Abstract
Background:
There is emerging evidence that supports a role for brain-derived neurotrophic factor (BDNF) in the risk and presence of serious cardiovascular conditions. However, few existing literature reviews methodically describe empirical findings regarding this relationship.
Objectives:
The purpose of this integrative review was to (a) evaluate BDNF (serum/plasma BDNF levels, BDNF Val66Met genotype) among humans at risk for or with serious cardiovascular conditions, and (b) investigate the relationship between BDNF and risk/presence of serious cardiovascular conditions in humans.
Methods:
A literature review with integrative review methodology was conducted. Articles in English included human subjects, a measure of BDNF levels or BDNF gene, involved serious cardiovascular conditions, and had quantitative data analyses. The search resulted in 475 unique titles with the final sample including 35 articles representing 30 studies. Articles that received “good” or “fair” ratings (n = 31) using the National Heart, Lung, and Blood Institute Study Quality Assessment Tools were included for synthesis.
Results:
The retrieved articles were largely non-experimental with sample sizes ranging from 20 to 5,510 participants (M = 546). Serum BDNF levels ranged from 0.8–43.2 ng/mL. Overall, BDNF levels were lower in patients with chronic heart failure and stroke, but higher in patients with unstable angina and recent myocardial infarction. Lower BDNF levels were associated with higher incidence of cardiovascular events in patients with a prior history of serious cardiovascular conditions and decreased cardiovascular risk in healthy samples. For BDNF genotype, on average 36.3% of participants had Met alleles. The frequency of the BDNF Met allele varied across race/ethnicity and cardiovascular conditions, and in terms of association with serious cardiovascular condition incidence/risk.
Discussion:
These findings indicate an emerging area of science. Future investigation is needed on serious cardiovascular condition phenotypes in relationship to BDNF in the same study conditions. Results also suggest for use of standardized BDNF measurement across studies, and additional investigation in cardiovascular inflammatory processes that impact BDNF. Moreover, within specific populations, it may be that the frequency of Met alleles is too low to be detected in sample sizes normally found in these types of studies.
Keywords: Brain-derived neurotrophic factor, cardiovascular diseases, heart diseases, scientific review
Serious cardiovascular conditions (e.g., coronary heart disease, heart failure) are highly prevalent among middle-age and older adults (Benjamin et al., 2019). Development of serious cardiovascular conditions occurs on a continuum, ranging from increased cardiovascular risk (e.g., metabolic syndrome, hypertension) to advanced stages of cardiovascular disease (e.g., heart failure; Rosin, 2007). In the United States (U.S.), 92.1 million adults are estimated to have a form of serious cardiovascular condition or after-effects of stroke (Benjamin et al., 2019). Serious cardiovascular conditions often contribute to high mortality rates and debilitating symptoms that diminish quality of life (Benjamin et al., 2019). There has been increased interest in examining key biomarkers that may be useful for early detection, prognosis, and symptom management in serious cardiovascular conditions (Page et al., 2018).
Brain-derived neurotrophic factor (BDNF) is a protein that is a member of the neurotrophin family and has a prominent role in neurological development and degeneration (Bathina & Das, 2015). Tropomyosin receptor kinase B (TrkB) is the high affinity receptor for BDNF, and is found in the cells and gray matter of the spinal cord (Fang et al., 2003). BDNF is well known for pleiotropic effects in the brain, including promoting the growth and survival of neurons and regulation of synaptic neuroplasticity that contributes to learning and memory (Erickson et al., 2013). In addition, BDNF appears to have a neuroprotective effect in the face of injury or disease, such as cerebral ischemia, hypoglycemia, and neurotoxicity (Bathina & Das, 2015). Circulating BDNF levels in the blood have been used to characterize cognitive dysfunction phenotypes and levels have been compared across populations. For example, compared with healthy persons, persons living with schizophrenia and Alzheimer’s disease had lower levels of serum BDNF (Fisher et al., 2016; Ng et al., 2019; Vinogradov et al., 2009) compared with control participants.
Recent studies suggest that BDNF also has independent and local effects in the cardiovascular system (Kermani & Hempstead, 2019). Specifically, BDNF is involved in angiogenesis, vasculogenesis, and increased cardiomyocyte contractility (Fulgenzi et al., 2015; Pius-Sadowska & Machaliński, 2017). In pre-clinical rodent models, episodes of sepsis may result in depletion of BDNF levels in cardiomyocytes and myocardial dysfunction (Zeng et al., 2017). In a rat model of post-myocardial infarction, exercise promoted the activation of BDNF-mediated trkB signaling, which led to improved cardiovascular functions as measured by ejection fraction, left ventricular end-diastolic pressure, stroke volume, and cardiac index (H. W. Lee et al., 2018). Other investigators have reported that in humans, BDNF may induce oxidative stress that may lead to instability of atherosclerotic plaques (Kaess et al., 2015) and lower serum BDNF levels may be associated with cardiac-related death (Taşçı et al., 2012). Overall, BDNF seems to play an important role in cardiovascular function and pathology, but relationships between its level of expression and functional correlates are less established in humans.
Regardless of brain or cardiac BDNF actions, the circulating levels of BDNF in the blood can reflect local levels of BDNF within those structures, and can be influenced by single nucleotide polymorphisms (SNPs) in the BDNF locus, which is located on chromosome 11 (Egan et al., 2003). These SNPs can be assessed to examine their frequencies across different populations and to evaluate associations between genotypes and phenotypes. For instance, the BDNF SNP (rs6265) produces a valine (Val)-to-methionine (Met) substitution in the pro-BDNF protein at codon 66 (Val66Met), which inhibits the production and/or release of BDNF. The presence of the Met allele is associated with disorders of learning and memory in adults living in the U.S. and Australia. (Dempster et al., 2005; Erickson et al., 2013; Hariri et al., 2003; Lim et al., 2014). In European Americans, approximately 20% of people have the BDNF Met allele (Val/Met or Met/Met genotype) and 80% do not have the BDNF Met allele (Val/Val genotype) (Zerbino et al., 2018). In contrast, East Asians have nearly a 50% Met allelic frequency while African Americans are less likely to have the Met allele (~5%; Zerbino et al., 2018). Thus, the percentage of people with the BDNF Met allele vary by population genetic structure, and ranges from 0 to 72% (Petryshen et al., 2010).
There were six previously published literature reviews in which either serum BDNF levels and/or BDNF genotype was evaluated in the context of the cardiovascular system or cardiovascular conditions (Finnell & Wood, 2016; Kermani & Hempstead, 2019; Motamedi et al., 2017; Pius-Sadowska & Machaliński, 2017; Rothman et al., 2012; Taşçı et al., 2012). The reviews provided new insights about associations between circulating BDNF levels, BDNF genotype and cardiovascular conditions but were limited in several ways. First, the previously published reviews were all “state of the science” reviews, and did not specify search strategies or comprehensiveness of the retrieved articles. Thus, the ability to draw conclusions was limited. Second, none of the reviews assessed the quality of articles in the review processes using a standard quality of bias assessment rating. Third, animal and human studies were combined in the reviews. Animal models may not accurately portray the human pathophysiology associated with BDNF and the effects of BDNF on the cardiovascular system in humans (Kermani & Hempstead, 2019). Thus, synthesis and conclusions are difficult to translate to humans. Taken together, no existing literature reviews were identified in which investigators methodically synthesized existing empirical literature regarding BDNF among humans with serious cardiovascular conditions. Due to this, it is also unclear how BDNF relates to the risk or presence of serious cardiovascular conditions among humans.
MATERIALS AND METHODS
The integrative review methodology outlined by Whittemore and Knafl (2005) was followed. This methodology was chosen because it allows investigators to compare and contrast studies with different types of designs, measures, and methods (Whittemore & Knafl, 2005). Integrative reviews are particularly useful when there are limited randomized controlled trials on specific phenomenon. A rigorously conducted integrative review will provide synthesis of knowledge to inform future studies, and ultimately, clinical practice (Russell, 2005). In addition, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to guide the processes for the literature search results reporting to ensure a rigorous methodology (Moher et al., 2009).
Problem Identification
The present review was conducted to address the concerns in prior literature reviews. The purpose of this integrative review was to (a) evaluate BDNF (serum/plasma BDNF levels, BDNF Val66Met genotype) among humans at risk for or with serious cardiovascular conditions, and (b) investigate the relationship between BDNF and risk/presence of serious cardiovascular conditions in humans. We also aimed to identify future priority areas of investigation.
Literature Search
The search strategy process consisted of three steps (Moher et al., 2009). First, a search was conducted of three electronic databases, PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and SciVerse Scopus, in July 2019. Second, the reference lists of the six previously published literature reviews were examined to identify relevant articles not retrieved in the electronic database search. Third, the reference lists of all of the retrieved articles were hand searched to identify relevant articles not retrieved in the electronic database search.
The database search was conducted using controlled vocabulary terms and synonymous free-text words for the two categories of BDNF and cardiovascular conditions. The search terms of the first category were brain-derived neurotrophic factor (MeSH), BDNF (text word), trkB (MeSH), pro-BDNF (text word), and NTRK2 (text word). The search terms of the second category were the following MeSH terms: cardiovascular disease, coronary disease, heart failure, heart diseases, atrial fibrillation, myocardial infarction, cardiac myocytes, and myocardial contractility. A medical librarian verified the search strategy. To ensure a thorough retrieval of the existing literature, the search was not limited by time parameters.
Data Evaluation
Inclusion criteria were as follows: (a) English language; (b) human subjects; (c) BDNF measured as serum, plasma, or BDNF gene; (d) study involved risk or presence of serious cardiovascular conditions; and (e) quantitative data analysis. Studies were excluded if the focus was on a co-morbid condition (e.g., schizophrenia) not directly related to serious cardiovascular conditions or risk for serious cardiovascular conditions.
Data Analysis
The following data points were extracted independently by two co-authors and entered in narrative tables for all articles in the final sample: (a) first authors; (b) publication year; (c) country (sampling area); (d) purpose and hypothesis as stated by the authors; (e) study design and follow-up time points (if applicable); (f) sample characteristics; (g) setting; (h) primary dependent variable measures; (i) BDNF measure; and (j) main findings specific to BDNF. If discrepancies were noted between the reviews of the two co-authors, the article was discussed by research team members and consensus achieved through review of the full-text article.
Data quality was assessed using the National Heart, Lung, and Blood Institute’s (NHLBI) Quality Assessment Tools (National Heart, Lung, and Blood Institute, 2019). The appropriate tool was chosen based on the article authors’ reported study design (i.e., Quality Assessment of Case-Control Studies, Quality Assessment for Observational Cohort and Cross-Sectional Studies, Quality Assessment Tool for Before-After Studies With No Control Group). Articles were scored on 12–14 criteria, depending on the specific tool that was selected based on study design, and assigned an overall rating of “poor”, “fair”, or “good” quality based on the proportion of criteria met (poor: < 30% criteria met; fair: 30%–60% criteria met; good: > 60% criteria met; Sheehan et al., 2018).
Presentation
Results from the data extraction of the final sample were organized into tabular format and synthesized based on the type of serious cardiovascular condition. To address the aim of the present review, narrative tables were categorized based on the primary dependent variable of each article: (a) dependent variable related to BDNF, or (b) dependent variable related to risk or presence of serious cardiovascular condition. The quality appraisal results informed the synthesis of evidence; articles rated as “good” or “fair” were included in the presentation and discussion of the main outcomes.
