Abstract
Cell-based therapies hold great promise for brain repair after stroke. While accumulating evidence confirms the preclinical and clinical benefits of cell therapies, the underlying mechanisms by which they promote brain repair remain unclear. Here, we briefly review endogenous mechanisms of brain repair after ischaemic stroke and then focus on how different stem and progenitor cell sources can promote brain repair.
Specifically, we examine how transplanted cell grafts contribute to improved functional recovery either through direct cell replacement or by stimulating endogenous repair pathways. Additionally, we discuss recently implemented preclinical refinement methods, such as preconditioning, microcarriers, genetic safety switches and universal (immune evasive) cell transplants, as well as the therapeutic potential of these pharmacologic and genetic manipulations to further enhance the efficacy and safety of cell therapies.
By gaining a deeper understanding of post-ischaemic repair mechanisms, prospective clinical trials may be further refined to advance post-stroke cell therapy to the clinic.
Keywords: stem cells, ischaemia, brain injury, iPSC, regeneration, therapy
Rust et al. review mechanisms of brain repair after ischaemic stroke, focusing on the potential of stem cells to enhance recovery. They discuss the challenges faced by cell therapies in recent clinical trials and consider how advances in preclinical research might pave the way for next-generation cell therapies.
Introduction
Stroke remains a leading cause of disability and death, affecting one in four people after the age of 25.1-3 Approximately 87% of strokes are ischaemic, in which a blood vessel supplying the brain is obstructed.1 More than half of stroke survivors experience various degrees of impairments that affect their somatosensory function, movement, speech and/or cognitive function, establishing an unmet medical need. Therefore, the development of novel regenerative therapies outside the confines of conventional treatments (Box 1) is critical.4
Box 1. Current status of clinically approved stroke treatments.
The only US Food and Drug Administration (FDA)-approved treatments in stroke focus on re-establishing reperfusion of the occluded vessel to restore normal cerebral blood flow levels. To achieve recanalization, two distinct strategies are available: (i) mechanical clot retrieval via endovascular thrombectomy; and (ii) pharmacological thrombolysis using tissue plasminogen activator (t-PA). Although these approaches have shown substantial clinical trial success, only a small percentage of stroke patients benefit from them due to the narrow therapeutic window of 4.5-h after stroke onset. This leaves behind a substantial portion of untreated stroke patients with no medical therapy to promote repair and recovery. Long-term management of ischaemic stroke is often limited to monitoring vital parameters and standard emergency care, providing preventative measures such as antithrombotic treatment with aspirin or low-molecular-weight heparins.
During the chronic phase, stroke treatments involve long-term neurorehabilitation to prevent the development of further complications, such as progressive stiffening of the limbs, and stimulate neural plasticity. Despite advances in stroke patient care, rehabilitation remains insufficient to induce complete functional recovery. To date, no clinical trials have succeeded in alleviating stroke survivors’ neurological impairment.
Today, it is critical to develop new therapies that promote brain repair and functional recovery after stroke. Among all the therapeutic strategies currently in development, cell therapy has shown promise in enhancing brain repair in preclinical models. The crucial advantage of transplanted cells is their ability to activate multiple endogenous repair processes after stroke.
Stem cell therapy holds great promise for the treatment of many disorders of the CNS, including stroke.5-7 Although preclinical findings in animal models of stroke provide a large body of evidence for functional recovery (reviewed by Sandu et al.,8 Laso-García et al.9 and Zhang et al.10), clinical translation of cell-based treatments has proven to be challenging due to several reasons such as high cell mortality rate after transplantation, poor integration with the host tissue and lack of control over cell differentiation in the injured brain.11,12
Several cell types have been proposed to enhance post-ischaemic brain repair, including primary mesenchymal stem cells (MSCs), neural stem and progenitor cells (NSC/NPCs), glia and pericytes.13 Apart from MSCs (abundant and easily obtained from peripheral blood), cell grafts are commonly generated from patient or donor-induced pluripotent stem cells (iPSCs). Since iPSCs can be derived from blood cells, expanded to large quantities and differentiated into many cell types, they represent an ideal resource for therapeutic purposes with fewer ethical concerns than embryonic stem cells (ESCs). A detailed overview of the therapeutic potential of different cell sources for stroke has recently been reviewed elsewhere.5,14-17
Unlike single-target pharmaceuticals, cell-based therapies have pleiotropic effects as they can activate multiple cellular and molecular mechanisms in both the transplanted graft and the host tissue, which makes efforts to decipher stem cells’ mode of action exceptionally challenging. Cell therapies have been proposed to either contribute to brain repair through direct replacement of host brain cells and integration into the damaged brain circuits and/or by the secretion of pro-regenerative signalling molecules that stimulate endogenous regeneration.18-21 For instance, indirect effects of cell-based therapies include not only modulation of the inflammatory response to stroke injury22 and glial scar formation,18 but also stimulation of post-stroke angiogenesis,23-25 neurogenesis26-28 and blood–brain barrier (BBB) integrity.29-32
Here, we present a summary of recent progress in the development of stem cell therapies for long-term treatment of post-ischaemic stroke treatment, along with our current understanding of brain repair mechanisms by which cell grafts can enhance brain regeneration and functional recovery after stroke. The overall focus of this review is to critically appraise the therapeutic potentials of cell-based therapies for stroke recovery and to provide suggestions for future research direction.
Pathophysiology and brain repair after stroke
Ischaemic strokes are defined by the interruption of blood flow in a brain artery. Patients experiencing large vessel occlusions lose, on average, 120 million neurons, 830 billion synapses and 714 km of myelinated fibres, ageing the brain by 3.6 years per hour.33,34 Apart from neural injury, stroke causes a massive local and systemic inflammatory response and severe damage to components of the BBB. These detrimental events are followed by a neuroplasticity phase which activates endogenous mechanisms of repair and tissue remodelling in an attempt to regenerate the damaged tissue and restore the lost functions.
Molecular and cellular tissue damage after stroke
The abrupt reduction of blood flow during a stroke episode (below 20% of baseline blood flow levels35) causes immediate and irreversible neuronal damage characterized by hyperacute excitotoxic and necrotic cell death in the stroke core, known as infarct. Owing to its perfusion by collateral blood arteries, the region directly adjacent to the infarct, also called the penumbra, is an area of intermediate blood flow (below 30%–40% of baseline blood flow levels35) sufficient to maintain cellular integrity but not electric activity of neurons or their metabolic functions. Since collateral circulation is inadequate to maintain the neuronal demand for oxygen and glucose long-term, neurons in the penumbra die if reperfusion is not established during the early hours, spreading the necrotic core towards the healthy tissue.
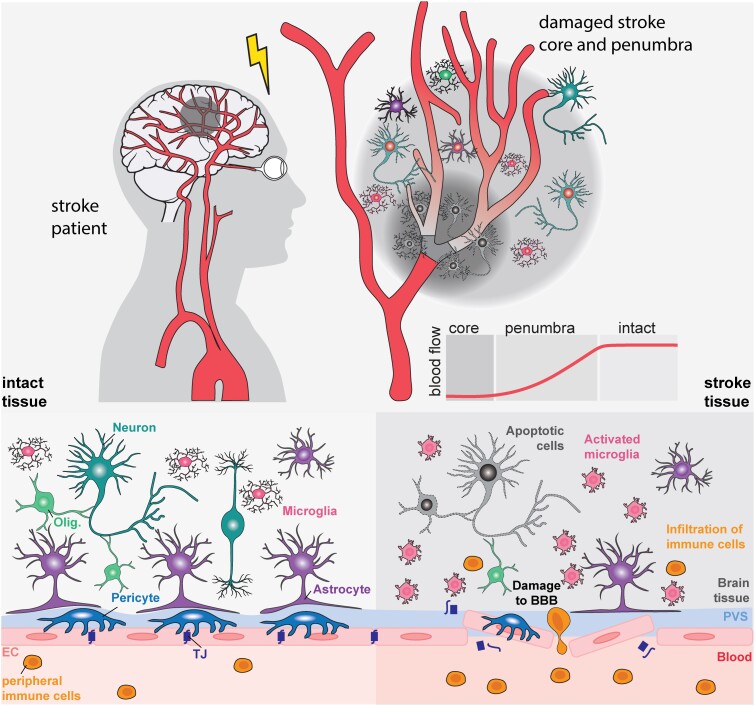
Interestingly, while neurons are very sensitive to hypoxia, non-neural cells like endothelial cells and pericytes are more resistant and can survive within the stroke-injured areas, even after a permanent ischaemic stroke.36 At the vascular level, stroke induces significant changes to endothelial cell receptor expression, matrix degradation, detachment of astrocyte end-feet,37 and pericytes,38,39 all of which lead to BBB damage within the first 30 min and up to weeks after stroke onset in preclinical animal models.37,40 The injury-induced vascular dysfunction is further aggravated during the acute phase by a series of cellular and molecular events. The acute secretion of the vascular endothelial growth factor (VEGF) in neurons, astrocytes and endothelial cells (ECs) of the penumbra can increase BBB permeability and elevate the risk of hemorrhagic transformation.41-43 High levels of VEGF lead to the formation of new, immature vessels that lack tight junction proteins and pericyte coverage.40,44,45 Activation of microglia, infiltration of peripheral immune cells through the opened BBB and release of pro-inflammatory cytokines (e.g. TNFα, IL1β, IL6, IFN-γ) and metalloproteases (MMPs) such as MMP-2 and MMP-9 further exacerbates vascular permeability, neuroinflammation and cell death.46-48 (Fig. 1).
Figure 1.
Pathophysiology of stroke. An ischaemic stroke usually occurs when a brain artery becomes blocked, leading to a reduction in oxygen and nutrients in the affected brain areas. These regions can be divided into: the stroke core, an area with severe cerebral blood flow reduction and irreversible necrotic cell death (<10 ml/100 g/min); the penumbra, an area with reversible damage (17–10 ml/100 g per min); and normal, intact tissue (>17 ml/100 g per min). Damage to stroke tissue includes necrotic and apoptotic cell death, damage to the blood–brain barrier, activation of resident microglia and infiltration of peripheral immune cells. EC = endothelial cell; olig = oligodendrocyte; TJ = tight junction.
