Abstract
Post-traumatic stress disorder (PTSD) is related with myocardial injury and cardiac dysfunction, while the molecular mechanism has not been clear. This study investigated whether TLR4/MyD88/NF-κB-mediated inflammation involved in myocardial injury of PTSD. Adult male Wistar rats were exposed to single-prolonged stress (SPS), which was used broadly as a animal model of PTSD. Morris Water Maze (MWM) test and forced swimming test (FST) was carried out for behavioral testing. The protein expression of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) in the left ventricular of heart and TLR4/MyD88/NF-κB-mediated inflammation were examined. Our results showed that there were obvious increased in the protein expression of ANP and BNP in heart after exposure to SPS, SPS also significantly enhanced the serum level of IL-1β and TNF-α, and meanwhile, the TLR4/MyD88/NF-κB pathway were activated. These results demonstrated that the TLR4/MyD88/NF-κB pathway were involved in the myocardial injury of PTSD, which might be one of possible molecular mechanism contributed to the pathogenesis of cardiac dysfunction in PTSD.
Keywords: Single-prolonged stress, Post-traumatic stress disorder, TLR4, MyD88, Nf-κb
Introduction
Post-traumatic stress disorder (PTSD) is one of mental illness caused by traumatic events. Previous studies have found that up to 60–90% of individuals during their lifetime would suffer to at least one traumatic event (Kilpatrick et al. 2013; Gomez-Beneyto et al. 2006; Georgopoulos et al. 2018), and part of them might experience PTSD symptoms (American Psychiatric Association 2013; Georgopoulos et al. 2018), including: (1) re-experiencing traumatic event, such as nightmares; (2) avoidance of reminders of the event, (3) negative alterations in cognition and mood, (4) alterations in arousal and reactivity. And besides the above symptoms, PTSD patients also suffer some cardiovascular symptoms, such as increased blood pressure and basal heart rate (Kibler 2009; Kubzansky and Koenen 2009; von Kanel et al. 2011). Some recent population studies have been reported that PTSD is related to greater mean systolic blood pressure (Dyball et al. 2019) and reduced left ventricular diastolic function in women (Hieda et al. 2019), and PTSD with greater body mass index and poor health behavior have higher risk for development to hypertension (Hoerster et al. 2019). A 15 year postwar analysis (Britvic et al. 2015) have found that veterans with PTSD suffered from arrhythmias for a long period compared with who not exposed to combat experience. Hence, PTSD has been considered as a contributory factor to cardiovascular disease and positively related with the development of cardiovascular disease.
Animal experiments also have found intimate relationship between PTSD and heart injury. Cho et al. verified PTSD-like mouse induced by aggressor-exposed social stress caused transitory heart injury (Cho et al. 2014), and Liu et al. demonstrated a long-term effect of PTSD exposed to single prolonged stress on myocardial injury with morphologic changes of apoptosis and activation of endoplasmic reticulum stress (Liu et al. 2016). In addition, a recent animal experiment on a predator-based model of PTSD showed that chronic predator stress induced myocardial injury characterized by collagen deposition, mononuclear infiltration, and accompanied by cardiac remodeling (Rorabaugh et al. 2019). Although there are many studies focused on relationship between PTSD and heart injury, until now the exact mechanisms have been elusive, and based on these study backgrounds, this present study goes on investigating the effects and mechanisms of PTSD on myocardial injury.
Toll-like receptor 4 (TLR4) is a transmembrane receptor which have a key role in immune system activation (Shi et al. 2013; Wang et al. 2016). TLR4 activation leads to the recruitment of its downstream via the adaptor protein myeloid differentiation primary response protein 88 (MyD88) to activate NF-κB and increase the level of several pro-inflammatory factors such as IL-1β and TNF-α (Miller et al. 2005; Kuzmich et al. 2017). Thus, TLR4 is generally taken as a regulator of inflammation and tissue injury in different animal models, such as ischemia/reperfusion (Wang et al. 2016), Alzheimer's diseases (Azam et al. 2019), and the TLR4 signaling is involved in many diseases, including autoimmune disorders (Xu et al. 2015), metabolic diseases (de Laat et al. 2014), cardiovascular diseases(Yang et al. 2016). A recent study has reported that the level of TLR4 increased in the basolateral amygdala of PTSD-like rats (Lai et al. 2018). However, there are still no findings on the role of TLR4 signaling in the myocardial injury of PTSD.
