Abstract
Molecular and clinical research based on isocitrate dehydrogenase (IDH) mutations is much sought after in glioma research since a decade of its discovery in 2008. IDH enzyme normally catalyzes isocitrate to α-keto-glutarate (α-KG), but once the gene is mutated it produces an ‘oncometabolite’, 2-hydroxyglutarate (2-HG). 2-HG is proposed to inhibit α-KG-dependent dioxygenases and also blocks cellular differentiation. Here, we discuss the role of the IDH1 mutation in gliomagenesis. The review also focuses on the effect of 2-HG on glioma epigenetics, the cellular signaling involved in IDH1 mutant glioma cells and the therapeutic response seen in mutant IDH1(mIDH1) harboring glioma patients in comparison to the patients with wild-type IDH1. The review encompasses the debatable impacts of the mutation on immune microenvironment a propos of various mIDH1 inhibitors in practice or in trials. Recent studies revealing the relation of IDH mutation with the immune microenvironment and inflammatory status in untreated versus treated glioblastoma patients are highlighted with respect to prospective therapeutic targets. Also at the molecular level, the association of mIDH1/2-HG with the intracellular components such as mitochondria and other neighboring cells is discussed.
Keywords: Mutant IDH1, Glioma, 2-HG, Tumorigenesis
Introduction
Gliomas arising from the glia of the central nervous system (CNS) are the common primary CNS tumors in adults. Depending on the cell of origin, there are various types of gliomas—astrocytomas, oligodendrogliomas and ependymomas, of which astrocytoma is the most common subtype. This categorization is based on the phenotypic and genotypic parameters established by the World Health Organization (WHO) in the 2016 classification of tumors of the central nervous system (CNS). In addition to glioma-typing, the WHO system also assigns ‘grades’ to the gliomas depending on their behavior, with higher grades signifying more aggressive behavior. There are four WHO grades of astrocytomas: “I (pilocytic astrocytoma, subependymal giant cell astrocytoma), II (diffuse astrocytoma), III (anaplastic astrocytoma) and IV (glioblastoma)” (Louis et al. 2016).
Of all the four grades, glioblastomas (GB) is the most malignant, aggressive and incurable astrocytomas with a median survival of approximately 15 months (Stupp et al. 2009). There are two subtypes of glioblastomas—primary glioblastomas (which originate de novo) and secondary glioblastomas (that arise from a pre-existing low-grade astrocytoma). The poor prognosis of this tumor is due to resistance to treatment and tumor recurrence even after surgical removal (Park et al. 2010; Osuka and Van Meir 2017). Glioblastomas are primarily treated by surgical resection followed by adjuvant therapy. Surgery for gliomas is complicated because of their infiltrative nature. Therefore, it is difficult to cure them with surgery alone, necessitating adjuvant radiotherapy and chemotherapy. However, none of the treatment modalities have significantly increased the survival. The commonly used chemotherapeutic agent for gliomas is Temozolomide (TMZ), the use of which is limited by its acquired resistance (Chen et al. 2018).
Several genetic and epigenetic alterations have been identified in the pathogenesis of gliomas in adults (Richterová and Kolarovszki 2016). The genetic alterations include gene mutations, amplifications and deletions. The frequent genetic alterations seen in astrocytomas include mutations in isocitrate dehydrogenase genes 1 and 2 (IDH1/2), TP53 and TERT promoter (pTERT); EGFR amplification and pRB deletion (Bralten and French 2011). But there are some genetic alterations that make primary glioblastomas distinct from secondary glioblastomas. Some of the prominent mutations seen in primary glioblastomas are EGFR amplification or mutation, PTEN deletion or mutation, CDKN2A-p16INK4a deletion. Frequently seen alterations in secondary glioblastomas are TP53 mutations, IDH1/2 mutations and (Platelet-derived growth factor receptor) PDGFR gene mutations (Crespo et al. 2015). IDH mutation was the frequently observed one and was reported in glioma in the year 2008 (Parsons et al. 2008) and later in some other tumors like acute myeloid lymphoma and solid tumors like cholangicarcinoma and chondrosarcoma (Dang et al. 2016). Even the current WHO classification of gliomas, especially glioblastomas is based on the status of IDH (wt/mutant) (Louis et al. 2016), which is due to the prevalence of IDH mutation in glioma (Parsons et al. 2008; Nasser and Mehdipour 2018).
IDH mutation is a factor that is responsible for the generation of elevated levels of reactive oxygen species (ROS) in glioma cells (Shi et al. 2014a, b). This review mainly focuses on IDH1 mutations in diffuse glioma (which includes astrocytomas, oligodendrogliomas and oligoastrocytomas, ranging from grade II–IV), mutant IDH1 product 2-HG, the effect of 2-HG on factors involved in gliomagenesis (formation of glioma) like HIF-1α (Hypoxia-Inducible Factor 1α), p53, VEGF (Vascular Endothelial Growth Factor), genomic instability, mitochondrial function (Fig. 1) and immunomodulation, and the association of IDH1 with metabolic flexibility, and therapeutic response on gliomas with mIDH1. A very recent book chapter has described certain above-mentioned aspects of IDH-mutant gliomas pertaining to role in tumorigenesis and therapeutic targets and hence out of the scope of the current review (Tateishi and Yamamoto 2019). Thus, we have shifted the focus to studies on the molecular effects of 2-HG in vitro and in vivo.
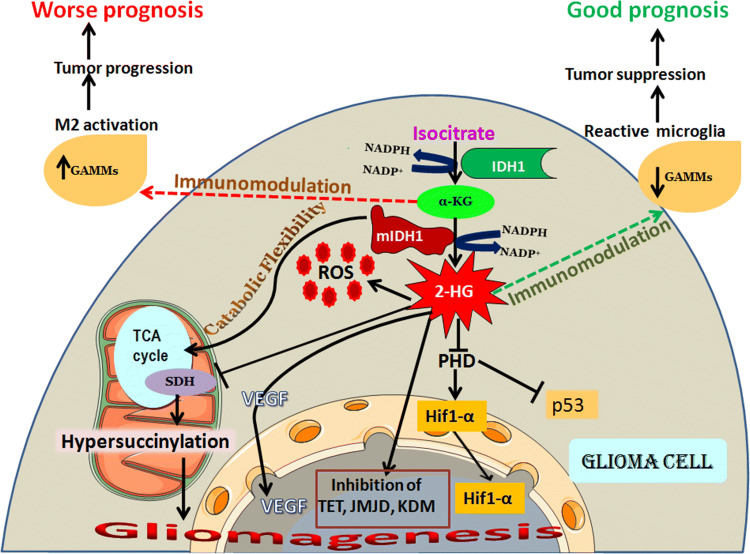
Fig. 1.
