Abstract
Traumatic brain injury (TBI) and autism spectrum disorder (ASDs) share several same biochemical mechanisms and symptoms, such as learning memory impairments and communication deficits. Chromodomain helicase DNA binding protein 8 (CHD8), a member of the CHD family of ATP-dependent chromatin remodeling factors, is one of the top risk genetic factors in ASDs and is highly associated with Wnt/β-catenin signaling. Yet, the possible effect of CHD8 on TBI remains poorly understood. In vivo, we found that Chd8 co-localized in neurons, astrocytes, and microglia, but predominantly presented in neurons in the prefrontal cortex, hippocampus, and cortex. Both Chd8 and β-catenin expression peaked at 12 h and shared the similar change tendency after TBI. Chd8 knockdown inhibited wnt pathway, promoted the activation of apoptosis and autophagy, and caused learning and memory impairments both at normal and TBI condition. In addition, overexpression of Chd8 via 17β-estrogen (E2) treatment enhanced wnt signaling pathway and suppressed TBI-induced apoptosis and autophagic activation. In vitro, a significant increase of Chd8 and β-catenin expression was observed in HT22 cells after lipopolysaccharide (lps) treatment or mechanical injury, respectively. Chd8 knockdown inhibited wnt signaling pathway and increased apoptosis and autophagy activation in lps-stimulated HT22 cells. But activation of wnt signaling inverted the effects of Chd8-siRNA. Our results demonstrated that Chd8 exerted neuroprotection and promoted cognitive recovery through inhibiting apoptosis and autophagy activation following TBI, at least partially by wnt signaling pathway.
Electronic supplementary material
The online version of this article (10.1007/s10571-020-00806-5) contains supplementary material, which is available to authorized users.
Keywords: Chromodomain helicase DNA binding protein 8, Traumatic brain injury, Wnt, Apoptosis, Autophagy
Introduction
Traumatic brain injury (TBI), one of the leading causes of disability and death in modern society, has become an enormous economic burden worldwide. TBI affects 2.8 million people annually in the U.S and over 10 million people worldwide (DeKosky and Asken 2017). TBI might cause a variety of deficits in intelligence, memory, balance coordination, emotional regulation, and social judgment. Patients suffering from TBI are associated with worse cognitive performance and higher disability, which makes them fail to return to regular life. However, the underlying molecular and cellular mechanisms post TBI are still elusive (Gardner and Zafonte 2016; Zhao et al. 2017).
Autism spectrum disorder (ASDs) is a heterogeneous disease characterized by social deficits, communication difficulties, stereotyped behaviors, and cognitive delays. Previous studies showed that TBI and ASDs may have similar symptoms, such as gastrointestinal problems, learning difficulties, seizures, anxiety, and self-regulation behavior impairments, and share several same biochemical mechanisms which cause the above symptoms in nervous system. Changes on areas closely associated with social interaction and communication in brain have been found both in TBI and ASDs. In addition, Lucke Wold reported that lessons from each field might be combined to provide better guidelines for patients suffering from TBI or ASDs (Singh et al. 2016). Therefore, investigating the function of ASDs-related genes in TBI may benefit to understand the pathophysiologic mechanisms following TBI.
Chromodomain helicase DNA binding protein 8 (CHD8), a member of the CHD family of ATP-dependent chromatin remodeling factors, is one of the top risk genetic factors in ASDs. Interestingly, Chd8-regulated genes are enriched for neural development, Wnt/β-catenin signaling, cell cycle, chromosome organization, and RNA processing. Chd8 binds to the promoters of cyclin E2 (ccne2) and thymidylate synthase (TYMS), two genes which highly express during G1/S transition of the cell cycle, and serves as a transcriptional activator (Durak et al. 2016). Chd8 also interacts with endogenous RE-1 silencing transcription factor (REST), a protein that inhibits the transcription of many neuronal genes, and Chd8 knockdown may result in abnormal activation of REST, which accounts for the neurodevelopment deficits (Katayama et al. 2016). In addition, Chd8 regulates β-catenin by recruiting histone H1 to the promoters of wnt targeting genes. Recently, chd8+/− mice exert hallmark features including increased anxiety, repetitive behavior, and social deficits, which are similar to the symptoms of ASD patients (Platt et al. 2017). However, little is known about the function of Chd8 and the underling mechanisms such as wnt signaling pathway in the experimental TBI model. Based on the previous studies of Chd8 in ASDs, we hypothesized that Chd8 might improve cognitive function by regulating wnt pathway following TBI.
In this work, we determined the distribution of Chd8 in the brain by double immunofluorescence and investigated the relationship between Chd8 and wnt pathway post TBI by Western blot and RT-qPCR both in vivo and in vitro. Then we examined the effects of Chd8 on neurological impairments and behavior deficits at normal and TBI conditions by employing Chd8-siRNA. Our data suggested that Chd8 could exert neuroprotective effects and improve cognitive recovery through wnt signaling pathway following TBI.
Materials and Methods
Animal Traumatic Injury Model and Experimental Groups
All experiments were performed on mature male ICR mice (20–25 g) which were obtained from SLAC Company (Shanghai, China). Adult mice were initially anesthetized with 4% chloral hydrate (0.4 mg/g) and fixed in a stereotaxic frame equipped with a heating pad. A middle incision was performed on the scalp to expose the skull, and a 4-mm-diameter craniotomy was made over the left somatosensory cortex centered at 3.5 mm posterior and 4 mm lateral to the bregma. To induce TBI, a weight-drop model, a 40 g weight dropped from 20 cm onto a 2-mm-diameter footplate, was set to produce an impact on the exposed cortex. After TBI injury, the scalp incision was sutured, and the mice were placed in a 37 °C incubator until they recovered from anesthesia. The mice in the sham group received all procedures except TBI. A pathogen-free and light-cycled house with abundant water and food was provided for all adult male mice. All protocols were carried out in accordance with the Guidelines of Animal Use and Care of the National Institutes of Health (NIH) in aseptic conditions. (Luo et al. 2011).
The experimental design and groups in animals were shown as follows: (1) To confirm the expression pattern of Chd8 and β-catenin post TBI, ICR mice were randomly divided into sham and TBI groups. In TBI group, mice were sacrificed at 3 h, 6 h, 12 h, 1 day, 3 day, and 7 day after TBI. (2) To explore the function of Chd8 in the central nervous system at normal condition, ICR mice were randomly divided into sham, si-control, and si-Chd8 groups. To control for the off-target and non-specific effects of siRNA, a scramble siRNA control (si-control) was designed. In si-control group, intracerebroventricular injection of si-control (1 μl/mouse, RiboBio) was performed on the mice and mice were sacrificed at 3 days post si-control treatment; in si-Chd8 group, the sequence of the Chd8-siRNA is GCAAGATTCGGGAATTTAA and the concentration of Chd8-siRNA is 4 nmol/μl. The intracerebroventricular injection of Chd8-siRNA (1ul/ mouse, RiboBio) was made in the parietal region (1.0 mm posterior and 1.0 mm lateral to the bregma, 2.5 mm in depth) and the flow rate of the Chd8-siRNA injection is 0.1 μl/min using microsyringe. Intracerebroventricular injection of si-Chd8 (1 μl/mouse, RiboBio) was performed on the mice and mice were sacrificed at 3 days post si-Chd8 treatment. (3) To determine the relationship between 17β-estrogen (E2, 1 mg/kg, sigma) and Chd8 post TBI, ICR mice were randomly divided into sham, E2, TBI, and TBI + E2 groups. In E2 groups, mice were sacrificed at 30 min post E2 (1 mg/kg, sigma) treatment. In TBI groups, mice were sacrificed at 3 h, 6 h, 12 h, 1 day, 3 day, and 7 day after TBI. In TBI + E2 groups, mice were injected intraperitoneally with E2 (1 mg/kg, sigma) 30 min post TBI and sacrificed at 3 h, 6 h, 12 h, 1 day, 3 days, and 7 days post TBI. (4) To confirm the role of Chd8 following TBI, ICR mice were randomly divided into sham, TBI, TBI + E2 group, TBI + si-Chd8, and TBI + si-Chd8 + E2 groups. In TBI group, mice were sacrificed at 12 h after TBI. In TBI + E2 group, mice were subjected to TBI and injected intraperitoneally with E2 (1 mg/kg, sigma) 30 min post TBI, then mice were sacrificed at 12 h following TBI. In TBI + si-Chd8 group, intracerebroventricular injection of si-Chd8 (1 μl/ mouse, RiboBio) was performed on the mice. After 3 days, mice were subjected to TBI and sacrificed at 12 h following TBI. In TBI + si-Chd8 + E2 group, intracerebroventricular injection of si-Chd8 (1 μl/mouse, RiboBio) was performed on the mice. After 3 days, mice were subjected to TBI and injected intraperitoneally with E2 (1 mg/kg, sigma) 30 min post TBI, then mice were sacrificed at 12 h following TBI.
Immunofluorescent Staining and Quantitative Analysis
Tissues from the prefrontal cortex, hippocampus, and cortex were removed after being perfused with 4% paraformaldehyde. Then the brains were immersed in 20% sucrose for 1 day followed by 30% sucrose for 2–3 days and cut into 10 μm sections. The sections were treated by 0.3% triton X-100 for 5 min and 1% bovine serum albumin (BSA) for 2 h at room temperature. Then the sections were incubated with primary antibodies for anti-Chd8 (1:200; Abcam), anti-LC3 (1:200; Abcam), neuron marker Neun (1:500, Abcam), astrocyte marker GFAP (1:500, Abcam), and microglia marker Iba1 (1:500, Abcam) overnight at 4 °C. Following washing, the brain sections were then incubated with secondary antibodies including Alexa Flour 488 and Alexa Flour 555 at 4 °C for 2 h.