Thus, this present study was planned to investigate whether TLR4 signaling are involved in the myocardial injury of PTSD. Adult male wistar rats were exposed to single-prolonged stress (SPS), which was used broadly as a animal model of PTSD (Souza et al. 2017; Yamamoto et al. 2009). First, we use morris water maze (MWM) test and forced swimming test (FST) to know the rats’ behavioral changes after SPS, and then detected the protein expression of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) in the left ventricular of heart and the heart weight indexes. And meanwhile, the TLR4/MyD88/NF-κB-mediated inflammation were also examined. The present study not only demonstrated the earlier findings, but also extended a new possible mechanism underlying the cardiac dysfunction in PTSD.
Methods
Animals
Male Wistar rats (n = 20) were obtained, aged 9 weeks and weighed 180–220 g. The rats were housed in standardized cage (each cage n = 5) under a reversed 12 h:12 h light/dark cycle (lights off at 10.00 a.m), and given clean water and normal diet. The experiments were carried out according the Guidance Suggestions for the Care and Use of Laboratory Animals, the Ministry of Science and Technology of the People’s Republic of China.
Groups Assignment and SPS Procedure
After 7 days of habituation, all rats were randomly assigned to two groups (each n = 10). One was called the control group (con), and the other was SPS groups (SPS). The SPS group received the SPS procedure (Liberzon et al. 1999; Takahashi et al. 2006), which described in our previous studies (Xie et al. 2015).
Morris Water Maze Test
Morris water maze (MWM) was applied to measure the learning and memory of the rats at the 8th day after SPS exposure. The test of MWM included two trials: a visible platform trial and a probe trial, and both trials were carried out according our previous studies (Yu et al. 2014). The rats were randomly placed to swim for 120 s to find the platform for 1 time for 5 days. If the rat failed, it will be guided and stand on the platform for 20 s. And on the 13th day, we removed the platform and rats was tested in the probe trial.
Forced Swim Test
On the 14th day after SPS, the forced swimming test (FST) was carried out to know the rats’ behavioral despair (Castagne et al. 2011). Rats were forced to swim in a transparent glass cylinders (30 cm diameter × 70 cm high), and could not touch the bottom of the cylinder when they swim in water. After 15 min of training session, the 5-min recorded session was carried out 24 h later and the total time of immobility behavior was analyzed between two groups.
ELISA Assay of IL-1β and TNF-α
The serum concentrations of IL-1β and TNF-α were estimated using ELISA kits (Shanghai MLBIO Biotechnology Co. Ltd) in accordance with the manufacturer’s instructions.
Tissue Preparation and Western Blot
After FST, rats were weighed and decapitated. Their hearts were quickly removed and the left ventricle of rats was token out immediately, then weighed and stored at − 80 °C. The heart weight indexes: heart weight/body weight (HW/BW), left ventricle weight (LVW/BW) and left ventricle weight/heart weight (LVW/HW) were measured. The proteins in the left ventricle were extracted (Beyotime Biotechnology, China) and was separated by 8% sodium dodecyl sulfate–polyacrylamide gels. After transferred to PVDF membrane and blocked with 5% skim milk, the membranes were incubated with anti-brain natriuretic peptide (BNP) (1:500, ABclonal, China), anti-atrial natriuretic peptide (ANP) (1:500, ABclonal, China), anti-TLR4 (1:500, ABclonal, China), anti-TLR4 (1:500, ABclonal, China), anti-MyD88 (1:500, ABclonal, China), anti-NF-κB p65 (1:500, ABclonal, China),and anti-GADPH (1:500, Zhongshan Glodenbridge Biotechnology, China) overnight at 4 °C, and later incubated by secondary antibody for 1–1.5 h at room temperature. After 3–4 times of washing in TBST (each time for 15 min), blots were taken to the autoradiography by ECL reagents, and the densitometry of the bands were normalized against GADPH and analyzed by Image J software.
