Abstract
Objectives:
To evaluate the effectiveness of COVID-19 vaccinations (initial and booster) during pre-Delta, Delta, and Omicron dominant periods among pregnant people via (1) COVID-19 incident and severe infections among pregnant people who were vaccinated vs. unvaccinated and (2) post-COVID-19 vaccination breakthrough infections and severe infections among vaccinated females who were pregnant vs. non-pregnant.
Design:
Retrospective cohort study using nationally sampled electronic health records data from the National COVID Cohort Collaborative (N3C), December 10, 2020, to June 07, 2022.
Participants:
Cohort 1 included pregnant people (15-55 years), and Cohort 2 included vaccinated females of reproductive age (15-55 years).
Exposures:
(1) COVID-19 vaccination and (2) pregnancy.
Main outcome measures:
Adjusted hazard ratios (aHRs) for COVID-19 incident or breakthrough infections and severe infections (i.e., COVID-19 infections with related hospitalizations).
Results:
In Cohort 1, 301,107 pregnant people were included. Compared to unvaccinated pregnant people, the aHRs for pregnant people with initial vaccinations during pregnancy of incident COVID-19 were 0.77 (95% CI: 0.62, 0.96) and 0.88 (95%CI: 0.73, 1.07) and aHRs of severe COVID-19 infections were 0.65 (95% CI: 0.47, 0.90) and 0.79 (95% CI: 0.51, 1.21) during the Delta and Omicron periods, respectively. Compared to pregnant people with full initial vaccinations, the aHR of incident COVID-19 for pregnant people with booster vaccinations was 0.64 (95% CI: 0.58, 0.71) during the Omicron period. In Cohort 2, 934,337 vaccinated people were included. Compared to vaccinated non-pregnant females, the aHRs of severe COVID-19 infections for people with initial vaccinations during pregnancy was 2.71 (95% CI: 1.31, 5.60) during the Omicron periods.
Conclusions:
Pregnant people with initial and booster vaccinations during pregnancy had a lower risk of incident and severe COVID-19 infections compared to unvaccinated pregnant people across the pandemic stages. However, vaccinated pregnant people still had a higher risk of severe infections compared to non-pregnant females.
Keywords: vaccine effectiveness, pregnant people, COVID-19 pandemic
INTRODUCTION
COVID-19 vaccines are considered one of the best tools in the global fight against the COVID-19 pandemic. Increasing data suggest that the incidence and severity of COVID-19 infections are lower among vaccinated vs. unvaccinated people.[1] While breakthrough infections still occurred among vaccinated individuals, particularly during Delta and Omicron variant periods in the U.S., their lower frequency and severity may be attributable largely to vaccine effectiveness.[2], [3]
Pregnant people with COVID-19 infections appear to be at higher risk of severe COVID-19 outcomes, such as a higher risk of severe pneumonia and intensive care unit (ICU) admission, than non-pregnant people.[4], [5] Immunological and physiological changes during pregnancy may be driving factors for the overall increased risk.[6] Pregnant people with COVID-19 infection may also face a higher risk of adverse pregnancy outcomes, including preeclampsia/eclampsia, preterm birth, and mortality, than pregnant people without COVID-19 infection.[7], [8] Furthermore, infections during pregnancy may be associated with potentially adverse long-term outcomes in infants.[9] Thus, either avoiding or attenuating severe COVID-19 infections is important for pregnant people.
Generally, vaccinations are well tolerated, safe, and effective in pregnancy. For instance, influenza vaccinations have demonstrated effectiveness at preventing influenza infection and are not related to adverse maternal and infant outcomes, such as preterm birth or low birth weight.[10], [11], [12] Also, emerging data suggests COVID-19 vaccinations are safe for pregnant people without major adverse birth outcomes.[13], [14], [15], [16], [17] Studies in different settings have also shown COVID-19 vaccines had a protective effect on pregnant people.[18], [19], [20], [21] However, whether the COVID-19 vaccines work similarly for both pregnant and non-pregnant people has been rarely investigated. While previous research suggested that pregnant people had a higher risk of breakthrough infection compared to non-pregnant females, COVID-19 severity, the stage of the pandemic, and the timing of vaccination relative to pregnancy were not accounted for in the analysis. [22] With the emergence of different viral variants and evolving immunological changes during pregnancy, the pandemic stages and the timing of vaccination regarding pregnancy may impact the effectiveness of vaccines.