Cell quantification was performed in an unbiased manner. Approximately 6 to 8 images per section and five microscopic fields per image were examined and photographed at × 10 magnification and × 20 magnification by Nikon fluorescence microscope (Germany). The exposed times of primary antibody combining the Alexa Flour 488 in all images was 200 ms, and the exposed times of primary antibody combining the Alexa Flour 555 in all images was 500 ms. In addition, the exposed times of Dapi in all images was 50 ms. To identify the proportion of Chd8-positive cells, the number of cells labeled for Chd8 and Dapi was quantified (Chd8+Dapi+/Dapi+). To identify the proportion of each phenotype-specific marker-positive cells expressing Chd8, the number of cells double labeled for Chd8 and the other phenotypic markers used in the experiment was quantified (Chd8+Neun+/Neun+, Chd8+GFAP+/GFAP+, and Chd8+Iba1+/Iba1+). The number of cells was measured using Image-Pro Plus 6.0 (Wang et al. 2020).
HT22 Cell Injury Model and Experimental Groups
HT22 cells were obtained from Dr. Xingshun Xu (Institute of Neuroscience, Soochow University, China). The HT22 cells were seeded onto 6-well plates and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) with 10% fetal bovine serum (FBS, Gibco), 100 U/mL penicillin (Invitrogen), and 100 µg/ml streptomycin (Invitrogen) (37 °C, 5% CO2). (1) To further confirm the expression pattern of Chd8 and β-catenin in vitro injury model, cells were divided into control, lipopolysaccharide (lps), and mechanical injury groups. In lps group, HT22 cells were treated with lps (1 μg/ml, Sigma) and then the culture medium was collected at 0.5 h, 1 h, 3 h, 6 h, 12 h, and 24 h after lps treatment. In mechanical injury group, HT22 cells were subjected to severe mechanical stretch and then the culture medium was collected at 0.5 h, 1 h, 3 h, 6 h, 12 h, and 24 h after mechanical injury treatment. (2) To determine the function of Chd8 in HT22 cells, cells were divided into control, si-control, and si-Chd8 groups. In si-control group, HT22 cells were treated with control siRNA (50 nM, RiboBio) for 24 h, and then the culture medium was collected. In si-Chd8 group, HT22 cells were treated with Chd8-siRNA (50 nM, RiboBio) for 24 h, and then the culture medium was collected. (3) To confirm the role of Chd8 and wnt signaling pathway in lps-stimulated HT22 cells, cells were divided into control, lps, lps + si-Chd8 ,and lps + si-Chd8 + lici groups. In lps group, HT22 cells were treated with lps (1 μg/ml, Sigma) for 6 h and then the culture medium was collected. In lps + si-Chd8 group, HT22 cells were treated with lps (1 μg/ml, Sigma) for 6 h after the treatment of si-Chd8 (50 nM, RiboBio) for 24 h and then the culture medium was collected. In lps + si-Chd8 + lici group, HT22 cells were treated with lps (1 μg/ml, Sigma) for 6 h after the treatment of si-Chd8 (50 nM, RiboBio) for 24 h. Then HT22 cells were treated with Lici (30 mmol/l, Sigma), the wnt/β‐catenin signaling pathway activator, for 0.5 h and the culture medium was collected (Ma et al. 2017).
Western Blot Analysis
Adult male mice were sacrificed at indicated time points, and tissues from the prefrontal cortex, hippocampus, and injured cortex were collected. The samples were then homogenized in an ice-cold RIPA lysis buffer (Beyotime Institute of Biotechnology) and centrifuged at 12,000×g for 20 min at 4 °C. After the protein concentration of the resulting supernatant was detected by Bradford assay (BioRad), the resulting supernatant (50 lg of protein) was subjected to sodium polyacrylamide gel electrophoresis (SDS-PAGE).
Protein samples were subjected to 8%, 10%, and 12% SDS-PAGE gels and then transferred to polyvinylidene fluoride membranes. The membrane was blocked with 5% non-fat milk for 2 h and then incubated with antibody against Chd8 (1:1000, Abcam), β-catenin (1:1000, Abcam), LC3-II (1:1000, Abcam), cleaved Caspase-3 (1:1000, Abcam), p62 (1:1000, Abcam), Bax (1:1000, Bio-world), Bcl-2 (1:1000, Bio-world), GAPDH (1:1000, SantaCruz), or β-Tubulin (1:1000, SantaCruz) overnight at 4 ℃, respectively. Then the protein samples were incubated with anti-rabbit secondary antibody (1:1000, SantaCruz) or anti-mouse secondary antibody (1:1000, SantaCruz) for 1 h at room temperature. Finally, the signal was detected by using an enhanced chemiluminescence system (Pierce Company). The relative quantity of proteins was analyzed using Image J and normalized to that of loading controls (Gao et al. 2018).
RNA Isolation and RT-qPCR
Total RNA was extracted from HT22 cells by using TRIzol reagent (Invitrogen). The quality and quantity of the RNA in the samples was measured by Nanodrop 2000 based on the 260/280 nm absorbance. RNA was reversely transcribed by using a Reverse Transcription Kit (Takara). Respective target genes were amplified by Quantitative real-time PCR with specific primers. The detailed qPCR primer sequences were listed in supplement data 1. PCR reaction was performed in the following steps: an initial polymerase activation and DNA denaturation for 5 min at 94 °C; 30 cycles of denaturation step at 94 °C for 30 s, annealing step at 56 °C for 30 s and elongation step at 72 °C for 1 min; and a final extension for 10 min at 72 °C. PCR product was run in a 1% agarose gel and visualized under UV light. The relative level of mRNA was calculated by formula 2−ΔΔCt method and normalized to the GAPDH expression (Fang et al. 2018).
Assessment of Motor Performance
According to the motor test protocol, the motor test was performed on day 1 before and days 1–7 post TBI. Motor function was evaluated by wire grip test. Mice were picked up by the tail and placed on a 35 cm wire that suspended between two wooden poles. The time and manner in which the mouse grasped on the wire were detected on a degree of 0–5: 0 indicates mouse fails to remain on the wire for less than 30 s; 1 indicates mouse holds on to the wire for more than 30 s, not both sets of fore paws and hind paws together on the wire; 2 indicates mouse holds on to the wire with all paws but not the tail; 3 indicates mouse holds on to the wire by using its tail along with all paws, but without movement on the wire; 4 indicates mouse moves along the wire with all four paws plus tail; 5 indicates mouse ambulates down one of the posts used to support the wire. Each mouse was trailed three consecutive times a day and the score of each trial was calculated based on the average of these scores at each time point. (Petraglia et al. 2014).
Evaluation of Morris Water Maze Function
To ensure recovery from motor deficits, the Morris water maze (MWM) test was performed on days 8–15 after TBI. The MWM test was used to assess the spatial memory acquisition of adult male mice. A large round pool (a depth of 50 cm) was filled with water. A round plexiglass escape platform (10 cm in diameter) was placed in the center of southwest of the round pool and positioned 1 cm beneath the water surface. For each trial, the adult male mice were placed at each of the four possible quadrants (N, S, E, W) randomly into the circular pool facing the wall. Each mouse was allowed to search the escape platform and remained for 15 s and then was placed in a warmed box. If they failed within 90 s, the mice were guided to the platform. All tracks and speed data were recorded and analyzed using video analysis system (Leung et al. 2018).
Statistical Analysis
All data were expressed as mean ± standard error (S.E.M.). The data of Western blot, RT-qPCR ,and Immunofluorescence were analyzed using one-way ANOVA followed by post hoc Tukey's test to compare the differences between groups. The behavioral data were analyzed using repeated measures ANOVA followed by Tukey’s post hoc test across groups. Each experiment was repeated at least three times. Differences were considered statistically significant at P < 0.05. All statistical analyses were carried out using SPSS 20 software (Zhang et al. 2017).
Results
In Vivo Study
Chd8 Predominantly Located in Neurons in the Prefrontal Cortex, Hippocampus, and Cortex
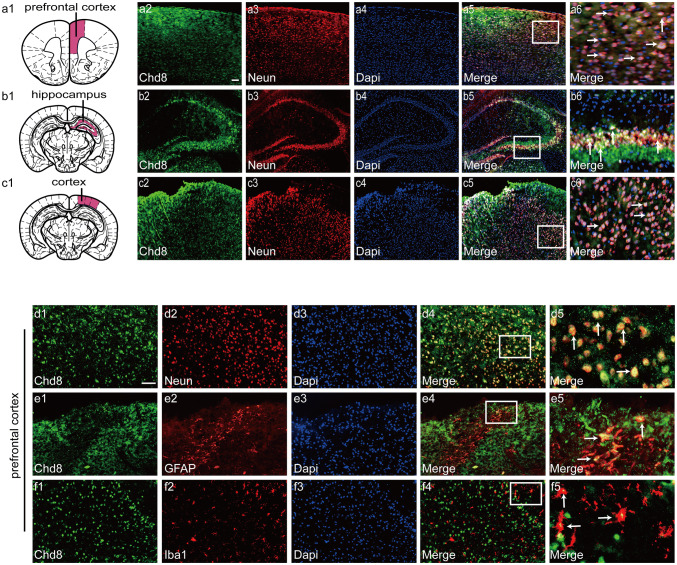
To confirm the cell types of Chd8 in normal mice brain, we analyzed the co-expression of Chd8 and neuronal marker (Neun), astrocyte marker (GFAP), and microglia marker (Iba1) in the prefrontal cortex, hippocampus, and cortex. Double immunofluorescent staining results showed that Chd8 highly expressed in the prefrontal cortex, hippocampus, and cortex (Fig. 1a1–6, b1–6, c1–6). In addition, Chd8 co-localized in neurons, astrocytes, and microglia, but predominantly presented in neurons in the prefrontal cortex (Fig. 1d1–5, e1–5, f1–5). These results suggested that Chd8 might play a significant role in the neurons in the prefrontal cortex, hippocampus, and cortex.
Fig. 1.
