Abstract
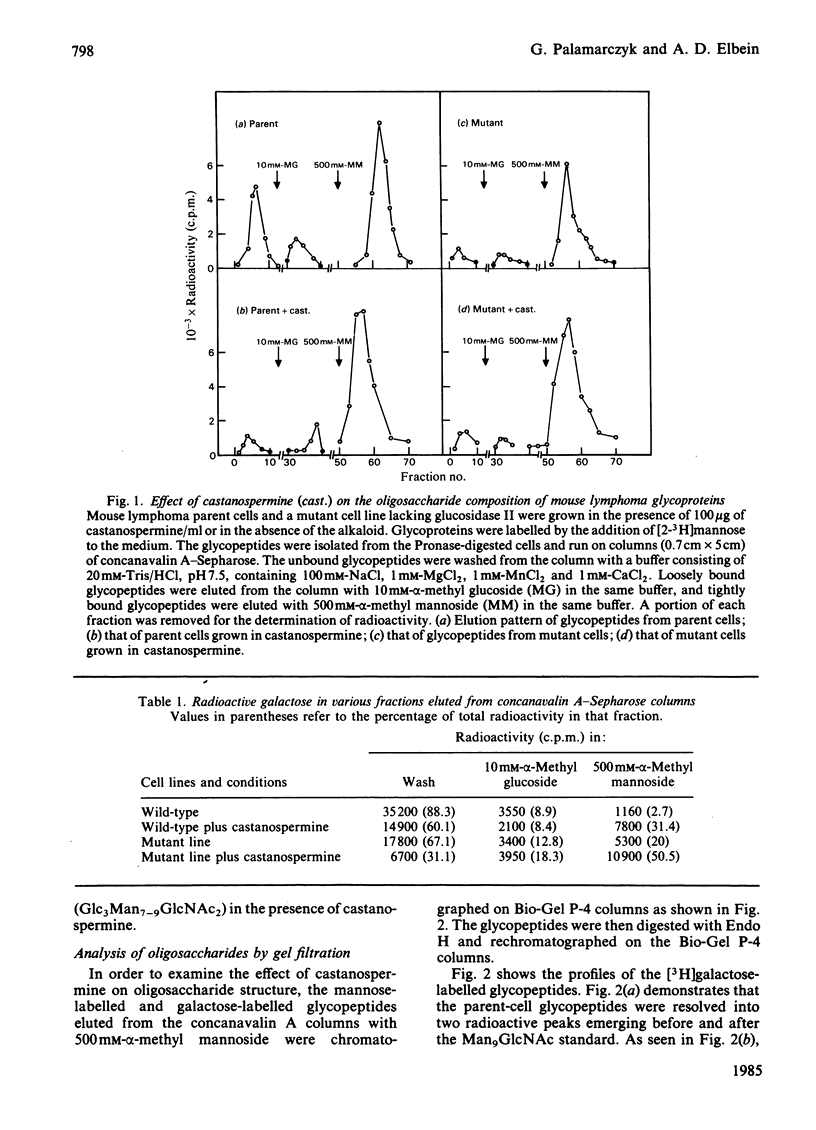
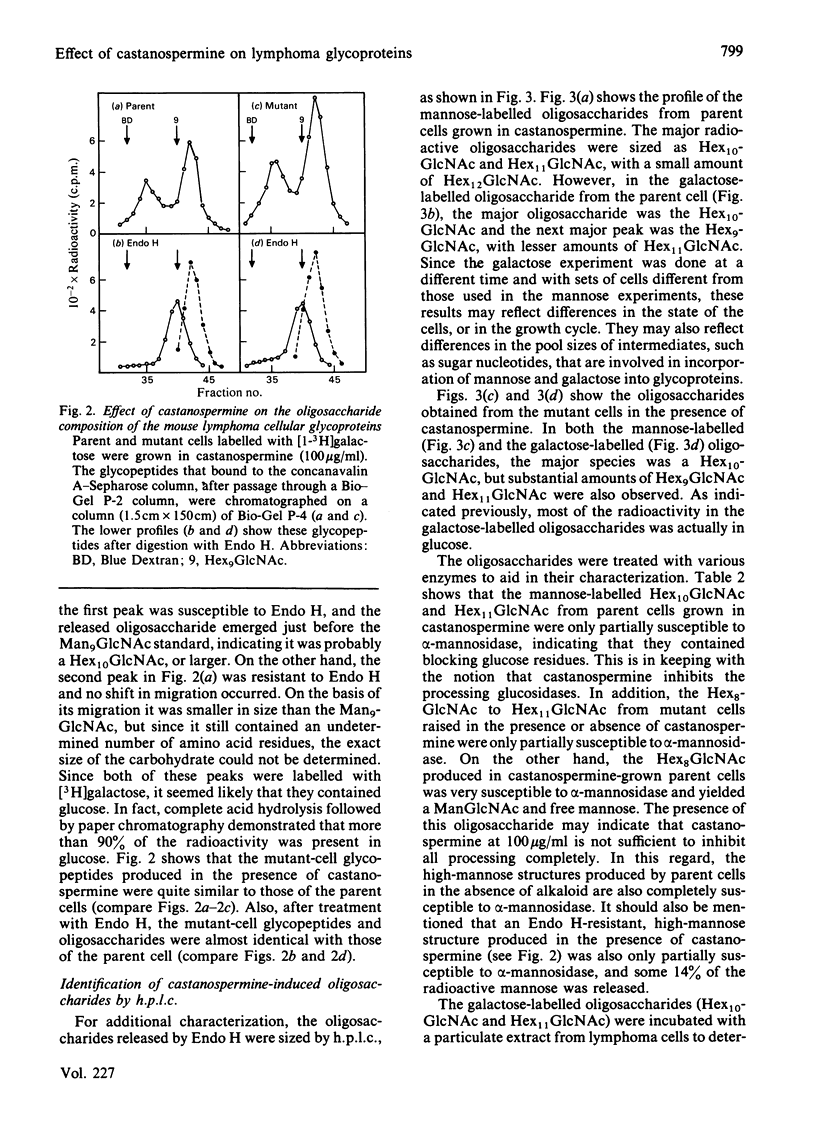
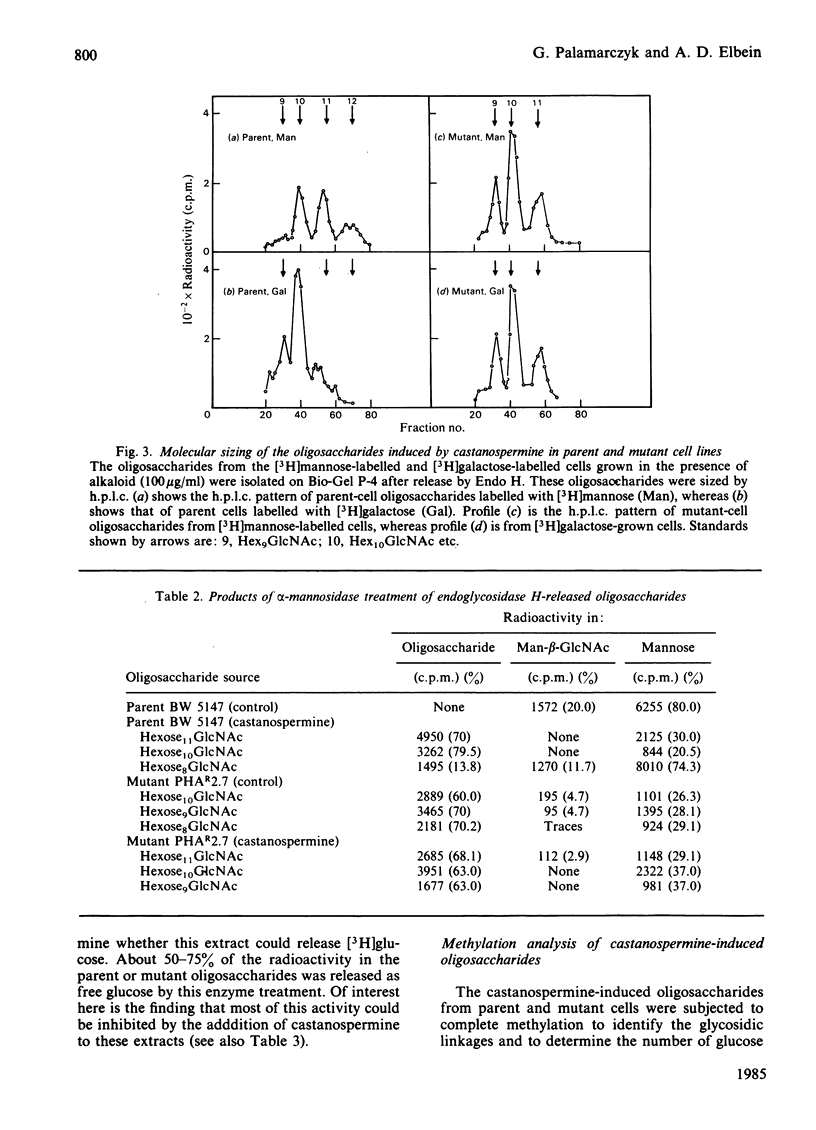
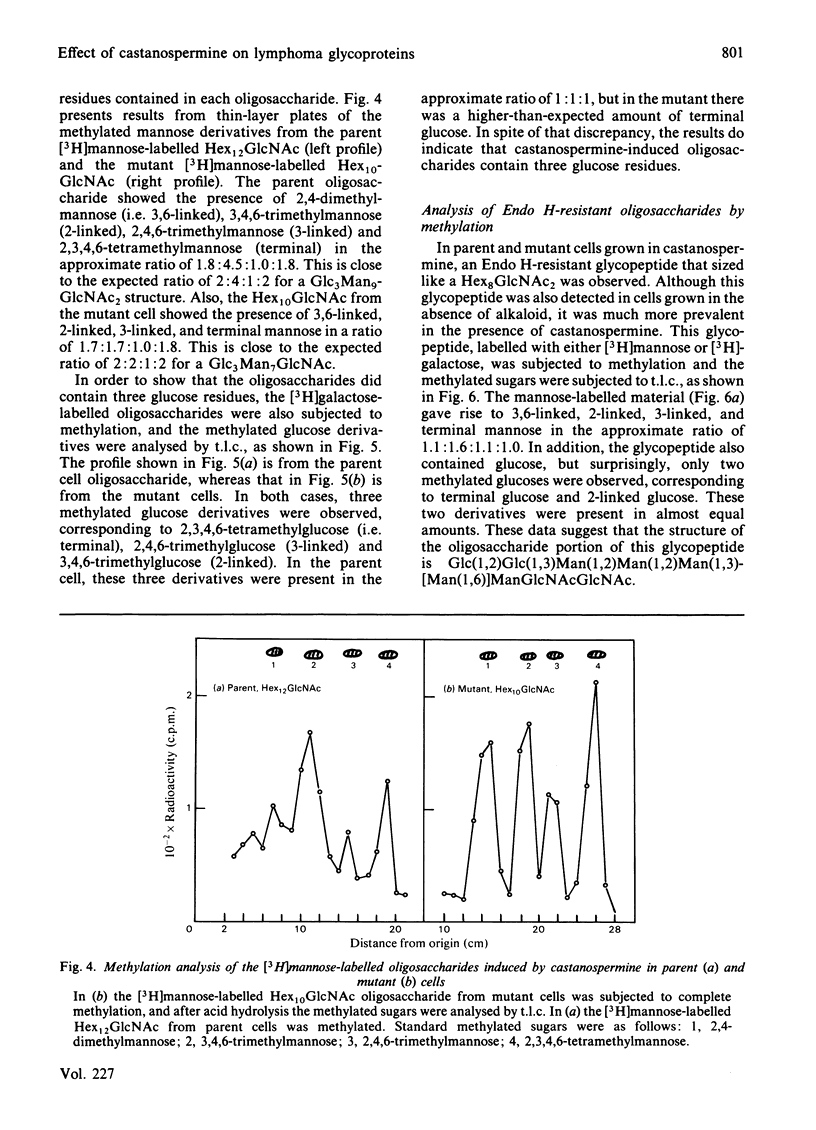
The effect of castanospermine on the processing of N-linked oligosaccharides was examined in the parent mouse lymphoma cell line and in a mutant cell line that lacks glucosidase II. When the parent cell line was grown in the presence of castanospermine at 100 micrograms/ml, glucose-containing high-mannose oligosaccharides were obtained that were not found in the absence of inhibitor. These oligosaccharides bound tightly to concanavalin A-Sepharose and were eluted in the same position as oligosaccharides from the mutant cells grown in the absence or presence of the alkaloid. The castanospermine-induced oligosaccharides were characterized by gel filtration on Bio-Gel P-4, by h.p.l.c. analysis, by enzymic digestions and by methylation analysis of [3H]mannose-labelled and [3H]galactose-labelled oligosaccharides. The major oligosaccharide released by endoglucosaminidase H in either parent or mutant cells grown in castanospermine was a Glc3Man7GlcNAc, with smaller amounts of Glc3Man8GlcNAc and Glc3Man9GlcNAc. On the other hand, in the absence of castanospermine the mutant produces mostly Glc2Man7GlcNAc. In addition to the above oligosaccharides, castanospermine stimulated the formation of an endoglucosaminidase H-resistant oligosaccharide in both cell lines. This oligosaccharide was characterized as a Glc2Man5GlcNAc2 (i.e., Glc(1,2)Glc(1,3)Man(1,2)Man(1,2)Man(1,3)[Man(1,6)]Man-GlcNAc-GlcNAc). Castanospermine was tested directly on glucosidase I and glucosidase II in lymphoma cell extracts by using [Glc-3H]Glc3Man9GlcNAc and [Glc-3H]Glc2Man9GlcNAc as substrates. Castanospermine was a potent inhibitor of both activities, but glucosidase I appeared to be more sensitive to inhibition.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhrem A. A., Avvakumov G. V., Sidorova I. V., Strel'chyonok O. A. Methylation analysis in glycoprotein chemistry. General procedure for quantification of the products of solvolysis of permethylated glycopeptides and glycoproteins. J Chromatogr. 1979 Nov 28;180(1):69–82. doi: 10.1016/s0021-9673(00)80175-2. [DOI] [PubMed] [Google Scholar]
- Bischoff J., Kornfeld R. Evidence for an alpha-mannosidase in endoplasmic reticulum of rat liver. J Biol Chem. 1983 Jul 10;258(13):7907–7910. [PubMed] [Google Scholar]
- Burns D. M., Touster O. Purification and characterization of glucosidase II, an endoplasmic reticulum hydrolase involved in glycoprotein biosynthesis. J Biol Chem. 1982 Sep 10;257(17):9990–10000. [PubMed] [Google Scholar]
- Chapman A., Fujimoto K., Kornfeld S. The primary glycosylation defect in class E Thy-1-negative mutant mouse lymphoma cells is an inability to synthesize dolichol-P-mannose. J Biol Chem. 1980 May 25;255(10):4441–4446. [PubMed] [Google Scholar]
- Chen W. W., Lennarz W. J. Enzymatic excision of glucosyl units linked to the oligosaccharide chains of glycoproteins. J Biol Chem. 1978 Aug 25;253(16):5780–5785. [PubMed] [Google Scholar]
