Abstract
Sustained activation of pro-apoptotic signaling due to a sudden and prolonged disturbance of cerebral blood circulation governs the neurodegenerative processes in prefrontal cortex (PFC) of rats whose common carotid arteries are permanently occluded. The adequate neuroprotective therapy should minimize the activation of toxicity pathways and increase the activity of endogenous protective mechanisms. Several neuroprotectants have been proposed, including progesterone (P4). However, the underlying mechanism of its action in PFC following permanent bilateral occlusion of common carotid arteries is not completely investigated. We, thus herein, tested the impact of post-ischemic P4 treatment (1.7 mg/kg for seven consecutive days) on previously reported aberrant neuronal morphology and amount of DNA fragmentation, as well as the expression of progesterone receptors along with the key elements of Akt/Erk/eNOS signal transduction pathway (Bax, Bcl-2, cytochrome C, caspase 3, PARP, and the level of nitric oxide). The obtained results indicate that potential amelioration of histological changes in PFC might be associated with the absence of activation of Bax/caspase 3 signaling cascade and the decline of DNA fragmentation. The study also provides the evidence that P4 treatment in repeated regiment of administration might be effective in neuronal protection against ischemic insult due to re-establishment of the compromised action of Akt/Erk/eNOS-mediated signaling pathway and the upregulation of progesterone receptors.
Electronic supplementary material
The online version of this article (10.1007/s10571-019-00777-2) contains supplementary material, which is available to authorized users.
Keywords: Progesterone, Neuroprotection, Cerebral hypoperfusion, Prefrontal cortex
Introduction
Brain damage due to a sudden and prolonged disturbance of cerebral blood flow underlies pathological conditions associated with neurodegeneration, cognitive decline, and motor dysfunction, including Alzheimer’s disease and vascular and aging dementia (De Jong et al. 1999; de la Torre 2002a, b; Farkas et al. 2007; Villapol et al. 2014; Nelson et al. 2016; Clark et al. 2017; Rius-Pérez et al. 2018). Since the first descriptions of these impairments, the treatment has remained challenging and numerous studies have focused on developing adequate therapeutic strategies that would minimize the activation of toxic pathways triggered by the insult and enhance the activity of endogenous neuroprotective mechanisms. During the past two decades, sex steroid hormones, such as progesterone (P4) and its metabolites, have attracted interest as promising therapeutic agents following ischemic brain injury. In prior studies of either transient or permanent ischemic insult, P4 has been shown to reduce lesions’ volume and cerebral edema along with promoting functional recovery (Allen et al. 2016; Perez-Alvarez et al. 2015; Zhao et al. 2015; Yousuf et al. 2014).
Within the brain, P4 easily diffuses across the cellular membranes due to its lipophilic nature, and it interacts with specific, widespread distributed receptors. Classic nuclear progesterone receptors (PRs) are present primarily as two isoforms, PRA and PRB. They are ligand-activated transcription factors that shuttle between the cytoplasm and the nucleus where they target gene promoters to activate or repress transcription (Brinton et al. 2008). In addition to genomic effects, the interaction of P4 with classic PRs and/or membrane PRs (mPRs) and progesterone membrane receptor component (PGMRC) initiates rapid non-genomic mechanisms (Guennoun et al. 2008; Brinton et al. 2008; Ishrat et al. 2012). Both genomic and non-genomic P4 actions may be responsible for the modulation of signaling cascades and the activation of Akt (Protein kinase B) and Erk (Extracellular signal-regulated kinases). This is accompanied with the upregulation of endothelial nitric oxide synathase (eNOS) (Khorram and Han 2009) and/or inactivation of apoptotic cascade (Singh 2001; Guerra-Araiza et al. 2009; Ishrat et al. 2012; Deutsch et al. 2013; Stanojlović et al. 2015a), including the pro-apoptotic members of Bcl-2 family and caspase 3 (Perez-Alvarez et al. 2015; Perez-Alvarez and Wandosell 2016; Yousuf et al. 2017).
Although numerous reports demonstrated the dose-dependent response and involvement of various molecular mechanisms and signaling pathways in P4-mediated neuroprotection in different brain injuries (Yao et al. 2005; Cai et al. 2008; Liu et al. 2010; Allen et al. 2016; Andrabi et al. 2017; Yousuf et al. 2017), little attention has been devoted to its actions in the rat model of permanent bilateral occlusion of common carotid arteries, the 2VO model. To our knowledge, only our previous study indicated that repeated P4 treatment induces modulation of activated apoptotic cascade in the hippocampus after 2VO, and it attenuates DNA fragmentation along with the reduction of the number of degenerating neurons (Stanojlović et al. 2015a). Thus, the aim of the present study was to evaluate the possible therapeutic efficacy of P4 in PFC of 2VO animals by assessing the following: (a) potential amelioration of histological changes; (b) expression and shuttle of PRs from cytoplasm to nucleus; (c) level of molecules involved in apoptotic signaling (Bcl-2, Bax, cytochrome C, caspase 3, PARP, Akt and Erk kinases, eNOS, and NO). The obtained data might help reveal some molecular mechanisms underlying the P4 action in 2VO-damaged brain using parameters that were shown to be modified in other models of brain injuries after P4 treatment.
Materials and Methods
Materials
Analytical grade salts and buffer reagents 4-Pregnene-3.20-dione (P4), bovine serum albumin (BSA), sulphanilamide, diphenylamine (DPA), Eukitt® Quick-hardening mounting medium, xylol, and N-(naphthyl)-ethylenediaminedihydrochloride were purchased from Sigma-Aldrich Co (St Louis, MO, USA). Polyvinylidene fluoride (PVDF) membrane was acquired from EMD Millipore Corporation (Billerica, MA, USA), while non-fat dry milk (NFM)/Blotto was obtained from Santa Cruz Biotechnology, Inc. (CA, USA). Protease and phosphatase inhibitors were provided from Roche (Penzberg, Germany) and Pierce Biotechnology (Rockford, IL, USA), respectively. Enhanced chemiluminescent (ECL) system was acquired from EMD Millipore Corporation (Billerica, MA, USA), while primary and secondary antibodies were gained from different suppliers (Table 1). From Invitrogen (Carlsbad, CA, USA), TRIzol Reagent and primers (presented in Table 2) were purchased, whereas High Capacity cDNA Reverse Transcription Kit and SYBR Green PCR Master Mix were from Thermo Fisher Scientific (Waltham, MA, USA).
Table 1.
List of primary and secondary antibodies used for Western blot analysis
| Antigen | Company | Product | Dilution |
|---|---|---|---|
| Bax | Santa Cruz Biotechnology Inc., CA, USA | sc-7480 | 1:1000 |
| Bcl-2 | Santa Cruz Biotechnology Inc., CA, USA | sc-492 | 1:1000 |
| Procaspase 3 | Santa Cruz Biotechnology Inc., CA, USA | sc-7148 | 1:1000 |
| Cytochrome C | Santa Cruz Biotechnology Inc., CA, USA | sc-13561 | 1:1000 |
| PR | Santa Cruz Biotechnology Inc., CA, USA | sc-810 | 1:1000 |
| eNOS (t-eNOS) | Santa Cruz Biotechnology Inc., CA, USA | sc-654 | 1:1000 |
| Phospho-eNOS (p-eNOSSer1177) | Santa Cruz Biotechnology Inc., CA, USA | sc-12972 | 1:1000 |
| Phospho-eNOS (p-eNOSThr495) | Santa Cruz Biotechnology Inc., CA, USA | sc-19827 | 1:1000 |
| β-actin | Santa Cruz Biotechnology Inc., CA, USA | sc-1615 | 1:1000 |
| c-jun | Santa Cruz Biotechnology Inc., CA, USA | sc-1694 | 1:1000 |
| mHsp60 | Santa Cruz Biotechnology Inc., CA, USA | sc-13115 | 1:1000 |
| Akt (t-Akt) | Cell Signaling Inc., Beverly, MA, USA | 9272 | 1:1000 |
| phospho-Akt (p-Akt) | Cell Signaling Inc., Beverly, MA, USA | 9271S | 1:1000 |
| Erk42/44 kDa (t-Erk42/44 kDa) | Cell Signaling Inc., Beverly, MA, USA | 9102 | 1:1000 |
| Phospho-Erk42/44 (p-Erk42/44 kDa) | Cell Signaling Inc., Beverly, MA, USA | 9101S | 1:1000 |
| PARP | Cell Signaling Inc., Beverly, MA, USA | 9542 | 1:1000 |
| α-tubulin | Sigma-Aldrich, St Louis, MO, USA | T9026 | 1:500 |
| Anti-rabbit IgG-HRP | Santa Cruz Biotechnology Inc., CA, USA | sc-2030 | 1:5000 |
| Anti-mouse IgG-HRP | Santa Cruz Biotechnology Inc., CA, USA | sc-2318 | 1:5000 |
| Anti-goat IgG-HRP | Santa Cruz Biotechnology Inc., CA, USA | sc-2033 | 1:5000 |
Table 2.