The schematic of prefrontal cortex (a1), hippocampus (b1), and cortex (c1) region in the brain. Double immunofluorescence for Chd8, neuronal marker (Neun), and Dapi in the prefrontal cortex, hippocampus, and cortex of adult male brain under physiological conditions (a2–6, b2–6, c2–6). Horizontal sections were labeled with Chd8 (green, a2, b2, c2) and neuronal marker (Neun) (red, a3, b3, c3). Nuclei were counter-stained with Dapi (blue, a4, b4, c4). Co-localization of Chd8, Neun, and Dapi are shown in (a5, b5, c5). (a6, b6, c6) are 16 times enlargement of the white boxed area in (a5, b5, c5); Double immunofluorescence for Chd8 and neuronal marker (Neun), astrocyte marker (GFAP), and microglia marker (Iba1) in the prefrontal cortex (d1–5, e1–5, f1–5) of adult male brain under physiological conditions. Horizontal sections labeled with Chd8 (green, d1, e1, f1). Horizontal sections labeled with neuronal marker (Neun) (red, d2). Horizontal sections labeled with astrocyte marker (GFAP) (red, e2). Horizontal sections labeled with microglia (red, f2). Nuclei were counter-stained with Dapi (blue, d3, e3, f3). Co-localization of Chd8 and Neun are shown in (d4). Colocalization of Chd8 and GFAP are shown in (e4). Colocalization of Chd8 and Iba1 are shown in (f4). d5, e5, f5 are 16 times enlargement of the white boxed area in (d4, e4, f4). The yellow color visualized in the merged images represented colocalization of Chd8 with Neun, GFAP, and Iba1. Scale bar is 100 μm. Experiments are representative of three independent experiments
The Time Course of Chd8 and β-Catenin Expression and the Changes of Chd8 in Different Cell Types Following TBI
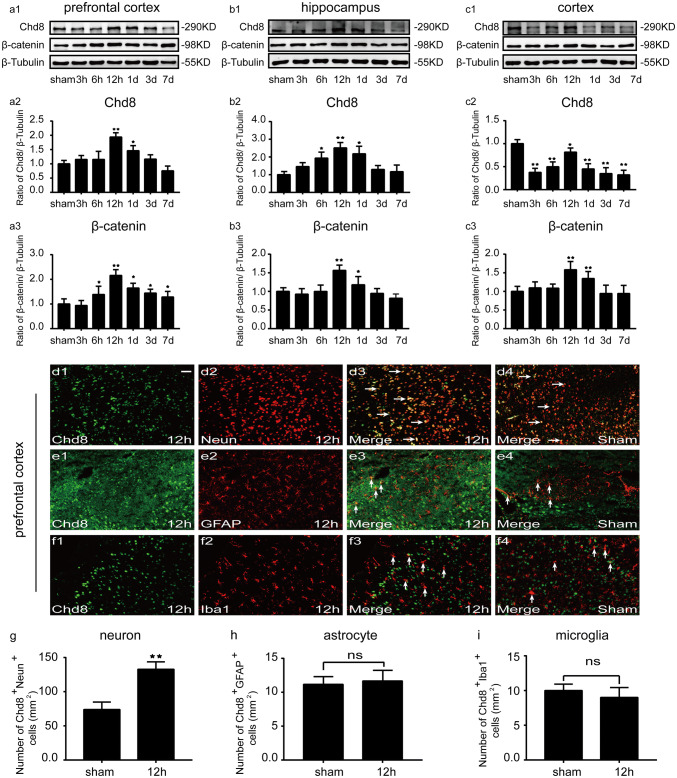
To confirm the expression pattern of Chd8 and β-catenin post TBI, the amount of Chd8 and β-catenin in the prefrontal cortex, hippocampus ,and injured cortex were measured by Western blot at a series of indicated time points after TBI. In the prefrontal cortex, Chd8 level was increased at 12 h and 1 days, peaking at 12 h post TBI compared to sham group (Fig. 2a1, a2). Similarly, the expression of β-catenin was remarkably upregulated from 6 h to 7 days and peaked at 12 h after TBI, compared with sham group (Fig. 2a1, a3). In addition, Chd8 level was elevated effectively from 6 h to 1 d and peaked at 12 h in the hippocampus after TBI (Fig. 2b1, b2). β-catenin was markedly increased at 12 h and 1 days, reaching its highest level at 12 h, compared to sham group following TBI (Fig. 2b1, b3). In the injured cortex, Chd8 expression reached a valley at 3 h, and gradually increased, peaked at 12 h, then slightly decreased afterward, but its highest level was still lower than the level of sham group (Fig. 2c1, c2). β-catenin increased at 12 h and 1 day with a peak accumulation at 12 h compared with sham group post TBI (Fig. 2c1, c3). Additionally, our immunofluorescent staining results also verified the changes of Chd8 post TBI in Western blot results (Fig. supplement. 1). The above results indicated that TBI induced a similar expression pattern of Chd8 and β-catenin in the prefrontal cortex, hippocampus, and inured cortex.
Fig. 2.
The expression of Chd8 and β-catenin after TBI (a1–3, b1–3, c1–3). (a1, b1, c1) The changes of Chd8 and β-catenin expression in the prefrontal cortex (a1), hippocampus (b1), and injured cortex (c1) at a series of indicated time points after TBI. a2–3, b2–3, and c2–3 Semiquantitative analysis (relative optical density) of the intensity of staining of Chd8 and β-catenin to β-Tubulin in the prefrontal cortex (a2, a3), hippocampus (b2, b3), and injured cortex (c2, c3) for each time point; Double immunofluorescence for Chd8 and neuronal marker (Neun), astrocyte marker (GFAP), and microglia marker (Iba1) in the prefrontal cortex at normal condition and at 12 h post TBI (d1–4, e1–4, f1–4). Horizontal sections labeled with Chd8 (green, d1, e1, f1). Horizontal sections labeled with neuronal marker (Neun) (red, d2), astrocyte marker (GFAP) (red, e2), and microglia marker (Iba1) (red, f2). Colocalization of Chd8 and Neun are shown in (d3, d4). Colocalization of Chd8 and GFAP are shown in (e3, e4). Colocalization of Chd8 and Iba1 are shown in (f3, f4). Quantitative analysis of the number of Chd8+Neun+ cells per mm2 (g). Quantitative analysis of the number of Chd8+GFAP+ cells per mm2 (h). Quantitative analysis of the number of Chd8+Iba1+ cells per mm2 (i). The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs. sham group). Scale bar is 100 μm. Experiments are representative of three independent experiments
To confirm the changes of Chd8 in different cell types, we analyzed the co-expression of Chd8 and neuronal marker (Neun), astrocyte marker (GFAP), and microglia marker (Iba1) in the prefrontal cortex at 12 h post TBI. Double immunofluorescent staining results showed that TBI led to a remarkable increase in the number of neurons expressing Chd8 (Fig. 2d1–4, g) and no difference in the number of astrocytes (Fig. 2e1–4, h) and microglia (Fig. 2f1–4, i) expressing Chd8, compared to sham group. The above results showed that the significant increase of Chd8 in the prefrontal cortex post TBI occurred totally in neurons.
Chd8 Knockdown Inhibited Wnt pathway, Promoted the Activation of Apoptosis and Autophagy Both at Normal and TBI Condition In Vivo
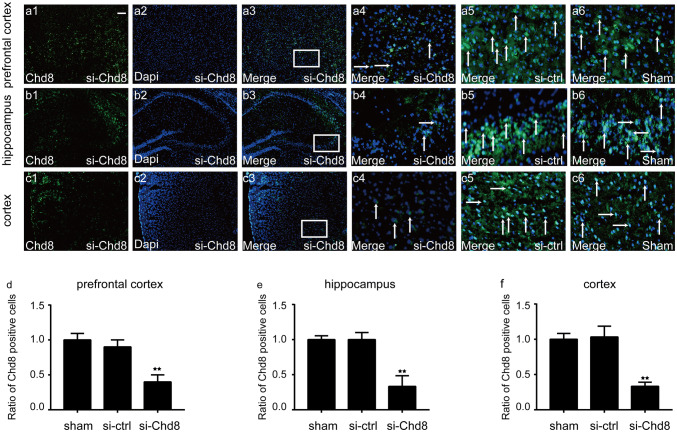
To explore the function of Chd8 in the central nervous system at normal condition, siRNA specifically targeting Chd8 was designed. As expected, immunofluorescence results showed that Chd8 siRNA transfection led to a significant decrease of Chd8 in prefrontal cortex, hippocampus, and cortex (Fig. 3), which was consistent with our Western blot results (Fig. 4a, b, c, d, h, l). In addition, β-catenin was decreased obviously in the group treated with Chd8 siRNA compared to sham and vehicle group (Fig. 4a, b, c, e, i, m). Moreover, Chd8 siRNA resulted in a remarkable increase of Caspase-3 (Fig. 4a, b, c, f, j, n) and LC3-II (Fig. 4a, b, c, g, k, o) in the prefrontal cortex, hippocampus, and cortex. The abovementioned data demonstrated that the knockdown of Chd8 may inhibit wnt signaling pathway and promote apoptosis and autophagy in prefrontal cortex, hippocampus, and cortex at normal condition in mice.
Fig. 3.
Double immunofluorescence for Chd8 and Dapi in the prefrontal cortex, hippocampus, and cortex at normal, si-control, and si-Chd8 mice. Horizontal sections labeled with Chd8 (green, a1, b1, c1). Horizontal sections labeled with Dapi (blue, a2, b2, b3). Colocalization of Chd8 and Dapi are shown in (a3–6, b3–6, c3–6). (a4, b4, c4) are 16 times enlargement of the white boxed area in (a3, b3, c3). The white color visualized in the merged images represented colocalization of Chd8 with Dapi. d–f Quantitative analysis of ratio of Chd8+/Dapi+ cells in the prefrontal cortex (d), hippocampus, (e) and cortex (f). The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs sham group). Scale bar is 100 μm. Experiments are representative of three independent experiments
Fig. 4.
