Abstract
In this article, we report the modification and photocatalytic evaluation of commercial TiO2-P25 under visible light for methyl orange (MO) dye degradation under visible light. The activity of materials doped with N, Pd, Pt and Au on to the TiO2-P25 was evaluated, with optimal photocatalytic performance achieved using Au nanoparticles doped on an N-functionalized titania surface. X-ray diffraction (XRD), physical nitrogen adsorption/desorption isotherm curves, transmission electron microscopy (TEM), diffuse reflectance spectroscopy, scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) were used to study the structural and textural properties of the samples. The chemical species present in the bulk and surface of the catalysts were identified using X-ray photoelectron spectroscopy (XPS) and microwave plasma-atomic emission spectroscopy. The results show that Au/N-TiO2 photocatalyst presents a remarkable enhanced activity for MO dye degradation, under visible light illumination, reaching 100% after 4 h. The enhanced photocatalytic activity using this composite is attributable to the well-dispersed and small size of Au nanoparticles, large surface area, reduction of band-gap energy and the interaction between nitrogen and Au which promoted a synergistic effect.
This article is part of the discussion meeting issue ‘Green carbon for the chemical industry of the future’.
Keywords: water pollution, visible light absorption, metallic nanoparticles
1. Introduction
A viable solution providing a renewable and sustainable energy source with the potential to meet continually growing energy demands, as well as the remediation of environmental pollutants can be achieved using photocatalysis. Synthetic dyes are widely employed in the textile industry, posing a significant environmental challenge when disposed of into effluents. Because synthetic dyes are complex, traditional biological treatments cannot effectively achieve both discolouration and wastewater degradation. In recent decades, there has been a surge of interest in advanced oxidation processes as a more efficient approach for eliminating aqueous and gaseous pollutants. Numerous studies have highlighted the photocatalytic activity of oxide semiconductors for oxidizing organic compounds in residual water. Since the seminal work of Fujishima & Honda [1], discussing hydrogen-photo-electrochemical production, titanium dioxide has been extensively utilized as photocatalyst, thanks to its chemical stability, durability, minimal toxicity and favourable production capacity. Furthermore, the high density of states in its bands allows TiO2 to efficiently convert photons to current, thereby enhancing its photocatalytic activity beyond that of other semiconductors [2]. However, for its large band gap of 3.2 eV, a TiO2 photocatalyst is only effective when irradiated by UV light (comprising only 5% of the solar spectrum), but the most significant impediment to its practical deployment is the quick recombination of its photogenerated electron–hole pairs [3]. This has resulted in many approaches to improve the catalytic activity, including the addition of metal-oxide heterojunctions [4], non-metal element doping [5] and noble-metal doping [6]. Furthermore, it has been demonstrated that a number of variables, including the concentration of the photocatalyst, the pH of the dye solution, the presence of oxidants and the light intensity, affect how quickly azo dyes photodegrade [7]. Therefore, modification of pH, addition of inorganic oxidants [8] (O3 or H2O2) or use of powerful UV light illumination sources are procedures commonly employed to obtain high degradation efficiencies.
The use of noble metals (Au, Ag, Rh and Pd) has shown to be an effective way to change physical parameters such as absorption edge, crystallite size, surface area and charge transfer process. Doping the surface of TiO2 with noble metals such as Ag [9], Au [10–12], Pt [13] and Pd [14,15] has been largely investigated by several researchers and shown to increase photocatalytic activity under visible light by acting as an electron trap, thereby augmenting the rates of photo-induced electron transfer at the interface. Additionally, they play a role in inhibiting the recombination of electron–hole pairs. The Fermi levels associated with TiO2 are higher than those for noble metals, and significant reductions in electron–hole recombination rates are seen by incorporating TiO2 with noble-metal dopants. Kim et al. [13] showed that photogenerated electrons become trapped upon modifying TiO2 with Pt, thus promoting interfacial charge transfer rates. Seery et al. [16] reported that Ag-modified TiO2 can extend the photo response of TiO2 towards visible light. During dye degradation studies, the addition of Ag was found to enhance the degradation rates of rhodamine 6G dye. In the same study, Ag+ ions were found to enhance the visible light absorption capacity of the TiO2 material by limiting the recombination rates of photogenerated electrons/holes. Camposeco et al. [17] reported a remarkable enhancement of photocatalytic activity in the water-splitting reaction to produce H2, where the presence of metallic nanoparticles improved the photocatalytic activity of TiO2 nanotubes. Recently, Motamedisade et al. [18] presented a novel catalyst for dye degradation fabricated with Au nanoclusters on nitrogen-functionalized TiO2. Clark et al. [19] have improved the selectivity of methyl orange (MO) and 4-chlorophenol degradation relative to TiO2 by using a distorted perovskite structure, CaCu3Ti4O12. The use of square planar Cu2+ and octohedral Ti4+ sites generates two individual band gaps. Recombination rate is decreased because an electron–hole trap is formed between localized Cu+ sites and the conduction band in Ti3+.
On the other hand, nitrogen-doped titania has gained significant attention in the field of photocatalysis. Numerous studies have identified it as a promising material for various environmental applications [20–22]. Characteristic features of nitrogen atoms, such as small ionization energy, similar atomic size and high stability compared to oxygen, encourage the incorporation of nitrogen dopants into the structure of TiO2 [22,23]. Upon incorporation of nitrogen impurities into the TiO2 material, the energy associated with oxygen vacancies decreases from 4.2 to 0.6 eV, thus nitrogen dopants favour oxygen vacancy formation [24]. This doping enhances the material’s photocatalytic efficiency by narrowing the band gap, allowing better utilization of visible light. Furthermore, nitrogen attached to the surface can serve as active sites, forming strong complexes between metallic nanoparticles and titania surfaces. This interaction increases the loading level of the nanoparticles and reduces their agglomeration [18], thereby enhancing the overall photocatalytic efficiency and stability of the material.
It is clear that the use of non-metals and metals on the surface of titania helps to enhance its photocatalytic performance in photocatalytic reactions. In this article, we show the synthesis of modified TiO2 photocatalysts for MO dye degradation. The aim was to evaluate the effect of three different metals on nitrogen-functionalized TiO2 support (Pd/N-TiO2, Pt/N-TiO2 and Au/N-TiO2) and its influence on the MO dye degradation under visible light illumination.
2. Experimental details
(a). Photocatalyst synthesis
(i). N-doped TiO2 support
Hexamethylenetetramine (HMT), C6H12N4(Sigma-Aldrich) was used as the nitrogen source. Manual grinding of TiO2 powder P25 (Degussa) and HMT was performed for 15 min using a pestle and mortar. The amount of HMT was 20 wt% and the sample was identified as N-TiO2. The resultant N-doped TiO2 was calcined at 400°C for 1 h in flowing air with a ramp rate of 5°C min−1.
(ii). Addition of metallic nanoparticles to N-TiO2 support
Gold, platinum and palladium nanoparticles were added to the N-doped TiO2 support by deposition–precipitation with the urea (DPU) method in the absence of light. The target metal concentration was 1 wt%. The metal precursors were hydrogen tetrachloroaurate (III) hydrate (HAuCl4·3H2O), potassium tetrachloroplatinate (K2PtCl4), palladium nitrate hydrate Pd(NO3)22H2O and urea (0.42 M), all supplied by Sigma-Aldrich. In all cases, the metal precursor and urea were dissolved in 20 ml of distilled water. Then, 500 mg of N-TiO2 (obtained by manual grinding) was added to the solution; subsequently, the suspension was vigorously stirred at 80°C for 16 h.
