB, Gensel JC (2018) Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics 15:541–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchi E, Frati A, Cantatore S et al (2019) Acute spinal cord injury: a systematic review investigating miRNA families involved. Int J Mol Sci. 10.3390/ijms20081841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires BRB, Silva R, Ferreira GM et al (2018) NF-kappaB: two sides of the same coin. Genes (Basel). 10.3390/genes9010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompunt E, Sanit J, Barrere-Lemaire S et al (2018) The cardioprotective effects of secretory leukocyte protease inhibitor against myocardial ischemia/reperfusion injury. Exp Ther Med 15:5231–5242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quabius ES, Gorogh T, Fischer GS et al (2015) The antileukoprotease secretory leukocyte protease inhibitor (SLPI) and its role in the prevention of HPV-infections in head and neck squamous cell carcinoma. Cancer Lett 357:339–345 [DOI] [PubMed] [Google Scholar]
- Ren H, Chen X, Tian M et al (2018) Regulation of inflammatory cytokines for spinal cord injury repair through local delivery of therapeutic agents. Adv Sci (Weinh) 5:1800529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq MM, Hannila SS (2015) Looking downstream: the role of cyclic AMP-regulated genes in axonal regeneration. Front Mol Neurosci 8:26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NA, Sousa N, Reis RL et al (2014) From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol 114:25–57 [DOI] [PubMed] [Google Scholar]
- Suh HS, Choi N, Tarassishin L et al (2012) Regulation of progranulin expression in human microglia and proteolysis of progranulin by matrix metalloproteinase-12 (MMP-12). PLoS ONE 7(4):e35115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson D, Aidoukovitch A, Anders E et al (2017) Secretory leukocyte protease inhibitor regulates human periodontal ligament cell production of pro-inflammatory cytokines. Inflamm Res 66:823–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart CC, Greene CM, McElvaney NG et al (2002) Secretory leucoprotease inhibitor prevents lipopolysaccharide-induced IκBα degradation without affecting phosphorylation or ubiquitination. J Biol Chem 277(37):33648–33653 [DOI] [PubMed] [Google Scholar]
- Taggart CC, Cryan SA, Weldon S et al (2005) Secretory leucoprotease inhibitor binds to NF-kappaB binding sites in monocytes and inhibits p65 binding. J Exp Med 202(12):1659–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C, Mutch J, Parent S et al (2015) The changing demographics of traumatic spinal cord injury: an 11-year study of 831 patients. J Spinal Cord Med 38:214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso ML, Chen Y-W, Scrimgeour AG et al (2007) Alterations in mRNA expression and protein products following spinal cord injury in humans. J Physiol 579(3):877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandooren J, Goeminne P, Boon L et al (2018) Neutrophils and activated macrophages control mucosal immunity by proteolytic cleavage of antileukoproteinase. Front Immunol 9:1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li X, Xu L et al (2003) Up-regulation of secretory leukocyte protease inhibitor (SLPI) in the brain after ischemic stroke: adenoviral expression of SLPI protects brain from ischemic injury. Mol Pharmacol 64(4):833–840 [DOI] [PubMed] [Google Scholar]
- Wang H, Wu Y, Han W et al (2018a) Hydrogen sulfide ameliorates blood-spinal cord barrier disruption and improves functional recovery by inhibiting endoplasmic reticulum stress-dependent autophagy. Front Pharmacol 9:858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jin Y, Li YX et al (2018b) Secretory leukocyte peptidase inhibitor expression and apoptosis effect in oral leukoplakia and oral squamous cell carcinoma. Oncol Rep 39:1793–1804 [DOI] [PubMed] [Google Scholar]
- Xu CB, Liu XS, Li JQ et al (2019) microRNA-539 functions as a tumor suppressor in papillary thyroid carcinoma via the transforming growth factor beta1/Smads signaling pathway by targeting secretory leukocyte protease inhibitor. J Cell Biochem 120:10830–10846 [DOI] [PubMed] [Google Scholar]
- Zakrzewicz A, Richter K, Zakrzewicz D et al (2019) SLPI inhibits ATP-mediated maturation of IL-1beta in human monocytic leukocytes: a novel function of an old player. Front Immunol 10:664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yao JL, Dong SC et al (2015) SLPI knockdown induced pancreatic ductal adenocarcinoma cells proliferation and invasion. Cancer Cell Int 15:37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YD, Zhu ZS, Zhang D et al (2017) Lentivirus-mediated silencing of the PTC1 and PTC2 genes promotes recovery from spinal cord injury by activating the Hedgehog signaling pathway in a rat model. Exp Mol Med 49:e412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang WX, Zhang YJ et al (2018) Melatonin for the treatment of spinal cord injury. Neural Regen Res 13(10):1685–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong QQ, Wang X, Li YF (2017) Secretory leukocyte protease inhibitor promising protective roles in obesity-associated atherosclerosis. Exp Biol Med (Maywood) 242:250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]