- Datema R., Schwarz R. T. Effect of energy depletion on the glycosylation of a viral glycoprotein. J Biol Chem. 1981 Nov 10;256(21):11191–11198. [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharides. CRC Crit Rev Biochem. 1984;16(1):21–49. doi: 10.3109/10409238409102805. [DOI] [PubMed] [Google Scholar]
- Elting J. J., Chen W. W., Lennarz W. J. Characterization of a glucosidase involved in an initial step in the processing of oligosaccharide chains. J Biol Chem. 1980 Mar 25;255(6):2325–2331. [PubMed] [Google Scholar]
- Forsee W. T., Schutzbach J. S. Purification and characterization of a phospholipid-dependent alpha-mannosidase from rabbit liver. J Biol Chem. 1981 Jul 10;256(13):6577–6582. [PubMed] [Google Scholar]
- Grinna L. S., Robbins P. W. Glycoprotein biosynthesis. Rat liver microsomal glucosidases which process oligosaccharides. J Biol Chem. 1979 Sep 25;254(18):8814–8818. [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Harpaz N., Schachter H. Control of glycoprotein synthesis. Processing of asparagine-linked oligosaccharides by one or more rat liver Golgi alpha-D-mannosidases dependent on the prior action of UDP-N-acetylglucosamine: alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase I. J Biol Chem. 1980 May 25;255(10):4894–4902. [PubMed] [Google Scholar]