After the DPU method was completed, all materials were filtered, washed with distilled water and dried at 80°C for 12 h. Following drying, the samples were vacuum-sealed and kept at room temperature in a desiccator, shielded from light, to prevent any alterations.
All samples underwent thermal activation treatments under H2 at 400°C for 2 h with a ramp rate of 5°C min−1.
(b). Characterization techniques
The crystalline structure of the materials was studied using X-ray diffraction (XRD) and a PANalytical X’pert Pro powder diffractometer operating at 40 kV, 40 mA using Cu Kα radiation (λ = 1.54 × 10–10 m) with a Ge (111) single crystal monochromator. The phases present were assigned using the International Centre for Diffraction Data database as a reference. Transmission electron microscopy (TEM) analysis was conducted using a JEOL JEM-2100 operating at 200 kV. Powders were deposited on to 300 mesh copper grids coated with holey carbon film for imaging. Scanning electron microscopy (SEM) was performed on a Tescan Maia3 field emission gun microscope. An Oxford Instruments XMAXN 80 energy dispersive X-ray (EDX) detector was used for elemental analysis, controlled by Aztec software. The samples were dispersed as powders on to adhesive carbon Leit discs fixed to 12.5 mm aluminium stubs. For imaging purposes, the samples were coated with a 10 nm coating of 80:20 Au:Pd atoms by a Quorum plasma coater to prevent charging, and images were acquired using the secondary electron detector. The uncoated samples were analysed for EDX using the backscattered electron detector. The optical properties of the photocatalysts were measured using a UV–vis spectrometer, CARY 4000, equipped with a Harrick Praying Mantis attachment.
The textural properties were obtained using a Quantachrome Nova 2200E. A Brunauer–Emmett–Teller (BET) equation based on N2 physisorption data was used to determine the specific surface area. The Barrett–Joyner–Halenda method was applied to calculate the pore size distribution from the desorption branch of the isotherm. The materials were dried for 4 h at 100°C and outgassed prior to nitrogen adsorption. The chemical composition of the materials was analysed on a Thermo Fisher Scientific K-Alpha+ photoelectron spectrometer utilizing micro-focussed monochromatic Al Kα radiation operating at 72 W power (6 mA × 12 kV), utilizing the 400 micron spot mode, which affords an elliptical analysis area of ca 400 x 600 microns. Data were calibrated to the Ti 2p peak for the TiO2 support (taken to be 458.8 eV) using an approach that avoids the uncertainties in calibration to the C1s signal, which can be affected by changes in the surface chemistry. Data were analysed using CasaXPS (v2.3.26 PR1.0N) [25]. Surface homogeneous equivalent concentrations were calculated using transmission-corrected spectra, with a combination of Scofield sensitivity factors and an electron escape depth according to the TPP-2M formulation after Shirley background subtraction. The chemical content of Au, Pd and Pt in the dried samples was achieved by microwave plasma-atomic emission spectroscopy (MP-AES). The metal catalysts were filtered and diluted to a metal concentration of approximately 10 ppm after being digested for 24 h (50 mg of catalyst and 5 ml of aqua regia). Metal concentrations were measured using an Agilent 4200 MP-AES 7900. Grams of metal per gram of material were used to express the metal weight loadings.
(c). Photocatalytic activity evaluation using visible light
The photocatalytic activity evaluation of the samples was performed in an open-air, glass photoreactor system containing 0.025 g of the catalyst and 50 ml of an aqueous solution of MO dye (10 ppm). The exposed-to-air suspension was kept at room temperature with no pH change (pH = 7), and with magnetic stirring at 350 r.p.m. A cold water bath was used to reduce thermal effects maintaining a temperature range between 25 and 30°C. The resultant solution was irradiated with visible light (λ > 380 nm; 68 mW cm−2) using a SOLIS-3C high-power LED lamp (Thorlabs) over 4 h. To guarantee the adsorption–desorption equilibrium, the suspension was held in the dark for 30 min prior to being exposed to visible light. The method used to measure the MO dye concentration in each case was to acquire a filtered aliquot of one millilitre at regular intervals, using a UV–vis spectrophotometer to measure the aliquot absorbance (with the MO dye absorption band at 464 nm).
Percentage degradation = (1 C/C o) × 100, where the MO solution absorbance maxima before and after the radiation period are denoted by C o and C, respectively. The Langmuir–Hinshelwood kinetic model, which is typically used to describe the photodegradation kinetics on semiconductors, was used to determine the reaction rate constant. Pseudo-first-order kinetics with an apparent first-order rate constant (k app) can be used to simplify the reduction rate (r) in cases where the adsorption or reactant concentration is low. The initial concentration (C o ) is the reactant concentration after reaching time (t), which can be expressed as
where k r is the rate constant and k ads is the adsorption equilibrium constant. The apparent rate constant (k app) is the slope of a straight line obtained by plotting ln(C o/C) against reaction time (t).
3. Results
(a). Structure of the photocatalysts
Figure 1 illustrates the XRD patterns of N-doped support decorated with Pd, Pt and Au. They were all showing signs the presence the anatase polymorph with reflections at 25.3° (101), 36.9° (103), 37.8° (004), 38.6° (112), 48.1° (200), 53.9° (105), 55.1° (204), 62.14° (213), 62.7° (204), 68.8° (116), 70.30° (220), 75.0° (215) and 76.0° (301), agreed with the JCPDS 01-075-2547 card; also the reflections related to the rutile phase at 27.4° (110), 36.1° (101), 41.3° (111) and 44.1° (210) agreed with the JCPDS 01-079-6031 card. No evidence of nitrogen was observed in the N-TiO2 pattern. However, in the Pd/N-TiO2 pattern, a reflection related to Pd (111) was identified according to matching JCPDS 01-087-0645. The absence of reflections correlating to Pt and Au phases in the XRD pattern of Pt/N-TiO2 and Au/N-TiO2 indicates that these metal particles are too small to be detected on the surface of N-TiO2 surface. The crystallite size of the TiO2 in each catalyst was determined using the Scherrer equation at 2θ = 25.3° (101) for the anatase phase and 2θ = 27.4° (110) for the rutile phase. The findings indicate that the addition of metallic nanoparticles on the surface of N-TiO2 did not drastically affect the crystallite size for either phase, when compared to pristine P25 TiO2, with the exception of Au/N-TiO2 catalyst for rutile phase (see table 1). Figure 1b shows that the XRD reflections at 2θ = 25.3° for Pt and Au/N-TiO2 catalyst are shifted to larger angles in comparison with pristine TiO2. Table 1 indicates that for the materials, the measured loadings by MP-AES of Pd, Pt and Au metals were quite similar to the nominal one (1 wt%), indicating that DPU has good metal deposition efficiency.
Figure 1.
XRD pattern of (a) N-TiO2, Pd/N-TiO2, Pt/N-TiO2 and Au/N-TiO2 catalysts. (b) Magnified XRD peak at 25.3◦ (101) for all catalysts.
Table 1.
Textural properties of the compounds and their metal load.
| catalyst |
S
BET
(m2g−1) |
average pore size (Å) | crystallite size of anatase (nm) | crystallite size of rutile (nm) | anatase (101) peak position (2θ °) | d-spacing (nm) | actual M (M = Pd, Pt or Au) content wt% |
|---|---|---|---|---|---|---|---|
| TiO2 | 54 | 45.1 | 21 | 31 | 25.3 | 35.1 | — |
| N-TiO2 | 56 | 54.9 | 21 | 30 | 25.3 | 35.1 | — |
| Pd/N-TiO2 | 48 | 17.9 | 21 | 31 | 25.3 | 35.2 | 1.2 |
| Pt/N-TiO2 | 63 | 141 | 20 | 32 | 25.7 | 34.6 | 1.1 |
| Au/N-TiO2 | 95 | 162 | 21 | 35 | 25.5 | 34.8 | 1.1 |
Metal loading determined by MP-AES.