Within days to weeks, the lesion site turns into an empty cavity devoid of structural features necessary for cell infiltration and tissue growth.25,49,50 The morphological remodelling of perilesional astrocytes into an astrocytic scar creates a physical barrier to tissue growth and compartmentalizes the cavity. Importantly, the length of the remodelling and involved processes can vary considerably depending on the sex, species and stroke size.4,51,52 This compartmentalized cavity can accept a large volume of transplant without further damaging the surrounding healthy parenchyma,53,54 which makes extracellular matrix (ECM)-mimetic engineered biomaterials55 and stem cell transplantation within the lesion site promising approaches for stroke treatment.56,57 The subacute phase after injury is also characterized by progressive injury affecting neurons that survived the initial injury and leads to axon and glial degeneration due to the altered energy metabolism in preclinical models.58,59 Moreover, a persistent secondary neurodegeneration process occurs, leading to neuronal loss, neuroinflammation and accumulation of neurotoxic proteins in distant brain regions that are anatomically connected to the infarct site but were initially unaffected by the cerebral blood flow (CBF) reduction from the stroke.60,61
This phase of acute cell death and tissue remodelling is followed by a phase of neuroplasticity and repair, during which neuronal and synaptic formation and remapping, gliogenesis, neurogenesis, vascular repair and restoration of the BBB happen. In patients, the neuroplasticity window is estimated to peak during the first 3 months62 and to weaken after 6–12 months after stroke.63,64 Although partial functional recovery in chronic stroke is possible,65 it often requires intensive rehabilitation therapy and predominantly occurs through compensatory processes rather than ‘true’ regeneration.66,67
Endogenous stroke recovery
Post-stroke neural plasticity, also referred to neuroplasticity, can be defined as the ability of the brain to rewire and reorganize its neural circuits in response to injury in an attempt to adapt to the structural, metabolic and cellular changes associated with the tissue and functional loss. Plasticity involves both the de novo creation and modifications of existing neurons, axons, synapses and neuronal connections, persist for several months after stroke and primarily occurs in the peri-lesional area that surrounds the infarct.68 During this phase, the transcriptional signature indicates an upregulation of genes and signalling pathways involved in axonal growth, vascular development, dendritic sprouting, synapse formation, adhesion molecules and cytoskeletal rearrangements in experimental rodent models of stroke.50,69-72
Neuroplasticity after stroke
Studies using rodent models of stroke indicate that genes associated with neuroplasticity, such as Srr1, Gap43, Cap23 and Marcks, c-Jun, and genes coding for neurotrophic factors, such as Ngf, Bdnf, Nt-3, Fgf-2, Igf-1 and Gdnf, are upregulated in sequential waves from Day 3 after injury.69,71,73 Subsequently, cell adhesion molecules and cytoskeletal reorganization-associated genes such as L1cam, Cdkn1a, Scg10 and Sclip are expressed. A solid body of evidence indicates that neuroplasticity is also associated with the reduction of growth inhibitory factors. The main axonal growth inhibitors in the adult and damaged CNS are myelin-associated proteins (e.g. Nogo-A/RNT4A, MAG) and axonal guidance molecules (e.g. netrins, ephrins, semaphorins). The regulation of these factors shows unique expression at different stages after stroke,50,71,74 and appears to be age-dependent.69,75 More recently some of the axonal growth inhibitors have been shown to be release and exhibit long-range signalling.76,77 Loss- and gain-of-function studies indicate that the inhibition of pro-repair genes and activation of inhibitory genes impair functional recovery in animal models of stroke.50,68,78-82
Recent studies have shown a high heterogeneity of neuroplasticity in different brain areas. In rodents, non-human primates and humans, stroke lesions in the sensorimotor cortex were found to induce substantial remapping of sensorimotor functions over a period of 8 weeks.4,83 As an example, the forelimb M1 area responds to stroke by inducing axonal sprouting from corticospinal fibres in the intact hindlimb M1 areas, converting a hindlimb neuron into a forelimb-projecting neuron. In addition, neurological functions from large infarcted areas can be recovered through unaffected regions of the contralateral hemisphere where sufficient capacity for structural remodelling is still present. Studies in both rodents and humans show that other brain circuits such as the ones in the somatosensory areas, may have weaker neuroplasticity potential with less remapping observed after stroke.84-86 Even within the motor cortex, experimental studies have shown that spontaneously recovered mice after stroke have a distinct ipsi- and contralesional motor cortex transcriptome to littermates that show no recovery involving Adora2a, Drd2 and Pde10a-mediated cyclic AMP (cAMP) signalling.73
The formation of new neurons, or neurogenesis, may also contribute to neuroplasticity and repair after stroke. Early experimental data suggests that NPCs from the subventricular zone can proliferate, migrate towards the lesion site and differentiate into mature neurons. Additionally, experimental stroke can activate NPCs within and around the ischaemic areas, extending beyond the conventional neurogenic zones like the subventricular zone and the dentate gyrus.87-89 A solid body of evidence shows that experimental enhancement of neurogenesis is associated with improved recovery,90-93 while its inhibition is linked to impaired recovery.93 Although NPCs have been well characterized throughout the years, their migration pattern from the neurogenic niche towards the lesion site is still poorly understood, as animal studies show that the route, distance and efficiency of migration are highly variable and depend on the severity, location and cellular activity of the lesion.90
Various other cell types play a significant role in stimulating neurogenesis and plasticity after injury, including immune and vascular cells.94,95 Experimental studies have shown that endothelial cells are capable of releasing growth factors that stimulate vascular growth and remodelling in the peri-infarct area,96,97 which in turn enhances NSC proliferation and migration towards the lesion site, while guiding their infiltration in the peri-infarct area49,98 to stimulate neuronal plasticity.98,99 In turn, NPCs increase angiogenesis via the secretion of VEGF,100 demonstrating a bidirectional relationship between vascular and neuronal remodelling.101,102
Other important cell types for plasticity are pericytes and glial cells. For instance, pericytes as part of the neurovascular unit have been shown to regulate angiogenesis, CBF and repairment of the BBB after stroke,103,104 and their absence leads to neurovascular uncoupling.105,106 On the other hand, a subset of pericytes, termed type A pericytes, has recently been described to form a fibrotic scar tissue inhibiting neural plasticity in several neurological injuries, including stroke.107,108
Astrocytes not only have the capability to eliminate neurotransmitters and other molecules associated with excitotoxicity but are also important in neurovascular coupling and enhancing synaptogenesis by promoting the formation of new functional synapses.109-111 Oligodendrocyte progenitors differentiate into mature oligodendrocytes, facilitating axon remyelination and improving white matter integrity,112,113 while microglia have been shown to reduce inflammation and release trophic factors such as BDNF, thereby promoting synaptogenesis.114
Despite the many advances made in recent years, the exact mechanism by which all these neuroplasticity processes interact with each other to promote the repair and recovery of lost functions is not fully elucidated. As an example, although neurogenesis was evidenced to contribute to healing in animal models, whether it can also contribute to stroke patients' functional recovery remains uncertain, as the vast majority of newly generated NPCs die within their migratory path before reaching the lesion site or integrating into pre-existing functional neuronal networks. Furthermore, adult neurogenesis is more robust in rodents, which have shorter turnover and maturation cycles, compared to primates, including humans.115 The age-dependent decline of adult neurogenesis in the mouse116 and human brain117,118 may further limit the therapeutic impact for stroke patients with an average age of 70 years.119 Overall, in humans, evidence of any functional contribution of adult neurogenesis to recovery remains limited and under-investigated.120-122
Such experimental uncertainties continue to hinder the development of clinically relevant solutions for stroke treatment. Over 50% of stroke survivors suffer from persistent motor and/or cognitive deficiencies that significantly affect their quality of life and increase their vulnerability to other comorbidities such as hypertension, cancer, diabetes and congestive heart failure.123 With the current therapeutic approach, most improvement occurs in the first few weeks post-stroke, with a plateau after 3–6 months with not much motor recovery subsequently. Cognitive dysfunction, including those of the language domains, can still achieve some improvements with training even at the chronic phase, after 6 months. The process of functional recovery involves not only the enlargement of dendritic trees and the creation of new connections by ipsilateral neurons but also the sprouting of contralateral cells to support the damaged brain area. However, such extreme responses can even be detrimental and hinder functional recovery.124 With the biology of recovery involving multiple interrelated pathways not yet fully known, the road toward a full recovery remains challenging; and newer approaches, including cell therapy, are essential to explore and advance the understanding and treatment of stroke recovery.
Vascular formation and repair of the blood–brain barrier
During the subacute phase after stroke, the vascular system and the associated components of the BBB and the peri-vascular ECM undergo extensive remodelling in the peri-lesional area. During this phenomenon, known as post-stroke angiogenesis, new blood vessels are formed, pericytes are recruited to the vessel wall and tight junctions are re-established to partially restore BBB integrity. Increasing evidence shows that the efficiency of post-stroke angiogenesis is age-dependent as EC proliferation and remodelling of brain vessels weakens with age in patients125 and rodents.75
Animal studies indicate that all major angiogenesis-associated signalling factors are transcriptionally upregulated after stroke, e.g. Vegfa, Vegfr2, Tie2, Angpt2, Pdgf, Cxcl1 and Pecam1.50,75 VEGF secretion and activation of its receptor VEGFR2 are required for post-stroke angiogenesis.49,50 Increased peri-lesional vascular growth has been shown to be associated with functional recovery in experimental and human stroke.49,50,126,127 As an example, increased CBF is correlated with the upregulation of the VEGF-eNOS-NO pathway and glucose intake in the peri-lesional area,128 VEGF expression is increased in the serum and the brain of stroke patients129,130 and increased microvascular density around the lesion is correlated with longer survival in patients.126,131
Cerebral arteries and capillaries are supported by smooth muscle cells and pericytes, which play a major role in the BBB permeability and integrity105,132,133 through their cellular crosstalk with ECs. During post-stroke angiogenesis, EC-secreted PDGFB binds to its receptor PDGFRB on the pericyte's surface, while pericyte-secreted Angpt1 binds to its receptor Tie2 on the EC's surface.134,135 Enhanced pericyte coverage of peri-lesional vascular walls has been shown to improve BBB integrity and contribute to tissue repair and functional recovery.49,50,136
Despite clear evidence of the role VEGF plays in brain repair and neuroplasticity, developing VEGF-based therapies remains challenging due to the effect of VEGF on BBB permeability. Indeed, multiple studies have shown that the brain injection of VEGF can worsen the inflammatory response to stroke by increasing the risk of haemorrhagic transformation in newly formed, immature vessels,43 known to have impaired tight junction protein and incomplete pericyte coverage.40,44,45 Indeed, sustained BBB permeability after stroke is correlated with worse neurological outcome in rodents and stroke patients.137 Adverse effects include enhanced peripheral immune cell infiltration, neurotoxicity and vasogenic oedema, which all lead to enhanced neuronal loss and infarct volume.138,139
Learning from clinical trials: efficacy and challenges of clinical cell therapy in stroke
Cell therapies have emerged as a promising therapeutic strategy for stroke and reached clinical testing two decades ago.11,12 Initially, immortalized neural cell lines, fetal cell sources and adult MSCs were used as a cell source (Fig. 2). Cell therapies for stroke are generally considered safe and a large body of evidence from preclinical and larger phase 2 and phase 3 clinical trials reported only sporadic adverse events and generally a good safety profile (Table 1 and Supplementary Table 1).18,29,142,146,151,152 Comprehensive reviews and meta-analyses on the clinical efficacy and safety of cell therapy for stroke have been discussed elsewhere.11,153-155
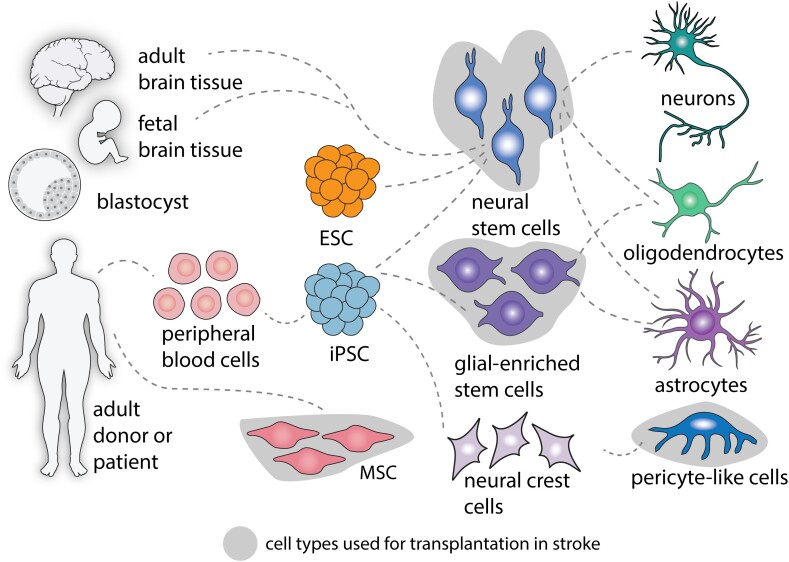
Figure 2.
Cell types for cell therapy after stroke. Cell transplants can be derived either from the embryonic/fetal stages or the adult human. Adult stem cells comprise mesenchymal stem cells (MSCs) or differentiated cells from the peripheral blood reprogrammed to generate induced pluripotent stem cells (iPSCs). Both embryonic stem cells (ESC) and iPSCs can be differentiated into neural or glial-enriched stem cells to generate neurons, oligodendrocytes or astrocytes. Pericyte-like cells can be generated from iPSCs through neural crest cell intermediates.
Table 1.
Selected recently completed clinical trials with results from using stem cells to treat stroke
| Route | Cell type | Cell origin | Cell dose | Transplanted patients, n | Control patients, n | Stroke phase | Adverse events | Major findings | Year |
|---|---|---|---|---|---|---|---|---|---|
| IV | BM multipotent adult progenitor cell (TREASURE)140 | Allogenic | 1.2 × 109 | 104 | 102 | Acute/subacute | No | No improvement in ‘excellent’ outcomes at 90 days compared with placebo measured in mRS, NHSS, Barthe index | 2024 |
| IV | BM-MSC141 | Autologous | – | 31 | 13 | Subacute | Not measured | Significant better FM and improved connectivity | 2022 |
| IV | BM-MSC142 | Autologous | – | 39 | 15 | Acute/subacute | No | No significant improvement in mRS, but better leg motor function | 2021 |
| IV | BM-MSC143 | Autologous | 100, 300 × 106 | 16 | 15 | Subacute | No | Improvement in motor-NIHSS, motor FM score and task-related fMRI. But no significant improvement in NIHSS, mRS. BI | 2020 |
| IC | NSC (cell line CTX0E03)144 (PISCES-II) | Allogenic | 2 × 107 | 21 | 0 | Subacute/chronic | Transient procedural events | Action research arm test improvement (only in patients with upper limb function) | 2020 |
| IV | BM-MSC145 | Allogenic | 0.5, 1, 1.5 × 106 | 15 + 21 | 0 | Chronic | Only mild adverse events possibly related to procedure | Improvement in BI, NIHSS compared to baseline | 2019 |
| IA | BM-aldehyde dehydrogenase bright stem cells (RECOVER)146 | Autologous | 3.08 × 106 | 60 | 40 | Subacute | Only mild/moderate adverse events | Demonstrated safety, no significant improvement in mRS, NIHSS, BI | 2019 |
| IC | Modified BM-MSC (SB623)147,148 | Allogenic | 2.5, 5, 10 × 106 | 18 | 0 | Chronic | Adverse events e.g. headache, caused by surgical procedure | ESS, NIHSS, FM, FMMS improvement. No difference in mRS | 2018 |
| IA | BM-MNC149 | Autologous | 5 × 108 | 10 | 10 | Subacute | No | Improving trends in NIHSS, mRS, BI compared to baseline | 2018 |
| IV | BM multipotent adult progenitor cell (MASTERS)150 | Allogenic | 400, 1200 × 106 | 67 | 62 | Acute | No | No significant improvement in NIHSS, mRS, BI | 2017 |
Stroke phase: acute, <7 days; subacute, 7 days–6 months; chronic, >6 months. AD-MSC = adipose-derived mesenchymal stem cells; BI = Barthel Index; BM-MNC = bone marrow mononuclear cells; BM-MSC = bone marrow mesenchymal stem cells; CD34+ HSC = CD34+ haematopoietic stem cells; ESS = European Stroke Scale; FM = Fugl-Meyer Assessment; FMMS = Fugl-Meyer Motor Score; IA = intra-arterial; IC = intracranial; ICV = intracerebroventricular; IT = intrathecal; IV = intravenous; mRS = modified Rankin Scale; NIHSS = National Institute of Health Stroke Scale; NSC = neural stem cells; UCB = umbilical cord blood.
Although many cell sources proved effective in preclinical models, their subsequent translation to clinical applications has been met with inconsistent outcomes. Within the clinical landscape, challenges included limited efficacy, inefficient delivery methods, poor graft survival and differentiation rate and/or ethical concerns.13 Recent advances in genetic cell manipulation and the iPSC technology have enhanced the potential and applications of cell therapy.156 Cell sources for iPSCs can be isolated from donors, expanded indefinitely and differentiated into well-characterized cell types such as NSCs and glia (Fig. 2).