Gliomagenesis, prognosis and immunomodulation rendered by IDH1 status. IDH1 resides in the cytosol catalysesisocitrate to alpha-keto glutarate via oxidative decarboxylation generating NADPH. IDH1 mutation is prevalent in glioma and is considered as an early event in gliomagenesis. Mutation in IDH1 produces an oncometabolite 2-HG which favors gliomagenesis. 2-HG is produced from α-KG by consuming NADPH. The decline in NADPH causes a reduction in the antioxidant GSH level which elevates ROS in the glioma cell. 2-HG competitively inhibits various α-KG-dependent dioxygenases like lysine histone demethylases (KDMs), TET (Ten Eleven Translocase) family of DNA hydroxylases, JMJDs (Jumonji C-domain-containing enzymes) and prolyl hydroxylase domain (PHD) and thus is involved in tumorigenesis. In wild-type IDH1 cells, PHD acts on its substrate HIF-1α (Hypoxia-Inducible Factor-1 α) and promotes HIF degradation, because HIF accumulation results in tumorigenesis. 2-HG inhibits HIF degradation and p53 downregulation by inhibiting PHD, promoting gliomagenesis. 2-HG is also involved in the hypersuccinylation of TCA enzymes in the mitochondria via inhibition of succinate dehydrogenase (SDH), which leads to apoptotic resistance in tumor cells and thus results in tumorigenecity. 2-HG favors VEGF (Vascular Endothelial Growth Factor) upregulation leading to angiogenesis. mIDH1 cells have higher proportion of pro-inflammatory GAMMs (Glioma Associated Microglia and Macrophages), although fewer in total. wt-IDH1 and mIDH1 shows distinct immunomodulatory functions which may render opposite prognostic values
IDH1 Mutation, 2-Hydroxyglutarate Production and Gliomagenesis
IDH1 Mutation
The discovery of IDH mutations was a landmark in the history of gliomas. It is a good diagnostic and prognostic biomarker in gliomas. Numerous studies conducted over the last decade on various aspects of IDH mutations has led to the accumulation of a wealth of data. IDH gene encodes Isocitrate Dehydrogenase, an NAD(P)+-dependent enzyme which catalyzes the formation of α-KG via an oxidative decarboxylation step involving NAD(P)H generation (Shi et al. 2014b). Of the 3 isoforms of IDH, IDH1 resides in the cytosol and in peroxisomes, whereas IDH2/3 in the mitochondria (Krell et al. 2011). IDH1/2 are homodimeric enzymes, whereas IDH3 is a heterodimer, which is not related to the other two isoforms structurally (Mellai et al. 2013). In astrocytomas and oligodendrogliomas, mutations in idh1/2 genes occur, but the predominant one is IDH1 mutation (Yan et al. 2009). Out of all gliomas, higher prevalence of IDH1 mutation occurs in lower grade astrocytomas and oligodendrogliomas, and in secondary glioblastomas (more than 75%). It is a point mutation, which occurs either at the first or second base of the codon 132 of the IDH1 gene, where histidine is formed instead of arginine (R132H). Several other types of mutations also occur at the same codon where arginine is replaced by glutamine, cysteine, serine or lysine (Watanabe et al. 2009). IDH1 mutation is an early event in gliomagenesis and precedes the acquisition of other genetic alterations like TP53 mutation or 1p19q co-deletion (OHBA and HIROSE 2016). Two recent reviews give the frequencies of IDH1/2 mutations in different types of glioma and also the frequency of specific IDH mutations in glioma (Waitkus et al. 2016; OHBA and HIROSE 2016).
IDH mutations were first identified in gliomas by Parsons et al, when they sequenced human glioma tissue samples. The mutation was observed in all but one of the secondary GBM, although none of the primary exhibited IDH mutation (Parsons et al. 2008). Secondary glioblastomas which develop on a background of low-grade astrocytoma possess IDH1 mutation (Ohgaki and Kleihues 2013). The IDH1 mutation is a heterozygous mutation where one allele is a mutant one and the other is the wild-type. The IDH1 catalyzes isocitrate to α-KG and the mutant allele further converts α-KG to 2-HG (Navis et al. 2013). Homozygous IDH1 mutation was also found recently in a few number of patients, but the prognostic significance and effects of the 2-HG produced by the same is not fully understood (Singh et al. 2017). It remains to be understood whether the quantity of 2-HG produced be directly linked to the heterozygous or homozygous nature of the mutation. In addition, mIDH1 harboring tumor cells are metabolically different from the tumor cells with wild-type IDH1 (Garrett et al. 2018).
Similarly, mutation in IDH2 is also seen in gliomas even though it is less common compared with IDH1, where Arginine at codon 172 is replaced by lysine (R172K) (Xu et al. 2004; Cohen et al. 2013). This NADP+-dependent mitochondrial enzyme has the same function as that of IDH1 and it participates in the Kreb’s cycle for energy production (Xu et al. 2004). The IDH2 enzyme helps in maintaining the mitochondrial redox balance and it protects the cell from oxidative stress by producing NADPH which is the precursor of the GSH anti-oxidant pool (Smolková and Ježek 2012). IDH2 mutations too show similar effects as that of IDH1 mutations in glioma (Cohen et al. 2013).
2-HG “an Oncometabolite”
Normally, wild-type IDH1 produces α-KG in the cytoplasm, which enters the mitochondria where NAD(P)H is generated, which in turn helps in the formation of glutathione (GSH), a major antioxidant. GSH is responsible for preventing the damage caused by oxidative stress and also has anti-apoptotic functions (Circu and Aw 2012). Once mutation occurs in the IDH1 gene, the production of NADPH that helps in the synthesis of GSH gets reduced. Moreover, NADPH will be consumed by the mutant form of IDH1(mIDH1) to produce 2-HG which leads to the decreased production of GSH, thereby increasing the cellular ROS level (Shi et al. 2014a).
Dang et al. first depicted that 2-HG is synthesized by the mutant form of IDH1 and it has properties that enable us to refer to it as an ‘oncometabolite’. 2-HG is a metabolite that is present in micromolar levels even in normal cells. Elevated level of 2-HG in blood, other body fluids like plasma and urine, or tissues is an indication of metabolic disorders. 2-HG in the mitochondria is produced by the enzyme, hydroxyacid-oxoacid-transhydrogenase (HOT) and in the cytosol by phosphoglycerate dehydrogenase from a common substrate, α-KG. 2-HG dehydrogenase catalyzes the conversion of 2-HG into α-KG, and any impairment in this enzyme leads to the accumulation of 2-HG (Struys 2006).
2-HG can be present either in the S-form (L-2-HG) or in the R-form (D-2-HG). Out of the two, D-2-HG is the one that accumulates in IDH1-mutant glioma cells (Dang et al. 2009). Patients with D-2-HG have increased risk of brain tumors. Normally, D-2-HG is converted by the enzyme D-2-HGDH (D-2-HG Dehydrogenase) to α-KG and thereby decreasing its level in the cell. The increased L-2-HG level is seen in a disease condition called 2-Hydroxyaciduria (a rare neurological disorder) (Madala et al. 2018). L-2-HG patients do not have brain tumors, because they all die in infancy. This is in contrast to D-2-HG which accumulates in gliomas with IDH mutation. It is the presence of 2-HG that helps in identifying the IDH mutation in glioma patients (Kranendijk et al. 2012). 2-HG is found to impair maturation of collagen, which results in basement membrane aberration that has a major role in glioma progression. Mutant IDH overexpression in glioma cells produced increased levels of 2-HG and high levels of 5mC (5-methylcytosine). 2-HG is found to inhibit mTOR signaling and ATP synthase (Tateishi and Yamamoto 2019).