(b). Textural properties
The values of the specific surface areas for N-TiO2, Pd/N-TiO2, Pt/N-TiO2 and Au/N-TiO2 catalysts were 56, 48, 63 and 95 m2 g−1, respectively. The specific surface area of N-TiO2 did not drastically change, while for the Pd/N-TiO2 catalyst it decreased with respect to pristine TiO2-P25 (54 m2 g−1), probably as a result of Pd deposition causing partial occlusion of the support’s pores. On the other hand, the deposition of Pt and Au increased the specific surface area of these compounds compared to the N-TiO2 support, which was more evident in the Au/N-TiO2 catalyst. The findings of the adsorption/desorption analysis for N-TiO2, Pd/N-TiO2, Pt/N-TiO2 and Au/N-TiO2 are shown in figure 2. All of the photocatalysts presented N2 adsorption/desorption isotherm curves type IV with an H3-type hysteresis loop, indicating a distinctive mesoporous structure. The average pore sizes for the N-TiO2, Pd/N-TiO2, Pt/N-TiO2 and Au/N-TiO2 catalysts were 54.9, 17.9, 141 and 162 Å, respectively. A general trend was observed, with samples having a larger specific surface area also showing a larger pore size, probably owing to the influence of pH on the etching N-TiO2 surface during the deposition precipitation method using urea [26,27].
Figure 2.
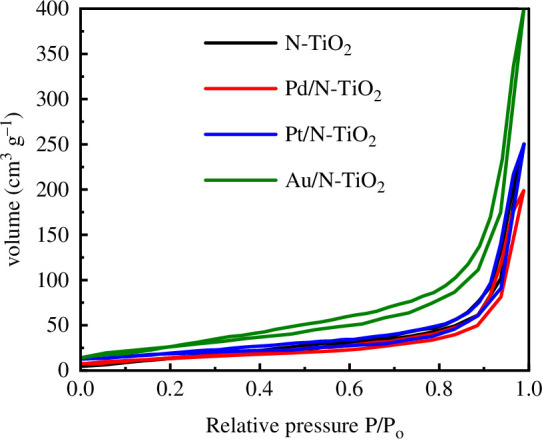
N2 adsorption/desorption isotherms of N-TiO2, Pd/N-TiO2, Pt/N-TiO2 and Au/N-TiO2 catalysts.
(c). TEM and XPS
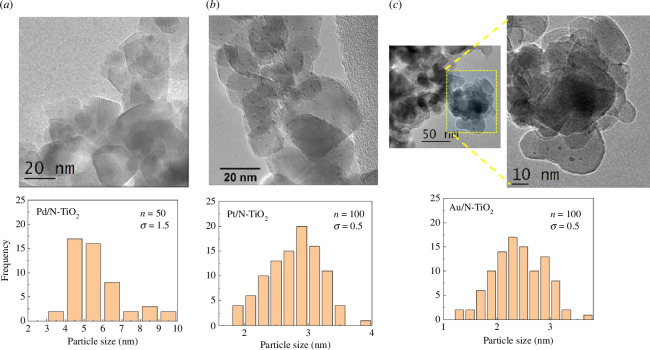
Figure 3a–c shows the TEM images and the associated dispersion of metal particle sizes for Pd/N-TiO2, Pt/N-TiO2 and Au/N-TiO2 catalysts. Semi-spherical metallic nanoparticles of Pt and Au were well-dispersed on the N-TiO2 surface with an average diameter of 3 and 2 nm, respectively. In contrast, the metallic nanoparticles of Pd were not as well dispersed on the support surface, with an average diameter of 6 nm and with a higher standard deviation (1.5) with respective to Pt and Au nanoparticles (0.5). These observations of particle size are in line with results from XPS, where the full-width-half-maximum of the nanoparticles are ca. 0.3–0.4 eV broader than their bulk metal equivalents (recorded under identical conditions) and have some differences in peak asymmetry for the Pt and Pd nanoparticles [28–30].
Figure 3.
TEM images and particle size distribution for (a) Pd/N-TiO2, (b) pt/N-TiO2 and (c) Au/N-TiO2.
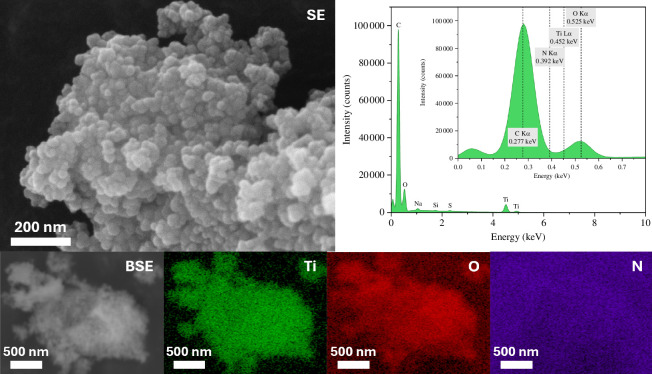
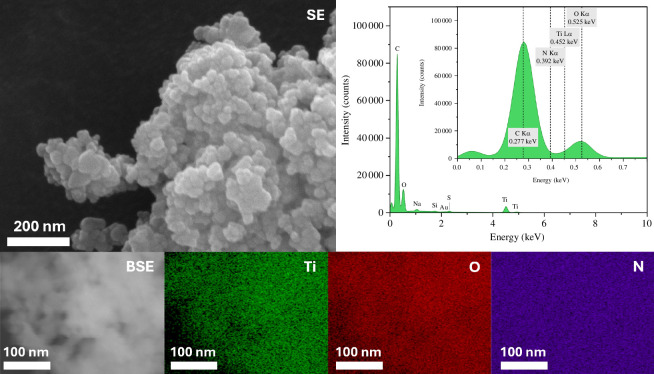
The surface elemental composition of the photocatalysts was studied using XPS to determine the extent of surface dopant inclusion. Table 2 lists the values for all the chemical elements present on the surface of the catalysts. In each case, nitrogen was observed. SEM-EDX was used to further investigate the existence of nitrogen in the catalysts. In contrast to the findings from XPS, nitrogen was not clearly observed by EDX in either N-TiO2 (figure 4) or Au/N-TiO2 (figure 5); however, this is believed to be due to the close overlap in the energies of the Kα peaks for carbon (present from the adhesive carbon discs) and nitrogen, at 0.277 keV and 0.392 keV, respectively (see inset EDX spectrum). The presence of S is also explained by the adhesive used in the carbon discs, and Si is an artefact of the silicon drift detector used in EDX.
Table 2.
Surface elemental composition of all catalysts as determined by XPS.
| sample | C 1s (% atom) | N 1s (% atom) | O 1s (% atom) | Ti 2p (% atom) | Pd 3d (% atom) | Pt 4f (% atom) | Au 4f (% atom) |
|---|---|---|---|---|---|---|---|
| N-TiO2 | 27.9 | 0.29 | 50.67 | 21.13 | — | — | — |
| Pd/N-TiO2 | 25.39 | 0.26 | 52.07 | 21.99 | 0.15 | — | — |
| Pt/N-TiO2 | 26.04 | 0.19 | 51.78 | 21.67 | — | 0.18 | — |
| Au/N-TiO2 | 25.74 | 0.26 | 52.27 | 21.41 | — | — | 0.18 |
Figure 4.
SEM imaging and EDX mapping of N-TiO2. Secondary electron image taken at 250 kx. Backscattered electron image taken at 41 kx. Elemental maps show titanium, oxygen and nitrogen in area of BSE image, with EDX spectrum showing intensities of the elements detected.