RESULTS
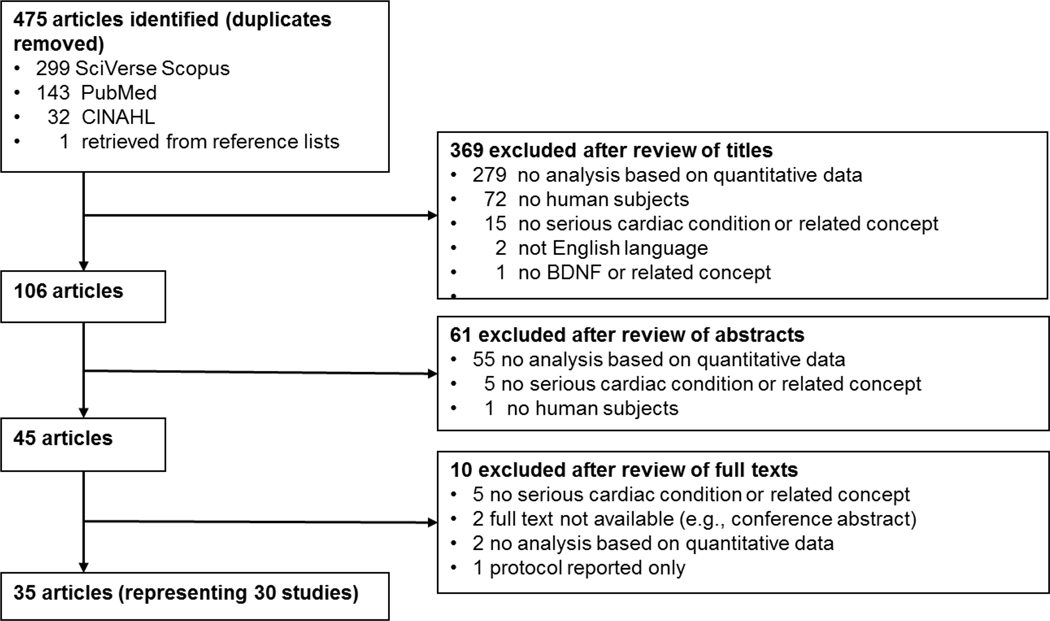
The initial search resulted in 475 unique titles (Figure 1). The authors independently reviewed the retrieved titles, followed by abstracts, and then full-text articles to evaluate whether they met the inclusion criteria. The authors reached a mutual consensus by discussion and further review wherein review decisions were incongruent. Based on title review, 369 articles were excluded. The majority of exclusions (n = 279) occurred because the studies did not include quantitative data analysis (e.g., case reports, clinical reviews). Next, the abstracts of the 106 remaining articles were evaluated and 61 of these articles were excluded. The majority of these articles (n = 55) were excluded because they did not have quantitative data analysis. Full-text review was completed for the remaining 45 articles and 10 articles were excluded because they did not focus on risk or presence of serious cardiovascular conditions (n = 5), no full text was available (e.g., conference abstract; n = 2), they did not include an analysis based on quantitative data (n = 2), and they reported the study protocol only (n = 1). The final sample resulting from this search strategy was 35 articles representing 30 studies. In four cases, there were two articles published that used data from the same study (H. Jiang et al., 2009, 2011; Kadoya et al., 2014; Kurajoh et al., 2017; Pressler et al., 2015, 2017; Vuren et al., 2019). In addition, two articles used a data set from the Framingham Heart Study (Kaess et al., 2015; Rahman et al., 2017). The majority of the articles had quality assessment ratings that were categorized as fair (n = 24), while 7 were categorized as good, and 4 as poor (Table 1). Most frequently, articles received lower ratings because of the following items: (a) unclear specification of a purpose statement and/or hypothesis, (b) lack of justification of sample size, (c) lack of inclusion and exclusion criteria for sample and control groups, and (d) lack of recruitment details.
Figure 1.
Flow chart of search and retrieval process and results.
Table 1.
Quality Assessment Ratings of the 35 Articles by the National Heart, Blood, and Lung Institute Study Quality Assessment Tool Type
| First Author, Year | Quality Rating |
|---|---|
| Controlled Intervention Studies | |
| Pressler, 2015 | Good |
| Before-After (Pre-Post) Studies with No Control Group | |
| Pressler, 2017 | Fair |
| Case-Control Studies | |
| Bozzini, 2009 | Fair |
| Chaldakov, 2004 | Poor |
| Costa, 2014 | Fair |
| Jiang, H, 2009 | Good |
| Kadowaki, 2016 | Fair |
| Lee, 2012 | Fair |
| Lorgis, 2010 | Good |
| Stanne, 2016 | Fair |
| Sustar, 2016 | Fair |
| Takashio, 2015 | Fair |
| Wu, 2019 | Fair |
| Zembron-Lacny, 2016 | Poor |
| Zhang, 2015 | Fair |
| Observational Cohort and Cross-Sectional Studies | |
| Bus, 2011 | Poor |
| Fukushima, 2015 | Good |
| Golden, 2010 | Fair |
| Greisenegger, 2015 | Fair |
| Lasek-Bal, 2015 | Good |
| Ejiri, 2005 | Fair |
| Jiang, R, 2017 | Fair |
| Jiang, H, 2011 | Fair |
| Jung, 2011 | Poor |
| Kadoya, 2014 | Poor |
| Kaess, 2015 | Fair |
| Kurajoh, 2017 | Fair |
| Lee, 2018 | Fair |
| Pedersen, 2017 | Fair |
| Rahman, 2017 | Fair |
| Sanchez-DeToledo, 2014 | Good |
| Schutte, 2016 | Poor |
| Shibata, 2018 | Fair |
| Szabo, 2013 | Fair |
| van Vuren, 2019 | Fair |
Of the 35 retrieved articles, authors described their designs as experimental (n = 1), non-experimental (n = 33), and not reported (n = 1). The one article that reported an experimental design was a secondary analysis of a randomized controlled trial. Non-experimental designs included cross-sectional (n = 8), case control (n = 12), longitudinal ranging from 8 weeks to 10 years (n = 12), and one pre-post design (Tables 2 and 3). The articles included studies conducted in 16 countries representing the continents of Asia (n = 14), Europe (n = 10), North America (n = 8), Africa (n = 2), and South America (n = 1).
Table 2.
Sixteen Articles with BDNF-related Dependent Variables and Cardiovascular-related Independent Variables
| First Author | Year | Study Purpose/ Hypothesisa | Design/ Follow-up | Quality Ratingb | Sample | Setting | Primary Dependent Variablea | BDNF-related Findingsc |
|---|---|---|---|---|---|---|---|---|
| Metabolic syndrome | ||||||||
| Chaldakov | 2004 | To study the cardiovascular and metabolic biology of nerve growth factor, BDNF and mast cells. | Case control | Poor | 33 adults (23 with metabolic syndrome, 10 healthy without metabolic syndrome) Age M 44.3 (2.7) 81.8% women |
Clinic | Plasma BDNF levels Nerve growth factor Mast cells |
Plasma BDNF levels were lower in participants with metabolic syndrome compared to participants without metabolic syndrome. |
| Lee | 2012 | To examine the relationship of metabolic syndrome, adipose tissue and biomarkers with BDNF in men. | Case control | Fair | 58 men (34 with metabolic syndrome but not diabetes; 24 age-matched men without metabolic syndrome) Age M 40.2 (10.4) |
Clinic | Serum BDNF levels • Collected after an overnight fast |
There was no significant difference in serum BDNF levels between men with or without metabolic syndrome. There were no significant differences in the components of metabolic syndrome between those with low and those with high serum BDNF levels, except for lower systolic blood pressure in participants with high serum BDNF levels. |
| Coronary artery disease | ||||||||
| Bozzini | 2009 | To investigate the possible roles of BDNF Val66Met, 5-HTTLPR and −1438 G/A polymorphisms in CAD in patients with and without depression. | Case control | Fair | 242 adults (99 patients with CAD, 143 healthy control participants) 43.8% women 100% White |
Clinic | BDNF gene | The frequency of Met/Met genotype was significantly higher in the patients with CAD compared to the control cases. |
| Bus | 2011 | To examine the determinants (sampling characteristics, socio-demographic variables, lifestyle indicators, CAD, and metabolic syndrome) of serum BDNF in a large and well-defined cohort of people without current psychiatric or neurologic diseases. | Cross-sectional | Poor | 1168 adults with subthreshold depressive or anxiety symptoms, or had high risk Age M 42.5 (14.1) 65% women |
Clinic | Serum BDNF levels • Collected after an overnight fast • Immediately transferred and processed within 1 hour |
Higher serum BDNF levels were found in participants who were older, had higher degrees of urbanicity, current smokers, and had metabolic syndrome or CAD. Significance for both metabolic syndrome and cardiovascular disease was lost controlling for age. Eating prior to blood withdrawal resulted in a significantly lower serum BDNF levels. Sampling later in the morning resulted in lower serum BDNF levels and longer sample storage. |
| Sustar | 2016 | To evaluate the association between BDNF Val66Met (rs6265) polymorphism and CHD and/or BMI in patients with CHD and in healthy control participants. Hypothesis: Different BDNF Val66Met genotypes would be associated with CHD or with increased BMI in CHD patients and healthy control participants. | Case control | Good | 704 adults (206 with CHD, 498 healthy control participants) Age M 54.2 years 100% White |
Clinic | BDNF gene | There were no significant differences of BDNF genotype frequencies by sex and between adults with CHD and healthy control participants. BDNF rs6265 polymorphism was associated with BMI categories. |
| Myocardial infarction | ||||||||
| Lorgis | 2010 | To evaluate the relationship between BDNF, functional parameters and biological markers associated with inflammatory processes and platelet activation. | Case control | Good | 40 adults (20 with acute myocardial infarction, 20 age- and gender-matched with stable angina) Age Mdn 61.5 years (IQR = 54–78) 25% women |
Clinic | Serum BDNF levels • Collected in the morning after an overnight fast |
Median serum BDNF levels were significantly higher in the myocardial infarction group than in the stable angina group. In patients with myocardial infarction, a significant correlation was found between BDNF and sP-selectin. |
| Zhang | 2015 | To identify serum biomarkers, including BDNF, of patients with STEMI for use in diagnosis | Case control | Fair | 20 men (10 with STEMI, 10 healthy without STEMI) Age M 54.2 (5.8) |
Hospital | Serum BDNF levels | Serum BDNF levels were significantly higher in the STEMI group compared with control cases. |
| Heart failure | ||||||||
| Pressler | 2015 | To examine the efficacy of Brain Fitness cognitive training intervention on memory in patients with heart failure, and to examine changes in serum BDNF levels between patients who were randomized to Brain Fitness and health education who were BDNF gene Met negative (homozygous Val/Val) and patients who were BDNF gene Met positive (heterozygous Val/Met and homozygous Met/Met). Hypothesis: Compared with heart failure patients in the active control health education group, heart failure patients who receive Brain Fitness have improved memory and serum BDNF levels. | RCT testing a cognitive training intervention (Brain Fitness) 12 weeks |
Good | 27 adults with heart failure Age M = 61.0 (11.9) 22% women 85.2% White 11.1% Black 3.7% Asian |
Clinic | Serum BDNF levels BDNF gene |
Serum BDNF levels significantly increased among patients who completed the intervention and decreased among patients who completed health education control at 12 weeks. There were no significant differences in memory between the groups over time. The intervention was associated with increased serum BDNF levels regardless of BDNF Met status. |
| Pressler | 2017 | To characterize major allelic frequency of 2 variants in APOE gene in patients with heart failure, and evaluate differences in memory and serum BDNF levels based on APOE ε 4 allele(s). | One-group pre-post design testing a cognitive training intervention 8 weeks 12 weeks |
Fair | 26 adults with heart failure Age M 60.8 (12.1) |
Clinic | Serum BDNF levels | There were no significant differences in serum BDNF levels between those who had the APOEε4 allele and those who did not. None of the participants who had APOE ε 4 present had the BDNF Met allele. |
| Takashio | 2015 | To evaluate plasma BDNF levels in patients with heart failure and age- and gender-matched control participants. Hypothesis: Plasma BDNF levels would be decreased in patients with heart failure. | Case control | Fair | 242 heart failure patients, 80 case control participants Age M 71 (12) 34% women |
Hospital | Plasma BDNF levels | Plasma BDNF levels were significantly lower in patients with heart failure and lower in patients with heart failure with NYHA class III than class I. |
| Stroke | ||||||||
| Lasek-Bal | 2015 | To assess the role of BDNF concentration in the course of ischemic stroke and its effect on post-stroke disability prognosis. | Longitudinal 90 days |
Good | 87 adults with ischemic stroke Age M 71.7 (11.8) 48.3% women |
Hospital | Serum BDNF levels • On the first day of stroke |