List of primers used for real-time PCR
| Gene | Sequences of primers | Melting temperature (°C) | Product length (bp) |
|---|---|---|---|
| RPL-19 | F: TCGCCAATGCCAACTCTCGTC | 63 | 92 |
| R: AGCCCGGGAATGGACAGT | 65 | ||
| Bax | F: TGCTACAGGGTTTCATCCAG | 58 | 135 |
| R: CCAGTTCATCGCCAATTC | 57 | ||
| Bcl-2 | F: TGGAAAGCGTAGACAAGGAGATGC | 65 | 88 |
| R: CAAGGCTCTAGGTGGTCATTCAGG | 67 | ||
| PARP | F: CGCTCAAGGCTCAGAACGAG | 63 | 130 |
| R: CAGGATTGCGGACTCTCCA | 59 | ||
| caspase 3 | F: GATGTCGATGCAGCTAACC | 57 | 321 |
| R: TGTCTCAATACCGCAGTCC | 57 |
Animals
All experiments were performed on adult male rats (300–350 g, n = 81) of Wistar strain, obtained from the local colony and separate litters. Animals were housed (3–4/cage) under the standard conditions 12 h light/dark regime, constant ambient temperature (22 ± 2 °C), and ad libitum access to food (commercial pellet) and tap water. An appropriate action was taken to alleviate the pain and discomfort of the animals in accordance with the European Community Council Directive of 86/609/EEC and 010/63/EU for animal experiments. Research procedures were approved by the Ethical Committee for the Use of Laboratory Animals of VINČA Institute of Nuclear Sciences, University of Belgrade, Belgrade, Republic of Serbia (protocol authorization numbers 02/11 and 323-07-04253/2016-05).
Surgical Procedure and Treatment
Following the chloral hydrate anesthesia (5%, 400 mg/kg), both common carotid arteries were exposed by a neck ventral midline incision, and they were carefully separated from the carotid sheath, cervical sympathetic nerve, and vagus nerve. The following step included their double ligation with 5–0 silk suture (Stanojlović et al. 2015a, b). The animals were randomly assigned in two groups. The rats in the first injured group (n = 29) were injected subcutaneously with vehicle (commercial flax oil, dose 1 ml/kg/day, 2VO + V), while the animals in the second injured group (n = 29) received P4 dissolved in commercial flax oil in dose of 1.7 mg/kg/day (2VO + P4). The same surgical procedure was performed on rats in the sham group (n = 23), but without actual ligation of carotid arteries. Sham-operated animals were subjected to vehicle (commercial flax oil, Sham + V) and used as a control, an uninjured group.
Males were used due to their low and stable levels of circulating P4 and considering that protective treatment for ischemic injuries should also be applicable to men who exhibit a higher risk and poorer outcome compared to women (Liu et al. 2009, 2012). The P4 dose and regiment of administration used in current experimental setup were determined according to our previous study showing that 1.7 mg/kg/day provides P4 ameliorative effect following ischemic injury in hippocampus (Stanojlović et al. 2015a). The treatments in all experimental groups were administrated from 09.00 to 10.00 AM for seven consecutive days and animals were sacrificed 4 h following the last treatment (Stanojlović et al. 2015a).
Tissue and Sample Preparation
To prepare for Nissl and Fluoro-Jade C (FJC) staining, the rats (n = 5 per each injured group and n = 5 per uninjured group) were deeply anesthetized with an overdose i.p. injection of 5% chloral hydrate, perfused transcardially with saline (4 °C, 200 ml), and then with 4% paraformaldehyde (PFA). The isolated whole brains were then fixed in 4% PFA for 24 h at 4 °C and cryoprotected in ascending sucrose series gradient in 0.2 M phosphate-buffered saline (10, 20 and 30% for 24 h at 4 C).
The animals for the other analyses (for DNA fragmentation assay (n = 8 per each injured group and n = 6 per uninjured group), immunoblotting of proteins in cytosolic, nuclear, and mitochondrial extracts (n = 8 per each injured group and n = 6 per uninjured group), and qRT-PCR analysis (n = 8 per each injured group and n = 6 per uninjured group) were quickly decapitated with guillotine (Harvard Apparatus, Holliston, MA, USA), and prefrontal cortici (PFC) were isolated, immediately frozen in liquid nitrogen, and stored at − 70 °C for subsequent processing.
Nissl and Fluoro-Jade C Staining
For Nissl staining, coronal sections (20 μm) including PFC were cut and immersed in dH2O and incubated for 20 min in 0.5% thionine solution. Excessive thionine was washed out with dH2O and ascending ethanol series (70, 95, and 99.9%). The sections were cleared in xylol and covered with Eukitt® Quick-hardening mounting medium (Zaric et al. 2018) and examined under a light Olympus AX70 microscope (Olympus AX70 Fluorescence Microscope, Analytical Instruments LLC, Minneapolis, Minnesota, USA).
To perform FJC staining, before transferring for 10 min in 0.0001% FJC solution with 0.1% acetic acid, coronal sections were immersed in PBS for 5 min and then in 0.06% KMnO4 solution for 10 min. Following the washing in dH2O for 1 min three times, slides were dried, incubated with xylol for 2 min, and cover slipped. The sections were examined under Zeiss Axiovert fluorescent microscope (Zeiss, Jena, Germany) (Zaric et al. 2018).
The number of cells with potential morphological changes assessed by the Nissl staining and degenerating neurons and labeled by the Fluoro-Jade C were counted in three fields under the area of the screen (0.38 mm2) in five region sections of interest per animal. Sections from all groups were run in the same assay.
DNA Fragmentation Assay
DPA colorimetric assay was carried out as previously described (Drakulić et al. 2013; Stanojlović et al. 2015b) and results are obtained by the formula: % of fragments = [OD600 nm T/OD600 nm (T + B)] × 100, where T is fragmented DNA and B is intact DNA obtained from Sham + V rats as well as 2VO + V or 2VO + P4 animals. The level of DNA fragmentation in each sample was expressed as a percentage of total DNA appearing in the supernatant fraction and obtained values are presented as a percentage of DNA fragmentation detected in Sham + V.
Preparation of Subcellular Fractions for Western Blot Analysis
To estimate the impact of P4 treatment on the protein expression and activation in mitochondrial (mth), nuclear (nuc), and cytosolic (cyt) subcellular fractions, the fractions were prepared as previously described (Stanojlovic et al. 2015a, b).
Western Blotting
Equal amounts of total proteins (20 μg of cytosolic, nuclear or mitochondrial extracts) were resolved on 10% or 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), depending on the protein molecular mass, and transferred on PVDF membranes. Membranes were blocked in TBS-T (TRIS buffered saline pH 7.4, 0.1% Tween 20) containing 5% NFM or BSA for 1 h and incubated overnight at 4 °C with primary antibodies (dilutions and specifications are given in Table 1). After washing, the membranes were incubated for 2 h with appropriate secondary antibodies (Table 1). The antigen–antibody complex was detected using an enhanced ECL system and densitometric analysis was performed using Image J software package. The relative protein abundance of investigated proteins in Sham + V group was normalized to β-actin and arbitrarily defined as 100%, whereas levels in 2VO + V and 2VO + P4 groups were also normalized to β-actin and calculated with respect to this value.
The additional experiments confirming the purity of the cell fractions using specific antibodies have been performed to exclude the possibility of cross-contamination. Namely, samples (20 μg of proteins per lane) were loaded onto a SDS–polyacrylamide gel, and following the transfer, PVDF membranes were incubated with anti-c-jun, anti-α-tubulin, and anti-mHsp60 (Table 1) used as nuclear, cytosolic, and mitochondrial markers, respectively, and β-actin as loading control (Supplementary data 1).
Measurement of Nitric Oxide Content
Production of nitric oxide (NO) was estimated by measuring nitrite (NO2) levels as a stable NO product, using the Griess reagent as reported previously (Boyadjieva and Andreeva-Gateva 2011). Briefly, 50 µl of sample/standard/blank was mixed with an equal volume of Griess reagent, was incubated for 10 min in the dark, and the absorbance was measured spectrophotometrically (WALLAC 1420-Victor2 Multilabel Counter, PerkinElmer, Waltham, MA, USA). The increasing concentrations of sodium nitrite were used to generate a standard curve from which the NO2 concentration in the sample was calculated. The level of NO2 in Sham + V group was taken as 100%, whereas the changes in other experimental groups were calculated with respect to this value.
Quantitative Real-Time PCR
The total RNA was extracted using TRIzol Reagent according to the manufacturer’s instructions, while 2 μg of total RNA was used for cDNA synthesis. Real-time PCR amplifications were performed in duplicate, using a mixture of SYBR Green PCR Master Mix, cDNA samples, and designate primers (sequences are given in Table 2). Reactions were conducted in the 7500 Real-time PCR System (Applied Biosystems, Foster City, CA, USA). The relative changes in mRNA expression were analyzed as described previously (Stanojlović et al. 2015a) using the 2−ΔΔCt method and RPL-19 as an internal control.
Data Analysis
The results are presented as a percentage of the mean of the values in Sham + V ± SEM. One way analysis of variance (ANOVA) test was used to assess the differences between the means of the groups followed by Tukey's post hoc test using Origin 7.5 software package. The values of p < 0.05 or less were considered statistically significant.
