Abstract
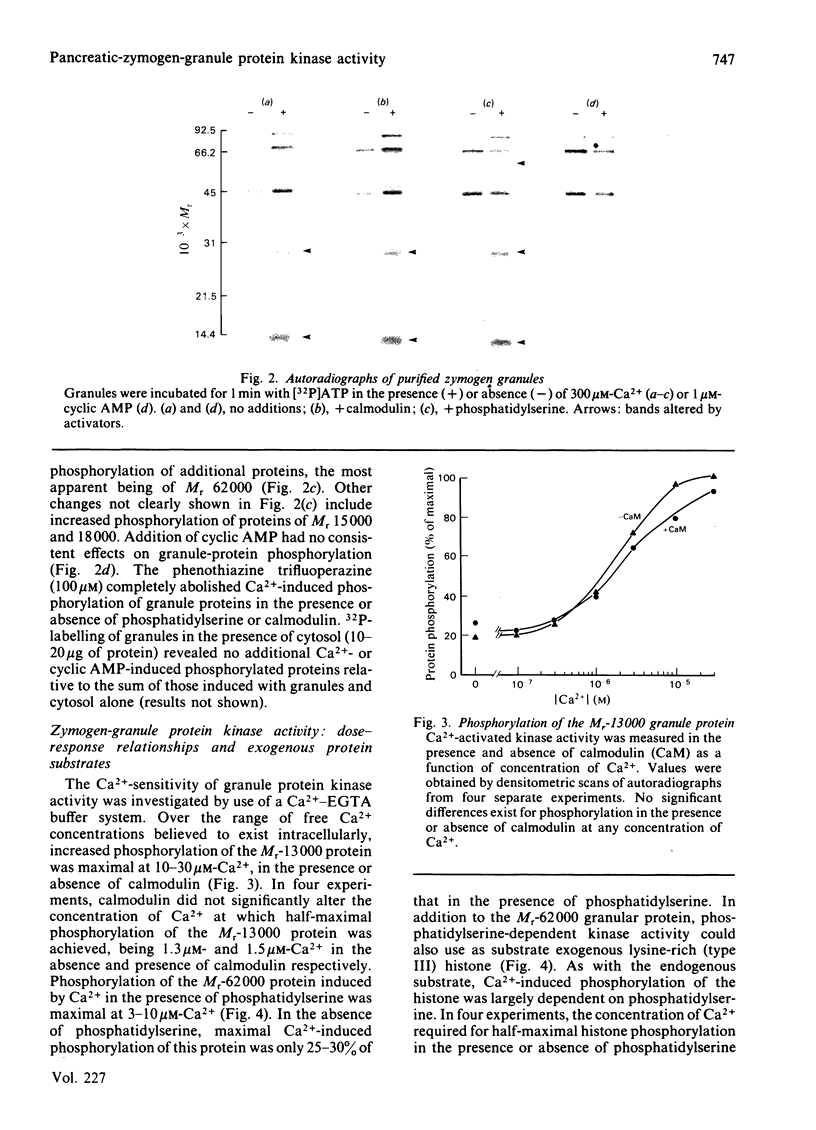
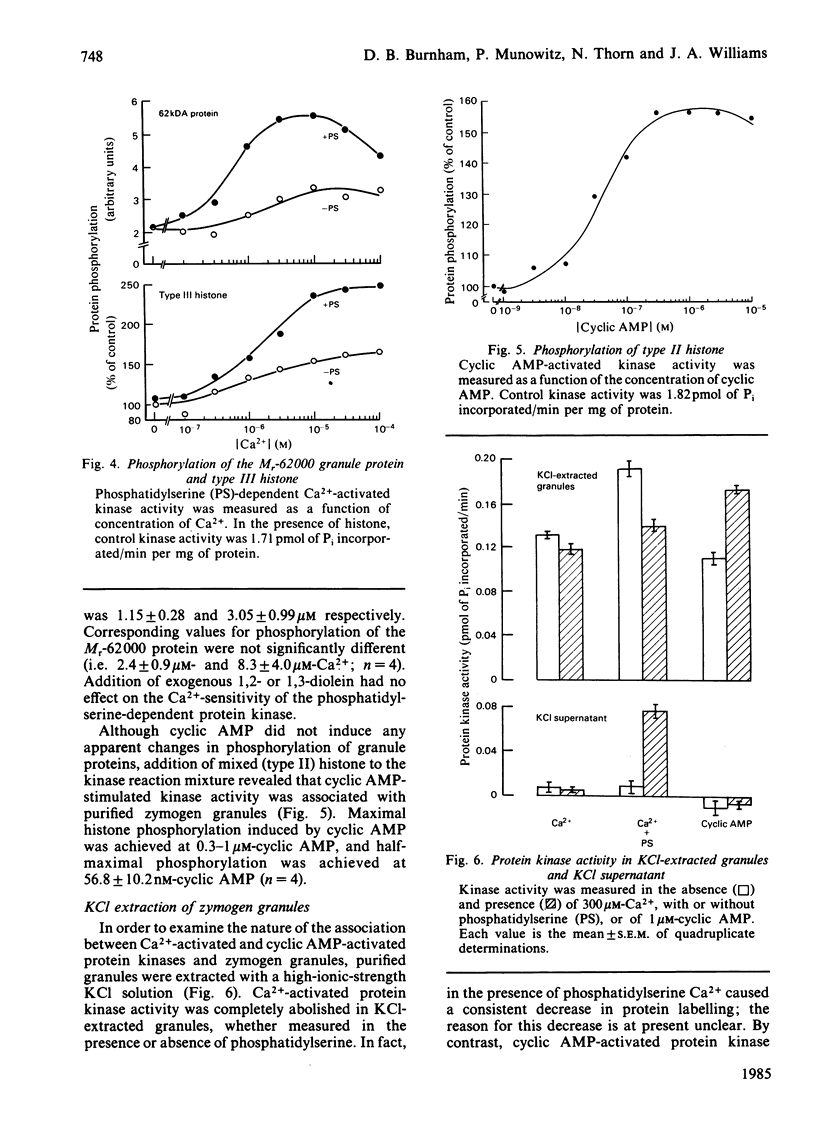
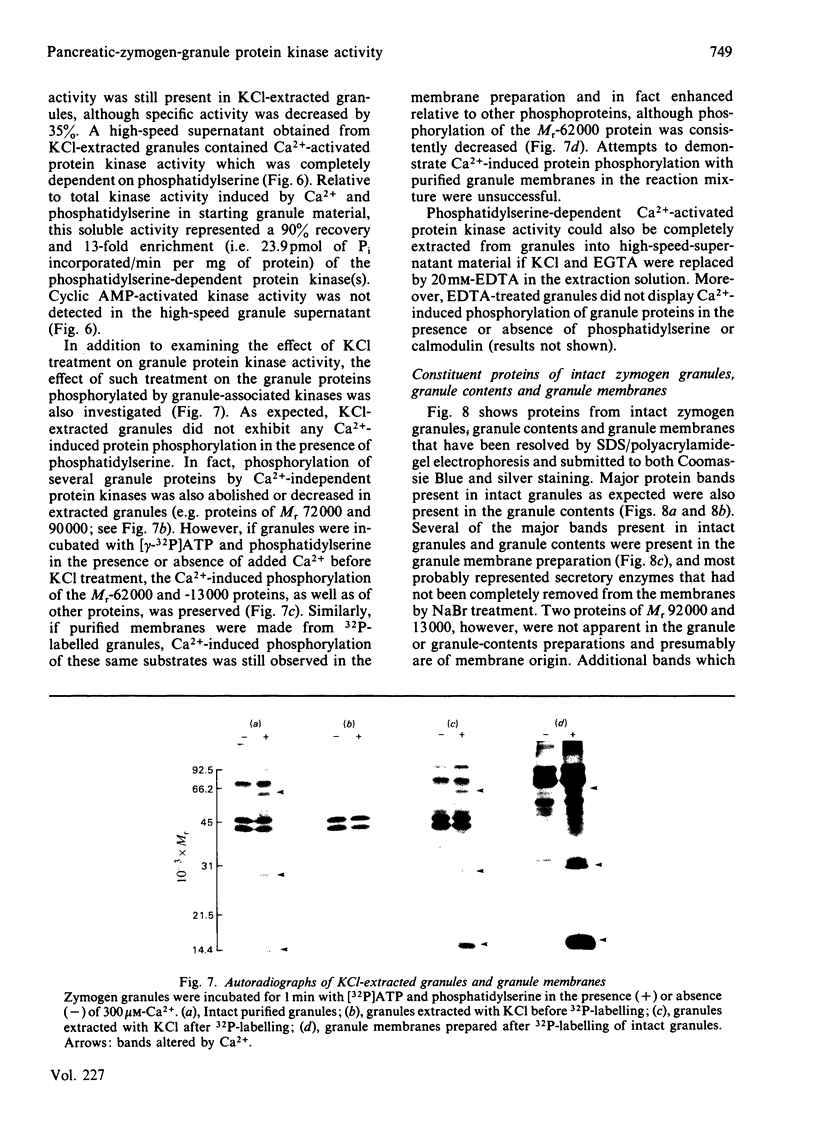
Purified zymogen granules were prepared from rat pancreas by using an iso-osmotic Percoll gradient. In the presence of [gamma-32P]ATP, phosphorylation of several granule proteins was induced by Ca2+, most notably a Mr-13 000 protein, whereas addition of cyclic AMP was without effect. When phosphatidylserine was also added, Ca2+ increased the phosphorylation of additional proteins, with the largest effect on a protein of Mr 62 000. Purified granules were also able to phosphorylate exogenous substrates. Ca2+-induced phosphorylation of lysine-rich histone was enhanced over 3-fold in the presence of phosphatidylserine, and cyclic AMP-activated protein kinase activity was revealed with mixed histone as substrate. The concentrations of free Ca2+ and cyclic AMP required for half-maximal phosphorylation of both endogenous and exogenous proteins were 1-3 microM and 57 nM respectively. Treatment of granules with 0.25 M-KCl resulted in the release of phosphatidylserine-dependent kinase activity into a high-speed granule supernatant. In contrast, granule-protein substrates of Ca2+-activated kinase activity were resistant to KCl extraction, and in fact were present in purified granule membranes. Kinase activity activated by cyclic AMP was not extracted by KCl treatment. It is concluded that phosphorylation of integral membrane proteins in the zymogen granule can be induced by one or more Ca2+-activated protein kinases. Such a reaction is a potential mechanism by which exocytosis may be regulated in the exocrine pancreas by Ca2+-mediated secretagogues.
Full text
PDF



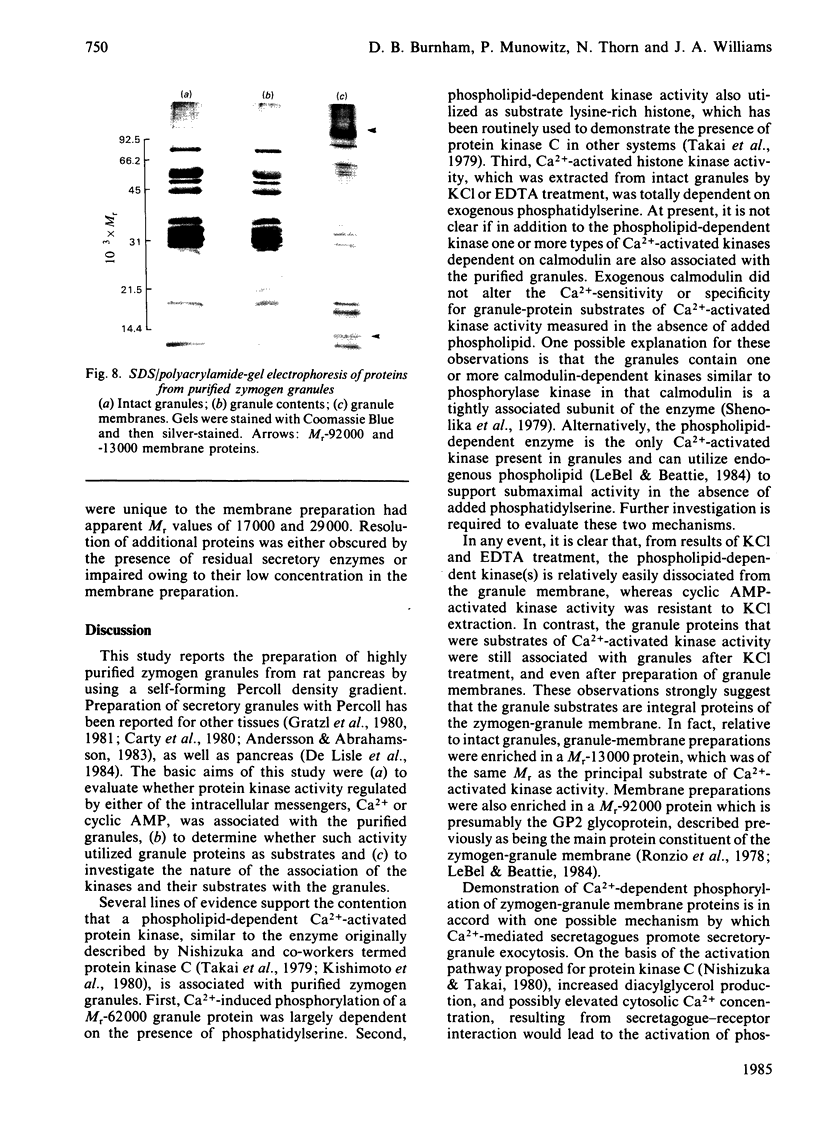