There were no significant differences between adults who had mild vs. moderate/severe neurological deficits on the first day post-stroke. Serum BDNF levels were significantly lower in adults with lower functional status at 90 days after the onset of stroke. |
| Stanne | 2016 | To investigate whether circulating concentrations of BDNF are altered in the acute phase of ischemic stroke and whether they are associated with short- or long-term functional outcomes. Hypothesis: Circulating BDNF concentrations are lowered in the acute phase of ischemic stroke and low BDNF concentrations are associated with poor short- and long-term functional outcomes | Case control | Fair | 491 adults after stroke, 513 case control participants (1004 total) Age Mdn 58.5 (IQR = 51–64) 36% women 100% White |
Clinic | • Serum BDNF levels | Serum BDNF levels were significantly lower in adults after ischemic stroke compared with healthy control participants. Serum BDNF levels were not significantly associated with 3-month outcomes. In adults after ischemic stroke, those with the lowest tertile of BDNF had an increased risk of experiencing poor outcomes both at follow-up at 2 and 7 years. |
| Other | ||||||||
| Costa | 2014 | To evaluate the acute effect of aerobic exercise on the BDNF levels in adults with Chagas heart disease and the relationship between BDNF and ventricular dysfunction and exercise intensity. | Case control | Fair | 30 patients with Chagas heart disease (16 non-dilated, 14 dilated) Age M 47.9 (8.7) 33.0 % women |
Clinic | Serum BDNF levels • Collected immediately after exercise |
There was a significant decrease in serum BDNF levels after acute exercise across groups. The non-dilated group showed a significant decrease in serum BDNF levels. Patients who completed exercise at a high intensity had a significant decrease in serum BDNF levels compared to patients at moderate intensity. |
| Ejiri | 2005 | To examine neurotrophin plasma levels in development of CAD and compare the levels in coronary circulation in with stable and unstable angina and without CAD | Cross-sectional | Fair | 107 adults (45 with stable angina, 38 with unstable angina, 24 without CAD) Age M 64.8 36% women |
Hospital | Plasma BDNF levels • Obtained from human coronary arteries |
The difference in BDNF levels between the coronary sinus and aorta was significantly greater in adults with unstable angina group compared with adults the stable angina and adults without CAD. |
| Sanchez-DeToledo | 2014 | To determine the utility of non-invasive bedside neuromonitoring, including serum biomarkers (including serum BDNF), in identifying children at risk from adverse neurological outcome after heart surgery. Hypothesis: Two non-invasive neurological monitoring tools (including serum BDNF) would predict short-term adverse neurological outcomes. | Longitudinal 16 hours, 12 months |
Good | 36 children undergoing heart surgery with cardiopulmonary bypass Age Mdn 8 months (IQR = 1–20) |
Hospital | Serum BDNF levels • Collected at baseline, immediately after and 16 hours after surgery |
There were no significant differences in the levels of serum BDNF at baseline and immediately after and 16 hours after surgery between children with adverse neurological outcomes and children with good neurological outcomes at 12 months after surgery. |
| Schutte | 2016 | To investigate associations between cardiometabolic risk markers, cortisol and cortisol:BDNF ratio in a bi-ethnic cohort | Cross-sectional | Fair | 406 adults Age M 44.7 (9.5) 50.7% women 51.5% White 58.5% African |
Clinic | Cortisol:serum BDNF ratio | Cortisol levels were lower in African men. BDNF was lower in African women compared to White women. In Africans (particularly men), higher cortisol:BDNF ratios were significantly associated with hyperglycemia, elevated 24-hour blood pressure, and silent ischemia. |
As reported in the study publications.
Quality ratings were determined by the National Heart, Lung, and Blood Institute Study Quality Assessment Tools, with the appropriate tool chosen by the design.
Adjusted model findings reported as available.
Note. BDNF = brain-derived neurotrophic factor. CAD = coronary artery disease. NYHA = New York Heart Association Classification. STEMI = ST-Elevation Myocardial Infarction. BMI = body mass index. CHD = coronary heart disease
Table 3.
Nineteen Articles with Cardiovascular-related Dependent Variables and BDNF Independent Variables
| First Author | Year | Study Purpose/Hypothesisa | Design/Follow-up | Quality Ratingb | Sample | Setting | Primary Dependent Variablea | BDNF Measure | BDNF-related Findingsc |
|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular Events | |||||||||
| Greisenegger | 2015 | To validate the association of a panel of biomarkers with all-cause death, despite the absence of associations with risk of non-fatal vascular events | Longitudinal Mdn = 6.4 years (IQR = 3.6–8.4) |
Fair | 929 patients with TIA or minor ischemic stroke Age Mdn 74 51.0% women |
Hospital and/or clinic | Vascular-related death | Serum BDNF levels | Serum BDNF levels were not significantly associated with vascular death. Serum BDNF levels were not predictive of recurrent non-fatal ischemic stroke or myocardial infarction. There was no significant difference in serum BDNF levels between survivors and non-survivors. |
| Jiang, R | 2017 | To examine the association of the Val66Met single-nucleotide polymorphism with CVD clinical outcomes. Hypothesis: We hypothesized that the Val/Val genotype would be associated with increased CAD and incidence of CVD mortality. |
Longitudinal Mdn = 5.9 years |
Fair | 5510 adults Age M 62.5 (SD = 11.5) 34.0% women 100% White |
Hospital (cardiac catheterize-tion laboratory) | CVD event incidence (death from any cause or MI) Severity of CAD |
BDNF gene | The Val/Val genotype was not significantly associated with CVD events. CAD severity was significantly higher in adults with Val/Val genotype than having at least one Met allele. |
| Jiang, H | 2011 | To examine the association of plasma levels of BDNF and c-reactive protein, and risk factors of cardiovascular dysfunction and prognosis in patients with angina pectoris. | Longitudinal Mdn = 48 months |
Fair | 885 adults with angina pectoris Age M 62.1 (SD = 10.5) 32.6% women |
Clinic | Major coronary event (acute myocardial infarction, sudden cardiac death, sudden death) | Plasma BDNF levels • Collected in the morning |
Lower plasma BDNF levels were significantly associated with 4-year major coronary events. The lowest tertile of plasma BDNF level was associated with a significantly higher probability of all-cause mortality than the middle and high tertiles. |
| Jiang, H | 2009 | To examine whether the BDNF Val66Met polymorphism is associated with CAD. | Case control | Good | 1168 adults (628 unstable angina pectoris, 276 stable angina pectoris, 513 control participants) Age M 62.8 (10.1) 33.3% women |
Hospital | Major coronary event (acute myocardial infarction, sudden cardiac death, sudden death) | Plasma BDNF levels BDNF gene |
Patients with unstable angina had lower plasma BDNF levels than other groups. The Met/Met genotype was significantly associated with lower occurrence of unstable angina. No significant difference of plasma BDNF levels were found between Met/Met and Val alleles (Val/Met, Val/Val) in all groups. |
| Kadowaki | 2016 | To evaluate whether serum BDNF levels can effectively provide prognostic information in CHF patients. | Case control | Fair | 157 adults (134 with chronic heart failure, 23 age matched control participants) Age M 70.6 (SD = 12.2) 39.5% women |
Clinic | Cardiac events (sudden cardiac death and death from worsening CHF, and rehospitalization for progressive heart failure) | Serum BDNF levels • Collected on admission |
Lower serum BDNF levels were significantly associated with cardiac events. Patients with CHF had significantly lower serum BDNF levels than the control participants. |
| Kaess | 2015 | To investigate the association of circulating BDNF levels with cardiovascular events and mortality in the community-based Framingham Heart Study cohort. | Longitudinal Mdn =8.5 years for CVD events, 8.9 years for all-cause mortality |
Fair | 3687 adults (669 Framingham Original cohort, 3018 offspring cohort participants) Age M 65.0 (SD = 11.0) 56.0% women |
Community | Cardiovascular events All-cause mortality |
Serum BDNF levels BDNF gene |
Higher serum BDNF levels were significantly associated with fewer cardiovascular events and lower rates of mortality. Individuals in the highest quartile of BDNF had a significantly lower adjusted risk for future CVD events and death compared with those in the lowest quartile. |
| Rahman | 2017 | To examine if serum BDNF was associated with risk of incident AF in the Framingham Heart Study. Hypothesis: lower BDNF concentrations would be associated with increased risk of developing AF prospectively. |
Longitudinal 10 years |
Fair | 3457 adults (Framingham Original and offspring cohort participants) Age M = 64.5 (SD = 11.3) 58.0% women |
Community | Atrial fibrillation incidence | Serum BDNF levels | In adjusted models, there were no statistically significant associations of serum BDNF concentrations and risk of incident AF. |
| Shibata | 2018 | To investigate whether the serum BDNF levels at discharge could predict the prognosis in patients with heart failure, and examine the relationship between BDNF and exercise tolerance. | Longitudinal 18 months |
Fair | 94 patients who were hospitalized for worsening heart failure Age M 68.0 (SD = 14.5) 36.2% women |
Hospital | Cardiac events Rehospitalization |
Serum BDNF levels • Collected in the morning |
Higher serum BDNF levels were significantly associated with a lower risk of cardiac events. Serum BDNF levels were not directly associated with peak VO2, but the combination of peak VO2 and serum BDNF levels strongly predicted early rehospitalization due to worsening HF. |
| Wu | 2019 | To see whether increased values of serum CA125 and BDNF on acute MI act as predictor for acute HF. | Case control | Fair | 160 patients with acute MI (78 with acute HF, 82 without acute HF) Age M 65.0 (SD = 8.0) 34.4% women |
Hospital | Acute HF | Serum BDNF • Collected in the morning after an overnight fast |
Combined CA125 and BDNF had high sensitivity and specificity to predict acute HF. The serum BDNF levels in the acute HF group were significantly higher than in the non-acute HF group. |
| Cardiovascular Risk Factors | |||||||||
| Fukushima | 2015 | To investigate whether serum BDNF levels were associated with prognosis in patients with heart failure. | Longitudinal Mdn = 20.3 months |
Good | 58 patients with heart failure Age M 59.2 (SD = 13.7) 25.0% women |
Hospital | Cardiorespiratory fitness (peak VO2) Adverse outcomes (all cardiac death, heart failure readmission) |
Serum BDNF levels • Collected in the morning |
Higher serum BDNF levels were significantly associated with higher peak VO2. There was a lower incidence of adverse outcomes after hospitalization related to heart failure. |