Figure 5.
SEM imaging and EDX mapping of Au/N-TiO2. Secondary electron image taken at 250 kx. Backscattered electron image taken at 320 kx. Elemental maps show titanium, oxygen and nitrogen in area of BSE image, with EDX spectrum showing intensities of the elements detected.
(d). Diffuse reflectance spectroscopy (DRS)
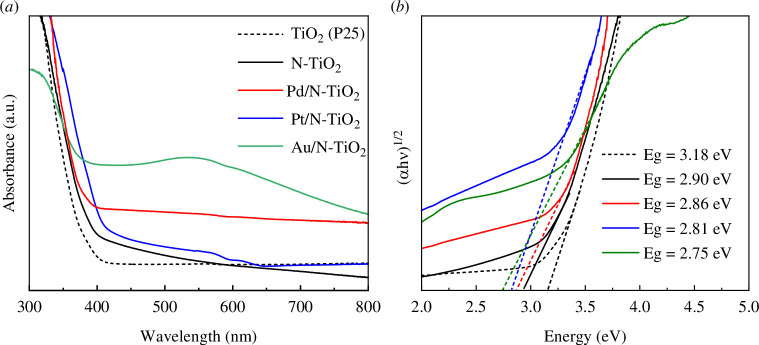
Figure 6a shows the results of DRS of N-TiO2, Pd/N-TiO2, Pt/N-TiO2 and Au/N-TiO2 catalysts. The DRS of commercial TiO2-P25 was also included as reference material. The band-gap energies of 3.2 and 3.0 eV, respectively, are associated with the well-known absorption of TiO2 anatase and rutile phases, which are, respectively, approximately 387 and 413 nm in wavelength [31,32]. In comparison to TiO2-P25, all of the surface-modified composites exhibited a shift in absorption to longer wavelengths, which was caused by the extra energy levels produced by N, Pd, Pt and Au on the surface of pristine TiO2-P25. Additionally, the surface plasmon resonance (SPR) in the Au/N-TiO2 photocatalyst reveals a unique behaviour concerning the absorption of gold nanoparticles in the visible spectrum (500−600 nm), verifying that the Au nanoparticles achieved the zero-valence state after being thermally treated in an H2 environment [33]. The SPR phenomenon of metallic nanoparticles is dependent on several parameters, including the size, shape, loading and dielectric characteristics of the surrounding medium [34,35]. Optical band-gap (E g ) energies obtained from diffuse reflectance spectra are shown in figure 6b . The addition of nitrogen on the TiO2-P25 surface helps to decrease its band gap from 3.18 to 2.9 eV. For Pd-, Pt- and Au-decorated photocatalysts, the reduction in band-gap energies was notably larger, measured at 2.86, 2.81 and 2.75 eV, respectively.
Figure 6.
(a) Diffuse reflectance spectra and (b) diffuse absorbance spectra of the (αhν)1/2 plots for N-TiO2, Pd/N-TiO2, Pt/N-TiO2, Au/N-TiO2 and TiO2-P25.
(e). Photocatalytic activity evaluation
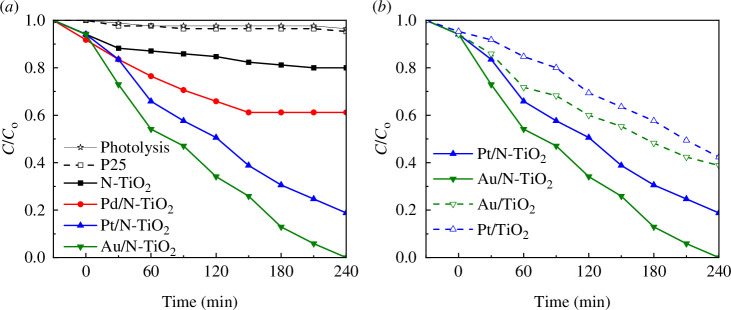
Figure 7a shows the relative concentration profile (C/C o) of the MO dye during both the photocatalytic and photolysis processes for TiO2-P25, N-TiO2, Pd/N-TiO2, Pt/N-TiO2, and Au/N-TiO2 materials. The C/C o graphs show the initial dye concentration (C/C o = 1), the data were obtained after 30 min in darkness with stirring (t = 0), and the subsequent values are post-illumination because, as was previously noted, the adsorption–desorption equilibrium was reached after 30 min.
Figure 7.
Evaluation of photocatalytic activity for (a) N-TiO2, Pd/N-TiO2, Pt/N-TiO2, Au/N-TiO2, TiO2-P25 and (b) photocatalytic evaluation of compounds with and without nitrogen.
Under the visible light illumination conditions applied in this study, it is evident that the dye concentration remained unchanged during dark conditions, indicating negligible adsorption of the dye on to the powder surfaces. Furthermore, the photolysis exhibited only marginal decreases in the C/C o ratio, confirming the stability of the MO dye throughout the irradiation. This observation, coupled with the absence of dye adsorption on the powder surfaces and minimal photolysis, allows us to classify the decrease in time of the C/C o ratio as a discolouration process caused by photocatalytic activity in semiconductor materials, which is not the same as mineralization of the dye. Mineralization refers to the complete conversion of dye molecules to inorganic substances such as CO2 and H2O.
In the photocatalytic reaction using the TiO2-P25, it was observed that the C/C o of the dye is very similar to the photolysis, which was expected owing to the value of its band gap (3.18 eV). In addition, the N-TiO2 powder achieved a notable discolouration of the MO dye after 4 h. Incorporating Pd, Pt and Au nanoparticles on to the surface of N-TiO2 notably enhanced its photocatalytic performance under visible light, culminating in complete discolouration of the MO dye by Au/N-TiO2 within 4 h. Furthermore, as shown figure 7b , the photocatalytic activity of Pt/N-TiO2, Au/N-TiO2, Pt/TiO2 and Au/TiO2 indicates that the combination of nitrogen and metallic nanoparticles on the TiO2-P25 surface exhibited greater efficiency compared to the presence of metallic nanoparticles alone. This indicates a synergistic effect between the N and the Au/Pt. The apparent kinetic rate constants (K app) calculated using the pseudo-first-order model for N-TiO2, Pd/N-TiO2, Pt/N-TiO2 and Au/N-TiO2 were 0.0007, 0.0028, 0.0054 0.0083 min−1, respectively.
The stability of the best catalyst (Au/N-TiO2) was tested by performing three consecutive cycles for MO dye degradation. The percentage value of MO dye degradation obtained in each cycle is shown in figure 8. The sample was gathered and centrifuged following each cycle to assess its potential for reuse. The photodegradation efficacies of MO dye were 99.9, 89 and 73% for first, second and third cycles, respectively. The photodegradation effectiveness of the Au/N-TiO2 catalyst was diminished as the cycle number increased; powder loss during suspension and centrifugation treatment may be the primary cause of this.
Figure 8.
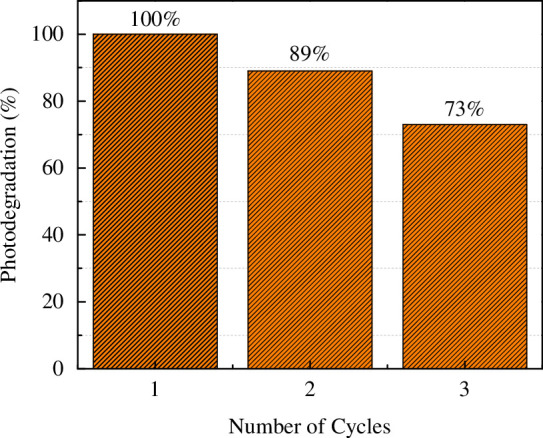
Percentage degradation values of MO dye obtained from the stability test using Au/TiO2 catalyst.
