Abstract
Meningiomas are the most common primary intracranial tumors in adults and are increasing in incidence due to the aging population and increased access to neuroimaging. While most exhibit nonmalignant behavior, a subset of meningiomas are biologically aggressive and are associated with treatment resistance, resulting in significant neurologic morbidity and even mortality. In recent years, meaningful advances in our understanding of the biology of these tumors have led to the incorporation of molecular biomarkers into their grading and prognostication. However, unlike other central nervous system (CNS) tumors, a unified molecular taxonomy for meningiomas has not yet been established and remains an overarching goal of the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy-Not Official World Health Organization (cIMPACT-NOW) working group. Additionally, clinical equipoise still remains on how specific meningioma cases and patient populations should be optimally managed. To address these existing gaps, members of the International Consortium on Meningiomas including field-leading experts, have prepared this comprehensive consensus narrative review directed toward clinicians, researchers, and patients. Included in this manuscript are detailed overviews of proposed molecular classifications, novel biomarkers, contemporary treatment strategies, trials on systemic therapies, health-related quality-of-life studies, and management strategies for unique meningioma patient populations. In each section, we discuss the current state of knowledge as well as ongoing clinical and research challenges to road map future directions for further investigation.
Keywords: extra-axial, meningioma, methylation, molecular, neurofibromatosis 2, nonmalignant, radiotherapy
Meningioma is the most common primary intracranial tumor in adults. Historically, investigation into its molecular biology and pathogenesis has trailed other central nervous system (CNS) tumors. Since 2016, through the efforts of independent research groups and consortia including but not limited to the International Consortium on Meningiomas (ICOM) and the German Consortium on Aggressive Meningiomas (KAM), there has been a surge in molecular studies on meningiomas that have uncovered novel diagnostic and prognostic alterations. Despite these advances, meningioma treatments are still largely limited to surgery and radiotherapy (RT). Systemic medical therapies are reserved for otherwise treatment-refractory meningiomas in the context of clinical trials. There is a pressing need to translate findings from the current molecular era of meningioma research into meaningful improvements in decision-making and novel therapies. In this comprehensive consensus review, key advances in the understanding of meningioma biology will be discussed, with a focus on recent breakthroughs. Each section will also discuss ongoing controversies, critical knowledge gaps and areas of unmet need for clinicians, researchers, and patients that could be targeted for future research and investigation.
Epidemiology and Risk Factors
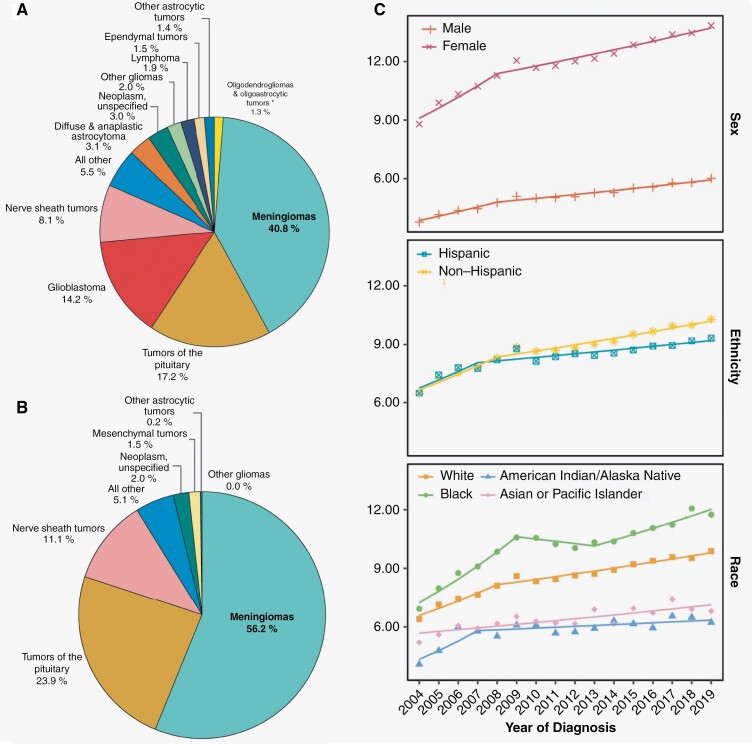
Meningiomas make up 40.8% of all primary brain tumors in the United States and 56.2% of “nonmalignant” primary brain tumors (Figure 1A and B).1 Incidence rates of nonmalignant meningioma are the highest amongst all CNS tumors at 9.73 per 100 000 population in the United States. These rates increase after the age of 65 years and again after the age of 85. Age-adjusted incidence rates of nonmalignant meningiomas continue to increase across different sexes, ethnicities, and races (Figure 1C). Meningiomas also account for the largest proportion of intradural spinal tumors in patients 20 years of age and older (39.9%), although spinal meningiomas represent only 4.2% of all diagnosed meningiomas.1
Figure 1.
(A) Distribution of all primary brain tumors (malignant and nonmalignant combined; 5-year total = 453 623; annual average cases = 90 725) by histopathology. (B) Distribution of all nonmalignant primary brain tumors (5-year total = 326 894; average annual cases = 65 379) by histopathology. (C) Annual age-adjusted incidence rates of meningioma based on sex, ethnicity, and race. (D) Incidence rate ratios by sex (female-to-male) for selected primary brain and other central nervous system (CNS) tumor histopathologies with malignant and nonmalignant meningiomas highlighted. (E) Female-to-male incidence rate ratios and 95% confidence intervals (CI) for meningioma, by age group at diagnosis and stratified by WHO tumor grade. (F) Incidence rate ratios by race (white:black and white:Asian or Pacific Islander [API]) for selected primary brain and other CNS tumor histopathologies with malignant and nonmalignant meningiomas highlighted. (G) Average annual age-adjusted incidence rate and 95% confidence interval (CI) for meningioma by race/ethnicity and stratified by grade. Incidence rate ratios (IRR) and their 95% CI appear above bars and are calculated relative to non-Hispanic White individuals as the reference. Rates are age-adjusted to the 2000 US standard population. CBTRUS statistical report: US Cancer Statistics—National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology, and End Results (SEER), 2016–2020.1,2 Image panels A–D, F reused with permission from Ostrom et al. (2023).1 Image panels E, G reused with permission from Walsh et al. (2023).2 GBM, glioblastoma; CBTRUS, Central Brain Tumor Registry of the United States.
By World Health Organization (WHO) 2021 grading, 80.1% of reported meningiomas are CNS WHO grade 1, 18.3% are grade 2, and 1.5% are grade 3.1,3 “Nonmalignant” meningiomas (represented in the SEER database in the following proportions: 81.4% CNS WHO grade 1 and 18.4% CNS WHO grade 2) are 2.3 times more commonly diagnosed in females than in males, and this disparity is the largest between the ages of 35 and 44 (Figure 1D and E). Ten-year relative survival for nonmalignant meningioma is 83.4%. Although the SEER database quotes a 10-year survival rate of 60% for “malignant” meningiomas, this group is not exclusively comprised of CNS WHO grade 3 cases (63.6%) but also includes a sizeable proportion of CNS WHO grade 1 (20.4%) and grade 2 (15%) meningiomas. Notably, the designation of a “malignant” meningioma in this context is imprecisely defined and based on ICD coding instead of central neuropathological review. Non-registry data of exclusively CNS WHO grade 3 malignant or anaplastic meningiomas show far more dismal outcomes with a 5-year overall survival rate of 66% in one cohort and an estimated 10-year overall survival of only 14–24%.1,2,4,5
The incidence of intracranial meningiomas is higher in black patients compared to white patients and this disparity increases with higher tumor grade (Figure 1F and G).2,6 In turn, the incidence of “nonmalignant” meningiomas is higher in white patients compared to Asian-Pacific Islanders, although there may be a higher incidence of “malignant meningiomas” in the latter group (Figure 1F). The reasons behind these racial and ethnic differences remain unknown and the limitations of reporting based on population-based epidemiological data need to be considered, particularly for comparisons between different countries and continents.
Heritable genetic polymorphisms in MLLT10 (MLLT10 histone lysine methyltransferase DOT1L cofactor) have also been robustly associated with increased meningioma risk.7,8 Distinct from germline variants that cause hereditary syndromes associated with meningiomas, MLLT10 risk alleles are common at the population level and confer a comparatively modest increase in meningioma risk. These variants also increase the risk for ovarian cancer and estrogen receptor-positive breast cancer, and pan-cancer analyses implicate a potential estrogenic mechanism connecting MLLT10 variation to the risk of diverse tumor types.9
Despite progress in identifying exogeneous and endogenous factors associated with risk of meningioma development, relatively few modifiable risk factors have been definitively identified. These few include ionizing radiation, elevated body mass index, methotrexate treatment, and cigarette smoking (where the increased risk is restricted to male sex only).10–21 The most well-validated of these risk factors is cranial irradiation, with a linear dose–response association between the radiation dose received and the risk of subsequent meningioma development, particularly in patients who were treated under the age of 10 (to be further discussed in the Radiation-Induced Meningiomas [RIMs] section later in the article).19,20 Despite the fact that meningiomas are known to commonly express progesterone receptors, estrogen receptor expression is rare and there are conflicting results on the risk of meningioma growth or development in response to endogenous and exogenous sex hormones.22–28 Several large retrospective studies have demonstrated a positive association between current or past use of hormone replacement therapy and the diagnosis of a meningioma.21,29 On a population-level, pregnancy does not appear to be a risk factor for meningioma development, although accelerated growth of an existing meningioma during pregnancy has long been described.30–32There is currently insufficient evidence to support a standardized screening approach such as germline genetic testing or routine neuroimaging, even in higher risk cohorts such as female relatives of meningioma patients with the MLLT10 risk allele, or women on hormone replacement therapy. This remains an area of active investigation and guidelines may evolve with emerging evidence.
Genomics and Biology
The neurofibromatosis-2 (NF2) gene was the first gene to be implicated in meningioma development (Table 1). It remains the most common genetic abnormality in sporadic meningiomas, inclusive of short structural or copy number variants, and is found in up to 60% of all meningioma cases. As a tumor suppressor gene on chromosome 22q12.2, NF2 encodes the protein Merlin which has been implicated in the inhibition of signals from the PI3K/Akt, Raf/MEK/ERK, and mTOR signaling pathways in non-meningioma cells.33–35 In meningioma cells, loss of Merlin may also be associated with overexpression of yes-associated protein 1 (YAP1) and deregulation of the Hippo signaling pathway, leading to increased cell proliferation and anchorage-independent growth.36 NF2/Merlin loss may also increase the apoptotic threshold of meningioma cells and decrease susceptibility to cytotoxic therapies through interferon regulatory factor-mediated gene expression pathways.37 Consequently, meningiomas with NF2 alterations have an increased risk of being higher grade and more biologically aggressive, although benign NF2-mutant cases are still observed. The rate of NF2 mutations in meningiomas in one large study were found to be 37% in CNS WHO grade 1 cases (81/220), 60% of grade 2 cases (265/441), and 69% of grade 3 tumors (122/176).38
Table 1.
Recurrent Mutations Associated With Meningiomas
| Gene | Location | Protein | Gene status | Frequency (%) | WHO grade | Modification | Associated meningioma phenotype | Effect of modification | Reference |
|---|---|---|---|---|---|---|---|---|---|
| AKT1 | 14q32.33 | Protein kinase B alpha, beta, and gamma | Oncogene | 10 | 1 | Point mutation | Anterior/middle skull base location, NF2-wild-type meningiomas | Conformational change in protein, altering its localization from the cytoplasm to the plasma membrane, resulting in constitutive activation of the AKT1 kinase and activation of mTOR and ERK1/2 signaling pathways | 38,39,41,383–387 |
| ARID1A | 1p36.11 | AT-rich interaction domain 1A | Tumor suppressor | 5 | 2, 3 | Frameshift mutation | Recurrent and high-grade cases | Destabilizes SWI/SNF complex which normally modulates DNA accessibility for cellular processes involved in chromatin structure including transcription, DNA replication, and repair, resulting in global deregulation of gene transcription | 38,59–61,89 |
| BAP1 | 3p21.1 | BRCA1-associated protein 1 | Tumor suppressor | <1 | 2, 3 | Splice site, nonsense, frameshift mutation | Rhabdoid and papillary histology | Impaired nuclear localization of a ubiquitin carboxy-terminal hydrolase that normally has tumor suppressor activity when bound to BRCA1 or BARD1 | 38,45,53,62,70,71,388 |
| BRAF (V600E) | 7p34 | B-Raf proto-oncogene | Oncogene | <1 | 3 | Point mutation | Rhabdoid histology | Mimics phosphorylation of nearby serine and threonine residues resulting in BRAF activation and subsequent activation of the MAP kinase/ERK-signaling pathway | 43,93,389,390 |
| CDH1 | 16q22 | E-cadherin | Tumor suppressor | 30 | 1–3 | Deletion, nonsense mutation | Unknown | Activation of wnt signaling via dysregulation of β-catenin and the APC protein, resulting in upregulation of cell cycling programs including c-myc and cyclin D1 pathways | 391–393 |
| CDKN2A/B | 9p21.3 | p16, p15INK4b | Tumor suppressor | 1–5 | 3 | Deletion | Anaplastic, biologically aggressive meningiomas | Loss of inhibition of CDK4 and CDK6 mediated phosphorylation of retinoblastoma family of proteins, leading to unchecked cell cycle progression from G1 to S-phase | 3,38,92,93,96,98,101,119,394–398 |
| CDKN2C | 1p32.3 | Cyclin-dependent kinase 4 inhibitor C (p18) | Tumor suppressor | 1 | 2 | Nonsense mutation | Atypical and anaplastic meningiomas | Activation of CDK4 and CDK6 resulting in loss of control of cell growth regulation and subsequent cell cycle G1 progression | 92,96 |
| CHEK2 | 22q12.1 | Checkpoint kinase 2 (Chk2) | Tumor suppressor | 50 | 1–3 | Deletion, frameshift mutation | NF2-altered meningiomas | Defects in DNA homologous recombination or nonhomologous end-joining pathways following DNA damage including double-stranded DNA breaks resulting in increased chromosomal instability | 349,399 |
| Dal-1 | 18p11.3 | Protein 4.1B | Tumor suppressor | 50–80 | 1–3 | Deletion, nonsense mutation | Multiple meningiomas, sporadic meningiomas | Dysregulation of cell-cell contact inhibition growth arrest normally mediated through actin cytoskeletal-associated proteins resulting in similar downstream effects as NF2 inactivation | 354,400–402 |
| EZH2 | 7q36.1 | Enhancer of zeste homolog 2 | Oncogene | 1–5 | 2–3 | Point mutation, amplification | Higher grade meningiomas | Dysregulation of catalytic subgroup of PRC2 complex, upregulation of proliferative genes in the cell cycle-retinoblastoma-E2F pathway | 403–405 |
| FBXW7 | 4q31.3 | F-box and WD repeat domain containing 7 | Tumor suppressor | 1–5 | 1–3 | Frameshift mutation | NF2-altered meningiomas | Deregulation of ubiquitin-mediated proteolysis of oncoproteins such as cyclin E, notch, c-Jun, c-Myc, mTOR resulting in increased tumorigenesis | 406,407 |
| FGFR3 | 4p16.3 | Fibroblast growth factor receptor 3 | Oncogene | 15 | 1–2 | Missense mutation | Skull base location, NF2-wild-type meningiomas | Increased mitogenic signaling from FGF receptors/kinases via activation of the PI3K-Akt-p70S6K pathway and activation of STAT3 | 408,409 |
| KDM6A | Xp11.3 | Lysine demethylase 6A (UTX) | Tumor suppressor | 5 | 2–3 | Deletion | NF2-altered, higher-grade meningiomas | Dysregulation of polycomb repressive complex (PRC2) catalyzed histone methylation including of H3K27 | 38,410 |
| KLF4 | 9p31 | Krüppel-like factor 4 | Tumor suppressor | 10–15 | 1 | Missense mutation | Skull base location, secretory meningiomas (when combined with TRAF7 mutation) | Deactivation of CDKN1A (cyclin-dependent kinase inhibitor p21) resulting in cell proliferation, and loss of inhibition of CDK1 transcription | 10,39,45,132,411–415 |
| NF2 | 22q12.2 | Merlin | Tumor suppressor | 40–60 | 1–3 | Deletion, nonsense mutation | Non-skull base location | Dysregulation of several essential cell proliferation and survival pathways including loss of cell-to-cell contact inhibition, activation of hippo pathway, mTOR/PI3K/AKT pathway, and receptor tyrosine kinases | 33–35,37,64,91,92,109,175,354,395,402,416–418 |
| PIK3CA | 3q26.32 | Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (p110α protein) | Oncogene | 5 | 1 | Point, missense mutation | Skull base location, benign WHO grade 1, progestin-related meningiomas | Activation of PI3 kinase and PI3K/AKT pathway resulting in downstream signaling cascades mediating cell survival. Apoptosis, transformation, metastasis, and cell migration | 42–44,419,420 |
| POLR2A | 17p13.1 | RNA polymerase II subunit A | Oncogene | 5 | 1 | Missense mutation | Skull base, tuberculum sellae location, benign WHO grade 1 meningiomas, meningothelial histology | Activation of catalytic subunit of RNA polymerase II, hijacking enzyme and driving cell proliferation and neoplastic progression | 40,45,415 |
| PBRM1 | 3p21.1 | Protein polybromo-1 | Tumor suppressor | 1 | 3 | Nonsense mutation, deletion | Papillary histology | Dysregulation of SWI/SNF chromatin remodeling complex, dysfunctional repair of DNA double-stranded breaks via ATM phosphorylation | 38,54 |
| PTEN | 10q23.31 | Phosphatase and tensin homolog | Tumor suppressor | 2–6 | 2, 3 | Frameshift mutation, deletion, rearrangement | Biologically aggressive, proliferative meningiomas | Dysregulation of AKT/PI3K pathway in the cell cytoplasm resulting from loss of feedback inhibition of AKT and subsequent uncontrolled cell cycle progression | 38,62–64 |
| SMARCB1 | 22q11.23 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 | Tumor suppressor | <5 | 2, 3 | Missense mutation | NF2-altered, atypical meningiomas | Inactivation of core subunit of SWI/SNF chromatin remodeling complex resulting in aberrant enhancer and promoter regulation and subsequent loss of transcriptional control | 61,421,422 |
| SMARCE1 | 17q21.2 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily E member 1 | Tumor suppressor | <1 | 1 | Splice site, nonsense, frameshift mutation | Clear cell histology | Inactivation of subunit of SWI/SNF chromatin remodeling complex resulting in loss of apoptosis induction via tumor suppressor gene CYLD and other pathways | 47–51,69 |
| SMO | 7p32.1 | Smoothened | Oncogene | 3–5 | 1 | Point mutation | Anterior medial skull base location | Activation of the sonic hedgehog (SHH) signaling pathway resulting in cell proliferation, differentiation, and angiogenesis | 39,41,42,387,423,424 |
| SUFU | 10q24.32 | Suppressor of fused homolog | Tumor suppressor | 1–2 | 2–3 | Frameshift mutation | Familial multiple meningiomas | Abnormal constitutive upregulation of downstream Gli-mediated transcription factors in SHH pathway | 74,75,425,426 |
| TERTp | 5p15.33 | Telomerase reverse transcriptase promoter | Oncogene | 5.5 | 3 | Point mutation | Biologically aggressive, high-grade meningiomas | Activation of telomerase-mediated telomere stabilization resulting in delayed replicative senescence and increased telomere-driven genomic instability | 56–59,427–429 |
| TRAF7 | 16p13.3 | TNF receptor-associated factors 7 | Tumor suppressor | 20–25 | 1 | Missense mutation | Skull base location, brain invasion | Disruption of catalytic activity of E3 ubiquitin ligase interaction with MAPK pathway and RAS GTPases, altering actin dynamics and promoting anchorage-independent growth | 39,132,430 |
While some genes and their alterations have been well investigated by multiple independent groups, others may have been identified in only single or small cohorts of meningiomas. Consequently, the overall frequencies of less studied mutations may be confounded by smaller cohorts biased to include either more benign or clinically aggressive cases from which the data is obtained.
More recently, recurrent mutations in TRAF7 (tumor necrosis factor receptor-associated factor 7), KLF4 (Kruppel-like factor 4), AKT1 (AKT serine/threonine kinase 1), SMO (Smoothened), SUFU (Suppressor of fused homolog), PRKAR1A (protein kinase cAMP-dependent type I regulatory subunit alpha), PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha), and POLR2A (RNA Polymerase II Subunit A) have been discovered in meningiomas without any NF2 alterations (Table 1).39–44 Compared to NF2-altered meningiomas, meningiomas with these “non-NF2” mutations tend to be lower WHO grade, have fewer chromosomal abnormalities, and generally have better clinical outcomes with standard therapies.
The anatomic location of meningiomas also appear to have genomic underpinnings. Meningiomas with NF2 loss tend to be located along the cerebral convexities or in the posterior/lateral skull base. Those with non-NF2 mutations (eg, TRAF7, SMO, SUFU, and PRKAR1A) are more common around the anterior skull base. Meningiomas with combined NF2/SMARCB1 mutations (2 genes in close physical proximity to one another on chromosome 22q) may be more commonly found along the anterior falx.39,45 Alterations in several meningioma driver genes (including NF2 and TRAF7) have also been found in normal leptomeninges with similar anatomic predilection.46
Mutations in SMARCE1 (SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1), BAP1 (BRCA1 associated protein-1), and PBRM1 are associated with different meningioma histologic subtypes. SMARCE1 loss is found in almost all clear cell meningioma, which are currently CNS WHO grade 2 by the 2021 classification.47–51SMARCE1 encodes for a protein involved in the SWI/SNF chromatin remodeling complex and consequently SMARCE1-deficient cells may be susceptible to SWI/SNF inhibition.50 Inactivation of BAP1 has been associated with rhabdoid and papillary histology and is almost universally associated with poor prognosis, although fewer than 30 of these cases have been reported in the literature.52,53PBRM1 mutations often co-occur with BAP1 mutations and are associated with papillary or sometimes rhabdoid histology.54 Notably, alterations in SMARCE1 and BAP1 appear to be independent of NF2 mutation or loss, identifying a small, rare group of NF2-wild-type meningiomas that are unusually aggressive.
Finally, mutations in the TERT (telomerase reverse transcriptase) promoter (TERTp) have been added to the most recent iteration of the WHO classification as an independent marker of grade 3 meningiomas.3 While rare in meningiomas, this alteration is associated with significantly worse progression-free survival (PFS) and overall survival when present.55,56TERT functions to maintain DNA telomere ends, resulting in immortalization of cancer cells. Successful downstream blockade of TERTp activity via E26 transformation-specific (ETS) transcription factor inhibition is a potential therapeutic strategy for these tumors.57,58 Other rare mutations associated with higher-grade meningiomas include ARID1A, PTEN, and PBRM1.38,54,59–64
Some of these recurrent mutations identified in meningiomas may occur in the germline and correspond with hereditary meningioma syndromes. The most common of these is germline mutation of NF2 resulting in what was historically referred to as syndromic Neurofibromatosis type 2, an autosomal dominant condition characterized by the growth of multiple schwannomas and meningiomas.65 Due to the overlapping phenotypes of Neurofibromatosis type 2 and schwannomatosis, the latter being a tumor predisposition syndrome also characterized by the development of multiple schwannomas, the diagnostic criteria and disease nomenclature for NF2 and schwannomatosis was updated in 2022. “Schwannomatosis” is now an umbrella term referring to the phenotype of multiple schwannomas, and the individual syndromes are named by their underlying genetic mutation. The previously defined "NF2 syndrome" has now been renamed "NF2-related schwannomatosis" (NF2-SWN) and this is the term which will be used in the rest of this article. Schwannomatosis is designated as SMARCB1-related, LZTR1-related, or 22q-related.66 Meningiomas are uncommon in non-NF2-related schwannomatosis and not part of the diagnostic criteria of LZTR1-related schwannomatosis and SMARCB1-related schwannomatosis despite the presence of SMARCB1 mutations in sporadic clear cell meningiomas.67 Meningiomas in NF2-SWN patients will be discussed in more detail in a later section.
Other hereditary syndromes associated with meningiomas are less common and there is an overall lack of data to support these germline mutations driving meningioma tumorigenesis. A rare autosomal dominant inheritance pattern of SMARCE1 mutations predisposing to intracranial and spinal meningiomas with clear cell histology has been reported.68,69 Germline BAP1 loss causes a hereditary cancer predisposition syndrome phenotypically associated with mesothelioma and uveal melanoma. As mentioned above, sporadic and hereditary germline BAP1 mutations have been linked to the development of rhabdoid and papillary meningiomas in small case series, which may also have an increased risk of extracranial metastasis.53,70–73 Other meningioma-associated tumor predisposition syndromes include: Werner syndrome, an autosomal recessive condition caused by biallelic loss of WRN, characterized by premature aging; Gorlin syndrome (or familial multiple meningiomas), an autosomal dominant condition resulting from germline mutations in Sonic Hedgehog (Hh) pathway genes including PTCH1 or SUFU, characterized by multiple basal cell carcinomas and biologically aggressive meningiomas74–76; and Cowden syndrome, another autosomal dominant condition resulting from germline PTEN mutation, characterized by multiple cancers including breast and thyroid.62 Notably, these are all rare entities and only a subset of patients with each syndrome will develop meningiomas. The overall prevalence of these syndromes is estimated to be between 1 in 20 000 (in some Japanese populations) to as few as 1 in 1 000 000 for Werner syndrome, between 1 in 30 000 to 1 in 250,000 for Gorlin syndrome, and between 1 in 200 000 to 1 in 250 000 for Cowden syndrome.77–79 The specific prevalence of germline SMARCE1 and BAP1 mutations is less clear given their rarity.
In addition to single-gene alterations, somatic copy number alterations (other than loss of 22q) have also been implicated in meningioma development (Table 2). Deletions of chromosome arm 1p were identified early in meningiomas, where it was associated with significantly shorter progression free survival (PFS).80–86 Multiple genomic targets of 1p loss have been proposed including CDKN2C, RAD54, EPB41, GADD45A, ALPL, MUTYH, PRDX1, FOXD2, FOXE3, and PTCH2, but their independent prognostic contributions to a more aggressive meningioma phenotype remain relatively unknown and remains an area of study study.87,88 Losses of chromosomal arms 6p, 10q, 14q, 18q, and gains of 17q and 20q were found to be recurrent across high-grade meningiomas and additional studies have also linked losses of 4q, 6, and 19p with poorer PFS (Table 2).89–94 In cases without chromosome 22q loss, several unique somatic copy number alterations including those affecting chromosomes 2q and 7q were found to be associated with dysregulated Hh signaling activation in otherwise mutation-negative meningiomas.95
Table 2.