| Golden | 2010 | To measure plasma BDNF levels in a cohort of healthy middle age and older adults enrolled in the Baltimore Longitudinal Study of Aging (BLSA), and identify physiological and pathological parameters that may be correlated with plasma BDNF levels. |
Cross-sectional | Fair | 496 healthy midlife and older adults Age M 70.1 (SD = 0.7) 50.6% women |
Community | Cardiovascular risk factors (blood pressure, BMI, plasma triglycerides, total cholesterol) | Plasma BDNF levels | Higher plasma BDNF levels were found in women and older adults. In women, higher plasma BDNF levels were significantly associated with increased diastolic blood pressure, increased LDL, increased cholesterol, increased fat mass, and increased BMI. In men, higher plasma BDNF levels were associated with increased diastolic blood pressure and increased triglycerides. |
| Jung | 2011 | To examine the association between basal levels of whole circulating BDNF and cardiorespiratory fitness levels and cardiovascular disease risk factors. | Cross-sectional | Fair | 955 healthy men | Community | Cardiorespiratory fitness (peak VO2) Cardiovascular risk factors |
Serum BDNF levels | Higher serum BDNF levels were significantly associated with lower relative peak VO2 and less heart rate reserve. Higher serum BDNF levels were significantly associated with lower body mass index, lower total cholesterol, and lower triglycerides. |
| Kadoya | 2014 | To investigate the mutual relationships among plasma BDNF, patterns of nocturnal blood pressure changes (terms: “dippers, non-dippers, extra-dippers, and reverse-dippers” p. e105977), and cardiac autonomic function as determined by heart rate variability. | Cross-sectional | Poor | 250 adults with ≥1 cardiovascular risk factor Age M 58.8 (SD = 13.1) 44.1% women |
Clinic | Nocturnal blood pressure changes Heartrate variability Cardiovascular risk factors |
Plasma BDNF levels • Collected in the morning |
Lower plasma BDNF levels were significantly associated with a larger decrease in nocturnal blood pressure, and were significantly lower in reverse-dippers compared to dippers. Plasma BDNF levels were significantly positively associated with all of the time-domain measurements of heartrate variability. |
| Kurajoh | 2017 | To examine the relationship between plasma BDNF concentration and future development of chronic kidney disease in patients with cardiovascular risk factors as part of the Hyogo Sleep Cardio-Autonomic Atherosclerosis cohort study. | Longitudinal Mdn = 37 months |
Fair | 324 adults with cardiovascular risk factors Age M 57.9 (SD = 13.0) 45.7% women |
Clinic | Chronic kidney disease development | Plasma BDNF levels | Higher plasma BDNF levels were significantly associated with lower risk of chronic kidney disease development. |
| Lee | 2018 | To examine the relationship between serum BDNF levels and changes in pulse pressure after a one-hour rest period in women nurses working night shifts. | Longitudinal 8 weeks |
Fair | 48 women nurses Age M 29.0 (SD = 5) |
Hospital | Resting pulse pressure | Serum BDNF levels • Collected in the morning |
Higher serum BDNF levels were significantly associated with narrower pulse pressure. Serum BDNF levels during the first month of night shift were significantly and inversely correlated with the change in pulse pressure. Higher serum BDNF was significantly associated with a narrowing of in pulse pressure after a one-hour rest. |
| Pedersen | 2017 | To examine the association between serum BDNF and a composite z-score consisting of six cardiovascular risk factors. A secondary aim was to examine the associations between serum BDNF and each of the six risk factors. | Cross-sectional | Fair | 447 adolescents in grades 6 to 11 Age M 14.1 (SD = 1.3) 48% women |
School | Combined score of the following cardiovascular risks: • HDL (inverse) • Blood pressure • Insulin resistance • Waist circumference • Total cholesterol • Cardiorespirato-ry fitness (inverse) |
Serum BDNF levels • Collected in the morning after an overnight fast |
Higher serum BDNF levels were significantly associated with a higher composite score of increased cardiovascular risk. When the analyses were dichotomized by sex, BDNF levels were positively associated with the composite score for males, but the association was not significant for female. Gender and serum BDNF did not interact in predicting the composite score. |
| Van Vuren | 2019 | To assess prospective associations between BDNF, cardiac myocyte injury (cTnT) and cognitive interference in a bi-ethnic cohort. Hypothesis: Inverse associations will exist between changes in BDNF, changes in cTnT and cognitive interference in adults of Black race. |
Longitudinal 3 years |
Fair | 338 adults (186 White, 152 Black) Age M 41.3 (SD = 9.2) 55.0% White 45.0% Black |
Clinic | Serum cTnT | Serum BDNF • Collected in the morning after an overnight fast |
Adults of White race had significantly higher BDNF levels, cTnT levels, and cognitive interference scores compared to adults of Black race. No significant associations existed between BDNF, cTnT, or cognitive inference in whites. In adults of Black race, increased cTnT levels were positively associated with percent change in BDNF in adults of Black race. In adults of Black race only, increases in BDNF increased the likelihood for chronic lower cTnT levels. |
| Zembron-Lacny | 2016 | To identify the age-related changes in peripheral BDNF and its relationship to oxidative damage and conventional health markers in active and inactive men. | Case control | Poor | 34 (17 older men and 17 young men) Age m 71.2 (SD = 5.1) in older men Age m 21.5 (SD = 1.5) in young men |
University | CVD risk factors (Framingham CVD risk scores, lipid profile, cardiorespiratory fitness, and oxidative stress markers) | Plasma BDNF levels • Collected in the morning |
Higher plasma BDNF levels were significantly related with fewer CVD risk factors. Young men had higher plasma BDNF than older men. Across both age groups, active men had higher plasma BDNF than inactive peers. |
| Other | |||||||||
| Szabo | 2013 | To examine the associations of BDNF genotype with cognitive function among individuals with CVD. | Cross-sectional | Fair | 110 adults with CVD Age M 68.0 (7.6) 41% women |
Clinic | Cognition: • General cognition • Memory • Language • Attention • Executive function • Visuospatial |
BDNF gene | Having at least one Met allele was significantly associated with better attention, executive function, and memory. There were no between group differences between men and women. |
As reported in the study publications.
Quality ratings were determined by the National Heart, Lung, and Blood Institute Study Quality Assessment Tools, with the appropriate tool chosen by the design.
Adjusted model findings reported as available.
Note. BDNF = brain-derived neurotrophic factor. CVD = cardiovascular disease. TIA = transient ischemic attack. MI = myocardial infarction. CAD = coronary artery disease. CHF = chronic heart failure. HF = heart failure. AF = atrial fibrillation. CA125 = protein expressed in response to inflammatory mediators. BMI = body mass index. LDL = low-density lipoproteins. HDL = high-density lipoproteins. cTnT = cardiac Troponin T.
In the 35 articles, sample sizes ranged from 20 to 5,510 participants (M = 546 participants, SD = 119). Of the 35 articles, 33 included adult participants (M age = 61.2 years) and 2 included pediatric patients (M age = 12.8 years; Pedersen et al., 2017; Sanchez-de-Toledo et al., 2014). Twenty-five of the 35 articles included both men and women, while 4 articles included only men and 1 article included only women. If both genders were included, on average there were 46.4% women (range = 22–81.8% women). Five articles reported race or ethnicity, and the majority of those samples were 89.1% White on average. Study settings were clinic (n = 17, 52%), hospital (n = 10, 30%), community (n = 4, 12%), and school (n = 2, 6%).
BDNF Levels and BDNF Genotype Frequency
Results of measures of BDNF levels (serum or plasma) and BDNF genotype frequency in the 35 retrieved articles are displayed in Table 4. Across studies, serum BDNF levels ranged from 0.8 ng/ml among patients with minor ischemic stroke (Greisenegger et al., 2015) to 43.2 ng/ml among a sample of healthy men (I.-T. Lee et al., 2012). Across 9 studies, the BDNF genotype frequency for Val allele homozygotes (Val/Val) ranged from 26.4% among participants with stable angina of Han descent in China (H. Jiang et al., 2009) to 70% among participants with cardiovascular disease and primarily of White race in the U.S. (Szabo et al., 2013). The BDNF genotype frequency for Val allele heterozygotes (Val/Met) ranged from 22.2% among participants with heart failure and in studies conducted in predominantly White race in the U.S. (Pressler et al., 2017) to 54.6% among participants with unstable angina of Han descent in China (H. Jiang et al., 2009). The BDNF genotype frequency for Met allele homozygotes (Met/Met) ranged from 3.9% among participants with coronary artery disease and White race in Croatia (Sustar et al., 2016) to 25.0% among healthy control participants of Han descent in China (H. Jiang et al., 2009).
Table 4.
BDNF Levels and BDNF Genotype Frequency in the Retrieved Articles (N = 35)
| First Author | Year | Country (sampling area) | Sample | BDNF M (SD)/Mdn (IQR) | BDNF Genotype N (%) | SNPs Location |
|---|---|---|---|---|---|---|
| Serum BDNF | ||||||
| Bus | 2011 | Netherlands | 1168 adults with subthreshold depressive or anxiety symptoms, or had high risk | Total sample M (SD): 8.98 (3.1) ng/mL | N/A | N/A |
| Chaldakov | 2004 | Bulgaria | 33 adults (23 with metabolic syndrome, 10 healthy without metabolic syndrome) | N/A | N/A | N/A |
| Costa | 2014 | Brazil | 30 patients with Chagas heart disease (16 non-dilated, 14 dilated) | Patients with non-dilated Chagas M (SD): 15.1 (4.5) ng/mL at rest 12.8 (4.6) ng/mL after exercise Patients with dilated Chagas M (SD): 13.2 (3.5) ng/mL at rest 10.9 (5.4) ng/mL after exercise |
N/A | N/A |
| Fukushima | 2015 | Japan | 58 patients with heart failure | Total sample M (SD): 19.0 (5.6) ng/mL | N/A | N/A |
| Greisenegger | 2015 | United Kingdom | 929 patients with TIA or minor ischemic stroke | Total sample: Mdn (IQR) 0.8 (0.5–1.3) ng/mL | N/A | N/A |
| Jung | 2011 | South Korea | 955 healthy men | 1st quintile of VO2max M (SD) : 14.7 (3.2) ng/mL 2nd quintile of VO2max M (SD): 13.9 (3.4) ng/mL 3rd quintile of VO2max M (SD): 12.9 (4.0) ng/mL 4th quintile of VO2max M (SD): 12.4 (3.7) ng/mL 5th quintile of VO2max: 10.4 (4.0) ng/mL |
N/A | N/A |
| Kadowaki | 2016 | Japan | 157 adults (134 with chronic heart failure, 23 age matched control participants) | Patients with chronic heart failure M (SD): 25.8 (8.4) ng/mL Control participants M (SD): 14.7 (8.4) ng/mL |
N/A | N/A |
