Abstract
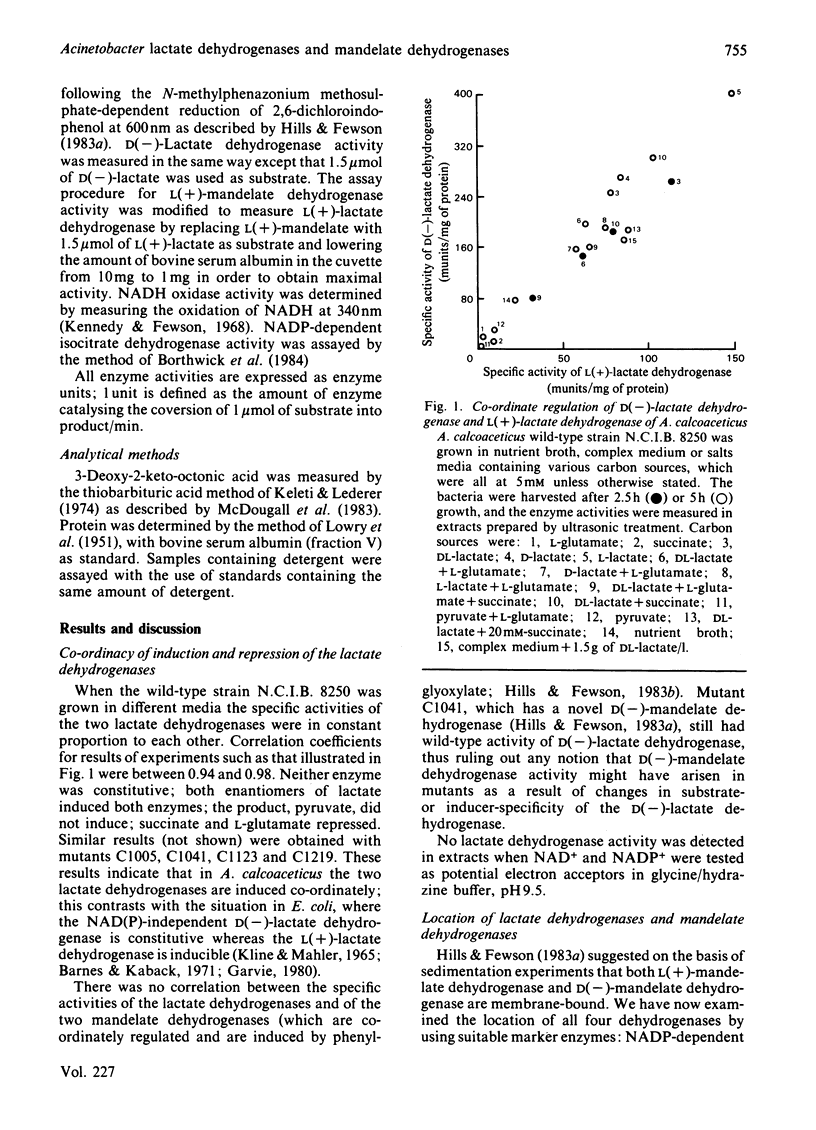
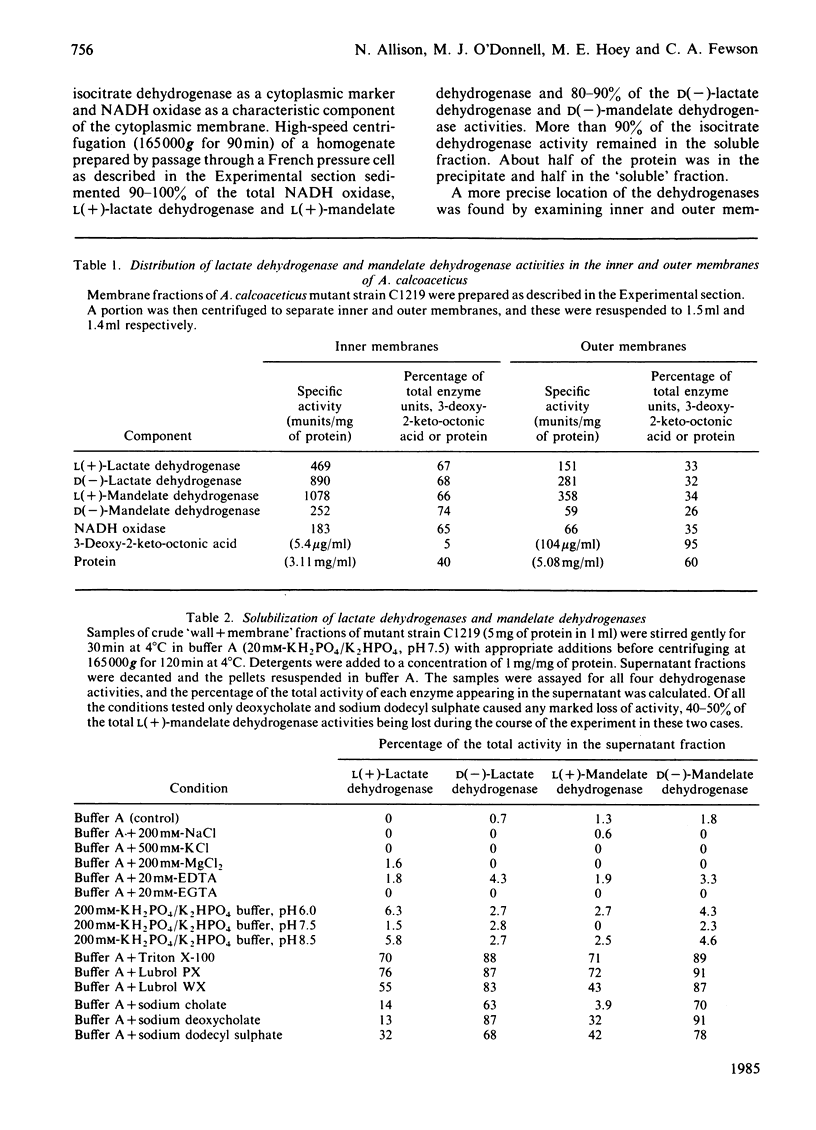
Acinetobacter calcoaceticus possesses an L(+)-lactate dehydrogenase and a D(-)-lactate dehydrogenase. Results of experiments in which enzyme activities were measured after growth of bacteria in different media indicated that the two enzymes were co-ordinately induced by either enantiomer of lactate but not by pyruvate, and repressed by succinate or L-glutamate. The two lactate dehydrogenases have very similar properties to L(+)-mandelate dehydrogenase and D(-)-mandelate dehydrogenase. All four enzymes are NAD(P)-independent and were found to be integral components of the cytoplasmic membrane. The enzymes could be solubilized in active form by detergents; Triton X-100 or Lubrol PX were particularly effective D(-)-Lactate dehydrogenase and D(-)-mandelate dehydrogenase could be selectively solubilized by the ionic detergents cholate, deoxycholate and sodium dodecyl sulphate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borthwick A. C., Holms W. H., Nimmo H. G. Isolation of active and inactive forms of isocitrate dehydrogenase from Escherichia coli ML 308. Eur J Biochem. 1984 Jun 1;141(2):393–400. doi: 10.1111/j.1432-1033.1984.tb08204.x. [DOI] [PubMed] [Google Scholar]
- Futai M., Kimura H. Inducible membrane-bound L-lactate dehydrogenase from Escherichia coli. Purification and properties. J Biol Chem. 1977 Aug 25;252(16):5820–5827. [PubMed] [Google Scholar]
- Futai M. Membrane D-lactate dehydrogenase from Escherichia coli. Purification and properties. Biochemistry. 1973 Jun 19;12(13):2468–2474. doi: 10.1021/bi00737a016. [DOI] [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills C. A., Fewson C. A. Mutant strains of Acinetobacter calcoaceticus possessing additional mandelate dehydrogenases. Identification and preliminary characterization of the enzymes. Biochem J. 1983 Feb 1;209(2):379–386. doi: 10.1042/bj2090379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills C. A., Fewson C. A. Regulation of expression of novel mandelate dehydrogenases in mutants of Acinetobacter calcoaceticus. J Gen Microbiol. 