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T., Abrahamsson H. Purification of mitochondria and secretory granules isolated from pancreatic beta cells using Percoll and Sephacryl S-1000 superfine. Anal Biochem. 1983 Jul 1;132(1):82–88. doi: 10.1016/0003-2697(83)90428-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnham D. B., Williams J. A. Activation of protein kinase activity in pancreatic acini by calcium and cAMP. Am J Physiol. 1984 May;246(5 Pt 1):G500–G508. doi: 10.1152/ajpgi.1984.246.5.G500. [DOI] [PubMed] [Google Scholar]
- Burnham D. B., Williams J. A. Effects of carbachol, cholecystokinin, and insulin on protein phosphorylation in isolated pancreatic acini. J Biol Chem. 1982 Sep 10;257(17):10523–10528. [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Carty S. E., Johnson R. G., Scarpa A. The isolation of intact adrenal chromaffin granules using isotonic Percoll density gradients. Anal Biochem. 1980 Aug;106(2):438–445. doi: 10.1016/0003-2697(80)90545-x. [DOI] [PubMed] [Google Scholar]
- De Lisle R. C., Schulz I., Tyrakowski T., Haase W., Hopfer U. Isolation of stable pancreatic zymogen granules. Am J Physiol. 1984 Apr;246(4 Pt 1):G411–G418. doi: 10.1152/ajpgi.1984.246.4.G411. [DOI] [PubMed] [Google Scholar]
- Freedman S. D., Jamieson J. D. Hormone-induced protein phosphorylation. II. Localization to the ribosomal fraction from rat exocrine pancreas and parotid of a 29,000-dalton protein phosphorylated in situ in response to secretagogues. J Cell Biol. 1982 Dec;95(3):909–917. doi: 10.1083/jcb.95.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D. Regulation of pancreatic exocrine function in vitro: initial steps in the actions of secretagogues. Annu Rev Physiol. 1979;41:55–66. doi: 10.1146/annurev.ph.41.030179.000415. [DOI] [PubMed] [Google Scholar]