2-HG is also associated with various other processes of the cell including the epigenetic regulation and cellular signaling and repression of the tumor-associated immune system in glioma (Gagné et al. 2017). In a recent report, it shows that 2-HG inhibits the pathways involved in complement activation, with a reduction in proliferation of tumor infiltrating and activated T cells, phagocytosis and cytokine secretion in human glioma tissue samples. This suppression of host immune system by 2-HG in mIDH1 tumors helps in the escape of tumors from the action of immune system against tumor cells. Thus, this could be one of the answers for the frequently asked question, how 2-HG favors tumorigenesis (Zhang et al. 2018). Philip et al. have shown that IDH1R132H promotes gliomagenesis in vivo. According to their observations, R132H IDH1 mutation cannot induce tumorigenesis alone, but it cooperates with PDGFA and also the loss of some of the tumor suppressors including Cdkn2a, Atrx, and Pten to induce tumor development. This study also demonstrated that tumors of mutant IDH1 mouse shows increased proliferation and abnormal vasculature and a 100-fold greater increase in 2-HG production than the cells with wild-type IDH1 (Philip et al. 2018).
2-HG in Gliomagenesis
IDH1 mutation is a gain-of-function mutation, as it results in the production of a different metabolite, 2-HG in excess than the normal α-KG (Liu et al. 2016). Koivunen et al. have shown that R132H IDH1 cells proliferate faster than their wild-type counterparts. The epigenetic alterations occurring due to IDH1 mutation is an essential factor of tumorigenesis in glioma. For example, the TET2 (Ten Eleven Translocase 2) and the HIF1-α are the important molecules involved in the mutant IDH1 pathway, where the high level of latter in the cell can lead to cancer (Koivunen et al. 2012).
The real driver of gliomagenesis in mIDH1 gliomas is the most confusing part of the story. It could be the oncogenic properties shown by the mIDH1 product 2-HG on the α-KG-dependent enzymes and further effects on HIF1-α and VEGF. The increased levels of ROS in mIDH1 could be another factor that may contribute to gliomagenesis.
2-HG in Epigenetic Regulation
The production of 2-HG in IDH1 mutant cases is responsible for majority of the epigenetic changes seen in gliomas. Since 2-HG is a structural analogue of α-KG and thus it competitively inhibit α-KG-dependent dioxygenase enzymes such as lysine histone demethylases (KDM), TET (Ten Eleven Translocase) family of DNA hydroxylases, JMJDs (Jumonji C-domain-containing enzymes) and prolyl hydroxylase domain (PHD) and hence, contributes to tumorigenesis and glioma progression (Yang et al. 2012; Wong et al. 2017). TET enzymes helps in DNA demethylation process by converting 5-methylcytosine (5mC) to 5-hydroxylmethylcytosine (5hmC). Any kind of negative regulation of the TET enzymes is associated with abnormal DNA hypermethylation. Such an aberrant DNA methylation pattern is a major hallmark of cancer development. The conversion of 5mC to 5hmC is blocked by the action of 2-HG on TET, where it inhibits the DNA hydroxylation leading to DNA hypermethylation. This leads to the loss of 5hmC, which plays an important role in gene expression, in IDH1 mutated tumors (Shim et al. 2014). DNA hypermethylation causes complete gene dysregulation which leads to initiation and progression of cancer in mutant IDH1 cases (Gagné et al. 2017). However the exact impact of TET inhibition by mutant IDH1 product 2-HG on cell transformation, tumor progression and maintenance is not well understood (Rasmussen and Helin 2016). PHD plays a role in HIF-1α degradation and its inhibition due to increased 2-HG results in accumulation and stabilization of HIF-1α, which favors gliomagenesis (Kaur et al. 2005).
Like DNA methylation, methylation of lysine groups present on histone proteins is yet another important factor that influences chromatin structure and gene expression regulation. The oncometabolite also acts on the Jumonji histone demethylase, which is an α-KG-dependent dioxygenase that helps in the removal of methyl groups from the lysine residues present on the histone tail. KDM2A, KDM4A KDM4B, KDM4C, KDM5B, etc. are some of the lysine demethylases that act on histone. But it was found that lysine-methylated histones were elevated in mutant IDH1 oligodendrogliomas than the oligodendrogliomas with wild-type IDH1. The demethylase depletion in 2-HG accumulated tumors impairs the genes (TET2, PHD, KDM4A, ALKBH2/3) involved in cell differentiation, thereby blocking differentiation and promoting tumorigenesis in mutant IDH1 condition (Gagné et al. 2017).
2-HG-Associated Genomic Instability
Alkylating agents are commonly used as chemotherapeutics in glioma patients and the DNA lesions made by those drugs are repaired by DNA repair enzymes such as MGMT [O-(6)-methylguanine-DNA methyltransferase] and alkB homolog (ALKBH) proteins. Since, 2-HG directly affects the MGMT methylation and ALKBHs are α-KG-dependent enzymes, the accumulation of 2-HG in mutant IDH1 cells, therefore, inhibits the function of these enzymes involved in DNA break repair. This forms the basis of sensitization of IDH1-mutant tumor cells to alkylating agents compared to the wildtype. Inhibition of α-KG-dependent dioxygenenases, like, KDM4A and KDM4B via the accumulation of 2-HG in IDH1 mutations also have been reported to cause a defect in homologous recombination (HR). Mutant IDH1 cells showed selective sensitivity to PARP inhibition through synthetic lethality interactions. The above said HR defect is reversed by the mutant IDH1 treatment and thus eliminated the PARP inhibitor sensitivity. All these together shows that IDH1 mutations are associated with genomic instability, thereby greater susceptibilities to treatment leading to further DNA damage or defect in DNA repair (Han and Batchelor 2017). This could be one of the possible explanations for the better outcome for IDH1 mutant gliomas. This condition parallels to the use of PARP inhibitors in BRCA-mutated tumors, where it shows the property of synthetic lethality; the presence of mutations in IDH1 gene imparts sensitivity to therapeutic interventions and thus offering a survival advantage in glioma.
2-HG in Cellular Signaling
Effect of Mutant IDH1 on HIF-1α and VEGF
There are contradictory reports about the effect of mutant IDH1 on HIF and VEGF (Williams et al. 2011; Kickingereder et al. 2015; Semukunzi et al. 2017). There are reports that mutant IDH1 in glioma inactivates the enzyme IDH1 and thus helps in HIF-1α induction (Zhao et al. 2009; Wang et al. 2014). The overexpression of mutant IDH1 and the knockdown of wild-type IDH1 in U87 glioblastoma cells showed an increase in the expression levels of HIF-1α. Normally, the α-KG produced due to the activity of IDH1 results in increased level of PHD, which in turn leads to the degradation of HIF-1α. Therefore, a decrease in IDH1 activity due to IDH1 mutation leads to the stabilization of HIF-1α through the decline in α-KG. Moreover, this stabilization of HIF-1α leads to a rise in VEGF levels, along with other HIF-1α target genes in gliomas with mutant IDH1, when compared to those without this mutation (Yalaza et al. 2017). These findings suggest that IDH1 is a tumor suppressor gene in its wild-type form and once the gene becomes mutated, it acts as an oncogene inducing the tumorigenic and angiogenic pathways in glioma (Zhao et al. 2009). In contrast to these observations, the study by Polívka et al. in U251 glioblastoma cells in vitro showed a decrease in the levels of HIF-1α and VEGF in the presence of IDH1 mutation. However, this finding was not corroborated in glioblastomas in vivo, where the expression of HIF-1α and VEGF was not dependent on the status of IDH1 (Polívka et al. 2018).