Chd8 activated wnt pathway and inhibited apoptosis and autophagy in normal mice. a–c Chd8, β-catenin, Caspase-3, and LC3-II protein levels were detected with immunoblotting in the prefrontal cortex (a), hippocampus, (b) and injured cortex (c) post si-chd8 treatment. d–o Semiquantitative analysis (relative optical density) of the intensity of staining of Chd8, β-catenin, Caspase-3, and LC3-II to β-Tubulin in the prefrontal cortex (d, e, f, g), hippocampus (h, i, j, k), and injured cortex (l, m, n, o). The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs. si-control group. ns, P > 0.05). Experiments are representative of three independent experiments
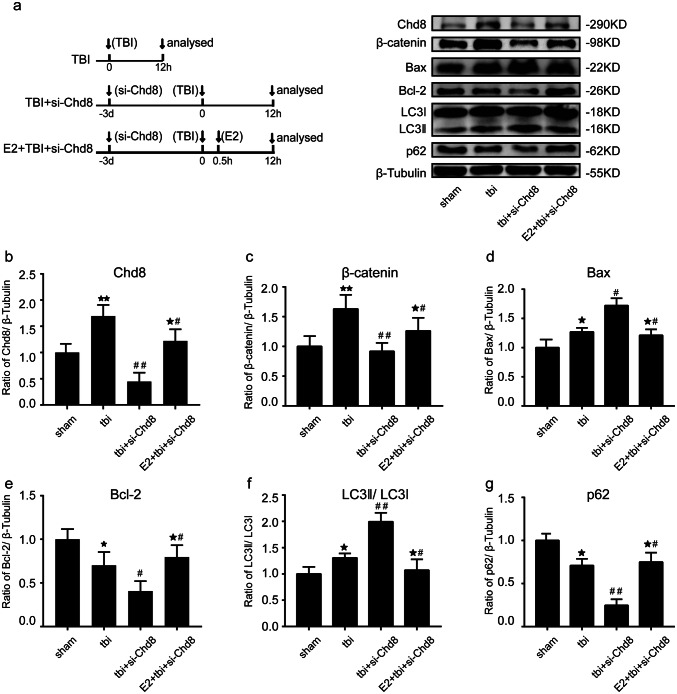
Western blot results showed that TBI induced the increase of Chd8, wnt signaling pathway, apoptosis, and autophagy at 12 h post TBI at prefrontal cortex (Fig. 5a–g) and there was no difference between TBI group and TBI + si-control group (Fig. supplement. 2a, d–i). Furthermore, we found that Chd8-siRNA resulted in a significant decline of TBI-induced increase of Chd8 (Fig. 5a, b) and β-catenin (Fig. 5a, c) in the prefrontal cortex following TBI. Immunoblotting results showed that Chd8-siRNA evidently increased Bax expression (Fig. 5a, d), but decreased the Bcl-2 level (Fig. 5a, e) at 12 h after TBI, compared to TBI alone group in the prefrontal cortex. To determine whether Chd8 regulates autophagy post TBI, Western blot analysis was performed to reveal that Chd8-siRNA transfection led to a significant upregulation of LC3-II (Fig. 5a, f) and a remarkable downregulation of p62 (Fig. 5a, g) at 12 h following TBI in the prefrontal cortex, when compared with TBI group. The abovementioned data demonstrated that the Chd8 knockdown might inhibit wnt signaling pathway and activate apoptosis and autophagy in the prefrontal cortex following TBI. Interestingly, we found the same results about the relationship between Chd8 and apoptosis and autophagy in the hippocampus post TBI (Fig. supplement. 3).
Fig. 5.
A 17β-estrogen (E2) activated Chd8 to enhance wnt signaling pathway and suppress apoptosis and autophagy activation in the prefrontal cortex post TBI. a The design of experiment: The (TBI) at time 0 means that the mice were subjected to TBI at time 0. The (analyzed) at time 12 h means that mice were sacrificed and analyzed at 12 h following TBI. The (si-Chd8) at time − 3 days means that intracerebroventricular injection of si-Chd8 was performed on the mice three days before TBI. The (E2) at time 0.5 h means that Intraperitoneal injection of E2 (1 mg/kg) was performed on the mice at 0.5 h post TBI. Chd8, β-catenin, Caspase-3, Bcl-2, LC3-II, and p62 protein levels were detected with immunoblotting in the prefrontal cortex. b–f Semiquantitative analysis (relative optical density) of the intensity of staining of Chd8 (b), β-catenin (c), Bax (d), Bcl-2, (e) LC3-II (f) and p62 (g) to β-Tubulin in the prefrontal cortex. The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs sham group. #P < 0.05 and ##P < 0.01, vs tbi group. *#P < 0.05, vs tbi + si-Chd8 group. ns, P > 0.05). Experiments are representative of three independent experiments
3.1.4. Overexpression of Chd8 Via 17β-Estrogen (E2) Administration Enhanced Wnt Signaling Pathway and Suppressed Apoptosis and Autophagy Activation Post TBI in Mice
To explore the relationship between Chd8 and 17β-estrogen (E2), the Western blot results showed that E2 treatment led to a profound increase of Chd8 both at normal condition (Fig. supplement. 2c, l) and at 3 h (Fig. 6a, g), 6 h (Fig. 6b, h), 12 h (Fig. 6c, i), 1 day (Fig. 6d, j), 3 day (Fig. 6e, k), and 7 day (Fig. 6f, l) post TBI in the prefrontal cortex. Therefore, we then used E2 as an agent to induce Chd8 expression. More importantly, E2 treatment inverted the Chd8-siRNA-induced decrease of wnt signaling pathway (Fig. 5a, c) and activation of apoptosis (Fig. 5a, d, e) and autophagy (Fig. 5a, f, g) compared to tbi + si-Chd8 group. The similar results were observed in the hippocampus at 12 h after TBI (Fig. Supplement. 3). These data suggested that increase of Chd8 by E2 treatment might enhance wnt signaling pathway and suppress apoptosis and autophagy activation in the prefrontal cortex and hippocampus following TBI. However, E2 is not a specific activator of chd8, indicating a possibility that the inhibition of apoptosis and autophagy by E2 treatment is caused by other effects of E2 rather than overexpression of chd8. In order to test this hypothesis, we examined the following experiment. Then, we found that E2 resulted in a significant inhibition of apoptosis and autophagy compared with TBI group, and Chd8-siRNA inverted the E2 and induced the decline of Bax and LC3-II compared to TBI + E2 group (Fig. Supplement. 2b, j, k), which revealed that although the relationship between Chd8 and E2 was not specific, Chd8 was also essential in the specific mechanism of the neuroprotective effects of E2 post TBI. It means that Chd8 overexpression might promote neurological recovery via inhibiting apoptosis and autophagy following TBI.
Fig. 6.
Effect of 17β-estrogen (E2) treatment on Chd8 expression in the prefrontal cortex at a series of indicated time points post TBI. a–f Chd8 protein level was detected with immunoblotting at 3 h (a), 6 h (b), 12 h (c), 1 d (d), 3 d, (e) and 7 d (f) in the prefrontal cortex following TBI. (g-l) Semiquantitative analysis (relative optical density) of the intensity of staining of Chd8 to β-Tubulin in the prefrontal cortex at 3 h (g), 6 h (h), 12 h (i), 1 day (j), 3 days, (k) and 7 days (l) following TBI. The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs. sham group. #P < 0.05 and ##P < 0.01, vs tbi group. ns, P > 0.05). Experiments are representative of three independent experiments
Chd8 Knockdown Caused Learning and Memory Impairments at Normal and TBl Condition in Mice
In order to investigate whether Chd8 is associated with motor function and learning and memory performance at normal and TBl condition, Wire grip test and Morris water maze (MWW) were performed. No difference in baseline motor function was observed between sham, si-control, and si-Chd8 groups (Fig. 7a). However, si-Chd8 induced significant cognitive deficits as evidenced by the increased latency to reach hidden platform compared to sham group and si-control group (Fig. 7b). No difference in baseline motor function was also observed between TBI alone and TBI + si-Chd8 group (Fig. 7c). Whereas, si-Chd8 treatment led to a remarkable increase of latency to find the hidden platform compared with TBI alone group (Fig. 7d). The above results indicated that the knockdown of Chd8 had no significant effect on motor function, but caused learning and memory impairments at normal and TBl condition.
Fig. 7.
Chd8 knockdown induced behavior deficits at normal condition and post TBI. a, b Mice were randomly assigned to sham, si-control, and si-Chd8 group. a Wire grip test was performed at 1–7 days after si-control and si-Chd8 treatment. b MWM testing was performed on days 8–15 after si-control and si-Chd8 treatment, and the animals in each group were those who had suffered above wire grip test. c, d Mice were randomly assigned to sham, tbi, and tbi + si-Chd8 group. c Wire grip test was performed 1–7 d after TBI. d MWM testing was performed on days 8–15 following TBI, and the animals in each group were those who had suffered above wire grip test. The data are mean ± SEM (n = 15. *P < 0.05, vs. sham group. #P < 0.05, vs. tbi group). Experiments are representative of three independent experiments
In Vitro Study
Expression Changes of Chd8 and β-Catenin in HT22 Cells After lps Treatment and Mechanical Injury
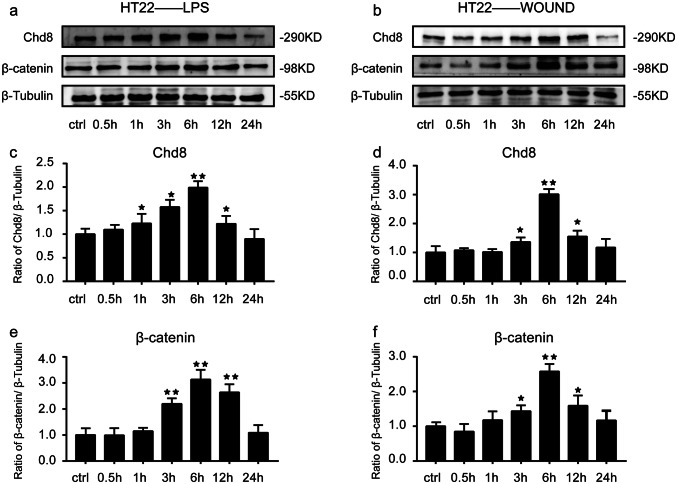
In order to confirm the above results in vivo, we detected the expression of Chd8 and β-catenin in HT22 cells after lps treatment and mechanical injury. We found that Chd8 expression was upregulated from 1 to 12 h and peaked at 6 h after lps treatment in HT22 cells compared to control group (Fig. 8a, c). Similarly, β-catenin expression was increased at 1 h and reached the peak at 6 h post lps treatment in HT22 cells (Fig. 8a, e). In parallel, in the mechanical injury group, both Chd8 (Fig. 8b, d) and β-catenin (Fig. 8b, f) expression was increased from 3 to 12 h, peaking at 6 h, compared to the control group. The above results suggested that the expression of Chd8 and β-catenin had the similar change tendency following injury in HT22 cells.