- Gorelick F. S., Cohn J. A., Freedman S. D., Delahunt N. G., Gershoni J. M., Jamieson J. D. Calmodulin-stimulated protein kinase activity from rat pancreas. J Cell Biol. 1983 Oct;97(4):1294–1298. doi: 10.1083/jcb.97.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzl M., Krieger-Brauer H., Ekerdt R. Latent acetylcholinesterase in secretory vesicles isolated from adrenal medulla. Biochim Biophys Acta. 1981 Dec 7;649(2):355–366. doi: 10.1016/0005-2736(81)90425-9. [DOI] [PubMed] [Google Scholar]
- Gratzl M., Torp-Pedersen C., Dartt D., Treiman M., Thorn N. A. Isolation and characterization of secretory vesicles from bovine neurohypophyses. Hoppe Seylers Z Physiol Chem. 1980 Nov;361(11):1615–1628. doi: 10.1515/bchm2.1980.361.2.1615. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Hootman S. R., Philpott C. W. Ultracytochemical localization of Na+,K+-activated ATPase in chloride cells from the gills of a euryhaline teleost. Anat Rec. 1979 Jan;193(1):99–129. doi: 10.1002/ar.1091930107. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Gardner J. D. Cyclic nucleotide-dependent protein kinase activity in acinar cells from guinea pig pancreas. Gastroenterology. 1978 Nov;75(5):806–816. [PubMed] [Google Scholar]
- Jung D. H. Preparation and application of Procion Yellow starch for amylase assay. Clin Chim Acta. 1980 Jan 1;100(1):7–11. doi: 10.1016/0009-8981(80)90179-5. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Lam K. S., Kasper C. B. Selective phosphorylation of a nuclear envelope polypeptide by an endogenous protein kinase. Biochemistry. 1979 Jan 23;18(2):307–311. doi: 10.1021/bi00569a012. [DOI] [PubMed] [Google Scholar]