4. Discussion
In this work, nitrogen and metallic nanoparticles (Pd, Pt and Au) were used to modify commercial TiO2-P25 and to improve its photocatalytic performance under visible light to degrade the MO dye. Interestingly, these combinations (metal and non-metal) have been used to enhance visible light absorption of TiO2 previously [6]. The findings of this investigation reveal that the best photocatalyst for MO dye degradation under visible light illumination was the Au/N-TiO2 composite, followed in effectiveness by the Pt/N-TiO2 composite, with the least effective catalyst being the Pd/N-TiO2 composite.
The improved photocatalytic activity, using visible light illumination, of the Pt/N-TiO2 and Au/N-TiO2 photocatalysts is associated with their fundamental physical characteristics. It is commonly recognized that photocatalysts with large surface areas and/or small crystal sizes exhibit enhanced photocatalytic performance when it comes to the breakdown of organic compounds. The results demonstrate that the inclusion of all dopants on the TiO2-P25 increased the surface area in all cases except for palladium. The largest increase in surface area was from 54 to 95 m2 g−1 for Au/N-TiO2, while the crystalline sizes did not change with the addition of N, Pd, Pt or Au with respect to bare TiO2. Furthermore, the increased size and well-defined mesoporous structure of the Pt and Au catalysts enabled MO molecules (6–8 nm) to transfer and diffuse into the interior pores (14.1 nm for Pt/N-TiO2 and 16.2 nm for Au/N-TiO2), thus increasing the MO-photocatalyst contact interface and providing more reactive sites, resulting in an increase of MO dye degradation. The majority of previous research has concentrated on metallic nanoparticles with sizes ranging from 5 to 100 nm and found that as size decreased, catalytic activity increased [36–42]. On a few occasions, nanoparticle catalytic activity may decrease with decreasing size or remain unchanged [43,44]. It has been shown that small gold nanoclusters (<1.5 nm) exhibit a quantized electronic structure similar to a molecule owing to the quantum confinement phenomenon [45,46]. In this study, the nanoparticle sizes obtained were 5, 3 and 2 nm for Pd, Pt and Au, respectively.
All the diffraction patterns exhibited both the anatase and rutile phases related to commercial TiO2-P25 except the Pd/N-TiO2 material, where one small reflection was observed indicating the presence of metallic Pd. This observation suggests a larger particle size for Pd nanoparticles compared to the Pt and Au. Figure 1b shows that in comparison to TiO2-P25, the XRD reflections of Pt/N-TiO2 and Au/N-TiO2 are displaced to higher angles, indicating a lattice contraction. The synergy observed between Pt and Au ions with TiO2 is undoubtedly crucial, given that doped titania reveals diverse benefits to its photocatalytic activities by modifying its surface features and reducing the band gap. This agrees with the estimated optical band-gap (E g ) energies obtained from DRS for all materials that suffered a decrease with respect to E g values for bare TiO2. Specifically, Ti 3d and O 2p orbitals constitute the majority of the conduction and valence bands in the TiO2 matrix [47,48]. Introducing additional metal ions into the structure of TiO2 leads to interactions between the outer shell orbitals of these ions and the energy states in the bands, resulting in the creation of impurity levels and alterations in the band structures. Indeed, the influence of dopants on the electronic structures of semiconductors is closely linked to several factors, including the dopant atomic numbers, ionic radii and oxidation states [2,17,48]. These characteristics determine the extent of modifications in the band structure, impurity levels and, ultimately, the photocatalytic performance of the doped material. In summary, the presence of Pt and Au nanoparticles on the N-TiO2 surface leads to a significant distortion of the octahedral geometry, causing the conduction-band minimum to shift downward to lower energy levels, modifying its electronic properties and catalytic behaviour.
The surface chemical composition was obtained using XPS (table 2). It is important to emphasize that in each case nitrogen was detected on the surface of the TiO2-P25, and so we propose the near-surface is N functionalized, which, with a nitrogen binding energy of 399.7 eV, is characteristic of NHx species; however, the insertion of nitrogen at interstitial sites cannot be excluded [49]. Studies have demonstrated that co-catalysts (metallic nanoparticles) and N-functionalized TiO2 surfaces can form strong complexes with N bound on the surface, increasing loading levels and reducing co-catalyst agglomeration [18,50–52]. This can be seen in figure 5b , where the photocatalytic activity of the composites with N and Pt or Au nanoparticles presents much better photocatalytic performance than the composites with only Pt or Au nanoparticles on the surface, suggesting a synergistic effect between N and metallic nanoparticles.
There are some reports related to the use of non-metal and metal ions to improve the photocatalytic efficiency of TiO2 for dye degradation [4,53,54]. Nonetheless, the Au/N-TiO2 photocatalyst prepared in this study showed comparable or even better photocatalytic performance for MO dye degradation than those reported in previous works (table 3) without altering the pH, adding H2O2 or using UV illumination. The apparent kinetic rate constant (k app) for the Au/N-TiO2 photocatalyst was 0.0083 min−1, which is more than ten times larger than the K app of N-TiO2 composite (0.0007 min−1), two times larger than that estimated by Kader et al. [59] (0.004 min−1) and more than five times larger than the one reported by Ellouzi et al. [53] (0.0015 min−1) under similar experimental conditions. However, it is important to mention that the lack of standardization makes it difficult to reliably compare and reproduce findings across photocatalytic studies. Kisch & Bahnemann [64] suggest that for solid/liquid photocatalytic systems, an apparent optimal quantum yield should be obtained, which improves the comparability of photocatalytic activity results across different laboratories.
Table 3.
A comparison of the photocatalytic activity between the current study and other published works.
| material | dye | catalyst amount (g) | dye conc. (ppm) |
pH | type of light | illumination time (min) | effectiveness (%) | ref. |
|---|---|---|---|---|---|---|---|---|
| TiO2 NPsMO | MO | 0.15 | 15 | 3 | 100 W UV lamp | 300 | 99.8 | [55] |
| TiO2 NPs | MO | 0.10 | 5 | — | 182 W UV lamp | 240 | 41.1 | [56] |
| TiO2 spheres | MO | 0.02 | 10 | 7 | 32 W Hg lamp | 140 | 89.7 | [57] |
| TiO2/ASS | MO | 0.2 | 25 | 7 | UV lamp | 360 | 90 | [58] |
| Ag/MoO3/TiO2 | MO | 0.12 | 10 | 7 | UV irradiation | 330 | 75.8 | [59] |
| C, N, S–Fe–TiO2 | MO | 0.5 | 10 | — | 6 W visible lamp | 180 | 25 | [53] |
| M/TiO2 (M = Mn, Ni, Co) | MO | — | 8 | — | 300 W lamp that simulates solar radiation | 600 | 60 | [60] |
| NiSO4/TiO2 | MO | 1 g l−1 | 10 | 5.8 | UV irradiation | 120 | 31 | [54] |
| TiO2–Ag–WO3 | methylene blue | 0.5 | 10 | 7 | 121 W visible light source | 60 | 72 | [61] |
| TiO2–Au–WO3 | methylene blue | 0.02 | 30 | — | 300 W xenon lamps | 240 | 94.5 | [62] |
| TiO2-F–WO3 | MO | 0.12 | 10 | 3 | 100 W UV irradiation | 330 | 99.68 | [63] |
| Au/N–TiO2 | MO | 0.025 | 10 | 7 | visible light | 240 | 99.9 | this work |
5. Conclusion
This work demonstrates that doping TiO2 with nitrogen and metallic nanoparticles (Pd/N-TiO2, Pt/N-TiO2 and Au/N-TiO2) enhances the photocatalytic decomposition of MO mediated by visible light. The optimum photocatalytic activity in MO dye degradation was achieved using Au/N-TiO2 photocatalyst with 99.9% degradation observed after 4 h with an apparent first-order constant rate of k app = 0.0083 min−1. The photocatalytic performance during visible light illumination of Au/N-TiO2 with respect to the other composites is explained by the well-dispersed and smaller nanoparticle size (2 nm), largest surface area (95 m2g−1, 1.7 times larger than bare TiO2), lowest band-gap energy (2.75 eV) and synergistic effect at the interface sites between N and Au nanoparticles, with all of these confirmed by XRD, TEM, BET, DRS and XPS characterization techniques.