Recurrent Copy Number Alterations Observed in Meningiomas and Their Association With Clinical Prognosis (When Known)
| Chromosome arm/gene | Loss/gain | Approximate frequency in all meningiomas | Associated clinical prognosis |
|---|---|---|---|
| 1p | Loss | 30%–50% | Intermediate to poor |
| 1q | Gain | 5% | Poor |
| 3p | Loss | 10%–15% | Intermediate |
| 4p/q | Loss | 5%–10% | Intermediate to poor |
| 5p/q | Gain | 2%–3% | Good |
| 6q | Loss | 15%–20% | Poor |
| 7p | Loss | <5% | Intermediate-poor |
| 8p | Gain | <5% | Unknown |
| 10q | Loss | 10% | Poor |
| 11q | Loss | 5% | Intermediate |
| 12p/q | Gain | 2%–3% | Good |
| 14q | Loss | 20% | Poor |
| 15q | Gain | <5% | Unknown |
| 16q | Gain | 5% | Unknown |
| 17q | Gain | 5%–10% | Unknown |
| 18q | Loss | 15%–20% | Poor |
| 20q | Gain | 10% | Unknown |
| 22q | Loss | 50%–60% | Intermediate to poor |
Importantly, homozygous loss of the CDKN2A/B (cyclin-dependent kinase inhibitor 2A/B) locus on chromosome 9p21 was incorporated into the 2021 WHO classification as a defining feature of CNS WHO grade 3 meningioma.3CDKN2A/B encodes for multiple tumor suppressor proteins including p16, which inhibits the G1-to-S transition in the cycle cell through the inactivation of CDK4 and CDK6. Its loss has been implicated in dysregulated cell cycle progression in multiple cancers.96,97 In meningioma, homozygous deletion of CDKN2A/B is associated with significantly shorter PFS, and even heterozygous deletions have been found to be associated with similarly poor outcomes in some studies.98–101 In meningiomas with an intact CDKN2A/B locus, higher mRNA expression of CDKN2A was also associated with significantly shorter PFS and increased rates of resistance to CDK inhibitors.98
The integration of prognostic copy number alterations with contemporary histological grading has resulted in the development of “integrated” or “molecular-morphologic” grading schemes. For example, a nomogram was developed whereby one point is assigned to each of the following copy number alterations if present: 1p-, 3p-, 4p/q-, 6p/q-, 10p/q-, 14q-, 18p/q-, 19p/q-, CDKN2A/B- in addition to one point for 4–19 mitoses per 10 high-powered fields or 2 points for more than 20 mitoses. A total of 0–1 points in this proposed grading paradigm would constitute an "Integrated grade 1" meningioma, 2–3 points for an "Integrated grade 2" case, and 4 or more points for an "Integrated grade 3" case. This integrated grading system was able to predict tumor recurrence/progression more accurately than standard WHO grading alone.92 Similar models have been developed by assigning scores based on combining WHO grade (histologic grade), DKFZ methylation-class family (benign, intermediate, or malignant; to be described further below), and the presence of 3 prognostic CNVs: 1p-, 6q-, and/or 14q-. This “integrated molecular morphologic risk” also had significantly better accuracy for outcome prediction compared to WHO grade or any of these molecular criteria alone, particularly for meningiomas bordering the threshold between CNS WHO grades 1 and 2.93
Histopathologic Classification
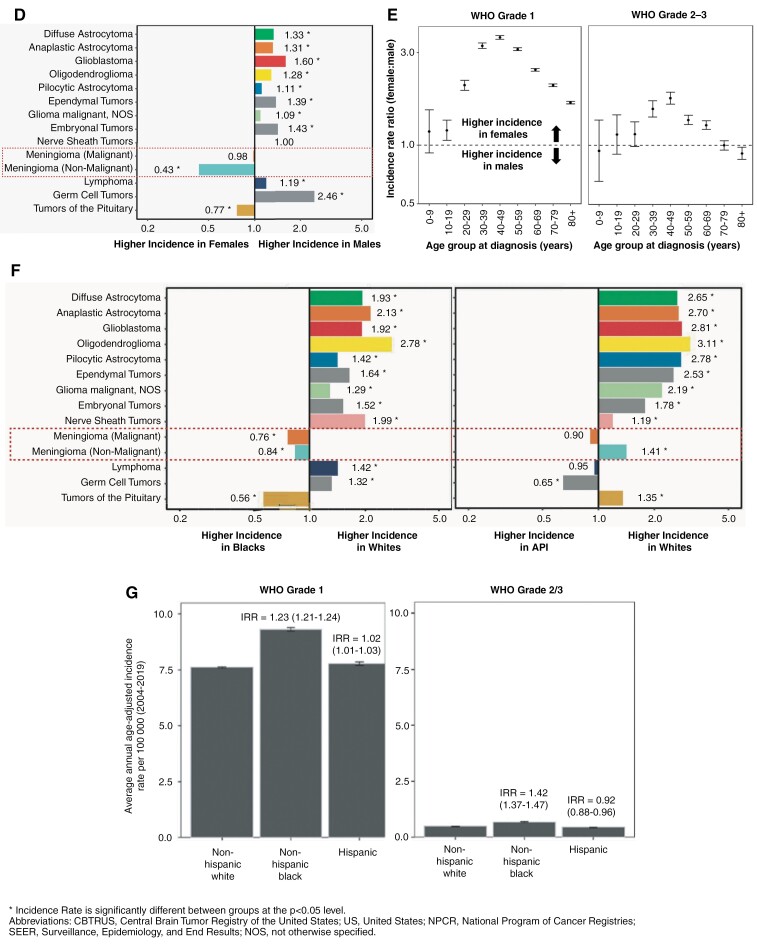
The histopathologic characteristics of meningioma have been the main correlate to outcome for decades and still form the basis of contemporary WHO grading. Released in 2021, the 5th edition of the WHO CNS classification is the first to include molecular criteria for the definition of a CNS WHO grade 3 meningioma: presence of a TERTp hotspot mutation or homozygous loss of CDKN2A/B. These molecular alterations are rare in meningiomas, particularly in cases that do not have other worrisome histologic findings. In the absence of these alterations, which automatically impart a CNS WHO grade 3 designation, grading is assigned based on histopathologic features such as the number of mitotic figures or identification of at least 3 out of 5 “soft” criteria for atypia (sheeting architecture, hypercellularity, small cell formation, macronucleoli, spontaneous necrosis; Figure 2).3,102
Figure 2.
Updated 2021 World Health Organization (WHO) Grading criteria for meningiomas including histological subtypes for CNS WHO grade 1 cases: (A) meningothelial, (B) fibrous, (C) transitional, (D) psammomatous, (E) secretory, (F) angiomatous, (G) microcystic, (H) lymphoplasmacyte-rich, (I) metaplastic; CNS WHO grade 2 cases: (J) atypical, (K) clear cell, (L) chordoid; and CNS WHO grade 3 cases: (M) anaplastic, (N) papillary, (O) rhabdoid. HPF- high-powered fields; N:C, nuclear to cytoplasm. Histological image panels (A–O) used with permission from Bi et al. (2016).91 CNS, central nervous system; HPF, high-powered fields; N:C, nuclear to cytoplasm; TERT, telomerase reverse transcriptase.
While the presence of brain invasion alone is now sufficient for a designation of CNS WHO grade 2 meningioma, its association with outcome in the absence of any other higher grade histopathological features (eg, brain invasion without elevated mitotic index, hypercellularity, loss of architecture, small cell change, spontaneous necrosis, or prominent nucleoli) remains unclear.103–105 Given that cases of brain invasion alone as a solitary atypical finding is rare, only a minority of meningioma cases will likely require retrospective re-grading based solely on this feature.104 More work is needed to understand the biological significance and mechanism of brain invasion in meningioma.106 Additionally, current intraoperative sampling methods to identify brain invasion vary significantly between neurosurgical departments worldwide and this too requires standardization given that many pathology samples for this extra-axial tumor may lack brain tissue altogether.107,108 A systematic, structured method of safely sampling areas suspicious for brain invasion during surgery may be needed to optimize the diagnostic yield for detecting CNS invasion.
While chordoid or clear cell histology still mandates a CNS WHO grade 2 classification by the 2021 criteria, rhabdoid or papillary histology alone without other features of anaplasia or malignancy are now insufficient to render a CNS WHO grade 3 designation (Figure 2).3,102
Biomarkers and Molecular Classification
Given the prognostic alterations uncovered in meningiomas, significant efforts have been made to develop a unified molecular classification system similar to those that exist for glioma and medulloblastoma.3 In 2017, the first landmark studies on DNA methylation-based classification systems for meningioma were published. These models were capable of stratifying meningiomas into groups at high- or low-risk of recurrence/progression and further identified 6 unique methylation-defined subgroups of meningioma (benign-1, benign-2, benign-3, intermediate-A, intermediate-B, and malignant) that appeared to reflect tumor biology more accurately than WHO grade alone.109,110 The DNA methylation profiles of meningiomas could be further combined with prognostic clinical variables including histologic grade and extent of resection to robustly predict clinical outcome and help guide decisions on adjuvant treatment after surgery.111
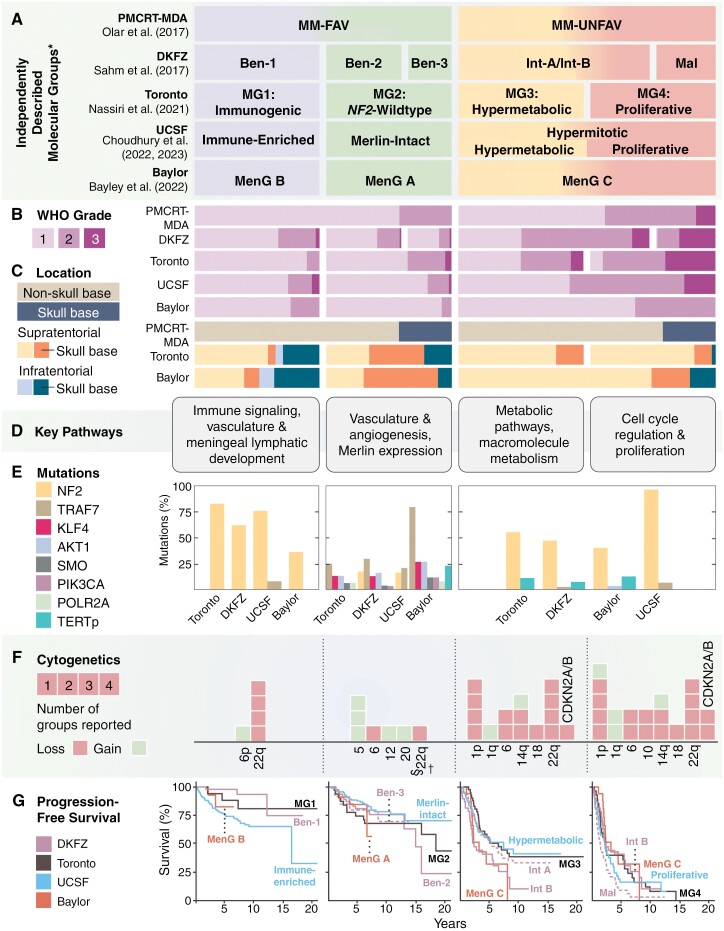
Subsequently, the integration of genome-wide DNA methylation, mRNA expression, and copy number alterations resulted in the discovery of 4 stable molecular groups (MGs) of meningioma (Figure 3).64 Classification by MG was found to have improved prognostication potential and biological relevancy compared to WHO grade and classification using any single epigenomic or genomic platform alone. MG1 or “immunogenic” meningiomas were defined as NF2-mutant, copy-number neutral cases enriched in immune-related transcriptomic pathways. MG2 meningiomas were found to be enriched for non-NF2 mutations and angiogenic processes, earning the “NF2-wild type” designation. MG3 and MG4 meningiomas were enriched for prognostically unfavorable alterations including TERTp mutation and homozygous loss of CDKN2A/B, in addition to novel somatic mutations in KDM6A, CHD2, and PTEN, and these tumors had a significantly higher degree of chromosomal instability. On transcriptomic analysis, several metabolic pathways including those involved in nucleotide and lipid metabolism were upregulated in MG3 meningiomas, giving this group its “hypermetabolic” name. MG4 or “proliferative” meningiomas were found to be enriched for cell cycling pathways including MYC, FOXM1, and E2F pathways, had the highest mutational and copy number burden, and were associated with the worst clinical outcomes.64,112–116
Figure 3.
(A) Different meningioma molecular/methylation classifications discovered by independent groups arranged based on approximately how they correlate with one another based on common biology, alterations, and outcome (read from top to bottom). (B) Relative distribution of meningiomas belonging to each WHO grade in each molecular or methylation group. (C) Relative proportion of meningiomas based on location in either a skull base or non-skull base location in the supratentorial or infratentorial compartment in datasets where tumor location was available. (D) Key transcriptomic pathways found to be overexpressed in meningiomas belonging to each molecular or methylation group, grouped into 4 main sets of pathways. (E) Relative distribution of common meningioma driver mutations found in cases with more benign biology (left) and more biologically aggressive cases (right). (F) Proportion of different chromosomal alterations seen in each molecular or methylation group. (G) progression-free survival (PFS) of meningiomas belonging to each recently published molecular or methylation group based on the original publication’s cohort. *Importantly to note, these groups may not correlate with one another precisely on a one-to-one basis and as a result, the PFS curves of different groups may be repeated in different panels. †For instance, while many meningiomas from the Ben-3 methylation subclass share commonalities with Merlin-intact or NF2-wild-type cases (eg, absence of 22q deletions, presence of chromosome 5 gain, angiomatous histology), some cases may classify into other molecular groups eg, immunogenic or hypermetabolic groups. Similarly, some cases of Ben-3 do have 22q deletions as well. Int-A and Int-B meningiomas may not precisely separate into hypermetabolic and proliferative cases. PMCRT, Princess Margaret Cancer Research Tower; DKFZ, German Cancer Research Center; UCSF, University of California San Francisco; MM-FAV, meningioma methylation group favorable; MM-UNFAV, meningioma methylation group unfavorable; Ben, benign; Int, intermediate; Mal, malignant; MG, Molecular Group; MenG, Meningioma Group; NF2, neurofibromatosis 2; TRAF7, Tumor necrosis factor receptor-associated factor 7; KLF4, Krüppel-like factor 4; AKT1, RAC(Rho family)-alpha serine/threonine-protein kinase; SMO, Smoothened; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha; DNA-directed RNA polymerase II subunit RPB1; TERTp, Telomerase reverse transcriptase promoter; CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B.
Around the same period of time, other molecular classification schemes were discovered by others in independent cohorts. Choudhury et al. uncovered 3 stable methylation groups with unique clinical outcomes and biology: Merlin-intact (MI), immune-enriched (IE), and hypermitotic (HM). MI meningiomas, analogous to MG2 cases (NF2-wild type), were largely benign tumors enriched for non-NF2 mutations such as TRAF7, AKT1, and KLF4. IE meningiomas, similar to MG1 (Immunogenic), were found to have significant immune cell infiltration and increased expression of HLA and meningeal lymphatic genes including LYVE1, CCL21, and CD3E. HM meningiomas were clinically aggressive cases with poor outcomes enriched for FOXM1 cell proliferation pathways.37 Subsequent reanalysis of the HM group revealed 2 distinct subgroups within it: one subgroup enriched in pathways related to macromolecule metabolism (resembling the MG3 Hypermetabolic meningiomas with intermediate to poor outcomes) and the other enriched for cell cycle pathways that had the worst clinical outcomes (similar to the MG4 Proliferative meningiomas). These findings seem to support the concept of either 4 distinct MGs matching those discovered by Nassiri et al. or 3 epigenetic groups with one group that could be further split into 2 subgroups with distinct clinical outcomes and gene expression signatures.117 Bayley et al. also found 3 methylation groups of meningioma based on integration of DNA methylation, RNA expression, NF2 status, and degree of chromosomal instability in a cohort of primary CNS WHO grade 1 and 2 meningiomas. By their classification, MenG A meningiomas were almost entirely CNS WHO grade 1, had no cytogenetic changes, and were NF2-wild type, corresponding to the MG2 and MI groups described above. MenG B meningiomas were all NF2-deficient, had a low degree of chromosomal instability, and had overall good clinical outcomes, seemingly matching the MG1 and IE groups. MenG C meningiomas were NF2-deficient, had a high burden of copy number alterations including 1p loss, and like the MG3, MG4, and HM groups, have the worst clinical outcomes.118 Each of these molecular classification systems tends to complement and/or outperform contemporary WHO grading alone in predicting clinical outcomes. Despite differences in nomenclature and classification, which may be attributed to the use of different epigenomic/genomic platforms and bioinformatic methods utilized in these separate studies, these molecular classifications share a meaningful degree of common biology, particularly when considering they were discovered in completely non-overlapping, independent cohorts (Figure 3; Supplementary Table 1). These studies together have not only demonstrated the value of utilizing orthogonal bioinformatic methods to independently produce stable molecular/methyation groups, but have also generated a wealth of genomic/epigenomic data as a valuable resource for future studies. An important caveat is that these classifiers may be insufficiently powered to include rare subsets of poor-performing NF2-wild-type tumors including meningiomas with BAP1 mutations, and management of these unusual, but clinically important cases should be carefully considered on an individual patient basis. Upcoming efforts, including those by the cIMPACT-NOW group, will focus on reconciling the nomenclature of these different molecular classifications to reach a consensus that can be implemented into a future unified grading system.
One of the additional challenges hindering the routine implementation of these molecular classifications is the requirement for sequencing and/or methylation array technology that may not be accessible at all centers. This is in addition to other barriers to genomic testing that include but are not limited to: financial reimbursement, site-dependent experience in data analysis and interpretation, and uncertainty in selecting the specific assays or tests to perform. One method of addressing these challenges may be with the use of proteomics to identify immunohistochemical (IHC) markers enriched in each molecular group or specific combination of markers such that tumors may be molecularly subtyped in the future without genomic data at all. For this to be clinically validated, IHC stains will need to be multiplexed in large, molecularly annotated meningioma cohorts, ideally in a prospective manner, and analyzed by experienced neuropathologists blinded to molecular classification and each other’s annotations.
Additional uncertainty may arise in deciding on which molecular classification or the above referenced integrated grading system to use. While there are notable differences in classification or prognostication in models that are trained on clinical endpoints (eg, integrated molecular-morphological meningioma classification or integrated WHO grade) versus the unbiased molecular group classifications detailed above, these methods all provide some degree of additive prognostic information to traditional grading and for the time being, may be utilized interchangeably based on the available resources of each institution.64,92,93,117–119 Efforts to expand access to genomic and methylation testing for meningiomas will not only aid in prognostication, but help ensure continued progress in better understanding the biology of these tumors. To this point, while the DNA methylation and gene expression patterns of some meningiomas appear to remain stable between the primary and recurrent case, the effect of accumulating epigenetic and genomic alterations including progressive chromosomal instability with multiply recurrent cases (including cases that were completely resected at one point), metastatic meningiomas, and cases following receipt of radiotherapy (RT) still need to be further investigated.109,120,121
An emerging area of interest for meningiomas is the use of liquid biopsy for diagnosis and subtyping. The use of cell-free methylated DNA immunoprecipitation and high throughput sequencing (cfMeDIP) on patient plasma was able to effectively differentiate meningiomas from other radiographic mimickers such as solitary fibrous tumors, dural-based metastases, and chordomas.122,123 Extracellular vesicles from the plasma of meningioma patients quantitatively correlated with the extent of resection and their contents were found to reliably recapitulate the methylation signatures of the parent tumor, including copy number and mutational profile.124 Additional work has found that plasma-based DNA methylation signatures of meningioma patients may have similar prognostic potential as in the tumor tissue for differentiating between high- and low-risk cases. These findings collectively need to be further validated in larger, external validation cohorts with matched tissue profiling before clinical translation may be feasible.125
Diagnosis and Imaging
Many meningiomas are diagnosed when patients become symptomatic from either mass effects or seizures.126–128 On non-contrast computed tomography (CT), up to 25% of meningiomas will have some degree of calcification, which may be sometimes associated with slower tumor growth and lower WHO grade.129 Magnetic resonance imaging (MRI) is the preferred modality for confirming the radiographic diagnosis with most meningiomas being isodense to cortex on all sequences, and approximately 50% may be associated with some perilesional edema.130 Secretory, microcystic, angiomatous, and lymphoplasmacyte-rich meningiomas are histologic subtypes known to cause a disproportionately large degree of edema relative to tumor size and may portend increased risk of postoperative complications.131,132 Almost all meningiomas avidly enhance with gadolinium contrast and up to 72% have a dural tail.133 Whether the dural tail consistently contains neoplastic meningioma cells requiring treatment or simply represents reactive or inflammatory dural thickening is controversial.134–138 Vascular imaging, often CT- or MR-angiogram (CTA, MRA) and/or CT/MR-venogram (CTV, MRV) can help assess the involvement of nearby vascular structures for treatment planning, which is particularly important around the skull base or dural venous sinuses. Formal cerebral angiography is more rarely performed but may be indicated if noninvasive vascular imaging provides insufficient information or if preoperative embolization is planned. There remains controversy around whether preoperative embolization reliably leads to decreased blood loss intraoperatively and its use may be associated with an increased risk of postoperative venous thromboembolism.139,140 Therefore, preoperative embolization is not a recommended strategy for all meningiomas and decisions surrounding its use must be made on a case-by-case basis.
There are currently no standardized response criteria or clinical trial endpoints for meningioma studies. Previous trials have used a modification of the Macdonald criteria (initially developed for high-grade gliomas), the Response Assessment in Neuro-Oncology (RANO) criteria for high-grade gliomas, or the Response Evaluation Criteria in Solid Tumors (RECIST) criteria for systemic cancers.141–146 While some trials have used a reduction in lesion size as a radiographic endpoint, meningioma control is better encapsulated by lack of growth (size stabilization) as a decrease in size occurs in only a relative minority of cases treated with RT over time (approximately 20%–30% of cases).147–149 Additionally, while overall survival is often the gold standard for determining treatment efficacy, the long follow-up time required to reach this endpoint for all but the most aggressive meningiomas presents a significant challenge, particularly for clinical trials. The RANO Working Group instead proposed that 6-month PFS could be a viable endpoint for meningioma drug trials with a 25% increase in the tumor’s bidimensional product representing definitive progression.141 For patients enrolling in clinical trials, collection of pretreatment MRIs will be important to confirm adequate progression of the tumor during trial follow-up. In the future, measurement of tumor volume and assessing changes in the rate of tumor growth before and after treatment may be another method of evaluating the efficacy of novel therapies.150 For reporting in retrospective studies, the ICOM proposed the definition of tumor progression to be any radiographic progression that leads to a change in the clinical management of the tumor (eg, from observation to consideration for surgery, RT, or stereotactic radiosurgery [SRS]), thereby excluding cases of minimal radiographic growth or small volume increases followed by a plateau of stability that may not be clinically significant.151
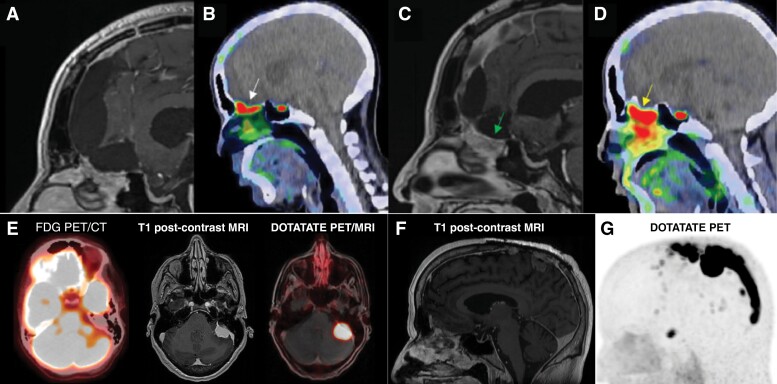
An emerging imaging tool for meningiomas is positron emission tomography (PET) using somatostatin receptor (SSTR) ligands such as Gallium-68-labeled DOTATATE given that nearly all meningiomas express SSTR1/2 (Figure 4).152 Recently published guidelines from the RANO Working Group suggest that [68Ga]Ga-DOTATATE PET can be used for diagnosis, surgical resection, and RT treatment volume planning, as well as post-treatment surveillance (Figure 4).153,154 When compared to conventional MRI, [68Ga]Ga-DOTATOC PET had improved sensitivity for detecting meningiomas, particularly in areas of tumor-invading bone, locations obscured by calcifications or radiographic abnormalities, tumors centered at the skull base, or those located next to the falx.155 The ability to image the entire body is also advantageous for detecting systemic metastases in multiply recurrent higher grade or malignant meningiomas that although rare, will dramatically influence patient prognosis and treatment planning if identified. When correlated with SSTR2 immunohistochemistry and tumor histology, [68Ga]Ga-DOTATATE PET was found to be capable of differentiating between meningioma and tumor-free tissue with high accuracy, suggesting that it can be reliably used to demarcate tumor-invaded bone that may require additional drilling to maximize extent resection (particularly in the skull base; Figure 4) and also inform adjustments in RT planning in addition to response assessment after RT.152,156–160 Postoperatively, PET imaging may also better define residual tumor more accurately than traditional MRI and may also differentiate true tumor progression/recurrence from treatment effect.153,161,162 Recently, Fluorine-18-labeled SSTR-tracers such as [18F]SiTATE have been developed which demonstrate similarly high uptake in meningiomas while boasting lower radiation exposure and less logistic constraints for transport and clinical use compared to [68Ga]Ga-DOTATATE PET given its longer half-life (110 vs 68 minutes).163,164 Although PET imaging is a promising addition to the armamentarium for meningioma diagnosis and treatment, its limitations include the still sparse data on cost-effectiveness, physiologic uptake near certain anatomic structures such as the pituitary gland, and tracer uptake by other tumors or non-neoplastic diseases that may also express SSTR.153,165 Furthermore, additional prospective work and multicentre clinical trials are needed to link these positive findings from often single-institution retrospective studies with demonstrable improvements in clinical outcomes.152
Figure 4.