| Kaess | 2015 | United States | 3687 adults (669 Framingham Original cohort, 3018 offspring cohort participants) | Total sample M (SD): 23.5 (8.3) ng/mL | N/A | N/A |
| Lasek-Bal | 2015 | Poland | 87 adults with ischemic stroke | Total sample M (SD): 9.96 (5.21) ng/mL Men: 10.16 (4.94) ng/mL Women: 9.77 (5.51) ng/mL |
N/A | N/A |
| Lee | 2012 | Taiwan | 58 men (34 with metabolic syndrome but not diabetes; 24 age-matched men without metabolic syndrome) | Men with metabolic syndrome M (SD): 40.9 (8.0) ng/mL Control participants M (SD): 43.2 (6.1) ng/mL |
N/A | N/A |
| Lee | 2018 | Taiwan | 48 women nurses | Total sample M (SD): 21.6 (6.2) ng/mL | N/A | N/A |
| Lorgis | 2010 | France | 40 adults (20 with acute myocardial infarction, 20 age- and gender-matched with stable angina) | Patients post myocardial infarction M (SD): 1.7 (0.7) ng/mL Patients with stable angina M (SD): 0.9 (0.4) ng/mL |
N/A | N/A |
| Pedersen | 2017 | Denmark | 447 adolescents in grades 6 to 11 | Women M (SD): 26.98 (6.05) ng/mL Men M (SD): 27.01 (6.34) ng/mL |
N/A | N/A |
| Pressler | 2015 | United States | 27 adults with heart failure | Intervention group M (SD): 8.4 (4.6) ng/mL Control group M (SD): 9.5 (3.5) ng/mL |
Val/Val 17 (63%) Val/Met 6 (22.2%) Met/Met 2 (7.4%) 2 (7.4%) could not be analyzed |
rs6265
Val66Met |
| Pressler | 2017 | United States | 26 adults with heart failure | Participants with APOE
ε4 absent M (SD): 8.7 (3.7) ng/mL at baseline 8.9 (4.7) ng/mL at 8 weeks Participants with APOE ε4 present M (SD): 10.5 (4.0) ng/mL at baseline 11.1 (6.7) ng/mL 8 weeks |
Participants with APOE
ε4 absent: Val/Val 10 (55.6%) Val/Met + Met/Met 8 (44.4%) Participants with APOE ε4 present: Val/Val 7 (100%) Val/Met + Met/Met 0 (0%) |
rs6265
Val66Met |
| Rahman | 2017 | United States | 3457 adults (Framingham Original and offspring cohort participants) | Total sample Mdn: 23.4 ng/mL | N/A | N/A |
| Sanchez-DeToledo | 2014 | United States | 36 children undergoing heart surgery with cardiopulmonary bypass | Total sample at baseline M (SE): 3.2 (0.7) ng/mL | N/A | N/A |
| Schutte | 2016 | South Africa | 406 adults | White adults M (SD): 1.7 (0.9) ng/mL Black adults M (SD): 1.4 (0.7) ng/mL |
N/A | N/A |
| Shibata | 2018 | Japan | 94 patients who were hospitalized for worsening heart failure | Patients with cardiac events at discharge M (SD): 18.3 ng/mL | N/A | N/A |
| Stanne | 2016 | Sweden | 491 post-stroke cases, 513 case control participants (1004 total) |
Patients post-stroke M (SD): 18.3 ng/mL Control participants M (SD): 23.9 ng/mL |
N/A | N/A |
| Van Vuren | 2019 | South Africa | 338 adults (186 White, 152, Black) | White adults M (SD): 1.7 (0.9) ng/mL Black adults M (SD): 1.5 (0.7) ng/mL |
N/A | N/A |
| Wu | 2019 | China | 160 patients with acute MI (78 with acute HF, 82 without acute HF) | N/A | N/A | N/A |
| Zhang | 2015 | China | 20 men (10 patients with STEMI, 10 healthy controls) | N/A | N/A | N/A |
| Plasma BDNF | ||||||
| Ejiri | 2005 | Japan | 107 adults (24 adults without CAD, 45 adults with stable angina, 38 adults with unstable angina) | Adults with stable angina Mdn (IQR): Coronary sinus 1.2 ng/mL (0.7–1.6) Aortic root 1.2 ng/mL (0.8–1.8) Peripheral vein 1.3 ng/mL (0.8–1.7) Adults with unstable angina Mdn (IQR): Coronary sinus 1.5 ng/mL (1.1–2.4) Aortic root 1.3 ng/mL (0.8–1.8) Peripheral vein 1.3 ng/mL (0.8–1.7) Adults without CAD Mdn (IQR): Coronary sinus 1.3 ng/mL (0.8–1.7) Aortic root 1.3 ng/mL (0.8–1.7) Peripheral vein 1.3 ng/mL (0.7–1.9) |
N/A | N/A |
| Golden | 2010 | United States | 496 healthy midlife and older adults | N/A | N/A | N/A |
| Jiang, H | 2011 | China | 885 adults with angina pectoris | N/A | N/A | N/A |
| Kadoya | 2014 | Japan | 250 adults with ≥1 cardiovascular risk factor | Reverse blood pressure dippers: ln(BDNF)a 7.2 (0.7) pg/mL Non-dippers: ln(BDNF) 7.7 (0.9) pg/mL |
N/A | N/A |
| Kurajoh | 2017 | Japan | 324 adults with cardiovascular risk factors | Total sample: ln(BDNF) 7.7 (0.8) pg/mL | N/A | N/A |
| Takashio | 2015 | Japan | 242 heart failure patients, 80 case control participants | Patients with heart failure M (SD): 3.7 ng/mL Control participants M (SD): 7.3 ng/mL |
N/A | N/A |
| Zembron-Lacny | 2016 | Poland | 34 (17 older men and 17 young men) | Young men M (SD): 1.6 (0.4) ng/mL Older men M (SD): 1.4 (0.3) ng/mL |
N/A | N/A |
| BDNF Genotype | ||||||
| Bozzini | 2009 | Italy | 242 adults (99 patients with CAD, 143 healthy control participants) | N/A | Adults with coronary artery disease (n = 99): Val/Val 53 (53.5%) Val/Met 30 (30.3%) Met/Met 16 (16.1%) |
rs6265
Val66Met |
| Jiang, H | 2009 | China | 1168 adults (628 unstable angina pectoris, 276 stable angina pectoris, 513 control participants) | N/A | Adults with stable angina: Val/Val 73 (26.4%) Val/Met 135 (48.9%) Met/Met 68 (24.6%) Adults with unstable angina: Val/Val 181 (28.8%) Val/Met 343 (54.6%) Met/Met 104 (16.6%) Control participants: Val/Val 139 (27.1%) Val/Met 246 (48.0%) Met/Met 128 (25.0%) |
rs6265
Val66Met |
| Jiang, R | 2017 | United States | 5510 adults | N/A | Val/Val 3627 (65.8%) Val/Met + Met/Met 1883 (34.2%) |
rs6265
Val66Met |
| Pressler | 2015 | United States | 27 adults with heart failure | Intervention group M (SD): 8.4 (4.6) ng/mL Control group M (SD): 9.5 (3.5) ng/mL |
Val/Val 17 (63%) Val/Met 6 (22.2%) Met/Met 2 (7.4%) 2 (7.4%) could not be analyzed |
rs6265
Val66Met |
| Pressler | 2017 | United States | 26 adults with heart failure | Participants with APOE
ε4 absent M (SD): 8.7 (3.7) ng/mL at baseline 8.9 (4.7) ng/mL at 8 weeks Participants with APOE ε4 present M (SD): 10.5 (4.0) ng/mL at baseline 11.1 (6.7) ng/mL 8 weeks |
Participants with APOE
ε4 absent: Val/Val 10 (55.6%) Val/Met + Met/Met 8 (44.4%) Participants with APOE ε4 present: Val/Val 7 (100%) Val/Met + Met/Met 0 (0%) |
rs6265
Val66Met |
| Sustar | 2016 | Croatia | 704 adults (206 with CAD, 498 healthy control participants) | N/A | Patients with coronary artery disease: Val/Val 143 (69.4%) Val/Met 55 (26.7%) Met/Met 8 (3.9%) Healthy control participants: Val/Val 337 (67.7%) Val/Met 140 (28.1%) Met/Met 21 (4.2%) |
rs6265
Val66Met |
| Szabo | 2013 | United States | 110 adults with CVD | N/A | Val/Val 77 (70%) Val/Met + Met/Met 33 (30%) |
rs6265
Val66Met |
natural logarithm.
Note. BDNF = brain-derived neurotrophic factor. CVD = cardiovascular disease. TIA = transient ischemic attack. MI = myocardial infarction. HF = heart failure. STEMI = ST-Elevation Myocardial Infarction. CAD = coronary artery disease.
BDNF Examined as a Dependent Variable
BDNF-related measures were the primary dependent variables in 16 articles (Table 2). In these 16 articles, 10 articles had measures of serum BDNF levels (Bus et al., 2011; Costa et al., 2014; Lasek-Bal et al., 2015; I.-T. Lee et al., 2012; Lorgis et al., 2010; Pressler et al., 2015, 2017; Sanchez-de-Toledo et al., 2014; Stanne et al., 2016; Zhang et al., 2015), 3 had measures of plasma BDNF levels (Chaldakov et al., 2004), and 3 had measures of BDNF gene (Bozzini et al., 2009; Pressler et al., 2015; Sustar et al., 2016). The BDNF measures were investigated in diverse cardiovascular conditions and the results are presented below by the conditions including metabolic syndrome (n = 2; Chaldakov et al., 2004; I.-T. Lee et al., 2012), heart failure (n = 3; (Pressler et al., 2015, 2017; Takashio et al., 2015), coronary artery disease (n = 2; Bozzini et al., 2009; Bus et al., 2011; Sustar et al., 2016), myocardial infarction (n = 2; Lorgis et al., 2010; Zhang et al., 2015), stroke (n = 2; Lasek-Bal et al., 2015; Stanne et al., 2016), angina (n = 1; (Ejiri et al., 2005), cardiometabolic risk factors (n = 1; Schutte et al., 2016), Chagas heart disease (n = 1; Costa et al., 2014), or pediatric heart surgery (n = 1; Sanchez-de-Toledo et al., 2014). Of these 16 articles, 14 were rated as “good” or “fair” and were included in the synthesis of main findings.
Serum and Plasma BDNF Levels
Metabolic syndrome. There was no significant difference in serum BDNF levels between patients with elevated risk for cardiovascular disease due to metabolic syndrome and healthy control participants (I.-T. Lee et al., 2012). Myocardial infarction. In two articles, serum BDNF levels were significantly higher in patients who had myocardial infarction within the last 24 hours compared to control participants (Lorgis et al., 2010; Zhang et al., 2015). Heart failure. Among patients with heart failure, plasma BDNF levels were significantly lower compared with control participants, and levels were even lower with increased disease severity (Takashio et al., 2015). Among patients with heart failure, Pressler and colleagues (2015) found significantly increased BDNF levels among patients who completed a cognitive training intervention compared to patients who completed the education attention control intervention, regardless of BDNF Met status (Pressler et al., 2015). Stroke. Among patients with stroke, lower serum BDNF levels were related to lower functional status 90 days post-stroke (Lasek-Bal et al., 2015), and to higher risk of poor outcomes at two and seven years (Stanne et al., 2016). Angina. Among patients with unstable angina, plasma BDNF levels obtained from the coronary sinuses and aortas were significantly higher compared to patients with stable angina and healthy control participants (Ejiri et al., 2005). Cardiometabolic risk factors. Schutte and colleagues (2016) found that higher cortisol:BDNF ratios were associated with increased cardiometabolic risk factors (i.e., blood pressure, silent ischemia). Chagas heart disease. Among patients with Chagas heart disease, patients who underwent a single exercise session had lower serum BDNF levels post-exercise. Pediatric heart surgery. There were no significant differences in BDNF levels before or after heart surgery in pediatric patients with differing neurological post-operative outcomes (Sanchez-de-Toledo et al., 2014).
BDNF Genotype
Coronary artery disease. Among patients with coronary artery disease, Bozzini and colleagues (2009) found the percentage of patients with Met/Met genotype was significantly higher compared to healthy control participants, while Sustar and colleagues (2016) found no significant difference between patients with coronary artery disease and healthy control participants. Heart failure. Among a small sample of 26 patients with heart failure, increases in serum BDNF following a cognitive training intervention did not differ between genotypes (Pressler et al., 2017).
In sum, serum BDNF was the most commonly measured form of BDNF in articles with BDNF-related dependent variables. BDNF levels were significantly lower among samples of patients with heart failure and patients with poor stroke-related outcomes, and significantly higher among patients with unstable angina and recent myocardial infarction. Articles in which the BDNF Val66Met polymorphism were examined in persons with serious cardiovascular conditions were few and results were variable across studies, mainly due to differences in race/ethnicity of the measured populations, limiting ability for synthesis.