Effect of Mutant IDH1 on p53 Level
The process of cancer formation begins either due to the activation of oncogenes or the inhibition of tumor suppressor genes. p53 is a transcription factor known as the gatekeeper of cells by playing a major role as a tumor suppressor gene. Mutation/inactivation of TP53 gene is a common feature of many cancers (Muller and Vousden 2013). TP53 gene mutation is a frequently seen alteration in glioblastomas and other diffuse astrocytomas. It is also suggested that the mutation in this gene helps progression of astrocytomas from lower grades to the higher ones (Nagpal et al. 2006). IDH1 and TP53 mutations co-occur in astrocytomas. Jiang et al. have shown that IDH1 mutation in glioma downregulates p53 via activation of the microRNA, miR-380-5p, which leads to tumor formation. This correlation is as follows: as mentioned previously, 2-HG production due to mutant IDH1 in gliomas inhibits the enzyme PHD, which stabilizes HIF-1α, as well as, HIF-2α. The latter inhibits p53 through the activation of miR-380-5p. The tumor formation in IDH1-mutant gliomas may be due to the downregulation of p53 due to the increased 2-HG production via PHD inhibition (Jiang et al. 2018). In contrast, there are other studies which suggest that p53 expression does not significantly correlate with the increased progression-free survival (PFS)and overall survival (OS) in glioma patients, thus warranting more such studies (SongTao et al. 2012).
IDH1 Mutation and Metabolic Flexibility/Reprogramming
A recent study on cells containing IDH1 mutations have showed that it inhibited the fatty acid oxidation (FAO) and elevated glucose consumption by elevating GLUT 2 and downregulating CPT1 in HCT 116 cells. The mitochondrial content remained the same, but the cytochrome c and cytochrome 4 were downregulated. While this may be true in colon cells, IDH1 mutation may not be related to fatty acid metabolism in brain cells, as they do not have fatty acid metabolizing machinery in them, whether there could be elevation of glucose utilization remains to be known (Li et al. 2019). Metabolic changes such as the depleted glutathione metabolites, copious TCA metabolites, diminished levels of NAAG (N-acetyl-aspartyl-glutamate, a neurotransmitter metabolite) and increased glutaminolysis has been reported already in AML(Acute Myeloid Leukemia) too (Waitkus et al. 2016; Stuani et al. 2017). In AML, it has also been found that IDH1 mutation and 2-HG production confers catabolic flexibility and hence enhances the capacity of mutant IDH1 cells to consume more substrates like glutamine (Stuani et al. 2017). Mutant IDH cells show altered amino acid and TCA cycle intermediates. IDH-mutant cells alters pyruvate metabolism which results in replenishing the TCA cycle intermediates (Tateishi and Yamamoto 2019).
2-HG and Mitochondrial Function
Earlier reports show that IDH1 mutations cause alterations in cellular metabolism under hypoxic conditions in IDH1 mutant cell lines, making those cells depend more on oxidative mitochondrial metabolism as well as induce reductive glutamine metabolism. This indicates these cells to be more sensitive to inhibitors of mitochondrial metabolism (Grassian et al. 2014). Further studies have provided evidence that IDH1 mutant cells are vulnerable to mitochondrial metabolism inhibition as these cells were unable to generate energy from various carbon substrates (Han and Batchelor 2017). A recent study has demonstrated that 2-HG competitively inhibits the mitochondrial TCA cycle enzyme succinate dehydrogenase (SDH) resulting in impaired mitochondrial respiration via accumulation of succinyl-CoA and hypersuccinylation (Li et al. 2015). IDH1 mutations resulting in mitochondrial dysfunction may thereby contribute to the tumorigenicity and apoptotic resistance of cancer cells by certain unknown pathways (Li et al. 2019). Although with respect to disruption of mitochondrial ETC, data is unavailable for glioma cells, a phase 1 clinical trial exploring the combination of metformin and chloroquine in IDH1 mutant tumors is underway (Han and Batchelor 2017) and will open new treatment options for these tumors.
2-HG and Immunomodulation
To date, several clinical trials have recruited patients with GBM to investigate the efficacy of various brain tumor immunotherapies, mostly with wild type IDH1 genotype with few protocols with low-grade and anaplastic glioma. The immunomodulating effects of IDH mutation and its application in immune-based therapies have been reviewed in detail elsewhere by Berghoff et al. and Choi and Curry (Berghoff et al. 2017; Choi and Curry 2017). As our understanding develops regarding the interactions between the IDH1 mutant tumors and the host immunity further, it is becoming increasingly evident that mutant IDH1 confers an immunologically quiescent phenotype onto glial cell tumors. They have fewer tumor infiltrating lymphocytes (TILs) and decreased expression of programmed cell death ligand (PD-L1) when compared to their wild type counterparts. Moreover, the persistence of IDH1 mutant allele expression throughout the entire stages and variants of cancer cells has instigated few on-going trials of vaccination-based immunotherapy targeting IDH1 mutations. In such a study, IDHR132H mice harboring intracranial tumors were subjected to an IDHR132H specific peptide vaccine that retarded the intracranial tumor expansion (Pellegatta et al. 2015). Zhang et al. have shown that glioma cells harboring IDH1 mutation were found to be resistant to natural killer (NK) cell-mediated lysis due to the silencing of NKG2D-L (NK group 2D ligand) by 2-HG induced hypermethylation (Zhang et al. 2016). The data obtained from TCGA studies have indicated that IDH-mutant-mediated immune suppression through reduced expression of CD8 + T cell and interferon-γ associated genes, crucial for the recruitment and activation of a robust antitumor T cell response. Further accumulation of 2-HG leads to hypermethylation of key immune-related genes resulting in their repression. In a preclinical model that leads to the overproduction of 2-HG, it led to the decreased leukocyte production of C-X-C motif chemokine (CXCL)like CXCL9 and CXCL10 through inhibition of signal transducer and activator of transcription1 (STAT1), in turn resulting in reduced infiltration of CD8 + T cells into tumors. It is still a matter of investigation whether these observed effects are due to epigenetic regulation of crucial genes involved or by the direct effect of 2-HG on tumor infiltrating lymphocytes or both (Richardson et al. 2019). A clear understanding of how 2-HG is affecting T cells have emerged from the studies done by Bunse et al., which has shown that 2-HG is imported by the T cells from the tumor cells leading to the inhibition of T cell, both activation and proliferation (Bunse et al. 2018).