Results
Impact of P4 Treatment on Cell Survival and Function in PFC of Rats Subjected to 2VO
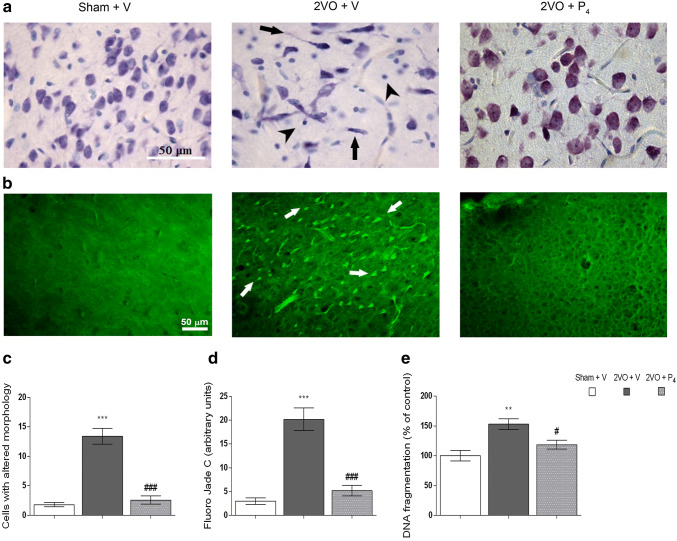
A routine Nissl staining was used to evaluate the structural changes of cortical neurons. The uninjured rats, Sham + V, exhibited the regular anatomical structure of the entire cortex and individual cells appeared to be morphologically intact with a well-defined cell soma, round vesicular nucleus, prominent nucleolus, and visible Nissl bodies (Fig. 1a, left panel). In the injured group that received vehicle, morphologically abnormal neurons with irregularly arranged Nissl substance were detected (Fig. 1a, middle panel). As presented in Fig. 1a, (right panel) in 2VO + P4 group, neurons with regular cell morphology and visible Nissl substance were observed, although some neurons exhibited darker staining, resembling to the ones observed in 2VO + V. Nissl staining was further used for quantitative analysis of cells with potential changes in morphology. As presented in Fig. 1c, ischemic insult and vehicle treatment induced the appearance of shrunken cells that could barely be observed in the Sham + V group (p < 0.001, Sham + V vs. 2VO + V) and 2VO + P4 group (p < 0.001, 2VO + V vs. 2VO + P4). No difference regarding the number of cells with potential morphological changes could be observed between Sham + V animals and injured group treated with P4 (Fig. 1c).
Fig. 1.
Progesterone promotes cell survival in prefrontal cortex of rats subjected to permanent common carotid occlusion (2VO). Nissl staining indicates the typical morphology of the cells in prefrontal cortex of sham-operated animals treated with vehicle (Sham + V, left panel). Following permanent ligation of carotid arteries and vehicle treatment (2VO + V, middle panel), the slices showed the presence of aberrant cell morphology (arrows indicate dark, shrunken cells). When treated with progesterone (2VO + P4, right panel), 2VO animals exhibited regular cell morphology although some cells were darker. A thionine-stained cortical sections were viewed at 63 × magnification (20 μm thick sections), while arrows indicate degenerative neuronal cells and arrowheads indicate glial cells (a). Fluoro-Jade C (FJC) staining of degenerative neuronal cells in prefrontal cortex of rats subjected to sham operation and vehicle treatment (Sham + V, left panel), and animals subjected to permanent occlusion of common carotid arteries and either vehicle (2VO + V, middle panel) or P4 treatment (2VO + P4, right panel). FJC-stained cortical sections were viewed at 200 × magnification (20-μm-thick sections), while arrows indicate FJC-positive degenerative neuronal cells (b). Quantitative analysis of cells with aberant morphology in prefrontal cortex of rats, assessed by Nissl staining (c). Quantitative analysis of degenerative neuronal cells in prefrontal cortex of rats, assessed by FJC staining (d). Measurement of DNA fragments with the diphenylamine (DPA) colorimetric assay (e). Data are presented as the mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test (**p < 0.01, ***p < 0.001 Sham + V vs. 2VO + V, and #p < 0.05, ###p < 0.001 2VO + V vs. 2VO + P4)
FJC staining was performed to label and identify degenerating neurons in PFCs of all experimental groups. As illustrated in Fig. 1b, FJC-positive cells were barely observed in this brain region of sham-operated animals treated with vehicle. In rats that were subjected to permanent bilateral occlusion of common carotid arteries and vehicle treatment, a large number of FJC-positive structures were found. Namely, FJC-positive cells showed a bright green color and exhibited neuronal profile, and they were distributed in layers III and IV. In animals subjected to permanent bilateral occlusion of common carotid arteries and P4 treatment, FJC-positive structures were still observed within layers III and IV. However, these structures were far fewer in number than in the other injured group and at the similar level as in the sham-operated group. Moreover, the quantitative analysis of FJC staining revealed that in 2VO + V rats, the number of degenerating neurons was higher comparing to Sham + V (p < 0.001, Sham + V vs. 2VO + V) and also to the other injured group treated with P4 (p < 0.001, 2VO + V vs. 2VO + P4) (Fig. 1d). P4 treatment following ischemic insult restored the number of degenerating neurons to the one observed in Sham + V group, indicating that P4 treatment is able to downscale neurodegenerative processes (Fig. 1d).
DPA colorimetric assay was used to assess the neuroprotective potential of P4 in the state of chronic cerebral hypoperfusion. Following P4 treatment, the content of apoptosis-specific DNA fragments in injured group treated with vehicle was reduced by 22% (p < 0.05, 2VO + V vs. 2VO + P4) and it returned to the values detected in the sham group (Sham + V vs. 2VO + P4) (Fig. 1e), suggesting that P4 might be capable of diminishing apoptotic-related changes in this type of brain injury.
PRs are Upregulated After P4 Treatment
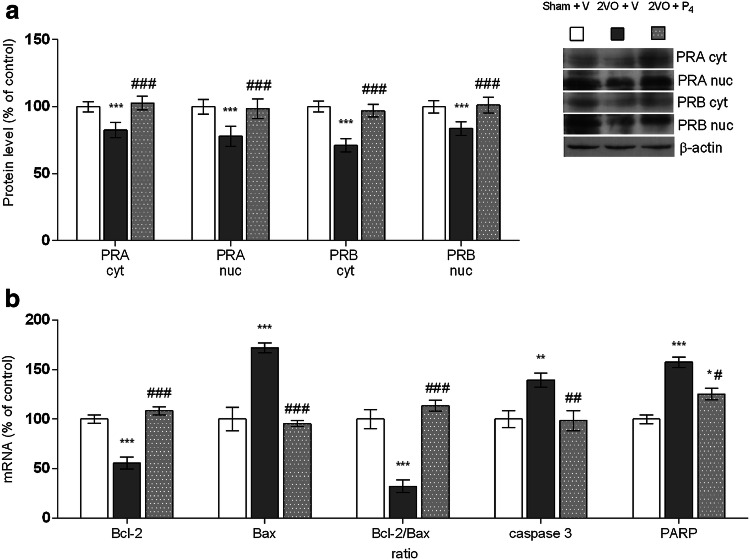
Since there is limited knowledge about how classical PRs respond after neural injury and hormonal treatment, herein, we evaluated the expression and redistribution of PRA and PRB in PFCs of rats that had been subjected to permanent ligation of common carotid arteries and P4 treatment. In the injured group after vehicle treatment, levels of PRA were reduced by 17% in cytosolic and 22% in nuclear fraction compared to sham-operated animals (p < 0.001, Sham + V vs. 2VO + V). The similar pattern of expression was observed for PRB where a decline of 29% in cytosolic and 16% in nuclear fraction was detected in comparison to controls (p < 0.001, Sham + V vs. 2VO + V). Following P4 treatment, the levels of PRA were increased by 25% in both cytoplasm and nucleus, with respect to the other injured group (p < 0.001, 2VO + V vs. 2VO + P4), and the values were restored to those observed in the sham-operated animals (Sham + V vs. 2VO + P4) (Fig. 2a). In parallel, the expression of PRB showed similar trend in both investigated subcellular fractions in contrast to the injured group treated with vehicle (increment of 20% in nucleus, p < 0.001, and 37% in cytoplasm, p < 0.001, 2VO + V vs. 2VO + P4) and relative to the control group (Sham + vs. 2VO + P4) (Fig. 2a).
Fig. 2.
Progesterone modulates progesterone receptor (PRA and PRB) protein levels in cytosolic (cyt) and nuclear (nuc) fractions obtained from prefrontal cortex of rats subjected to permanent common carotid occlusion (2VO) (a). Progesterone regulates PARP, caspase 3, Bcl-2, Bax, and Bcl-2-to-Bax mRNAs levels after 2VO (b). Data are presented as mean ± SEM, whereas the values of the sham-operated group treated with vehicle (Sham + V) were set as 100%. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test (*p < 0.05, **p < 0.01, ***p < 0.001 Sham + V vs. 2VO + V, and #p < 0.05, ##p < 0.01, ###p < 0.001 2VO + V vs. 2VO + P4)
Changes in Bcl-2, Bax, Caspase 3, and PARP mRNA Levels
A quantitative real-time PCR analysis showed a constitutive expression of Bcl-2, Bax, caspase 3, and PARP mRNAs in the PFC of control animals. Ischemic insult and vehicle treatment provoked decrement of Bcl-2 mRNA levels of 44% (p < 0.001, Sham + V vs. 2VO + V) and dramatic increment of Bax of 72% compared to controls (p < 0.001, Sham + V vs. 2VO + V). Furthermore, the relative ratio of Bcl-2 and Bax mRNAs indicated that observed modifications of Bcl-2 and Bax mRNA led to a decrease of Bcl-2/Bax mRNA ratio by 68% (p < 0.001, Sham + V vs. 2VO + V). On the other hand, P4 treatment increased Bcl-2 gene expression by 93% and decreased Bax by 44% with respect to the injured group treated with vehicle (p < 0.001, 2VO + V vs. 2VO + P4). This caused a shift in the ratio between apoptotic molecules within Bcl-2 family towards the anti-apoptotic ones (p < 0.001, 2VO + V vs. 2VO + P4), while at the same time, Bcl-2/Bax ratio remained unchanged in comparison to the sham-operated animals (Sham + V vs. 2VO + P4) (Fig. 2b).