As an example, iPSC-derived dopaminergic precursor cells and iPSC-derived retinal epithelial cells were found to be safe and effective in treating Parkinson's disease157,158 and age-related macular degeneration.159,160 In stroke research, there is a preferential use of iPSC-derived cell sources in preclinical studies18,29,152,161 that has not yet been translated into clinical applications. The majority of clinical trials instead used primary MSCs as a cell source, especially when choosing a systemic route of administration in acute stroke patients. Immortalized NSC cell lines were primarily administered directly into the brain, mostly in chronic stroke patients.144,162,163
Over the past 5 years, a limited number of smaller studies have demonstrated that the application of MSCs via intravenous or intraarterial administration leads to notable improvements in the National Institutes of Health Stroke Scale (NHSS), modified Rankin Scale (mRS) and/or Barthel Index (BI) when compared to control groups of stroke patients in the acute/subacute141,143,145,147,149,164 and chronic phase147,148 post stroke. The efficacy of MSCs has only been partially confirmed by a more extensive phase 3 randomized controlled trial, STARTING-2, enrolling 60 stroke patients. Intravenous application of MSCs led to diminished corticospinal tract degeneration and enhanced neural network connectivity, as assessed through neuroimaging, along with improved lower extremity motor function according to the Fugl-Meyer Assessment.141 However, the primary outcome in STARTING-2, as measured by mRS after 90 days, reported no significant differences between the groups.142 No significant functional improvement was observed in a more recent randomized, controlled, multicentre trial (IBIS trial) that recruited 77 patients. The intraarterial transplantation of autologous bone marrow mononuclear cells in stroke patients at 180 days showed no improvement in mRS scores.151 Parallel results were seen using allogenic bone marrow-derived adult progenitor cells, specifically MultiStem. In the phase 2, multicentre, double-blind, randomized and controlled MASTERS trial, intravenous MultiStem administration 24–48 h post-stroke failed to enhance the neurological outcome at 90 days.150 However, the follow-up trials MASTERS-2 (a phase-3 study recruiting 300 patients) and TREASURE (a phase 2/3 study that exclusively recruited 206 patients from Japan165) are currently exploring the impact of the same MultiStem cell source but administered within a tighter window of 18–36 h after stroke. The TREASURE trial recently reported enhanced functional outcomes in an exploratory subgroup analysis with no corrections for multiple comparisons in the mRS and BI.166 However, the trial did not meet its primary end point of achieving an ‘excellent outcome’—characterized by combined improvements in mRS, NIHSS and BI scores—at the 90-day mark.140 The MASTERS-2 trial is still in the recruitment phase and has not yet been finalized. As of now, no detailed data from the MASTERS-2 trials have been released for peer-reviewed publication.
Besides MSCs, a genetically modified neural stem cell line, CTX0E03, has been tested in chronic stroke patients through the PISCES series of trials. Both PISCES-I and PISCES-II involved the administration of CTX0E03 cells to chronic stroke patients via stereotactic injection.144,163 The larger PISCES-II trial, a phase 2a, multicentre trial, administered 20 million cells to 23 patients, resulting in improved upper limb function at 3, 6 and 12 month intervals.144 However, the subsequent randomized, controlled phase 2b trial, PISCES-III, began but was prematurely terminated, yielding no results.167
An overview of major clinical trials with results using stem cells for stroke is summarized in Table 1 and Supplementary Table 1. Comprehensive analysis and excellent reviews on the efficacy of cell therapies for stroke in clinical trials have been performed in recent studies.154,155,168,169
Despite the large body of preclinical and clinical data, only a small fraction of studies looked into the mechanistic insights and molecular crosstalk between grafted stem cells and stroke-injured brains.170,171 Clinical trials have produced mixed outcomes so far. This suggests that a more comprehensive understanding of the underlying mechanisms could refine various aspects of cell therapies. Two general beneficial mechanisms have been suggested for cell therapies in stroke. The first mechanism is the direct cell replacement that allows grafted cells to differentiate into mature neurons or glia and functionally integrate into damaged host's brain circuits. Depending on the cell source, the graft homing can directly contribute to e.g. restoration of brain function, BBB integrity or CBF in the damaged regions. Second, grafted cells may contribute indirectly through e.g. supportive release of pro-regenerative factors that enhance or prolong endogenous regeneration programs and ultimately lead to improved recovery.10,172
Mechanistic insights from preclinical studies on cell therapy for stroke
Cell replacement after stroke
Multiple animal studies have investigated the integration of NPC grafts into the host circuits through viral-assisted tracing of their synaptic connections,173 the monosynaptic inputs from grafted neurons,173,174 or through the inhibition of their neuronal activity (using chemogenetic or light-induced inhibition), which results in partial impairment of gained motor recovery.18,25,173 When transplanted into the damaged visual cortex, neural grafts exhibited functional calcium (Ca2+) activity in response to visual stimuli175 and, similarly, showed responses to physiological sensory stimuli when grafted in the somatosensory cortex.174 Both studies demonstrate the successful functional integration of grafted neurons.
Enhanced functional integration of iPSC-NPCs grafts has recently been shown by optochemogenetic activation. After transplanting genetically modified iPSC-NPCs with the optochemogenetics fusion protein (LMO3) into the post-stroke brains of young and old mice, repeated stimulation improved graft integration in the peri-infarct regions and fostered recovery.176 Earlier mechanistic studies have shown that an important pathway guiding migration and integration is CXCR4/SDF1-a. CXCR4-expressing NSC migrate/move towards astrocytic and endothelial SDF-1a expression that is upregulated within the injured brain regions.177 Newer studies have also found that CXCR4 genetic up-regulation, preconditioning of cell grafts and loss of LRP1, a regulator of CXCR4 can further enhance the homing process towards the stroke area.178-180
Direct functional replacements in cell therapy have also been investigated for non-neural cells. In contrast to NPC grafts, genetic ablation (induction of cell death after transplantation using diphtheria toxin18,25) of glial-enriched progenitor cells did not contribute to loss of functional benefits after transplantation in a rodent model of white matter stroke, suggesting that these cells promote recovery through an indirect mechanism.18 Transplanted iPSC-derived pericyte-like cells were localized within the vasculature in the ischaemic border zone and were associated with reduced BBB opening and improved functional recovery.29 In addition, multiple studies suggest that, although MSCs can survive in the brain over 2 weeks post-transplantation, they likely do not have the ability to transdifferentiate to neural lineages in vivo,181 despite conflicting in vivo studies in which unspecific uptake of fluorescent and DNA tracers were found in astrocytes and neurons.182
The extent of direct cell replacement depends not only on the selected cell source but may also be considerably influenced by the timing of cell therapy. Generally, it is believed that the capacity to integrate new cells into a damaged circuitry is lower in chronic stroke due to the substantial remodelling in the transition from the acute to the chronic phase of stroke.183
One of the most important limitations of direct cell replacement lies in the short survival of newly generated neurons that are thought to protect the infarcted brain by releasing factors rather than by replacing lost tissue. Survival of transplanted cells is usually not an outcome of clinical trials limiting our knowledge. Also, heterogeneity in differentiation capacity is reported depending on tissue sources and donor characteristics (i.e. age, gender, comorbidities), which may hamper the functional properties of MSCs. Preclinical evidence suggests a role for pro-survival treatment in this field, including preconditioning, pretreatment or overexpression of antiapoptotic, neurotrophic and homing mediators.13,156 Also, to improve survival and facilitate replacement, microcarriers have been successfully used in preclinical studies. As compared to cell culture media or saline, hydrogels or scaffolds protect cells from the hostile stroke environment and carries neurotrophic and protective factors, further improving graft survival.184-187 Lastly, the use of structured tissue (i.e. brain organoids) rather than dissociated cells may offer cell-cell and cell-matrix protection and improve graft survival and vascularization after transplantation.161,188-190
Indirect beneficial effects of cell therapies
Although cellular integration into the host tissue is possible, the majority of cell transplantation studies in stroke have found that the beneficial effects on repair and recovery are stimulated through indirect mechanisms that include immunomodulation, release of pro-regenerative factors that stimulate vascular and neural growth and ECM remodelling in the peri-infarct tissue, all of which activate the endogenous repair machinery of the brain and promote repair and recovery after stroke (Fig. 3). The main findings from preclinical studies that have used cell therapy for stroke treatments are summarized in Table 2 and Supplementary Table 2.
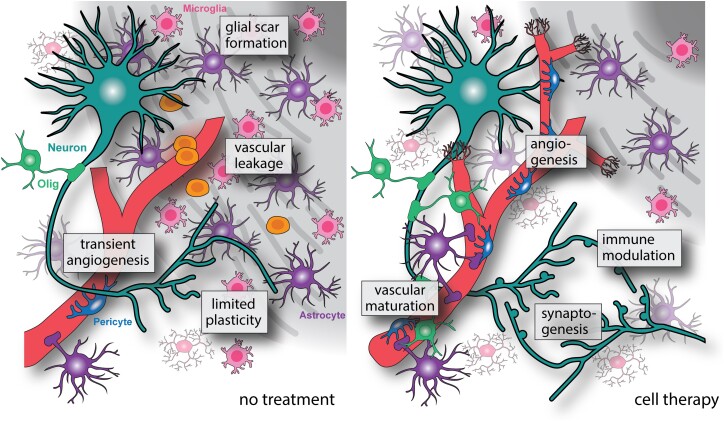
Figure 3.
Indirect mechanisms of cell therapy for brain repair. Indirect effects of cell therapy include improved vascular repair and maturation through the release of pro-angiogenic factors in the peri-infarct region. Immunomodulation and an increased anti-inflammatory signature reduce secondary damage and decrease glial scar formation. The release of neurotrophic factors by cell grafts also increases axonogenesis and synaptogenesis in damaged areas.
Table 2.
Selected recent preclinical studies using cell therapy for stroke
| Route | Cell source | Ischaemia | Species | Animals, n | Sex | Stroke phase | Dose | Major findings | Year |
|---|---|---|---|---|---|---|---|---|---|
| IP | iPSC-NSC171 | Permanent | Mouse | 40 | Male and female | Chronic | 2.5 × 105 | Improved angiogenesis, reduced inflammation, axonogenesis, reduced BBB leakage, improved functional outcome | 2024 |
| IP | iPSC-organoids161 | Permanent injury on visual cortex | Rat | 36 | Male | Chronic | Organoids | Survival, differentiation, neuronal projections to visual system and spontaneous and visually-evoked neural activity of engrafted organoid cells (GFP, IHC, rabies virus retrograde tracing, herpes simplex virus anterograde tracing, electrophysiological recordings) | 2023 |
| IP | Human brain-derived iSCs191 | Permanent | Mouse | 118 | N/A | Chronic | 1.0 × 104 | Improved behavioural recovery (wire hang, basket, open-field, hot plate, Y-maze, passive avoidance-learning, sucrose preference and tail suspension tasks); homing and differentiation of engrafted cells (GFP, IHC); activation of endogenous NSCs (IHC) | 2023 |
| 1.0 × 105 | |||||||||
| IP | NSC19 | Temporary | Mouse | 75 | Male | Subacute | 1.0 × 105 | Reduced infarct size (TTC); reduced inflammation TNFα, IL6, IL1β, ICAM1 and extracellular matrix disassembly MMP3, MMP9 (PCR); brain transcriptome (bulk RNAseq) | 2022 |
| IP | Glia-enriched progenitors18 | Temporary | Mouse | 114 | Male | Acute–chronic | 0.9 × 105 | Reduced infarct size (BDA); improved behavioural recovery (grid walking, cylinder test, novel object recognition, fear conditioning); homing, survival, proliferation and differentiation of engrafted cells (GFP, IHC); improved axon growth and connection (BDA, IHC); improved remyelination and oligodendrocyte proliferation (IHC) | 2021 |
| IP | Control and p5-primed ADMSCs23 | Temporary | Rat | 40 | Male | – | 1.0 × 106 | No change in infarct volume (IHC NeuN); improved behavioural recovery (beam walking, sticky-tape, cylinder test, water maze); reduced microglia apoptosis (IHC, p5-ADMSCs only); increased angiogenesis (IHC); survival of engrafted cells (IHC) |
2021 |
| IA | MSCs20 | Temporary | Rat | 30 | Female | Acute | 1.0 × 105 | Reduced infarct volume (TTC); improved behavioural recovery (NDS, Rotarod); reduced brain inflammasome activity caspase 1, ASC, IL1β, ASIC1a, NLRP1, NLRP3 (WB) | 2021 |
| IV | hPSC-pericytes29 | Temporary | Mouse | 110 | Male | Subacute | 1.0 × 106 | Reduced infarct size (TTC); improved behavioural recovery (NDS, corner test, sticky-tape, Rotarod); reduced neuron apoptosis (TUNEL, IHC); improved BBB (water content, permeability assay, TEM, WB, IHC); homing, vessel investment and pericytic differentiation of engrafted cells (DsRedE2, IHC); greater benefits by hPSC-PC than fibroblast, similar to brain pericyte; the role of midline in neuroprotection by hPSC-PC | 2020 |
Stroke phase at time point of cell transplantation: acute, <1 day; subacute, 1 day–7 days; chronic, >7 days. Animal numbers in some studies were estimated based on the methods section and figure legends. ADMSCs = adipose-derived mesenchymal stem cell; BBB = blood–brain barrier; BDA = biotinylated dextran amine; fMRI = functional MRI; IA = intra-arterial; IP = intraparenchymal; IHC = immunohistochemistry; iPSCs = Induced pluripotent stem cells; iSC = induced multipotent stem cells; IV = intravenous; MSCs = marrow stromal cells; NDS = neurologic deficit score; NPCs = neural progenitor cells; NSCs = neural stem cells; TTC = 5-triphenyltetrazolium chloride staining; WB = western blot.