The National COVID Cohort Collaborative (N3C) was developed in 2020 to provide an aggregated and harmonized platform for de-identified electronic health record (EHR) data from numerous healthcare sites from all geographic regions in the U.S. for use in COVID-19 research. Our group has phenotyped pregnant people, their pregnancy episodes as well as gestational aging, and their COVID-19 outcomes in N3C.[23] With the largest harmonized EHR data in the U.S., we focused on two interconnected questions for this study. First, we aimed to evaluate the extent to which COVID-19 vaccination could reduce the risk of incident and severe COVID-19 infections among pregnant people who were vaccinated vs. unvaccinated. Second, we aimed to evaluate whether and how the risk of breakthrough and severe COVID-19 infections would vary among vaccinated females comparing those who were pregnant vs. non-pregnant. We stratified both analyses by the stages of the pandemic and vaccination timing regarding pregnancy.
MATERIALS AND METHODS
Overall study setting and design
We conducted a retrospective cohort analysis of a prospectively collected EHR repository. The N3C consortium is a high-granularity EHR data repository of de-identified, patient-level data from medical centers across the U.S. The overall N3C design, data ingestion and harmonization, and sampling approach have been described previously.[24], [25] Additional details about N3C and ethical review are in supplementary materials.
Our dataset included COVID-19 vaccines currently given Food and Drug Administration (FDA) authorization, including two mRNA vaccines (Pfizer-BioNTech [BNT162b2] and Moderna [mRNA-1273]) and a viral vector vaccine (Johnson & Johnson/Janssen [JNJ-784336725]), as well as other vaccines (e.g., AstraZeneca). We categorized initial full vaccination as completion of the recommended dosing regimen of any vaccine (i.e., at least two doses for mRNA or other vaccines and one dose for the Janssen vaccine) and partial vaccination as one dose of mRNA vaccine. We categorized any additional doses after 90 days of initial dosing (e.g., a 3rd mRNA dose) as booster vaccinations and only accounted for the first booster vaccination in this analysis.[26] The concepts and codes to define vaccinations can be found in supplementary table 1.
Study period and analytic cohort selection
Our study observation period occurred from December 10, 2020, to June 7, 2022. We used N3C data version 84 (released July 7, 2022), noting that we truncated the observation period one month earlier to allow for adequate time for data reporting. We defined the beginning of the observation period, December 10, 2020, based on the date the FDA first approved COVID-19 vaccination27. We chose the end date, June 7, 2022, to allow 90 days prior to when the bivalent booster became available (September 9, 2022) so that this analysis amply precludes bivalent booster impact (separate analysis forthcoming).