- Hettkamp H., Legler G., Bause E. Purification by affinity chromatography of glucosidase I, an endoplasmic reticulum hydrolase involved in the processing of asparagine-linked oligosaccharides. Eur J Biochem. 1984 Jul 2;142(1):85–90. doi: 10.1111/j.1432-1033.1984.tb08253.x. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Gregory W., Chapman A. Class E Thy-1 negative mouse lymphoma cells utilize an alternate pathway of oligosaccharide processing to synthesize complex-type oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11649–11654. [PubMed] [Google Scholar]
- Lehle L., Tanner W. Polyprenol-linked sugars and glycoprotein synthesis in plants. Biochem Soc Trans. 1983 Oct;11(5):568–574. doi: 10.1042/bst0110568. [DOI] [PubMed] [Google Scholar]
- Michael J. M., Kornfeld S. Partial purification and characterization of the glucosidases involved in the processing of asparagine-linked oligosaccharides. Arch Biochem Biophys. 1980 Jan;199(1):249–258. doi: 10.1016/0003-9861(80)90278-7. [DOI] [PubMed] [Google Scholar]
- Opheim D. J., Touster O. Lysosomal alpha-D-mannosidase of rat liver. Purification and comparison with the golgi and cytosolic alpha-D-mannosidases. J Biol Chem. 1978 Feb 25;253(4):1017–1023. [PubMed] [Google Scholar]
- Pan Y. T., Hori H., Saul R., Sanford B. A., Molyneux R. J., Elbein A. D. Castanospermine inhibits the processing of the oligosaccharide portion of the influenza viral hemagglutinin. Biochemistry. 1983 Aug 2;22(16):3975–3984. doi: 10.1021/bi00285a038. [DOI] [PubMed] [Google Scholar]
- Rearick J. I., Chapman A., Kornfeld S. Glucose starvation alters lipid-linked oligosaccharide biosynthesis in Chinese hamster ovary cells. J Biol Chem. 1981 Jun 25;256(12):6255–6261. [PubMed] [Google Scholar]