As Fig. 2b shows, caspase 3 mRNA level was augmented for 40% in injured group after vehicle treatment when compared to the sham-operated animals (p < 0.01, Sham + V vs. 2VO + V). P4 treatment restored it to the control values (Sham + V vs. 2VO + P4) and kept it lower by 29% than in the other injured group (p < 0.01, 2VO + V vs. 2VO + P4). Further, the expression of PARP mRNA in injured group treated with vehicle was increased by 58% regarding to controls (p < 0.001, Sham + V vs. 2VO + V) and 21% comparing to the injured group treated with P4 (p < 0.05, 2VO + V vs. 2VO + P4). Following P4 treatment, PARP mRNA was still elevated by 25% relative to the controls (p < 0.05, Sham + V vs. 2VO + P4) (Fig. 2b).
P4 Affects Regulators of Apoptotic Signaling in PFC of 2VO Rats
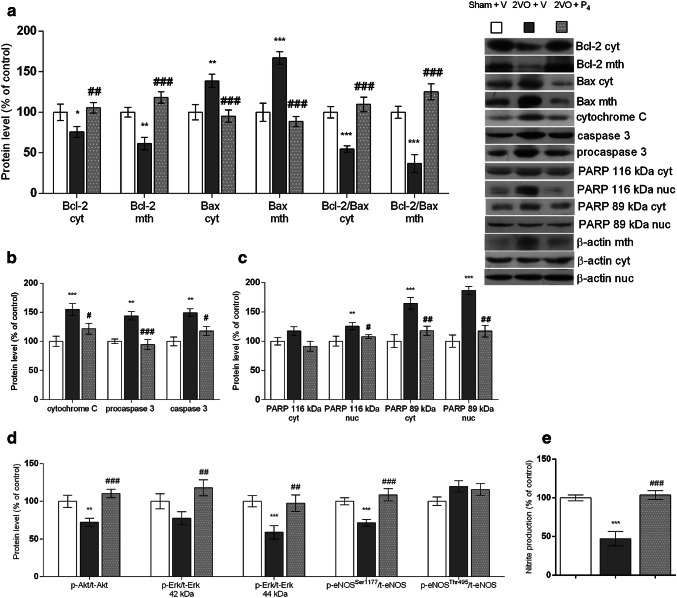
To evaluate the potential relation between P4 treatment and 2VO-induced mitochondria-triggered apoptosis in cortical brain area, we estimated the expression and intracellular redistribution between cytoplasm and mitochondria of pro- and anti-apoptotic members of Bcl-2 family, Bax, and Bcl-2. Namely, in the vehicle-treated injured group, Bcl-2 expression was significantly decreased in both investigated subcellular fractions compared to control (24%, p < 0.05 for cytosolic and 39%, p < 0.01 for mitochondrial fraction, Sham + V vs. 2VO + V). In comparison to the injured group treated with vehicle, the one treated with P4 had Bcl-2 levels higher by 39% in cytosolic (p < 0.01, 2VO + V vs. 2VO + P4) and by 93% in mitochondrial fraction (p < 0.001, 2VO + V vs. 2VO + P4) (Fig. 3a). In parallel, 2VO induced upregulation of Bax in both subcellular fractions (39%, p < 0.01 for cytosolic and 67%, p < 0.001 for mitochondrial fraction, Sham + V vs. 2VO + V), while its expression decreased by 32% after P4 treatment in cytosolic (p < 0.001, 2VO + V vs. 2VO + P4) and by 47% in mitochondrial fraction (p < 0.001, 2VO + V vs. 2VO + P4). Correspondingly, under the influence of P4, when the mitochondrial/cytoplasmic ratios of Bcl-2-to-Bax were analyzed, a significant increase in the cytoplasm and more prominent enhancement in mitochondrial fraction (p < 0.001, 2VO + V vs. 2VO + P4) were detected (Fig. 3a).
Fig. 3.
Progesterone alters protein expression of apoptotic molecules in cytosolic (cyt), nuclear (nuc), or mitochondrial (mth) fraction and nitrite level (NO) in cyt obtained from prefrontal cortex of rats subjected to permanent common carotid occlusion (2VO). Graphics represent representative Western blots and protein levels of Bcl-2, Bax, and Bcl-2/Bax protein ratio (a), cytochrome C, procaspase 3, and cleaved caspase 3 (b), PARP (full length (116 kDa) and cleaved (89 kDa)) (c), ratios of p-Akt to t-Akt, p-Erk 42/44 kDa to t-Erk42/44 kDa, and p-eNOSSer1177 or p-eNOSThr495 to t-eNOS (d). Bands were quantified by densitometry and normalized to β-actin as a loading control. Progesterone treatment increased NO content in the prefrontal cortex after 2VO (e). Data are presented as the mean ± SEM, whereas the values of the sham-operated group treated with vehicle (Sham + V) were set as 100%. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test (*p < 0.05, **p < 0.01, ***p < 0.001 Sham + V vs. 2VO + V, and #p < 0.05, ##p < 0.01, ###p < 0.001 2VO + V vs. 2VO + P4)
We also monitored whether the P4 treatment following permanent ligation of the common carotid arteries could cause the changes in the levels of pro-apoptotic proteins, like cytochrome C and caspase 3. The abundance of cytochrome C was enhanced by 55% in the injured rats treated with vehicle when compared to the sham group (p < 0.01, Sham + V vs. 2VO + V), while its level decreased almost to the control one due to P4 treatment (21%, p < 0.05, 2VO + V vs. 2VO + P4) (Fig. 3b). Regarding the expression of procaspase 3 and cleaved caspase 3, when compared to the control, brain injury provoked an increase in their levels by 44% and 49%, respectively (p < 0.01, Sham + V vs. 2VO + V). The P4 treatment decreased expression of these pro-apoptotic markers in comparison to the other injured group (35% p < 0.001 for procaspase 3 and 21% for cleaved caspase 3, p < 0.05, 2VO + V vs. 2VO + P4), and restored them to the level detected in sham-operated animals (Sham + V vs. 2VO + P4) (Fig. 3b). Moreover, the cleavage of PARP, from the full length protein of 116 kDa into an inactive 89 kDa fragment by activated caspase 3, was assessed. No significant difference in the expression of PARP 116 kDa was observed in cytosolic fraction among all experimental groups (Fig. 3c). As illustrated in Fig. 3c, in nuclear fraction of the injured group treated with vehicle, the level of PARP 116 kDa was increased by 26% compared to the control (p < 0.01, Sham + V vs. 2VO + V), and decreased by 19% following P4 treatment (p < 0.05, 2VO + V vs. 2VO + P4). Further, induced brain injury elevated protein content of PARP 89 kDa in both cytosolic and nuclear fractions with respect to the sham-operated group (65%, p < 0.001 in cytosolic and 87%, p < 0.001 in nuclear fraction, Sham + V vs. 2VO + V). In comparison to vehicle treatment, P4 caused the decrease of its expression by 29% in cytosol and 37% in nucleus (p < 0.01, 2VO + V vs. 2VO + P4) (Fig. 3c).