(A) Postoperative magnetic resonance imaging suggesting a gross total resection with contrast-enhancing reactive changes only. (B) Positron emission tomography (PET) imaging showing focal uptake along the cribriform plate (standardized uptake value 7.43, white arrow) suspicious for residual disease. (C) Follow-up MRI 2 years later after patient declined to pursue recommended adjuvant RT with increased enhancing soft tissue signal (arrow). (D) Increased focal PET uptake in the cribriform plate suggests progression of residual disease (standardized uptake value 8.96, yellow arrow). (E) Axial brain MRI of a different patient: a 54-year-old woman with newly diagnosed breast cancer metastatic to axillary lymph nodes who was noted to have asymmetric photopenia in the left cerebellum on a staging fluorodeoxyglucose (FDG)-position emission tomography (PET) and computer tomography (CT) scan (left). T1 post-contrast brain magnetic resonance imaging (MRI) showed a multilobulated, homogeneously enhancing extra-axial mass adjacent to the left petrous temporal bone with associated edema and mass effect in the left cerebellum and cerebellar peduncle (middle). Leading differential diagnoses included a distant metastasis or a meningioma. DOTATATE PET/MRI showed markedly avid uptake in the intracranial mass (right), but not in the right breast or ipsilateral lymph nodes (not shown). A diagnosis of synchronous meningioma and locoregionally advanced breast cancer was made. The meningioma was treated with stereotactic radiosurgery (SRS). The patient underwent lumpectomy, sentinel lymph node biopsy, and adjuvant whole breast radiotherapy. At 24 months after meningioma treatment and 13 months after breast cancer treatment, the patient had no evidence of disease. (F) Sagittal T1 post-contrast brain MRI (left) and DOTATATE PET (right) of a 61-year-old male with recurrent atypical meningioma, CNS WHO grade 2, status post resection and stereotactic radiosurgery 8 years before developing multiple vertex recurrences that were treated with subtotal resection. Planning DOTATATE PET imaging revealed extensive tumor infiltration of the sagittal sinus from the vertex to the torcula. Part of this figure was originally published in The International Journal of Radiation Oncology, Biology, Physics. Prasad et al. 68Ga-DOTATATE PET: The Future of Meningioma Treatment (2022).152 FDG, fluorodeoxyglucose; MRI, magnetic resonance imaging; PET, positron emission tomography; used with permission.
While many meningiomas are diagnosed symptomatically, approximately 20% are found incidentally, a proportion likely to increase with an aging population with increased access to neuroimaging.128,166 Incidental meningiomas can be a source of significant anxiety for patients, an economic burden due to the need for regular follow-up imaging, and a clinical dilemma for clinicians due to their unpredictable biology given the absence of diagnostic tissue.128,166–168 Natural history studies on incidental meningiomas typically only extend to the 10-year follow-up mark and most have found a relatively slow rate of growth (average < 5% volumetric increase per year). Approximately 5–8% of patients will develop new symptoms during a mean follow-up period of 4.1 years (standard deviation 2.4 years).167 Imaging features that may portend a higher risk of progression of an incidental meningioma include: lack of calcification, hyperintensity on T2-weighted MRI, presence of peritumoral edema, large tumor volume at diagnosis (>10 cm3), non-skull base location, and closer proximity to a dural venous sinus.39,114,167,169–175 There are currently no standardized guidelines for the interval or duration of monitoring for incidental meningiomas. Although most meningiomas that progress will do so within 5 years of observation, some cases can remain indolent for a longer period before demonstrating accelerated recurrence or growth. Consequently, many clinicians may follow incidental meningiomas in younger patients for a longer duration of time, progressively lengthening the interval between neuroimaging while elderly patients may be discharged from follow-up earlier after a confirmatory period of radiographic stability.128,167 Several prognostic models such as IMPACT (Incidental Meningioma: Prognostic Analysis Using Patient Comorbidity and MRI Tests) have been developed to assist clinicians in tailoring follow-up to a specific patient based on individualized clinical and tumor factors but these models all require prospective validation.128,166,176–178 Upfront treatment of incidental meningiomas is also an option, with surgical resection for often larger tumors, and SRS as a reasonable option for smaller volume cases or for patients with contraindications to surgery.179–181 Decisions to treat usually hinges on a combination of patient wishes, clinician preference, and tumor factors including proximity of the meningioma to critical neurovascular structures such that further enlargement or growth could make later resection more challenging or higher risk. Newer technologies such as liquid biopsy or 18F-FLT PET could be used to help predict the risk of recurrence non-invasively and better individualize management for these cases.125,182 While SRS improves radiographic local control of asymptomatic meningiomas compared to observation, this may not translate to a reduced risk of developing new symptoms over time.181 Furthermore, even though a subset of incidental meningiomas will grow radiographically, these changes may not become clinically significant until tumor size reaches a certain threshold or nears eloquent brain areas. As usual, treatment decisions should weigh the risks of progression versus the risks of intervention, while also taking into consideration the psychosocial, neurocognitive, and socioeconomic effects of active surveillance for the patient versus upfront treatment.183,184
Surgical Management
Surgery remains the mainstay of treatment for growing or symptomatic meningiomas (Figure 5). Goals of surgery, as defined by the 2021 EANO guidelines, are predominantly to obtain a tissue diagnosis, relieve mass effect, and alleviate neurologic symptoms if present.127 Notably, extent of resection is an important correlate of outcome, and maximal safe resection should be sought while minimizing neurologic morbidity in all symptomatic cases. To this end, surgical adjuncts including neuronavigation, ultrasonography, and intraoperative neuromonitoring are critical for tumors located in highly eloquent areas such as the cerebellopontine angle or foramen magnum, to reduce the risk of incurring permanent neurologic injury. Since some meningiomas are intimately associated with critical neurovascular structures, complete resection without unacceptable morbidity is not always possible; it is, therefore, important to standardize a maximally beneficial degree of resection for these cases in a meaningful way.
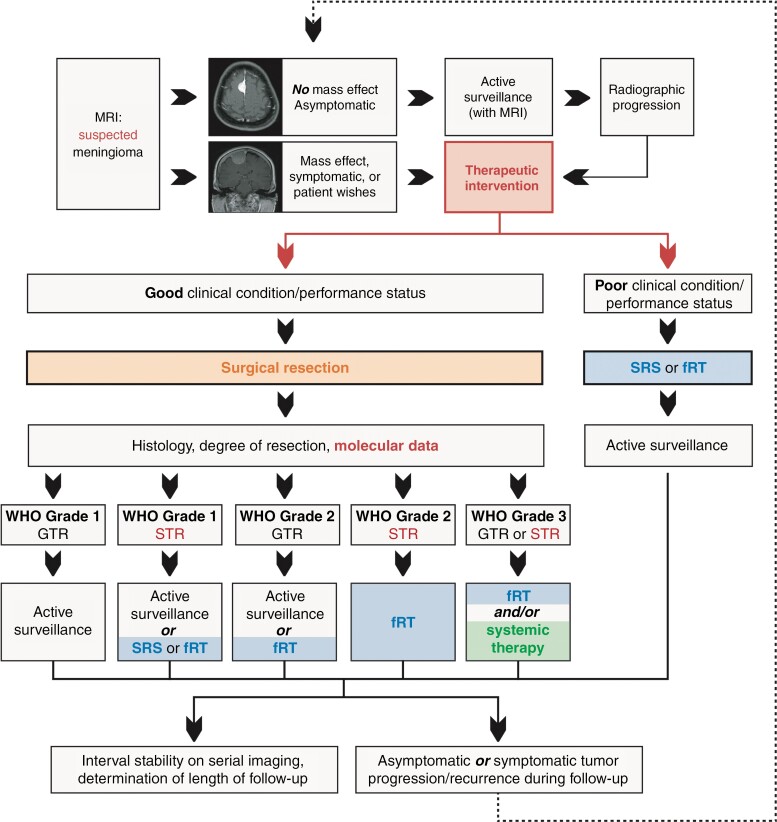
Figure 5.
Summary of most contemporary treatment guidelines for the management of meningiomas based on WHO grade, extent of resection, with the incorporation of molecular data if available. Content for this figure was partly adopted from Goldbrunner et al. EANO Guideline on The Diagnosis and Treatment of Meningiomas (2021) published in Neuro-Oncology.127 Used with permission. MRI, magnetic resonance imaging; SRS, stereotactic radiosurgery; fRT, fractionated external beam radiotherapy; GTR, gross total resection; STR, subtotal resection.
The Simpson grade, first introduced in 1957, describes the surgeons’ assessment of the extent of resection for meningioma. It ranges from Simpson grade 1 (complete resection of tumor, affected dural attachment, and bone) to 5 (decompression/biopsy only) with higher grades associated with higher rates of recurrence.185–188 Complete tumor resection may be designated as Simpson grades 1, 2, or 3 depending on whether the underlying dura is resected, coagulated in situ, or left intact, respectively. While the Simpson grade has historically been a major predictor of postoperative PFS, its role in modern meningioma surgery has become somewhat controversial.161,186,187,189–191 For example, recent studies have shown that resecting the underlying dura (Simpson grade 1 resection) may not be associated with improved outcomes compared to other Simpson grades.190,191 This is important in cases of meningiomas originating from the skull base, where aggressive dural resection may be associated with increased risk of complications such as CSF leak or for meningiomas involving dural venous sinuses where hemorrhage, venous infarct, or air embolism are notable risks when pursuing aggressive resection.186,190 In these cases, achieving maximal tumor resection without excising the underlying dura may decrease morbidity without meaningfully affecting PFS. Additionally, skull base meningiomas are more likely to exhibit more benign biology, which is used as evidence for opposing viewpoints. On one hand, striving for a Simpson grade 1 resection in these complex cases may confer unnecessary surgical risk, thereby supporting a more conservative philosophy. On the other hand, complete resection in the context of a meningioma with more benign biology may provide an opportunity for robust oncologic cure, obviating the need for further surgery or adjuvant RT and this view supports a more aggressive surgical approach.192–194 The optimal strategy in these cases will depend largely on the surgeon’s comfort level, experience, and of course, the patient’s wishes and their risk tolerance for neurologic deficits, temporary or permanent, that may be incurred in an effort to achieve a potential cure. As an additive step to a Simpson grade 1 excision, a “Simpson grade 0” resection, whereby an additional 2-cm margin of surrounding dura is removed, has been proposed primarily for convexity-located meningiomas where this is most feasible.195 However, there are currently no well-established guidelines for the extent of dural resection recommended to optimally prolong time to recurrence and adjunctive technologies such as Raman spectroscopy or SSTR PET may help to better define this moving forward.152,156,161,196–198
Considering these limitations in Simpson grading, there has been movement towards defining extent of resection as simply either gross total resection (GTR), indicating cases where all tumor is removed regardless of how the underlying dura is handled (analogous to Simpson grades 1–3), and subtotal resection (STR), indicating cases where a portion of gross tumor is left behind (Simpson grades 4/5). This definition has been adopted by organizations such as the European Organization for Research and Treatment of Cancer (EORTC) and the Radiation Therapy Oncology Group (RTOG).127 However, the role of either Simpson grading or extent of resection in the context of meningioma molecular classifications has yet to be adequately explored.
The different surgical approaches to intracranial meningiomas are vast and a comprehensive review of each approach is beyond the scope of this article. The latest evolution in surgical techniques for meningiomas emerged with improvements in endoscopic technologies, permitting expanded endonasal approaches (EEA) to the anterior skull base including olfactory groove and tuberculum sella meningiomas (or, less commonly, tumors in the middle fossa, posterior fossa, or orbit) for appropriately selected patients. Tuberculum sellae meningiomas are the prototypical candidates for endoscopic resection through an EEA and a trend towards better visual outcomes at the cost of higher CSF leak rates for these patients has been found when compared to open, transcranial approaches.199–201 Tuberculum sellae meningiomas selected for EEA often tend to be smaller in size with less perilesional edema and no vascular encasement (which is a contraindication for most surgeons using an endoscopic approach).201 Overall, there is insufficient evidence demonstrating the universal superiority of one surgical approach over another, and each case should be individualized based on patient and tumor factors in addition to the surgeon’s comfort level and expertise.127
External Beam Radiotherapy for Meningiomas
In addition to surgical resection, RT is the only widely accepted treatment modality for meningiomas. RT may be prescribed as primary treatment or as an adjunct to surgery, either immediately following surgery as adjuvant therapy or delayed as salvage treatment at the time of tumor progression/recurrence. The optimal timing of adjuvant RT is currently unknown. The recent EANO guidelines suggest primary fractionated RT as a treatment option for symptomatic patients or those with sufficiently large meningiomas beyond the treatment limits of stereotactic radiosurgery (SRS) who cannot undergo surgery due to underlying comorbidities, unacceptably high surgical risk, or patient preference. These same guidelines also recommend RT as an adjunct to surgery in all patients with CNS WHO grade 3 meningiomas or CNS WHO grade 2 cases following subtotal resection. Recent evidence has suggested that RT may have a role even for patients with CNS WHO grade 1 meningiomas that cannot be completely resected, a cohort that had worse PFS than completely resected and irradiated CNS WHO grade 2 meningiomas in the non-randomized RTOG-0539 phase II clinical trial (Figure 5).127,202
The use of adjuvant RT in all CNS WHO grade 3 meningiomas and partially resected CNS WHO grade 2 meningiomas (so-called “high-risk” cases) is supported by the same RTOG-0539 trial, which treated these cases with intensity-modulated RT (IMRT) with 60 Gy over 30 fractions.203 This achieved a 3-year PFS of 58.8% and overall survival of 78.6% in 51 enrolled patients, with minimal adverse effects (one grade 5 necrosis-related complication in a patient with a large RT treatment field, all others being grades 1–3 adverse events). Additionally, EORTC 22042-26042, a non-randomized phase II study of patients with WHO grade 2 meningioma who underwent complete resection and postoperative RT (60 Gy), achieved an encouraging 3-year PFS of 88.7%.204 With improvements in RT technology, dose escalation has been proposed as a strategy for higher-grade (WHO grade 2 or 3) meningiomas. The phase II MARCIE trial utilized a carbon-ion (C12) boost of 18 Gy over 6 fractions combined with IMRT or fractionated stereotactic RT of 50.4 Gy/28 fractions for incompletely resected WHO grade 2 meningiomas, with resultant 3-year PFS and local control rates of 80.3% and 86.7%, respectively. However, a higher-than-expected proportion of patients developed radiation-induced contrast enhancement post-treatment and the study was prematurely terminated due to one treatment-associated death.205 A large, single-center retrospective study from Toronto found that dose escalation of conventional photon-based RT to 66–70 Gy over 33–35 fractions for WHO grades 2 and 3 meningiomas (both as adjuvant and salvage treatment) led to improvements in local control and PFS compared to standard dose RT regimens (59.4–60 Gy/30–33 Fr), without a significant difference in treatment-related adverse events, although the authors acknowledged likely underreporting of these toxicities.206 Given these uncertainties, a randomized controlled trial may be needed to answer the question of optimal RT dosing for higher-grade meningiomas. Several other retrospective studies also supported the use of adjuvant RT in CNS WHO grade 2 and 3 meningiomas but these studies were often limited by small sample sizes, non-standardized RT doses/techniques, lack of distinction between local and out-of-field treatment failures, and evolving WHO criteria.207,208 There continues to be controversy surrounding the benefit of adjuvant RT in patients with completely resected CNS WHO grade 2 meningiomas, a group wherein the guidelines remain equivocal. This critical question is being addressed with the ongoing phase III randomized trials NRG BN-003 (NCT03180268) and ROAM/EORTC-1308 (ISRCTN71502099) with results of both trials pending.209–212
The conventional use of WHO grading to stratify meningiomas into different treatment arms should also be considered. The WHO criteria for CNS WHO grade 2 and 3 meningiomas (the cases that are most often selected for adjuvant RT clinically) have undergone several updates of from 2000 to 2021.3,213–215 Clinical trials that accrue over several years may require central pathological review and regrading or be limited by this confounder. Furthermore, apart from the most recent 2021 classification, all previous WHO grading systems were entirely based on histopathology and in some instances may be susceptible to differences in interpretation between pathologists.216,217 In this emergent molecular era of meningioma classification, the WHO grade has been shown to be less predictive of outcome than nearly all molecular classification systems although robust, large-scale validation of these classifications are still needed, particularly as it pertains to response to RT. Despite the associated challenges, it will be important to consider prognostic molecular alterations when it comes to future selection of patients for adjuvant RT. When DNA methylation was performed on 38 CNS WHO grades 2 and 3 meningiomas from the phase II EORTC 22042–26042 clinical trial that received different degrees of surgical resection, loss of chromosome 1p and unfavorable DKFZ methylation class were found to be associated with worse 3-year PFS, although statistical significance was not met.218 Recently, a 34-gene expression signature was developed that appeared to outperform WHO grade and several other molecular prognostic systems in accurately predicting 5-year PFS. Using this prognostic signature, meningiomas were able to be stratified into cases at high- and low-risk of recurrence following surgery.219 Although this gene expression biomarker was robustly validated in large external cohorts where postoperative management for up to 29.8% of cases could be refined, these cohorts spanned multiple decades of time and included only 210 patients who actually received postoperative RT. Therefore, further validation is needed to translate this signature to specifically RT-treated meningioma cases before its utility for determining response to RT can be definitively established.220
Finally, patients undergoing primary RT for meningioma in lieu of surgery may undergo either SRS or fractionated external beam RT. While both have been associated with high rates of tumor control, the latter may be preferred for larger tumors (typically larger than 2–3 cm in maximum diameter but may be institution-dependent) or those close to radiation-sensitive structures such as the brainstem or optic nerves since fractionation optimizes normal tissue tolerance.221–224 Nevertheless, recent non-randomized evidence suggests that larger meningiomas may have worse outcomes with fractionated RT.223,225,226 Small cavernous sinus meningiomas and optic nerve sheath meningiomas; however, tend to be well controlled with primary fractionated RT and have similarly high rates of symptomatic improvement after treatment.223,227,228
Stereotactic Radiosurgery for Meningiomas
SRS is defined as treatment with a single fraction of radiation, typically using doses ranging from 12–18 Gy to the 50% isodose line for Gamma Knife, 60%–70% isodose line for CyberKnife, or up to 80% for other linear accelerator (LINAC) based methods. Delivery of SRS in multiple fractions using frameless image-guided SRS systems, termed hypofractionated stereotactic radiotherapy (HSRT), has also been implemented and typically applies a dose per fraction of ≥5 Gy not exceeding 5 fractions. The multicenter retrospective IMPASSE study (Incidental Meningioma Progression During Active Surveillance or After Stereotactic Radiosurgery) on small asymptomatic/incidental meningiomas demonstrated that in a large cohort matched for patient age, tumor volume, location, and imaging follow-up, meningiomas that received SRS had a tumor control rate of 99.4% compared to 62.1% in the observation arm. This suggests that SRS likely does change the natural history of some meningiomas, with the caveat that most incidental and asymptomatic meningiomas do not demonstrate clinically significant growth on long-term follow-up and can be safely observed without any treatment.128,167,177 Treatment may be warranted in meningiomas that are adjacent to critical structures where growth may lead to neurologic deficits or higher risk of subsequent intervention, particularly in younger patients, although this decision too must be balanced against long-term RT-associated adverse events such as cognitive decline.208
In a meta-analysis of non-cavernous sinus CNS WHO grade 1 meningiomas treated with SRS or HSRT, local control rates ranged from 71% to 100% (median 94.2%) while PFS ranged from 55% to 97% (median 89.4%) with a median follow-up of at least 3 years.229 Factors associated with improved tumor control included smaller tumor volume and patient age under 65 years.229 Local control and PFS rates for cavernous sinus meningiomas appear to be more favorable, with 5-year PFS rates ranging from 86% to 99% and 10-year PFS rates from 69% to 97%.229 Factors associated with improved local control following SRS included higher marginal dose, small-to-medium sized tumors (generally < 10 cc), CNS WHO grade 1, primary SRS (vs adjuvant), treatment within 1 year of symptom onset, female sex, younger age, and less conformal plans. By contrast, tumor volume >10 cc, parasagittal/parafalcine tumor location, and venous sinus invasion were associated with worse tumor control and an increased rate of complications after SRS.207,208,224,230–232
The evidence on SRS for higher grade meningiomas (CNS WHO grades 2 and 3) is limited. However, tumor control in histologically confirmed higher-grade meningiomas is typically poor, with one series reporting rates as low as 50% and 17% at 2–2.5 years for WHO grade 2 and 3 meningiomas respectively.233 A recent multicenter study of 233 WHO grade 2 meningiomas found a similar 3-year PFS rate of 53.9% after SRS, with a 5-year PFS rate of 33.1%. When recursive partitioning analysis was performed, 2 subgroups were identified with divergent prognoses. Poor outcomes were associated with patient age over 50 years, multiple prior resections or prior RT, and treatment volume >11.5 cm3.234 There are limited data on whether higher SRS doses or hypofractionated treatment regimens are advantageous for higher grade meningiomas and existing evidence is confounded by clinical factors such as pretreatment clinical history, treatment timing, and RT field. Therefore, prospective studies are needed, particularly for cases with treatment equipoise. Importantly, as with external beam RT, given the lack of molecular stratification in the current SRS literature, future studies should focus on incorporating molecular criteria into retrospective and prospective analyses.
SSTR-Targeted Peptide Receptor Radionuclide Therapy
Given the fact that SSTR2 ligands can be utilized for either diagnostic (eg, 68Ga) or therapeutic purposes (eg, 177Lu or Y), the concept of theranostics has gained traction in meningiomas.235 Several mostly single- or bi-center retrospective studies have been completed with promising results in terms of achieving stable disease in progressive, pretreated meningiomas.236,237 The uptake of the diagnostic tracer might be suitable as a prognostic marker for the efficacy of this therapy given its usually high sensitivity and specificity for its target.238 Recently, an EMA- and FDA-approved radiopharmaceutical for SSTR2-radioligand therapy became available for the treatment of neuroendocrine tumors, which like meningiomas, are characterized by high SSTR expression.239 A recent single-arm phase II study (NCT03971461) on the use of 177Lu-DOTATATE for progressive, intracranial meningiomas saw 6/14 patients achieving the PFS-6 threshold required for the study to progress to its second stage, currently open for enrollment in the United States.240,241 A randomized clinical trial to evaluate the efficacy of 177Lu-DOTATATE in recurrent meningioma is in preparation within the EORTC Brain Tumor Group network. Other radioligands are also currently being developed for similar applications.
Systemic Therapies for Meningiomas
Classically, meningioma treatment has centered on surgical resection and RT. However, novel systemic agents have emerged as a possible option for recurrent or aggressive subtypes, all of which remain under investigation.242 These include tyrosine kinase inhibitors and monoclonal antibodies targeting vascular endothelial growth factor (VEGF) signaling pathways.243–247 A phase II trial of the multikinase inhibitor sunitinib which targets VEGF and platelet-derived growth factor receptors, among others, in CNS WHO grade 2 and 3 meningiomas showed a PFS-6 rate of 42%, meeting the primary endpoint.243 A phase II trial of bevacizumab (a monoclonal antibody against VEGF-A) in recurrent meningiomas reported a PFS-6 rate of 77% in grade 2 and 46% in grade 3 meningiomas, suggesting anti-tumor activity.248 The Alliance A071401 trial is the first genomic-driven phase II study in which patients with recurrent meningiomas are genotyped and assigned to treatment with vismodegib for tumors with SMO mutations, abemaciclib for cases with CDK alterations, capivasertib for tumors with AKT or PI3K mutations and a FAK inhibitor (GSK2256098) for NF2-mutant cases. GSK2256098 was well tolerated and demonstrated promise in achieving a PFS-6 of 83% in progressive CNS WHO grade 1 meningiomas and 33% in CNS WHO grades 2/3 cases. Cytotoxic and hormonal agents, including trabectedin, somatostatin agonists, and progesterone antagonists, have demonstrated less clinical efficacy.247,249–257
Immunotherapy has shown promise in treating solid organ tumors, and recently there has been growing interest in its role in meningiomas despite the challenges of their usually immunologically quiet microenvironment and low tumor mutational burden.258 In a single-arm, open-label phase II trial (NCT03279692), patients with progressive CNS WHO grade 2 and 3 meningiomas were treated with pembrolizumab, a PD-1 inhibitor, which met the primary endpoint and achieved a PFS-6 of 48% with a median PFS of 7.6 months.259 In the same trial, 20% of patients experienced one or more grade-3 or higher adverse events associated with treatment. A trial of nivolumab monotherapy in similarly progressive high-grade meningiomas failed to demonstrate improvement in PFS-6 (PFS-6 42.4%); however, 2 patients with high tumor mutational burden had increased immune cell proliferation and were long-term survivors.260
Thus far, more trials are needed to identify better systemic therapies for meningioma patients. Results from several published and completed clinical trials are summarized in Table 3. Given the lack of current options for treatment-refractory meningiomas, additional agents are needed. The results from ongoing trials may highlight the importance of molecular classification on patient selection for targeted therapies as opposed to stratification based on WHO grade alone. There are several ongoing clinical immunotherapy trials on the use of nivolumab, ipilimumab, and avelumab (NCT03173950, NCT04659811, and NCT03267836) for meningiomas and other CNS tumors, the results of which may yield interesting treatment insights for the future (Table 4).
Table 3.