Fig. 8.
The expression of Chd8 and β-catenin after injury in vitro. a The changes of Chd8 and β-catenin expression in lps-stimulated HT22 cells. b The changes of Chd8 and β-catenin expression in mechanical trauma-induced HT22 cells. c–f Semiquantitative analysis (relative optical density) of the intensity of staining of Chd8 and β-catenin to β-Tubulin in lps-stimulated HT22 cells (c, d) and in mechanical trauma-induced HT22 cells (e, f). The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs. control group). Experiments are representative of three independent experiments
Chd8 Activated Wnt Signaling Pathway and Inhibited Apoptosis and Autophagy Activation in HT22 Cells
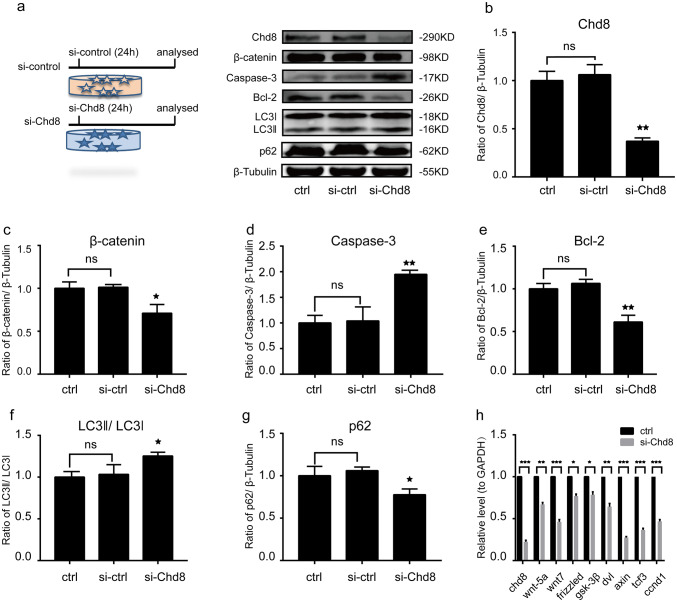
Immunoblotting results showed that Chd8 siRNA provoked a dramatic loss in the expression of Chd8 compared to control and si-control group (Fig. 9a, b), which was consistent with our RT-qPCR results (Fig. 9h), and also led to a downregulation of β-catenin (Fig. 9a, c). In addition, our RT-qPCR results revealed that Chd8 siRNA led to a significant decrease of many wnt signaling pathway-related components including wnt-5a, wnt7, frizzled, dvl, gsk, tcf3, axin, and ccnd1 (wnt signaling target gene) (Fig. 9h). Moreover, we found that Chd8-siRNA significantly increased the Caspase-3 expression (Fig. 9a, d), reduced Bcl-2 level (Fig. 9a, e), elevated LC3-II (Fig. 9a, f), and decreased p62 (Fig. 9a, g). These results suggested that Chd8 may activate wnt signaling pathway and inhibit apoptosis and autophagy activation in HT22 cells, which was consistent with our data in vivo.
Fig. 9.
Chd8 activated wnt signaling pathway and inhibited apoptosis and autophagy in HT22 cells. a The design of experiment: The (si-control 24 h) means that HT22 cells were treated with si-control for 24 h. The (si-Chd8 24 h) means that HT22 cells were treated with si-Chd8 for 24 h. Chd8, β-catenin, Caspase-3, Bcl-2, LC3-II, and p62 protein levels were detected with immunoblotting. b–g Semiquantitative analysis (relative optical density) of the intensity of staining of Chd8 (b), β-catenin (c), Caspase-3 (d), Bcl-2 (e), LC3-II, (f) and p62 (g) to β-Tubulin in HT22 cells. h The mRNA level of wnt-5a, wnt7, frizzled, dvl, gsk-3β, tcf3, axin, and ccnd1 in control and si-Chd8 group. The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs. si-control group). Experiments are representative of three independent experiments
Chd8 Inhibited Apoptosis and Autophagy Activation Via Wnt Signaling Pathway in lps-Stimulated HT22 Cells
To confirm the effectiveness of Chd8 siRNA in vitro injury model, we found that Chd8-siRNA induced a remarkable decline of Chd8 at 6 h post lps treatment in HT22 cells (Fig. 10a, b). To further investigate whether wnt signaling pathway is involved in the effects of Chd8-siRNA in vitro, lici, a specific activator of wnt signaling pathway, was utilized to treat HT22 cells. And we found that lici rescued the Chd8-siRNA-induced decrease of β-catenin at 6 h post lps treatment in HT22 cells (Fig. 10a, c), which verified the effectiveness of lici. More importantly, the results showed that activation of wnt signaling inverted the Chd8-siRNA-induced increase of Bax (Fig. 10a, d) and LC3-II (Fig. 10a, f) and decrease of Bcl-2 (Fig. 10a, e) and p62 (Fig. 10a, g). These data suggested that Chd8 might inhibit apoptosis and autophagy activation via wnt signaling pathway in vitro injury model.
Fig. 10.
Chd8 inhibited the activation of apoptosis and autophagy through wnt signaling pathway in lps-stimulated HT22 cells. a The design of experiment: Chd8, β-catenin, Caspase-3, Bcl-2, LC3-II, and p62 protein levels were detected with immunoblotting. b–f Semiquantitative analysis (relative optical density) of the intensity of staining of Chd8 (b), β-catenin (c), Bax (d), Bcl-2 (e), LC3-II, (f) and p62 (g) to β-Tubulin in HT22 cells. The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs. control group; #P < 0.05 and ##P < 0.01 vs. lps group; *#P < 0.05, vs. lps + si-Chd8 group). Experiments are representative of three independent experiments
Discussion
In this study, our finding demonstrated that Chd8 predominantly co-located in neurons in the prefrontal cortex, hippocampus, and cortex, and the increase of Chd8 by 17β-estrogen (E2) might reduce TBI-induced apoptosis and autophagy activation and improve learning and memory performance following TBI. Otherwise, the Chd8-siRNA leads to an opposite result. Our study in vitro further revealed that the neuroprotection of Chd8 post TBI may act through activating wnt signaling pathway.
Given that Chd8 presents as a central role in ASDs which is a developmental disorder with social interaction impairments and repetitive and stereotypic actions emerging at early childhood, previous studies about the function of Chd8 are almost concentrated on neural progenitor cells (NPCs), neural stem cells (NSCs), and embryonic cortex (Cotney et al. 2015). However, the location of Chd8 in the adult mice has not been fully elucidated. Platt reported that Chd8 co-localized in neurons, astrocytes, and microglia in the somatosensory cortex from male mice, which is consistent with our result (Platt et al. 2017). Furthermore, by double immunofluorescent staining, we found that Chd8 also expressed in the neurons in the prefrontal cortex and hippocampus, which are the most vulnerable areas to be influenced by secondary brain injury in TBI model, indicating that Chd8 might play an important role in memory and emotion.
Previous studies have proved that TBI induced severe dysregulation of pathways essential for cell survival, neuroplasticity, and protein homeostasis in both hippocampus and injured cortex. However, prefrontal cortex, a region critically key for higher order cognitive function, is also damaged post TBI. Being interconnected densely with hippocampus, thalamus, striatum, and other cortical areas, the prefrontal cortex is considered to be a key area integrating numerous sensory modalities. Therefore, in addition to the injured cortex and hippocampus, investigating the changes on prefrontal cortex could be useful for a better understanding of the neural circuitry of behavioral dysfunctions following TBI (Ewing-Cobbs et al. 2019; Nolan et al. 2018; Rogers and McKinlay 2019).
To understand the expression pattern of Chd8 and β-catenin post TBI, we determined the amount of Chd8 and β-catenin both in vivo (prefrontal cortex, hippocampus, and injured cortex) and in vitro (HT22 cells) after injury. In vivo, TBI induced a significant increase of Chd8 and β-catenin expression in the prefrontal cortex and hippocampus, which both peaked at 12 h post TBI. However, Chd8 expression in the injured cortex valleyed at 3 h, peaked at 12 h, and decreased afterward, but its peak level was still lower than that of the sham group. The reason of difference in Chd8 expression in injured cortex to that in prefrontal cortex and hippocampus is that the injured cortex is the primary lesion site where massive cell death occurs immediately after the mechanical impact and Chd8 may be exhausted to rescue the dying cells at early time. Whereas prefrontal cortex and hippocampus, in which the mechanism differs from that in the primary mechanical injury site following TBI, mainly suffer from secondary injury, which provides chance for Chd8 to rescue more neurons from death. So, we speculated that the different mechanism between primary and secondary injury might be the possible reason for decrease in Chd8 expression in the injured cortex, but it was increased in prefrontal cortex and hippocampus. In order to explore the underlying mechanism of Chd8 on neurons post TBI, we used lps and mechanical injury to treat HT22 cells to mimic our in vivo TBI model, respectively. A remarkable increase of Chd8 and β-catenin expression was observed, and their expression both reached a peak at 6 h post injury in our in vitro model. Interestingly, Chd8 and β-catenin expression almost has the same change tendency post TBI in both in vivo and in vitro TBI models. These findings may provide an important clue to learn the relationship between Chd8 and wnt signaling pathway following TBI.