Reports on the consequences of 2-HG on the activity of immune cells have furthermore revealed pointers about their effects on infiltrating myeloid cells and macrophage polarization, and thereby shaping the tumor microenvironment or the niches harboring the glioma stem cells (GSCs)vide post. Niches are described as the spots of paramount expression of the tumor microenvironment (TME), and include immune cells, microglia/macrophages (Schiffer et al. 2018). Resident microglia-derived cells and blood-borne macrophages constitute the glioma-associated microglia and macrophages (GAMMs). GAMMs are construed to favor tumor progression in glioma and found to be more polarized to the pro-tumoral and anti-inflammatory M2 phenotype particularly in IDH1 mutant gliomas (Szulzewsky et al. 2015; Wang et al. 2018; Annovazzi et al. 2018; Schiffer et al. 2018). There is already a report that α-KG prevents the M1 activation and augments M2 activation via suppressing NFκB, thereby helping in tumor progression and development in glioma. Thus, the decline in α-KG levels in gliomas with IDH1 mutation may reverse the above said scenario, thus favoring M1 activation leading to tumor suppression (Liu et al. 2017). Currently only limited data is available on the association between macrophages and IDH1 status in glioma. Nevertheless, Poon and colleagues have recently emerged with an observation that although there are less GAMMs in mutant IDH1 cells than wt-IDH cells, the pro-inflammatory microglia population is more in the former when compared to predominance of anti-inflammatory macrophages in the latter. Through this probably landmark finding the authors have suggested the use of differential treatment modalities based on IDH1 status and pro-inflammatory scores, i.e., abolition of the GAMM and immunostimulation in wt-IDH1 GBMs, whereas suppression of the GBM-mediated immunomodulation in mIDH1 GBMs. Thus, they emphasized on the need of patient stratification while deciding the kind of therapies to individual patients. Another interesting observation was that the robust anti-inflammatory macrophages outnumbered the pro-inflammatory microglia in treated mIDH1 GBMs, thereby highlighting the discrepancy in IDH1 prognosis a propos of the native immune microenvironment and the inflammatory status (Poon et al. 2019).
IDH1 Mutation and GSCs
Factors which regulate the subtle balance between the stemness and differentiation of GSCs might be key players in determining such a tumor suppressor function. For instance, the canonical Wnt/β-catenin signaling is proved to be involved in the cell division and death of glioblastoma cells in vitro. Stimulation of Wnt/β-catenin pathway has been shown to reduce the cell proliferation in glial tumor cells and via GSK-3 inhibition; it further inhibits their invasive property. (Gao et al. 2017). IDH R132H mutation has been found to be activating the negative regulators of Wnt/β-catenin signaling in human glioblastomas (Cui et al. 2016). To add to this, downregulation of upstream and downstream players of Wnt/β-catenin signaling and consequent reduction of self-renewal, migration, invasion and induction of apoptosis was observed inIDH R132H transfected GSCs (Yao et al. 2018). In vitro data as well as human data from serial magnetic resonance imaging demonstrated that the mutation was also associated with a reduction in cellular fitness as there were reduced cell proliferation, migration and increased radiosensitivity caused by mIDH1 (Kessler et al. 2015; Rosiak et al. 2016). Thus, presence of a mIDH1 imparts a weakened hostility to the GSCs pertaining to tumor progression. Recently, it was found that tumor initiating stem cell numbers were less in mutant IDH1 in comparison with the wild-type IDH1(Núñez et al. 2019). Thus the lower frequency of GSCs in mutant IDH1 than the wild-type could be another reason for a better prognosis of mIDH1 patients than the wild-type IDH1 since GSCs have the properties of angiogenesis and metastasis.
Response to Therapy
IDH1 mutation in glioma patients is also a predictor of better response to certain drug treatments (Hartmann et al. 2011). For example, glioblastoma with oligodendroglioma component shows prolonged survival periods in mIDH1 patients than the wild-type, when on TMZ regimen (Myung et al. 2014). As per the findings of Houillier et al. in the year 2010, it was reported that IDH1 mutation has a positive effect on the TMZ treatment response in glioma and it can contribute to the increased survival rates (OS and PFS) (Houillier et al. 2010). The actual reason for this remains unclear, but since mutant IDH1 reduces cellular anti-oxidant activity via the depletion of NADPH, and hence fails to protect cells from the damage caused by oxidative stress, leading to glioma cell apoptosis during the course of therapy (SongTao et al. 2012). There is a strong correlation between IDH1 mutation and MGMT methylation. In addition, another reason for the increased patient survival in gliomas with mIDH1 could be the methylation of the MGMT promoter by 2-HG which leads to the lower expression of MGMT whereby the DNA damage repair in glioma cells will be affected, which makes the patients more sensitized to TMZ treatment (Molenaar et al. 2014).
As mentioned above, there are various co-occuring mutations along with IDH1 in gliomas, of which, studies related to IDH1 mutation and methylation of MGMT promoter have been done widely (Combs et al. 2011; Zhang et al. 2013; Myung et al. 2014; Oyan et al. 2015). MGMT is a prominent DNA repair enzyme which removes the DNA lesions generated by TMZ in glioma patients (Chen et al. 2018). The methylation of the promoter region of the enzyme helps in silencing the enzyme, thus reducing the DNA repair activity in glioma cells. This leads to an improved response to chemotherapy, particularly TMZ, thereby increasing patient survival. Hence, MGMT methylation is considered as a strong positive prognostic factor in glioma, particularly in glioblastomas (Combs et al. 2011). There are reports stating that the presence of MGMT promoter methylation along with IDH1 mutation outdo the effect of either of them alone (Molenaar et al. 2014). The actual reason for the correlation of IDH1 mutation and patient survival in glioma is yet to be elucidated. More scientific validations are required to prove mIDH1 is a good prognostic biomarker of glioma.
A study by Rohle et al. have shown that one of the mutant IDH inhibitor (AGI-5198) delays tumor growth and helps in glioma cell differentiation (Rohle et al. 2013). Some of the commonly used mutant IDH1 inhibitors are AG-120 (Ivosidenib), AGI-5198, which target mIDH1 activity specifically and AG-221 (Enasidenib) is a mIDH2 inhibitor (Urban et al. 2017). AG-881 or Vorasidenib, is the only pan-mutant IDH inhibitor (acts on both mIDH1 and mIDH2) in glioma with supporting clinical data. Most of the mIDH inhibitors are in Phase 1 clinical trial. Several mIDH inhibitors have been developed for the past few years. Almost all are in clinical trials and the two of the FDA approved inhibitors are Enasidenib and Ivosidenib (Golub et al. 2019). A recent study by Costantini et al. has shown that a DNA methyltransferase inhibitor, 5-Azacytidine in combination with TMZ reduces cell growth in mutant IDH1 glioma. Both the drugs individually reduces cell proliferation, when in combination were showing increased DNA damage and apoptosis (Costantini et al. 2013) (Fig. 2).
Fig. 2.

Complementary action of mutant IDH1 inhibitor and Temozolomide in mIDH1 glioma. AG-5198 is an IDH1 mutant inhibitor that specifically inhibits the R132H and R132C IDH1 mutation in gliomas. AG-5198 blocks the production of 2-HG from mutant IDH1. This inhibitor was found to be effective when tested in various cell lines including U87MG human glioblastoma cells. It induces the demethylation of H3K9me3 and helps in tumor growth suppression in mutant IDH1 gliomas. Thus it blocks the tumorigenic properties shown by 2-HG. On one hand, blocking 2-HG is beneficial for the glioma patients harboring mutant IDH1. But on the other hand, 2-HG is important while treatment with drugs like Temozolomide (TMZ). TMZ is an alkylating drug used against glioma, where it damages the cancer cell DNA by adding methyl groups to the cell’s DNA, finally causing cell death. Since cancer cells have multiple DNA repair machineries and mechanisms, they quickly acquire resistance to therapeutic agents. Such a repair enzyme in gliomas is MGMT [O-(6)-methylguanine-DNA methyltransferase]. MGMT helps cancer cell survival by removing the methyl groups on the cancer cell DNA. So glioma patients with wild-type IDH1 when receive TMZ, MGMT confers TMZ resistance. But in case of patients harboring mutant IDH1, 2-HG inhibits MGMT, thereby the DNA damage repair gets blocked and hence, TMZ treatment becomes effective
Mutant IDH1: Better Prognosis in Glioma?