Selected Completed and Published Clinical Trials on Systemic Therapy in Meningiomas
| Corresponding author | Year | Study title | Study population and key eligibility criteria | Total patients | Systemic/experimental agent | Primary endpoint | Outcome | +/− Primary outcome | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Chamberlain | 2004 | Temozolomide for treatment-resistant recurrent meningioma | Progressive WHO grade 1 meningiomas and KPS ≥ 60 | 16 | Temozolomide (75 mg/m2 for 42 days followed by 28-day break) | PFS-6 | 0% | − | 261 |
| Wen | 2009 | Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01–08) | Recurrent meningiomas with KPS ≥ 60 | 23 (13 benign (WHO grade 1), 5 atypical (WHO grade 2), 5 malignant (WHO grade 3)) | Imatinib (600–800 mg PO daily) | PFS-6 | 29.4% (45% for benign, 0% for atypical or malignant) | − | 262 |
| Wen | 2010 | Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma | Recurrent meningiomas and KPS ≥ 60 | 25 (16 gefitinib, 9 erlotinib) | Gefitinib (500–1000 mg PO daily), erlotinib (150 mg PO daily) | PFS-6 | 28% (25% gefitinib, 33% erlotinib) | − | 263 |
| Reardon | 2011 | Phase II study of Gleevec® plus hydroxyurea (HU) in adults with progressive or recurrent meningioma | Progressive/recurrent meningioma and KPS ≥ 60 | 21 | Hydroxyurea (500 mg PO BID) and Imatinib (400–800 mg PO daily) | PFS-6 | 61.9% (87.5% WHO grade 1, 46.2% WHO grade 2/3) | + | 264 |
| Wen | 2014 | Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma | Recurrent or progressive meningioma with KPS ≥ 60 | 34 (18 cohort A/malignant, 16 cohort B/benign) | Pasireotide (60 mg LAR IM monthly) | PFS-6 | 17% cohort A, 50% cohort B | − | 253 |
| Kaley | 2014 | Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma | Refractory recurrent WHO grades 2–3 meningiomas | 36 | Sunitinib 50 mg PO daily | PFS-6 | 42% | + | 243 |
| Raizer | 2014 | A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas | Surgery and radiotherapy refractory recurrent meningiomas and KPS ≥ 60 | 22 (14 WHO grade 2, 8 WHO grade 3) | PTK787/ZK 22585 (500 mg PO BID) | PFS-6 | 54.4% (64.3% WHO grade 2, 37.5% WHO grade 3) | + | 244 |
| Verschraegen | 2015 | Double-BLIND PHASE III randomized trial of the antiprogestin agent mifepristone in the treatment of unresectable Meningioma: SWOG S9005 | Progressive or refractory meningioma with prior radiotherapy, PS 0–2 | 164 (80 mifepristone, 84 placebo) | Mifepristone (200 mg PO daily) | PFS-24 | 30% mifepristone, 33% placebo | − | 265 |
| Jensen | 2016 | Combined hydroxyurea and verapamil in the clinical treatment of refractory meningioma: Human and orthotopic xenograft studies | Refractory recurrent/progressive meningiomas with KPS ≥ 90 | 7 | Hydroxyurea (20 mg/kg/day PO BID), Verapamil (120–240 mg PO daily) | PFS-6 | 85% | − | 266 |
| Graillon | 2020 | Everolimus and octreotide for patients with recurrent meningioma: results from the phase II CEVOREM trial | Progressive meningioma ineligible for further surgery/radiotherapy with KPS > 50 | 20 | Everolimus (10 mg PO daily) and octreotide (30 mg LAR IM monthly) | PFS-6 | 55% | + | 256 |
| Reardon | 2021 | Activity of PD-1 blockade with nivolumab among patients with recurrent atypical/anaplastic meningioma: Phase II trial results | WHO grade 2 or 3 recurrent meningiomas with KPS ≥ 70 | 25 | Nivolumab (240mg IV q2weeks) | PFS-6 | 42.40% | − | 260 |
| Preusser | 2022 | Trabectedin for recurrent WHO grade 2 or 3 meningioma: A randomized phase II study of the EORTC brain tumor group (EORTC-1320-BTG) | Recurrent WHO grade 2 or 3 meningiomas with PS 0–2 | 90 (61 trabectedin, 29 standard of care) | Trabectedin (1.5 mg/m2 q3weeks) | PFS-6 | 2.4 months Trabectedin, 4.2 months standard of care | − | 247 |
| Kumthekar | 2022 | A multi-institutional phase II trial of bevacizumab for recurrent and refractory meningioma | Progressive meningiomas with KPS ≥ 60 | 42 (10 WHO grade 2, 20 WHO grade 2, 12 WHO grade 3) | Bevacizumab (10 mg/kg IV q2weeks for 6 months, then 15 mg/kg IV q3weeks) | PFS-6 | 90% WHO grade 1, 76% WHO grade 2, 45% WHO grade 3 | + | 248 |
| Brastianos | 2022 | Alliance A071401: phase II trial of focal adhesion kinase inhibition in meningiomas with somatic NF2 mutations | WHO grade 1–3 recurrent or progressive meningiomas with NF2 mutation | 36 (12 WHO grade 1, 24 WHO grade 2/3) | GSK2256098 750 mg PO BID | PFS-6 | 83% WHO grade 1, 33% WHO grades 2/3 | + | 267 |
| Plotkin | 2023 | Prospective phase II trial of the dual mTORC1/2 inhibitor vistusertib for progressive or symptomatic meningiomas in persons with neurofibromatosis 2 | NF2 patients with progressive or symptomatic meningiomas | 18 | Vistusertib (125 mg PO BID for 2 days per week) | Volume decrease > 20% | 6% partial response, 94% stable disease | − | 268 |
WHO, World Health Organization; KPS, Karnofsky performance score; PFS-6/24, progression-free survival at 6/24 months; PO, per os; IM, intramuscular; IV, intravenous; EORTC, European Organization for Research and Treatment of Cancer; NF2, neurofibromatosis-2 (NF2-SWN); BID, twice per day
Table 4.
Selected Ongoing Clinical Trials on Systemic Therapies in Meningiomas
| Principal investigator | Estimated completion | Study design | Study population and key eligibility criteria | Systemic/experimental agent | Primary endpoint | NCT # |
|---|---|---|---|---|---|---|
| Jiayi Huang | 2023 | Neoadjuvant avelumab and hypofractionated proton radiation therapy followed by surgery for recurrent radiation-refractory meningioma | WHO grade 1–3 meningioma which has failed maximal safe resection + radiation therapy | Avelumab (10 mg/kg IV q2weeks for 3 months), proton therapy (20 CGE/5 daily fractions of 4 CGE per day) | CD8+/CD4 + tumor-infiltrating lymphocytes | NCT03267836 |
| David A. Reardon | 2024 | An open-label phase II study of nivolumab and ipilimumab in adult participants with progressive/recurrent meningioma | Progressive or recurrent meningiomas with KPS ≥ 70 | Nivolumab (240 mg q2 weeks), Ipilimumab 1 mg/kg q3weeks) | PFS-6 | NCT02648997 |
| Priya Kumthekar | 2024 | Optune delivered electric field therapy and bevacizumab in treating patients with recurrent or progressive grade 2 or 3 meningioma | Progressive or recurrent meningiomas KPS ≥ 60 | Bevacizumab IV dose not specified, electric field therapy using Optune daily over 18 hours | PFS-6 | NCT02847559 |
| Priscilla K. Brastianos | 2024 | Vismodegib, capivasertib, and abemaciclib in treating patients with progressive meningiomas | Progressive or recurrent meningiomas | Vismodegib (PO once daily), capivasertib (PO BID days 1–4, treatment q7days), abemaciclib (PO q12h), FAK inhibitor GSK2256098 (PO BID) | PFS-6 | NCT02523014 |
| Erik P. Sulman | 2025 | A phase II trial of 177Lu-DOTATATE for recurrent/progressive meningioma | Progressive meningioma (any grade) with KPS ≥ 60 | 177Lu-DOTATATE intravenously every 8 weeks up to 4 cycles | PFS-6 | NCT03971461 |
| Recursion Pharmaceuticals | 2027 | Efficacy and safety of REC-2282 in patients with progressive neurofibromatosis type 2 (NF2) mutated meningiomas (POPLAR-NF2) | Progressive and recurrent NF2 meningiomas | Small molecule HDAC inhibitor REC 2282 (30–60 mg PO 3 times per week, for 3 of the 4 weeks) | PFS-6 | NCT05130866 |
| Marta Penas-Prado | 2027 | Phase II trial of the immune checkpoint inhibitor nivolumab in patients with recurrent select rare CNS cancers | Atypical or malignant meningioma | Nivolumab (240 mg IV q2weeks for cycles 1–2, then 480 mg q4weeks for 14 additional doses) | PFS-6, CR/PR | NCT03173950 |
| Rupesh R. Kotecha | 2028 | A phase II study of cabozantinib for patients with recurrent or progressive meningioma | Progressive or recurrent meningiomas with KPS ≥ 50 | Cabozantinib (60 mg PO daily for 28 days) | PFS-6 | NCT05425004 |
| Nancy Ann Oberheim Bush | 2028 | Stereotactic radiosurgery (SRS) and immunotherapy (Pembrolizumab) for the treatment of recurrent meningioma | Recurrent WHO grade 2 or 3 meningioma | SRS (15–20 Gy/1 Fr or 25–30 Gy/5 Fr) combined with pembrolizumab (200 mg IV on day 1 to −1 of radiation then q3weeks) | PFS-12 | NCT04659811 |
WHO, World Health Organization; NCT, National Clinical Trial; CGE, Cobalt Gray Equivalent; KPS, Karnofsky Performance Score; PFS-6/12, Progression-free survival at 6/12 months; CR, complete response; PR, partial response; CNS, central nervous system; PO, per os; BID, twice per day.
Quality of Life for Meningioma Patients
The impact of a meningioma diagnosis on patients is often underestimated and standardized methods or tools to assess health-related quality of life (HRQoL) are still lacking. Many patients present with symptoms that can profoundly impair HRQoL including but not limited to: seizures, motor and sensory deficits, cognitive impairment, cranial neuropathies, neuropsychiatric symptoms, and systemic symptoms such as fatigue.269,270 Many of these symptoms may persist long after treatment, with some patients reporting considerable effects on HRQoL as long as 10 years after surgery.271 Therefore, even if long-term tumor control is achieved, patients may require additional targeted interventions to help them return to their premorbid quality of life. Even surveillance imaging may be associated with significant anxiety and negative effects on HRQoL.
After surgery, patients with symptomatic meningiomas often experience a significant improvement in their symptoms and improved HRQoL in the short term. However, most demonstrate persistently reduced long-term HRQoL when compared to healthy controls.272–274 Of note, achieving seizure control plays a significant role in improving HRQoL, whereas multiple surgical resections and the use of adjuvant RT are associated with reduced HRQoL scores, although these results may be confounded by tumor location and extent of resection. Other predictors of lower postoperative HRQoL include lower preoperative HRQoL, larger tumor size, skull base location, and the presence of peritumoral edema.275 Fatigue is the most common symptom that has been reported to worsen in the post-treatment period following either surgery or SRS.275,276 Patients who receive RT may demonstrate improvement in some domains of HRQoL in the short term but may also experience delayed and progressive cognitive decline in the long-term. Much of this data, however, is based on older RT treatment paradigms, with modern treatment plans likely to show more favorable long-term cognitive outcomes.274,277–279 Increased patient support resources and counseling should be directed towards patients at high risk of persisting impairments in HRQoL both before and after treatment.276 There is currently a critical lack of a standardized, externally validated questionnaire targeting HRQoL for meningioma patients as well as specific interventions outside of standard tumor treatments designed to improve different HRQoL domains.274 Correlation between the quality of life and molecular biomarkers also remain largely unexplored despite key differences in tumor behavior between molecular groups (eg, NF2-wild-type meningiomas may be more likely to grow in the skull base resulting in cranial nerve deficits whereas NF2-altered meningiomas are more common along the convexity and may be more commonly associated with seizures). Molecular subgroup-specific quality-of-life metrics may provide an opportunity for further improvements in personalized and patient-centric care.
Seizures in Meningioma Patients
Seizures are a common presenting symptom in patients with meningioma, occurring in up to 30% of cases preoperatively.280,281 Risk factors associated with increased seizure risk include recurrent disease, larger tumor size, non-skull base location, higher WHO grade, presence of peritumoral edema, and receipt of postoperative RT.4,282,283 The presence of brain invasion and peritumoral edema are associated with neurotransmitter alterations, ionic channel changes, and blood-brain barrier disruption, all of which may contribute to the development of a cortical epileptogenic focus.284–287 One study found that NF2-mutated meningiomas had an increased risk of preoperative seizures but only when associated with atypical histology and peritumoral edema. It was additionally found that mutations in Hedgehog signaling pathway genes (SMO, PRKAR1A, SUFU) in CNS WHO grade 1 meningiomas were associated with increased risk of postoperative seizures.282 Additional work is needed to ascertain whether specific molecular alterations can independently predict perioperative seizure risk or if the postoperative course is mainly affected by the anatomic location associated with meningiomas harboring SMO, PRKAR1A, or SUFU mutations.
The primary treatment for meningioma-related seizures is surgical resection, ideally GTR of the tumor.288 For patients in whom surgery or GTR may not be feasible, or for those whose seizures persist despite surgical resection, antiepileptic medications are often used following a similar treatment protocol as prescribed for idiopathic epilepsy. There are currently no recommendations regarding the first-line antiepileptic drug of choice for these patients, although levetiracetam is the most commonly prescribed option.288,289 The optimal duration of perioperative antiepileptic therapy for meningioma patients with seizures is also highly variable and there are no current guidelines or level 1 evidence to support specific practices that currently are institution or provider-specific. In general, the literature supports continuing seizure medications postoperatively for 1–2 years before tapering off in the setting of ongoing seizure freedom.288,290,291 The use of antiepileptic medication as prophylaxis is controversial but is generally not recommended given a lack of conclusive evidence demonstrating reduction in postoperative seizure risk.292,293 Nevertheless, it may be considered in patients with one or more seizure risk factors such as significant peritumoral edema.280,294 Furthermore, results from the ongoing multicentre, randomized controlled trial STOP ‘EM (Surgeons Trial Of Prophylaxis for Epilepsy in seizure naive patients with Meningioma) in the United Kingdom on prophylactic levetiracetam for seizure naïve meningioma patients may help inform significant changes in clinical practice.295
Meningiomas in Patients With NF2-Related Schwannomatosis
Pathogenic germline alterations in the NF2 gene, whether inherited or acquired (eg, new germline variant), result in the development of the tumor predisposition syndrome NF2-SWN.66 NF2-SWN is a highly penetrant autosomal dominant condition with an incidence of 1 in 25 000 to 33 000.296–298 While classically characterized by the development of bilateral vestibular schwannomas, 48%–75% of patients with NF2-SWN will develop meningiomas at some point in their clinical course.299 Compared to patients with sporadic meningiomas, patients with NF2-SWN typically develop meningiomas at a younger age and are at higher risk for developing multiple meningiomas.75,300 Therefore patients with these phenotypes should be screened for germline NF2 and SMARCE1 mutations. The majority of NF2-SWN-associated meningiomas are asymptomatic and often diagnosed during the work up for NF2-SWN or over the course of routine radiographic surveillance. When present, approximately 10% of these meningiomas will grow rapidly (defined as ≥ 2 cm3/year by one study) while the remainder will demonstrate no or very slow growth. New meningiomas will develop in 20% of NF2-SWN patients.301–303 Importantly, patients with NF2-SWN who have a meningioma have been found to have a significantly increased risk of death compared to those who do not.304
Given their complexity, the management of patients with NF2-SWN by multidisciplinary teams at high-volume centers has been demonstrated to improve both their quality of life and life expectancy.304 The majority of NF2-SWN-associated meningiomas can be safely observed including those that demonstrate slow, clinically silent growth. Surgery remains the primary treatment for symptomatic or rapidly enlarging tumors, although its risks must be weighed against the anticipated risks of additional or future operative procedures that NF2-SWN patients may need to undergo for their other neoplasms.304 Maximizing extent of resection remains important but even moreso here must be balanced against the risk of incurring a significant neurologic deficit that may irreparably impair their quality of life or make them ineligible for other required interventions.302 NF2-SWN-associated meningiomas tend to be more biologically aggressive than sporadic cases (52% are WHO grades 2 or 3), although this statistic may be confounded by a relative hesitancy on the part of most surgeons to resect the presumably more benign, slow-growing tumors in these patients.298,301
SRS has also been proposed as a viable treatment option for enlarging meningiomas in NF2-SWN patients with 5-year local control rates generally greater than 90%. However, distant failure rates range from 27% to 51%, and studies on the topic have been limited to small institutional case series.305–308 Additionally, malignant transformation remains a rare but important concern with SRS in this patient population with a significantly higher risk of malignant progression in previously benign tumors from NF2-SWN patients (up to 5–6% absolute risk increase) compared to patients with irradiated sporadic disease.309 Furthermore, the quality of life of NF2-SWN patients may be negatively impacted by the use of RT.278,310,311 It is because of these considerations that RT should be used judiciously in this patient population and in many instances, are more commonly reserved for recurrent meningiomas or tumors with unacceptably high surgical risk.298
Given the challenges related to NF2-SWN-associated tumors, several clinical trials have been undertaken to identify better treatment options. The AZD2014 trial in NF2-SWN patients with progressive or symptomatic meningiomas (NCT02831257) used a dual mTORC1/mTORC2 inhibitor but 12 out of 18 patients withdrew due to adverse effects. The RAD001 trial (NCT01419639) was a phase II clinical trial utilizing everolimus, an mTOR inhibitor, as monotherapy for NF2-SWN patients which although appeared to slow tumor growth, did not demonstrate any significant reduction in size. The phase II CEVOREM trial (NCT02333565) combined octreotide, a somatostatin analog, with everolimus for the treatment of aggressive and otherwise treatment-refractory meningiomas, although only 4 of the 20 patients had a germline NF2 mutation. This trial demonstrated a significant reduction in the median tumor growth rate at 3- and 6-months, with 4 patients withdrawing due to adverse effects.256 A retrospective review also found slowing of meningioma tumor growth with the EGFR/ErbB2 inhibitor lapatinib (NCT00973739) in 8 NF2-SWN patients with 17 tumors.312 The phase II INTUITT-NF2 trial (NCT04374305) utilized brigatinib, a potent anaplastic lymphoma kinase inhibitor in combination with INK-128, a dual mTORC1/2 inhibitor for NF2-SWN associated tumors, with interim results of the brigatinib arm demonstrating radiographic response of 28% in meningiomas.313 While some of these treatments show promise, additional prospective trials are needed before any systemic therapies are adopted into standard of care treatment guidelines. Furthermore, translational studies incorporating radiography, molecular biomarkers (including noninvasive biomarkers), histopathology, and quality of life will be critical for improving treatment paradigms for NF2-SWN patients.
Radiation-Induced Meningiomas
Exposure to ionizing radiation is a well-known risk factor for meningioma development. While RT improves the survival of many childhood cancers, some long-term survivors are left with secondary neoplasms as a consequence of their treatment, most commonly RIMs.314,315 Other rarer patient populations with RIMs include those who received low-dose cranial RT for tinea capitis in the first half of the 20th century and in survivors of the atomic bombs of World War 2.13,15,316,317 The latency period between initial radiation exposure and the development of RIMs typically range between 10-40 years and may be inversely associated with the initial exposure dose (higher initial dose = shorter latency period).11,13,15,318 Given that RIMs in childhood cancer survivors may be diagnosed 40 years after their initial treatment, imaging follow-up at fixed intervals, with more frequent follow-up for those who received high-intensity treatment may be warranted.314,315
RIMs are biologically and clinically distinct from their sporadic counterparts and while rare (making up only 1–2% of all meningiomas), present significant clinical challenges due to their increased biological aggressiveness, multiplicity, and resistance to standard therapies. RIMs have a much higher burden of cytogenetic changes compared to sporadic meningiomas including frequent losses of chromosomes 1p (over 50% of cases), 9p, 19q, 18q, 10p, and 22q.10,14,319 Notably, RIMs were less frequently found to have loss of chromosome 22q or NF2 point mutations compared to sporadic meningiomas but had more frequent NF2 gene fusion events. These fusions are likely secondary to misrepair of RT-associated double-stranded DNA breaks and are an alternative mechanism of NF2 disruption. Non-NF2 recurrent mutations including in AKT1, SMO, TRAF7, and KLF4 were generally not observed in RIMs.10,41
Standard treatment guidelines for RIMs do not currently differ from sporadic meningiomas, with surgical resection as first line therapy for symptomatic cases. When multiple RIMs are present in the same patient, surgery should target the largest and/or symptomatic tumors first. Otherwise, active surveillance remains a safe initial strategy for these tumors, with a low rate of neurologic morbidity.320 The role of adjuvant RT for RIMs is unclear but may be utilized in cases of subtotally resected meningiomas or those that are higher WHO grade. However, even CNS WHO grade 1 RIMs can demonstrate aggressive behavior and many of these cases are RT-resistant. SRS in select cases however appears to be safe and well tolerated for RIMs that are not amenable to surgical resection or in patients with multiple RIMs that require treatment. Overall, tumor control rates following SRS are lower for RIMs than for sporadic meningiomas, and larger treatment volume is associated with worse PFS.321–324
Spinal Meningiomas
Although rarer than their intracranial counterparts (with an incidence of approximately 0.193–0.33 cases per 100 000), spinal meningiomas are the most common intradural spinal tumors, representing 25–45% of these cases.325–327 CNS WHO grades 2 and 3 cases are also comparatively less common in the spine.326–328 Spinal meningiomas also appear to differ from intracranial meningiomas on a molecular basis and are generally more biologically benign.329–331 A novel molecular classification was recently proposed for spinal meningiomas with 2 major subgroups: one with predominantly NF2 mutations and the other with AKT1 mutations (mutually exclusive to NF2 mutations). While both subgroups were predominantly comprised of meningiomas from benign methylation subclasses, the NF2-mutated subgroup was associated with intermediate outcomes and were more strongly associated with female sex, thoracic spine location, and frequent tumor calcification.109,329,330AKT1-mutated spinal meningiomas had no sex predilection and were associated with meningothelial subtype, cervical spine location, and the absence of tumor calcification.329,330 Interestingly, there was a small subset of spinal meningiomas with a much higher degree of cytogenetic changes that did not show a clear association with either of the above subgroups and instead more closely resembled intermediate and malignant methylation subclasses of intracranial meningiomas.330 This suggests that as more clinically aggressive spinal meningiomas are profiled, additional molecular subgroups may be elucidated, potentially mirroring those that have been discovered for intracranial cases.
Treatment guidelines for spinal meningiomas are the same as for intracranial meningiomas, with GTR as the usual goal of surgery. In cases where a Simpson grade 1 resection (including dural resection with patch reconstruction) may not be feasible such as for tumors with a ventral origin, a Simpson grade 2 resection with extensive dural coagulation may have comparable outcomes.327,328,332 Careful anatomic planning of surgical corridors may avoid the need for instrumentation in many of these cases.333 The role of RT for spinal meningiomas is unclear, particularly given their largely benign behavior. A review of the National Cancer Database showed that only 2.5% of 10 458 patients with spinal meningiomas received RT. Older patients with higher WHO grade tumors, larger tumors, and recurrent cases were more likely to receive RT. Interestingly, this study also reported an increase in mortality risk among “borderline” (CNS WHO grade 2) and malignant (CNS WHO grade 3) tumors that received RT following surgery compared to those that did not.334 Further study is needed in order to fully resolve the role of adjuvant RT or primary stereotactic body RT for spinal meningiomas.335–337
Pediatric Meningiomas
Unlike in adults, meningiomas are rare in the pediatric population and account for only 2.2–3.6% of all brain tumors in this group and 0.4%–2.5% of all diagnosed meningiomas.338–342 Also dissimilar to their adult counterparts, pediatric meningiomas affect males and females relatively equally with a greater incidence of tumors in intraventricular and spinal locations.341–343 There are also a higher proportion of CNS WHO grade 2 and 3 meningiomas in pediatric patients compared to adults338,341,344 and a larger proportion with clear cell (CNS WHO grade 2) or papillary (associated with CNS WHO grade 3) histology.343–345 However, grading appeared to be less predictive of overall outcome in these cases.338,341,346 Patients under 3-years of age or over 12 years may have worse overall survival outcomes; the former group may be associated with higher operative morbidity and mortality while the latter have meningiomas that more closely resemble adult cases.338 Pediatric patients with meningiomas are more likely to have NF2-SWN than adults and these cases have a much shorter time to progression and higher mortality rate compared to non-NF2-SWN cases.338,343 Therefore, all pediatric patients who are diagnosed with a meningioma should be screened for NF2 mutations and other associated rare genetic conditions that may predispose them to meningioma development.347 Given that spinal meningiomas are also more common in pediatric patients, full craniospinal imaging at the time of diagnosis should be considered.340,341,343,348,349
As in adult cases, extent of resection appears to be the most important prognostic factor, with GTR conferring both a PFS and overall survival benefit.338,347,348 The role of adjuvant RT is controversial, with insufficient literature to assess its utility. One meta-analysis showed no clear demonstrated benefit for PFS or overall survival, though there was likely a high degree of selection bias for irradiating aggressive tumors.338 Clinical decisions should be made with multidisciplinary discussion on a case-by-case basis, keeping in mind that cranial irradiation is associated with significant morbidity in children. Some clinicians advocate for second-look surgery if residual tumor is detected on postoperative imaging although evidence to support this approach is not well established.340,350–352
Similar to adult meningiomas, NF2 mutations and loss of chromosome 22 are the most common alterations in pediatric meningiomas, found in 47%–72% of cases.344,349,353 However, other classical non-NF2 driver mutations such as AKT1, SMO, KLF4, and TRAF7 have not been described in the pediatric population.344,349,354 Instead, a number of different YAP1-fusions have been described in non-NF2 altered pediatric meningiomas (YAP1-MAML2; YAP1-PYGO1; and YAP1-LMO1) and have been proposed as an alternative oncogenic driver to NF2 inactivation.355,356 Preclinical studies have shown that the YAP component of these gene fusions is likely the critical driver of these tumors and the YAP1-MAML2 fusion may be targetable through pharmacologic disruption of the YAP1-TEAD interaction.356
The majority of clear cell meningiomas in this population expectedly harbor SMARCE1 mutations.47,49–51 DNA methylation profiling largely segregates pediatric meningioma cases from adult cases and may further separate them into 3 methylation subgroups: one group comprised almost exclusively of SMARCE1-mutated clear cell meningiomas, one group driven by NF2 or chromosome 22q loss, and another mixed group containing all cases with rhabdoid histology, allelic loss of chromosome 11 and rare loss of chromosome 22.344 The prognostic significance of these groups remain uncertain given the rarity of cases with both molecular profiling and well annotated clinical data.