- Reitman M. L., Trowbridge I. S., Kornfeld S. A lectin-resistant mouse lymphoma cell line is deficient in glucosidase II, a glycoprotein-processing enzyme. J Biol Chem. 1982 Sep 10;257(17):10357–10363. [PubMed] [Google Scholar]
- Saul R., Chambers J. P., Molyneux R. J., Elbein A. D. Castanospermine, a tetrahydroxylated alkaloid that inhibits beta-glucosidase and beta-glucocerebrosidase. Arch Biochem Biophys. 1983 Mar;221(2):593–597. doi: 10.1016/0003-9861(83)90181-9. [DOI] [PubMed] [Google Scholar]
- Spiro R. G., Spiro M. J., Bhoyroo V. D. Processing of carbohydrate units of glycoproteins. Characterization of a thyroid glucosidase. J Biol Chem. 1979 Aug 25;254(16):7659–7667. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11655–11663. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]
- Turco S. J. Modification of oligosaccharide-lipid synthesis and protein glycosylation in glucose-deprived cells. Arch Biochem Biophys. 1980 Dec;205(2):330–339. doi: 10.1016/0003-9861(80)90115-0. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Robbins P. W. The initial stages of processing of protein-bound oligosaccharides in vitro. J Biol Chem. 1979 Jun 10;254(11):4560–4567. [PubMed] [Google Scholar]
- Ugalde R. A., Staneloni R. J., Leloir L. F. Action of glycosidases on the saccharide moiety of the glucose--containing dolichyl diphosphate oligosaccharide. FEBS Lett. 1978 Jul 15;91(2):209–212. doi: 10.1016/0014-5793(78)81174-0. [DOI] [PubMed] [Google Scholar]