Justin Cotney et al. reported that Chd8 targeting of ASD risk genes was highly conserved during mammalian neurodevelopment (Cotney et al. 2015). Because of the conservation of Chd8 in brain development (Cotney et al. 2015), investigating the regulatory mechanism and biological circuitry of Chd8 may contribute to understanding of molecular and cellular mechanism in human post TBI. Chd8, an ATP-dependent chromatin remodeling factor, is initially isolated as a negative regulator of Wnt/β-catenin signaling pathway which also plays a significant role in normal brain development and neuropsychiatric disorders (Sawada et al. 2013). Masaaki Nishiyama et al. reported that Chd8 controlled negatively β-catenin by recruiting histone H1 to the promoters of Wnt targeting genes (Nishiyama et al. 2009). However, other literatures reported that Chd8 bound to β-catenin directly and positively regulated wnt signaling pathway in both mice and human NPCs (Durak et al. 2016). Consistent with the latter, our study showed that Chd8 knockdown resulted in a decrease of β-catenin both in vivo (prefrontal cortex, hippocampus, cortex) and in vitro (HT22 cells). In addition to β-catenin, our RT-qPCR results revealed that Chd8-siRNA led to a significant downregulation of many wnt signaling pathway components including wnt-5a, wnt-7, frizzled, dvl, gsk, tcf3, axin, and wnt signaling target gene ccnd1, which all play crucial roles in the function of wnt signaling pathway. However, the relationship between Chd8 and Wnt/β-catenin pathway is controversial. Three possible reasons may account for this discrepancy: Firstly, Chd8 might play cell-specific roles in regulating wnt signaling, suggesting that Chd8 may regulate wnt signaling positively in neural progenitors and negatively regulate wnt pathway in non-neuronal cells; the second reason might be spatiotemporal variation of the phenotype, which means that Chd8 may have specific function in each region because of different cellular requirements in different brain regions; time difference might be the third reason. For example, the effects of Chd8 on early progenitors differs from that on late progenitors (Breuss and Gleeson 2016).
TBI not only produces the primary injury caused by an external force, but also gives rise to the development of delayed secondary brain damage with progressive morphological and functional deterioration (DeKosky and Asken 2017; Fan et al. 2018). Apoptotic cell death plays an important role in the secondary brain damage (Vlodavsky et al. 2005). However, the relationship between Chd8 and apoptosis post TBI has not been elucidated yet. Kiyotaka Oshikawa et al. reported that chd8−/− mice died early during embryogenesis resulting from widespread apoptosis, and Chd8 could suppress apoptosis by inhibiting p53 transactivation activity rather than regulating wnt signaling pathway during early embryogenesis (Nishiyama et al. 2009). However, in our study, we found that Chd8 may inhibit apoptosis, but the activation of wnt signaling pathway may rescue Chd8-siRNA-induced apoptosis. In response to this discrepancy, Breuss et al. explained that two distinct employed methodologies might be the reason. For example, Kiyotaka Oshikawa employed germline mutation mice, which could allow neural progenitors to find a steady state during development and before neurogenesis. However, our laboratories employed adult wild-type mice that treated with Chd8 siRNA, leaving little time for homeostatic correction (Breuss and Gleeson 2016).
Autophagy, a highly conserved intracellular mechanism, plays an important role in eliminating cytoplasmic organelles, abnormal or damaged proteins, and maintaining physiological homeostasis (Zhang and Wang 2018) and is considered to be cytoprotective at most circumstances. However, the expression pattern and consequences of autophagy post TBI has remained obscure, which means that the expression of autophagy might be either decreased or increased, and the consequences may be either beneficial or detrimental. Different TBI models or injury severity might cause diverse influence in various cell types of distinct regions. However, it is recognized that the restoration of autophagy could promote cell survival and reduce damage to cells. So, autophagy pathway is thought to be a potential therapeutic target for treating TBI (Lipinski et al. 2015). In addition, previous studies in our laboratory has showed that our TBI model might lead to a significant increase of autophagy and inhibiting autophagy may produce protective effects on neuronal function and stimulate neuronal regeneration following TBI (Gao et al. 2017; Shen et al. 2016; Zhang et al. 2014). Interestingly, autophagy deficiency induced by mTOR hyperactivation might result in the disruption of excitatory–inhibitory (E–I) balance in the brain, which contributes to the ASDs pathology (Hui et al. 2018). Given that Chd8 plays a key role in the pathogenesis of ASDs and intranasal application of recombinant Wnt3a reduces cell death and lesion volume by modulating negatively the autophagy activity following TBI (Zhang et al. 2018), so we hypothesized that Chd8 might regulate autophagy to promote neuroprotection and neural regeneration via wnt signaling pathway. Supporting our hypothesis, the results in our study showed that Chd8 knockdown led to a significant increase of LC3II level, and activation of wnt signaling pathway using lici might inhibit Chd8-siRNA-induced autophagic activation. For the first time, we found that Chd8 might inhibit activation of autophagy via wnt signaling pathway, which is an innovation in this research.
ASDs are approximately four times more common in males than in females (Menon 2018). Jung et al. reported that Chd8 mutant young male mice, when separated from their mother, displayed a series of abnormal behaviors, including increased mother-seeking ultrasonic vocalization, enhanced attachment to reunited mother (Jung et al. 2018), and showed remarkable increased neuronal activity in the prefrontal cortex (PFC), an important brain area which is highly involved in emotion processing, cognitive control, value-based decision-making, and error detection (Gluck et al. 2017), while female mice did not. These findings suggest that the actual effects of Chd8 on the brain are gender dependent. Interestingly, many laboratories have proved that systemic progesterone and estrogen treatment provide neuroprotective function after TBI (Brotfain et al. 2016). In addition, Ceballos-Chávez et al. reported that Chd8 is strongly required for the transcription of the progesterone-dependent enhancers (Ceballos-Chavez et al. 2015). However, the relationship between estrogen and Chd8 in the PFC post TBI has not been elucidated. In this study, we found that estrogen treatment increased the expression of Chd8 from 3 h to 7 days in the PFC following TBI. More importantly, E2 administration could activate Chd8 to inhibit apoptosis and autophagy activation in the PFC and hippocampus. E2 plays an important role in eliminating free radicals, reducing the BBB permeability, and the production of inflammatory cytokines. Although it was not a specific activator of Chd8, mediating Chd8 to suppress apoptosis and autophagy was a necessary part in the specific mechanism of the neuroprotective effects of E2 post TBI. The above results suggested that E2 may also be used as an activator of Chd8 and it can induce upregulation of Chd8 to protect vulnerable neurons, enhance existing neuronal function, and stimulate neuronal regeneration following TBI.
TBI Patients often experience cognitive deficits including loss of executive function, dysfunction in communication, and difficulties in intellectual processing, which leads to the persistent detrimental effects in daily life (Dash et al. 2015; Shang et al. 2014). In addition, cognitive impairments on children suffering from TBI might be remained for many years and was found to worsen rather than recover over time. More importantly, the level of cognitive deficits may be a sensitive predictor of long-term outcomes. The changes in the structural properties of neurons (such as the number of dendritic spines or synapses) and gray matter volume (based on neurogenesis, glial cell proliferation, and synaptogenesis) might be the possible reasons for the impairments on cognitive processing and emotion regulating post TBI (Catharine et al. 2019). The anatomical, neurophysiological, functional neuroimaging, and neuropsychological evidence reveled that there are interactions between emotion and memory system, indicating emotion might affect the memory performance (Rolls 2015). Furthermore, regulating emotion contributes to removing the negative contents of working memory (Flores and Berenbaum 2017). Chd8 is highly associated with cognitive impairments and emotion processing; we hypothesized that Chd8 might influence the learning and memory performance post TBI. Supporting our hypothesis, the results showed that Chd8 knockdown had no effect on motor behavior, but increased TBI-induced learning and memory impairments, and the effects of Chd8 on promoting cognitive recovery after TBI might be attributed to the inhibition of apoptosis and autophagy in the hippocampus and prefrontal cortex.
TBI and ASDs share some similar deficits in social interaction and communication. For example, TBI and ASDs both induced emotional perception difficulties, facial affect recognition impairments, and sensory processing disruption. Importantly, these deficits are highly associated with impairments in PFC and hippocampus. Interestingly, our results showed that overexpressed Chd8 via E2 produced beneficial effects, and Chd8 knockdown induced serious neurological damage by regulating wnt signaling pathway in the PFC and hippocampus post TBI (Fig. 11). Thus, Chd8 maybe a good therapeutic gene target for both TBI and ASDs patients. However, this work mainly focused on the function of Chd8 on neurons after TBI. Future work should be done to investigate the function of Chd8 in other cell types such as microglia and astrocytes following TBI.
Fig. 11.
Chd8 overexpression via 17β-estrogen (E2) treatment inhibited the activation of apoptosis and autophagy via wnt signaling pathway and promoted learning and memory recovery post TBI
Conclusion
In conclusion, Chd8 inhibited the activation of apoptosis and autophagy via wnt signaling pathway and promoted learning and memory recovery after experimental TBI both in vivo and in vitro. Chd8 predominantly co-localized in neurons in the prefrontal cortex, hippocampus, and parietal cortex. Both Chd8 and β-catenin expression had the similar change tendency post TBI. More importantly, Chd8 suppressed apoptosis and autophagy activation by regulating wnt signaling pathway following TBI. In addition, knockdown of Chd8 had no significant effect on motor function, but caused learning and memory impairments at normal and TBl conditions. Our results demonstrated that Chd8 might play an important role in promoting neuroprotection and cognitive recovery after TBI.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (TIF 14685 kb)
Supplement figure 1: (a) The schematic of cortex region in the brain. (b1-7, c1-7, d1-7) Double immunofluorescence for Chd8 and Dapi in the cortex at a series of time points post TBI. Horizontal sections labeled with Chd8 (green, b1-7) and Dapi (blue, c1-7). Colocalization of Chd8 and Dapi are shown in (d1-7); (e) The schematic of prefrontal cortex region in the brain. Double immunofluorescence for Chd8 and Dapi in the prefrontal cortex at 12h post TBI (f1-3, g1-3). Horizontal sections labeled with Chd8 (green, f1, g1) and Dapi (blue, f2, g2). Colocalization of Chd8 and Dapi are shown in (f3, g3); (h) The schematic of hippocampus region in the brain. Double immunofluorescence for Chd8 and Dapi in the cortex at 12h post TBI (i1-3, j1-3). Horizontal sections labeled with Chd8 (green, i1, j1) and Dapi (blue, i2, j2). Colocalization of Chd8 and Dapi are shown in (i3, j3); (k, l, m) Quantitative analysis of the ratio of Chd8+/Dapi+ cells in the cortex (k), prefrontal cortex (l) and the hippocampus (m) at a series of time points following TBI. The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs sham group). Experiments are representative of three independent experiments.