Effects of P4 treatment in the state of cerebral hypoperfusion were also evaluated based on the level of activation of Akt and Erk by analyzing their protein expression and the extent of phosphorylation in contrast to the control. In all investigated groups, the abundance of total Akt (t-Akt) was not significantly affected by brain injury or by treatment (Sham + V vs. 2VO + V, Sham + V vs. 2VO + P4, and 2VO + V vs. 2VO + P4) (Table 3). In contrast to vehicle treatment, P4 following brain injury provoked a significant increase in Akt activation that is associated with phosphorylation of Akt on Ser473(p-Akt) (38%, p < 0.01, 2VO + V vs. 2VO + P4) (Table 3). Moreover, a similar level of its phosphorylation was detected between the control and injured rats treated with P4 (Sham + V vs. 2VO + P4) (Table 3). To determine whether the ratio of p-Akt to t-Akt, as an indicator of Akt activation, was modified, p-Akt levels were normalized to t-Akt gained on the same membrane. According to our results, in injured groups normalized Akt levels decreased by 35% following vehicle in comparison to P4 treatment (p < 0.001, 2VO + V vs. 2VO + P4) and by 28% with respect to the control (p < 0.01, Sham + V vs. 2VO + V) (Fig. 3d). This implies that 2VO-induced damage could cause inactivation of Akt which in contrast might be ameliorated by P4 treatment. Furthermore, concerning the expression of other investigated pro-survival kinase, Erk, the levels of total Erk isoforms (t-Erk42 kDa and t-Erk 44 kDa) were unchanged among investigated groups (Sham + V vs. 2VO + V, Sham + V vs. 2VO + P4, and 2VO + V vs. 2VO + P4) (Table 3). In contrast, both phosphorylated isophorms, p-Erk44 kDa and p-Erk42 kDa, were reduced in the injured group that received vehicle in comparison to the control group (44%, p < 0.001 for p-Erk44 kDa and 25%, p < 0.05 for p-Erk42 kDa, Sham + V vs. 2VO + V) and this was annulled following P4 treatment (augmentation by 70%, p < 0.001 for p-Erk44 kDa and 41%, p < 0.01 for p-Erk42 kDa, 2VO + V vs. 2VO + P4) (Table 3). Moreover, as an indicator of Erk activation, we assessed the ratio of both isophorms of p-Erk to t-Erk and the obtained levels of phosphorylated isophorms were normalized to total isophorms gained on the same membrane. Obtained results indicate that both normalized Erk isophorms were significantly increased following P4 treatment when compared to vehicle-treated injured group (64% for Erk 44 kDa, p < 0.001 and 51% for Erk42 kDa, p < 0.001, 2VO + V vs. 2VO + P4). With respect to the control, the Erk 44 kDa ratio was reduced by 41% (p < 0.001, Sham + V vs. 2VO + V), while for Erk 42 kDa this alteration, although notable, was not significant (Fig. 3d). Modulation of Erk ratio under the influence of brain damage along with its activation in the state of cerebral hypoperfusion following P4 treatment might suggest that Erk is an important protein involved in the effects observed in current experimental setup.
Table 3.
Quantitative analysis of several investigated proteins expression
| protein | Sham + V | 2VO + V | 2VO + P4 |
|---|---|---|---|
| t-Akt | 100 ± 8.75 | 105.63 ± 7.93 | 95.11 ± 8.34 |
| p-Akt | 100 ± 6.15 | 76.26 ± 5.66** | 105.03 ± 9.35## |
| t-Erk 44 kDa | 100 ± 10.05 | 77.51 ± 8.64 | 117.80 ± 10.41 |
| p-Erk 44 kDa | 100 ± 6.90 | 56.21 ± 9.45*** | 94.96 ± 9.02### |
| t-Erk 42 kDa | 100 ± 5.30 | 96.09 ± 7.54 | 90.34 ± 6.83 |
| p-Erk 42 kDa | 100 ± 6.64 | 74.48 ± 4.87* | 106.42 ± 7.82## |
| t-eNOS | 100 ± 4.00 | 104.35 ± 5.31 | 96.01 ± 5.28 |
| p-eNOSSer1177 | 100 ± 2.22 | 72.40 ± 3.40*** | 97.87 ± 4.46### |
| p-eNOSThr495 | 100 ± 3.19 | 119.88 ± 3.46** | 105.49 ± 3.78# |
Optical densities of bands expressed as percentages of sham-operated animals treated with vehicle. Data are presented as means ± SEM. One way analysis of variance (ANOVA) test is used to assess the differences between the means of the groups followed by post hoc Tukey's test
Sham + V sham-operated animals treated with vehicle, 2VO + V animals subjected to bilateral common carotid artery occlusion and vehicle treatment, 2VO + P4 animals subjected to bilateral common carotid artery occlusion and progesterone treatment
*p < 0.05, **p < 0.01, ***p < 0.001; #p < 0.05, ##p < 0.01, ###p < 0.001
Phosphorylation of eNOS at Ser1177 through kinase-mediated pathways promotes eNOS activation, while phosphorylation on Thr495, usually provoked by oxidative stress, induces its inactivation, consequently modulating the NO synthesis. Thus, the expression and activation of another important molecule of pro-survival pathway, eNOS, was also monitored under current experimental conditions. The total eNOS (t-eNOS) abundance was at the similar level in all investigated groups (Sham + V vs. 2VO + V, Sham + V vs. 2VO + P4, and 2VO + V vs. 2VO + P4) (Table 3). The level of p-eNOSSer1177 was decreased for 28% in the injured group that received vehicle compared to the control (p < 0.001, Sham + V vs. 2VO + V) (Table 3), while p-eNOSThr495 increased by 20% (p < 0.01, Sham + V vs. 2VO + V) (Table 3). Following P4 treatment, investigated eNOS isophorms showed the opposite pattern of expression. Namely, with respect to vehicle-treated group, p-eNOSSer1177 levels rose by 36% (p < 0.001, 2VO + V vs. 2VO + P4) and the expression of p-eNOSThr495 dropped by 19% (p < 0.05, 2VO + V vs. 2VO + P4) (Table 3), while in comparison to sham-operated animals, they were unaltered (Sham + V vs. 2VO + P4) (Table 3). To determine whether the ratio of p-eNOSSer1177 to t-eNOS as an indicator of eNOS activation as well as p-eNOSThr495 to t-eNOS as an indicator of its inactivation was modified, the levels of both phosphorylated isophorms were normalized to t-eNOS obtained on the same membrane. Namely, p-eNOSSer 1177/t-eNOS ratio was decreased by 29% in injured rats subjected to vehicle when compared to the sham group (p < 0.001, Sham + V vs. 2VO + V), while P4 treatment restored it to the control level (Sham + V vs. 2VO + P4). The p-eNOSThr 495/t-eNOS ratio was unaltered in both injured groups relative to the control (Sham + V vs. 2VO + V and Sham + V vs. 2VO + P4) (Fig. 3d). Moreover, we estimated the level of NO as a downstream effector of eNOS. As shown in Fig. 3e, the level of NO in PFC of rats subjected to brain injury and vehicle treatment was decreased by 53% compared to the control (p < 0.001, Sham + V vs. 2VO + V), while its level was declined by 55% following P4 (p < 0.001, 2VO + V vs. 2VO + P4).
Discussion
Recent studies from our laboratory were focused on understanding tissue and gender differences, with the emphasis on time-specific response to mildly reduced cerebral blood flow (Stanojlovic et al. 2014a, b), as well as the effects of repeated P4 treatment in the hippocampus (Stanojlović et al. 2015a). The recognized time window of the pro-apoptotic signaling activation following permanent ligation of carotid arteries along with the observed attenuation of 2VO-induced damage after applied therapy in the hippocampus were used as the base to test the protective P4 properties in PFC in rat model of global ischemia. Herein, presented results demonstrate that repeated P4 therapy is able to improve cognitive function, limit the activation of Bax/caspase 3 signaling cascade, decline DNA fragmentation and, potentially ameliorate histological changes, most likely by re-establishing the Akt/Erk/eNOS signaling and upregulation of PRs.
Previous reports have shown the rise of P4 levels in human serum following the traumatic brain injury (TBI) (Wagner et al. 2011). The same was observed in mice serum and brain after the transient middle artery occlusion (MCAO) (Liu et al. 2012) and in the rat brain in response to TBI (Meffre et al. 2007). Liu et al. (2012) emphasize that endogenous P4 protects brain shortly after the brain injury due to brain steroidogenesis and PR activation, which are not sufficient for providing protection for no longer than 48 h. They also indicate that for the long-term neuroprotective outcome, the additional treatment with exogenous P4 and the presence of PR are necessary (Liu et al. 2012). Moreover, studies with PR knockout and heterozygous mice demonstrate that not only the presence but also the levels of PR are relevant for the post-injury P4 performance (Ghoumari et al. 2003; Hussain et al. 2011; Liu et al. 2012; Allen et al. 2016). Under the current experimental conditions, P4 upregulated the expressions of both PRA and PRB compared to the injured group that received vehicle and restored them to the control level most likely because exogenous P4 managed to rehabilitate PFC to more “normal” condition. This was reflected through the re-establishment of cellular functions and morphology.
P4 exhibits its actions through several different mechanisms. Many of them include binding of P4 to classical PRs which act as transcriptional factors that regulate expression of target genes (Lange 2008; Hanna et al. 2010). In mammals, numerous gene promoters contain regulatory sequence for both types of PRs, PRA and PRB (Guennoun et al. 2008; Brinton et al. 2008). PRB has been shown to be a strong transcriptional activator, while the transactivational activity of PRA is cell- and gene-specific (Giangrande and McDonnell 1999). PRA negatively controls not only the PRB-mediated transcription, but also the one mediated by the other steroid receptors, like estradiol and glucocorticoid receptors (Vegeto et al. 1993; Giangrande and McDonnell 1999; Singh et al. 2013). Thus, it is most likely that the exogenous P4 in PFC of 2VO animals induces transcriptional activation of several genes that corresponds to the increase in mRNA level of Bcl-2 and PARP and suppresses a few others, like Bax and caspase 3. Furthermore, this was accompanied by significantly reduced expression of Bax molecule in both investigated subcellular fractions and the increment of protein expression of Bcl-2 and Bcl-2/Bax ratio, as well as by Bcl-2 translocation from cytoplasm to mitochondria. These findings, besides declined levels of cytochrome C and procaspase 3/cleaved caspase 3 in cytosol, might be associated with the suppression of DNA fragmentation and disturbance in cells morphology. Herein, presented data for beneficial effects of P4 treatment in the rat 2VO model are in accordance with studies that proved the reduction of apoptosis in neurons and involvement of Bcl-2 in prevention of cytochrome C release from the mitochondrial membrane and subsequent suppression of detrimental caspase 3 activity following P4 treatment (Djebaili et al. 2004, 2005; Yao et al. 2005; Kaur et al. 2007; Stein 2008; Guerra-Araiza et al. 2009; Ishrat et al. 2012; Yousuf et al. 2017).