- Lambert M., Camus J., Christophe J. Phosphorylation of protein components of isolated zymogen granule membranes from the rat pancreas. FEBS Lett. 1974 Dec 15;49(2):228–232. doi: 10.1016/0014-5793(74)80518-1. [DOI] [PubMed] [Google Scholar]
- LeBel D., Beattie M. The integral and peripheral proteins of the zymogen granule membrane. Biochim Biophys Acta. 1984 Feb 15;769(3):611–621. doi: 10.1016/0005-2736(84)90060-9. [DOI] [PubMed] [Google Scholar]
- Lewis D. S., Ronzio R. A. An assessment of the role of protein kinase and zymogen granule phosphorylation during secretion by the rat exocrine pancreas. Biochim Biophys Acta. 1979 Apr 3;583(4):422–433. doi: 10.1016/0304-4165(79)90059-x. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Ronzio R. A. Phosphorylation of a zymogen granule membrane polypeptide from rat pancreas. FEBS Lett. 1974 Mar 15;40(1):203–206. doi: 10.1016/0014-5793(74)80928-2. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Jamieson J. D., Palade G. E. Composition of cellular membranes in the pancreas of the guinea pig. I. Isolation of membrane fractions. J Cell Biol. 1971 Apr;49(1):109–129. doi: 10.1083/jcb.49.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs D. L., Korenbrot J. I., Williams J. A. Intracellular free calcium concentrations in isolated pancreatic acini; effects of secretagogues. Biochem Biophys Res Commun. 1983 Nov 30;117(1):122–128. doi: 10.1016/0006-291x(83)91549-8. [DOI] [PubMed] [Google Scholar]