Acknowledgements
J.K.E. would like to acknowledge the Harmonized Impact Acceleration Account for Funding IG and Sêr Cymru programme, Welsh Government for the provision of the solar simulator. This publication is based on research funded by (or in part by) the Bill & Melinda Gates Foundation. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation.
X-ray photoelectron (XPS) data were acquired at the EPSRC National Facility for XPS (‘HarwellXPS’, EP/Y023587/1, EP/Y023609/1, EP/Y023536/1, EP/Y023552/1 and EP/Y023544/1).
For the purpose of open access, the author has applied a CC BY public copyright license (where permitted by UKRI, ‘Open Government License’ or ‘CC BY-ND public copyright license’ may be stated instead) to any Author Accepted Manuscript version arising.
Contributor Information
J. C. Medina, Email: MedinaJ@cardiff.ac.uk.
Eleanor Warren, Email: warrene4@cardiff.ac.uk.
David Morgan, Email: MorganDJ3@cardiff.ac.uk.
Isla E. Gow, Email: GowI@cardiff.ac.uk.
Jennifer Edwards, Email: edwardsjk@cardiff.ac.uk.
Data accessibility
Information on the data underpinning the results presented here, including how to access them, can be found in the Cardiff University data catalogue at [65].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
J.C.M.: conceptualization, data curation, investigation, methodology, writing—original draft; E.W.: conceptualization, investigation, methodology; D.M.: data curation, formal analysis, investigation, methodology, writing—original draft; I.E.G.: formal analysis, investigation, writing—original draft; J.E.: conceptualization, formal analysis, funding acquisition, methodology, project administration, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interests
We declare we have no competing interests.
Funding
This work was supported, in whole or in part, by the Bill & Melinda Gates Foundation (INV-048434). Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
References
- 1. Fujishima A, Honda K. 1972. Electrochemical photolysis of water at a semiconductor electrode. Nature 238 , 37–38. ( 10.1038/238037a0) [DOI] [PubMed] [Google Scholar]
- 2. Chang S m., Liu W s. 2014. The roles of surface-doped metal ions (V, Mn, Fe, Cu, Ce, and W) in the interfacial behavior of TiO2 photocatalysts. Appl. Catal. B: Environ. 156–157 , 466–475. ( 10.1016/j.apcatb.2014.03.044) [DOI] [Google Scholar]
- 3. Li D, Song H, Meng X, Shen T, Sun J, Han W, Wang X. 2020. Effects of particle size on the structure and photocatalytic performance by alkali-treated TiO(2). Nanomaterials 10 , 546. ( 10.3390/nano10030546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muscetta M, Jitan SA, Palmisano G, Andreozzi R, Marotta R, Cimino S, Di Somma I. 2022. Visible light – driven photocatalytic hydrogen production using Cu2O/TiO2 composites prepared by facile mechanochemical synthesis. J. Environ. Chem. Eng. 10 , 107735. ( 10.1016/j.jece.2022.107735) [DOI] [Google Scholar]
- 5. Devi LG, Kavitha R. 2013. A review on non metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal. B Environ. 140–141 , 559–587. ( 10.1016/j.apcatb.2013.04.035) [DOI] [Google Scholar]
- 6. Nagaveni K, Hegde MS, Madras G. 2004. Structure and photocatalytic activity of TiMO (M = W, V, Ce, Zr, Fe, and Cu) synthesized by solution combustion method. J. Phys. Chem. B 108 , 20204–20212. ( 10.1021/jp047917v) [DOI] [Google Scholar]
- 7. Reza KM, Kurny ASW, Gulshan F. 2017. Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl. Water Sci. 7 , 1569–1578. ( 10.1007/s13201-015-0367-y) [DOI] [Google Scholar]
- 8. Habib IY, Burhan J, Jaladi F, Lim CM, Usman A, Kumara NTRN, Tsang SCE, Mahadi AH. 2021. Effect of Cr doping in CeO2 nanostructures on photocatalysis and H2O2 assisted methylene blue dye degradation. Catal. Today 375 , 506–513. ( 10.1016/j.cattod.2020.04.008) [DOI] [Google Scholar]
- 9. Ansari M, Sajjadi SA, Sahebian S, Heidari EK. 2020. Photocatalytic and antibacterial activity of silver/titanium dioxide/zinc oxide nanoparticles coated on cotton fabrics. ChemistrySelect 5 , 8370–8378. ( 10.1002/slct.202001655) [DOI] [Google Scholar]
- 10. Calzada LA, Louis C, Wan Han C, Ortalan V, Zanella R. 2020. Au-Ru/TiO2 prepared by deposition-precipitation with urea: relevant synthesis parameters to obtain bimetallic particles. Appl. Catal. B Environ. 264 , 118503. ( 10.1016/j.apcatb.2019.118503) [DOI] [Google Scholar]
- 11. Padikkaparambil S, Narayanan B, Yaakob Z, Viswanathan S, Tasirin SM. 2013. Au/TiO2 reusable photocatalysts for dye degradation. Int. J. Photoenergy 2013 , 1–10. ( 10.1155/2013/752605) [DOI] [Google Scholar]
- 12. Yang J, et al. 2023. Enhancing visible-light photocatalytic performance of Au/TiO2catalysts through light reflection-promoted optical absorption with oriented anatase mesocrystals. J. Mater. Chem. A 11 , 4751–4757. ( 10.1039/D2TA09982A) [DOI] [Google Scholar]
- 13. Kim S, Hwang SJ, Choi WY. 2005. Visible light active platinum-ion-doped TiO2 photocatalyst. J. Phys. Chem. B 109 , 24260–24267. ( 10.1021/jp055278y) [DOI] [PubMed] [Google Scholar]
- 14. Nguyen CH, Fu CC, Juang RS. 2018. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: efficiency and degradation pathways. J. Clean. Prod. 202 , 413–427. ( 10.1016/j.jclepro.2018.08.110) [DOI] [Google Scholar]
- 15. Nkambule TI, Kuvarega AT, Krause RWM, Haarhoff J, Mamba BB. 2012. Synthesis and characterisation of Pd-modified N-doped TiO2 for photocatalytic degradation of natural organic matter (NOM) fractions. Environ. Sci. Pollut. Res. 19 , 4120–4132. ( 10.1007/s11356-012-0872-6) [DOI] [PubMed] [Google Scholar]
- 16. Seery MK, George R, Floris P, Pillai SC. 2007. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A Chem. 189 , 258–263. ( 10.1016/j.jphotochem.2007.02.010) [DOI] [Google Scholar]
- 17. Camposeco R, Hinojosa-Reyes M, Zanella R. 2022. Behavior of the energy levels of hydrogen titanate nanotubes decorated with Au, Ag, Mn, and Ni and their effect on the H2 evolution. Top. Catal. 65 , 989–999. ( 10.1007/s11244-022-01639-w) [DOI] [Google Scholar]