Additionally, the effect of P4 treatment in response to 2VO-induced brain injury was tested by evaluating the expression and activation of Akt and Erk kinases. Akt and Erk are central mediators that regulate signal transduction pathways by a dual mechanism involving the post-translational modification and suppressing actions of components of the cell death machinery and by enhancing the transcription of pro-survival genes (Ghoumari et al. 2003; Yao et al. 2005; Cargnello and Roux 2011; Ishrat et al. 2012). Namely, the activations of these kinases are shown to restrain Bax in cytoplasm due to inhibition of its conformational changes required for translocation to mitochondria. Moreover, they increase the expression of Bcl-2 and preserve the permeability of mitochondrial membrane, maintaining cytochrome C within mitochondrial membranous inter space, and inhibiting caspase 3 activation/PARP cleavage. In that manner, they prevent apoptosis and neuronal death (Terada et al. 2000; Ghoumari et al. 2003; Djebaili et al. 2004, 2005; Yao et al. 2005; Stein 2008; Ishrat et al. 2012; Yousuf et al. 2017). Consistent with previous studies on ischemia (Ghoumari et al. 2003; Yao et al. 2005; Ishrat et al. 2012; Stanojlović et al. 2015a), our results demonstrate significant changes in the expression and activation of these anti-apoptotic markers following P4 treatment in the 2VO rat model. Since the expression and phosphorylation of both investigated kinases were simultaneously affected in both injured groups, it could be assumed that in the imposed experimental conditions Akt and Erk might exhibit a cooperative response that has been previously reported in focal cerebral ischemia (Tang et al. 2014), rat model of global cerebral ischemia (Friguls et al. 2002), and in hippocampus of the 2VO rats exposed to P4 (Stanojlović et al. 2015a).
It is incompletely elucidated how the activation of Akt and/or Erk following P4 treatment increases neuronal resistance to neurodegenerative processes in 2VO rat model. The literature highlights that eNOS acts as a protective molecule that is phosphorylated at different amino acids by various members of multiple signaling cascades, like Akt and Erk (Chang et al. 2014; Khorram and Han 2009). Phosphorylation at Ser1177 through kinase-mediated pathways involving both Akt and Erk provokes eNOS activation, while phosphorylation on Thr495, usually induced by oxidative stress, initiates its inactivation. These events could lead to modulation of NO synthesis (Chen et al. 2008; Forstermann and Sessa 2012; Khorram and Han 2009; Yousuf et al. 2017). Several studies have shown that reduced eNOS activity in knockout mice (eNOS–/–) or use of eNOS-specific inhibitors diminishes NO levels and leads to hypertension-prone organism. This significantly reduces cerebral blood flow and subsequently results in larger infarct size and more severe ischemic injury (Huang et al. 1996; Wei et al. 1999; Ito et al. 2010; Chen et al. 2017). On the other hand, NO by scavenging oxygen-free radicals, inhibiting the expression of inflammatory and adhesion molecules, promoting the platelet aggregation and lymphocyte adhesion (Kuhlencordt et al. 2004; Nabah et al. 2005; Moore et al. 2011; Hossain et al. 2012; Chen et al. 2017) regulates cerebral microvascular tone, protects the blood–brain barrier, alleviates oxidative stress, and mitigates pro-coagulant stimulation (Moro et al. 2004; Duckles and Miller 2010; Pang et al. 2015). Thus, it consequently contributes to neuroprotective effects after brain ischemia (Moro et al. 2004). NO has also been associated with the suppression of apoptosis by inhibiting caspase-dependent Bcl-2 cleavage and sequentially, the release of cytochrome C (Kim et al. 1998) as well as with nitrosation of the downstream apoptotic machinery acting either at executioner caspases 3, 6, 7, or initiator caspases, like caspase 8, 9 (Kim et al. 1999). Our results showed that P4 in injured brain completely reversed the reduction of eNOS phosphorylation at Ser1177 and the level of NO. Moreover, together with cooperative actions of Akt, Erk, and eNOS, both PRs in the extranuclear location might be the candidates for mediating this non-genomic P4 action in the 2VO rat model since the modified levels of their transcripts were detected in the injured brain.
The studies from the other authors, regarding the cerebral hypoperfusion model suggest lasting cognitive changes observed by object recognition test (Wang et al. 2016; Mehla et al. 2017; Patel et al. 2017), while findings obtained by different behavior tests coincide with a solid evidence that P4 treatment could improve memory functions in different brain injury models (Shear et al. 2002; Gibson and Murphy 2004; Casas et al. 2011; Smith et al. 2013; Watts et al. 2014; Si et al. 2014; Yousuf et al. 2014, 2015, 2017; Lei et al. 2016; Wali et al. 2016; Montes et al. 2019). Combined with results from our previous publication in which P4 annulled 2VO-induced decrease in general locomotion and enhanced levels of anti-anxiety-like related behavior in animals (Stanojlovic et al. 2015a); herein, presented results (Supplementary data Fig. 2) demonstrate that P4 is also able to ameliorate 2VO-induced deficits in object recognition memory. Moreover, this might be associated with the attenuation of 2VO-induced alterations in DNA fragmentation and neuronal morphology, along with cellular dysfunctions due to the upregulation of PRs and activation of Akt/Erk/eNOS signaling pathway.
Although presented results indicate that P4-repeated administration may be a viable therapeutic treatment for mild reduction of cerebral blood flow, some issues need to be addressed, since the present study has several limitations: Firstly, the absence of a sham-operated group treated with P4 for examining its non-specific effects in animals whose carotid arteries are not compromised. Nevertheless, various animal models of brain injury have revealed that the use of P4 is safe and with no negative outcome on any currently investigated parameter even when P4 is applied in higher dose, different regime of administration, strain and/or species (Mani 2008; Singh et al. 2013; Watts et al. 2014; Geddes et al. 2014). Furthermore, the presented results imply that upregulation of PR and re-establishment of compromised Akt/Erk/eNOS signaling pathway represent, at least partially, the molecular basis of observed neuroprotective P4 effects in PFC in rat 2VO model. Our assumption originated from the notion that their levels are modified in current experimental setup. Although we have not evaluated their involvement in P4- induced response following the treatments with their specific inhibitors or antagonists, the involvement of P4 in activation/inactivation of investigated molecules was revealed earlier. Namely, previous reports proved that wortmannin, a selective PI3K/Akt inhibitor, compromised the positive P4 outcome due to inactivation of PI3K/Akt signaling pathway (Ishrat et al. 2012) and its downstream effector, eNOS (Khorram and Han 2009). U0126, the Erk pathway blocker, diminished its phosphorylation when two PR-negative non-tumorigenic breast epithelial cell lines are treated with P4 and its metabolites (Salazar et al. 2016), and it completely blocked the effect of P4 on eNOS phosphorylation (Khorram and Han 2009). PR antagonists, protein and RNA synthesis inhibitors, antisense oligonucleotides to PR, and mutant mice with targeted deletion of PR gene were shown to modify cell’s response following P4 treatment (Khorram and Han 2009; Mani 2008). Thus, in the 2VO model, new sets of experiments probing the impact of repeated P4 treatment on separate components of Akt/Erk/eNOS signaling besides PRA and PRB would demonstrate and clearly define the extent of their involvement in P4 neuroprotective actions within the injured brain. Our findings might provide the basis for further research into the potential therapeutic use of P4, solely or co-administrated with other agents, in patients experiencing mild but persistent reduction of cerebral blood flow. Thus, unequivocally, additional testing is necessary to confirm obtained results in animals of different age, ovariectomized or non-ovariectomized females in distinctive periods of the menstrual cycle, and higher level species.
Conclusion
Although it is unlikely that a single agent is sufficient to initiate neuroprotection, the current study provides evidence that post-ischemic repeated P4 treatment is capable of provoking activation of anti-apoptotic molecules through coordinated regulation of cytosolic, mitochondrial, and nuclear events in PFC. According to herein presented results, P4 actions in 2VO rat model are, at least partially, accomplished by PRs and Akt/Erk/eNOS.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (DOCX 71 kb)
Electronic supplementary material 2 (DOCX 5854 kb)
Acknowledgements
We thank Milorad Dragić for providing his unselfish technical support with Nissl staining and image analyses. We show gratitude to our retired colleague dr Anica Horvat who provided the original funding, knowledge, and expertise that greatly assisted the research.
Author Contributions
MS and IGS performed experiments and analyzed data. MZ assisted during biochemical measurements. IGS, MZ, JM, NM, and IG edited the manuscript. DD designed and supervised experimental setup, participated in the interpretation of obtained results, and wrote the manuscript. All authors approved the final version of the manuscript.
Funding
This work was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia, grants 173044 and 41014.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Miloš Stanojlović and Ivana Guševac Stojanović contributed equally to this work.