BDNF Examined as an Independent Variable
A total of 19 articles examined the BDNF-related measure as the independent variable to explain or phenotype cardiovascular conditions (Table 3). In these 19 articles, 11 articles had measures of serum BDNF levels (Fukushima et al., 2015; Greisenegger et al., 2015; Jung et al., 2011; Kadowaki et al., 2016; Kaess et al., 2015; I.-T. Lee et al., 2018; Pedersen et al., 2017; Rahman et al., 2017; Shibata et al., 2018; Vuren et al., 2019; Wu et al., 2019), 6 had measures of plasma BDNF levels (Golden et al., 2010; H. Jiang et al., 2009; Kadoya et al., 2014; Kurajoh et al., 2017; Zembron-Lacny et al., 2016), and 3 had measures of BDNF gene (H. Jiang et al., 2009; R. Jiang et al., 2017; Szabo et al., 2013). Of the 19 articles, 10 were focused on possible influences of BDNF on cardiovascular events (e.g., cardiovascular disease event incidence or atrial fibrillation incidence), 8 on cardiovascular risk factors (e.g., blood pressure, body mass index), and 1 on cognitive function in adults with cardiovascular disease. Of these 19 articles, 17 were rated as “good” or “fair” and were included in the synthesis of main findings.
Serum and Plasma BDNF Levels
Cardiovascular events.
Lower serum BDNF levels were associated with higher incidence of cardiovascular events among in a sample of 3687 persons from the Framingham Heart Study cohort (Kaess et al., 2015). Among patients with heart failure, lower serum BDNF levels were related to higher risk of cardiac events (Kadowaki et al., 2016; Shibata et al., 2018). Among patients with angina, H. Jiang and colleagues (2009, 2011) found that lower levels of plasma BDNF were predictive of a major coronary event among persons living in China. Among patients with acute myocardial infarction, higher levels of both BDNF and CA125 (high-molecular weight binding mucin related to inflammatory processes) predicted acute heart failure. Two studies did not have significant findings. Serum BDNF levels were not predictive of atrial fibrillation incidence in the Framingham cohort (Rahman et al., 2017), or of vascular-related death among patients with minor ischemic stroke (Greisenegger et al., 2015).
Cardiovascular risk factors.
Among samples of healthy individuals (adults and adolescents), higher levels of serum or plasma BDNF were associated with a higher index of cardiovascular risk factors, including body composition and cholesterol (Golden et al., 2010; Pedersen et al., 2017). Moreover, higher serum BDNF levels were related to narrowing pulse pressure in nurses working night shifts (I.-T. Lee et al., 2018). Among adults that had elevated cardiovascular risk, higher plasma BDNF levels were significantly associated with decreased risk of developing chronic kidney disease (Kurajoh et al., 2017). Among healthy men, lower BDNF levels were associated with better cardiorespiratory fitness (Jung et al., 2011). Conversely, higher serum BDNF levels were associated with better cardiorespiratory fitness among patients with heart failure (Fukushima et al., 2015).
BDNF Genes
Among patients with angina, H. Jiang and colleagues (2009, 2011) found that the BDNF Met/Met genotype was associated with the lower rates of unstable angina among people of Chinese Han descent. Similarly, in a sample of 5510 adult patients, the Val/Val genotype (that inhibits BDNF production and release) was associated with a higher incidence of major coronary events (R. Jiang et al., 2017). One cross-sectional study focused on cognition-related outcomes (i.e., neurocognitive measures of attention/executive function, memory, and language) in participants with cardiovascular disease, and Szabo and colleagues (2013) found that having at least one Met allele was significantly associated with better attention/executive function and memory among people in the U.S. with cardiovascular disease, but not with global cognitive function, which was acknowledged as contrary to past studies.
In sum, serum BDNF was the most commonly measured form of BDNF in articles with cardiovascular-related dependent variables. Findings were overall mixed, and 17 articles had significant findings while two did not. The articles with significant findings reported that lower BDNF levels were predictive of cardiovascular events, specifically major coronary events (e.g., myocardial infarction, acute ischemic heart failure). Conversely, in patients with myocardial infarction, authors speculated that higher serum BDNF levels were related to inflammatory processes predicting acute heart failure. Higher serum BDNF levels were significantly associated with higher cardiovascular risk in adults and adolescents without existing serious cardiovascular conditions, though findings on the association between BDNF levels and cardiorespiratory fitness were mixed.
Summary of Synthesis
Overall, the synthesized results of the 31 articles support three main conclusions. First, BDNF levels appeared to be higher in acute cardiovascular conditions, such as myocardial infarction and unstable angina, but lower in chronic cardiovascular conditions, such as heart failure. Second, higher BDNF levels were related to increases in certain cardiovascular risk factors (e.g., cholesterol, body composition) in healthy participants, while lower levels were related to increased likelihood of cardiovascular events in participants with existing cardiovascular conditions. Finally, the variations in BDNF genotype frequencies across samples may reflect differences in population genetic structure among people in different countries and of different races and ethnicities.
DISCUSSION
In this integrative review, we identified 35 articles representing 30 studies that had the following results: (a) different levels of serum or plasma BDNF or BDNF genotypes among diverse serious cardiovascular conditions; and (b) BDNF was a predictor of cardiovascular outcomes, including events or risk factors. Findings from the 31 articles that received “good” or “fair” quality ratings were synthesized, while the 4 articles that received “poor” quality ratings were excluded due to potential methodological issues and potential bias. Overall, compared with control participants, patients with heart failure and stroke had lower BDNF levels while patients with unstable angina and myocardial infarction had higher BDNF levels. In addition, lower BDNF levels were predictive of cardiovascular events, specifically major coronary events. In healthy samples, however, higher BDNF levels appeared to be generally associated with increased cardiovascular risk (e.g., body mass index, blood pressure). Findings regarding BDNF genotype were mixed across race/ethnicity and cardiovascular conditions.
In two of the retrieved articles, investigators examined the role of BDNF in acute inflammatory processes that may be related to serious cardiovascular conditions. In these studies, BDNF levels were higher in patients with myocardial infarction compared to patients with stable angina. Higher BDNF levels were positively associated with higher soluble platelet selectin (sP-selectin (Lorgis et al., 2010), a cell adhesion molecule that has been proposed as a biomarker for acute inflammation (Schrijver et al., 2017). Also in patients after myocardial infarction, Wu and colleagues (2019) found that patients who developed acute heart failure had higher BDNF and CA125 (protein expressed in response to inflammatory mediators) levels, potentially indicating increased inflammatory processes. This finding is consistent with earlier pre-clinical animal models that showed BDNF levels in the heart were acutely elevated following myocardial infarction (Hang et al., 2015). Other pre-clinical models of myocardial infarction showed that BDNF assists in repair processes (Calabrese et al., 2014), and higher BDNF levels after cardiac injury were related to less chronic cardiac dysfunction (Halade et al., 2013; Okada et al., 2012). Thus, this acute elevation of BDNF as a response to cardiac injury may be beneficial for recovery, but this proposition needs further testing. Alternatively, chronic elevation of BDNF because of long periods of inflammation may be related to increased cardiovascular risk. In clinical populations, chronic inflammation that can occur in obesity is potentially associated with the development of metabolic syndrome and cardiovascular disease (Park et al., 2017; Saltiel & Olefsky, 2017). Taken together, there is likely an interrelationship between both acute and chronic elevation BDNF and inflammation with the potential for important effects on cardiovascular health and outcomes.
Synthesis of the results from this integrative review must be interpreted cautiously because the majority of articles received quality ratings of “fair”. Many articles lacked a clear purpose statement and/or hypothesis. Related to this lack of purpose and/or hypothesis, some articles had underdeveloped scientific premise for the aims. Although scientific premise is not specifically rated in the NHLBI quality assessment methodology, an underdeveloped scientific premise may lead to poor specification of aims and lack of scientific rigor. In addition, not all articles reported detailed inclusion and exclusion criteria for the sample and/or control groups. No studies specifically addressed the importance of differences in genetic population structure that may have influenced results. Moreover, most articles considered confounders in analyses, but the selected confounders differed across studies. Confounders in the retrieved articles were commonly limited to demographics only (e.g., age, ethnic group, sex), and not all articles controlled for depression or pain, which deleteriously alters BDNF levels in adult populations (Phillips, 2017; Zhao et al., 2018). None of the retrieved articles considered physical activity level as a confounder, which is associated with increased levels of serum and plasma BDNF (Coelho et al., 2013; Dinoff et al., 2017). Finally, BDNF was analyzed as both a predictor and an outcome of cardiovascular conditions across the articles, highlighting the emerging nature of the science surrounding the biology of BDNF in serious cardiovascular conditions.
Importantly, most of the studies that addressed the frequency distributions of the BDNF rs6265 SNP Val and Met alleles did not interpret results in the context of differences across population genetic structure (Petryshen et al., 2019) that could account for differences in allelic frequency. The rather large differences in the BDNF Met allele frequencies ranging from 0 to 72% have implications for sample selection, BDNF measurement, and interpretation of results. Moreover, while we know there are differences in the genotype, it is not well understood how this might affect the phenotype across populations. Thus, additional studies are needed to fully understand the differences in genetic population structure of BDNF and its phenotypic expression and relationship to outcomes. Most importantly, acknowledgement of the frequency distribution of the Met allele within the population under study is crucial for interpretation of the results.
A strength across the retrieved articles was that most followed validated methods for assessing BDNF levels. However, the reported methods varied across studies, which limited the ability to synthesize results. There are multiple methods available to measure protein levels of BDNF in serum or plasma, including western blot analysis, enzyme-linked immunoabsorbant assay (ELISA), mass spectrometry and others. Of these, mass spectrometry is the most sensitive detection method, but may not be practical for many labs due to cost and resources. The next most sensitive measure is ELISA, which many consider the standard for BDNF measurement. For measurement of gene expression and SNP detection, standard methods are used including qPCR or next-generation sequencing for gene expression and either candidate Taqman probes for SNP detection or exome/whole genome sequencing (Git et al., 2010). Plasma BDNF levels have shown diurnal fluctuation in healthy human participants (Begliuomini et al., 2008; Pluchino et al., 2009). Thus, researchers must use consistent BDNF measurement across studies, including consistent time of day and serum BDNF analysis, using ELISA methodology (Polacchini et al., 2015).
This integrative review had three limitations. First, this review was not a systematic review. Although this integrative review methodology is advantageous given the developing nature of the topic of BDNF roles in serious cardiovascular conditions, there is a risk that we did not exhaustively capture studies from all databases. In addition, the 35 retrieved articles represented 30 studies, with multiple articles utilizing data from a single study, which may have increased bias. Second, the differing designs and methods, particularly the varied dependent variables and BDNF measures, made it difficult to draw conclusions across studies. These articles examined BDNF in a variety of serious cardiovascular conditions and cardiovascular risk. Third, the samples of the studies retrieved were limited to mainly adult patients in the clinic and hospital settings. The majority of samples were composed of White midlife adults (80% or more were White).
To our knowledge, this is one of the first reviews to examine the existing literature focused on the relationship between BDNF and serious cardiovascular conditions in human participants. Our exploratory synthesis of results points to important gaps in the current knowledge. First, there is an urgent need for additional investigation on the cardiovascular condition phenotypes in relationship to both BDNF levels and BDNF genotypes within the same study conditions. Second, researchers should assess change in BDNF levels as a response to interventions in randomized controlled trials with cardiovascular populations, which would also help elucidate the complexity of BDNF among serious cardiovascular conditions. Third, there is a need to investigate the interrelationships of BDNF and inflammatory biomarkers in cardiovascular patient populations with and without inflammatory processes. Finally, all future studies should include detailed information about the sample and population genetic structure, standardized measures of BDNF, including time of day collected, and should control for confounders commonly known to influence BDNF, such as depression and pain.
Footnotes
Conflict of Interest: The authors have no conflicts of interest.
Contributor Information
Shannon Halloway, College of Nursing, Rush University, Chicago, IL.