1983 Jul;129(7):2009–2015. doi: 10.1099/00221287-129-7-2009. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem J. 1968 Apr;107(4):497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline E. S., Mahler H. R. The lactic dehydrogenases of E. coli. Ann N Y Acad Sci. 1965 Jul 31;119(3):905–919. doi: 10.1111/j.1749-6632.1965.tb47451.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- O'Donnell M. J., Fewson C. A. Solubilization and stabilization of L(+)-mandelate dehydrogenase from Acinetobacter calcoaceticus. Biochem Soc Trans. 1980 Oct;8(5):653–654. doi: 10.1042/bst0080653. [DOI] [PubMed] [Google Scholar]
- Olson S. T., Massey V. Purification and properties of the flavoenzyme D-lactate dehydrogenase from Megasphaera elsdenii. Biochemistry. 1979 Oct 16;18(21):4714–4724. doi: 10.1021/bi00588a036. [DOI] [PubMed] [Google Scholar]
- Pratt E. A., Fung L. W., Flowers J. A., Ho C. Membrane-bound D-lactate dehydrogenase from Escherichia coli: purification and properties. Biochemistry. 1979 Jan 23;18(2):312–316. doi: 10.1021/bi00569a013. [DOI] [PubMed] [Google Scholar]
- SNOSWELL A. M. OXIDIZED NICOTINAMIDE-ADENINE DINUCLEOTIDE-INDEPENDENT LACTATE DEHYDROGENASES OF LACTOBACILLUS ARABINOSUS 17.5. Biochim Biophys Acta. 1963 Sep 3;77:7–9. doi: 10.1016/0006-3002(63)90464-5. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., GUNSALUS I. C., GUNSALUS C. F. The enzymatic conversion of mandelic acid to benzoic acid. II. Properties of the particulate fractions. J Bacteriol. 1953 Nov;66(5):543–547. doi: 10.1128/jb.66.5.543-547.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. C., Makula S. R., Finnerty W. R. Isolation and characterization of membranes from a hydrocarbon-oxidizing Acinetobacter sp. J Bacteriol. 1976 Jul;127(1):469–480. doi: 10.1128/jb.127.1.469-480.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