- 18. Motamedisade A, Heydari A, Yin YT, Alotabi AS, Andersson GG. 2024. Enhanced photocatalytic degradation of methyl orange using nitrogen-functionalized mesoporous TiO2 decorated with Au9 nanoclusters. Sol. Rrl. ( 10.1002/solr.202300943) [DOI] [Google Scholar]
- 19. Clark JH, Dyer MS, Palgrave RG, Ireland CP, Darwent JR, Claridge JB, Rosseinsky MJ. 2011. Visible light photo-oxidation of model pollutants using CaCu3Ti4O12: an experimental and theoretical study of optical properties, electronic structure, and selectivity. J. Am. Chem. Soc. 133 , 1016–1032. ( 10.1021/ja1090832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang B, Li X, Akiyama K, Bingham PA, Kubuki S. 2022. Elucidating the mechanistic origin of a spin state-dependent FeN x –C catalyst toward organic contaminant oxidation via peroxymonosulfate activation. Environ. Sci. Technol. 56 , 1321–1330. ( 10.1021/acs.est.1c05980) [DOI] [PubMed] [Google Scholar]
- 21. Asahi R, Morikawa T, Irie H, Ohwaki T. 2014. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: designs, developments, and prospects. Chem. Rev. 114 , 9824–9852. ( 10.1021/cr5000738) [DOI] [PubMed] [Google Scholar]
- 22. Bakar SA, Ribeiro C. 2016. Nitrogen-doped titanium dioxide: an overview of material design and dimensionality effect over modern applications. J. Photochem. Photobiol. C Photochem. Rev. 27 , 1–29. ( 10.1016/j.jphotochemrev.2016.05.001) [DOI] [Google Scholar]
- 23. Schneider J, Matsuoka M, Takeuchi M, Zhang J, Horiuchi Y, Anpo M, Bahnemann DW. 2014. Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev. 114 , 9919–9986. ( 10.1021/cr5001892) [DOI] [PubMed] [Google Scholar]
- 24. Daghrir R, Drogui P, Robert D. 2013. Modified TiO2for environmental photocatalytic applications: a review. Ind. Eng. Chem. Res. 52 , 3581–3599. ( 10.1021/ie303468t) [DOI] [Google Scholar]
- 25. Fairley N, et al. 2021. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 5 , 100112. ( 10.1016/j.apsadv.2021.100112) [DOI] [Google Scholar]
- 26. Liu H, Bansal S. 2023. Metal halide perovskite nanostructures and quantum dots for photocatalytic CO2 reduction: prospects and challenges. Mat. Today Energy 32 , 101230. ( 10.1016/j.mtener.2022.101230) [DOI] [Google Scholar]
- 27. García-Domínguez ÁE, Torres-Torres G, Arévalo-Pérez JC, Silahua-Pavón A, Sánchez-Trinidad C, Godavarthi S, Ojeda-López R, Sierra-Gómez UA, Cervantes-Uribe A. 2022. Urea assisted synthesis of TiO2–CeO2 composites for photocatalytic acetaminophen degradation via simplex-centroid mixture design. Res. Eng. 14 , 100443. ( 10.1016/j.rineng.2022.100443) [DOI] [Google Scholar]
- 28. Wertheim GK. 1987. Core-electron binding energies in free and supported metal clusters. Z. Physik B Condensed Matter 66 , 53–63. ( 10.1007/BF01312762) [DOI] [PubMed] [Google Scholar]
- 29. Morgan DJ. 2023. XPS insights: asymmetric peak shapes in XPS. Surf. Interface Anal. 55 , 567–571. ( 10.1002/sia.7215) [DOI] [Google Scholar]
- 30. Cheung TTP. 1986. X-ray photoemission studies of Pt·Sn and Pt·Pb bimetallic systems. Surf. Sci. 177 , 493–514. ( 10.1016/0039-6028(86)90029-4) [DOI] [Google Scholar]
- 31. Jang HD, Kim SK, Kim SJ. 2001. Effect of particle size and phase composition of titanium dioxide nanoparticles on the photocatalytic properties. J. Nanopart. Res. 3 , 141–147. ( 10.1023/A:1017948330363) [DOI] [Google Scholar]
- 32. Scanlon DO, et al. 2013. Band alignment of rutile and anatase TiO2 . Nat. Mater. 12 , 798–801. ( 10.1038/nmat3697) [DOI] [PubMed] [Google Scholar]
- 33. Medina JC, Garcia-Perez VI, Zanella R. 2021. Metallic composites based on Ag, Cu, Au and Ag-Cu nanoparticles with distinctive bactericidal effect on varied species. Mat. Today. Commun. 26 , 102182. ( 10.1016/j.mtcomm.2021.102182) [DOI] [Google Scholar]
- 34. Dodekatos G, Schünemann S, Tüysüz H. 2016. Surface plasmon-assisted solar energy conversion. In Solar energy for fuels (eds H. Tüysüz J, Chan CK), pp. 215–252. Cham: Springer International Publishing. ( 10.1007/128_2015_642) [DOI] [PubMed] [Google Scholar]
- 35. Du LC, Furube A, Yamamoto K, Hara K, Katoh R, Tachiya M. 2009. Plasmon-induced charge separation and recombination dynamics in gold−TiO2 nanoparticle systems: dependence on TiO2 particle size. J. Phys. Chem. C. 113 , 6454–6462. ( 10.1021/jp810576s) [DOI] [Google Scholar]
- 36. Jin RC, Li G, Sharma S, Li YW, Du XS. 2021. Toward active-site tailoring in heterogeneous catalysis by atomically precise metal nanoclusters with crystallographic structures. Chem. Rev. 121 , 567–648. ( 10.1021/acs.chemrev.0c00495) [DOI] [PubMed] [Google Scholar]
- 37. Du YX, Sheng HT, Astruc D, Zhu MZ. 2020. Atomically precise noble metal nanoclusters as efficient catalysts: a bridge between structure and properties. Chem. Rev. 120 , 526–622. ( 10.1021/acs.chemrev.8b00726) [DOI] [PubMed] [Google Scholar]
- 38. Li S, Du X, Liu Z, Li Y, Shao Y, Jin R. 2023. Size effects of atomically precise gold nanoclusters in catalysis. Precis. Chem. 1 , 14–28. ( 10.1021/prechem.3c00008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Y, Zhang P, Wan D, Xue C, Zhao J, Shao G. 2020. Direct evidence of 2D/1D heterojunction enhancement on photocatalytic activity through assembling MoS2 nanosheets onto super-long TiO2 nanofibers. Appl. Surf. Sci. 504 , 144361. ( 10.1016/j.apsusc.2019.144361) [DOI] [Google Scholar]
- 40. Dong C, Lian C, Hu S, Deng Z, Gong J, Li M, Liu H, Xing M, Zhang J. 2018. Size-dependent activity and selectivity of carbon dioxide photocatalytic reduction over platinum nanoparticles. Nat. Commun. 9 , 1252. ( 10.1038/s41467-018-03666-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raza MA, Kanwal Z, Rauf A, Sabri AN, Riaz S, Naseem S. 2016. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 6 , 74. ( 10.3390/nano6040074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ydollahi M, Ahari H, Anvar AA. 2016. Antibacterial activity of silver-nanoparticles against staphylococcus aureus. Afr. J. Microbiol. Res. 10 , 850–855. ( 10.5897/AJMR2016.7908) [DOI] [Google Scholar]
- 43. Seong H, Efremov V, Park G, Kim H, Yoo JS, Lee D. 2021. Atomically precise gold nanoclusters as model catalysts for identifying active sites for electroreduction of CO2 Angew. Chem. Int. Ed. Engl. 60 , 14563–14570. ( 10.1002/anie.202102887) [DOI] [PubMed] [Google Scholar]