Immunohistochemical Staining for TLR4
Heart tissues were fixed in 10% polyoxymethylene at 4 °C, after 24 h the heart tissues were embedded in paraffin wax and sectioned into slices (5um). Sections were dewaxed and pretreated by 0.3% hydrogen peroxide for 10 min, then incubated with anti-TLR4 (1:100, ABclonal) overnight at 4 °C. Then, incubated with two-step IHC detection reagent (Company of Zhongshan Goldenbridge, China) for 30 min at room temperature. The DAB-peroxidase reaction was carried out for about 1–3 min until the slices displayed brown color granules. Then, slices were dehydrated with alcohol, cleared with xylene, and mounted with neutral balsam.
Statistical Analysis
Data were expressed as the means ± standard deviations (SD) and evaluated using the Student's t test. A P value < 0.05 was considered significant.
Results
SPS Impaired the Ability of Learning and Memory and Induced Depression
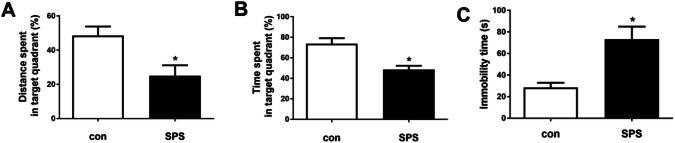
The results of MVM and FST were showed in Fig. 1. Relative to the control group, in MVM test, the distance spent in target quadrant (%) and the time spent in target quadrant (%) both significantly decreased in the SPS group. Meanwhile, the total immobility time in FST increased obviously after SPS exposure. These data suggested that SPS led to an injury of learning and memory and depression. SPS model was successfully copied of PTSD.
Fig. 1.
SPS induced the decrease of learning and memory and the increase of immobility time. a Comparison of the distance spent in target quadrant (%) in the probe trial; b comparison of the time spent in target quadrant (%) in the probe trial; c the total immobility time in two groups in FST. Statistical analysis was carried out by Student's t test, *P < 0.05 vs. the control group
SPS Caused Heart Remodel by Upregulation of ANP and BNP
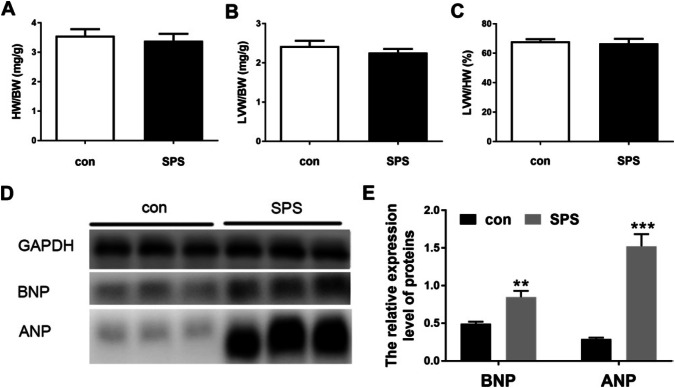
As previous study (Rorabaugh et al. 2019) has been reported that PTSD induced myocardial injury characterized by collagen deposition, mononuclear infiltration, and cardiac remodeling in morphologically, in this study, we detected the protein expression of ANP and BNP besides the weight indexes of heart. From the Fig. 2a–c, we found that there were no obvious differences in the heart weight indexes, including the heart weight/body weight ratio, the left ventricle weight/body weight ratio and the left ventricle weight/the heart weight ratio. However, the protein expression of ANP and BNP significantly upregulated after SPS, as shown in Fig. 2d and e. These findings extended the previous study and indicated that SPS caused heart injury characterized of myocardial remodel.
Fig. 2.