Preclinical Models of Meningioma
Historically, there has been a paucity of cell models for meningiomas due to the slow growth rates of most primary cell lines and the tendency of most cell lines to senesce after several passages. More recently, there have been renewed efforts by laboratories worldwide to optimize primary meningioma cell cultures to better study the functional impact of specific genomic alterations and create higher fidelity preclinical models.357,358
There are however, several well established malignant meningioma cell lines that are commercially available. One of these such lines is IOMM-Lee,established from an intraosseous CNS WHO grade 3 meningioma, and is still commonly utilized due to its ability to readily form heterotopic and orthotopic xenografts.359–361 Although it harbors CDKN2A/B loss as a hallmark of proliferation in addition to a high burden of copy number changes, it lacks the biallelic NF2 inactivation seen in the majority of biologically aggressive meningiomas and also contains unusual chromosomal gains of 3q, 5, and 9 that are not commonly found in meningiomas. The NCH93 meningioma cell line was similarly derived from a CNS WHO grade 3 meningioma, but unlike IOMM-Lee, contains an NF2 frameshift mutation making it a potentially more serviceable aggressive NF2-mutant meningioma model that also reliably forms xenografts.362,363 KT21-MG1 is another aggressive meningioma cell line with monosomy of chromosome 22 established from a human malignant meningioma that demonstrates c-myc amplification and can produce xenografts in nude mice.116,364,365 MN3 is another serially transplantable orthotopic cell model derived from a recurrent, malignant meningioma which has also been demonstrated to produce xenografts in nude mice while harboring several pathologic hallmarks of aggressive meningiomas including elevated Ki-67, vimentin expression, and NF2 inactivation.366
One of the first benign meningioma cell lines, Ben-Men-1, was derived from a CNS WHO grade 1 meningioma by transducing tumor cells with the human TERT gene to overcome cellular senescence. However, while Ben-Men-1 proliferated rapidly in vitro, orthotopic xenografts using this cell line grew slowly, making it suboptimal for testing therapeutic agents.367 Furthermore, introducing alterations such as the human TERT gene or disruption of p53 and pRb pathways necessary to immortalize meningioma cell lines may also alter how closely these cell lines recapitulate their tumor of origin.368
To date, orthotopic xenograft models have been the gold standard for evaluating treatment efficacy in vivo, with tumor volumes readily evaluable by MRI or bioluminescence methods.369,370 However, these models are limited by the requirement for immunodeficient mice as hosts, which may be missing important immune cell populations for the study of tumor microenvironment interactions. Genetically engineered mouse models have attempted to overcome this shortcoming and different groups have leveraged conditional homozygous NF2 knockout models, with and without CDKN2A/B loss or SMO activation, amongst other models. However, most of these models are hindered by similar limitations of prolonged tumor formation time, reduced animal survival due to secondary malignancies, low rates of induction, and complex time- and resource-intensive methodologies.371,372
Recently, 2 other novel ex vivo meningioma models have gained popularity: organotypic tumor slices and patient-derived spherical cell culture models (which include spheroids and organoids), both of which represent patient-derived 3D tumor models that closely recapitulate the genetic and epigenetic alterations of its parent tumor while also preserving cell type heterogeneity including immune and endothelial cells. While the former method enables meningioma tumor to remain in its original structure and within its native microenvironment, it requires larger amounts of intact tissue that cannot be damaged during sectioning and must account for the potential confounding effect of intratumoral heterogeneity within and between slices, making this technique challenging to standardize.373,374 Meningioma organoids, however, can be established from smaller amounts of tissue and can be readily multiplexed in order to perform rapid drug screening and other molecular assays such as DNA- or RNA-sequencing, flow cytometry, and immunohistochemistry.375–378 They can also be successfully established in 60–100% of cases from primary meningioma cells. Moreover, meningioma organoids and spheroids tend to express tumor markers such as progesterone receptor, more closely mimic the cell proliferation rate of human meningiomas compared to two-dimensional cultures, and may have increased transcriptomic markers for the epithelial-to-mesenchymal transition compared to traditional monolayer cultures.379,380 Variants identified in meningioma organoids such as NF2 or TRAF7 alterations are found at similar allele frequencies to their parental tumors, and the CNV profiles of the organoids also closely resemble those of the original tumor.381 The intratumoral heterogeneity and tumor microenvironment of the parental meningioma can also be recapitulated in organoid models, including retainment of immune cells (CD68 + macrophages, CD3 + T cells) and specific neoplastic cell subpopulations.376 Importantly, for organoids or spheroids, specific cell culture conditions need to be established in order to maintain the tumor microenvironment cells over time. While these innovations are promising, additional studies are needed to fully characterize both existing and novel preclinical meningioma models to assess their capability for replicating response to novel therapies.
Future Clinical Trial Design and Other Future Directions
The design of meningioma clinical trials face several challenges largely related to tumor heterogeneity. One issue is that despite a growing body of evidence demonstrating the value of molecular classification systems, there has yet to be a unified molecular taxonomy that can be readily adopted by the WHO as standard of care classification. The ideal classification scheme should have a strong biological foundation and be readily implementable across most pathology laboratories. This standardization will ensure that patients are grouped based on clinically meaningful biological criteria rather than histopathology alone. Immunohistochemical correlates that can reliably identify molecular groups on a one-to-one basis would also be helpful, particularly in expanding trial access beyond tertiary referral centers in high-income countries that have access to sequencing technology. These measures may ultimately improve the uniformity of meningioma cases included in clinical trials treatment arms and decrease biologic heterogeneity that could confound treatment response, particularly amongst CNS WHO grade 2 cases that are a current cohort of interest in randomized trials.
In addition to classification, standardization of outcomes reporting is crucial.151,382 For instance, while PFS-6 has been considered the primary endpoint in most meningioma trials, this control benchmark is largely based on historical cases which may be graded differently today. To account for this, there is a need to retrospectively determine PFS-6 amongst molecularly defined meningioma cohorts, which would contribute to establishing a set of modern control cases for future prospective trial cases (which will likely also be molecularly driven), to benchmark against. Additionally, the relative improvement in PFS-6 that can be considered clinically meaningful should be standardized and these criteria will require subsequent validation. Lastly, as suggested by the RANO group, if PFS is to be used as the primary endpoint, neuroimaging over the last 6–12 months prior to trial enrollment is critical to establish a baseline rate of growth for comparison.141 Defining the appropriate interval and type of surveillance needed for each meningioma subgroup will also enable clinicians to detect tumor progression or recurrence at an early stage, leading to timely interventions and improved outcomes. Given the poor reliability of historical benchmarks such as PFS-6, ideally randomized trials should be conducted whenever possible. The implementation of adaptive clinical trial designs, which allow for real-time adjustments based on novel data, could also enhance the efficiency of clinical trials and accelerate the identification of novel treatments. Addressing these challenges through collaborative efforts among researchers, clinicians, and regulatory bodies will pave the way for more robust meningioma clinical trials, enhancing the precision and impact of future treatments.
Summary
Meningiomas are the most common primary intracranial tumor type, and recent years have seen an increasing number of important discoveries that have shed light into their molecular underpinnings and heterogeneity. However, some unique groups of meningioma patients including those with NF2-associated schwannomatosis, RIMs, and pediatric patients have been largely excluded from most contemporary molecular studies. Furthermore, lack of access to sequencing resources and a relative dearth of high-fidelity preclinical models have also contributed to slower translation of encouraging benchtop findings to the bedside. While surgery and RT remain the only current standard of care options for patients, several promising systemic therapies have demonstrated evidence of efficacy in progressive or recurrent meningiomas with several important, ongoing clinical trials expected to report their results soon. Molecular profiling of meningiomas from these clinical trials including both responders and non-responders may uncover novel insights that can be leveraged for future study. Furthermore, in this current molecular era of meningioma research, there is a need to unify the many molecular classification schemes that have been discovered and utilize them to drive clinical trial design. Achieving this will require a data-driven approach and a consensus amongst experts in the field, including cooperation amongst those who originally developed these classification systems. This remains a high-priority goal of the cIMPACT-NOW working group and consortia such as ICOM and KAM. Implementation of a standardized molecular taxonomy for these tumors will ultimately serve as an important benchmark for future discovery and have a significant beneficial impact on the future care of patients with meningiomas.
Supplementary material
Supplementary material is available online at Neuro-Oncology (https://academic.oup.com/neuro-oncology).
Contributor Information
Justin Z Wang, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada; Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; MacFeeters Hamilton Neuro-Oncology Program, Princess Margaret Cancer Centre, University Health Network and University of Toronto, Toronto, Ontario, Canada.
Alexander P Landry, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada; Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; MacFeeters Hamilton Neuro-Oncology Program, Princess Margaret Cancer Centre, University Health Network and University of Toronto, Toronto, Ontario, Canada.
David R Raleigh, Department of Radiation Oncology, Neurological Surgery, and Pathology, University of California San Francisco, San Francisco, California, USA.
Felix Sahm, Department of Neuropathology, University Hospital Heidelberg and German Consortium for Translational Cancer Research (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany.
Kyle M Walsh, Department of Neurosurgery, Duke University, Durham, North Carolina, USA.
Roland Goldbrunner, Center of Neurosurgery, Department of General Neurosurgery, University of Cologne, Cologne, Germany.
Leeor S Yefet, Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada.
Jörg C Tonn, Department of Neurosurgery, University Hospital Munich LMU, Munich, Germany.
Chloe Gui, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada; Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; MacFeeters Hamilton Neuro-Oncology Program, Princess Margaret Cancer Centre, University Health Network and University of Toronto, Toronto, Ontario, Canada.
Quinn T Ostrom, Duke Cancer Institute, Duke University School of Medicine, Durham, North Carolina, USA; Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Department of Neurosurgery, Duke University, Durham, North Carolina, USA.
Jill Barnholtz-Sloan, Center for Biomedical Informatics & Information Technology (CBIIT), National Cancer Institute, Bethesda, Maryland, USA; Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, Maryland, USA; Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Arie Perry, Department of Pathology, University of California San Francisco, San Francisco, California, USA.
Yosef Ellenbogen, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada; Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; MacFeeters Hamilton Neuro-Oncology Program, Princess Margaret Cancer Centre, University Health Network and University of Toronto, Toronto, Ontario, Canada.
C Oliver Hanemann, Peninsula Schools of Medicine, University of Plymouth University, Plymouth, UK.
Gerhard Jungwirth, Division of Experimental Neurosurgery, Department of Neurosurgery, Heidelberg University, Heidelberg, Germany.
Michael D Jenkinson, Department of Neurosurgery, The Walton Centre NHS Foundation Trust, Liverpool, UK; Institute of Translational Medicine, University of Liverpool, UK.
Ghazaleh Tabatabai, Department of Neurology and Interdisciplinary Neuro-Oncology, University Hospital Tübingen, Hertie Institute for Clinical Brain Research, Eberhard Karls University Tübingen, Tübingen, Germany; Cluster of Excellence (EXC 2180) “Image Guided and Functionally Instructed Tumor Therapies,” Eberhard Karls University Tübingen, Tübingen, Germany; Center for Neuro-Oncology, Comprehensive Cancer Center Tübingen-Stuttgart, University Hospital Tübingen, Tübingen, Germany.
Tiit I Mathiesen, Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden; Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Michael W McDermott, Division of Neuroscience, Herbert Wertheim College of Medicine, Florida International University, Miami, Florida, USA; Miami Neuroscience Institute, Baptist Health of South Florida, Miami, Florida, USA.
Marcos Tatagiba, Department of Neurosurgery, University of Tübingen, Tübingen, Germany; Center for Neuro-Oncology, Comprehensive Cancer Center Tübingen-Stuttgart, University Hospital Tübingen, Tübingen, Germany.
Christian la Fougère, Nuclear Medicine and Clinical Molecular Imaging, University Hospital Tübingen, Germany; Cluster of Excellence (EXC 2180) “Image Guided and Functionally Instructed Tumor Therapies,” Eberhard Karls University Tübingen, Tübingen, Germany.
Sybren L N Maas, Department of Pathology, Erasmus Medical Center, Rotterdam, The Netherlands; Department of Pathology, Leiden University Medical Center, Leiden, The Netherlands.
Norbert Galldiks, Department of Neurology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Institute of Neuroscience and Medicine (IMN-3), Research Center Juelich, Juelich, Germany.
Nathalie L Albert, Department of Nuclear Medicine, Ludwig Maximilians-University of Munich, Munich, Germany.
Priscilla K Brastianos, Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, Massachusetts, USA.
Felix Ehret, Department of Radiation Oncology, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany; Berlin Institute of Health, Charité - Universitätsmedizin Berlin, Berlin, Germany.
Giuseppe Minniti, Department of Radiological Sciences, Oncology and Anatomical Pathology, Sapienza University of Rome, Rome, Italy.
Katrin Lamszus, Laboratory for Brain Tumor Biology, University Hospital Eppendorf, Hamburg, Germany.
Franz L Ricklefs, Department of Neurosurgery, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Jens Schittenhelm, Department of Neuropathology, University Hospital Tübingen, Eberhard-Karls-University Tübingen, Tübingen, Germany; Center for Neuro-Oncology, Comprehensive Cancer Center Tübingen-Stuttgart, University Hospital Tübingen, Tübingen, Germany.
Katharine J Drummond, Department of Neurosurgery, The Royal Melbourne Hospital, Melbourne, Victoria, Australia.
Ian F Dunn, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA.
Omar N Pathmanaban, Division of Neuroscience and Experimental Psychology, Manchester Centre for Clinical Neurosciences, Geoffrey Jefferson Brain Research Centre, University of Manchester, Manchester, UK.
Aaron A Cohen-Gadol, Department of Neurological Surgery, Indiana University, Indianapolis, Indiana, USA.
Erik P Sulman, Department of Radiation Oncology, NYU Grossman School of Medicine, New York, New York, USA.
Emeline Tabouret, CNRS, INP, Inst Neurophysiopathol, Aix-Marseille University, Marseille, France.
Emelie Le Rhun, Department of Neurology & Brain Tumor Center, University Hospital and University of Zurich, Zurich, Switzerland.
Christian Mawrin, Department of Neuropathology, University Hospital Magdeburg, Magdeburg, Germany.
Jennifer Moliterno, Department of Neurosurgery, Yale School of Medicine, New Haven, Connecticut, USA.
Michael Weller, Department of Neurology and Brain Tumor Center, University Hospital and University of Zurich, Zurich, Switzerland.
Wenya (Linda) Bi, Department of Neurosurgery, Brigham and Women’s Hospital, Dana-Farber Cancer Institute, Harvard Medical School, Boston, Massachusetts, USA.
Andrew Gao, Department of Laboratory Medicine and Pathobiology, University Health Network, Toronto, Ontario, Canada.
Stephen Yip, Department of Pathology & Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada; Department of Radiation Oncology, University Hospital, Munich, Germany; German Cancer Consortium (DKTK), Munich, Germany.
Maximilian Niyazi, Bavarian Cancer Research Center (BZKF), Munich, Germany; Center for Neuro-Oncology, Comprehensive Cancer Center Tübingen-Stuttgart, University Hospital Tübingen, Tübingen, Germany.
Kenneth Aldape, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland, USA.
Patrick Y Wen, Dana-Farber Cancer Institute, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Susan Short, Leeds Institute of Medical Research, St James’s University Hospital, Leeds, UK.
Matthias Preusser, Division of Oncology, Department of Medicine I, Medical University of Vienna, Vienna, Austria.
Farshad Nassiri, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada; Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; MacFeeters Hamilton Neuro-Oncology Program, Princess Margaret Cancer Centre, University Health Network and University of Toronto, Toronto, Ontario, Canada.
Gelareh Zadeh, Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada; Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; MacFeeters Hamilton Neuro-Oncology Program, Princess Margaret Cancer Centre, University Health Network and University of Toronto, Toronto, Ontario, Canada.
References
- 1. Ostrom QT, Price M, Neff C, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2016—2020. Neuro-Oncology. 2023;25(suppl_412 suppl 2):iv1–iv99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walsh KM, Price M, Neff C, et al. The joint impacts of sex and race/ethnicity on incidence of grade 1 versus grades 2-3 meningioma across the lifespan. Neurooncol. Adv. 2023;5(suppl 1):i5–i12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maier AD, Mirian C, Haslund-Vinding J, et al. Granular clinical history and outcome in 51 patients with primary and secondary malignant meningioma. J Neurosurg. 2022;137(5):1347–1357. [DOI] [PubMed] [Google Scholar]
- 5. Tosefsky K, Rebchuk AD, Wang JZ, et al. Grade 3 meningioma survival and recurrence outcomes in an international multicenter cohort. J Neurosurg. 2023;140(2):393–403. [DOI] [PubMed] [Google Scholar]
- 6. Kshettry VR, Hsieh JK, Ostrom QT, et al. Descriptive epidemiology of spinal meningiomas in the United States. Spine (Phila Pa 1976). 2015;40(15):E886–E889. [DOI] [PubMed] [Google Scholar]
- 7. Claus EB, Cornish AJ, Broderick P, et al. Genome-wide association analysis identifies a meningioma risk locus at 11p15.5. Neuro Oncol. 2018;20(11):1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dobbins SE, Broderick P, Melin B, et al. Common variation at 10p12.31 near MLLT10 influences meningioma risk. Nat Genet. 2011;43(9):825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walsh KM, Zhang C, Calvocoressi L, et al. Pleiotropic MLLT10 variation confers risk of meningioma and estrogen-mediated cancers. Neurooncol. Adv. 2022;4(1):vdac044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agnihotri S, Suppiah S, Tonge PD, et al. Therapeutic radiation for childhood cancer drives structural aberrations of NF2 in meningiomas. Nat Commun. 2017;8(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Mefty O, Topsakal C, Pravdenkova S, Sawyer JR, Harrison MJ.. Radiation-induced meningiomas: clinical, pathological, cytokinetic, and cytogenetic characteristics. J Neurosurg. 2004;100(6):1002–1013. [DOI] [PubMed] [Google Scholar]
- 12. Yamanaka R, Hayano A, Kanayama T.. Radiation-induced meningiomas: An exhaustive review of the literature. World Neurosurg. 2017;97:635–644.e8. [DOI] [PubMed] [Google Scholar]
- 13. Wang JZ, Agnihotri S, Zadeh G.. Radiation-induced meningiomas. Adv Exp Med Biol. 2023;1416:159–173. [DOI] [PubMed] [Google Scholar]
- 14. Shoshan Y, Chernova O, Jeun S-S, et al. Radiation-induced meningioma: A distinct molecular genetic pattern? J Neuropathol Exp Neurol. 2000;59(7):614–620. [DOI] [PubMed] [Google Scholar]
- 15. Umansky F, Shoshan Y, Rosenthal G, Fraifeld S, Spektor S.. Radiation-induced meningioma. Neurosurg Focus. 2008;24(5):E7. [DOI] [PubMed] [Google Scholar]
- 16. Schildkraut JM, Calvocoressi L, Wang F, et al. Endogenous and exogenous hormone exposure and the risk of meningioma in men. J Neurosurg. 2014;120(4):820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Claus EB, Calvocoressi L, Bondy ML, et al. Exogenous hormone use, reproductive factors, and risk of intracranial meningioma in females. J Neurosurg. 2013;118(3):649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Claus EB, Walsh KM, Calvocoressi L, et al. Cigarette smoking and risk of meningioma: The effect of gender. Cancer Epidemiol Biomarkers Prev. 2012;21(6):943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor AJ, Little MP, Winter DL, et al. Population-based risks of CNS tumors in survivors of childhood cancer: The British Childhood Cancer Survivor Study. J Clin Oncol. 2010;28(36):5287–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Withrow DR, Anderson H, Armstrong GT, et al. Pooled analysis of meningioma risk following treatment for childhood cancer. JAMA Oncol. 2022;8(12):1756–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blitshteyn S, Crook JE, Jaeckle KA.. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26(2):279–282. [DOI] [PubMed] [Google Scholar]
- 22. Schlehofer B, Blettner M, Wahrendorf J.. Association between brain tumors and menopausal status. J Natl Cancer Inst. 1992;84(17):1346–1349. [DOI] [PubMed] [Google Scholar]
- 23. Ryan P, Lee MW, North JB, McMichael AJ.. Risk factors for tumors of the brain and meninges: Results from the Adelaide Adult Brain Tumor Study. Int J Cancer. 1992;51(1):20–27. [DOI] [PubMed] [Google Scholar]
- 24. Jhawar BS, Fuchs CS, Colditz GA, Stampfer MJ.. Sex steroid hormone exposures and risk for meningioma. J Neurosurg. 2003;99(5):848–853. [DOI] [PubMed] [Google Scholar]
- 25. Wigertz A, Lönn S, Mathiesen T, et al. ; Swedish Interphone Study Group. Risk of brain tumors associated with exposure to exogenous female sex hormones. Am J Epidemiol. 2006;164(7):629–636. [DOI] [PubMed] [Google Scholar]
- 26. Hatch EE, Linet MS, Zhang J, et al. Reproductive and hormonal factors and risk of brain tumors in adult females. Int J Cancer. 2005;114(5):797–805. [DOI] [PubMed] [Google Scholar]
- 27. Custer B, Longstreth W, Phillips LE, Koepsell TD, Van Belle G.. Hormonal exposures and the risk of intracranial meningioma in women: A population-based case-control study. BMC Cancer. 2006;6(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee E, Grutsch J, Persky V, et al. Association of meningioma with reproductive factors. Int J Cancer. 2006;119(5):1152–1157. [DOI] [PubMed] [Google Scholar]
- 29. Weill A, Nguyen P, Labidi M, et al. Use of high dose cyproterone acetate and risk of intracranial meningioma in women: cohort study. BMJ. 2021;372:n37. [DOI] [PubMed] [Google Scholar]
- 30. Pettersson-Segerlind J, Mathiesen T, Elmi-Terander A, et al. The risk of developing a meningioma during and after pregnancy. Sci Rep. 2021;11(1):9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laviv Y, Ohla V, Kasper EM.. Unique features of pregnancy-related meningiomas: Lessons learned from 148 reported cases and theoretical implications of a prolactin modulated pathogenesis. Neurosurg Rev. 2018;41(1):95–108. [DOI] [PubMed] [Google Scholar]
- 32. Lusis EA, Scheithauer BW, Yachnis AT, et al. Meningiomas in pregnancy: A clinicopathologic study of 17 cases. Neurosurgery. 2012;71(5):951–961. [DOI] [PubMed] [Google Scholar]
- 33. Li W, You L, Cooper J, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140(4):477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xiao GH, Chernoff J, Testa JR.. NF2: The wizardry of merlin. Genes Chromosomes Cancer. 2003;38(4):389–399. [DOI] [PubMed] [Google Scholar]
- 35. Bachir S, Shah S, Shapiro S, et al. Neurofibromatosis Type 2 (NF2) and the implications for vestibular schwannoma and meningioma pathogenesis. Int J Mol Sci . 2021;22(2):690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baia GS, Caballero OL, Orr BA, et al. Yes-associated protein 1 is activated and functions as an oncogene in meningiomas. Mol Cancer Res. 2012;10(7):904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choudhury A, Magill ST, Eaton CD, et al. Meningioma DNA methylation groups identify biological drivers and therapeutic vulnerabilities. Nat Genet. 2022;54(5):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams EA, Santagata S, Wakimoto H, et al. Distinct genomic subclasses of high-grade/progressive meningiomas: NF2-associated, NF2-exclusive, and NF2-agnostic. Acta Neuropathol Commun. 2020;8(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clark VE, Erson-Omay EZ, Serin A, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339(6123):1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clark VE, Harmancı AS, Bai H, et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet. 2016;48(10):1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brastianos PK, Horowitz PM, Santagata S, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45(3):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abedalthagafi M, Bi WL, Aizer AA, et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro Oncol. 2016;18(5):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bujko M, Kober P, Tysarowski A, et al. EGFR, PIK3CA, KRAS and BRAF mutations in meningiomas. Oncol Lett. 2014;7(6):2019–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zadeh G, Karimi S, Aldape KD.. PIK3CA mutations in meningioma. Neuro Oncol. 2016;18(5):603–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Youngblood MW, Duran D, Montejo JD, et al. Correlations between genomic subgroup and clinical features in a cohort of more than 3000 meningiomas. J Neurosurg. 2019;133(5):1345–1354. [DOI] [PubMed] [Google Scholar]