Supplementary file2 (TIF 5439 kb)
Supplement figure 2: (a) Chd8, β-catenin, Bax, Bcl-2, LC3-II and p62 protein levels were detected with immunoblotting in the prefrontal cortex. (d-i) Semiquantitative analysis (relative optical density) of the intensity of staining of Chd8 (d), β-catenin (e), Bax (f), Bcl-2 (g), LC3 II (h), p62 (i) to β-Tubulin in the prefrontal cortex. The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs sham group. ns, p > 0.05). Experiments are representative of three independent experiments; (b) Bax and LC3-II protein levels were detected with immunoblotting in the prefrontal cortex. (j, k) Semiquantitative analysis (relative optical density) of the intensity of staining of Bax (j) and LC3-II (k) to β-Tubulin in the prefrontal cortex. The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs sham group. #P < 0.05 and ##P < 0.01, vs tbi group. *#P < 0.05, vs tbi+E2 group. ns, p > 0.05). Experiments are representative of three independent experiments; (c) Chd8 protein levels were detected with immunoblotting in the prefrontal cortex. (l) Semiquantitative analysis (relative optical density) of the intensity of staining of Chd8 to β-Tubulin in prefrontal cortex. The data are mean ± SEM (n = 6. *P < 0.05, vs sham group. ns, p > 0.05). Experiments are representative of three independent experiments.
Supplementary file3 (TIF 3516 kb)
Supplement figure 3: (a-e) 17β-estrogen (E2) activated Chd8 to reduce the activation of apoptosis and autophagy in the hippocampus post TBI. (a) Caspase-3, Bcl-2, LC3-II and p62 protein levels were detected with immunoblotting in the hippocampus. (b-e) Semiquantitative analysis (relative optical density) of the intensity of staining of Caspase-3 (b), Bcl-2 (c) and LC3-II (d) and p62 (e) to β-Tubulin in the hippocampus. The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs sham group. #P < 0.05 and ##P < 0.01, vs tbi group. *#P < 0.05, vs tbi+si-Chd8 group. ns, p > 0.05). Experiments are representative of three independent experiments.
Supplementary file4 (DOC 38 kb)
Supplement data 1: PCR primer sequences (sense and antisense, respectively) of chd8, ccnd1, gsk, frizzled, dvl, gapdh, wnt5a, wnt7, tcf3 and axin were in supplement data 1.
Acknowledgements
This study was supported by the National Nature Science Foundation of China (Grant Nos. 81530062, 81871536, 81971800) and PAPD.
Abbreviations
- TBI
Traumatic brain injury
- ASDs
Autism spectrum disorder
- CHD8
Chromodomain helicase DNA binding protein 8
- lps
Lipopolysaccharide
- TYMS
Thymidylate synthase
- REST
RE-1 silencing transcription factor
- E2
17β-Estrogen
- MWW
Morris water maze
- NPCs
Neural progenitor cells
- NSCs
Neural stem cells
- PFC
Prefrontal cortex
Author Contributions
CXP and TLY contributed to the conception and design. CJ and WHC performed the main part of the experiments. GC, ZYL, CW, GZY, and CG participated in the acquisition and analysis of data. LCL wrote the manuscript and supervised the study. All authors critically reviewed and approved the submitted version of the manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chen Jie and Wang Haochen have contributed equally to this work.
Contributor Information
Xiping Chen, Email: xipingchen@suda.edu.cn.
Luyang Tao, Email: taoluyang@suda.edu.cn.
References
- Breuss MW, Gleeson JG (2016) When size matters: CHD8 in autism. Nat Neurosci 19(11):1430–1432. 10.1038/nn.4431 [DOI] [PubMed] [Google Scholar]
- Brotfain E, Gruenbaum SE, Boyko M, Kutz R, Zlotnik A, Klein M (2016) Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Curr Neuropharmacol 14(6):641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catharine VL, Helena V, Ellen D, Guy V, Karel D, Karen C (2019) Exploration of gray matter correlates of cognitive training benefit in adolescents with chronic traumatic brain injury. Neuroimage Clin 23:101827. 10.1016/j.nicl.2019.101827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Chavez M, Subtil-Rodriguez A, Giannopoulou EG, Soronellas D, Vazquez-Chavez E, Vicent GP, Elemento O, Beato M, Reyes JC (2015) The chromatin remodeler CHD8 is required for activation of progesterone receptor-dependent enhancers. PLoS Genet 11(4):e1005174. 10.1371/journal.pgen.1005174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotney J, Muhle RA, Sanders SJ, Liu L, Willsey AJ, Niu W, Liu W, Klei L, Lei J, Yin J, Reilly SK, Tebbenkamp AT, Bichsel C, Pletikos M, Sestan N, Roeder K, State MW, Devlin B, Noonan JP (2015) The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat Commun 6:6404. 10.1038/ncomms7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Hylin MJ, Hood KN, Orsi SA, Zhao J, Redell JB, Tsvetkov AS, Moore AN (2015) Inhibition of eukaryotic initiation factor 2 alpha phosphatase reduces tissue damage and improves learning and memory after experimental traumatic brain injury. J Neurotrauma 32(20):1608–1620. 10.1089/neu.2014.3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Asken BM (2017) Injury cascades in TBI-related neurodegeneration. Brain Inj 31(9):1177–1182. 10.1080/02699052.2017.1312528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durak O, Gao F, Kaeser-Woo YJ, Rueda R, Martorell AJ, Nott A, Liu CY, Watson LA, Tsai LH (2016) Chd8 mediates cortical neurogenesis via transcriptional regulation of cell cycle and Wnt signaling. Nat Neurosci 19(11):1477–1488. 10.1038/nn.4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, DeMaster D, Watson CG, Prasad MR, Cox CS, Kramer LA, Fischer JT, Duque G, Swank PR (2019) Post-Traumatic Stress Symptoms after Pediatric Injury: Relation to Pre-Frontal Limbic Circuitry. J Neurotrauma. 10.1089/neu.2018.6071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan XX, Hao YY, Guo SW, Zhao XP, Xiang Y, Feng FX, Liang GT, Dong YW (2018) Knockdown of RTN1-C attenuates traumatic neuronal injury through regulating intracellular Ca(2+) homeostasis. Neurochem Int 121:19–25. 10.1016/j.neuint.2018.10.018 [DOI] [PubMed] [Google Scholar]
- Fang J, Wang H, Zhou J, Dai W, Zhu Y, Zhou Y, Wang X, Zhou M (2018) Baicalin provides neuroprotection in traumatic brain injury mice model through Akt/Nrf2 pathway. Drug Des Devel Ther 12:2497–2508. 10.2147/DDDT.S163951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores LE, Berenbaum H (2017) The social regulation of emotion and updating negative contents of working memory. Emotion 17(4):577–588. 10.1037/emo0000265 [DOI] [PubMed] [Google Scholar]
- Gao Y, Ma L, Luo CL, Wang T, Zhang MY, Shen X, Meng HH, Ji MM, Wang ZF, Chen XP, Tao LY (2017) IL-33 Exerts neuroprotective effect in mice intracerebral hemorrhage model through suppressing inflammation/apoptotic/autophagic pathway. Mol Neurobiol 54(5):3879–3892. 10.1007/s12035-016-9947-6 [DOI] [PubMed] [Google Scholar]
- Gao YY, Zhang ZH, Zhuang Z, Lu Y, Wu LY, Ye ZN, Zhang XS, Chen CL, Li W, Hang CH (2018) Recombinant milk fat globule-EGF factor-8 reduces apoptosis via integrin beta3/FAK/PI3K/AKT signaling pathway in rats after traumatic brain injury. Cell Death Dis 9(9):845. 10.1038/s41419-018-0939-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner AJ, Zafonte R (2016) Neuroepidemiology of traumatic brain injury. Handb Clin Neurol 138:207–223. 10.1016/B978-0-12-802973-2.00012-4 [DOI] [PubMed] [Google Scholar]
- Gluck ME, Viswanath P, Stinson EJ (2017) Obesity, appetite, and the prefrontal cortex. Curr Obes Rep 6(4):380–388. 10.1007/s13679-017-0289-0 [DOI] [PubMed] [Google Scholar]
- Hui K, Katayama Y, Nakayama KI, Nomura J, Sakurai T (2018) Characterizing vulnerable brain areas and circuits in mouse models of autism: towards understanding pathogenesis and new therapeutic approaches. Neurosci Biobehav Rev. 10.1016/j.neubiorev.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Jung H, Park H, Choi Y, Kang H, Lee E, Kweon H, Roh JD, Ellegood J, Choi W, Kang J, Rhim I, Choi SY, Bae M, Kim SG, Lee J, Chung C, Yoo T, Park H, Kim Y, Ha S, Um SM, Mo S, Kwon Y, Mah W, Bae YC, Kim H, Lerch JP, Paik SB, Kim E (2018) Sexually dimorphic behavior, neuronal activity, and gene expression in Chd8-mutant mice. Nat Neurosci 21(9):1218–1228. 10.1038/s41593-018-0208-z [DOI] [PubMed] [Google Scholar]
- Katayama Y, Nishiyama M, Shoji H, Ohkawa Y, Kawamura A, Sato T, Suyama M, Takumi T, Miyakawa T, Nakayama KI (2016) CHD8 haploinsufficiency results in autistic-like phenotypes in mice. Nature 537(7622):675–679. 10.1038/nature19357 [DOI] [PubMed] [Google Scholar]
- Leung LY, Cardiff K, Yang X, Srambical Wilfred B, Gilsdorf J, Shear D (2018) Selective brain cooling reduces motor deficits induced by combined traumatic brain injury hypoxemia and hemorrhagic shock. Front Neurol 9:612. 10.3389/fneur.2018.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski MM, Wu J, Faden AI, Sarkar C (2015) Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid Redox Signal 23(6):565–577. 10.1089/ars.2015.6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo CL, Li BX, Li QQ, Chen XP, Sun YX, Bao HJ, Dai DK, Shen YW, Xu HF, Ni H, Wan L, Qin ZH, Tao LY, Zhao ZQ (2011) Autophagy is involved in traumatic brain injury-induced cell death and contributes to functional outcome deficits in mice. Neuroscience 184:54–63. 10.1016/j.neuroscience.2011.03.021 [DOI] [PubMed] [Google Scholar]