It was found that IDH1 mutation is related to prolonged survival in gliomas, however, other cancers like AML and chondrosarcoma do not showcase such a link (Molenaar et al. 2018). IDH1 mutation along with many other factors like expression of p53, co-deletion of 1p19q, MGMT promoter methylation, is strongly linked to the prolonged survival in glioma patients. For both OS and PFS, IDH1 mutation is purported to be an important deciding factor (Liu et al. 2016). The IDH1 mutant patients have four-fold increased survival rates compared to the patients who harbor the wild-type IDH1, irrespective of the grade (Nobusawa et al. 2009). Meanwhile, there is also a unique report, nonetheless proposing that IDH1 mutation is a “weak predictor” of prolonged survival in glioma, especially glioblastomas (Amelot et al. 2015). But on the other hand, the prognostic significance does not show any sort of constancy in tumor types. The median survival of glioma patients harboring wt-IDH1 is 15 months and it increases to approximately 30 months in mIDH1 patients (Yan et al. 2009; Losman and Kaelin 2013). Mutant IDH1 inhibits the cytoprotection and reduces apoptosis resistance thereby eliciting a tumor suppressor role, but the actual mechanism remains incompletely elucidated (Houillier et al. 2010).
Conclusions
No promising and long-lasting therapy targeting against the most malignant grade IV gliomas without causing any harm to the neighboring normal cells of the brain have been yet developed. It is hitherto unclear as to how and what factors contribute to gliomagenesis in IDH1 mutant tumor. mIDH1 is a positive prognostic factor for glioma patients, but at the same time, its product 2-HG contributes to tumorigenesis. Mutant IDH1 inhibitors for glioma therapy are undergoing clinical trials. They selectively target the 2-HG production in mutant IDH1 cases leading to the reduction in the levels of this oncometabolite and the tumorigenic effects caused by them. Various therapies are in trial to suppress the tumorigenicity caused by 2-HG. Immunotherapy is one among them, where the antitumor response of the host immune system is enhanced by the use of agents like vaccines or cytokines. Combination therapy of mutant IDH1-specific inhibitor along with immunotherapy may help block tumorigenesis to an extent. Meanwhile, the outcomes of IDH1 mutation and/or 2-HG on the tumor microenvironment and tumor immune response are unclarified key questions that need to be addressed while using a mutant IDH1 inhibitor. Why a mutation that phenotypically favors tumorigenesis, has positive effect on the survival of glioma patients than the wild-type IDH patients still intrigues the scientific community and forms the basis of the enigmatic title for this review. The speed at which the research towards unearthing molecular pathways that is responsible for the tumorigenesis and unraveling those targets that confer therapeutic benefits in glioma will hold the answer to the question in dilemma “To be wild or mutant”.
Acknowledgements
The authors would like to acknowledge Dr. Cibin TR for his valuable inputs and suggestions towards the final drafting of the manuscript. BB would like to thank Department of Science and Technology, India for providing the Inspire Fellowship (IF150784). ACR acknowledges Council of Scientific and Industrial Research (09/523(0082)/2014-EMR-1) Government of India for Research Fellowship.
Author Contributions
BB and ACR were the major contributors (designing, searching and writing of the manuscript). MUK, DAN, RP and SG discussed ideas, helped in the outline of the review, and corrected the text. HVE, KK and SG made critical revision for important intellectual content and final approval of the review. The conception of the review was done by SG.
Funding
No funding was used for the manuscript completion.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Human and Animal Rights
This is a review and does not involve human participants and/or animals.
Informed Consent
No informed consent is needed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bharathan Bhavya and C. R. Anand have contributed equally to this work.
References
- Amelot A, Cremoux PD, Quillien V et al (2015) IDH-mutation is a weak predictor of long-term survival in glioblastoma patients. PLoS ONE 10:e0130596. 10.1371/journal.pone.0130596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annovazzi L, Mellai M, Bovio E et al (2018) Microglia immunophenotyping in gliomas. Oncol Lett 15:998–1006. 10.3892/ol.2017.7386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghoff AS, Kiesel B, Widhalm G et al (2017) Correlation of immune phenotype with IDH mutation in diffuse glioma. Neuro-oncology 19:1460–1468. 10.1093/neuonc/nox054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bralten LBC, French PJ (2011) Genetic Alterations in Glioma. Cancers (Basel) 3:1129–1140. 10.3390/cancers3011129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunse L, Pusch S, Bunse T et al (2018) Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med 24:1192. 10.1038/s41591-018-0095-6 [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang M, Gan H et al (2018) A novel enhancer regulates MGMT expression and promotes temozolomide resistance in glioblastoma. Nat Commun 9:2949. 10.1038/s41467-018-05373-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BD, Curry WT (2017) IDH mutational status and the immune system in gliomas: A tale of two tumors? Transl Cancer Res 6:S1253-S1256–S1256. 10.21037/16582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu ML, Aw TY (2012) Glutathione and modulation of cell apoptosis. Biochem Biophys Acta 1823:1767. 10.1016/j.bbamcr.2012.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Holmen S, Colman H (2013) IDH1 and IDH2 Mutations in Gliomas. Curr Neurol Neurosci Rep 13:345. 10.1007/s11910-013-0345-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs SE, Rieken S, Wick W et al (2011) Prognostic significance of IDH-1 and MGMT in patients with glioblastoma: One step forward, and one step back? Radiat Oncol 6:115. 10.1186/1748-717X-6-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini B, Kordasti SY, Kulasekararaj AG et al (2013) The effects of 5-azacytidine on the function and number of regulatory T cells and T-effectors in myelodysplastic syndrome. Haematologica 98:1196–1205. 10.3324/haematol.2012.074823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo I, Vital AL, Gonzalez-Tablas M et al (2015) Molecular and genomic alterations in glioblastoma multiforme. Am J Pathol 185:1820–1833. 10.1016/j.ajpath.2015.02.023 [DOI] [PubMed] [Google Scholar]
- Cui D, Ren J, Shi J et al (2016) R132H mutation in IDH1 gene reduces proliferation, cell survival and invasion of human glioma by downregulating Wnt/β-catenin signaling. Int J Biochem Cell Biol 73:72–81. 10.1016/j.biocel.2016.02.007 [DOI] [PubMed] [Google Scholar]
- Dang L, White DW, Gross S et al (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462:739. 10.1038/nature08617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, Yen K, Attar EC (2016) IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol 27:599–608. 10.1093/annonc/mdw013 [DOI] [PubMed] [Google Scholar]