- 46. Boetto J, Plu I, Ducos Y, et al. ; Brainbank Neuro-CEB Neuropathology Network. Normal meninges harbor oncogenic somatic mutations in meningioma-driver genes. Acta Neuropathol. 2023;146(6):833–835. [DOI] [PubMed] [Google Scholar]
- 47. Evans LT, Van Hoff J, Hickey WF, et al. SMARCE1 mutations in pediatric clear cell meningioma: Case report. J Neurosurg Pediatr. 2015;16(3):296–300. [DOI] [PubMed] [Google Scholar]
- 48. Gerkes E, Fock J, den Dunnen W, et al. A heritable form of SMARCE1-related meningiomas with important implications for follow-up and family screening. Neurogenetics. 2016;17(2):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tauziede-Espariat A, Parfait B, Besnard A, et al. Loss of SMARCE1 expression is a specific diagnostic marker of clear cell meningioma: A comprehensive immunophenotypical and molecular analysis. Brain Pathol. 2018;28(4):466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. St. Pierre R, Collings CK, Samé Guerra DD, et al. SMARCE1 deficiency generates a targetable mSWI/SNF dependency in clear cell meningioma. Nat Genet. 2022;54(6):861–873. [DOI] [PubMed] [Google Scholar]
- 51. Sievers P, Sill M, Blume C, et al. ; German Consortium “Aggressive Meningiomas”. Clear cell meningiomas are defined by a highly distinct DNA methylation profile and mutations in SMARCE1. Acta Neuropathol. 2021;141(2):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Landry AP, Wang JZ, Nassiri F, et al. BAP1-deficient meningioma presenting with trabecular architecture and cytokeratin expression: A report of two cases and review of the literature. J Clin Pathol. 2021;76(5):315–319. [DOI] [PubMed] [Google Scholar]
- 53. Shankar GM, Santagata S.. BAP1 mutations in high-grade meningioma: Implications for patient care. Neuro-Oncology. 2017;19(11):1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Williams EA, Wakimoto H, Shankar GM, et al. Frequent inactivating mutations of the PBAF complex gene PBRM1 in meningioma with papillary features. Acta Neuropathol. 2020;140(1):89–93. [DOI] [PubMed] [Google Scholar]
- 55. Mirian C, Duun-Henriksen AK, Juratli T, et al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: An individual patient data meta-analysis. J Neurol Neurosurg Psychiatry. 2020;91(4):378–387. [DOI] [PubMed] [Google Scholar]
- 56. Sahm F, Schrimpf D, Olar A, et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016;108(5):djv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chiba K, Lorbeer FK, Shain AH, et al. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science. 2017;357(6358):1416–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spiegl-Kreinecker S, Lötsch D, Neumayer K, et al. TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma. Neuro-Oncology. 2018;20(12):1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Abedalthagafi MS, Bi WL, Merrill PH, et al. ARID1A and TERT promoter mutations in dedifferentiated meningioma. Cancer Gen. 2015;208(6):345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chaluts D, Dullea JT, Ali M, et al. ARID1A mutation associated with recurrence and shorter progression-free survival in atypical meningiomas. J Cancer Res Clin Oncol. 2023;149(8):5165–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gill CM, Loewenstern J, Rutland JW, et al. SWI/SNF chromatin remodeling complex alterations in meningioma. J Cancer Res Clin Oncol. 2021;147(11):3431–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kerr K, Qualmann K, Esquenazi Y, Hagan J, Kim DH.. Familial syndromes involving meningiomas provide mechanistic insight into sporadic disease. Neurosurgery. 2018;83(6):1107–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pain M, Darbinyan A, Fowkes M, Shrivastava R.. Multiple meningiomas in a patient with cowden syndrome. J Neurol Surg Rep. 2016;77(3):e128–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nassiri F, Liu J, Patil V, et al. A clinically applicable integrative molecular classification of meningiomas. Nature. 2021;597(7874):119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Evans DG. Neurofibromatosis type 2 (NF2): A clinical and molecular review. Orphanet J Rare Dis. 2009;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Plotkin SR, Messiaen L, Legius E, et al. ; International Consensus Group on Neurofibromatosis Diagnostic Criteria (I-NF-DC). Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: An international consensus recommendation. Genet Med. 2022;24(9):1967–1977. [DOI] [PubMed] [Google Scholar]
- 67. Merker VL, Esparza S, Smith MJ, Stemmer-Rachamimov A, Plotkin SR.. Clinical features of schwannomatosis: A retrospective analysis of 87 patients. Oncologist. 2012;17(10):1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Smith MJ, Wallace AJ, Bennett C, et al. Germline SMARCE1 mutations predispose to both spinal and cranial clear cell meningiomas. J Pathol. 2014;234(4):436–440. [DOI] [PubMed] [Google Scholar]
- 69. Smith MJ, O’Sullivan J, Bhaskar SS, et al. Loss-of-function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas. Nat Genet. 2013;45(3):295–298. [DOI] [PubMed] [Google Scholar]
- 70. Prasad RN, Gardner UG, Yaney A, et al. Germline BAP1 mutation in a family with multi-generational meningioma with rhabdoid features: A case series and literature review. Front Oncol. 2021;11:721712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shankar GM, Abedalthagafi M, Vaubel RA, et al. Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. Neuro Oncol. 2017;19(4):535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Biczok A, Thorsteinsdottir J, Karschnia P, et al. Mutational signature of extracranial meningioma metastases and their respective primary tumors. Acta Neuropathol Commun. 2023;11(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Carbone M, Ferris LK, Baumann F, et al. BAP1 cancer syndrome: Malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med. 2012;10:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aavikko M, Li S-P, Saarinen S, et al. Loss of SUFU function in familial multiple meningioma. Am J Hum Genet. 2012;91(3):520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pathmanaban ON, Sadler KV, Kamaly-Asl ID, et al. Association of genetic predisposition with solitary schwannoma or meningioma in children and young adults. JAMA Neurol. 2017;74(9):1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Smith MJ, Beetz C, Williams SG, et al. Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol. 2014;32(36):4155–4161. [DOI] [PubMed] [Google Scholar]
- 77. Oshima J, Martin GM, Hisama FM.. Werner Syndrome. In: Adam MP, Feldman J, Mirzaa GM, et al. , ed. GeneReviews(®). Seattle, WA: University of Washington, SeattleĹCopyright © 1993-2024, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 1993. [Google Scholar]
- 78. Fujii K, Miyashita T.. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56(5):667–674. [DOI] [PubMed] [Google Scholar]
- 79. Farooq A, Walker LJ, Bowling J, Audisio RA.. Cowden syndrome. Cancer Treat Rev. 2010;36(8):577–583. [DOI] [PubMed] [Google Scholar]
- 80. Lindblom A, Ruttledge M, Collins VP, Nordenskjöld M, Dumanski JP.. Chromosomal deletions in anaplastic meningiomas suggest multiple regions outside chromosome 22 as important in tumor progression. Int J Cancer. 1994;56(3):354–357. [DOI] [PubMed] [Google Scholar]
- 81. Sulman EP, Dumanski JP, White PS, et al. Identification of a consistent region of allelic loss on 1p32 in meningiomas: Correlation with increased morbidity. Cancer Res. 1998;58(15):3226–3230. [PubMed] [Google Scholar]
- 82. Cai DX, Banerjee R, Scheithauer BW, et al. Chromosome 1p and 14q FISH analysis in clinicopathologic subsets of meningioma: Diagnostic and prognostic implications. J Neuropathol Exp Neurol. 2001;60(6):628–636. [DOI] [PubMed] [Google Scholar]
- 83. Espinosa AB, Tabernero MD, Maíllo A, et al. The cytogenetic relationship between primary and recurrent meningiomas points to the need for new treatment strategies in cases at high risk of relapse. Clin Cancer Res. 2006;12(3 Pt 1):772–780. [DOI] [PubMed] [Google Scholar]
- 84. Maillo A, Orfao A, Sayagues JM, et al. New classification scheme for the prognostic stratification of meningioma on the basis of chromosome 14 abnormalities, patient age, and tumor histopathology. J Clin Oncol. 2003;21(17):3285–3295. [DOI] [PubMed] [Google Scholar]
- 85. Ketter R, Rahnenführer J, Henn W, et al. Correspondence of tumor localization with tumor recurrence and cytogenetic progression in meningiomas. Neurosurgery. 2008;62(1):61–9; discussion 69. [DOI] [PubMed] [Google Scholar]
- 86. Ketter R, Urbschat S, Henn W, et al. Application of oncogenetic trees mixtures as a biostatistical model of the clonal cytogenetic evolution of meningiomas. Int J Cancer. 2007;121(7):1473–1480. [DOI] [PubMed] [Google Scholar]
- 87. Sulman EP, White PS, Brodeur GM.. Genomic annotation of the meningioma tumor suppressor locus on chromosome 1p34. Oncogene. 2004;23(4):1014–1020. [DOI] [PubMed] [Google Scholar]
- 88. Choy W, Kim W, Nagasawa D, et al. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011;30(5):E6. [DOI] [PubMed] [Google Scholar]
- 89. Bi WL, Greenwald NF, Abedalthagafi M, et al. Genomic landscape of high-grade meningiomas. npj Genomic Med. 2017;2(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lee Y, Liu J, Patel S, et al. Genomic landscape of meningiomas. Brain Pathol. 2010;20(4):751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bi WL, Abedalthagafi M, Horowitz P, et al. Genomic landscape of intracranial meningiomas. J Neurosurg. 2016;125(3):525–535. [DOI] [PubMed] [Google Scholar]
- 92. Driver J, Hoffman SE, Tavakol S, et al. A molecularly integrated grade for meningioma. Neuro Oncol. 2021;24(5):796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Maas SLN, Stichel D, Hielscher T, et al. ; German Consortium on Aggressive Meningiomas (KAM). Integrated molecular-morphologic meningioma classification: A multicenter retrospective analysis, retrospectively and prospectively validated. J Clin Oncol. 2021;39(34):3839–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Urbschat S, Rahnenführer J, Henn W, et al. Clonal cytogenetic progression within intratumorally heterogeneous meningiomas predicts tumor recurrence. Int J Oncol. 2011;39(6):1601–1608. [DOI] [PubMed] [Google Scholar]
- 95. Youngblood MW, Erson-Omay Z, Li C, et al. Super-enhancer hijacking drives ectopic expression of hedgehog pathway ligands in meningiomas. Nat Commun. 2023;14(1):6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Boström J, Meyer-Puttlitz B, Wolter M, et al. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol. 2001;159(2):661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhao R, Choi BY, Lee MH, Bode AM, Dong Z.. Implications of genetic and epigenetic alterations of CDKN2A (p16(INK4a)) in Cancer. EBioMedicine. 2016;8:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang JZ, Patil V, Liu J, et al. ; International Consortium on Meningiomas (ICOM). Increased mRNA expression of CDKN2A is a transcriptomic marker of clinically aggressive meningiomas. Acta Neuropathol. 2023;146(1):145–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Khan AB, English CW, Chen WC, et al. Even heterozygous loss of CDKN2A/B greatly accelerates recurrence in aggressive meningioma. Acta Neuropathol. 2023;145(4):501–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Perry A, Banerjee R, Lohse CM, Kleinschmidt-DeMasters BK, Scheithauer BW.. A role for chromosome 9p21 deletions in the malignant progression of meningiomas and the prognosis of anaplastic meningiomas. Brain Pathol. 2002;12(2):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sievers P, Hielscher T, Schrimpf D, et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020;140(3):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Torp SH, Solheim O, Skjulsvik AJ.. The WHO 2021 Classification of Central Nervous System tumours: A practical update on what neurosurgeons need to know-a minireview. Acta Neurochir (Wien). 2022;164(9):2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Brokinkel B, Hess K, Mawrin C.. Brain invasion in meningiomas-clinical considerations and impact of neuropathological evaluation: A systematic review. Neuro Oncol. 2017;19(10):1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rebchuk AD, Chaharyn BM, Alam A, et al. The impact of brain invasion criteria on the incidence and distribution of WHO grade 1, 2, and 3 meningiomas. Neuro-Oncology. 2022;24(9):1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li HY, Ying YZ, Zheng D, et al. Is brain invasion sufficient as a stand-alone criterion for grading atypical meningioma? J Neurosurg. 2023;139(4):953–964. [DOI] [PubMed] [Google Scholar]
- 106. von Spreckelsen N, Kesseler C, Brokinkel B, et al. Molecular neuropathology of brain-invasive meningiomas. Brain Pathol. 2022;32(2):e13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Behling F, Fodi C, Gepfner-Tuma I, et al. CNS invasion in meningioma-how the intraoperative assessment can improve the prognostic evaluation of tumor recurrence. Cancers (Basel). 2020;12(12):3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Behling F, Hempel JM, Schittenhelm J.. Brain invasion in meningioma-a prognostic potential worth exploring. Cancers (Basel). 2021;13(13):3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sahm F, Schrimpf D, Stichel D, et al. DNA methylation-based classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. [DOI] [PubMed] [Google Scholar]
- 110. Olar A, Wani KM, Wilson CD, et al. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol. 2017;133(3):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nassiri F, Mamatjan Y, Suppiah S, et al. ; International Consortium on Meningiomas. DNA methylation profiling to predict recurrence risk in meningioma: Development and validation of a nomogram to optimize clinical management. Neuro Oncol. 2019;21(7):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vasudevan HN, Braunstein SE, Phillips JJ, et al. Comprehensive molecular profiling identifies FOXM1 as a key transcription factor for meningioma proliferation. Cell Rep. 2018;22(13):3672–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kim H, Park KJ, Ryu BK, et al. Forkhead box M1 (FOXM1) transcription factor is a key oncogenic driver of aggressive human meningioma progression. Neuropathol Appl Neurobiol. 2020;46(2):125–141. [DOI] [PubMed] [Google Scholar]
- 114. Patel AJ, Wan YW, Al-Ouran R, et al. Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors. Proc Natl Acad Sci U S A. 2019;116(43):21715–21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Parada CA, Osbun J, Kaur S, et al. Kinome and phosphoproteome of high-grade meningiomas reveal AKAP12 as a central regulator of aggressiveness and its possible role in progression. Sci Rep. 2018;8(1):2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tanaka K, Sato C, Maeda Y, et al. Establishment of a human malignant meningioma cell line with amplified c-myc oncogene. Cancer. 1989;64(11):2243–2249. [DOI] [PubMed] [Google Scholar]
- 117. Choudhury A, Chen WC, Lucas CG, et al. Hypermitotic meningiomas harbor DNA methylation subgroups with distinct biological and clinical features. Neuro Oncol. 2022;25(3):520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bayley JC, Hadley CC, Harmanci AO, et al. Multiple approaches converge on three biological subtypes of meningioma and extract new insights from published studies. Sci Adv. 2022;8(5):eabm6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hielscher T, Sill M, Sievers P, et al. Clinical implementation of integrated molecular-morphologic risk prediction for meningioma. Brain Pathol. 2022;33(3):e13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Maier AD, Meddis A, Mirian C, et al. Gene expression analysis during progression of malignant meningioma compared to benign meningioma. J Neurosurg. 2023;138(5):1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. San-Miguel T, Megías J, Monleón D, et al. Matched paired primary and recurrent meningiomas points to cell-death program contributions to genomic and epigenomic instability along tumor progression. Cancers (Basel). 2022;14(16):4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nassiri F, Chakravarthy A, Feng S, et al. Detection and discrimination of intracranial tumors using plasma cell-free DNA methylomes. Nat Med. 2020;26(7):1044–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zuccato JA, Patil V, Mansouri S, et al. DNA methylation-based prognostic subtypes of chordoma tumors in tissue and plasma. Neuro Oncol. 2022;24(3):442–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ricklefs FL, Maire CL, Wollmann K, et al. Diagnostic potential of extracellular vesicles in meningioma patients. Neuro Oncol. 2022;24(12):2078–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Herrgott GA, Snyder JM, She R, et al. Detection of diagnostic and prognostic methylation-based signatures in liquid biopsy specimens from patients with meningiomas. Nat Commun. 2023;14(1):5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chamoun R, Krisht KM, Couldwell WT.. Incidental meningiomas. Neurosurg Focus. 2011;31(6):E19. [DOI] [PubMed] [Google Scholar]
- 127. Goldbrunner R, Stavrinou P, Jenkinson MD, et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021;23(11):1821–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Islim AI, Millward CP, Mills SJ, et al. The management of incidental meningioma: An unresolved clinical conundrum. Neurooncol. Adv.. 2023;5(suppl 1):i26–i34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zeng L, Liang P, Jiao J, Chen J, Lei T.. Will an asymptomatic meningioma grow or not grow? A meta-analysis. J Neurol Surg A Cent Eur Neurosurg. 2015;76(5):341–347. [DOI] [PubMed] [Google Scholar]
- 130. Lyndon D, Lansley JA, Evanson J, Krishnan AS.. Dural masses: meningiomas and their mimics. Insights Imaging. 2019;10(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Regelsberger J, Hagel C, Emami P, et al. Secretory meningiomas: A benign subgroup causing life-threatening complications. Neuro Oncol. 2009;11(6):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Reuss DE, Piro RM, Jones DT, et al. Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathol. 2013;125(3):351–358. [DOI] [PubMed] [Google Scholar]
- 133. O’Leary S, Adams WM, Parrish RW, Mukonoweshuro W.. Atypical imaging appearances of intracranial meningiomas. Clin Radiol. 2007;62(1):10–17. [DOI] [PubMed] [Google Scholar]
- 134. Bourekas EC, Wildenhain P, Lewin JS, et al. The dural tail sign revisited. AJNR Am J Neuroradiol. 1995;16(7):1514–1516. [PMC free article] [PubMed] [Google Scholar]
- 135. Slot KM, Verbaan D, Uitdehaag BM, et al. Can excision of meningiomas be limited to resection of tumor and radiologically abnormal dura mater? Neuronavigation-guided biopsies of dural tail and seemingly normal dura mater, with a review of the literature. World Neurosurg. 2014;82(6):e832–e836. [DOI] [PubMed] [Google Scholar]
- 136. Sotoudeh H, Yazdi HR.. A review on dural tail sign. World J Radiol. 2010;2(5):188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wallace EW. The dural tail sign. Radiology. 2004;233(1):56–57. [DOI] [PubMed] [Google Scholar]
- 138. Wen M, Jung S, Moon KS, et al. Immunohistochemical profile of the dural tail in intracranial meningiomas. Acta Neurochir (Wien). 2014;156(12):2263–2273. [DOI] [PubMed] [Google Scholar]
- 139. Wirsching HG, Richter JK, Sahm F, et al. Post-operative cardiovascular complications and time to recurrence in meningioma patients treated with versus without pre-operative embolization: A retrospective cohort study of 741 patients. J Neurooncol. 2018;140(3):659–667. [DOI] [PubMed] [Google Scholar]
- 140. Iacobucci M, Danieli L, Visconti E, et al. Preoperative embolization of meningiomas with polyvinyl alcohol particles: The benefits are not outweighed by risks. Diagn Interv Imaging. 2017;98(4):307–314. [DOI] [PubMed] [Google Scholar]
- 141. Huang RY, Bi WL, Weller M, et al. Proposed response assessment and endpoints for meningioma clinical trials: Report from the Response Assessment in Neuro-Oncology Working Group. Neuro Oncol. 2019;21(1):26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Watanabe S, Nonaka T, Maeda M, et al. Efficacy endpoints in phase II clinical trials for meningioma: An analysis of recent clinical trials. Ther Innov Regul Sci. 2023;57(3):603–610. [DOI] [PubMed] [Google Scholar]
- 143. Huang RY, Unadkat P, Bi WL, et al. Response assessment of meningioma: 1D, 2D, and volumetric criteria for treatment response and tumor progression. Neuro Oncol. 2019;21(2):234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kaley T, Barani I, Chamberlain M, et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: A RANO review. Neuro Oncol. 2014;16(6):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Macdonald DR, Cascino TL, ScholdSC, Jr, Cairncross JG.. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 146. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 147. Hall JT, Opalak CF, Carr MT, Harris TJ, Broaddus WC.. The effect of radiation on meningioma volume change. World Neurosurg. 2021;153:e141–e146. [DOI] [PubMed] [Google Scholar]
- 148. Fega KR, Fletcher GP, Waddle MR, et al. Analysis of MRI volumetric changes after hypofractionated stereotactic radiation therapy for benign intracranial neoplasms. Adv Radiat Oncol. 2019;4(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Harrison G, Kano H, Lunsford LD, Flickinger JC, Kondziolka D.. Quantitative tumor volumetric responses after Gamma Knife radiosurgery for meningiomas. J Neurosurg. 2016;124(1):146–154. [DOI] [PubMed] [Google Scholar]
- 150. Tabouret E, Furtner J, Graillon T, et al. 3D volume growth rate evaluation in the EORTC-BTG-1320 clinical trial for recurrent WHO grade 2 and 3 meningiomas. J Clin Oncol. 2023;41(16_suppl):2075–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Nassiri F, Wang JZ, Au K, et al. Consensus core clinical data elements for meningiomas (v2021.1). Neuro Oncol. 2022;24(5):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Prasad RN, Perlow HK, Bovi J, et al. (68)Ga-DOTATATE PET: The future of meningioma treatment. Int J Radiat Oncol Biol Phys. 2022;113(4):868–871. [DOI] [PubMed] [Google Scholar]
- 153. Galldiks N, Albert NL, Sommerauer M, et al. PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro Oncol. 2017;19(12):1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kong MJ, Yang AF, Vora SA, Ross JS, Yang M.. The complementary role of (68)Ga-DOTATATE PET/CT in diagnosis of recurrent meningioma. J Nucl Med Technol. 2022:jnmt.122.263949. [DOI] [PubMed] [Google Scholar]
- 155. Afshar-Oromieh A, Giesel FL, Linhart HG, et al. Detection of cranial meningiomas: Comparison of ⁶⁸Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur J Nucl Med Mol Imaging. 2012;39(9):1409–1415. [DOI] [PubMed] [Google Scholar]
- 156. Rachinger W, Stoecklein VM, Terpolilli NA, et al. Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med. 2015;56(3):347–353. [DOI] [PubMed] [Google Scholar]
- 157. Perlow HK, Siedow M, Gokun Y, et al. (68)Ga-DOTATATE PET-based radiation contouring creates more precise radiation volumes for patients with meningioma. Int J Radiat Oncol Biol Phys. 2022;113(4):859–865. [DOI] [PubMed] [Google Scholar]
- 158. Kessel KA, Weber W, Yakushev I, et al. Integration of PET-imaging into radiotherapy treatment planning for low-grade meningiomas improves outcome. Eur J Nucl Med Mol Imaging. 2020;47(6):1391–1399. [DOI] [PubMed] [Google Scholar]
- 159. Mahase SS, Roth O’Brien DA, No D, et al. [68Ga]-DOTATATE PET/MRI as an adjunct imaging modality for radiation treatment planning of meningiomas. Neurooncol. Adv.. 2021;3(1):vdab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kowalski ES, Khairnar R, Gryaznov AA, et al. (68)Ga-DOTATATE PET-CT as a tool for radiation planning and evaluating treatment responses in the clinical management of meningiomas. Radiat Oncol. 2021;16(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ueberschaer M, Vettermann FJ, Forbrig R, et al. Simpson grade revisited–intraoperative estimation of the extent of resection in meningiomas versus postoperative somatostatin receptor positron emission tomography/computed tomography and magnetic resonance imaging. Neurosurgery. 2021;88(1):140–146. [DOI] [PubMed] [Google Scholar]
- 162. Teske N, Biczok A, Quach S, et al. Postoperative [(68)Ga]Ga-DOTA-TATE PET/CT imaging is prognostic for progression-free survival in meningioma WHO grade 1. Eur J Nucl Med Mol Imaging. 2023;51(1):206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Unterrainer M, Kunte SC, Unterrainer LM, et al. Next-generation PET/CT imaging in meningioma-first clinical experiences using the novel SSTR-targeting peptide [(18)F]SiTATE. Eur J Nucl Med Mol Imaging. 2023;50(11):3390–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wängler C, Beyer L, Bartenstein P, et al. Favorable SSTR subtype selectivity of SiTATE: New momentum for clinical [(18)F]SiTATE PET. EJNMMI Radiopharm Chem. 2022;7(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Rodriguez J, Martinez G, Mahase S, et al. Cost-effectiveness analysis of (68)Ga-DOTATATE PET/MRI in radiotherapy planning in patients with intermediate-risk meningioma. AJNR Am J Neuroradiol. 2023;44(7):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Islim AI, Kolamunnage-Dona R, Mohan M, et al. A prognostic model to personalize monitoring regimes for patients with incidental asymptomatic meningiomas. Neuro Oncol. 2020;22(2):278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Islim AI, Mohan M, Moon RDC, et al. Incidental intracranial meningiomas: A systematic review and meta-analysis of prognostic factors and outcomes. J Neurooncol. 2019;142(2):211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Bui KT, Liang R, Kiely BE, et al. Scanxiety: A scoping review about scan-associated anxiety. BMJ Open. 2021;11(5):e043215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Niiro M, Yatsushiro K, Nakamura K, Kawahara Y, Kuratsu J.. Natural history of elderly patients with asymptomatic meningiomas. J Neurol Neurosurg Psychiatry. 2000;68(1):25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Go RS, Taylor BV, Kimmel DW.. The natural history of asymptomatic meningiomas in Olmsted County, Minnesota. Neurology. 1998;51(6):1718–1720. [DOI] [PubMed] [Google Scholar]
- 171. Nakamura M, Roser F, Michel J, Jacobs C, Samii M.. The natural history of incidental meningiomas. Neurosurgery. 2003;53(1):62–70; discussion 70. [DOI] [PubMed] [Google Scholar]
- 172. Nakasu S, Nakasu Y, Fukami T, Jito J, Nozaki K.. Growth curve analysis of asymptomatic and symptomatic meningiomas. J Neurooncol. 2011;102(2):303–310. [DOI] [PubMed] [Google Scholar]
- 173. Kim KH, Kang SJ, Choi J-W, et al. Clinical and radiological outcomes of proactive Gamma Knife surgery for asymptomatic meningiomas compared with the natural course without intervention. J Neurosurg. 2018;130(5):1740–1749. [DOI] [PubMed] [Google Scholar]
- 174. Romani R, Ryan G, Benner C, Pollock J.. Non-operative meningiomas: Long-term follow-up of 136 patients. Acta Neurochir (Wien). 2018;160(8):1547–1553. [DOI] [PubMed] [Google Scholar]
- 175. Suppiah S, Nassiri F, Bi WL, et al. ; International Consortium on Meningiomas. Molecular and translational advances in meningiomas. Neuro-Oncology. 2019;21(suppl_1):i4–i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lee EJ, Kim JH, Park ES, et al. A novel weighted scoring system for estimating the risk of rapid growth in untreated intracranial meningiomas. J Neurosurg. 2017;127(5):971–980. [DOI] [PubMed] [Google Scholar]