- Ma J, Chen L, Song D, Zhang Y, Chen T, Niu P (2017) SIRT1 attenuated oxidative stress induced by methyl tert-butyl ether in HT22 cells. Toxicol Res (Camb) 6(3):290–296. 10.1039/c7tx00016b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2018) Extracting new insights from bulk transcriptomics. Nat Neurosci 21(9):1142–1144. 10.1038/s41593-018-0218-x [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Oshikawa K, Tsukada Y, Nakagawa T, Iemura S, Natsume T, Fan Y, Kikuchi A, Skoultchi AI, Nakayama KI (2009) CHD8 suppresses p53-mediated apoptosis through histone H1 recruitment during early embryogenesis. Nat Cell Biol 11(2):172–182. 10.1038/ncb1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan A, Hennessy E, Krukowski K, Guglielmetti C, Chaumeil MM, Sohal VS, Rosi S (2018) Repeated mild head injury leads to wide-ranging deficits in higher-order cognitive functions associated with the prefrontal cortex. J Neurotrauma 35(20):2425–2434. 10.1089/neu.2018.5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia AL, Plog BA, Dayawansa S, Chen M, Dashnaw ML, Czerniecka K, Walker CT, Viterise T, Hyrien O, Iliff JJ, Deane R, Nedergaard M, Huang JH (2014) The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J Neurotrauma 31(13):1211–1224. 10.1089/neu.2013.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt RJ, Zhou Y, Slaymaker IM, Shetty AS, Weisbach NR, Kim JA, Sharma J, Desai M, Sood S, Kempton HR, Crabtree GR, Feng G, Zhang F (2017) Chd8 mutation leads to autistic-like behaviors and impaired striatal circuits. Cell Rep 19(2):335–350. 10.1016/j.celrep.2017.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, McKinlay A (2019) The long-term effects of childhood traumatic brain injury on adulthood relationship quality. Brain Inj 33(5):649–656. 10.1080/02699052.2019.1567936 [DOI] [PubMed] [Google Scholar]
- Rolls ET (2015) Limbic systems for emotion and for memory, but no single limbic system. Cortex 62:119–157. 10.1016/j.cortex.2013.12.005 [DOI] [PubMed] [Google Scholar]
- Sawada G, Ueo H, Matsumura T, Uchi R, Ishibashi M, Mima K, Kurashige J, Takahashi Y, Akiyoshi S, Sudo T, Sugimachi K, Doki Y, Mori M, Mimori K (2013) CHD8 is an independent prognostic indicator that regulates Wnt/beta-catenin signaling and the cell cycle in gastric cancer. Oncol Rep 30(3):1137–1142. 10.3892/or.2013.2597 [DOI] [PubMed] [Google Scholar]
- Shang JL, Cheng Q, Yang WF, Zhang M, Cui Y, Wang YF (2014) Possible roles of COX-1 in learning and memory impairment induced by traumatic brain injury in mice. Braz J Med Biol Res 47(12):1050–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Ma L, Dong W, Wu Q, Gao Y, Luo C, Zhang M, Chen X, Tao L (2016) Autophagy regulates intracerebral hemorrhage induced neural damage via apoptosis and NF-kappaB pathway. Neurochem Int 96:100–112. 10.1016/j.neuint.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Singh R, Turner RC, Nguyen L, Motwani K, Swatek M, Lucke-Wold BP (2016) Pediatric Traumatic Brain Injury and Autism: Elucidating Shared Mechanisms. Behav Neurol 2016:8781725. 10.1155/2016/8781725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky E, Palzur E, Feinsod M, Soustiel JF (2005) Evaluation of the apoptosis-related proteins of the BCL-2 family in the traumatic penumbra area of the rat model of cerebral contusion, treated by hyperbaric oxygen therapy: a quantitative immunohistochemical study. Acta Neuropathol 110(2):120–126. 10.1007/s00401-004-0946-8 [DOI] [PubMed] [Google Scholar]
- Wang CF, Zhao CC, Liu WL, Huang XJ, Deng YF, Jiang JY, Li WP (2020) Depletion of microglia attenuates dendritic spine loss and neuronal apoptosis in the acute stage of moderate traumatic brain injury in mice. J Neurotrauma 37(1):43–54. 10.1089/neu.2019.6460 [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang H (2018) Autophagy in traumatic brain injury: a new target for therapeutic intervention. Front Mol Neurosci 11:190. 10.3389/fnmol.2018.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Shan H, Chang P, Wang T, Dong W, Chen X, Tao L (2014) Hydrogen sulfide offers neuroprotection on traumatic brain injury in parallel with reduced apoptosis and autophagy in mice. PLoS ONE 9(1):e87241. 10.1371/journal.pone.0087241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Shan H, Chang P, Ma L, Chu Y, Shen X, Wu Q, Wang Z, Luo C, Wang T, Chen X, Tao L (2017) Upregulation of 3-MST relates to neuronal autophagy after traumatic brain injury in mice. Cell Mol Neurobiol 37(2):291–302. 10.1007/s10571-016-0369-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Lee JH, Gu X, Wei ZZ, Harris MJ, Yu SP, Wei L (2018) Intranasally delivered Wnt3a improves functional recovery after traumatic brain injury by modulating autophagic, apoptotic, and regenerative pathways in the mouse brain. J Neurotrauma 35(5):802–813. 10.1089/neu.2016.4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZA, Zhao Y, Ning YL, Yang N, Peng Y, Li P, Chen XY, Liu D, Wang H, Chen X, Bai W, Chen JF, Zhou YG (2017) Adenosine A2A receptor inactivation alleviates early-onset cognitive dysfunction after traumatic brain injury involving an inhibition of tau hyperphosphorylation. Transl Psychiatry 7(5):e1123. 10.1038/tp.2017.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 (TIF 14685 kb)
Supplement figure 1: (a) The schematic of cortex region in the brain. (b1-7, c1-7, d1-7) Double immunofluorescence for Chd8 and Dapi in the cortex at a series of time points post TBI. Horizontal sections labeled with Chd8 (green, b1-7) and Dapi (blue, c1-7). Colocalization of Chd8 and Dapi are shown in (d1-7); (e) The schematic of prefrontal cortex region in the brain. Double immunofluorescence for Chd8 and Dapi in the prefrontal cortex at 12h post TBI (f1-3, g1-3). Horizontal sections labeled with Chd8 (green, f1, g1) and Dapi (blue, f2, g2). Colocalization of Chd8 and Dapi are shown in (f3, g3); (h) The schematic of hippocampus region in the brain. Double immunofluorescence for Chd8 and Dapi in the cortex at 12h post TBI (i1-3, j1-3). Horizontal sections labeled with Chd8 (green, i1, j1) and Dapi (blue, i2, j2). Colocalization of Chd8 and Dapi are shown in (i3, j3); (k, l, m) Quantitative analysis of the ratio of Chd8+/Dapi+ cells in the cortex (k), prefrontal cortex (l) and the hippocampus (m) at a series of time points following TBI. The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs sham group). Experiments are representative of three independent experiments.
Supplementary file2 (TIF 5439 kb)
Supplement figure 2: (a) Chd8, β-catenin, Bax, Bcl-2, LC3-II and p62 protein levels were detected with immunoblotting in the prefrontal cortex. (d-i) Semiquantitative analysis (relative optical density) of the intensity of staining of Chd8 (d), β-catenin (e), Bax (f), Bcl-2 (g), LC3 II (h), p62 (i) to β-Tubulin in the prefrontal cortex. The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs sham group. ns, p > 0.05). Experiments are representative of three independent experiments; (b) Bax and LC3-II protein levels were detected with immunoblotting in the prefrontal cortex. (j, k) Semiquantitative analysis (relative optical density) of the intensity of staining of Bax (j) and LC3-II (k) to β-Tubulin in the prefrontal cortex. The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs sham group. #P < 0.05 and ##P < 0.01, vs tbi group. *#P < 0.05, vs tbi+E2 group. ns, p > 0.05). Experiments are representative of three independent experiments; (c) Chd8 protein levels were detected with immunoblotting in the prefrontal cortex. (l) Semiquantitative analysis (relative optical density) of the intensity of staining of Chd8 to β-Tubulin in prefrontal cortex. The data are mean ± SEM (n = 6. *P < 0.05, vs sham group. ns, p > 0.05). Experiments are representative of three independent experiments.
Supplementary file3 (TIF 3516 kb)
Supplement figure 3: (a-e) 17β-estrogen (E2) activated Chd8 to reduce the activation of apoptosis and autophagy in the hippocampus post TBI. (a) Caspase-3, Bcl-2, LC3-II and p62 protein levels were detected with immunoblotting in the hippocampus. (b-e) Semiquantitative analysis (relative optical density) of the intensity of staining of Caspase-3 (b), Bcl-2 (c) and LC3-II (d) and p62 (e) to β-Tubulin in the hippocampus. The data are mean ± SEM (n = 6. *P < 0.05 and **P < 0.01, vs sham group. #P < 0.05 and ##P < 0.01, vs tbi group. *#P < 0.05, vs tbi+si-Chd8 group. ns, p > 0.05). Experiments are representative of three independent experiments.
Supplementary file4 (DOC 38 kb)
Supplement data 1: PCR primer sequences (sense and antisense, respectively) of chd8, ccnd1, gsk, frizzled, dvl, gapdh, wnt5a, wnt7, tcf3 and axin were in supplement data 1.