- Gagné LM, Boulay K, Topisirovic I et al (2017) Oncogenic activities of IDH1/2 mutations: from epigenetics to cellular signaling. Trends Cell Biol 27:738–752. 10.1016/j.tcb.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Gao L, Chen B, Li J et al (2017) Wnt/β-catenin signaling pathway inhibits the proliferation and apoptosis of U87 glioma cells via different mechanisms. PLoS ONE. 10.1371/journal.pone.0181346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett M, Sperry J, Braas D et al (2018) Metabolic characterization of isocitrate dehydrogenase (IDH) mutant and IDH wildtype gliomaspheres uncovers cell type-specific vulnerabilities. Cancer Metab. 10.1186/s40170-018-0177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub D, Iyengar N, Dogra S et al (2019) Mutant isocitrate dehydrogenase inhibitors as targeted cancer therapeutics. Front Oncol. 10.3389/fonc.2019.00417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassian AR, Parker SJ, Davidson SM et al (2014) IDH1 mutations alter citric acid cycle metabolism and increase dependence on oxidative mitochondrial metabolism. Cancer Res 74:3317–3331. 10.1158/0008-5472.CAN-14-0772-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CH, Batchelor TT (2017) Isocitrate dehydrogenase mutation as a therapeutic target in gliomas. Chin Clin Oncol 6:33. 10.21037/cco.2017.06.11 [DOI] [PubMed] [Google Scholar]
- Hartmann C, Hentschel B, Tatagiba M et al (2011) Molecular markers in low-grade gliomas: Predictive or prognostic? Clin Cancer Res 17:4588–4599. 10.1158/1078-0432.CCR-10-3194 [DOI] [PubMed] [Google Scholar]
- Houillier C, Wang X, Kaloshi G et al (2010) IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology 75:1560–1566. 10.1212/WNL.0b013e3181f96282 [DOI] [PubMed] [Google Scholar]
- Jiang B, Zhao W, Shi M et al (2018) IDH1 Arg-132 mutant promotes tumor formation through down-regulating p53. J Biol Chem 293:9747–9758. 10.1074/jbc.RA117.001385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur B, Khwaja FW, Severson EA et al (2005) Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-oncol 7:134–153. 10.1215/S1152851704001115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J, Güttler A, Wichmann H et al (2015) IDH1R132H mutation causes a less aggressive phenotype and radiosensitizes human malignant glioma cells independent of the oxygenation status. Radiother Oncol 116:381–387. 10.1016/j.radonc.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Kickingereder P, Sahm F, Radbruch A et al (2015) IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci Rep. 10.1038/srep16238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivunen P, Lee S, Duncan CG et al (2012) Transformation by the R enantiomer of 2-hydroxyglutarate Linked to EglN activation. Nature 483:484–488. 10.1038/nature10898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranendijk M, Struys EA, Salomons GS et al (2012) Progress in understanding 2-hydroxyglutaric acidurias. J Inherit Metab Dis 35:571. 10.1007/s10545-012-9462-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell D, Assoku M, Galloway M et al (2011) Screen for IDH1, IDH2, IDH3, D2HGDH and L2HGDH mutations in glioblastoma. PLoS ONE. 10.1371/journal.pone.0019868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, He X, Ye D et al (2015) NADP(+)-IDH mutations promote hypersuccinylation that impairs mitochondria respiration and induces apoptosis resistance. Mol Cell 60:661–675. 10.1016/j.molcel.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Li S, Sun C, Gu Y et al (2019) Mutation of IDH1 aggravates the fatty acid-induced oxidative stress in HCT116 cells by affecting the mitochondrial respiratory chain. Mol Med Rep 19:2509–2518. 10.3892/mmr.2019.9903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Hou C, Chen H et al (2016) Genetics and epigenetics of glioblastoma: applications and overall incidence of IDH1 mutation. Front Oncol. 10.3389/fonc.2016.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P-S, Wang H, Li X et al (2017) α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol 18:985–994. 10.1038/ni.3796 [DOI] [PubMed] [Google Scholar]
- Losman J-A, Kaelin WG (2013) What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes Dev 27:836–852. 10.1101/gad.217406.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- Madala HR, Punganuru SR, Arutla V et al (2018) Beyond brooding on oncometabolic havoc in IDH-mutant gliomas and AML: current and future therapeutic strategies. Cancers (Basel). 10.3390/cancers10020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellai M, Caldera V, Annovazzi L, Schiffer D (2013) The distribution and significance of IDH mutations in gliomas. Evolut Mol Biol Brain Tumors Ther Implic. 10.5772/52357 [Google Scholar]
- Molenaar RJ, Verbaan D, Lamba S et al (2014) The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol 16:1263–1273. 10.1093/neuonc/nou005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar RJ, Maciejewski JP, Wilmink JW, Noorden CJF (2018) Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene 37:1949–1960. 10.1038/s41388-017-0077-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PAJ, Vousden KH (2013) p53 mutations in cancer. Nat Cell Biol 15:2–8. 10.1038/ncb2641 [DOI] [PubMed] [Google Scholar]
- Myung JK, Cho HJ, Kim H et al (2014) Prognosis of glioblastoma with oligodendroglioma component is associated with the IDH1 mutation and MGMT methylation status. Transl Oncol 7:712–719. 10.1016/j.tranon.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal J, Jamoona A, Gulati ND et al (2006) Revisiting the role of p53 in primary and secondary glioblastomas. Anticancer Res 26:4633–4639 [PubMed] [Google Scholar]
- Nasser MM, Mehdipour P (2018) Exploration of involved key genes and signaling diversity in brain tumors. Cell Mol Neurobiol 38:393–419. 10.1007/s10571-017-0498-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navis AC, Niclou SP, Fack F et al (2013) Increased mitochondrial activity in a novel IDH1-R132H mutant human oligodendroglioma xenograft model: in situ detection of 2-HG and α-KG. Acta Neuropathol Commun 1:18. 10.1186/2051-5960-1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobusawa S, Watanabe T, Kleihues P, Ohgaki H (2009) IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res 15:6002–6007. 10.1158/1078-0432.CCR-09-0715 [DOI] [PubMed] [Google Scholar]
- Núñez FJ, Mendez FM, Kadiyala P et al (2019) IDH1-R132H acts as a tumor suppressor in glioma via epigenetic up-regulation of the DNA damage response. Sci Transl Med 11:eaaq1427. 10.1126/scitranslmed.aaq1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba S, Hirose Y (2016) Biological significance of mutant isocitrate dehydrogenase 1 and 2 in gliomagenesis. Neurol Med Chir (Tokyo) 56:170–179. 10.2176/nmc.ra.2015-0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P (2013) The definition of primary and secondary glioblastoma. Clin Cancer Res 19:764–772. 10.1158/1078-0432.CCR-12-3002 [DOI] [PubMed] [Google Scholar]
- Osuka S, Van Meir EG (2017) Overcoming therapeutic resistance in glioblastoma: the way forward. J Clin Invest 127:415–426. 10.1172/JCI89587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyan B, Ozturk MA, Ozkan F et al (2015) The change in the status of MGMT methylation and IDH mutation in initial and recurrent glial tumors and its relation with prognosis. JCO 33:e13058–e13058. 10.1200/jco.2015.33.15_suppl.e13058 [Google Scholar]