- 177. Islim AI, Mohan M, Moon RDC, et al. Treatment outcomes of incidental intracranial meningiomas: Results from the IMPACT Cohort. World Neurosurg. 2020;138:e725–e735. [DOI] [PubMed] [Google Scholar]
- 178. Islim AI, Millward CP, Piper RJ, et al. ; IMPACT Study Investigators, International Consortium on Meningioma (ICOM) and British Neurosurgical Trainee Research Collaborative (BNTRC). External validation and recalibration of an incidental meningioma prognostic model - IMPACT: Protocol for an international multicentre retrospective cohort study. BMJ Open. 2022;12(1):e052705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Reinert M, Babey M, Curschmann J, et al. Morbidity in 201 patients with small sized meningioma treated by microsurgery. Acta Neurochir (Wien). 2006;148(12):1257–65; discussion 1266. [DOI] [PubMed] [Google Scholar]
- 180. Näslund O, Skoglund T, Farahmand D, Bontell TO, Jakola AS.. Indications and outcome in surgically treated asymptomatic meningiomas: A single-center case-control study. Acta Neurochir (Wien). 2020;162(9):2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Sheehan J, Pikis S, Islim AI, et al. An international multicenter matched cohort analysis of incidental meningioma progression during active surveillance or after stereotactic radiosurgery: The IMPASSE study. Neuro Oncol. 2022;24(1):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Bashir A, Vestergaard MB, Marner L, et al. PET imaging of meningioma with 18F-FLT: A predictor of tumour progression. Brain. 2020;143(11):3308–3317. [DOI] [PubMed] [Google Scholar]
- 183. Kalasauskas D, Keric N, Abu Ajaj S, et al. Psychological burden in meningioma patients under a wait-and-watch strategy and after complete resection Is high—results of a prospective single center study. Cancers. 2020;12(12):3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Maurer R, Daggubati L, Ba DM, et al. Mental health disorders in patients with untreated meningiomas: An observational cohort study using the nationwide MarketScan database. Neurooncol. Pract.. 2020;7(5):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Gousias K, Schramm J, Simon M.. The Simpson grading revisited: Aggressive surgery and its place in modern meningioma management. J Neurosurg. 2016;125(3):551–560. [DOI] [PubMed] [Google Scholar]
- 187. Nanda A, Bir SC, Maiti TK, et al. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J Neurosurg. 2017;126(1):201–211. [DOI] [PubMed] [Google Scholar]
- 188. Behling F, Fodi C, Hoffmann E, et al. The role of Simpson grading in meningiomas after integration of the updated WHO classification and adjuvant radiotherapy. Neurosurg Rev. 2021;44(4):2329–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Gallagher MJ, Jenkinson MD, Brodbelt AR, Mills SJ, Chavredakis E.. WHO grade 1 meningioma recurrence: Are location and Simpson grade still relevant? Clin Neurol Neurosurg. 2016;141:117–121. [DOI] [PubMed] [Google Scholar]
- 190. Schwartz TH, McDermott MW.. The Simpson grade: Abandon the scale but preserve the message. J Neurosurg. 2020;135(2):488–495.8. [DOI] [PubMed] [Google Scholar]
- 191. Sughrue ME, Kane AJ, Shangari G, et al. The relevance of Simpson Grade I and II resection in modern neurosurgical treatment of World Health Organization Grade I meningiomas. J Neurosurg. 2010;113(5):1029–1035. [DOI] [PubMed] [Google Scholar]
- 192. Mansouri A, Klironomos G, Taslimi S, et al. Surgically resected skull base meningiomas demonstrate a divergent postoperative recurrence pattern compared with non-skull base meningiomas. J Neurosurg. 2016;125(2):431–440. [DOI] [PubMed] [Google Scholar]
- 193. Zador Z, Landry AP, Balas M, Cusimano MD.. Landscape of immune cell gene expression is unique in predominantly WHO grade 1 skull base meningiomas when compared to convexity. Sci Rep. 2020;10(1):9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. McGovern SL, Aldape KD, Munsell MF, et al. A comparison of World Health Organization tumor grades at recurrence in patients with non-skull base and skull base meningiomas. J Neurosurg. 2010;112(5):925–933. [DOI] [PubMed] [Google Scholar]
- 195. Kinjo T, al-Mefty O, Kanaan I.. Grade zero removal of supratentorial convexity meningiomas. Neurosurgery. 1993;33(3):394–9; discussion 399. [DOI] [PubMed] [Google Scholar]
- 196. Chotai S, Schwartz TH.. The Simpson grading: Is it still valid? Cancers (Basel). 2022;14(8):2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Qi ST, Liu Y, Pan J, Chotai S, Fang LX.. A radiopathological classification of dural tail sign of meningiomas. J Neurosurg. 2012;117(4):645–653. [DOI] [PubMed] [Google Scholar]
- 198. Koljenović S, Schut TB, Vincent A, Kros JM, Puppels GJ.. Detection of meningioma in dura mater by Raman spectroscopy. Anal Chem. 2005;77(24):7958–7965. [DOI] [PubMed] [Google Scholar]
- 199. Qian K, Nie C, Zhu W, et al. Surgical management of tuberculum sellae meningioma: Transcranial approach or endoscopic endonasal approach? Front Surg. 2022;9:979940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH.. Endoscopic endonasal versus open transcranial resection of anterior midline skull base meningiomas. World Neurosurg. 2012;77(5–6):713–724. [DOI] [PubMed] [Google Scholar]
- 201. Magill ST, Schwartz TH, Couldwell WT, et al. International Tuberculum Sellae Meningioma Study: Surgical outcomes and management trends. Neurosurgery. 2023;96(3):1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Rogers L, Zhang P, Vogelbaum MA, et al. Intermediate-risk meningioma: Initial outcomes from NRG Oncology RTOG 0539. J Neurosurg. 2018;129(1):35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Rogers CL, Won M, Vogelbaum MA, et al. High-risk meningioma: Initial outcomes from NRG Oncology/RTOG 0539. Int J Radiat Oncol Biol Phys. 2020;106(4):790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Weber DC, Ares C, Villa S, et al. Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: A phase-II parallel non-randomized and observation study (EORTC 22042-26042). Radiother Oncol. 2018;128(2):260–265. [DOI] [PubMed] [Google Scholar]
- 205. Deng MY, Maas SLN, Hinz F, et al. Efficacy and toxicity of bimodal radiotherapy in WHO grade 2 meningiomas following subtotal resection with carbon ion boost: Prospective phase 2 MARCIE trial. Neuro Oncol. 2023;26(4):701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zeng KL, Soliman H, Myrehaug S, et al. Dose-escalated radiation therapy is associated with improved outcomes for high-grade meningioma. Int J Radiat Oncol Biol Phys. 2023;118(3):662–671. [DOI] [PubMed] [Google Scholar]
- 207. Chen WC, Perlow HK, Choudhury A, et al. Radiotherapy for meningiomas. J Neurooncol. 2022;160(2):505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Chen WC, Lucas C-HG, Magill ST, Rogers CL, Raleigh DR.. Radiotherapy and radiosurgery for meningiomas. Neurooncol. Adv.. 2023;5(suppl_1):i67–i83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Jenkinson MD, Javadpour M, Haylock BJ, et al. The ROAM/EORTC-1308 trial: Radiation versus Observation following surgical resection of Atypical Meningioma: Study protocol for a randomised controlled trial. Trials. 2015;16:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Jenkinson MD, Weber DC, Haylock BJ, et al. Radiotherapy versus observation following surgical resection of atypical meningioma (the ROAM trial). Neuro Oncol. 2014;16(11):1560–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Fleming CW, Parsai S, Suh JH.. A management dilemma: Adjuvant radiotherapy after gross total resection of atypical meningioma. Transl Cancer Res. 2019;8(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Okoye C, Rogers L.. Atypical and Malignant Meningioma. Intracranial Spinal Radiother. 2021:13–17. [Google Scholar]
- 213. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 214. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–25; discussion 226. [DOI] [PubMed] [Google Scholar]
- 216. Perry A. The definition and role of brain invasion in meningioma grading: Still controversial after all these years. Free Neuropathol. 2021;2:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Ammendola S, Bariani E, Eccher A, et al. The histopathological diagnosis of atypical meningioma: Glass slide versus whole slide imaging for grading assessment. Virchows Arch. 2021;478(4):747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Maas SLN, Sievers P, Weber DC, et al. Independent prognostic impact of DNA methylation class and chromosome 1p loss in WHO grade 2 and 3 meningioma undergoing adjuvant high-dose radiotherapy: Comprehensive molecular analysis of EORTC 22042-26042. Acta Neuropathol. 2023;146(6):837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Chen WC, Choudhury A, Youngblood MW, et al. Targeted gene expression profiling predicts meningioma outcomes and radiotherapy responses. Nat Med. 2023;29(12):3067–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Raleigh DR, Preusser M.. A 34-gene expression biomarker predicts meningioma outcomes and radiotherapy responses. Neuro Oncol. 2023;26(2):207–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Di Franco R, Borzillo V, Ravo V, et al. Radiosurgery and stereotactic radiotherapy with cyberknife system for meningioma treatment. Neuroradiol J. 2018;31(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. McGregor JM, Sarkar A.. Stereotactic radiosurgery and stereotactic radiotherapy in the treatment of skull base meningiomas. Otolaryngol Clin North Am. 2009;42(4):677–688. [DOI] [PubMed] [Google Scholar]
- 223. Wang JZ, Landry AP, Nassiri F, et al. Outcomes and predictors of response to fractionated radiotherapy as primary treatment for intracranial meningiomas. Clin Transl Radiat Oncol. 2023;41:100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Kaprealian T, Raleigh DR, Sneed PK, et al. Parameters influencing local control of meningiomas treated with radiosurgery. J Neurooncol. 2016;128(2):357–364. [DOI] [PubMed] [Google Scholar]
- 225. Onodera S, Aoyama H, Katoh N, et al. Long-term outcomes of fractionated stereotactic radiotherapy for intracranial skull base benign meningiomas in single institution. Jpn J Clin Oncol. 2011;41(4):462–468. [DOI] [PubMed] [Google Scholar]
- 226. Biau J, Khalil T, Verrelle P, Lemaire JJ.. Fractionated radiotherapy and radiosurgery of intracranial meningiomas. Neurochirurgie. 2018;64(1):29–36. [DOI] [PubMed] [Google Scholar]
- 227. Kheir V, Faouzi M, Borruat FX.. Visual outcomes of fractionated radiotherapy in optic nerve sheath meningioma: A Retrospective Study. Klin Monbl Augenheilkd. 2019;236(4):526–529. [DOI] [PubMed] [Google Scholar]
- 228. Lesser RL, Knisely JP, Wang SL, James BY, Kupersmith MJ.. Long-term response to fractionated radiotherapy of presumed optic nerve sheath meningioma. Br J Ophthalmol. 2010;94(5):559–563. [DOI] [PubMed] [Google Scholar]
- 229. Marchetti M, Sahgal A, De Salles AAF, et al. Stereotactic radiosurgery for intracranial noncavernous sinus benign meningioma: International stereotactic radiosurgery society systematic review, meta-analysis and practice guideline. Neurosurgery. 2020;87(5):879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. DiBiase SJ, Kwok Y, Yovino S, et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004;60(5):1515–1519. [DOI] [PubMed] [Google Scholar]
- 231. Park HR, Lee JM, Park KW, et al. Fractionated gamma knife radiosurgery as initial treatment for large skull base meningioma. Exp Neurobiol. 2018;27(3):245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Sheehan JP, Lee CC, Xu Z, et al. Edema following gamma knife radiosurgery for parasagittal and parafalcine meningiomas. J Neurosurg. 2015;123(5):1287–1293. [DOI] [PubMed] [Google Scholar]
- 233. Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62(1):53–8; discussion 58. [DOI] [PubMed] [Google Scholar]
- 234. Kowalchuk RO, Shepard MJ, Sheehan K, et al. Treatment of WHO Grade 2 meningiomas with stereotactic radiosurgery: Identification of an optimal group for SRS using RPA. Int J Radiat Oncol Biol Phys. 2021;110(3):804–814. [DOI] [PubMed] [Google Scholar]
- 235. Burkett BJ, Bartlett DJ, McGarrah PW, et al. A review of theranostics: Perspectives on emerging approaches and clinical advancements. Radiol Imaging Cancer. 2023;5(4):e220157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Bailey DL, Willowson KP, Harris M, et al. (64)Cu treatment planning and (67)Cu therapy with radiolabeled [(64)Cu/(67)Cu]MeCOSar-octreotate in subjects with unresectable multifocal meningioma: Initial results for human imaging, safety, biodistribution, and radiation dosimetry. J Nucl Med. 2023;64(5):704–710. [DOI] [PubMed] [Google Scholar]
- 237. Mirian C, Duun-Henriksen AK, Maier A, et al. Somatostatin receptor-targeted radiopeptide therapy in treatment-refractory meningioma: Individual patient data meta-analysis. J Nucl Med. 2021;62(4):507–513. [DOI] [PubMed] [Google Scholar]
- 238. Boursier C, Zaragori T, Bros M, et al. Semi-automated segmentation methods of SSTR PET for dosimetry prediction in refractory meningioma patients treated by SSTR-targeted peptide receptor radionuclide therapy. Eur Radiol. 2023;33(10):7089–7098. [DOI] [PubMed] [Google Scholar]
- 239. Strosberg J, El-Haddad G, Wolin E, et al. ; NETTER-1 Trial Investigators. Phase 3 trial of (177)Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Urso L, Nieri A, Uccelli L, et al. Lutathera(®) Orphans: State of the art and future application of radioligand therapy with (177)Lu-DOTATATE. Pharmaceutics. 2023;15(4):1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Kurz S, Zan E, Cordova C, et al. CTNI-57. Radionuclide therapy with 177Lu-DOTATATE (Lutathera) in adults with advanced intracranial meningioma- interim results of a single-arm open-label multicenter phase II study multicenter study. Neuro-Oncology. 2022;24(suppl_7):vii85–vii85. [Google Scholar]
- 242. Mair MJ, Berghoff AS, Brastianos PK, Preusser M.. Emerging systemic treatment options in meningioma. J Neurooncol. 2023;161(2):245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Kaley TJ, Wen P, Schiff D, et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015;17(1):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Raizer JJ, Grimm SA, Rademaker A, et al. A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. J Neurooncol. 2014;117(1):93–101. [DOI] [PubMed] [Google Scholar]
- 245. Nayak L, Iwamoto FM, Rudnick JD, et al. Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol. 2012;109(1):187–193. [DOI] [PubMed] [Google Scholar]
- 246. Shih KC, Chowdhary S, Rosenblatt P, et al. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J Neurooncol. 2016;129(2):281–288. [DOI] [PubMed] [Google Scholar]
- 247. Preusser M, Silvani A, Le Rhun E, et al. Trabectedin for recurrent WHO grade 2 or 3 meningioma: A randomized phase II study of the EORTC Brain Tumor Group (EORTC-1320-BTG). Neuro Oncol. 2022;24(5):755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Kumthekar P, Grimm SA, Aleman RT, et al. A multi-institutional phase II trial of bevacizumab for recurrent and refractory meningioma. Neurooncol. Adv.. 2022;4(1):vdac123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Chamberlain MC. Hydroxyurea for recurrent surgery and radiation refractory high-grade meningioma. J Neurooncol. 2012;107(2):315–321. [DOI] [PubMed] [Google Scholar]
- 250. Chamberlain MC, Tsao-Wei DD, Groshen S.. Salvage chemotherapy with CPT-11 for recurrent meningioma. J Neurooncol. 2006;78(3):271–276. [DOI] [PubMed] [Google Scholar]
- 251. Belanger K, Ung TH, Damek D, Lillehei KO, Ormond DR.. Concomitant Temozolomide plus radiotherapy for high-grade and recurrent meningioma: A retrospective chart review. BMC Cancer. 2022;22(1):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Chamberlain MC. Adjuvant combined modality therapy for malignant meningiomas. J Neurosurg. 1996;84(5):733–736. [DOI] [PubMed] [Google Scholar]
- 253. Norden AD, Ligon KL, Hammond SN, et al. Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology. 2015;84(3):280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Simó M, Argyriou AA, Macià M, et al. Recurrent high-grade meningioma: A phase II trial with somatostatin analogue therapy. Cancer Chemother Pharmacol. 2014;73(5):919–923. [DOI] [PubMed] [Google Scholar]
- 255. Marincek N, Radojewski P, Dumont RA, et al. Somatostatin receptor-targeted radiopeptide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in progressive meningioma: lLong-term results of a phase II clinical trial. J Nucl Med. 2015;56(2):171–176. [DOI] [PubMed] [Google Scholar]
- 256. Graillon T, Sanson M, Campello C, et al. Everolimus and octreotide for patients with recurrent meningioma: Results from the phase II CEVOREM trial. Clin Cancer Res. 2020;26(3):552–557. [DOI] [PubMed] [Google Scholar]
- 257. Cossu G, Levivier M, Daniel RT, Messerer M.. The role of mifepristone in meningiomas management: A systematic review of the literature. Biomed Res Int. 2015;2015:267831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Du Z, Abedalthagafi M, Aizer AA, et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget. 2015;6(7):4704–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Brastianos PK, Kim AE, Giobbie-Hurder A, et al. Phase 2 study of pembrolizumab in patients with recurrent and residual high-grade meningiomas. Nat Commun. 2022;13(1):1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Bi WL, Nayak L, Meredith DM, et al. Activity of PD-1 blockade with nivolumab among patients with recurrent atypical/anaplastic meningioma: phase II trial results. Neuro Oncol. 2022;24(1):101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Chamberlain MC, Tsao-Wei DD, Groshen S.. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004;62(7):1210–1212. [DOI] [PubMed] [Google Scholar]
- 262. Wen PY, Yung WK, Lamborn KR, et al. Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01-08). Neuro Oncol. 2009;11(6):853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Norden AD, Raizer JJ, Abrey LE, et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. 2010;96(2):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Reardon DA, Norden AD, Desjardins A, et al. Phase II study of Gleevec® plus hydroxyurea (HU) in adults with progressive or recurrent meningioma. J Neurooncol. 2012;106(2):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Ji Y, Rankin C, Grunberg S, et al. Double-blind phase III randomized trial of the antiprogestin agent mifepristone in the treatment of unresectable meningioma: SWOG S9005. J Clin Oncol. 2015;33(34):4093–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Karsy M, Hoang N, Barth T, et al. Combined hydroxyurea and verapamil in the clinical treatment of refractory meningioma: Human and Orthotopic Xenograft Studies. World Neurosurg. 2016;86:210–219. [DOI] [PubMed] [Google Scholar]
- 267. Brastianos PK, Twohy EL, Gerstner ER, et al. Alliance A071401: Phase II trial of focal adhesion kinase inhibition in meningiomas with somatic NF2 mutations. J Clin Oncol. 2023;41(3):618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Jordan JT, Orr CC, Thalheimer RD, et al. Prospective phase II trial of the dual mTORC1/2 inhibitor vistusertib for progressive or symptomatic meningiomas in persons with neurofibromatosis 2. Neurooncol. Adv.. 2023;5(1):vdad041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Gyawali S, Sharma P, Mahapatra A.. Meningioma and psychiatric symptoms: An individual patient data analysis. Asian J Psychiatr. 2019;42:94–103. [DOI] [PubMed] [Google Scholar]
- 270. Wu A, Garcia MA, Magill ST, et al. Presenting symptoms and prognostic factors for symptomatic outcomes following resection of meningioma. World Neurosurg. 2018;111:e149–e159. [DOI] [PubMed] [Google Scholar]
- 271. Nassiri F, Price B, Shehab A, et al. ; International Consortium on Meningiomas. Life after surgical resection of a meningioma: A prospective cross-sectional study evaluating health-related quality of life. Neuro Oncol. 2019;21(suppl 1):i32–i43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Jakola AS, Gulati M, Gulati S, Solheim O.. The influence of surgery on quality of life in patients with intracranial meningiomas: A prospective study. J Neurooncol. 2012;110(1):137–144. [DOI] [PubMed] [Google Scholar]
- 273. Miao Y, Lu X, Qiu Y, Jiang J, Lin Y.. A multivariate analysis of prognostic factors for health-related quality of life in patients with surgically managed meningioma. J Clin Neurosci. 2010;17(4):446–449. [DOI] [PubMed] [Google Scholar]
- 274. Zamanipoor Najafabadi AH, Peeters MCM, Dirven L, et al. Impaired health-related quality of life in meningioma patients-a systematic review. Neuro Oncol. 2017;19(7):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Nassiri F, Suppiah S, Wang JZ, et al. How to live with a meningioma: Experiences, symptoms, and challenges reported by patients. Neurooncol. Adv.. 2020;2(1):vdaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Haider S, Taphoorn MJB, Drummond KJ, Walbert T.. Health-related quality of life in meningioma. Neurooncol. Adv.. 2021;3(1):vdab089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. van Nieuwenhuizen D, Klein M, Stalpers LJ, et al. Differential effect of surgery and radiotherapy on neurocognitive functioning and health-related quality of life in WHO grade I meningioma patients. J Neurooncol. 2007;84(3):271–278. [DOI] [PubMed] [Google Scholar]
- 278. Lisowski D, Trömel J, Lutyj P, et al. Health-related quality of life and clinical outcome after radiotherapy of patients with intracranial meningioma. Sci Rep. 2022;12(1):19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Henzel M, Fokas E, Sitter H, Wittig A, Engenhart-Cabillic R.. Quality of life after stereotactic radiotherapy for meningioma: A prospective non-randomized study. J Neurooncol. 2013;113(1):135–141. [DOI] [PubMed] [Google Scholar]
- 280. Chen WC, Magill ST, Englot DJ, et al. Factors associated with pre- and postoperative seizures in 1033 patients undergoing supratentorial meningioma resection. Neurosurgery. 2017;81(2):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Englot DJ, Magill ST, Han SJ, et al. Seizures in supratentorial meningioma: A systematic review and meta-analysis. J Neurosurg. 2016;124(6):1552–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Gupte TP, Li C, Jin L, et al. Clinical and genomic factors associated with seizures in meningiomas. J Neurosurg. 2020;135(3):835–844. [DOI] [PubMed] [Google Scholar]
- 283. Elbadry Ahmed R, Tang H, Asemota A, et al. Meningioma related epilepsy- pathophysiology, pre/postoperative seizures predicators and treatment. Front Oncol. 2022;12:905976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Dincer A, Jalal MI, Gupte TP, et al. The clinical and genomic features of seizures in meningiomas. Neurooncol. Adv. 2023;5(suppl 1):i49–i57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Kawaguchi T, Kameyama S, Tanaka R.. Peritumoral edema and seizure in patients with cerebral convexity and parasagittal meningiomas. Neurol Med Chir (Tokyo). 1996;36(8):568–73; discussion 573. [DOI] [PubMed] [Google Scholar]
- 286. Haglund MM, Berger MS, Kunkel DD, et al. Changes in gamma-aminobutyric acid and somatostatin in epileptic cortex associated with low-grade gliomas. J Neurosurg. 1992;77(2):209–216. [DOI] [PubMed] [Google Scholar]
- 287. Lobato RD, Alday R, Gómez PA, et al. Brain oedema in patients with intracranial meningioma. Correlation between clinical, radiological, and histological factors and the presence and intensity of oedema. Acta Neurochir (Wien). 1996;138(5):485–93; discussion 493. [DOI] [PubMed] [Google Scholar]
- 288. Peart R, Melnick K, Cibula J, et al. Clinical management of seizures in patients with meningiomas: Efficacy of surgical resection for seizure control and patient-tailored postoperative anti-epileptic drug management. Neurooncol. Adv.. 2023;5(suppl_1):ii58–ii66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Avila EK, Tobochnik S, Inati SK, et al. Brain tumor-related epilepsy management: A Society for Neuro-oncology (SNO) consensus review on current management. Neuro Oncol. 2023;26(1):7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Islim AI, McKeever S, Kusu-Orkar TE, Jenkinson MD.. The role of prophylactic antiepileptic drugs for seizure prophylaxis in meningioma surgery: A systematic review. J Clin Neurosci. 2017;43:47–53. [DOI] [PubMed] [Google Scholar]
- 291. Lieu AS, Howng SL.. Intracranial meningiomas and epilepsy: Incidence, prognosis and influencing factors. Epilepsy Res. 2000;38(1):45–52. [DOI] [PubMed] [Google Scholar]
- 292. Skardelly M, Rother C, Noell S, et al. Risk factors of preoperative and early postoperative seizures in patients with meningioma: A Retrospective Single-Center Cohort Study. World Neurosurg. 2017;97:538–546. [DOI] [PubMed] [Google Scholar]
- 293. Sughrue ME, Rutkowski MJ, Chang EF, et al. Postoperative seizures following the resection of convexity meningiomas: Are prophylactic anticonvulsants indicated? Clinical article. J Neurosurg. 2011;114(3):705–709. [DOI] [PubMed] [Google Scholar]
- 294. Islim AI, Ali A, Bagchi A, et al. Postoperative seizures in meningioma patients: Improving patient selection for antiepileptic drug therapy. J Neurooncol. 2018;140(1):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Jenkinson M, Helmy A, Huckey H, et al. RTID-10. Surgeons trial of prophylaxis for epilepsy in seizure naïve patients with meningioma: A randomized controlled trial (STOP ‘EM). Neuro-Oncology. 2020;22(suppl_2):ii195–ii195. [Google Scholar]
- 296. Evans DG, Huson SM, Donnai D, et al. A genetic study of type 2 neurofibromatosis in the United Kingdom. I. Prevalence, mutation rate, fitness, and confirmation of maternal transmission effect on severity. J Med Genet. 1992;29(12):841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Evans DG, Moran A, King A, et al. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: Higher incidence than previously thought. Otol Neurotol. 2005;26(1):93–97. [DOI] [PubMed] [Google Scholar]
- 298. Gregory GE, Islim AI, Hannan CJ, et al. The clinical, genetic, and immune landscape of meningioma in patients with NF2-schwannomatosis. Neurooncol. Adv.. 2023;5(suppl_1):ii94–i104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Mautner VF, Lindenau M, Baser ME, et al. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery. 1996;38(5):880–5; discussion 885. [DOI] [PubMed] [Google Scholar]
- 300. Hannan CJ, Hammerbeck-Ward C, Pathmanaban ON, et al. Multiple meningiomas as a criterion for the diagnosis of neurofibromatosis type 2 and other tumor predisposition syndromes. Neurosurgery. 2022;90(6):793–799. [DOI] [PubMed] [Google Scholar]
- 301. Abi Jaoude S, Peyre M, Degos V, et al. Validation of a scoring system to evaluate the risk of rapid growth of intracranial meningiomas in neurofibromatosis type 2 patients. J Neurosurg. 2020;134(5):1377–1385. [DOI] [PubMed] [Google Scholar]
- 302. Goutagny S, Bah AB, Henin D, et al. Long-term follow-up of 287 meningiomas in neurofibromatosis type 2 patients: Clinical, radiological, and molecular features. Neuro Oncol. 2012;14(8):1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Evers S, Verbaan D, Sanchez E, Peerdeman S.. 3D volumetric measurement of neurofibromatosis type 2-associated meningiomas: Association between tumor location and growth rate. World Neurosurg. 2015;84(4):1062–1069. [DOI] [PubMed] [Google Scholar]