Immunomodulation
Although acute inflammatory responses post-stroke can also be beneficial,137 as they facilitate tissue repair by secreting neurotrophic factors and clearing debris, persistent inflammation can contribute to secondary injury to viable tissue by releasing pro-inflammatory cytokines and other cytotoxic products.48,192,193 Besides the classical pro-inflammatory M1 and anti-inflammatory M2 classification, transcriptomic profiling of microglia has revealed a dynamic range of activation states, including mixed phenotypes, following stroke.70,193,194
Experimental studies have found that transplantation of MSC, iPSC and ESC-derived NSC results in gene and protein upregulation of anti-inflammatory cytokines such as IL-10 and reduction of pro-inflammatory cytokines including TNFα and IFN-γ,19,32,195,196 both of which have been linked to improved stroke outcome.19-21,171,197,198 Furthermore, various cell grafts have been shown to release neurotrophic factors such as NGF, GDNF and BDNF, known to inhibit T-cell activation and leucocyte infiltration, which overall modulate the immune response to injury.27,199 Transplanted MSCs were also associated with enhanced TGF-β expression, which inhibits MCP-1 secretion and limits CD68+ macrophage infiltration into the lesion.27,200 Transplanted NSCs have also been shown to reduce chronic CNS inflammation by reducing succinate levels in the CSF and decreasing immune cell infiltration and secondary damage.201 Reduced post-stroke inflammation is associated with reduced infarct volume and improved recovery.19,21,24,26 Similarly, comparable anti-inflammatory effects were observed when delivering stem cell-derived extracellular vesicles containing anti-inflammatory cytokines.202-205
A very recent alternative theory, termed the ‘bioreactor’ hypothesis, attempts to determine how systemically delivered cells induce immunomodulatory effects without integrating the brain.181 This theory lies in the cells’ ability to migrate and integrate peripheral organs such as lungs, spleen and other lymphoid tissue, interact with the host's immune cells and generate an anti-inflammatory environment that is amenable for tissue remodelling and repair in the injured brain.181 In experimental stroke research, pioneering studies demonstrated functional transplantation of primary human umbilical cord blood without graft entry into the CNS, indicating an alternative peripheral recovery mechanism, such as migration towards the spleen.206 More recent experimental stroke studies confirmed that multipotent adult progenitor cells improve post-stroke recovery by elevating anti-inflammatory cytokines such as Il-10 and increase the presence of regulatory T cells.207,208 Interestingly, the removal of spleens mitigated the effect of cell therapy in stroked rats, indicating the spleen's essential role in these therapeutic responses.207 Also, inflammation in other peripheral tissues, such as the gut, can regulate T-cell mediated inflammation and recovery after stroke.209
Vascular repair and remodelling
In recent years, a solid body of evidence from both preclinical and human studies has shown that post-stroke angiogenesis plays a major role in activating endogenous repair mechanisms and functional recovery after injury,43,102 and its inhibition is systematically associated with delayed or worsened repair and recovery.49,50 Once delivered into the injured brain, transplanted stem cells, including MSCs and NSCs, are known to produce pro-angiogenic factors such as VEGF, ANG1, FGF2, PDGFA and HIF-1a, resulting in increased vascular density and expression of angiogenic receptors in the peri-infarct tissue.23-25,28,195 Cell-mediated angiogenic responses are largely induced by the expression of VEGF by the transplants or by the tissue directly adjacent to the graft.210 Ang1 signalling in MSCs cell grafts was found to activate angiogenesis and CBF after stroke,211 and increased FGF2 levels were found to induce endothelial and pericyte proliferation and migration in the peri-infarct area after experimental stroke.212 Mechanistically, transplanted bone-marrow mononuclear cell grafts have been shown to activate endogenous angiogenesis by transferring small molecules including glucose via gap junctions, thereby providing metabolic support to the stroke-injured tissue.213,214
Functional vascular repair also requires the re-establishment of the neurovascular unit to restore the BBB integrity. This is clearly illustrated in studies in which preservation and restoration of the BBB via NSC transplantation is associated with increased expression of tight junction proteins (claudin-5, occludin, ZO-1) and dystroglycan, decreased BBB leakage171,210 and reduced stroke volume and neurological deficits after stroke.171,199,215 In addition, studies investigating the effect of MSC brain injection found a direct correlation with the inhibition of the vascular key adhesion molecule ICAM-1, known to play a major role in the transmigration of peripheral immune cells, through the AMP-activated protein kinase (AMPK) pathway.199 Transplantation of MSCs also reduced MMP-9 activity (but not the MMP-2 activity) and BBB leakage in stroked mice.199,216 Studies on MSC-derived extracellular vesicles (EVs) showed comparable angiogenic and neuroprotective effects to transplanted cell grafts.202,217 One suggested mechanism is that EVs can promote angiogenesis after experimental stroke by inhibiting autophagy through STAT3 activation.217 Similar results were also found in animal studies of spinal cord injury, in which MSC-derived EVs were associated with a reduction in pericyte detachment from capillaries through inactivation of the NF-kB pathway.218
Glia and glial scar formation
The glial scar forms a major physical barrier to axonal infiltration at the lesion site and limits the regenerative process. Reduced glial scar formation has been associated with improved stroke recovery49,50 and beneficial effects following post-stroke cell therapy.219 While the pharmacological degradation of glial scar components (such as chondroitin sulfate proteoglycans) prior to NSC transplantation enhances recovery following CNS injury,220 the presence of the scar remains necessary as its absence reduces MSC graft integration and beneficial effects on functional outcomes.221 Molecular studies point out astrocyte heterogeneity, indicating inflammatory states that can differentially regulate axonal sprouting and support the neuroinflammatory response to injury.222,223
Earlier preclinical studies in spinal cord injury and stroke animal models suggest that endogenous NSCs from neurogenic niches play a major role in producing astrocytes that contribute to scar formation through a Notch /Thrombospondin-4 (Thbs4) signalling mechanism.224,225 The inhibition of NSC proliferation, blockage of Notch signalling and deficiency in Nuclear factor I (a regulator of Thbs4) were associated with impaired glial scar formation, increased neuronal apoptosis, BBB defects and increased haemorrhagic transformation.224,225 Transplantation of CDK5-knockdown astrocytes in an experimental global cerebral ischaemia model showed recovery of the neurovascular unit integrity by stimulating the production of endogenous BDNF and improving functional recovery.226 Furthermore, the direct lesion environment may influence the therapeutic effect of astrocytes as NSCs transplanted in close proximity to the lesion preferentially differentiate towards a naturally occurring repair astroglial phenotype.227
Glial cells can further contribute to the proliferation and differentiation of oligodendrocyte progenitor cells (OPCs) to myelinating oligodendrocytes. One underlying mechanism is through astrocytic release of TIMP-1 and modulation of the surrounding ECM.228 In a subcortical white matter stroke model, transplanted glial progenitor cells promoted endogenous OPC proliferation and axonal sprouting that led to improved motor and cognitive recovery.18 Furthermore, local stereotaxic transplantation of OPCs into a stroke model reduced infarct volume and protected the BBB via Wnt/β-catenin signalling acutely after stroke.229
Neural repair
Indirect repair of neural circuits can occur through several mechanisms after cell therapy. Transplanted MSCs and NSCs have shown to induce endogenous neurogenesis and gliogenesis.191,230 For instance, the migration and differentiation of endogenous cells are enhanced following MSC cell therapy via the PI3K-Akt signalling pathway.231 The release of general chemokines such as SDF-1 and NRG1 was shown to increase the proliferation of endogenous NPCs.21 Furthermore, there is accumulating evidence that many signalling pathways overlap between angiogenic and neurogenic processes.50,102 For instance, the pro-angiogenic factor VEGF also regulates neurogenesis and survival of neural progenitor cells after stroke,49 and axon guidance molecules such as Nogo-A and Netrin 1 have been shown to be important for post-stroke angiogenesis,50,232-234 as guidance cues to direct newly formed NPCs and newly formed neurons towards the injury.
To improve engrafted cell survival and homing, NSC can be preconditioned or engineered to overexpress factors that are neurotrophic, antiapoptotic, improve differentiation, or facilitate revascularization. For instance, BDNF pretreatment of transplanted NSCs reduced stroke size and improved sensorimotor recovery after stroke,235 overexpression of neurotrophin-3 in NSC improves survival and reduces glial activation and results in smaller brain lesion volume.236 GDNF overexpressing NSCs promote neuronal survival and migration of transplanted cells by improving the injury-microenvironment.237,238 Over-expression of angiogenic factors such as VEGF, HIF-1a and angiopoietin in NSCs improve functional recovery by balancing the proapoptotic environment and facilitating angiogenesis.239 Other approaches to reducing graft apoptosis by overexpressing Akt, Bcl-2 or survivin have also proved effective at the experimental level.156,240
Molecular crosstalk between grafted stem cells and stroked brain tissue
Advances in transcriptomic technologies now allow the identification of molecular interactions between grafted cells and the stroke-injured host tissue. A recent study showed that transplantation of iPSC-derived NSC promotes long-term functional recovery after cerebral ischaemia.171 Single-cell profiling of grafted cells revealed that the graft primarily differentiated towards GABAergic-like cells and communicated with host tissue via regeneration-associated pathways such as Neurexin (NRXN), Neuregulin (NRG), Neural Cell Adhesion Molecule (NCAM) and SLIT signalling pathways.171
To further understand the molecular crosstalk and identify paracrine factors between graft and host after post-stroke cell therapy, a recent molecular study used translating ribosome affinity purification (TRAP) sequencing to separate and identify both transcriptomes 7 days post stroke.170 As expected, most enriched pathways from host tissue were associated with stroke, immune response, cell death and brain plasticity. Analysis of the graft transcriptome revealed more than 400 differentially expressed genes from NSC grafted in stroke-injured versus naïve brains. These genes were enriched in pathways linked to cell differentiation, neurogenesis, gliogenesis, synaptic plasticity and lipid metabolism,170 confirming findings from experimental cell therapy studies for stroke.18,19,26,219,241 The study also confirms the importance of different microenvironments after stroke depending on the proximity to the core.227
The interaction of secreted factors and identification of canonical pathways between graft and host revealed molecular candidates such as BMP6 from the stroked brain induce canonical BMP signalling in the graft and hNSCs secrete noggin, a factor that has been shown in earlier studies to be important for stroke brain recovery.242
Current challenges and prospective strategies for the clinical implementation of cell therapy in stroke
Cell therapies have been recognized by the Stroke Treatment Academic Industry Roundtable (STAIR) as one of the most promising approaches to improving stroke outcome.243 However, several limitations have also been recognized to hinder the translation of cell therapy for stroke into clinical practice, including age,8 comorbidities244,245 and medications,246 which are mostly not accounted for in preclinical trials. This disparity in the design of preclinical and clinical studies may contribute to the limited efficacy of cell therapies in stroke patients.247 To address these limitations and propose future research directions, guidelines were proposed by academic and industry experts in the field during Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS) meetings.248-250 These guidelines recommend, for example, the use of preclinical models that reflect the targeted patient population (sex, stroke model, age, disease comorbidities) and the combination of cell therapies with medications predominantly used by stroke patients (antiplatelets, antihypertensives, statins).250 For instance, the therapeutic effects of stem cells on stroke in diabetic rodents can vary considerably compared to non-diabetic rodents, potentially leading to adverse outcomes, including haemorrhage and BBB leakage.244,251
Furthermore, the majority of preclinical studies use transient models of stroke, which mimic the recanalization therapy in patients.252 However, many patients do not receive recanalization therapy due to the short therapeutic window or contraindications. Additionally, the success rate of recanalization therapy in removing occlusions is only 6–30%,253 suggesting that permanent occlusion models might better reflect the clinical scenario.
Inconsistent outcomes from preclinical and clinical trials can also be attributed to the functional outcome measures, as recognized by the STEPS consortium.250 Functional tests in rodents should be highly sensitive to detect long-term impairments and include tasks such as reaching, foot fault tests or deep-learning assisted gait analysis.62,254,255 Simpler tasks are often useful to identify acute stroke or exclude animals but may not be appropriate to quantify long-term therapeutic efficacy. Similar challenges are also present in clinical settings. The commonly used mRS score is a relatively gross measurement256 that may not be able to detect meaningful improvements, especially in small trials with a heterogeneous cohort of stroke patients. New concepts for parallelizing preclinical and clinical trial designs have been proposed by the STEPS consortium to accelerate clinical translation. In these studies, preclinical efficacy results can be tested in early-stage safety clinical trials. Next, information about the target patient population can then be incorporated into more advanced confirmative preclinical efficacy studies, which are followed by larger clinical efficacy trials.250 Additionally, beyond outcome measures, future trials may support their findings with recent advancements in fluid biomarkers.130,257-259
As the pathology of ischaemic stroke is very heterogeneous, the potential of different cell sources may strongly depend on the stroke subtype. Glial-enriched cells are likely more suitable for white matter strokes, whereas NPCs have been preferentially tested in cortical strokes mainly affecting the grey matter.18 The delivery route of cell grafts can occur either by local brain transplantations directly to the injury site or through a systemic blood injection. While during acute stroke there is evidence that the disrupted BBB may allow more effective migration of cell grafts to the brain,260 chronic strokes likely require a local transplantation to ensure delivery of cell grafts in sufficient quantities.
In parallel, recent advancements in the genetic engineering of cell grafts may facilitate more efficient migration across the restored BBB by overexpressing surface peptides known to facilitate the migration of peripheral immune cells to the brain.156 Cell graft delivery could also be further improved using recently developed self-assembling microcarriers based on microbubble propulsion.261,262 If cell grafts are not derived from the patient, the immune incompatibility with the recipient represents another limitation 0.263 To avoid graft rejection, long-term treatment with immunosuppressive drugs is usually required for successful allogenic cell therapies.158 However, new strategies are developing to enhance the immunocompatibility of cell grafts by genetic modification of human leukocyte antigen (HLA) genes to create ‘universal’ or HLA-matched cell lines and the generation of large iPSC biobanks.264-266
One of the biggest clinical concerns is likely the undesired transformation of transplanted stem cells into malignant tumours. Therefore, establishing a cell safety system to selectively eliminate the cell grafts following undesired transformation must be considered.267 For instance, a commonly used approach is the expression of herpes simplex virus thymidine kinase and treatment with the prodrug ganciclovir, which has been shown to selectively remove grafted cells in preclinical and clinical settings.156,268 Alternatively, the knock-in of inducible caspase 9 (iCasp9) has been successfully implemented in preclinical and clinical studies. Upon administration of chemical inducer AP1903, iCasp9 becomes activated, leading to apoptotic signalling and subsequent apoptosis of the transduced cell graft.156,268
In future clinical trials involving cell grafts for stroke, iPSC-based cells are likely to become the preferred source. This is due to their easy accessibility, expandability and ability to give rise to customized cell types, as has been demonstrated in recent studies on other neurological and ophthalmological diseases.159,269 To address the logistical, regulatory and cost challenges associated with using autologous iPSCs, allogenic cell sources that have undergone in-depth HLA characterization and are stored in a biobank are likely to offer a more practical solution. This approach could make off-the-shelf cell products more readily available to a broader population.270 The scope of HLA coverage may further be increased by using genetically engineered, universal iPSCs that facilitate immune evasion.264,266 These modifications will likely require safety measures for iPSC-therapies, which could be implemented using genetic safety switches, a method already applied, e.g. in clinical T-cell therapies for lymphoma.271 If the anticipated cell therapeutic mechanism relies on the bystander effect, current clinical trials, such as MASTERS, suggest that systemic transplantation of cell grafts should ideally occur within the first 36 h after stroke onset.150 However, clinical data from the PISCES trial series indicate that neural cell sources could also be beneficial for chronic stroke patients when locally transplanted into the brain.144 Looking forward, local transplantation might not even be necessary if newly developed cell graft delivery systems like genetic brain shuttles156 or navigating microrobots261,262 prove effective in guiding cell sources to injury sites. These advancements may also facilitate the translation of stem cell therapies for a broader range of neurological disorders involving neuronal loss.183,272,273
Conclusion
Cell therapy remains a promising regenerative strategy for stroke. The multimodal effects of cell grafts have great potential to improve long-term recovery in stroke patients. Despite this potential, the varied outcomes of existing clinical trials highlight the importance of a more comprehensive mechanistic understanding. Recent advancements in preclinical research offer insights into the molecular interactions between cell grafts and the host at a single-cell level. Other preclinical work indicates how genetic engineering of cell grafts can improve current clinical challenges such as immunocompatibility, graft delivery and safety of cell therapies. An increasing understanding of the underlying therapeutic mechanisms will also help to identify the ideal cell sources and tailor customized therapies to stroke patients. Future clinical trials may implement these recent preclinical advancements and investigate whether they produce more consistent beneficial effects, paving the way for the integration of cell-based therapies into standard stroke treatments in the future.