We stratified the analysis by variants predominant during the pandemic: pre-Delta (December 10, 2020, to June 20, 2021), Delta (June 21, 2021, to December 25, 2021), and Omicron (December 26, 2021, to June 7, 2022), with predominance periods defined based on CDC estimates for the US in general.[27]
Cohort construction and bias control
Figure 1 illustrates our cohort construction. We included data from 43 total contributing sites, excluding sites in the bottom quartile of vaccination rates. Next, we generated subsets of this cohort to identify pregnant people (Cohort 1) and vaccinated females (Cohort 2).
Figure 1.
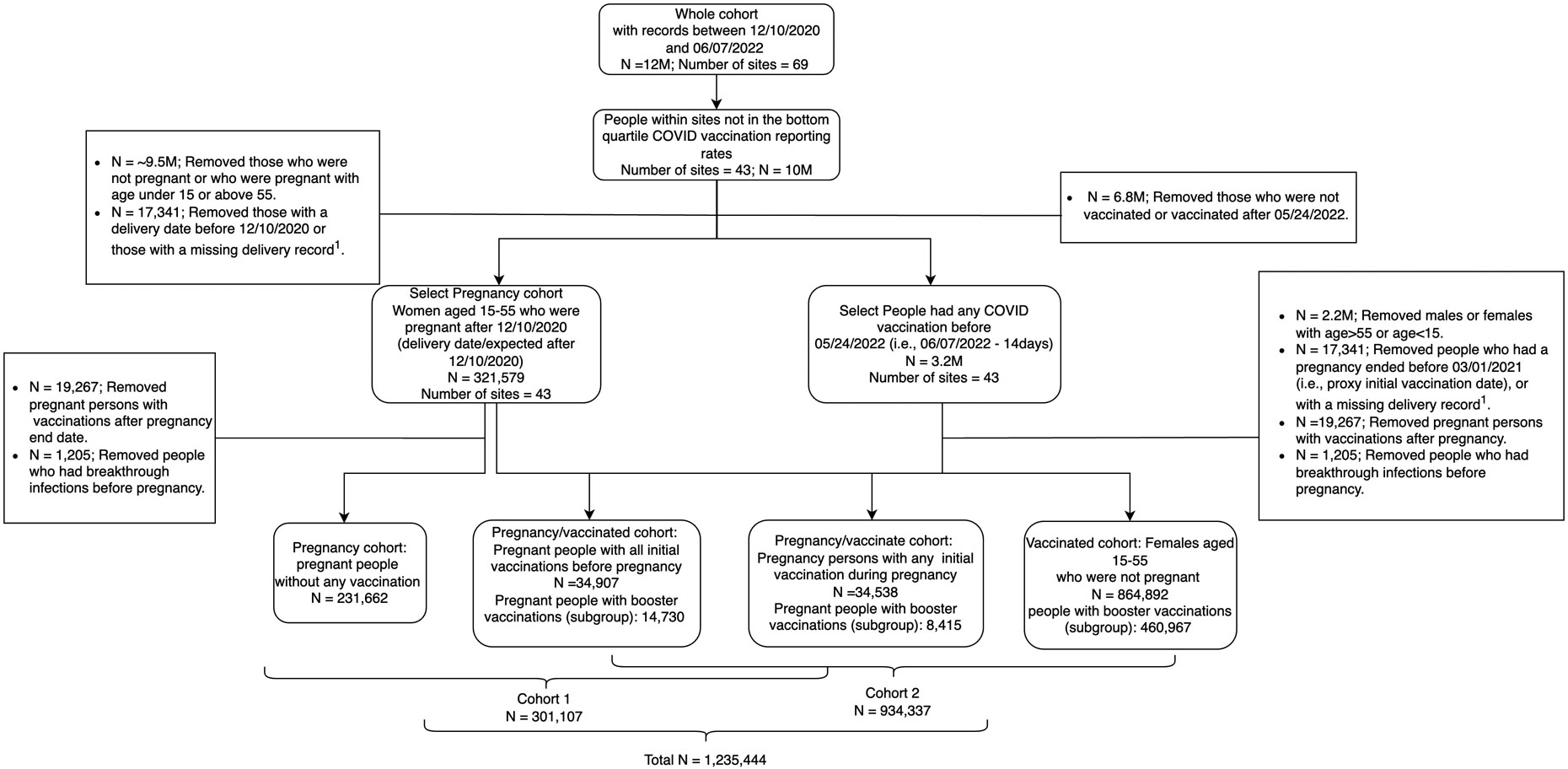
For the analysis of initial vaccinations with Cohort 1, we included pregnant people aged 15-55 with COVID-19 vaccinations during or before their pregnancy on or after December 10, 2020 (i.e., the earlies date that COVID-19 vaccination was approved by FDA). For unvaccinated pregnant people, we included those with pregnancy end dates after March 1, 2021. We used March 1, 2021, the first peak of the initial full vaccination date among vaccinated pregnant people, as the “proxy initial vaccination date.” Similarly, for the analysis of booster vaccinations with Cohort 1, we included pregnant people with a booster vaccination during or before their pregnancy after December 1, 2021. For unvaccinated pregnant people, we included those with pregnancy end dates after December 1, 2021. We used December 1, 2021, the peak of booster vaccination date among vaccinated pregnant people, as the “proxy booster vaccination date.” We defined proxy vaccination dates to avoid ascertainment bias in person-time at risk being longer in the unvaccinated vs. vaccinated groups.
For analysis of initial and booster vaccinations with Cohort 2, we included non-pregnant vaccinated (i.e., those initial vaccinations/ booster vaccinations) females of reproductive age to match with the pregnant vaccinated (i.e., those initial vaccinations/ booster vaccinations) females.
Cohort 1 analysis (all pregnant people)
In the analysis for initial and booster vaccinations in Cohort 1, we compared vaccinated vs. unvaccinated pregnant people aged 15-55 to evaluate the vaccine effectiveness in pregnancy via incident and severe COVID-19 infections. We categorized vaccinated pregnant people based on vaccination timing into four groups: received any initial or booster vaccination during pregnancy (vaccinated during pregnancy) and received all initial or booster vaccinations before pregnancy (vaccinated before pregnancy).
To further investigate the effectiveness of booster vaccinations, we compared pregnant people with full initial vaccinations and those with booster vaccinations via incident and severe COVID-19 infections.
Cohort 2 (all vaccinated females)
In the analysis for initial and booster vaccinations in Cohort 2, we compared pregnant people vaccinated during or before pregnancy (same as Cohort 1) vs. non-pregnant females aged 15-55 to examine the impact of pregnancy on COVID-19 vaccine effectiveness.