Miyeon Jung, Indiana University School of Nursing, Indianapolis, IN.
An-Yun Yeh, Hunter-Bellevue School of Nursing, New York, NY.
Jia Liu, Indiana University School of Nursing, Indianapolis, IN.
Ellen McAdams, Indiana University-Purdue University, Indianapolis, IN.
Maddison Barley, Indiana University School of Nursing, Indianapolis, IN.
Susan G. Dorsey, Department of Pain and Translational Symptom Science School of Nursing, University of Maryland, Baltimore, MD.
Susan J. Pressler, Sally Reahard Chair and Director of the Center for Enhancing Quality of Life in Chronic Illness, Indiana University School of Nursing, Indianapolis, IN.
References
- Bathina S, & Das UN (2015). Brain-derived neurotrophic factor and its clinical implications. Archives of Medical Science : AMS, 11(6), 1164–1178. 10.5114/aoms.2015.56342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begliuomini S, Lenzi E, Ninni F, Casarosa E, Merlini S, Pluchino N, Valentino V, Luisi S, Luisi M, & Genazzani AR (2008). Plasma brain-derived neurotrophic factor daily variations in men: Correlation with cortisol circadian rhythm. The Journal of Endocrinology, 197(2), 429–435. 10.1677/JOE-07-0376 [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway, Carson AP, … Virani SS (2019). Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation, 139(10), e56–e528. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- Bozzini S, Gambelli P, Boiocchi C, Schirinzi S, Falcone R, Buzzi P, Storti C, & Falcone C. (2009). Coronary artery disease and depression: Possible role of brain-derived neurotrophic factor and serotonin transporter gene polymorphisms. International Journal of Molecular Medicine, 24(6), 813–818. [DOI] [PubMed] [Google Scholar]
- Bus B. a. A., Molendijk ML, Penninx BJWH, Buitelaar JK, Kenis G, Prickaerts J, Elzinga BM, & Voshaar RCO (2011). Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology, 36(2), 228–239. 10.1016/j.psyneuen.2010.07.013 [DOI] [PubMed] [Google Scholar]
- Calabrese F, Rossetti AC, Racagni G, Gass P, Riva MA, & Molteni R. (2014). Brain-derived neurotrophic factor: A bridge between inflammation and neuroplasticity. Frontiers in Cellular Neuroscience, 8. 10.3389/fncel.2014.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaldakov GN, Fiore M, Stankulov IS, Manni L, Hristova MG, Antonelli A, Ghenev PI, & Aloe L. (2004). Neurotrophin presence in human coronary atherosclerosis and metabolic syndrome: A role for NGF and BDNF in cardiovascular disease? Progress in Brain Research, 146, 279–289. 10.1016/S0079-6123(03)46018-4 [DOI] [PubMed] [Google Scholar]
- Costa HS, Lima MMO, Silva MG, Alencar MCN, Nunes MCP, Camargos ERS, Martinelli PM, & Rocha MOC (2014). Effect of acute aerobic exercise on serum BDNF levels in patients with Chagas heart disease. International Journal of Cardiology, 174(3), 828–830. 10.1016/j.ijcard.2014.04.136 [DOI] [PubMed] [Google Scholar]
- Dempster E, Toulopoulou T, McDonald C, Bramon E, Walshe M, Filbey F, Wickham H, Sham PC, Murray RM, & Collier DA (2005). Association between BDNF val66 met genotype and episodic memory. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics, 134B(1), 73–75. 10.1002/ajmg.b.30150 [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, & Weinberger DR (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell, 112(2), 257–269. [DOI] [PubMed] [Google Scholar]
- Ejiri J, Inoue N, Kobayashi S, Shiraki R, Otsui K, Honjo T, Takahashi M, Ohashi Y, Ichikawa S, Terashima M, Mori T, Awano K, Shinke T, Shite J, Hirata K, Yokozaki H, Kawashima S, & Yokoyama M. (2005). Possible role of brain-derived neurotrophic factor in the pathogenesis of coronary artery disease. Circulation, 112(14), 2114–2120. 10.1161/CIRCULATIONAHA.104.476903 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Banducci SE, Weinstein AM, Angus W. MacDonald I, Ferrell RE, Halder I, … Manuck SB (2013). The brain-derived neurotrophic factor Val66Met polymorphism moderates an effect of physical activity on working memory performance. Psychological Science, 24(9), 1770–1779. 10.1177/0956797613480367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Chartier J, Sodja C, Desbois A, Ribecco-Lutkiewicz M, Walker PR, & Sikorska M. (2003). Transcriptional activation of the human brain-derived neurotrophic factor gene promoter III by dopamine signaling in NT2/N neurons. The Journal of Biological Chemistry, 278(29), 26401–26409. 10.1074/jbc.M211539200 [DOI] [PubMed] [Google Scholar]
- Finnell JE, & Wood SK (2016). Neuroinflammation at the interface of depression and cardiovascular disease: Evidence from rodent models of social stress. Neurobiology of Stress, 4, 1–14. 10.1016/j.ynstr.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Mellon SH, Wolkowitz O, & Vinogradov S. (2016). Neuroscience-informed auditory training in schizophrenia: A final report of the effects on cognition and serum brain-derived neurotrophic factor. Schizophrenia Research. Cognition, 3, 1–7. 10.1016/j.scog.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima A, Kinugawa S, Homma T, Masaki Y, Furihata T, Yokota T, Matsushima S, Takada S, Kadoguchi T, Oba K, Okita K, & Tsutsui H. (2015). Serum brain-derived neurotropic factor level predicts adverse clinical outcomes in patients with heart failure. Journal of Cardiac Failure, 21(4), 300–306. 10.1016/j.cardfail.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Fulgenzi G, Tomassoni-Ardori F, Babini L, Becker J, Barrick C, Puverel S, & Tessarollo L. (2015). BDNF modulates heart contraction force and long-term homeostasis through truncated TrkB.T1 receptor activation. The Journal of Cell Biology, 210(6), 1003–1012. 10.1083/jcb.201502100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Git A, Dvinge H, Salmon-Divon M, Osborne M, Kutter C, Hadfield J, Bertone P, & Caldas C. (2010). Systematic comparison of microarray profiling, real-time PCR, and next-generation sequencing technologies for measuring differential microRNA expression. RNA, 16(5), 991–1006. 10.1261/rna.1947110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden E, Emiliano A, Maudsley S, Windham BG, Carlson OD, Egan JM, Driscoll I, Ferrucci L, Martin B, & Mattson MP (2010). Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: Data from the Baltimore Longitudinal Study of Aging. PloS One, 5(4), e10099. 10.1371/journal.pone.0010099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greisenegger S, Segal HC, Burgess AI, Poole DL, Mehta Z, & Rothwell PM (2015). Biomarkers and mortality after transient ischemic attack and minor ischemic stroke: Population-based study. Stroke, 46(3), 659–666. 10.1161/STROKEAHA.114.007624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade GV, Ma Y, Ramirez TA, Zhang J, Dai Q, Hensler JG, Lopez EF, Ghasemi O, Jin Y-F, & Lindsey ML (2013). Reduced BDNF attenuates inflammation and angiogenesis to improve survival and cardiac function following myocardial infarction in mice. American Journal of Physiology-Heart and Circulatory Physiology, 305(12), H1830–H1842. 10.1152/ajpheart.00224.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang P, Zhao J, Cai B, Tian S, Huang W, Guo J, … Du Z. (2015). Brain-derived neurotrophic factor regulates TRPC3/6 channels and protects against myocardial infarction in rodents. International Journal of Biological Sciences, 11(5), 536–545. 10.7150/ijbs.10754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, & Weinberger DR (2003). Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(17), 6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Liu Y, Zhang Y, & Chen Z-Y (2011). Association of plasma brain-derived neurotrophic factor and cardiovascular risk factors and prognosis in angina pectoris. Biochemical and Biophysical Research Communications, 415(1), 99–103. 10.1016/j.bbrc.2011.10.020 [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang R, Liu Y, Zhang Y, & Chen Z-Y (2009). BDNF Val66Met polymorphism is associated with unstable angina. Clinica Chimica Acta; International Journal of Clinical Chemistry, 400(1–2), 3–7. 10.1016/j.cca.2008.10.017 [DOI] [PubMed] [Google Scholar]
- Jiang R, Babyak MA, Brummett BH, Hauser ER, Shah SH, Becker RC, Siegler IC, Singh A, Haynes C, Chryst-Ladd M, Craig DM, & Williams RB (2017). Brain-derived neurotrophic factor rs6265 (Val66Met) polymorphism is associated with disease severity and incidence of cardiovascular events in a patient cohort. American Heart Journal, 190, 40–45. 10.1016/j.ahj.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Kim J, Davis JM, Blair SN, & Cho H. (2011). Association among basal serum BDNF, cardiorespiratory fitness and cardiovascular disease risk factors in untrained healthy Korean men. European Journal of Applied Physiology, 111(2), 303–311. 10.1007/s00421-010-1658-5 [DOI] [PubMed] [Google Scholar]
- Kadowaki S, Shishido T, Honda Y, Narumi T, Otaki Y, Kinoshita D, Nishiyama S, Takahashi H, Arimoto T, Miyamoto T, Watanabe T, & Kubota I. (2016). Additive clinical value of serum brain-derived neurotrophic factor for prediction of chronic heart failure outcome. Heart and Vessels, 31(4), 535–544. 10.1007/s00380-015-0628-6 [DOI] [PubMed] [Google Scholar]
- Kadoya M, Koyama H, Kanzaki A, Kurajoh M, Hatayama M, Shiraishi J, Okazaki H, Shoji T, Moriwaki Y, Yamamoto T, Inaba M, & Namba M. (2014). Plasma brain-derived neurotrophic factor and reverse dipping pattern of nocturnal blood pressure in patients with cardiovascular risk factors. PloS One, 9(8), e105977. 10.1371/journal.pone.0105977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaess BM, Preis SR, Lieb W, Beiser AS, Yang Q, Chen TC, Hengstenberg C, Erdmann J, Schunkert H, Seshadri S, Vasan RS, CARDIoGRAM, Assimes TL, Deloukas P, Holm H, Kathiresan S, König IR, McPherson R, Reilly MP, … Stewart AFR (2015). Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. Journal of the American Heart Association, 4(3), e001544. 10.1161/JAHA.114.001544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermani P, & Hempstead B. (2019). BDNF actions in the cardiovascular system: Roles in development, adulthood and response to injury. Frontiers in Physiology, 10. 10.3389/fphys.2019.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurajoh M, Kadoya M, Morimoto A, Miyoshi A, Kanzaki A, Kakutani-Hatayama M, Hamamoto K, Shoji T, Moriwaki Y, Yamamoto T, Inaba M, Namba M, & Koyama H. (2017). Plasma brain-derived neurotrophic factor concentration is a predictor of chronic kidney disease in patients with cardiovascular risk factors—Hyogo Sleep Cardio-Autonomic Atherosclerosis study. PloS One, 12(6), e0178686. 10.1371/journal.pone.0178686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasek-Bal A, Jędrzejowska-Szypułka H, Różycka J, Bal W, Holecki M, Duława J, & Lewin-Kowalik J. (2015). Low concentration of BDNF in the acute phase of ischemic stroke as a factor in poor prognosis in terms of functional status of patients. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 21, 3900–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Ahmad M, Weldrick JJ, Wang H-W, Burgon PG, & Leenen FHH (2018). Effects of exercise training and TrkB blockade on cardiac function and BDNF-TrkB signaling post myocardial infarction in rats. American Journal of Physiology. Heart and Circulatory Physiology. 10.1152/ajpheart.00245.2018 [DOI] [PubMed] [Google Scholar]