Supplementary Material
Acknowledgements
We apologize to all authors whose important and relevant work we could not include due to the word and citation limitations.
Contributor Information
Ruslan Rust, Department of Physiology and Neuroscience, University of Southern California, Los Angeles, CA 90033, USA; Zilkha Neurogenetic Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA; Institute for Regenerative Medicine, University of Zurich, 8952 Schlieren, Switzerland.
Lina R Nih, Department of Brain Health, University of Nevada, Las Vegas, NV 89154, USA.
Luca Liberale, Department of Internal Medicine, University of Genoa, 16132 Genova, Italy; IRCCS Ospedale Policlinico San Martino, 16132 Genova, Italy.
Hao Yin, Robarts Research Institute, Schulich School of Medicine and Dentistry, Western University, London, ON N6A 3K7, Canada.
Mohamad El Amki, Department of Neurology, University Hospital and University of Zurich, 8091 Zurich, Switzerland.
Lin Kooi Ong, School of Health and Medical Sciences & Centre for Health Research, University of Southern Queensland, Toowoomba, QLD 4350, Australia.
Berislav V Zlokovic, Department of Physiology and Neuroscience, University of Southern California, Los Angeles, CA 90033, USA; Zilkha Neurogenetic Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA.
Funding
The authors acknowledge funding from the Mäxi-Stiftung Foundation, Swiss 3R Competence Centre (OC-2020-002) and the Swiss National Science Foundation (CRSK-3_195902) and (PZ00P3_216225).
Competing interests
L.L. is coinventor on the International Patent WO/2020/226993 filed in April 2020; the patent relates to the use of antibodies which specifically bind interleukin-1a to reduce various sequelae of ischemia-reperfusion injury to the central nervous system.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Tsao CW, Aday AW, Almarzooq ZI, et al. . Heart disease and stroke statistics-2023 update: A report from the American Heart Association. Circulation. 2023;147:e93–e621. [DOI] [PubMed] [Google Scholar]
- 2. Pu L, Wang L, Zhang R, Zhao T, Jiang Y, Han L. Projected global trends in ischemic stroke incidence, deaths and disability-adjusted life years from 2020 to 2030. Stroke. 2023;54:1330–1339. [DOI] [PubMed] [Google Scholar]
- 3. Feigin VL, Stark BA, Johnson CO, et al. . Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20:795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cirillo C, Brihmat N, Castel-Lacanal E, et al. . Post-stroke remodeling processes in animal models and humans. J Cereb Blood Flow Metab. 2020;40:3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kokaia Z, Llorente IL, Carmichael ST. Customized brain cells for stroke patients using pluripotent stem cells. Stroke. 2018;49:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parmar M, Grealish S, Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat Rev Neurosci. 2020;21:103–115. [DOI] [PubMed] [Google Scholar]
- 7. Genchi A, Brambilla E, Sangalli F, et al. . Neural stem cell transplantation in patients with progressive multiple sclerosis: An open-label, phase 1 study. Nat Med. 2023;29:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sandu RE, Balseanu AT, Bogdan C, Slevin M, Petcu E, Popa-Wagner A. Stem cell therapies in preclinical models of stroke. Is the aged brain microenvironment refractory to cell therapy? Exp Gerontol. 2017;94:73–77. [DOI] [PubMed] [Google Scholar]
- 9. Laso-García F, Diekhorst L, Gómez-de Frutos MC, et al. . Cell-based therapies for stroke: Promising solution or dead end? Mesenchymal stem cells and comorbidities in preclinical stroke research. Front Neurol. 2019;10:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang XL, Zhang XG, Huang YR, et al. . Stem cell-based therapy for experimental ischemic stroke: A preclinical systematic review. Front Cell Neurosci. 2021;15:628908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borlongan CV. Concise review: Stem cell therapy for stroke patients: Are we there yet? Stem Cells Transl Med. 2019;8:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krause M, Phan TG, Ma H, Sobey CG, Lim R. Cell-based therapies for stroke: Are we there yet? Front Neurol. 2019;10:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rust R, Tackenberg C. Stem cell therapy for repair of the injured brain: Five principles. Neuroscientist. 2024;30:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carmichael ST, Llorente IL. The ties that bind: Glial transplantation in white matter ischemia and vascular dementia. Neurotherapeutics. 2023;20:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li W, Shi L, Hu B, et al. . Mesenchymal stem cell-based therapy for stroke: Current understanding and challenges. Front Cell Neurosci. 2021;15:628940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou L, Zhu H, Bai X, et al. . Potential mechanisms and therapeutic targets of mesenchymal stem cell transplantation for ischemic stroke. Stem Cell Res Ther. 2022;13:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park YJ, Niizuma K, Mokin M, Dezawa M, Borlongan CV. Cell-based therapy for stroke: Musing with muse cells. Stroke. 2020;51:2854–2862. [DOI] [PubMed] [Google Scholar]
- 18. Llorente IL, Xie Y, Mazzitelli JA, et al. . Patient-derived glial enriched progenitors repair functional deficits due to white matter stroke and vascular dementia in rodents. Sci Transl Med. 2021;13:eaaz6747. [DOI] [PubMed] [Google Scholar]
- 19. Hamblin MH, Murad R, Yin J, Vallim G, Lee JP. Modulation of gene expression on a transcriptome-wide level following human neural stem cell transplantation in aged mouse stroke brains. Exp Neurol. 2022;347:113913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vats K, Sarmah D, Datta A, et al. . Intra-arterial stem cell therapy diminishes inflammasome activation after ischemic stroke: A possible role of acid sensing ion channel 1a. J Mol Neurosci. 2021;71:419–426. [DOI] [PubMed] [Google Scholar]
- 21. Tobin MK, Stephen TKL, Lopez KL, et al. . Activated mesenchymal stem cells induce recovery following stroke via regulation of inflammation and oligodendrogenesis. J Am Heart Assoc. 2020;9:e013583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Acosta SA, Tajiri N, Hoover J, Kaneko Y, Borlongan CV. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke. 2015;46:2616–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paudyal A, Ghinea FS, Driga MP, et al. . P5 peptide-loaded human adipose-derived mesenchymal stem cells promote neurological recovery after focal cerebral ischemia in a rat model. Transl Stroke Res. 2021;12:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin KC, Chai HT, Chen KH, et al. . Intra-carotid arterial transfusion of circulatory-derived autologous endothelial progenitor cells in rodent after ischemic stroke—Evaluating the impact of therapeutic time points on prognostic outcomes. Stem Cell Res Ther. 2020;11:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Zhao Z, Rege SV, et al. . 3K3A-APC stimulates post-ischemic neuronal repair by human neural stem cells in mice. Nat Med. 2016;22:1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noh JE, Oh SH, Lee S, et al. . Intracerebral transplantation of HLA-homozygous human iPSC-derived neural precursors ameliorates the behavioural and pathological deficits in a rodent model of ischaemic stroke. Cell Prolif. 2020;53:e12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamaguchi S, Horie N, Satoh K, et al. . Age of donor of human mesenchymal stem cells affects structural and functional recovery after cell therapy following ischaemic stroke. J Cereb Blood Flow Metab. 2018;38:1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ryu B, Sekine H, Homma J, et al. . Allogeneic adipose-derived mesenchymal stem cell sheet that produces neurological improvement with angiogenesis and neurogenesis in a rat stroke model. J Neurosurg. 2019;132:442–455. [DOI] [PubMed] [Google Scholar]
- 29. Sun J, Huang Y, Gong J, et al. . Transplantation of hPSC-derived pericyte-like cells promotes functional recovery in ischemic stroke mice. Nat Commun. 2020;11:5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang Y, Wang J, Cai J, et al. . Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood-brain barrier preservation. Theranostics. 2018;8:5929–5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang L, Graf I, Kuang Y, et al. . Neural progenitor cell-derived extracellular vesicles enhance blood-brain barrier integrity by NF-κB (nuclear factor-κB)-dependent regulation of ABCB1 (ATP-binding cassette transporter B1) in stroke mice. Arterioscler Thromb Vasc Biol. 2021;41:1127–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang B, Li W, Satani N, et al. . Protective effects of autologous bone marrow mononuclear cells after administering t-PA in an embolic stroke model. Transl Stroke Res. 2018;9:135–145. [DOI] [PubMed] [Google Scholar]
- 33. Saver JL. Time is brain–quantified. Stroke. 2006;37:263–266. [DOI] [PubMed] [Google Scholar]
- 34. Desai SM, Rocha M, Jovin TG, Jadhav AP. High variability in neuronal loss. Stroke. 2019;50:34–37. [DOI] [PubMed] [Google Scholar]
- 35. Amukotuwa S, Straka M, Aksoy D, et al. . Cerebral blood flow predicts the infarct core. Stroke. 2019;50:2783–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakagomi T, Tanaka Y, Nakagomi N, Matsuyama T, Yoshimura S. How long are reperfusion therapies beneficial for patients after stroke onset? Lessons from lethal ischemia following early reperfusion in a mouse model of stroke. Int J Mol Sci. 2020;21:6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tiedt S, Buchan AM, Dichgans M, Lizasoain I, Moro MA, Lo EH. The neurovascular unit and systemic biology in stroke — Implications for translation and treatment. Nat Rev Neurol. 2022;18:597–612. [DOI] [PubMed] [Google Scholar]
- 38. Özen I, Roth M, Barbariga M, et al. . Loss of regulator of G-protein signaling 5 leads to neurovascular protection in stroke. Stroke. 2018;49:2182–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Y, Kisler K, Nikolakopoulou AM, Fernandez JA, Griffin JH, Zlokovic BV. 3K3A-Activated protein C protects the blood-brain barrier and neurons from accelerated ischemic injury caused by pericyte deficiency in mice. Front Neurosci. 2022;16:841916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weber RZ, Grönnert L, Mulders G, et al. . Characterization of the blood brain barrier disruption in the photothrombotic stroke model. Front Physiol. 2020;11:586226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang ZG, Zhang L, Jiang Q, et al. . VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alfranca A. VEGF therapy: A timely retreat. Cardiovasc Res. 2009;83:611–612. [DOI] [PubMed] [Google Scholar]
- 43. Rust R. Insights into the dual role of angiogenesis following stroke. J Cereb Blood Flow Metab. 2020;40:1167–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sanchez-Bezanilla S, Hood RJ, Collins-Praino LE, et al. . More than motor impairment: A spatiotemporal analysis of cognitive impairment and associated neuropathological changes following cortical photothrombotic stroke. J Cereb Blood Flow Metab. 2021;41:2439–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hood RJ, Sanchez-Bezanilla S, Beard DJ, et al. . Leakage beyond the primary infarction: A temporal analysis of cerebrovascular dysregulation at sites of hippocampal secondary neurodegeneration following cortical photothrombotic stroke. J Neurochem. 2023;167:733–752. [DOI] [PubMed] [Google Scholar]
- 46. Edwards DN, Bix GJ. Roles of blood-brain barrier integrins and extracellular matrix in stroke. Am J Physiol Cell Physiol. 2018;316:C252–C263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Winkler L, Blasig R, Breitkreuz-Korff O, et al. . Tight junctions in the blood–brain barrier promote edema formation and infarct size in stroke – Ambivalent effects of sealing proteins. J Cereb Blood Flow Metab. 2021;41:132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: Friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nih LR, Gojgini S, Carmichael ST, Segura T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat Mater. 2018;17:642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rust R, Grönnert L, Gantner C, et al. . Nogo-A targeted therapy promotes vascular repair and functional recovery following stroke. Proc Natl Acad Sci USA. 116:14270–14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lipsanen A, Kalesnykas G, Pro-Sistiaga P, et al. . Lack of secondary pathology in the thalamus after focal cerebral ischemia in nonhuman primates. Exp Neurol. 2013;248:224–227. [DOI] [PubMed] [Google Scholar]
- 52. Raman-Nair J, Cron G, MacLeod K, Lacoste B. Sex-specific acute cerebrovascular responses to photothrombotic stroke in mice. eNeuro. 2024;11:ENEURO.0400-22.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nih LR, Carmichael ST, Segura T. Hydrogels for brain repair after stroke: An emerging treatment option. Curr Opin Biotechnol. 2016;40:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nih LR. Engineered biomaterials for tissue regeneration of innervated and vascularized tissues: Lessons learned from the brain. J Endod. 2020;46:S101–S104. [DOI] [PubMed] [Google Scholar]
- 55. Nih LR, Sideris E, Carmichael ST, Segura T. Injection of microporous annealing particle (MAP) hydrogels in the stroke cavity reduces gliosis and inflammation and promotes NPC migration to the lesion. Adv Mater. 2017;29:1606471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moshayedi P, Nih LR, Llorente IL, et al. . Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials. 2016;105:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nih LR, Moshayedi P, Llorente IL, et al. . Engineered HA hydrogel for stem cell transplantation in the brain: Biocompatibility data using a design of experiment approach. Data Brief. 2017;10:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pozo Devoto VM, Lacovich V, Feole M, et al. . Unraveling axonal mechanisms of traumatic brain injury. Acta Neuropathol Commun. 2022;10:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Norat P, Sokolowski JD, Gorick CM, et al. . Intraarterial transplantation of mitochondria after ischemic stroke reduces cerebral infarction. Stroke Vasc Interv Neurol. 2023;3:e000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stuckey SM, Ong LK, Collins-Praino LE, Turner RJ. Neuroinflammation as a key driver of secondary neurodegeneration following stroke? Int J Mol Sci. 2021;22:13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ong LK, Walker FR, Nilsson M, Ong LK, Walker FR, Nilsson M. Is stroke a neurodegenerative condition? A critical review of secondary neurodegeneration and amyloid-beta accumulation after stroke. AIMSMEDS. 2017;4:1–16. [Google Scholar]
- 62. Corbett D, Carmichael ST, Murphy TH, et al. . Enhancing the alignment of the preclinical and clinical stroke recovery research pipeline: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable translational working group. Neurorehabil Neural Repair. 2017;31:699–707. [DOI] [PubMed] [Google Scholar]