SPS caused heart remodel by upregulation of ANP and BNP. A the heart weight/body weight ratio; b the left ventricle weight/body weight ratio; c the left ventricle weight/the heart weight ratio; d representative Western blot photograph of ANP and BNP in two groups. e the relative expression of ANP and BNP. Statistical analysis was carried out by Student's t test, **P < 0.01 vs. the control group; ***P < 0.001 vs. the control group
SPS Upregulated the Level of Inflammatory Factors IL-1β and TNF-α
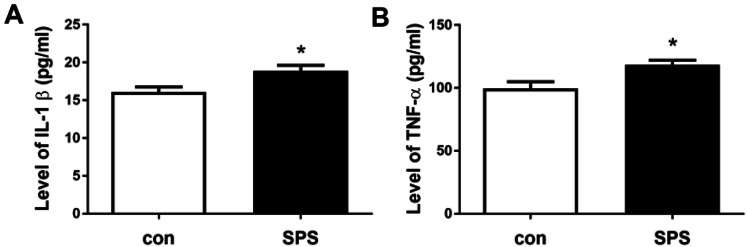
As inflammation is an important molecular mechanism in the pathogenesis of PTSD (Felger 2018; Girgenti et al. 2017; Michopoulos et al. 2017), we detected the serum concentrations of IL-1β and TNF-α. The results of ELISA kits were shown in Fig. 3, compared to the control group, the level of IL-1β and TNF-α obviously increased in SPS group, suggested SPS upregulated the expression of inflammatory factors IL-1β and TNF-α.
Fig. 3.
SPS increased the level of IL-1β and TNF-α. a the level of IL-1β; b the level of TNF-α. Statistical analysis was carried out by Student's t test, *P < 0.05 vs. the control group
Activation of TLR4 Signaling in the Left Ventricle Heart After SPS Exposure
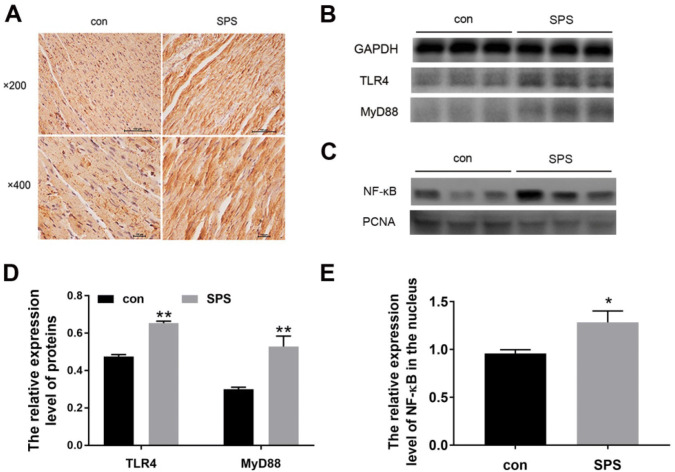
TLR4 signaling has a key role in inflammation (Shi et al. 2013; Wang et al. 2016). To investigate the possible molecule mechanism on the myocardial injury caused by SPS, we detected whether the TLR4/MyD88/NF-κB signaling were activated in the left ventricle. As shown in Fig. 4a, immunohistochemical staining-positive cells of TLR4 were brown, and the immunoreactivity of TLR4 after SPS exposure increased (data not shown). And from Fig. 4b, we found that the protein expression of TLR4 detected by western blot significantly increased in SPS groups, and its down-target MyD88 also upregulated compared to the control group, and besides, the expression of NF-κB in the nucleus of the left ventricle heart tissue increased after SPS exposure. These results suggested that SPS activated the TLR4/MyD88/NF-κB pathway.
Fig. 4.
SPS activated the TLR4-signaling pathway. a representative images for immune histochemical staining of TLR4 in the left ventricle heart tissues (from left to right: the normal control group, the SPS group; from up to down: × 200 magnification, × 400 magnification). b representative images of Western blot of TLR4/MyD88; c representative images of Western blot of NF-κB in the nucleus, PCNA as an internal reference for nucleus. d the relative expression level of TLR4/GAPDH and MyD88/GAPDH; e the relative expression level of NF-κB/PCNA. Statistical analysis was carried out by Student's t test, *P < 0.05 vs. the control group; **P < 0.01 vs. the control group
Discussion
PTSD is a psychiatric disorder after exposed to trauma, characterized of four clinical symptom clusters (American Psychiatric Association 2013; Georgopoulos et al. 2018). PTSD patients are more likely to be along with chronic medical conditions, especially cardiovascular responses, such as heightened sympathetic activation, elevated basal heart rate and blood pressure (Buckley and Kaloupek 2001; Morris et al. 2016). However, how exposure of traumatic stress influences heart and causes cardiovascular responses or cardiovascular disease in PTSD is poorly understood.