- Park JK, Hodges T, Arko L et al (2010) Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol 28:3838–3843. 10.1200/JCO.2010.30.0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812. 10.1126/science.1164382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegatta S, Valletta L, Corbetta C et al (2015) Effective immuno-targeting of the IDH1 mutation R132H in a murine model of intracranial glioma. Acta Neuropathol Commun 3:4. 10.1186/s40478-014-0180-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip B, Yu DX, Silvis MR et al (2018) Mutant IDH1 promotes glioma formation in vivo. Cell Rep 23:1553–1564. 10.1016/j.celrep.2018.03.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polívka J, Pešta M, Pitule P et al (2018) IDH1 mutation is associated with lower expression of VEGF but not microvessel formation in glioblastoma multiforme. Oncotarget 9:16462–16476. 10.18632/oncotarget.24536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CC, Gordon PMK, Liu K et al (2019) Differential microglia and macrophage profiles in human IDH-mutant and -wild type glioblastoma. Oncotarget 10:3129–3143. 10.18632/oncotarget.26863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen KD, Helin K (2016) Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev 30:733–750. 10.1101/gad.276568.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson LG, Choi BD, Curry WT (2019) (R)-2-hydroxyglutarate drives immune quiescence in the tumor microenvironment of IDH-mutant gliomas. Transl Cancer Res 8:S167–S170. 10.21037/tcr.2019.01.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richterová R, Kolarovszki B (2016) Genetic alterations of glioblastoma. Neurooncol Newer Dev. 10.5772/63127 [Google Scholar]
- Rohle D, Popovici-Muller J, Palaskas N et al (2013) An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340:626–630. 10.1126/science.1236062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosiak K, Smolarz M, Stec WJ et al (2016) IDH1R132H in neural stem cells: differentiation impaired by increased apoptosis. PLoS ONE. 10.1371/journal.pone.0154726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer D, Annovazzi L, Casalone C et al (2018) Glioblastoma: microenvironment and niche concept. Cancers (Basel). 10.3390/cancers11010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semukunzi H, Roy D, Li H et al (2017) IDH mutations associated impact on related cancer epidemiology and subsequent effect toward HIF-1α. Biomed Pharmacother 89:805–811. 10.1016/j.biopha.2017.02.083 [DOI] [PubMed] [Google Scholar]
- Shi J, Sun B, Shi W et al (2014a) Decreasing GSH and increasing ROS in chemosensitivity gliomas with IDH1 mutation. Tumor Biol 36:655–662. 10.1007/s13277-014-2644-z [DOI] [PubMed] [Google Scholar]
- Shi J, Zuo H, Ni L et al (2014b) An IDH1 mutation inhibits growth of glioma cells via GSH depletion and ROS generation. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 35:839–845. 10.1007/s10072-013-1607-2 [DOI] [PubMed] [Google Scholar]
- Shim E-H, Livi CB, Rakheja D et al (2014) l-2-hydroxyglutarate: an epigenetic modifier and putative oncometabolite in renal cancer. Cancer Discov 4:1290–1298. 10.1158/2159-8290.CD-13-0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Gurav M, Dhanavade S et al (2017) Diffuse glioma—Rare homozygous IDH point mutation, is it an oncogenetic mechanism? Neuropathology 37:582–585. 10.1111/neup.12401 [DOI] [PubMed] [Google Scholar]
- Smolková K, Ježek P (2012) The role of mitochondrial NADPH-dependent isocitrate dehydrogenase in cancer cells. Int J Cell Biol. https://www.hindawi.com/journals/ijcb/2012/273947/. Accessed 4 Mar 2019 [DOI] [PMC free article] [PubMed]
- SongTao Q, Lei Y, Si G et al (2012) IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci 103:269–273. 10.1111/j.1349-7006.2011.02134.x [DOI] [PubMed] [Google Scholar]
- Struys EA (2006) D-2-Hydroxyglutaric aciduria: unravelling the biochemical pathway and the genetic defect. J Inherit Metab Dis 29:21–29. 10.1007/s10545-006-0317-9 [DOI] [PubMed] [Google Scholar]
- Stuani L, Recher C, Portais J-C, Sarry J-E (2017) Utilization of a-Ketoglutarate for Synthesis of 2-Hydroxyglutarate Oncometabolite Promotes Catabolic Flexibility, Redox Perturbation and Mitochondrial Activity That Supports Chemoresistance in IDH1 Mutant Acute Myeloid Leukemia. Blood 130:5080–5080 [Google Scholar]
- Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. 10.1016/S1470-2045(09)70025-7 [DOI] [PubMed] [Google Scholar]
- Szulzewsky F, Pelz A, Feng X et al (2015) Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS ONE 10:e0116644. 10.1371/journal.pone.0116644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi K, Yamamoto T (2019) IDH-Mutant Gliomas. Brain and spinal tumors—primary and secondary. 10.5772/intechopen.84543
- Urban DJ, Martinez NJ, Davis MI et al (2017) Assessing inhibitors of mutant isocitrate dehydrogenase using a suite of pre-clinical discovery assays. Sci Rep 7:12758. 10.1038/s41598-017-12630-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitkus MS, Diplas BH, Yan H (2016) Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol 18:16–26. 10.1093/neuonc/nov136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Sai K, Gong F et al (2014) Mutation of isocitrate dehydrogenase 1 induces glioma cell proliferation via nuclear factor-κB activation in a hypoxia-inducible factor 1-α dependent manner. Mol Med Rep 9:1799–1805. 10.3892/mmr.2014.2052 [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang C, Zhang Z et al (2018) Specific clinical and immune features of CD68 in glioma via 1,024 samples. Cancer Manag Res 10:6409–6419. 10.2147/CMAR.S183293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Nobusawa S, Kleihues P, Ohgaki H (2009) IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 174:1149–1153. 10.2353/ajpath.2009.080958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SC, Karajannis MA, Chiriboga L et al (2011) R132H-mutation of isocitrate dehydrogenase-1 is not sufficient for HIF-1α upregulation in adult glioma. Acta Neuropathol 121:279–281. 10.1007/s00401-010-0790-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CC, Qian Y, Yu J (2017) Interplay between epigenetics and metabolism in oncogenesis: mechanisms and therapeutic approaches. Oncogene 36:3359–3374. 10.1038/onc.2016.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhao J, Xu Z et al (2004) Structures of human cytosolic NADP-dependent Isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J Biol Chem 279:33946–33957. 10.1074/jbc.M404298200 [DOI] [PubMed] [Google Scholar]
- Yalaza C, Ak H, Cagli MS et al (2017) R132H mutation in IDH1 gene is associated with increased tumor HIF1-alpha and serum VEGF levels in primary glioblastoma multiforme. Ann Clin Lab Sci 47:362–364 [PubMed] [Google Scholar]
- Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 Mutations in Gliomas. N Engl J Med 360:765–773. 10.1056/NEJMoa0808710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Ye D, Guan K-L, Xiong Y (2012) IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin Cancer Res 18:5562–5571. 10.1158/1078-0432.CCR-12-1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Yao Q, Cai G et al (2018) IDH1 mutation diminishes aggressive phenotype in glioma stem cells. Int J Oncol 52:270–278. 10.3892/ijo.2017.4186 [DOI] [PubMed] [Google Scholar]
- Zhang K, Wang X, Zhou B, Zhang L (2013) The prognostic value of MGMT promoter methylation in Glioblastoma multiforme: a meta-analysis. Fam Cancer 12:449–458. 10.1007/s10689-013-9607-1 [DOI] [PubMed] [Google Scholar]
- Zhang X, Rao A, Sette P et al (2016) IDH mutant gliomas escape natural killer cell immune surveillance by downregulation of NKG2D ligand expression. Neuro-oncology 18:1402–1412. 10.1093/neuonc/now061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Sorensen M, Kristensen BW et al (2018) The oncometabolite D-2-hydroxyglutarate is an intercellular mediator in IDH-mutant gliomas that inhibits both complement and T cells. Clin Cancer Res. 10.1158/1078-0432.ccr-17-3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Lin Y, Xu W et al (2009) Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science 324:261–265. 10.1126/science.1170944 [DOI] [PMC free article] [PubMed] [Google Scholar]