- 63. Stinear CM, Lang CE, Zeiler S, Byblow WD. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020;19:348–360. [DOI] [PubMed] [Google Scholar]
- 64. Cramer SC, Dodakian L, Le V, et al. . Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke: A randomized clinical trial. JAMA Neurol. 2019;76:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shin S, Lee Y, Chang WH, et al. . Multifaceted assessment of functional outcomes in survivors of first-time stroke. JAMA Network Open. 2022;5:e2233094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jones TA. Motor compensation and its effects on neural reorganization after stroke. Nat Rev Neurosci. 2017;18:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bailey RR, Klaesner JW, Lang CE. Quantifying real-world upper-limb activity in nondisabled adults and adults with chronic stroke. Neurorehabil Neural Repair. 2015;29:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li S, Nie E, Yin Y, et al. . GDF10 is a signal for axonal sprouting and functional recovery after stroke. Nat Neurosci. 2015;18:1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Androvic P, Kirdajova D, Tureckova J, et al. . Decoding the transcriptional response to ischemic stroke in young and aged mouse brain. Cell Rep. 2020;31:107777. [DOI] [PubMed] [Google Scholar]
- 70. Zheng K, Lin L, Jiang W, et al. . Single-cell RNA-seq reveals the transcriptional landscape in ischemic stroke. J Cereb Blood Flow Metab. 2022;42:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rust R. Ischemic stroke-related gene expression profiles across species: A meta-analysis. J Inflamm. 2023;20:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Han B, Zhou S, Zhang Y, et al. . Integrating spatial and single-cell transcriptomics to characterize the molecular and cellular architecture of the ischemic mouse brain. Sci Transl Med. 2024;16:eadg1323. [DOI] [PubMed] [Google Scholar]
- 73. Ito M, Aswendt M, Lee AG, et al. . RNA-Sequencing analysis revealed a distinct motor cortex transcriptome in spontaneously recovered mice after stroke. Stroke. 2018;49:2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kaiser J, Maibach M, Salpeter I, et al. . The spinal transcriptome after cortical stroke: In search of molecular factors regulating spontaneous recovery in the spinal cord. J Neurosci. 2019;39:4714–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jin C, Shi Y, Shi L, et al. . Leveraging single-cell RNA sequencing to unravel the impact of aging on stroke recovery mechanisms in mice. Proc Natl Acad Sci USA. 2023;120:e2300012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rust R, Holm MM, Egger M, et al. . Nogo-A is secreted in extracellular vesicles, occurs in blood and can influence vascular permeability. J Cereb Blood Flow Metab. 2024;44:938–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Glasgow SD, Labrecque S, Beamish IV, et al. . Activity-dependent netrin-1 secretion drives synaptic insertion of GluA1-containing AMPA receptors in the hippocampus. Cell Rep. 2018;25:168–182.e6. [DOI] [PubMed] [Google Scholar]
- 78. Hirt L, Fukuda AM, Ambadipudi K, et al. . Improved long-term outcome after transient cerebral ischemia in aquaporin-4 knockout mice. J Cereb Blood Flow Metab. 2017;37:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li F, Zhang Y, Li R, et al. . Neuronal Serpina3n is an endogenous protector against blood brain barrier damage following cerebral ischemic stroke. J Cereb Blood Flow Metab. 2023;43:241–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gillis HL, Kalinina A, Xue Y, et al. . VGF is required for recovery after focal stroke. Exp Neurol. 2023;362:114326. [DOI] [PubMed] [Google Scholar]
- 81. Yu R, Kim NS, Li Y, et al. . Vascular Sema3E-plexin-D1 signaling reactivation promotes post-stroke recovery through VEGF downregulation in mice. Transl Stroke Res. 2022;13:142–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liberale L, Bonetti NR, Puspitasari YM, et al. . Postischemic administration of IL-1α neutralizing antibody reduces brain damage and neurological deficit in experimental stroke. Circulation. 2020;142:187–189. [DOI] [PubMed] [Google Scholar]
- 83. Joy MT, Carmichael ST. Encouraging an excitable brain state: Mechanisms of brain repair in stroke. Nat Rev Neurosci. 2021;22:38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zeiger WA, Marosi M, Saggi S, Noble N, Samad I, Portera-Cailliau C. Barrel cortex plasticity after photothrombotic stroke involves potentiating responses of pre-existing circuits but not functional remapping to new circuits. Nat Commun. 2021;12:3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Merino-Serrais P, Plaza-Alonso S, Hellal F, et al. . Microanatomical study of pyramidal neurons in the contralesional somatosensory cortex after experimental ischemic stroke. Cereb Cortex. 2023;33:1074–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Olafson ER, Jamison KW, Sweeney EM, et al. . Functional connectome reorganization relates to post-stroke motor recovery and structural and functional disconnection. Neuroimage. 2021;245:118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shimada IS, Peterson BM, Spees JL. Isolation of locally derived stem/progenitor cells from the peri-infarct area that do not migrate from the lateral ventricle after cortical stroke. Stroke. 2010;41:e552–e560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shimada IS, LeComte MD, Granger JC, Quinlan NJ, Spees JL. Self-renewal and differentiation of reactive astrocyte-derived neural stem/progenitor cells isolated from the cortical peri-infarct area after stroke. J Neurosci. 2012;32:7926–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nishie H, Nakano-Doi A, Sawano T, Nakagomi T. Establishment of a reproducible ischemic stroke model in nestin-GFP mice with high survival rates. Int J Mol Sci. 2021;22:12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Liang H, Zhao H, Gleichman A, et al. . Region-specific and activity-dependent regulation of SVZ neurogenesis and recovery after stroke. Proc Natl Acad Sci USA. 2019;116:13621–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen D, Wei L, Liu ZR, et al. . Pyruvate kinase M2 increases angiogenesis, neurogenesis, and functional recovery mediated by upregulation of STAT3 and focal adhesion kinase activities after ischemic stroke in adult mice. Neurotherapeutics. 2018;15:770–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hao XZ, Yin LK, Tian JQ, et al. . Inhibition of notch1 signaling at the subacute stage of stroke promotes endogenous neurogenesis and motor recovery after stroke. Front Cell Neurosci. 2018;12:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Williamson MR, Le SP, Franzen RL, et al. . Subventricular zone cytogenesis provides trophic support for neural repair in a mouse model of stroke. Nat Commun. 2023;14:6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Walker TL, Schallenberg S, Rund N, et al. . T lymphocytes contribute to the control of baseline neural precursor cell proliferation but not the exercise-induced up-regulation of adult hippocampal neurogenesis. Front Immunol. 2018;9:2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wasielewska JM, Grönnert L, Rund N, et al. . Mast cells increase adult neural precursor proliferation and differentiation but this potential is not realized in vivo under physiological conditions. Sci Rep. 2017;7:17859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhu S, Nih L, Carmichael ST, Lu Y, Segura T. Enzyme-Responsive delivery of multiple proteins with spatiotemporal control. Adv Mater. 2015;27:3620–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li S, Nih LR, Bachman H, et al. . Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat Mater. 2017;16:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Williamson MR, Franzen RL, Fuertes CJA, Dunn AK, Drew MR, Jones TA. A window of vascular plasticity coupled to behavioral recovery after stroke. J Neurosci. 2020;40:7651–7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hoffmann CJ, Harms U, Rex A, et al. . Vascular signal transducer and activator of transcription-3 promotes angiogenesis and neuroplasticity long-term after stroke. Circulation. 2015;131:1772–1782. [DOI] [PubMed] [Google Scholar]
- 100. Červenka J, Tylečková J, Kupcová Skalníková H, et al. . Proteomic characterization of human neural stem cells and their secretome during in vitro differentiation. Front Cell Neurosci. 2021;14:612560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Denninger JK, Miller LN, Walters AE, et al. . Neural stem and progenitor cells support and protect adult hippocampal function via vascular endothelial growth factor secretion. bioRxiv. [Preprint] doi: 10.1101/2023.04.24.537801 [DOI] [Google Scholar]
- 102. Rust R, Grönnert L, Weber RZ, Mulders G, Schwab ME. Refueling the ischemic CNS: Guidance molecules for vascular repair. Trends Neurosci. 2019;42:644–656. [DOI] [PubMed] [Google Scholar]
- 103. Shrouder J, Filser S, Varga DP, et al. . Continued dysfunction of capillary pericytes promotes no-reflow after experimental stroke in vivo. Brain 2024;147:1057–1074. [DOI] [PubMed] [Google Scholar]
- 104. Buizza C, Enström A, Carlsson R, Paul G. The transcriptional landscape of pericytes in acute ischemic stroke. Transl Stroke Res. 2023;15:714–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nikolakopoulou AM, Montagne A, Kisler K, et al. . Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci. 2019;22:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kisler K, Nikolakopoulou AM, Sweeney MD, Lazic D, Zhao Z, Zlokovic BV. Acute ablation of cortical pericytes leads to rapid neurovascular uncoupling. Front Cell Neurosci. 2020;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dias DO, Kim H, Holl D, et al. . Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell. 2018;173:153–165.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dias DO, Kalkitsas J, Kelahmetoglu Y, et al. . Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun. 2021;12:5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gaidin SG, Zinchenko VP, Sergeev AI, Teplov IY, Mal’tseva VN, Kosenkov AM. Activation of alpha-2 adrenergic receptors stimulates GABA release by astrocytes. Glia. 2020;68:1114–1130. [DOI] [PubMed] [Google Scholar]
- 110. Robin LM, Oliveira da Cruz JF, Langlais VC, et al. . Astroglial CB1 receptors determine synaptic D-serine availability to enable recognition memory. Neuron. 2018;98:935–944.e5. [DOI] [PubMed] [Google Scholar]
- 111. Freitas-Andrade M, Comin CH, Van Dyken P, et al. . Astroglial Hmgb1 regulates postnatal astrocyte morphogenesis and cerebrovascular maturation. Nat Commun. 2023;14:4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Choi JY, Jin X, Kim H, Koh S, Cho HJ, Kim BG. High mobility group box 1 as an autocrine chemoattractant for oligodendrocyte lineage cells in white matter stroke. Stroke. 2023;54:575–586. [DOI] [PubMed] [Google Scholar]
- 113. Dai X, Chen J, Xu F, et al. . TGFα preserves oligodendrocyte lineage cells and improves white matter integrity after cerebral ischemia. J Cereb Blood Flow Metab. 2020;40:639–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen W, Zhang Y, Zhai X, et al. . Microglial phagocytosis and regulatory mechanisms after stroke. J Cereb Blood Flow Metab. 2022;42:1579–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Amrein I, Slomianka L, Poletaeva II, Bologova NV, Lipp HP. Marked species and age-dependent differences in cell proliferation and neurogenesis in the hippocampus of wild-living rodents. Hippocampus. 2004;14:1000–1010. [DOI] [PubMed] [Google Scholar]
- 116. Nishiyama R, Nakagomi T, Nakano-Doi A, Kuramoto Y, Tsuji M, Yoshimura S. Neonatal brains exhibit higher neural reparative activities than adult brains in a mouse model of ischemic stroke. Cells. 2024;13:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sorrells SF, Paredes MF, Cebrian-Silla A, et al. . Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sanai N, Nguyen T, Ihrie RA, et al. . Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Johnson CO, Nguyen M, Roth GA, et al. . Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:439–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kempermann G, Gage FH, Aigner L, et al. . Human adult neurogenesis: Evidence and remaining questions. Cell Stem Cell. 2018;23:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lucassen PJ, Fitzsimons CP, Salta E, Maletic-Savatic M. Adult neurogenesis, human after all (again): Classic, optimized, and future approaches. Behav Brain Res. 2020;381:112458. [DOI] [PubMed] [Google Scholar]
- 122. Ong LK, Ilicic M, Hood RJ, Warren KE, Coupland KG. Targeting adult neurogenesis for brain recovery after stroke: The next frontier in stroke medicine. In: Raza SS, ed. Regenerative therapies in ischemic stroke recovery. Springer Nature; 2022:1–30. [Google Scholar]
- 123. Cipolla MJ, Liebeskind DS, Chan SL. The importance of comorbidities in ischemic stroke: Impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab. 2018;38:2129–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Carmichael ST, Kathirvelu B, Schweppe CA, Nie EH. Molecular, cellular and functional events in axonal sprouting after stroke. Exp Neurol. 2017;287(Pt 3):384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Petcu EB, Smith RA, Miroiu RI, Opris MM. Angiogenesis in old-aged subjects after ischemic stroke: A cautionary note for investigators. J Angiogenes Res. 2010;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kufner A, Khalil AA, Galinovic I, et al. . Magnetic resonance imaging-based changes in vascular morphology and cerebral perfusion in subacute ischemic stroke. J Cereb Blood Flow Metab. 2021;41:2617–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Escudero C, Acurio J, López E, et al. . Vascular endothelial growth factor and poor prognosis after ischaemic stroke. Eur J Neurol. 2021;28:1759–1764. [DOI] [PubMed] [Google Scholar]
- 128. Zhang R, Wang L, Zhang L, et al. . Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–313. [DOI] [PubMed] [Google Scholar]
- 129. Prodjohardjono A, Vidyanti AN, Susianti NA, Sudarmanta SS, Setyopranoto I. Higher level of acute serum VEGF and larger infarct volume are more frequently associated with post-stroke cognitive impairment. PLoS One. 2020;15:e0239370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Matsuo R, Ago T, Kamouchi M, et al. . Clinical significance of plasma VEGF value in ischemic stroke - research for biomarkers in ischemic stroke (REBIOS) study. BMC Neurol. 2013;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. [DOI] [PubMed] [Google Scholar]
- 132. Armulik A, Genové G, Mäe M, et al. . Pericytes regulate the blood–brain barrier. Nature. 2010;468:557–561. [DOI] [PubMed] [Google Scholar]