Some animal researches showed that PTSD induced myocardial injury, characterized by collagen deposition, mononuclear infiltration, and cardiac remodeling in morphologically (Rorabaugh et al. 2019); PTSD exposed to SPS on myocardial injury with morphologic changes of apoptosis and activation of endoplasmic reticulum stress (Liu et al. 2016). In our experiment, our results showed that the protein expression of ANP and BNP significantly upregulated after SPS exposure, although there were no obvious differences in the heart weight indexes. Our results extended the previous study (Rorabaugh et al. 2019) and further verified that SPS caused heart injury characterized of myocardial remodel.
Recently, emerging and convincing evidences (Levine et al. 2014; Speer et al. 2018; Wang et al. 2017) show that PTSD may be an immunological disorder with a low-grade pro-inflammatory state, suggesting that inflammation is a key molecular mechanism in PTSD. In this research, we also found that the serum concentrations of IL-1β and TNF-α increased in SPS group, suggesting that SPS caused an pro-inflammatory state with characterized of increase of IL-1β and TNF-α level. Given inflammation also has an important role in the development of cardiovascular disease, many studies have been reported that inflammation is an intimate link between PTSD and cardiovascular disease (Ridker 2009; Ridker et al. 2017), we suspect inflammation might be associated with myocardial injury induced by SPS.
Given TLR is a key regulator of inflammation and TLR4-mediated inflammation signaling have been reported to involved in many disease (Yang et al. 2016), and a recent study has reported that the level of TLR4 increased in the basolateral amygdala of PTSD-like rats (Lai et al. 2018), suggesting TLR4 was associated with PTSD, thus we detected whether TLR4 was activated in the heart tissue after SPS in our this study. From Fig. 4a, b, d, we could find that the expression of TLR4 increased in SPS groups, which suggested that the activation of TLR was involved in the myocardial injury of PTSD.
TLR4 activation leads to the recruitment of MyD88 and activates NF-κB to translocate into the nucleus and then upregulate the expression of several pro-inflammatory factors such as IL-1β and TNF-α (Miller et al. 2005; Kuzmich et al. 2017; Zhang et al. 2019), in this paper we also detected the expression of MyD88 and NF-κB. As shown in Fig. 4b–e, the protein expression of MyD88 was upregulated compared to the control group, and the expression of NF-κB in the nucleus of the left ventricle heart tissue was also increased after SPS exposure. These results suggested that SPS activated the TLR4/MyD88 pathway, and TLR4/MyD88/NF-κB pathway were involved in the myocardial injury of PTSD.
In summary, in this paper, we found that SPS increased ANP and BNP at the level of protein expression in the left ventricle heart tissue, upregulated the level of IL-1β and TNF-α and activated the TLR4-signaling pathway. These results suggested us that SPS-induced heart injury of remodel through activation of TLR-mediated inflammation signaling. The present study demonstrated and extended the earlier findings by adding a new possible mechanism underlying the heart injury induced by PTSD. Targeting the TLR4 and its signaling might be feasible, however, the prevention of PTSD with TLR4 inhibitors has not been launched in this paper. And we will go further in this direction to find the effects and mechanisms of PTSD on myocardial injury.
Conclusion
Our study is the first to suggest that the heart injury of PTSD induced by SPS may be, at least in part, was associated with the activation of TLR-mediated inflammation signaling. These results provide a new possible direction to further explore the mechanism of PTSD on myocardial injury.
Acknowledgements
This work was supported by Doctoral Scientific Research Fund from Liao Ning Province (No. 20170520016); Doctoral Scientific Research Fund from Shenyang Medical College (No. 20163047; No. 20171005 and No. 20181005). The authors thank the Laboratory of Cardiology in the First Hospital of China Medical University for valuable help in this experiment.
Author Contributions
All authors mentioned in the paper have significantly contributed to the research. ML and YS conceived and designed the experiments, ML and JX performed the experiment and wrote the article, YS refined the article. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare no potential conflicts of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Juhua Xie, Email: xjh814@126.com.
Yingxian Sun, Email: Sunyingxian12@126.com.