- Price R. G., Dance N. The cellular distribution of some rat-kidney glycosidases. Biochem J. 1967 Nov;105(2):877–883. doi: 10.1042/bj1050877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr, Burgess G. M., Halenda S. P., McKinney J. S., Rubin R. P. Effects of secretagogues on [32P]phosphatidylinositol 4,5-bisphosphate metabolism in the exocrine pancreas. Biochem J. 1983 May 15;212(2):483–488. doi: 10.1042/bj2120483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pâquet M. R., St-Jean P., Roberge M., Beaudoin A. R. Isolation of zymogen granules from rat pancreas and characterization of their membrane proteins. Eur J Cell Biol. 1982 Aug;28(1):20–26. [PubMed] [Google Scholar]
- Roberts M. L., Butcher F. R. The involvement of protein phosphorylation in stimulus-secretion coupling in the mouse exocrine pancreas. Biochem J. 1983 Feb 15;210(2):353–359. doi: 10.1042/bj2100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzio R. A., Kronquist K. E., Lewis D. S., MacDonald R. J., Mohrlok S. H., O'Donnell J. J., Jr Glycoprotein synthesis in the adult rat pancreas. IV. Subcellular distribution of membrane glycoproteins. Biochim Biophys Acta. 1978 Mar 21;508(1):65–84. doi: 10.1016/0005-2736(78)90189-x. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Cohen P. T., Cohen P., Nairn A. C., Perry S. V. The role of calmodulin in the structure and regulation of phosphorylase kinase from rabbit skeletal muscle. Eur J Biochem. 1979 Oct 15;100(2):329–337. doi: 10.1111/j.1432-1033.1979.tb04175.x. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Takesue S., Omura T. Solubilization of NADH-cytochrome b5 reductase from liver microsomes by lysosomal digestion. J Biochem. 1970 Feb;67(2):259–266. doi: 10.1093/oxfordjournals.jbchem.a129249. [DOI] [PubMed] [Google Scholar]
- Williams J. A. Regulation of pancreatic acinar cell function by intracellular calcium. Am J Physiol. 1980 Apr;238(4):G269–G279. doi: 10.1152/ajpgi.1980.238.4.G269. [DOI] [PubMed] [Google Scholar]