- 133. Bell RD, Winkler EA, Sagare AP, et al. . Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Shen J, Xu G, Zhu R, et al. . PDGFR-β restores blood-brain barrier functions in a mouse model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2019;39:1501–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Teichert M, Milde L, Holm A, et al. . Pericyte-expressed tie2 controls angiogenesis and vessel maturation. Nat Commun. 2017;8:16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bosworth A, Griffin C, Chakhoyan A, et al. . Molecular signature and functional properties of human pluripotent stem cell-derived brain pericytes. bioRxiv. [Preprint] doi: 10.1101/2023.06.26.546577 [DOI] [Google Scholar]
- 137. Candelario-Jalil E, Dijkhuizen RM, Magnus T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. 2022;53:1473–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Feng Y, Liao S, Wei C, et al. . Infiltration and persistence of lymphocytes during late-stage cerebral ischemia in middle cerebral artery occlusion and photothrombotic stroke models. J Neuroinflammation. 2017;14:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ng FC, Churilov L, Yassi N, et al. . Microvascular dysfunction in blood-brain barrier disruption and hypoperfusion within the infarct posttreatment are associated with cerebral edema. Stroke. 2022;53:1597–1605. [DOI] [PubMed] [Google Scholar]
- 140. Houkin K, Osanai T, Uchiyama S, et al. . Allogeneic stem cell therapy for acute ischemic stroke: The phase 2/3 TREASURE randomized clinical trial. JAMA Neurol. 2024;81:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lee J, Chang WH, Chung JW, et al. . Efficacy of intravenous mesenchymal stem cells for motor recovery after ischemic stroke: A neuroimaging study. Stroke. 2022;53:20–28. [DOI] [PubMed] [Google Scholar]
- 142. Chung JW, Chang WH, Bang OY, et al. . Efficacy and safety of intravenous mesenchymal stem cells for ischemic stroke. Neurology. 2021;96:e1012–e1023. [DOI] [PubMed] [Google Scholar]
- 143. Jaillard A, Hommel M, Moisan A, et al. . Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: A randomized clinical trial. Transl Stroke Res. 2020;11:910–923. [DOI] [PubMed] [Google Scholar]
- 144. Muir KW, Bulters D, Willmot M, et al. . Intracerebral implantation of human neural stem cells and motor recovery after stroke: Multicentre prospective single-arm study (PISCES-2). J Neurol Neurosurg Psychiatry. 2020;91:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Levy ML, Crawford JR, Dib N, Verkh L, Tankovich N, Cramer SC. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke. 2019;50:2835–2841. [DOI] [PubMed] [Google Scholar]
- 146. Savitz SI, Yavagal D, Rappard G, et al. . A phase 2 randomized, sham-controlled trial of internal carotid artery infusion of autologous bone marrow–derived ALD-401 cells in patients with recent stable ischemic stroke (RECOVER-stroke). Circulation. 2019;139:192–205. [DOI] [PubMed] [Google Scholar]
- 147. Steinberg GK, Kondziolka D, Wechsler LR, et al. . Clinical outcomes of transplanted modified bone marrow–derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke. 2016;47:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Steinberg GK, Kondziolka D, Wechsler LR, et al. . Two-year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow-derived mesenchymal stem cells (SB623): A phase 1/2a study. J Neurosurg. 2018;131(5):1462–1472. [DOI] [PubMed] [Google Scholar]
- 149. Bhatia V, Gupta V, Khurana D, Sharma RR, Khandelwal N. Randomized assessment of the safety and efficacy of intra-arterial infusion of autologous stem cells in subacute ischemic stroke. Am J Neuroradiol. 2018;39:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hess DC, Wechsler LR, Clark WM, et al. . Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360–368. [DOI] [PubMed] [Google Scholar]
- 151. Moniche F, Cabezas-Rodriguez JA, Valverde R, et al. . Safety and efficacy of intra-arterial bone marrow mononuclear cell transplantation in patients with acute ischaemic stroke in Spain (IBIS trial): A phase 2, randomised, open-label, standard-of-care controlled, multicentre trial. Lancet Neurol. 2023;22:137–146. [DOI] [PubMed] [Google Scholar]
- 152. Rust R, Weber RZ, Generali M, et al. . Xeno-free induced pluripotent stem cell-derived neural progenitor cells for in vivo applications. J Transl Med. 2022;20:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kawabori M, Shichinohe H, Kuroda S, Houkin K. Clinical trials of stem cell therapy for cerebral ischemic stroke. Int J Mol Sci. 2020;21:7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Li Z, Dong X, Tian M, et al. . Stem cell-based therapies for ischemic stroke: A systematic review and meta-analysis of clinical trials. Stem Cell Res Ther. 2020;11:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lalu MM, Montroy J, Dowlatshahi D, et al. . From the lab to patients: A systematic review and meta-analysis of mesenchymal stem cell therapy for stroke. Transl Stroke Res. 2020;11:345–364. [DOI] [PubMed] [Google Scholar]
- 156. Achon Buil B, Tackenberg C, Rust R. Editing a gateway for cell therapy across the blood–brain barrier. Brain. 2023;46:823–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kikuchi T, Morizane A, Doi D, et al. . Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 2017;548:592–596. [DOI] [PubMed] [Google Scholar]
- 158. Takahashi J. iPS cell-based therapy for Parkinson’s disease: A Kyoto trial. Regen Ther. 2020;13:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mandai M, Watanabe A, Kurimoto Y, et al. . Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038–1046. [DOI] [PubMed] [Google Scholar]
- 160. Sharma R, Khristov V, Rising A, et al. . Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med. 2019;11:eaat5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Jgamadze D, Lim JT, Zhang Z, et al. . Structural and functional integration of human forebrain organoids with the injured adult rat visual system. Cell Stem Cell. 2023;30:137–152.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Negoro T, Okura H, Maehata M, et al. . Trends in clinical trials for stroke by cell therapy: Data mining ClinicalTrials.gov and the ICTRP portal site. npj Regen Med. 2019;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kalladka D, Sinden J, Pollock K, et al. . Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet. 2016;388:787–796. [DOI] [PubMed] [Google Scholar]
- 164. Laskowitz DT, Bennett ER, Durham RJ, et al. . Allogeneic umbilical cord blood infusion for adults with ischemic stroke: Clinical outcomes from a phase I safety study. Stem Cells Transl Med. 2018;7:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Osanai T, Houkin K, Uchiyama S, Minematsu K, Taguchi A, Terasaka S. Treatment evaluation of acute stroke for using in regenerative cell elements (TREASURE) trial: Rationale and design. Int J Stroke. 2018;13:444–448. [DOI] [PubMed] [Google Scholar]
- 166. Athersys Inc . Athersys announces that its partner, Healios KK, reported topline data from the treasure multistem ischemic stroke study. Athersys Inc; 2022. Accessed 13 October 2023. https://www.athersys.com/investors/press-releases/press-release-details/2022/Athersys-Announces-That-Its-Partner-HEALIOS-K.K.-Reported-Topline-Data-From-the-TREASURE-Multistem-Ischemic-Stroke-Study/default.aspx
- 167. ReNeuron . Phase 2b Clinical trial in stroke disability (PISCES III). ReNeuron; 2022. Accessed 13 October 2023. https://www.reneuron.com/clinical-trials/phase-iib-clinical-trial-in-stroke-disability-pisces-iii/
- 168. Mays RW, Savitz SI. Intravenous cellular therapies for acute ischemic stroke. Stroke. 2018;49:1058–1065. [DOI] [PubMed] [Google Scholar]
- 169. He JQ, Sussman ES, Steinberg GK. Revisiting stem cell-based clinical trials for ischemic stroke. Front Aging Neurosci. 2020;12:575990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Azevedo-Pereira RL, Manley NC, Dong C, et al. . Decoding the molecular crosstalk between grafted stem cells and the stroke-injured brain. Cell Rep. 2023;42:112353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Weber RZ, Buil BA, Rentsch NH, et al. . Human iPSC-derived cell grafts promote functional recovery by molecular interaction with stroke-injured brain. bioRxiv. [Preprint] doi: 10.1101/2024.04.03.588020 [DOI] [Google Scholar]
- 172. Zheng H, Zhang B, Chhatbar PY, et al. . Mesenchymal stem cell therapy in stroke: A systematic review of literature in Pre-clinical and clinical research. Cell Transplant. 2018;27:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Palma-Tortosa S, Tornero D, Grønning Hansen M, et al. . Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc Natl Acad Sci USA. 2020;117:9094–9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Tornero D, Tsupykov O, Granmo M, et al. . Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain. 2017;140:692–706. [DOI] [PubMed] [Google Scholar]
- 175. Falkner S, Grade S, Dimou L, et al. . Transplanted embryonic neurons integrate into adult neocortical circuits. Nature. 2016;539:248–253. [DOI] [PubMed] [Google Scholar]
- 176. Yu SP, Tung JK, Wei ZZ, et al. . Optochemogenetic stimulation of transplanted iPS-NPCs enhances neuronal repair and functional recovery after ischemic stroke. J Neurosci. 2019;39:6571–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Imitola J, Raddassi K, Park KI, et al. . Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Dietert K, Mahesula S, Hegde S, et al. . Loss of LRP1 in adult neural stem cells impairs migration to ischemic lesions. Stem Cells. 2023;41:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Li L, Chu L, Fang Y, et al. . Preconditioning of bone marrow-derived mesenchymal stromal cells by tetramethylpyrazine enhances cell migration and improves functional recovery after focal cerebral ischemia in rats. Stem Cell Res Ther. 2017;8:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Chau MJ, Deveau TC, Gu X, et al. . Delayed and repeated intranasal delivery of bone marrow stromal cells increases regeneration and functional recovery after ischemic stroke in mice. BMC Neurosci. 2018;19:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Savitz SI, Cox CS. Cell-based therapies for neurological disorders — The bioreactor hypothesis. Nat Rev Neurol. 2023;19:9–18. [DOI] [PubMed] [Google Scholar]
- 182. Coyne TM, Marcus AJ, Woodbury D, Black IB. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24:2483–2492. [DOI] [PubMed] [Google Scholar]
- 183. Wechsler LR, Bates D, Stroemer P, Andrews-Zwilling YS, Aizman I. Cell therapy for chronic stroke. Stroke. 2018;49:1066–1074. [DOI] [PubMed] [Google Scholar]
- 184. Wang H, Yang H, Shi Y, et al. . Reconstituting neurovascular unit with primary neural stem cells and brain microvascular endothelial cells in three-dimensional matrix. Brain Pathol. 2021;31:e12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Payne SL, Tuladhar A, Obermeyer JM, et al. . Initial cell maturity changes following transplantation in a hyaluronan-based hydrogel and impacts therapeutic success in the stroke-injured rodent brain. Biomaterials. 2019;192:309–322. [DOI] [PubMed] [Google Scholar]
- 186. Ghuman H, Matta R, Tompkins A, et al. . ECM hydrogel improves the delivery of PEG microsphere-encapsulated neural stem cells and endothelial cells into tissue cavities caused by stroke. Brain Res Bull. 2021;168:120–137. [DOI] [PubMed] [Google Scholar]
- 187. Somaa FA, Wang TY, Niclis JC, et al. . Peptide-based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell Rep. 2017;20:1964–1977. [DOI] [PubMed] [Google Scholar]
- 188. Daviaud N, Friedel RH, Zou H. Vascularization and engraftment of transplanted human cerebral organoids in mouse Cortex. eNeuro. 2018;5:ENEURO.0219-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Revah O, Gore F, Kelley KW, et al. . Maturation and circuit integration of transplanted human cortical organoids. Nature. 2022;610:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Cao SY, Yang D, Huang ZQ, et al. . Cerebral organoids transplantation repairs infarcted cortex and restores impaired function after stroke. npj Regen Med. 2023;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Nakagomi T, Nakano-Doi A, Kubo S, et al. . Transplantation of human brain-derived ischemia-induced multipotent stem cells ameliorates neurological dysfunction in mice after stroke. Stem Cells Transl Med. 2023;12:400–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18:1058–1066. [DOI] [PubMed] [Google Scholar]
- 193. Haupt M, Gerner ST, Doeppner TR. The dual role of microglia in ischemic stroke and its modulation via extracellular vesicles and stem cells. Neuroprotection. 2024;2:4–15. [Google Scholar]
- 194. Guo K, Luo J, Feng D, et al. . Single-cell RNA sequencing with combined use of bulk RNA sequencing to reveal cell heterogeneity and molecular changes at acute stage of ischemic stroke in mouse cortex penumbra area. Front Cell Dev Biol. 2021;9:624711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Kim J, Shin K, Cha Y, et al. . Neuroprotective effects of human neural stem cells over-expressing choline acetyltransferase in a middle cerebral artery occlusion model. J Chem Neuroanat. 2020;103:101730. [DOI] [PubMed] [Google Scholar]
- 196. Li X, Huang M, Zhao R, et al. . Intravenously delivered allogeneic mesenchymal stem cells bidirectionally regulate inflammation and induce neurotrophic effects in distal middle cerebral artery occlusion rats within the first 7 days after stroke. Cell Physiol Biochem. 2018;46:1951–1970. [DOI] [PubMed] [Google Scholar]
- 197. Piepke M, Clausen BH, Ludewig P, et al. . Interleukin-10 improves stroke outcome by controlling the detrimental interleukin-17A response. J Neuroinflammation. 2021;18:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zhang G, Chen L, Chen W, et al. . Neural stem cells alleviate inflammation via neutralization of IFN-γ negative effect in ischemic stroke model. J Biomed Nanotechnol. 2018;14:1178–1188. [DOI] [PubMed] [Google Scholar]
- 199. Cheng Z, Wang L, Qu M, et al. . Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice. J Neuroinflammation. 2018;15:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Park HW, Kim Y, Chang JW, et al. . Effect of single and double administration of human umbilical cord blood-derived mesenchymal stem cells following focal cerebral ischemia in rats. Exp Neurobiol. 2017;26:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Peruzzotti-Jametti L, Bernstock JD, Vicario N, et al. . Macrophage-Derived extracellular succinate licenses neural stem cells to suppress chronic neuroinflammation. Cell Stem Cell. 2018;22:355–368.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Wang C, Börger V, Sardari M, et al. . Mesenchymal stromal cell-derived small extracellular vesicles induce ischemic neuroprotection by modulating leukocytes and specifically neutrophils. Stroke. 2020;51:1825–1834. [DOI] [PubMed] [Google Scholar]