- 304. Forde C, King AT, Rutherford SA, et al. Disease course of neurofibromatosis type 2: A 30-year follow-up study of 353 patients seen at a single institution. Neuro Oncol. 2021;23(7):1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Gao F, Li M, Wang Z, et al. Efficacy and safety of gamma knife radiosurgery for meningiomas in patients with neurofibromatosis type 2: A long-term Follow-Up Single-Center Study. World Neurosurg. 2019;125:e929–e936. [DOI] [PubMed] [Google Scholar]
- 306. Mohammed N, Hung YC, Xu Z, et al. Neurofibromatosis type 2-associated meningiomas: An international multicenter study of outcomes after Gamma Knife stereotactic radiosurgery. J Neurosurg. 2022;136(1):109–114. [DOI] [PubMed] [Google Scholar]
- 307. Birckhead B, Sio TT, Pollock BE, Link MJ, Laack NN.. Gamma Knife radiosurgery for neurofibromatosis type 2-associated meningiomas: A 22-year patient series. J Neurooncol. 2016;130(3):553–560. [DOI] [PubMed] [Google Scholar]
- 308. Liu A, Kuhn EN, LucasJT, Jr, et al. Gamma Knife radiosurgery for meningiomas in patients with neurofibromatosis Type 2. J Neurosurg. 2015;122(3):536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Evans DG, Halliday D, Obholzer R, et al. ; English Specialist NF2 Research Group. Radiation treatment of benign tumors in NF2-related-schwannomatosis: A national study of 266 irradiated patients showing a significant increase in malignancy/malignant progression. Neurooncol. Adv.. 2023;5(1):vdad025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Ferner RE, Shaw A, Evans DG, et al. Longitudinal evaluation of quality of life in 288 patients with neurofibromatosis 2. J Neurol. 2014;261(5):963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Cosetti MK, Golfinos JG, RolandJT, Jr. Quality of Life (QoL) assessment in patients with neurofibromatosis Type 2 (NF2). Otolaryngol Head Neck Surg. 2015;153(4):599–605. [DOI] [PubMed] [Google Scholar]
- 312. Osorio DS, Hu J, Mitchell C, et al. Effect of lapatinib on meningioma growth in adults with neurofibromatosis type 2. J Neurooncol. 2018;139(3):749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Plotkin S, Allen J, Babovic-Vuksanovic D, et al. CTNI-65. INTUITT-NF2, an adaptive platform-basket trial for neurofibromatosis 2 patients with progressive tumors: Interim results of the brigatinib treatment arm. Neuro-Oncology. 2022;24(suppl_7):vii88–vii88. [Google Scholar]
- 314. Kok JL, Teepen JC, van Leeuwen FE, et al. ; DCOG-LATER Study Group. Risk of benign meningioma after childhood cancer in the DCOG-LATER cohort: Contributions of radiation dose, exposed cranial volume, and age. Neuro Oncol. 2019;21(3):392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Bowers DC, Moskowitz CS, Chou JF, et al. Morbidity and mortality associated with meningioma after cranial radiotherapy: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2017;35(14):1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Sadetzki S, Chetrit A, Freedman L, et al. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163(4):424–432. [DOI] [PubMed] [Google Scholar]
- 317. Maeda Y, Onishi S, Yamasaki F, et al. Secondary meningioma after cranial irradiation: Case series and comprehensive literature review. Jpn J Clin Oncol. 2023;53(3):212–220. [DOI] [PubMed] [Google Scholar]
- 318. Strojan P, Popović M, Jereb B.. Secondary intracranial meningiomas after high-dose cranial irradiation: Report of five cases and review of the literature. Int J Radiat Oncol Biol Phys. 2000;48(1):65–73. [DOI] [PubMed] [Google Scholar]
- 319. Bello MJ, Leone PE, Nebreda P, et al. Allelic status of chromosome 1 in neoplasms of the nervous system. Cancer Genet Cytogenet. 1995;83(2):160–164. [DOI] [PubMed] [Google Scholar]
- 320. Xu M, Lasocki A, Bressel M, et al. Favourable outcomes with an initial active surveillance strategy for asymptomatic radiation-induced meningiomas in long-term survivors of paediatric and young adult malignancies. Radiother Oncol. 2023;189(12):109916. [DOI] [PubMed] [Google Scholar]
- 321. Jensen AW, Brown PD, Pollock BE, et al. Gamma knife radiosurgery of radiation-induced intracranial tumors: Local control, outcomes, and complications. Int J Radiat Oncol Biol Phys. 2005;62(1):32–37. [DOI] [PubMed] [Google Scholar]
- 322. Bunevicius A, Suleiman M, Patel S, et al. Stereotactic radiosurgery for treatment of radiation-induced meningiomas: A multiinstitutional study. J Neurosurg. 2021;135(3):862–870. [DOI] [PubMed] [Google Scholar]
- 323. Kondziolka D, Kano H, Kanaan H, et al. Stereotactic radiosurgery for radiation-induced meningiomas. Neurosurgery. 2009;64(3):463–9; discussion 469. [DOI] [PubMed] [Google Scholar]
- 324. Kuhn EN, Chan MD, Tatter SB, Ellis TL.. Gamma knife stereotactic radiosurgery for radiation-induced meningiomas. Stereotact Funct Neurosurg. 2012;90(6):365–369. [DOI] [PubMed] [Google Scholar]
- 325. Helseth A, Mørk SJ.. Primary intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg. 1989;71(6):842–845. [DOI] [PubMed] [Google Scholar]
- 326. Elsamadicy AA, Reeves BC, Craft S, et al. A current review of spinal meningiomas: epidemiology, clinical presentation and management. J Neurooncol. 2023;161(2):395–404. [DOI] [PubMed] [Google Scholar]
- 327. Kobayashi K, Ando K, Matsumoto T, et al. Clinical features and prognostic factors in spinal meningioma surgery from a multicenter study. Sci Rep. 2021;11(1):11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Hua L, Zhu H, Deng J, et al. Clinical and prognostic features of spinal meningioma: A thorough analysis from a single neurosurgical center. J Neurooncol. 2018;140(3):639–647. [DOI] [PubMed] [Google Scholar]
- 329. Hua L, Alkhatib M, Podlesek D, et al. Two predominant molecular subtypes of spinal meningioma: Thoracic NF2-mutant tumors strongly associated with female sex, and cervical AKT1-mutant tumors originating ventral to the spinal cord. Acta Neuropathol. 2022;144(5):1053–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Ricklefs FL, Fita KD, Mohme M, et al. Genetic and epigenetic profiling identifies two distinct classes of spinal meningiomas. Acta Neuropathol. 2022;144(5):1057–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Sayagués JM, Tabernero MD, Maíllo A, et al. Microarray-based analysis of spinal versus intracranial meningiomas: Different clinical, biological, and genetic characteristics associated with distinct patterns of gene expression. J Neuropathol Exp Neurol. 2006;65(5):445–454. [DOI] [PubMed] [Google Scholar]
- 332. Nakamura M, Tsuji O, Fujiyoshi K, et al. Long-term surgical outcomes of spinal meningiomas. Spine (Phila Pa 1976). 2012;37(10):E617–E623. [DOI] [PubMed] [Google Scholar]
- 333. Misra SN, Morgan HW.. Avoidance of structural pitfalls in spinal meningioma resection. Neurosurg Focus. 2003;14(6):e1. [DOI] [PubMed] [Google Scholar]
- 334. Yolcu YU, Goyal A, Alvi MA, Moinuddin FM, Bydon M.. Trends in the utilization of radiotherapy for spinal meningiomas: Insights from the 2004-2015 National Cancer Database. Neurosurg Focus. 2019;46(6):E6. [DOI] [PubMed] [Google Scholar]
- 335. Golanov AV, Konovalov NA, Antipina NA, et al. Stereotactic radiotherapy for spinal meningiomas and neurinomas. Voprosy Neirokhirurgii Imeni N.N. Burdenko. 2015;79(1):4–13. [DOI] [PubMed] [Google Scholar]
- 336. Kufeld M, Wowra B, Muacevic A, Zausinger S, Tonn JC.. Radiosurgery of spinal meningiomas and schwannomas. Technol Cancer Res Treat. 2012;11(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Hwang L, Okoye CC, Patel RB, et al. Stereotactic body radiotherapy for benign spinal tumors: Meningiomas, schwannomas, and neurofibromas. J Radiosurg SBRT. 2019;6(3):167–177. [PMC free article] [PubMed] [Google Scholar]
- 338. Kotecha RS, Pascoe EM, Rushing EJ, et al. Meningiomas in children and adolescents: A meta-analysis of individual patient data. Lancet Oncol. 2011;12(13):1229–1239. [DOI] [PubMed] [Google Scholar]
- 339. Ostrom QT, Price M, Neff C, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 2022;24(suppl 5):v1–v95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Lin DD, Lin JL, Deng XY, et al. Trends in intracranial meningioma incidence in the United States, 2004-2015. Cancer Med. 2019;8(14):6458–6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Dudley RWR, Torok MR, Randall S, et al. Pediatric versus adult meningioma: Comparison of epidemiology, treatments, and outcomes using the Surveillance, Epidemiology, and End Results database. J Neurooncol. 2018;137(3):621–629. [DOI] [PubMed] [Google Scholar]
- 342. Tauziède-Espariat A, Pfister SM, Mawrin C, Sahm F.. Pediatric meningiomas: A literature review and diagnostic update. Neurooncol. Adv.. 2023;5(suppl_1):ii105–ii111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Thuijs NB, Uitdehaag BM, Van Ouwerkerk WJ, et al. Pediatric meningiomas in The Netherlands 1974-2010: A descriptive epidemiological case study. Childs Nerv Syst. 2012;28(7):1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Kirches E, Sahm F, Korshunov A, et al. Molecular profiling of pediatric meningiomas shows tumor characteristics distinct from adult meningiomas. Acta Neuropathol. 2021;142(5):873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Wang Y, Qin X, Liu M, et al. Clear cell meningioma in the central nervous system: Analysis of surveillance, epidemiology, and end results database. Front Oncol. 2020;10:592800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Cahill KS, Claus EB.. Treatment and survival of patients with nonmalignant intracranial meningioma: Results from the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. Clinical article. J Neurosurg. 2011;115(2):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Szychot E, Goodden J, Whitfield G, Curry S.. Children’s Cancer and Leukaemia Group (CCLG): Review and guidelines for the management of meningioma in children, teenagers and young adults. Br J Neurosurg. 2020;34(2):142–153. [DOI] [PubMed] [Google Scholar]
- 348. El Beltagy MA, Enayet AE, Atteya MME, et al. Management of pediatric CNS meningiomas: CCHE-57357 experience in 39 cases. Childs Nerv Syst. 2019;35(8):1323–1331. [DOI] [PubMed] [Google Scholar]
- 349. Toland A, McNulty SN, Pekmezci M, et al. Pediatric meningioma: A clinicopathologic and molecular study with potential grading implications. Brain Pathol. 2020;30(6):1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Blakeley JO, Evans DG, Adler J, et al. Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis type 2. Am J Med Genet A. 2012;158A(1):24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Norden AD, Drappatz J, Wen PY.. Advances in meningioma therapy. Curr Neurol Neurosci Rep. 2009;9(3):231–240. [DOI] [PubMed] [Google Scholar]
- 352. Wen PY, Quant E, Drappatz J, Beroukhim R, Norden AD.. Medical therapies for meningiomas. J Neurooncol. 2010;99(3):365–378. [DOI] [PubMed] [Google Scholar]
- 353. Battu S, Kumar A, Pathak P, et al. Clinicopathological and molecular characteristics of pediatric meningiomas. Neuropathol. 2018;38(1):22–33. [DOI] [PubMed] [Google Scholar]
- 354. Perry A, Giannini C, Raghavan R, et al. Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: A clinicopathologic study of 53 cases. J Neuropathol Exp Neurol. 2001;60(10):994–1003. [DOI] [PubMed] [Google Scholar]
- 355. Sievers P, Chiang J, Schrimpf D, et al. YAP1-fusions in pediatric NF2-wildtype meningioma. Acta Neuropathol. 2020;139(1):215–218. [DOI] [PubMed] [Google Scholar]
- 356. Szulzewsky F, Arora S, Arakaki AKS, et al. Both YAP1-MAML2 and constitutively active YAP1 drive the formation of tumors that resemble NF2 mutant meningiomas in mice. Genes Dev. 2022;36(13-14):857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Maze EA, Agit B, Reeves S, et al. Human endogenous retrovirus type K promotes proliferation and confers sensitivity to antiretroviral drugs in merlin-negative schwannoma and meningioma. Cancer Res. 2022;82(2):235–247. [DOI] [PubMed] [Google Scholar]
- 358. Ferluga S, Baiz D, Hilton DA, et al. Constitutive activation of the EGFR-STAT1 axis increases proliferation of meningioma tumor cells. Neurooncol. Adv.. 2020;2(1):vdaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Mawrin C. Animal models of meningiomas. Chin Clin Oncol. 2017;6(suppl 1):S6. [DOI] [PubMed] [Google Scholar]
- 360. Ragel BT, Couldwell WT, Gillespie DL, et al. A comparison of the cell lines used in meningioma research. Surg Neurol. 2008;70(3):295–307; discussion 307. discussion 307. [DOI] [PubMed] [Google Scholar]
- 361. Baia GS, Dinca EB, Ozawa T, et al. An orthotopic skull base model of malignant meningioma. Brain Pathol (Zurich, Switzerland). 2008;18(2):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Jungwirth G, Yu T, Moustafa M, et al. Identification of KIF11 as a novel target in meningioma. Cancers (Basel). 2019;11(4):545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Jungwirth G, Yu T, Liu F, et al. Pharmacological landscape of FDA-approved anticancer drugs reveals sensitivities to ixabepilone, romidepsin, omacetaxine, and carfilzomib in aggressive meningiomas. Clin Cancer Res. 2023;29(1):233–243. [DOI] [PubMed] [Google Scholar]
- 364. Negroni C, Hilton DA, Ercolano E, et al. GATA-4, a potential novel therapeutic target for high-grade meningioma, regulates miR-497, a potential novel circulating biomarker for high-grade meningioma. EBioMedicine. 2020;59:102941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Iwami K, Natsume A, Ohno M, et al. Adoptive transfer of genetically modified Wilms’ tumor 1–specific T cells in a novel malignant skull base meningioma model. Neuro-Oncology. 2013;15(6):747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Nigim F, Esaki S, Hood M, et al. A new patient-derived orthotopic malignant meningioma model treated with oncolytic herpes simplex virus. Neuro Oncol. 2016;18(9):1278–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Püttmann S, Senner V, Braune S, et al. Establishment of a benign meningioma cell line by hTERT-mediated immortalization. Lab Invest. 2005;85(9):1163–1171. [DOI] [PubMed] [Google Scholar]
- 368. Baia GS, Slocum AL, Hyer JD, et al. A genetic strategy to overcome the senescence of primary meningioma cell cultures. J Neurooncol. 2006;78(2):113–121. [DOI] [PubMed] [Google Scholar]
- 369. Ragel BT, Elam IL, Gillespie DL, et al. A novel model of intracranial meningioma in mice using luciferase-expressing meningioma cells. Laboratory investigation. J Neurosurg. 2008;108(2):304–310. [DOI] [PubMed] [Google Scholar]
- 370. Peyre M, Stemmer-Rachamimov A, Clermont-Taranchon E, et al. Meningioma progression in mice triggered by Nf2 and Cdkn2ab inactivation. Oncogene. 2013;32(36):4264–4272. [DOI] [PubMed] [Google Scholar]
- 371. Jungwirth G, Hanemann CO, Dunn IF, Herold-Mende C.. Preclinical models of meningioma. Adv Exp Med Biol. 2023;1416:199–211. [DOI] [PubMed] [Google Scholar]
- 372. Choudhury A, Raleigh DR.. Preclinical models of meningioma: Cell culture and animal systems. Handb Clin Neurol. 2020;169:131–136. [DOI] [PubMed] [Google Scholar]
- 373. Meijer TG, Naipal KA, Jager A, van Gent DC.. Ex vivo tumor culture systems for functional drug testing and therapy response prediction. Future Sci OA. 2017;3(2):FSO190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Sivakumar R, Chan M, Shin JS, et al. Organotypic tumor slice cultures provide a versatile platform for immuno-oncology and drug discovery. Oncoimmunology. 2019;8(12):e1670019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Chan HSC, Ng HK, Chan AK, et al. Establishment and characterization of meningioma patient-derived organoid. J Clin Neurosci. 2021;94:192–199. [DOI] [PubMed] [Google Scholar]
- 376. Huang M, Xu S, Li Y, et al. Novel human meningioma organoids recapitulate the aggressiveness of the initiating cell subpopulations identified by ScRNA-Seq. Adv Sci (Weinh). 2023;10(15):e2205525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Yamazaki S, Ohka F, Hirano M, et al. Newly established patient-derived organoid model of intracranial meningioma. Neuro Oncol. 2021;23(11):1936–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Magill ST, Vasudevan HN, Seo K, et al. Multiplatform genomic profiling and magnetic resonance imaging identify mechanisms underlying intratumor heterogeneity in meningioma. Nat Commun. 2020;11(1):4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Tonn JC, Ott MM, Bouterfa H, et al. Inverse correlation of cell proliferation and expression of progesterone receptors in tumor spheroids and monolayer cultures of human meningiomas. Neurosurgery. 1997;41(5):1152–1159. [DOI] [PubMed] [Google Scholar]
- 380. van de Weijer LL, Ercolano E, Zhang T, et al. A novel patient-derived meningioma spheroid model as a tool to study and treat epithelial-to-mesenchymal transition (EMT) in meningiomas. Acta Neuropathol Commun. 2023;11(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Kim D, Park J, Park HC, et al. Establishment of tumor microenvironment-preserving organoid model from patients with intracranial meningioma. Cancer Cell Int. 2024;24(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Millward CP, Armstrong TS, Barrington H, et al. ; EORTC BTG, ICOM, EANO, SNO, RANO-PRO, BNOS, SBNS, BIMS, TBTC, International Brain Tumour Alliance, Brainstrust, and Brain Tumour Foundation of Canada. Development of ‘Core Outcome Sets’ for Meningioma in Clinical Studies (The COSMIC Project): Protocol for two systematic literature reviews, eDelphi surveys and online consensus meetings. BMJ Open. 2022;12(5):e057384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Abedalthagafi M, Bi WL, Aizer AA, et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro-Oncology. 2016;18(5):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Bleeker F, Felicioni L, Buttitta F, et al. AKT1 E17K in human solid tumours. Oncogene. 2008;27(42):5648–5650. [DOI] [PubMed] [Google Scholar]
- 385. Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448(7152):439–444. [DOI] [PubMed] [Google Scholar]
- 386. Sahm F, Bissel J, Koelsche C, et al. AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. Acta Neuropathol. 2013;126(5):757–762. [DOI] [PubMed] [Google Scholar]
- 387. Strickland MR, Gill CM, Nayyar N, et al. Targeted sequencing of SMO and AKT1 in anterior skull base meningiomas. J Neurosurg. 2017;127(2):438–444. [DOI] [PubMed] [Google Scholar]
- 388. Cheung M, Testa JR.. BAP1, a tumor suppressor gene driving malignant mesothelioma. Transl Lung Cancer Res. 2017;6(3):270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Behling F, Barrantes-Freer A, Skardelly M, et al. Frequency of BRAF V600E mutations in 969 central nervous system neoplasms. Diagn Pathol. 2016;11(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. [DOI] [PubMed] [Google Scholar]
- 391. Bao Z, Hua L, Ye Y, et al. MEF2C silencing downregulates NF2 and E-cadherin and enhances Erastin-induced ferroptosis in meningioma. Neuro Oncol. 2021;23(12):2014–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Nikuseva-Martić T, Beros V, Pećina-Slaus N, Pećina HI, Bulić-Jakus F.. Genetic changes of CDH1, APC, and CTNNB1 found in human brain tumors. Pathol Res Pract. 2007;203(11):779–787. [DOI] [PubMed] [Google Scholar]
- 393. Collord G, Tarpey P, Kurbatova N, et al. An integrated genomic analysis of anaplastic meningioma identifies prognostic molecular signatures. Sci Rep. 2018;8(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Chapman EJ, Harnden P, Chambers P, Johnston C, Knowles MA.. Comprehensive analysis of CDKN2A status in microdissected urothelial cell carcinoma reveals potential haploinsufficiency, a high frequency of homozygous co-deletion and associations with clinical phenotype. Clin Cancer Res. 2005;11(16):5740–5747. [DOI] [PubMed] [Google Scholar]
- 395. Goutagny S, Yang HW, Zucman-Rossi J, et al. Genomic profiling reveals alternative genetic pathways of meningioma malignant progression dependent on the underlying NF2 status. Clin Cancer Res. 2010;16(16):4155–4164. [DOI] [PubMed] [Google Scholar]
- 396. Guyot A, Duchesne M, Robert S, et al. Analysis of CDKN2A gene alterations in recurrent and non-recurrent meningioma. J Neurooncol. 2019;145(3):449–459. [DOI] [PubMed] [Google Scholar]
- 397. Guyot A, Duchesne M, Robert S, et al. Analysis of CDKN2A gene alterations in recurrent and non-recurrent meningioma. J Neurooncol. 2019;145(3):449–459. [DOI] [PubMed] [Google Scholar]
- 398. Ruas M, Peters G.. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim Biophys Acta. 1998;1378(2):F115–F177. [DOI] [PubMed] [Google Scholar]
- 399. Yang HW, Kim TM, Song SS, et al. Alternative splicing of CHEK2 and codeletion with NF2 promote chromosomal instability in meningioma. Neoplasia. 2012;14(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Gutmann DH, Donahoe J, Perry A, et al. Loss of DAL-1, a protein 4.1-related tumor suppressor, is an important early event in the pathogenesis of meningiomas. Hum Mol Genet. 2000;9(10):1495–1500. [DOI] [PubMed] [Google Scholar]
- 401. Martinez-Glez V, Bello MJ, Franco-Hernandez C, et al. Mutational analysis of the DAL-1/4.1B tumour-suppressor gene locus in meningiomas. Int J Mol Med. 2005;16(4):771–774. [PubMed] [Google Scholar]
- 402. Perry A, Cai DX, Scheithauer BW, et al. Merlin, DAL-1, and progesterone receptor expression in clinicopathologic subsets of meningioma: A correlative immunohistochemical study of 175 cases. J Neuropathol Exp Neurol. 2000;59(10):872–879. [DOI] [PubMed] [Google Scholar]
- 403. Harmancı AS, Youngblood MW, Clark VE, et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun. 2017;8(2):14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Zeng J, Sun L, Huang J, Yang X, Hu W.. Enhancer of zeste homolog 2 is a negative prognostic biomarker and correlated with immune infiltrates in meningioma. Front Neurosci. 2022;16:1076530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Pereira BJA, Marcondes Lerario A, Sola PR, et al. Impact of a cell cycle and an extracellular matrix remodeling transcriptional signature on tumor progression and correlation with EZH2 expression in meningioma. J Neurosurg. 2023;138(3):649–662. [DOI] [PubMed] [Google Scholar]
- 406. Kar R, Jha SK, Ojha S, et al. The FBXW7-NOTCH interactome: A ubiquitin proteasomal system-induced crosstalk modulating oncogenic transformation in human tissues. Cancer Rep (Hoboken). 2021;4(4):e1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Barresi V, Simbolo M, Fioravanzo A, et al. Molecular profiling of 22 primary atypical meningiomas shows the prognostic significance of 18q heterozygous loss and CDKN2A/B homozygous deletion on recurrence-free survival. Cancers (Basel). 2021;13(4):903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Johnson MD, O’Connell MJ, Pilcher W, Reeder JE.. Fibroblast growth factor receptor-3 expression in meningiomas with stimulation of proliferation by the phosphoinositide 3 kinase-Akt pathway. J Neurosurg. 2010;112(5):934–939. [DOI] [PubMed] [Google Scholar]
- 409. AlSahlawi A, Aljelaify R, Magrashi A, et al. New insights into the genomic landscape of meningiomas identified FGFR3 in a subset of patients with favorable prognoses. Oncotarget. 2019;10(53):5549–5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Maggio I, Franceschi E, Tosoni A, et al. Meningioma: not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol. 2021;10(2):CNS72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Di Giammartino DC, Kloetgen A, Polyzos A, et al. KLF4 is involved in the organization and regulation of pluripotency-associated three-dimensional enhancer networks. Nat Cell Biol. 2019;21(10):1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Parada CA, Osbun JW, Busald T, et al. Phosphoproteomic and kinomic signature of clinically aggressive grade I (1.5) meningiomas reveals RB1 signaling as a novel mediator and biomarker. Clin Cancer Res. 2020;26(1):193–205. [DOI] [PubMed] [Google Scholar]
- 413. Tang H, Zhu H, Wang X, et al. KLF4 is a tumor suppressor in anaplastic meningioma stem-like cells and human meningiomas. J Mol Cell Biol. 2017;9(4):315–324. [DOI] [PubMed] [Google Scholar]
- 414. von Spreckelsen N, Waldt N, Poetschke R, et al. KLF4 K409Q–mutated meningiomas show enhanced hypoxia signaling and respond to mTORC1 inhibitor treatment. Acta Neuropathologica Commun. 2020;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Youngblood MW, Miyagishima DF, Jin L, et al. Associations of meningioma molecular subgroup and tumor recurrence. Neuro Oncol. 2021;23(5):783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Shapiro IM, Kolev VN, Vidal CM, et al. Merlin deficiency predicts FAK inhibitor sensitivity: A synthetic lethal relationship. Sci Transl Med. 2014;6(237):237ra268–237ra268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Stamenkovic I, Yu Q.. Merlin, a “magic” linker between the extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci. 2010;11(6):471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Leone PE, Bello MJ, de Campos JM, et al. NF2 gene mutations and allelic status of 1p, 14q and 22q in sporadic meningiomas. Oncogene. 1999;18(13):2231–2239. [DOI] [PubMed] [Google Scholar]
- 419. Cômes PC, Le Van T, Tran S, et al. Respective roles of Pik3ca mutations and cyproterone acetate impregnation in mouse meningioma tumorigenesis. Cancer Gene Ther. 2023;30(8):1114–1123. [DOI] [PubMed] [Google Scholar]
- 420. Peyre M, Miyagishima D, Bielle F, et al. Somatic PIK3CA mutations in sporadic cerebral cavernous malformations. N Engl J Med. 2021;385(11):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Hadfield KD, Smith MJ, Trump D, Newman WG, Evans D.. SMARCB1 mutations are not a common cause of multiple meningiomas. J Med Genet. 2010;47(8):567–568. [DOI] [PubMed] [Google Scholar]
- 422. Dhamija R, Plotkin S, Gomes A, Babovic-Vuksanovic D.. LZTR1- and SMARCB1-Related Schwannomatosis. In: Adam MP, Mirzaa GM, Pagon RA, et al. , eds. GeneReviews(®). Seattle, WA: University of Washington, Seattle Copyright © 1993-2023, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 1993. [Google Scholar]
- 423. Boetto J, Bielle F, Sanson M, Peyre M, Kalamarides M.. SMO mutation status defines a distinct and frequent molecular subgroup in olfactory groove meningiomas. Neuro-Oncology. 2017;19(3):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Findakly S, Choudhury A, Daggubati V, et al. Meningioma cells express primary cilia but do not transduce ciliary Hedgehog signals. Acta Neuropathol Commun. 2020;8(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Askaner G, Lei U, Bertelsen B, Venzo A, Wadt K.. Novel SUFU frameshift variant leading to meningioma in three generations in a family with gorlin syndrome. Case Rep Gen. 2019;2019:9650184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Smith MJ. Germline and somatic mutations in meningiomas. Cancer Genet. 2015;208(4):107–114. [DOI] [PubMed] [Google Scholar]
- 427. Biczok A, Kraus T, Suchorska B, et al. TERT promoter mutation is associated with worse prognosis in WHO grade II and III meningiomas. J Neurooncol. 2018;139(3):671–678. [DOI] [PubMed] [Google Scholar]
- 428. Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–961. [DOI] [PubMed] [Google Scholar]
- 429. Juratli TA, Thiede C, Koerner MV, et al. Intratumoral heterogeneity and TERT promoter mutations in progressive/higher-grade meningiomas. Oncotarget. 2017;8(65):109228–109237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Zotti T, Scudiero I, Vito P, Stilo R.. The emerging role of TRAF7 in tumor development. J Cell Physiol. 2017;232(6):1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.