- 44. Li S, Nagarajan AV, Du X, Li Y, Liu Z, Kauffman DR, Mpourmpakis G, Jin R. 2022. Dissecting critical factors for electrochemical CO2 reduction on atomically precise au nanoclusters. Angew. Chem. Int. Ed. 61 , e202211771. ( 10.1002/anie.202211771) [DOI] [PubMed] [Google Scholar]
- 45. Adnan RH, Madridejos JML, Alotabi AS, Metha GF, Andersson GG. 2022. A review of state of the art in phosphine ligated gold clusters and application in catalysis. Adv. Sci. (Weinh). 9 , e2105692. ( 10.1002/advs.202105692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mousavi H, Yin Y, Howard-Fabretto L, Sharma SK, Golovko V, Andersson GG, Shearer CJ, Metha GF. 2021. Au101-rGo nanocomposite: immobilization of phosphine-protected gold nanoclusters on reduced graphene oxide without aggregation. Nanoscale Adv. 3 , 1422–1430. ( 10.1039/d0na00927j) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kreibig U, Vollmer M. 1995. Optical properties of metal clusters. Springer Ser. Mater. Sci. 25 , 535. ( 10.1007/978-3-662-09109-8) [DOI] [Google Scholar]
- 48. Shough AM, Doren DJ, Ogunnaike B. 2009. Transition metal substitution in ETS-10: DFT calculations and a simple model for electronic structure prediction. Chem. Mater. 21 , 1232–1241. ( 10.1021/cm8021177) [DOI] [Google Scholar]
- 49. Dunnill CWH, Aiken ZA, Pratten J, Wilson M, Morgan DJ, Parkin IP. 2009. Enhanced photocatalytic activity under visible light in N-doped TiO2 thin films produced by APCVD preparations using T-butylamine as a nitrogen source and their potential for antibacterial films. J. Photochem. Photobiol. A 207 , 244–253. ( 10.1016/j.jphotochem.2009.07.024) [DOI] [Google Scholar]
- 50. Kitazawa N, Sato H, Watanabe Y. 2007. Effects of post-deposition chemical treatment on the formation of mesoporous titania films. J. Mater. Sci. 42 , 5074–5079. ( 10.1007/s10853-006-0479-8) [DOI] [Google Scholar]
- 51. Martínez‐Ferrero E, Sakatani Y, Boissière C, Grosso D, Fuertes A, Fraxedas J, Sanchez C. 2007. Nanostructured titanium oxynitride porous thin films as efficient visible‐active photocatalysts. Adv. Funct. Mater. 17 , 3348–3354. ( 10.1002/adfm.200700396) [DOI] [Google Scholar]
- 52. Viswanathan B, Krishanmurthy KR. 2012. Nitrogen incorporation in TiO2: does it make a visible light photo-active material?. Int. J. Photoenergy 2012 , 1–10. ( 10.1155/2012/269654) [DOI] [Google Scholar]
- 53. Ellouzi I, El Hajjaji S, Harir M, Schmitt Koplin P, Robert D, Laânab L. 2019. Synergistic effects of C, N, S, Fe-multidoped TiO2 for photocatalytic degradation of methyl orange dye under UV and visible light irradiations. SN Appl. Sci. 1 . ( 10.1007/s42452-019-0857-x) [DOI] [Google Scholar]
- 54. Regraguy B, Rahmani M, Mabrouki J, Drhimer F, Ellouzi I, Mahmou C, Dahchour A, Mrabet ME, Hajjaji SE. 2022. Photocatalytic degradation of methyl orange in the presence of nanoparticles NiSO4/TiO2 . Nanotechnol. Environ. Eng. 7 , 157–171. ( 10.1007/s41204-021-00206-0) [DOI] [Google Scholar]
- 55. Rashid Al-Mamun M, Hossain KT, Mondal S, Afroza Khatun M, Shahinoor Islam M, Zaved Hossain Khan DM. 2022. Synthesis, characterization, and photocatalytic performance of methyl orange in aqueous TiO2 suspension under UV and solar light irradiation. S. Afr. J. Chem. Eng. 40 , 113–125. ( 10.1016/j.sajce.2022.02.002) [DOI] [Google Scholar]
- 56. Mosquera EE, Herrera-Molina D, Diosa JE. 2021. Propiedades estructurales y ópticas de las nanopartículas de TiO2 y su comportamiento fotocatalítico bajo luz visible. Ing. Compet. 23 , e21310965. ( 10.25100/iyc.v23i2.10965) [DOI] [Google Scholar]
- 57. Kanjana N, Maiaugree W, Poolcharuansin P, Laokul P. 2020. Size controllable synthesis and photocatalytic performance of mesoporous TiO2 hollow spheres. J. Mater. Sci. Technol. 48 , 105–113. ( 10.1016/j.jmst.2020.03.013) [DOI] [Google Scholar]
- 58. Rashed MN, Eltaher MA, Abdou ANA. 2017. Adsorption and photocatalysis for methyl orange and Cd removal from wastewater using TiO2 . R. Soc. Open Sci. 4 , 170834. ( 10.1098/rsos.170834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kader S, Al-Mamun MR, Suhan MBK, Shuchi SB, Islam MS. 2022. Enhanced photodegradation of methyl orange dye under UV irradiation using MoO3 and Ag doped TiO2 photocatalysts. Environ. Technol. Inno. 27 , 102476. ( 10.1016/j.eti.2022.102476) [DOI] [Google Scholar]
- 60. Stojadinović S, Radić N, Vasilić R, Tadić N, Tsanev A. 2022. Photocatalytic degradation of methyl orange in the presence of transition metals (Mn, Ni, Co) modified TiO2 coatings formed by plasma electrolytic oxidation. Solid State Sci. 129 , 106896. ( 10.1016/j.solidstatesciences.2022.106896) [DOI] [Google Scholar]
- 61. Basumatary B, Basumatary R, Ramchiary A, Konwar D. 2022. Evaluation of Ag@TiO2/Wo3 heterojunction photocatalyst for enhanced photocatalytic activity towards methylene blue degradation. Chemosphere 286 , 131848. ( 10.1016/j.chemosphere.2021.131848) [DOI] [PubMed] [Google Scholar]
- 62. Yang X, Fu H, Wang W, Xiong S, Han D, Deng Z, An X. 2020. Enhanced solar light photocatalytic performance based on a novel Au-WO3@TiO2 ternary core–shell nanostructures. Appl. Surf. Sci. 505 , 144631. ( 10.1016/j.apsusc.2019.144631) [DOI] [Google Scholar]
- 63. Suhan MBK, Shuchi SB, Al-Mamun MR, Roy H, Islam MS. 2023. Enhanced UV light-driven photocatalytic degradation of methyl orange using MoO3/WO3-fluorinated TiO2 nanocomposites. Environ. Nanotechnol. Monit. Manag. 19 , 100768. ( 10.1016/j.enmm.2022.100768) [DOI] [Google Scholar]
- 64. Kisch H, Bahnemann D. 2015. Best practice in photocatalysis: comparing rates or apparent quantum yields? J. Phys. Chem. Lett. 6 , 1907–1910. ( 10.1021/acs.jpclett.5b00521) [DOI] [PubMed] [Google Scholar]
- 65. Gow I, Morgan D, Medina JCet al. 2024. Influence of Pd, Pt, and Au nanoparticles in the photocatalytic activity of N-TiO2 support under visible light: Data. Cardiff University ( 10.17035/d.2024.0316533542) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Information on the data underpinning the results presented here, including how to access them, can be found in the Cardiff University data catalogue at [65].