References
- Allen RS, Sayeed I, Oumarbaeva Y et al (2016) Progesterone treatment shows greater protection in brain vs. retina in a rat model of middle cerebral artery occlusion: progesterone receptor levels may play an important role. Restor Neurol Neurosci 34:947–963. 10.3233/RNN-160672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi SS, Parvez S, Tabassum H (2017) Neurosteroids and ischemic stroke: progesterone a promising agent in reducing the brain injury in ischemic stroke. J Environ Pathol Toxicol Oncol 36:191–205. 10.1615/JEnvironPatholToxicolOncol.2017017156 [DOI] [PubMed] [Google Scholar]
- Boyadjieva NI, Andreeva-Gateva P (2011) Effects of kainic acid on glutatione and nitrite in rat hippocampus. J IMAB 17(1):149–151. 10.5272/jimab.2011171.149 [Google Scholar]
- Brinton RD, Thompson RF, Foy MR et al (2008) Progesterone receptors: form and function in brain. Front Neuroendocrinol 29:313–339. 10.1016/j.yfrne.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Zhu Y, Furuya K et al (2008) Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology 55:127–138. 10.1016/j.neuropharm.2008.04.023 [DOI] [PubMed] [Google Scholar]
- Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75:50–83. 10.1128/MMBR.00031-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas S, García S, Cabrera R et al (2011) Progesterone prevents depression-like behavior in a model of Parkinson’s disease induced by 6-hydroxydopamine in male rats. Pharmacol Biochem Behav 99:614–618. 10.1016/j.pbb.2011.06.012 [DOI] [PubMed] [Google Scholar]
- Cechetti F, Pagnussat AS, Worm PV et al (2012) Chronic brain hypoperfusion causes early glial activation and neuronal death, and subsequent long-term memory impairment. Brain Res Bull 87:109–116. 10.1016/j.brainresbull.2011.10.006 [DOI] [PubMed] [Google Scholar]
- Chang C-M, Su Y-F, Chang C-Z et al (2014) Progesterone attenuates experimental subarachnoid hemorrhage-induced vasospasm by upregulation of endothelial nitric oxide synthase via Akt signaling pathway. Biomed Res Int 2014:1–6. 10.1155/2014/207616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-A, Druhan LJ, Varadharaj S et al (2008) Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J Biol Chem 283:27038–27047. 10.1074/jbc.M802269200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Mou R, Feng D et al (2017) The role of nitric oxide in stroke. Med Gas Res 7:194. 10.4103/2045-9912.215750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LR, Berman SE, Rivera-Rivera LA et al (2017) Macrovascular and microvascular cerebral blood flow in adults at risk for Alzheimer’s disease. Alzheimer’s Dementia Diagn, Assess Dis Monit 7:48–55. 10.1016/j.dadm.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong GI, Farkas E, Stienstra CM et al (1999) Cerebral hypoperfusion yields capillary damage in the hippocampal CA1 area that correlates with spatial memory impairment. Neuroscience 91:203–210. 10.1016/S0306-4522(98)00659-9 [DOI] [PubMed] [Google Scholar]
- de la Torre JC (2002a) Alzheimer disease as a vascular disorder: nosological evidence. Stroke 33:1152–1162. 10.1161/01.STR.0000014421.15948.67 [DOI] [PubMed] [Google Scholar]
- de la Torre JC (2002b) Alzheimer’s disease: how does it start? J Alzheimer’s Dis 4:497–512. 10.3233/JAD-2002-4606 [DOI] [PubMed] [Google Scholar]
- Deutsch ER, Espinoza TR, Atif F et al (2013) Progesterone’s role in neuroprotection, a review of the evidence. Brain Res 1530:82–105. 10.1016/j.brainres.2013.07.014 [DOI] [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG (2004) Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience 123:349–359 [DOI] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH et al (2005) The Neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma 22:106–118. 10.1089/neu.2005.22.106 [DOI] [PubMed] [Google Scholar]
- Drakulić D, Veličković N, Stanojlović M et al (2013) Low-dose dexamethasone treatment promotes the pro-survival signalling pathway in the adult rat prefrontal cortex. J Neuroendocrinol 25:605–616. 10.1111/jne.12037 [DOI] [PubMed] [Google Scholar]
- Duckles SP, Miller VM (2010) Hormonal modulation of endothelial NO production. Pflügers Archiv 459:841–851. 10.1007/s00424-010-0797-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Luiten PGM, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54:162–180. 10.1016/j.brainresrev.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33:829–837. 10.1093/eurheartj/ehr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friguls B, Petegnief V, Justicia C et al (2002) Activation of ERK and Akt signalling in focal cerebral ischemia: modulation by TGF-α and involvement of NMDA receptor. Neurobiol Dis 11:443–456. 10.1006/nbdi.2002.0553 [DOI] [PubMed] [Google Scholar]
- Geddes RI, Sribnick EA, Sayeed I, Stein DG (2014) Progesterone treatment shows benefit in a pediatric model of moderate to severe bilateral brain injury. PLoS ONE 9:e87252. 10.1371/journal.pone.0087252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoumari AM, Ibanez C, El-Etr M et al (2003) Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem 86:848–859 [DOI] [PubMed] [Google Scholar]
- Giangrande PH, McDonnell DP (1999) The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res 54:291–313 [PubMed] [Google Scholar]
- Gibson CL, Murphy SP (2004) Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab 24:805–813. 10.1097/01.WCB.0000125365.83980.00 [DOI] [PubMed] [Google Scholar]
- Guennoun R, Meffre D, Labombarda F et al (2008) The membrane-associated progesterone-binding protein 25-Dx: expression, cellular localization and up-regulation after brain and spinal cord injuries. Brain Res Rev 57:493–505. 10.1016/j.brainresrev.2007.05.009 [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Amorim MAR, Pinto-Almazán R et al (2009) Regulation of the phosphoinositide-3 kinase and mitogen-activated protein kinase signaling pathways by progesterone and its reduced metabolites in the rat brain. J Neurosci Res 87:470–481. 10.1002/jnr.21848 [DOI] [PubMed] [Google Scholar]
- Hanna RN, Daly SCJ, Pang Y et al (2010) Characterization and expression of the nuclear progestin receptor in zebrafish gonads and brain1. Biol Reprod 82:112–122. 10.1095/biolreprod.109.078527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M, Qadri SM, Liu L (2012) Inhibition of nitric oxide synthesis enhances leukocyte rolling and adhesion in human microvasculature. J Inflamm 9:28. 10.1186/1476-9255-9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Ma J et al (1996) Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab 16:981–987. 10.1097/00004647-199609000-00023 [DOI] [PubMed] [Google Scholar]
- Hussain R, El-Etr M, Gaci O et al (2011) Progesterone and nestorone facilitate axon remyelination: a role for progesterone receptors. Endocrinology 152:3820–3831. 10.1210/en.2011-1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F et al (2012) Progesterone is neuroprotective against ischemic brain injury through its effects on the phosphoinositide 3-kinase/protein kinase B signaling pathway. Neuroscience 210:442–450. 10.1016/j.neuroscience.2012.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Ohkubo T, Asano Y et al (2010) Nitric oxide production during cerebral ischemia and reperfusion in eNOS- and nNOS-knockout mice. Curr Neurovasc Res 7:23–31 [DOI] [PubMed] [Google Scholar]
- Kaur P, Jodhka PK, Underwood WA et al (2007) Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res 85:2441–2449. 10.1002/jnr.21370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorram O, Han G (2009) Influence of progesterone on endometrial nitric oxide synthase expression. Fertil Steril 91:2157–2162. 10.1016/j.fertnstert.2008.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Kim TH, Seol DW et al (1998) Nitric oxide suppression of apoptosis occurs in association with an inhibition of Bcl-2 cleavage and cytochrome C release. J Biol Chem 273:31437–31441 [DOI] [PubMed] [Google Scholar]
- Kim YM, Bombeck CA, Billiar TR (1999) Nitric oxide as a bifunctional regulator of apoptosis. Circ Res 84:253–256 [DOI] [PubMed] [Google Scholar]
- Kuhlencordt PJ, Rosel E, Gerszten RE et al (2004) Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. Am J Physiol-Cell Physiol 286:C1195–C1202. 10.1152/ajpcell.00546.2002 [DOI] [PubMed] [Google Scholar]
- Lange CA (2008) Integration of progesterone receptor action with rapid signaling events in breast cancer models. J Steroid Biochem Mol Biol 108:203–212. 10.1016/j.jsbmb.2007.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei B, Wang H, Jeong S et al (2016) Progesterone improves neurobehavioral outcome in models of intracerebral hemorrhage. Neuroendocrinology 103:665–677. 10.1159/000442204 [DOI] [PubMed] [Google Scholar]
- Liu M, Kelley MH, Herson PS, Hurn PD (2010) Neuroprotection of sex steroids. Minerva Endocrinol 35:127–143 [PMC free article] [PubMed] [Google Scholar]