References
- American Psychiatric Association (2013) The diagnostic and statistical manual of mental disorders, 5th edn. APA, Washington, DC [Google Scholar]
- Azam S, Jakaria M, Kim IS, Kim J, Haque ME, Choi DK (2019) Regulation of toll-like receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: focus on TLR4 signaling. Front Immunol 10:1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britvic D, Anticevic V, Kaliterna M, Lusic L, Beg A, Brajevic-Gizdic I, Kudric M, Stupalo Z, Krolo V, Pivac N (2015) Comorbidities with posttraumatic stress disorder (PTSD) among combat veterans: 15 years postwar analysis. Int J Clin Health Psychol 15:81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TC, Kaloupek DG (2001) A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom Med 63:585–594 [DOI] [PubMed] [Google Scholar]
- Castagne V, Moser P, Roux S, Porsolt RD (2011) Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci 55:8–10 [DOI] [PubMed] [Google Scholar]
- Cho JH, Lee I, Hammamieh R, Wang K, Baxter D, Scherler K, Etheridge A, Kulchenko A, Gautam A, Muhie S, Chakraborty N, Galas DJ, Jett M, Hood L (2014) Molecular evidence of stress-induced acute heart injury in a mouse model simulating posttraumatic stress disorder. Proc Natl Acad Sci USA 111:3188–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat MA, Gruntmeir KJ, Pollitt CC, McGowan CM, Sillence MN, Lacombe VA (2014) Hyperinsulinemia down-regulates TLR4 Expression in the mammalian heart. Front Endocrinol (Lausanne) 5:120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball D, Evans S, Boos CJ, Stevelink SAM, Fear NT (2019) The association between PTSD and cardiovascular disease and its risk factors in male veterans of the Iraq/Afghanistan conflicts: a systematic review. Int Rev Psychiatry 31:34–48 [DOI] [PubMed] [Google Scholar]
- Felger JC (2018) Imaging the Role of Inflammation in Mood and Anxiety-related Disorders. Curr Neuropharmacol 16:533–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, James LM, Christova P, Engdahl BE (2018) A two-hit model of the biological origin of posttraumatic stress disorder (PTSD). J Ment Health Clin Psychol 2:9–14 [PMC free article] [PubMed] [Google Scholar]
- Girgenti MJ, Hare BD, Ghosal S, Duman RS (2017) Molecular and Cellular Effects of Traumatic Stress: Implications for PTSD. Curr Psychiatry Rep 19:85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Beneyto M, Salazar-Fraile J, Marti-Sanjuan V, Gonzalez-Lujan L (2006) Posttraumatic stress disorder in primary care with special reference to personality disorder comorbidity. Br J Gen Pract 56:349–354 [PMC free article] [PubMed] [Google Scholar]
- Hieda M, Yoo JK, Badrov MB, Parker RS, Anderson EH, Wiblin JL, Kawalsky J, North CS, Suris A, Fu Q (2019) Reduced left ventricular diastolic function in women with posttraumatic stress disorder. Am J Physiol Regul Integr Comp Physiol 317:R108–R112 [DOI] [PubMed] [Google Scholar]
- Hoerster KD, Campbell S, Dolan M, Stappenbeck CA, Yard S, Simpson T, Nelson KM (2019) PTSD is associated with poor health behavior and greater Body Mass Index through depression, increasing cardiovascular disease and diabetes risk among US veterans. Prev Med Rep 15:100930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibler JL (2009) Posttraumatic stress and cardiovascular disease risk. J Trauma Dissociation 10:135–150 [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ (2013) National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress 26(5):537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC (2009) Is posttraumatic stress disorder related to development of heart disease? An update. Cleve Clin J Med 76(Suppl 2):S60–S65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmich NN, Sivak KV, Chubarev VN, Porozov YB, Savateeva-Lyubimova TN, Peri F (2017) TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines (Basel) 5:34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S, Wu G, Jiang Z (2018) Glycyrrhizin treatment facilitates extinction of conditioned fear responses after a single prolonged stress exposure in rats. Cell Physiol Biochem 45:2529–2539 [DOI] [PubMed] [Google Scholar]