- 203. Kuang Y, Zheng X, Zhang L, et al. . Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25. J Extracell Vesicles. 2020;10:e12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Xin H, Liu Z, Buller B, et al. . MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. J Cereb Blood Flow Metab. 2021;41:1131–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Barzegar M, Wang Y, Eshaq RS, et al. . Human placental mesenchymal stem cells improve stroke outcomes via extracellular vesicles-mediated preservation of cerebral blood flow. EBioMedicine. 2021;63:103161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Borlongan CV, Hadman M, Davis Sanberg C, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–2389. [DOI] [PubMed] [Google Scholar]
- 207. Yang B, Hamilton JA, Valenzuela KS, et al. . Multipotent adult progenitor cells enhance recovery after stroke by modulating the immune response from the spleen. Stem Cells. 2017;35:1290–1302. [DOI] [PubMed] [Google Scholar]
- 208. Nakajima M, Nito C, Sowa K, et al. . Mesenchymal stem cells overexpressing interleukin-10 promote neuroprotection in experimental acute ischemic stroke. Mol Ther Methods Clin Dev. 2017;6:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Lee J, d’Aigle J, Atadja L, et al. . Gut Microbiota–derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res. 2020;127:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Horie N, Pereira MP, Niizuma K, et al. . Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Barzegar M, Vital S, Stokes KY, et al. . Human placenta mesenchymal stem cell protection in ischemic stroke is angiotensin converting enzyme-2 and masR receptor-dependent. Stem Cells. 2021;39:1335–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Nakamura K, Arimura K, Nishimura A, et al. . Possible involvement of basic FGF in the upregulation of PDGFRβ in pericytes after ischemic stroke. Brain Res. 2016;1630:98–108. [DOI] [PubMed] [Google Scholar]
- 213. Takeuchi Y, Okinaka Y, Ogawa Y, et al. . Intravenous bone marrow mononuclear cells transplantation in aged mice increases transcription of glucose transporter 1 and Na+/K+-ATPase at hippocampus followed by restored neurological functions. Front Aging Neurosci. 2020;12:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kikuchi-Taura A, Okinaka Y, Takeuchi Y, et al. . Bone marrow mononuclear cells activate angiogenesis via gap junction–mediated cell-cell interaction. Stroke. 2020;51:1279–1289. [DOI] [PubMed] [Google Scholar]
- 215. Chung TN, Kim JH, Choi BY, Chung SP, Kwon SW, Suh SW. Adipose-derived mesenchymal stem cells reduce neuronal death after transient global cerebral ischemia through prevention of blood-brain barrier disruption and endothelial damage. Stem Cells Transl Med. 2015;4:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Yoshida Y, Takagi T, Kuramoto Y, et al. . Intravenous administration of human amniotic mesenchymal stem cells in the subacute phase of cerebral infarction in a mouse model ameliorates neurological disturbance by suppressing blood brain barrier disruption and apoptosis via immunomodulation. Cell Transplant. 2021;30:09636897211024183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Xia Y, Ling X, Hu G, et al. . Small extracellular vesicles secreted by human iPSC-derived MSC enhance angiogenesis through inhibiting STAT3-dependent autophagy in ischemic stroke. Stem Cell Res Ther. 2020;11:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Lu Y, Zhou Y, Zhang R, et al. . Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front Neurosci. 2019;13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Oh SH, Jeong YW, Choi W, et al. . Multimodal therapeutic effects of neural precursor cells derived from human-induced pluripotent stem cells through episomal plasmid-based reprogramming in a rodent model of ischemic stroke. Stem Cells Int. 2020;2020:4061516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Suzuki H, Ahuja CS, Salewski RP, et al. . Neural stem cell mediated recovery is enhanced by Chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS One. 2017;12:e0182339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Sekiya T, Holley MC, Hashido K, et al. . Cells transplanted onto the surface of the glial scar reveal hidden potential for functional neural regeneration. Proc Natl Acad Sci USA. 2015;112:E3431–E3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Hasel P, Rose IVL, Sadick JS, Kim RD, Liddelow SA. Neuroinflammatory astrocyte subtypes in the mouse brain. Nat Neurosci. 2021;24:1475–1487. [DOI] [PubMed] [Google Scholar]
- 223. Liddelow SA, Guttenplan KA, Clarke LE, et al. . Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Benner EJ, Luciano D, Jo R, et al. . Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497:369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Laug D, Huang TW, Huerta NAB, et al. . Nuclear factor I-A regulates diverse reactive astrocyte responses after CNS injury. J Clin Invest. 2019;129:4408–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Becerra-Calixto A, Posada-Duque R, Cardona-Gómez GP. Recovery of neurovascular unit integrity by CDK5-KD astrocyte transplantation in a global cerebral ischemia model. Mol Neurobiol. 2018;55:8563–8585. [DOI] [PubMed] [Google Scholar]
- 227. O’Shea TM, Ao Y, Wang S, et al. . Lesion environments direct transplanted neural progenitors towards a wound repair astroglial phenotype in mice. Nat Commun. 2022;13:5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Jiang P, Chen C, Liu XB, Pleasure DE, Liu Y, Deng W. Human iPSC-derived immature astroglia promote oligodendrogenesis by increasing TIMP-1 secretion. Cell Rep. 2016;15:1303–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Wang L, Geng J, Qu M, et al. . Oligodendrocyte precursor cells transplantation protects blood–brain barrier in a mouse model of brain ischemia via Wnt/β-catenin signaling. Cell Death Dis. 2020;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Shiota Y, Nagai A, Sheikh AM, et al. . Transplantation of a bone marrow mesenchymal stem cell line increases neuronal progenitor cell migration in a cerebral ischemia animal model. Sci Rep. 2018;8:14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. He J, Zhang N, Zhu Y, Jin R, Wu F. MSC spheroids-loaded collagen hydrogels simultaneously promote neuronal differentiation and suppress inflammatory reaction through PI3K-akt signaling pathway. Biomaterials. 2021;265:120448. [DOI] [PubMed] [Google Scholar]
- 232. Podjaski C, Alvarez JI, Bourbonniere L, et al. . Netrin 1 regulates blood-brain barrier function and neuroinflammation. Brain. 2015;138(Pt 6):1598–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Rust R, Weber RZ, Grönnert L, et al. . Anti-Nogo-A antibodies prevent vascular leakage and act as pro-angiogenic factors following stroke. Sci Rep. 2019;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Rust R, Gantner C, Schwab ME. Pro- and antiangiogenic therapies: Current status and clinical implications. FASEB J. 2019;33:34–48. [DOI] [PubMed] [Google Scholar]
- 235. Rosenblum S, Smith TN, Wang N, et al. . BDNF pretreatment of human embryonic-derived neural stem cells improves cell survival and functional recovery after transplantation in hypoxic-ischemic stroke. Cell Transplant. 2015;24:2449–2461. [DOI] [PubMed] [Google Scholar]
- 236. Wu K, Huang D, Zhu C, et al. . NT3P75-2 gene-modified bone mesenchymal stem cells improve neurological function recovery in mouse TBI model. Stem Cell Res Ther. 2019;10:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Wang Y, Geng T, Ni A, Yin H, Han B. Effects of transplanted GDNF gene modified marrow stromal cells on focal cerebral ischemia in rats. Front Integr Neurosci. 2011;5:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Gantner CW, de Luzy IR, Kauhausen JA, et al. . Viral delivery of GDNF promotes functional integration of human stem cell grafts in Parkinson’s disease. Cell Stem Cell. 2020;26:511–526.e5. [DOI] [PubMed] [Google Scholar]
- 239. Liu C, Yang ZX, Zhou SQ, et al. . Overexpression of vascular endothelial growth factor enhances the neuroprotective effects of bone marrow mesenchymal stem cell transplantation in ischemic stroke. Neural Regen Res. 2022;18:1286–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Korshunova I, Rhein S, García-González D, et al. . Genetic modification increases the survival and the neuroregenerative properties of transplanted neural stem cells. JCI Insight. 2020;5:e126268, 126268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Salehi MS, Pandamooz S, Safari A, et al. . Epidermal neural crest stem cell transplantation as a promising therapeutic strategy for ischemic stroke. CNS Neurosci Ther. 2020;26:670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Shin JA, Lim SM, Jeong SI, Kang JL, Park EM. Noggin improves ischemic brain tissue repair and promotes alternative activation of microglia in mice. Brain Behav Immun. 2014;40:143–154. [DOI] [PubMed] [Google Scholar]
- 243. Wechsler LR, Adeoye O, Alemseged F, et al. . Most promising approaches to improve stroke outcomes: The stroke treatment academic industry roundtable XII workshop. Stroke. 2023;54:3202–3213. [DOI] [PubMed] [Google Scholar]
- 244. Chen J, Ye X, Yan T, et al. . Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011;42:3551–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Möller K, Pösel C, Kranz A, et al. . Arterial hypertension aggravates innate immune responses after experimental stroke. Front Cell Neurosci. 2015;9:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Cui X, Chopp M, Zacharek A, et al. . Chemokine, vascular and therapeutic effects of combination simvastatin and BMSC treatment of stroke. Neurobiol Dis. 2009;36:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Cui LL, Golubczyk D, Tolppanen AM, Boltze J, Jolkkonen J. Cell therapy for ischemic stroke: Are differences in preclinical and clinical study design responsible for the translational loss of efficacy? Ann Neurol. 2019;86:5–16. [DOI] [PubMed] [Google Scholar]
- 248. Savitz SI, Cramer SC, Wechsler L, et al. . Stem cells as an emerging paradigm in stroke 3. Stroke. 2014;45:634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Savitz SI, Chopp M, Deans R, et al. . Stem cell therapy as an emerging paradigm for stroke (STEPS) II. Stroke. 2011;42:825–829. [DOI] [PubMed] [Google Scholar]
- 250. Boltze J, Modo MM, Mays RW, et al. . Stem cells as an emerging paradigm in stroke 4. Stroke. 2019;50:3299–3306. [DOI] [PubMed] [Google Scholar]
- 251. Venkat P, Chopp M, Chen J. Cell-based and exosome therapy in diabetic stroke. Stem Cells Transl Med. 2018;7:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Sutherland BA, Neuhaus AA, Couch Y, et al. . The transient intraluminal filament middle cerebral artery occlusion model as a model of endovascular thrombectomy in stroke. J Cereb Blood Flow Metab. 2016;36:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Saqqur M, Uchino K, Demchuk AM, et al. . Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948–954. [DOI] [PubMed] [Google Scholar]
- 254. Weber RZ, Mulders G, Kaiser J, Tackenberg C, Rust R. Deep learning-based behavioral profiling of rodent stroke recovery. BMC Biol. 2022;20:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Boltze J, Kowalski I, Förschler A, et al. . The stairway: A novel behavioral test detecting sensomotoric stroke deficits in rats. Artif Organs. 2006;30:756–763. [DOI] [PubMed] [Google Scholar]
- 256. Pożarowszczyk N, Kurkowska-Jastrzębska I, Sarzyńska-Długosz I, Nowak M, Karliński M. Reliability of the modified Rankin scale in clinical practice of stroke units and rehabilitation wards. Front Neurol. 2023;14:1064642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Rust R. Towards blood biomarkers for stroke patients. J Cereb Blood Flow Metab. 2021;41:914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Hansra GK, Jayasena T, Hosoki S, et al. . Fluid biomarkers of the neurovascular unit in cerebrovascular disease and vascular cognitive disorders: A systematic review and meta-analysis. Cereb Circ Cogn Behav. 2024;6:100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Hosoki S, Hansra GK, Jayasena T, et al. . Molecular biomarkers for vascular cognitive impairment and dementia. Nat Rev Neurol. 2023;19:737–753. [DOI] [PubMed] [Google Scholar]
- 260. Merali Z, Huang K, Mikulis D, Silver F, Kassner A. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS One. 2017;12:e0171558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Del Campo Fonseca A, Glück C, Droux J, et al. . Ultrasound trapping and navigation of microrobots in the mouse brain vasculature. Nat Commun. 2023;14:5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Janiak J, Li Y, Ferry Y, Doinikov AA, Ahmed D. Acoustic microbubble propulsion, train-like assembly and cargo transport. Nat Commun. 2023;14:4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. González BJ, Creusot RJ, Sykes M, Egli D. How safe are universal pluripotent stem cells? Cell Stem Cell. 2020;26:307–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Xu H, Wang B, Ono M, et al. . Targeted disruption of HLA genes via CRISPR-cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 2019;24:566–578.e7. [DOI] [PubMed] [Google Scholar]
- 265. Huang CY, Liu CL, Ting CY, et al. . Human iPSC banking: Barriers and opportunities. J Biomed Sci. 2019;26:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Deuse T, Hu X, Gravina A, et al. . Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 2019;37:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Liu Y, Yang Y, Suo Y, et al. . Inducible caspase-9 suicide gene under control of endogenous oct4 to safeguard mouse and human pluripotent stem cell therapy. Mol Ther Methods Clin Dev. 2022;24:332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Sahillioglu AC, Schumacher TN. Safety switches for adoptive cell therapy. Curr Opin Immunol. 2022;74:190–198. [DOI] [PubMed] [Google Scholar]
- 269. Schweitzer JS, Song B, Herrington TM, et al. . Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N Engl J Med. 2020;382:1926–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Yoshida S, Kato TM, Sato Y, et al. . A clinical-grade HLA haplobank of human induced pluripotent stem cells matching approximately 40% of the Japanese population. Med. 2023;4:51–66.e10. [DOI] [PubMed] [Google Scholar]
- 271. Zhang P, Raju J, Ullah MA, et al. . Phase I trial of inducible caspase 9 T cells in adult stem cell transplant demonstrates massive clonotypic proliferative potential and long-term persistence of transgenic T cells. Clin Cancer Res. 2019;25:1749–1755. [DOI] [PubMed] [Google Scholar]
- 272. Turnbull MT, Zubair AC, Meschia JF, Freeman WD. Mesenchymal stem cells for hemorrhagic stroke: Status of preclinical and clinical research. npj Regen Med. 2019;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Vaes JEG, Vink MA, de Theije CGM, Hoebeek FE, Benders MJNL, Nijboer CHA. The potential of stem cell therapy to repair white matter injury in preterm infants: Lessons learned from experimental models. Front Physiol. 2019;10:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.