- Liu A, Margaill I, Zhang S et al (2012) Progesterone receptors: a key for neuroprotection in experimental stroke. Endocrinology 153:3747–3757. 10.1210/en.2012-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S (2008) Progestin receptor subtypes in the brain: the known and the unknown. Endocrinology 149:2750–2756. 10.1210/en.2008-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre D, Pianos A, Liere P et al (2007) Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: analysis by gas chromatography/mass spectrometry. Endocrinology 148:2505–2517. 10.1210/en.2006-1678 [DOI] [PubMed] [Google Scholar]
- Mehla J, iere S, Stuart E et al (2017) Gradual cerebral hypoperfusion impair fear conditioning and object recognition learning and memory in mice: potential roles of neurodegeneration and cholinergic dysfunction. bioRxiv. 10.1101/177121 [DOI] [PubMed] [Google Scholar]
- Montes P, Vigueras-Villaseñor RM, Rojas-Castañeda JC et al (2019) Progesterone treatment in rats after severe global cerebral ischemia promotes hippocampal dentate gyrus neurogenesis and functional recovery. Neurol Res 41:429–436. 10.1080/01616412.2019.1576356 [DOI] [PubMed] [Google Scholar]
- Moore C, Sanz-Rosa D, Emerson M (2011) Distinct role and location of the endothelial isoform of nitric oxide synthase in regulating platelet aggregation in males and females in vivo. Eur J Pharmacol 651:152–158. 10.1016/j.ejphar.2010.11.011 [DOI] [PubMed] [Google Scholar]
- Moro MA, Cárdenas A, Hurtado O et al (2004) Role of nitric oxide after brain ischaemia. Cell Calcium 36:265–275. 10.1016/j.ceca.2004.02.011 [DOI] [PubMed] [Google Scholar]
- Nabah YNA, Mateo T, Cerdá-NicoláS M et al (2005) L-NAME induces direct arteriolar leukocyte adhesion, which is mainly mediated by angiotensin-II. Microcirculation 12:443–453. 10.1080/10739680590960962 [DOI] [PubMed] [Google Scholar]
- Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV (2016) Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim et Biophys Acta 1862:887–900. 10.1016/j.bbadis.2015.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Dong J, Thomas P (2015) Progesterone increases nitric oxide synthesis in human vascular endothelial cells through activation of membrane progesterone receptor-α. Am J Physiol-Endocrinol Metab 308:E899–E911. 10.1152/ajpendo.00527.2014 [DOI] [PubMed] [Google Scholar]
- Patel A, Moalem A, Cheng H et al (2017) Chronic cerebral hypoperfusion induced by bilateral carotid artery stenosis causes selective recognition impairment in adult mice. Neurol Res 39:910–917. 10.1080/01616412.2017.1355423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alvarez MJ, Wandosell F (2016) Stroke and neuroinflamation: role of sexual hormones. Curr Pharm Des 22:1334–1349. 10.2174/138161282210160304112834 [DOI] [PubMed] [Google Scholar]
- Perez-Alvarez MJ, Mateos L, Alonso A, Wandosell F (2015) Estradiol and progesterone administration after pMCAO stimulates the neurological recovery and reduces the detrimental effect of ischemia mainly in hippocampus. Mol Neurobiol 52:1690–1703. 10.1007/s12035-014-8963-7 [DOI] [PubMed] [Google Scholar]
- Rius-Pérez S, Tormos AM, Pérez S, Taléns-Visconti R (2018) Vascular pathology: cause or effect in alzheimer disease? Neurología (English Edition) 33:112–120. 10.1016/j.nrleng.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Salazar M, Lerma-Ortiz A, Hooks GM et al (2016) Progestin-mediated activation of MAPK and AKT in nuclear progesterone receptor negative breast epithelial cells: the role of membrane progesterone receptors. Gene 591:6–13. 10.1016/j.gene.2016.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear DA, Galani R, Hoffman SW, Stein DG (2002) Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol 178:59–67. 10.1006/exnr.2002.8020 [DOI] [PubMed] [Google Scholar]
- Si D, Yang P, Jiang R et al (2014) Improved cognitive outcome after progesterone administration is associated with protecting hippocampal neurons from secondary damage studied in vitro and in vivo. Behav Brain Res 264:135–142. 10.1016/j.bbr.2014.01.049 [DOI] [PubMed] [Google Scholar]
- Singh M (2001) Ovarian Hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine 14:407–416. 10.1385/ENDO:14:3:407 [DOI] [PubMed] [Google Scholar]
- Singh M, Su C, Ng S (2013) Non-genomic mechanisms of progesterone action in the brain. Front Neurosci. 10.3389/fnins.2013.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Saraswati M, Koehler R, Robertson C (2013) 182: the effect of progesterone on neurologic outcome in a rat model of pediatric traumatic brain injury. Crit Care Med 41:A40. 10.1097/01.ccm.0000439331.43555.fb [Google Scholar]
- Stanojlovic M, Gusevac I, Grkovic I et al (2014a) Time-related sex differences in cerebral hypoperfusion-induced brain injury. Arch Biol Sci 66:1673–1680. 10.2298/ABS1404673S [Google Scholar]
- Stanojlović M, Horvat A, Guševac I et al (2014b) Time course of cerebral hypoperfusion-induced neurodegenerative changes in the cortex of male and female rats. Folia Biol (Praha) 60:123–132 [DOI] [PubMed] [Google Scholar]
- Stanojlović M, Guševac I, Grković I et al (2015a) Effects of chronic cerebral hypoperfusion and low-dose progesterone treatment on apoptotic processes, expression and subcellular localization of key elements within Akt and Erk signaling pathways in rat hippocampus. Neuroscience 311:308–321. 10.1016/j.neuroscience.2015.10.040 [DOI] [PubMed] [Google Scholar]
- Stanojlović M, Zlatković J, Guševac I et al (2015b) Repeated low-dose 17β-estradiol treatment prevents activation of apoptotic signaling both in the synaptosomal and cellular fraction in rat prefrontal cortex following cerebral ischemia. Neurochem Int 83–84:1–8. 10.1016/j.neuint.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Stein DG (2008) Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev 57:386–397. 10.1016/j.brainresrev.2007.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Zhang Q, Yang L et al (2014) GPR30 mediates estrogen rapid signaling and neuroprotection. Mol Cell Endocrinol 387:52–58. 10.1016/j.mce.2014.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada K, Kaziro Y, Satoh T (2000) Analysis of Ras-dependent signals that prevent caspase-3 activation and apoptosis induced by cytokine deprivation in hematopoietic cells. Biochem Biophys Res Commun 267:449–455. 10.1006/bbrc.1999.1955 [DOI] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX et al (1993) Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 7:1244–1255. 10.1210/mend.7.10.8264658 [DOI] [PubMed] [Google Scholar]
- Villapol S, Byrnes KR, Symes AJ (2014) Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte–vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Front Neurol. 10.3389/fneur.2014.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, McCullough EH, Niyonkuru C et al (2011) Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury. J Neurotrauma 28:871–888. 10.1089/neu.2010.1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali B, Ishrat T, Stein DG, Sayeed I (2016) Progesterone improves long-term functional and histological outcomes after permanent stroke in older rats. Behav Brain Res 305:46–56. 10.1016/j.bbr.2016.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fan J, Wang J et al (2016) Chronic cerebral hypoperfusion induces long-lasting cognitive deficits accompanied by long-term hippocampal silent synapses increase in rats. Behav Brain Res 301:243–252. 10.1016/j.bbr.2015.12.047 [DOI] [PubMed] [Google Scholar]
- Watts L, Sprague S, Fletcher L et al (2014) Does progesterone show neuroprotective effects on traumatic brain injury through increasing phosphorylation of Akt in the hippocampus? Neural Regener Res 9:1891. 10.4103/1673-5374.145355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Dawson VL, Zweier JL (1999) Role of neuronal and endothelial nitric oxide synthase in nitric oxide generation in the brain following cerebral ischemia. Biochim Biophys Acta 1455:23–34 [DOI] [PubMed] [Google Scholar]
- Yao X-L, Liu J, Lee E et al (2005) Progesterone differentially regulates pro- and anti-apoptotic gene expression in cerebral cortex following traumatic brain injury in rats. J Neurotrauma 22:656–668. 10.1089/neu.2005.22.656 [DOI] [PubMed] [Google Scholar]
- Yousuf S, Atif F, Sayeed I et al (2014) Progesterone in transient ischemic stroke: a dose–response study. Psychopharmacology 231:3313–3323. 10.1007/s00213-014-3556-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf S, Atif F, Sayeed I et al (2015) Long-term behavioral deficits and recovery after transient ischemia in middle-aged rats: effects of behavioral testing. Restor Neurol Neurosci 33:251–261. 10.3233/RNN-140450 [DOI] [PubMed] [Google Scholar]
- Yousuf S, Brat DJ, Shu H-K et al (2017) Progesterone improves neurocognitive outcomes following therapeutic cranial irradiation in mice. Horm Behav 96:21–30. 10.1016/j.yhbeh.2017.08.004 [DOI] [PubMed] [Google Scholar]
- Zaric M, Drakulic D, Stojanovic IG et al (2018) Regional-specific effects of cerebral ischemia/reperfusion and dehydroepiandrosterone on synaptic NMDAR/PSD-95 complex in male Wistar rats. Brain Res 1688:73–80. 10.1016/j.brainres.2018.03.023 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang M, Liu H, Wang J (2015) Progesterone is neuroprotective by inhibiting cerebral edema after ischemia. Neural Regener Res 10:1076. 10.4103/1673-5374.160097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (DOCX 71 kb)
Electronic supplementary material 2 (DOCX 5854 kb)