- Levine AB, Levine LM, Levine TB (2014) Posttraumatic stress disorder and cardiometabolic disease. Cardiology 127:1–19 [DOI] [PubMed] [Google Scholar]
- Liberzon I, Lopez JF, Flagel SB, Vazquez DM, Young EA (1999) Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol 11:11–17 [DOI] [PubMed] [Google Scholar]
- Liu M, Xu F, Tao T, Song D, Li D, Li Y, Guo Y, Liu X (2016) Molecular mechanisms of stress-induced myocardial injury in a rat model simulating posttraumatic stress disorder. Psychosom Med 78:888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T (2017) Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 42:254–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SI, Ernst RK, Bader MW (2005) LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol 3:36–46 [DOI] [PubMed] [Google Scholar]
- Morris MC, Hellman N, Abelson JL, Rao U (2016) Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clin Psychol Rev 49:79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM (2009) The JUPITER trial: results, controversies, and implications for prevention. Circ Cardiovasc Qual Outcomes 2:279–285 [DOI] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377:1119–1131 [DOI] [PubMed] [Google Scholar]
- Rorabaugh BR, Mabe NW, Seeley SL, Stoops TS, Mucher KE, Ney CP, Goodman CS, Hertenstein BJ, Rush AE, Kasler CD, Sargeant AM, Zoladz PR (2019) Myocardial fibrosis, inflammation, and altered cardiac gene expression profiles in rats exposed to a predator-based model of posttraumatic stress disorder. Stress. 10.1080/10253890.2019.1641081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Dong L, Jiang J, Zhao J, Zhao G, Dang X, Lu X, Jia M (2013) Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 303:107–114 [DOI] [PubMed] [Google Scholar]
- Souza RR, Noble LJ, McIntyre CK (2017) Using the single prolonged stress model to examine the pathophysiology of PTSD. Front Pharmacol 8:615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer K, Upton D, Semple S, McKune A (2018) Systemic low-grade inflammation in post-traumatic stress disorder: a systematic review. J Inflamm Res 11:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Morinobu S, Iwamoto Y, Yamawaki S (2006) Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology 189:165–173 [DOI] [PubMed] [Google Scholar]
- von Kanel R, Hari R, Schmid JP, Wiedemar L, Guler E, Barth J, Saner H, Schnyder U, Begre S (2011) Non-fatal cardiovascular outcome in patients with posttraumatic stress symptoms caused by myocardial infarction. J Cardiol 58:61–68 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu S, Yu X, Zhou S, Ge M, Chi X, Cai J (2016) Dexmedetomidine protects rat liver against ischemia-reperfusion injury partly by the alpha2A-adrenoceptor subtype and the mechanism is associated with the TLR4/NF-kappaB pathway. Int J Mol Sci 17:e995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Caughron B, Young MRI (2017) Posttraumatic stress disorder: an immunological disorder? Front Psychiatry 8:222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Han F, Shi Y (2015) Single-prolonged stress activates the transcription factor ATF6alpha branch of the unfolded protein response in rat neurons of dorsal raphe nucleus. Mol Cell Biochem 399:209–216 [DOI] [PubMed] [Google Scholar]
- Xu D, Yan S, Wang H, Gu B, Sun K, Yang X, Sun B, Wang X (2015) IL-29 enhances LPS/TLR4-mediated inflammation in rheumatoid arthritis. Cell Physiol Biochem 37:27–34 [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I (2009) Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety 26:1110–1117 [DOI] [PubMed] [Google Scholar]
- Yang Y, Lv J, Jiang S, Ma Z, Wang D, Hu W, Deng C, Fan C, Di S, Sun Y, Yi W (2016) The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis 7:e2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Wen L, Xiao B, Han F, Shi Y (2014) Single Prolonged Stress induces ATF6 alpha-dependent endoplasmic reticulum stress and the apoptotic process in medial frontal cortex neurons. BMC Neurosci 15:115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xue C, Xu Q, Zhang Y, Li H, Li F, Liu Y, Guo C (2019) Caprylic acid suppresses inflammation via TLR4/NF-kappaB signaling and improves atherosclerosis in ApoE-deficient mice. Nutr Metab (Lond) 16:40 [DOI] [PMC free article] [PubMed] [Google Scholar]