- Lee I-T, Lee W-J, Tsai I-C, Liang K-W, Lin S-Y, Wan C-J, Fu C-P, & Sheu WH-H (2012). Brain-derived neurotrophic factor not associated with metabolic syndrome but inversely correlated with vascular cell adhesion molecule-1 in men without diabetes. Clinica Chimica Acta; International Journal of Clinical Chemistry, 413(9–10), 944–948. 10.1016/j.cca.2012.02.013 [DOI] [PubMed] [Google Scholar]
- Lee I-T, Sheu WH-H, Lee W-J, & Chen D-Y (2018). Serum brain-derived neurotrophic factor predicting reduction in pulse pressure after a one-hour rest in nurses working night shifts. Scientific Reports, 8(1), 5485. 10.1038/s41598-018-23791-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA, Harrington K, Bourgeat P, Bush AI, Martins RN, Masters CL, Rowe CC, Maruff P, & AIBL Research Group. (2014). Effect of BDNF Val66Met on memory decline and hippocampal atrophy in prodromal Alzheimer’s disease: A preliminary study. PloS One, 9(1), e86498. 10.1371/journal.pone.0086498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorgis L, Amoureux S, de Maistre E, Sicard P, Bejot Y, Zeller M, Vergely C, Sequeira-Le Grand A, Lagrost A-C, Berchoud J, Cottin Y, & Rochette L. (2010). Serum brain-derived neurotrophic factor and platelet activation evaluated by soluble P-selectin and soluble CD-40-ligand in patients with acute myocardial infarction. Fundamental & Clinical Pharmacology, 24(4), 525–530. 10.1111/j.1472-8206.2009.00790.x [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & Group TP (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamedi S, Karimi I, & Jafari F. (2017). The interrelationship of metabolic syndrome and neurodegenerative diseases with focus on brain-derived neurotrophic factor (BDNF): Kill two birds with one stone. Metabolic Brain Disease, 32(3), 651–665. 10.1007/s11011-017-9997-0 [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute. (2019). Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Ng TKS, Ho CSH, Tam WWS, Kua EH, & Ho RC-M (2019). Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s disease (AD): A systematic review and meta-analysis. International Journal of Molecular Sciences, 20(2). 10.3390/ijms20020257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Yokoyama M, Toko H, Tateno K, Moriya J, Shimizu I, Nojima A, Ito T, Yoshida Y, Kobayashi Y, Katagiri H, Minamino T, & Komuro I. (2012). Brain-derived neurotrophic factor protects against cardiac dysfunction after myocardial infarction via a central nervous system-mediated pathway. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(8), 1902–1909. 10.1161/ATVBAHA.112.248930 [DOI] [PubMed] [Google Scholar]
- Page GG, Corwin EJ, Dorsey SG, Redeker NS, McCloskey DJ, Austin JK, … Grady P. (2018). Biomarkers as common data elements for symptom and self-management science. Journal of Nursing Scholarship: An Official Publication of Sigma Theta Tau International Honor Society of Nursing, 50(3), 276–286. 10.1111/jnu.12378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HK, Kwak MK, Kim HJ, & Ahima RS (2017). Linking resistin, inflammation, and cardiometabolic diseases. The Korean Journal of Internal Medicine, 32(2), 239–247. 10.3904/kjim.2016.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NH, Tarp J, Andersen LB, Gejl AK, Huang T, Peijs L, & Bugge A. (2017). The association between serum brain-derived neurotrophic factor and a cluster of cardiovascular risk factors in adolescents: The CHAMPS-study DK. PloS One, 12(10), e0186384. 10.1371/journal.pone.0186384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner SF, Waggoner SG, Tahl AR, & Sklar P. (2010). Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Molecular Psychiatry, 15(8), 810–815. 10.1038/mp.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pius-Sadowska E, & Machaliński B. (2017). BDNF - A key player in cardiovascular system. Journal of Molecular and Cellular Cardiology, 110, 54–60. 10.1016/j.yjmcc.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Pluchino N, Cubeddu A, Begliuomini S, Merlini S, Giannini A, Bucci F, Casarosa E, Luisi M, Cela V, & Genazzani AR (2009). Daily variation of brain-derived neurotrophic factor and cortisol in women with normal menstrual cycles, undergoing oral contraception and in postmenopause. Human Reproduction (Oxford, England), 24(9), 2303–2309. 10.1093/humrep/dep119 [DOI] [PubMed] [Google Scholar]
- Polacchini A, Metelli G, Francavilla R, Baj G, Florean M, Mascaretti LG, & Tongiorgi E. (2015). A method for reproducible measurements of serum BDNF: Comparison of the performance of six commercial assays. Scientific Reports, 5, 17989. 10.1038/srep17989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler SJ, Harrison JM, Titler M, Koelling TM, Jung M, Dorsey SG, … Giordani B. (2017). APOE ε4 and memory among patients with heart failure. Western Journal of Nursing Research, 39(4), 455–472. 10.1177/0193945916670145 [DOI] [PubMed] [Google Scholar]
- Pressler SJ, Titler M, Koelling TM, Riley PL, Jung M, Hoyland-Domenico L, … Giordani B. (2015). Nurse-enhanced computerized cognitive training increases serum brain-derived neurotropic factor levels and improves working memory in heart failure. Journal of Cardiac Failure, 21(8), 630–641. 10.1016/j.cardfail.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Rahman F, Himali JJ, Yin X, Beiser AS, Ellinor PT, Lubitz SA, Vasan RS, Magnani JW, McManus DD, Seshadri S, & Benjamin EJ (2017). Serum brain-derived neurotrophic factor and risk of atrial fibrillation. American Heart Journal, 183, 69–73. 10.1016/j.ahj.2016.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin BL (2007). The progression of cardiovascular risk to cardiovascular disease. Reviews in Cardiovascular Medicine, 8 Suppl 4, S3–8. [PubMed] [Google Scholar]
- Rothman SM, Griffioen KJ, Wan R, & Mattson MP (2012). Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Annals of the New York Academy of Sciences, 1264, 49–63. 10.1111/j.1749-6632.2012.06525.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CL (2005). An overview of the integrative research review. Progress in Transplantation (Aliso Viejo, Calif.), 15(1), 8–13. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, & Olefsky JM (2017). Inflammatory mechanisms linking obesity and metabolic disease. The Journal of Clinical Investigation, 127(1), 1–4. 10.1172/JCI92035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-de-Toledo J, Chrysostomou C, Munoz R, Lichtenstein S, Sao-Avilés CA, Wearden PD, Morell VO, Clark RSB, Toney N, & Bell MJ (2014). Cerebral regional oxygen saturation and serum neuromarkers for the prediction of adverse neurologic outcome in pediatric cardiac surgery. Neurocritical Care, 21(1), 133–139. 10.1007/s12028-013-9934-y [DOI] [PubMed] [Google Scholar]
- Schrijver IT, Kemperman H, Roest M, Kesecioglu J, & de Lange DW (2017). Soluble p-selectin as a biomarker for infection and survival in patients with a systemic inflammatory response syndrome on the intensive care unit. Biomarker Insights, 12, 1177271916684823–1177271916684823. 10.1177/1177271916684823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte CE, Malan L, Scheepers JD, Oosthuizen W, Cockeran M, & Malan NT (2016). Cortisol:brain-derived neurotrophic factor ratio associated with silent ischaemia in a black male cohort: The SA BPA study. Cardiovascular Journal of Africa, 27(6), 387–391. 10.5830/CVJA-2016-065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan R, Strydom A, Brown E, Marston L, & Hassiotis A. (2018). Association of focused medication review with optimization of psychotropic drug prescribing: A systematic review and meta-analysis. JAMA Network Open, 1(6), e183750–e183750. 10.1001/jamanetworkopen.2018.3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata A, Hanatani A, Izumi Y, Kitada R, Iwata S, & Yoshiyama M. (2018). Serum brain-derived neurotrophic factor level and exercise tolerance complement each other in predicting the prognosis of patients with heart failure. Heart and Vessels, 33(11), 1325–1333. 10.1007/s00380-018-1174-9 [DOI] [PubMed] [Google Scholar]
- Stanne TM, Åberg ND, Nilsson S, Jood K, Blomstrand C, Andreasson U, … Jern C. (2016). Low circulating acute brain-derived neurotrophic factor levels are associated with poor long-term functional outcome after ischemic stroke. Stroke, 47(7), 1943–1945. 10.1161/STROKEAHA.115.012383 [DOI] [PubMed] [Google Scholar]
- Szabo AJ, Alosco ML, Miller LA, McGeary JE, Poppas A, Cohen RA, & Gunstad J. (2013). Brain-derived neurotrophic factor Val66Met polymorphism and cognitive function in persons with cardiovascular disease. Psychogeriatrics : The Official Journal of the Japanese Psychogeriatric Society, 13(4). 10.1111/psyg.12013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashio S, Sugiyama S, Yamamuro M, Takahama H, Hayashi T, Sugano Y, Izumiya Y, Hokimoto S, Minamino N, Yasuda S, Anzai T, & Ogawa H. (2015). Significance of low plasma levels of brain-derived neurotrophic factor in patients with heart failure. The American Journal of Cardiology, 116(2), 243–249. 10.1016/j.amjcard.2015.04.018 [DOI] [PubMed] [Google Scholar]
- Taşçı İ, Kabul HK, & Aydoğdu A. (2012). Brain derived neurotrophic factor (BDNF) in cardiometabolic physiology and diseases. Anadolu Kardiyoloji Dergisi: AKD = the Anatolian Journal of Cardiology, 12(8), 684–688. 10.5152/akd.2012.221 [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, & Mellon SH (2009). Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biological Psychiatry, 66(6), 549–553. 10.1016/j.biopsych.2009.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vuren EJ, Malan L, von Känel R, Magnusson M, Lammertyn L, & Malan NT (2019). BDNF increases associated with constant troponin T levels and may protect against poor cognitive interference control: The SABPA prospective study. European Journal of Clinical Investigation, 49(7), e13116. 10.1111/eci.13116 [DOI] [PubMed] [Google Scholar]
- Whittemore R, & Knafl K. (2005). The integrative review: Updated methodology. Journal of Advanced Nursing, 52(5), 546–553. 10.1111/j.1365-2648.2005.03621.x [DOI] [PubMed] [Google Scholar]
- Wu H, Cao G, Wang Y, Tian H, & Du R. (2019). Increased serum CA125 and brain-derived neurotrophic factor (BDNF) levels on acute myocardial infarction: A predictor for acute heart failure. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 25, 913–919. 10.12659/MSM.912642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembron-Lacny A, Dziubek W, Rynkiewicz M, Morawin B, & Woźniewski M. (2016). Peripheral brain-derived neurotrophic factor is related to cardiovascular risk factors in active and inactive elderly men. Brazilian Journal of Medical and Biological Research = Revista Brasileira De Pesquisas Medicas E Biologicas, 49(7). 10.1590/1414-431X20165253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng N, Xu J, Yao W, Li S, Ruan W, & Xiao F. (2017). Brain-derived neurotrophic factor attenuates septic myocardial dysfunction via eNOS/NO pathway in rats. Oxidative Medicine and Cellular Longevity, 2017, 1721434. 10.1155/2017/1721434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Girón CG, Gil L, Gordon L, Haggerty L, Haskell E, Hourlier T, Izuogu OG, Janacek SH, Juettemann T, To JK, … Flicek P. (2018). Ensembl 2018. Nucleic Acids Research, 46(D1), D754–D761. 10.1093/nar/gkx1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lin P, Jiang H, Xu J, Luo S, Mo J, Li Y, & Chen X. (2015). Extensive serum biomarker analysis in patients with ST segment elevation myocardial infarction (STEMI). Cytokine, 76(2), 356–362. 10.1016/j.cyto.2015.06.015 [DOI] [PubMed] [Google Scholar]