For both Cohorts 1 and 2, we excluded pregnant people with COVID-19 infections after their initial/booster vaccination (or proxy initial/booster vaccination dates) but before pregnancy. In the analysis for booster vaccinations, the included people were a subsample of the analysis cohort for initial vaccination.
Exposures definitions
In the analysis with Cohort 1 (all pregnant people), vaccination status is the key exposure category. In the analysis with Cohort 2 (all vaccinated females), pregnancy is the key exposure category.
Outcomes definitions
Our first co-primary outcome was incident and breakthrough COVID-19 infections among vaccinated and unvaccinated people, respectively. Our incident or breakthrough COVID-19 positivity definition only included positive results based on: 1) PCR-positivity, 2) antigen-positivity, or 3) ICD diagnosis condition, in that hierarchical order.[24] Breakthrough infection was defined by incident COVID-19 positivity after 14 days of the last vaccine received for initial/booster vaccination.[2], [22]
Our second co-primary outcome was severe COVID-19 infections. We defined severe COVID-19 infections as incident or breakthrough COVID-19 infections with hospitalization records within 14 days before or 45 days after infections that do not overlap within 7 days before or after the recorded delivery or pregnancy end date (to avoid confounding of hospitalization due to deliveries or other pregnancy outcomes).[2], [28] The concepts and codes to define COVID-19 infections and hospitalizations can be found in supplementary table 1.
Person-time at-risk for vaccinated people accrued from 14 days after their last date of vaccination (initial/booster) until the earliest date of COVID-19 incident or severe infections, death, transfer to hospice, or the date of their last record in N3C. Person-time at-risk for unvaccinated pregnant people accrued similarly from 14 days after the proxy initial or booster vaccination date. More details on person-time at risk are illustrated in supplementary figure 1. In the comparison of pregnant people with booster vaccinations vs. those with full initial vaccinations, we used the full initial vaccination date plus 90 days as the start date to calculate person-time-at-risk for pregnant people without booster vaccinations to avoid ascertainment bias. The 90-day time window was based on the definition of booster vaccinations, as mentioned in the previous section.
Other outcomes included: (1) ICU admission within 45 days after COVID-19 infection; (2) severe COVID-19 disease with invasive ventilation or extracorporeal membrane oxygenation (ECMO) treatment within 45 days after COVID-19 infection; and (3) 30-day mortality after COVID-19 infection [2], [29]. Note that ICU admission, ventilation, and ECMO outcomes are hospital-based and, therefore, subsumed in the second co-primary outcome of severe COVID-19 infection.
Statistical analysis
We present summary characteristics at the time of the first initial COVID-19 vaccination date and proxy vaccination date for vaccinated and unvaccinated people, respectively. We used descriptive statistics only for outcomes with low counts in various groups.
We applied Cox proportional hazard models to estimate adjusted hazard ratios (aHR) with 95% confidence intervals (CI). We clustered standard errors at data partner sites to account for heterogeneity across sites. We adjusted for individual-level sociodemographic characteristics, including age, race, ethnicity, specific clinical comorbidities, Charlson Comorbidity Index (CCI), and whether people had any prior COVID-19 infections before initial/booster or proxy vaccinations.
We stratified the models by the variant predominance periods (i.e., pre-Delta, Delta, and Omicron) to allow for different hazard ratio estimations during our observation period, and we assessed model assumptions accordingly. Person-time at-risk was also calculated for each variant stratum (i.e., one within each stratum). We further separated the analysis for initial and booster vaccinations.
Of note, given the timing of booster vaccinations occurring mainly during the Omicron period, we only analyzed booster vaccinations during the Omicron period. Since we anticipated pregnant people to possibly undergo more frequent COVID-19 testing than non-pregnant people (for instance, for the delivery hospitalization), we conducted an a priori sensitivity analysis restricting non-pregnant people in Cohort 2 to people with at least one recorded COVID-19 test. Due to the concern of different effectiveness of mRNA and non-mRNA vaccines,[30] we additionally conducted sensitivity analyses only including people with mRNA vaccinations (i.e., Pfizer and Moderna).
Details about covariates and modeling can be found in the supplementary materials. All analyses were conducted in the N3C Data Enclave using PySpark and SparkR/R31.
Patient and Public Involvement: This is a retrospective observational study, patients were not involved.
RESULTS
Cohort characteristics
A total of over 12 million people had records included in N3C from December 10, 2020, to June 7, 2022 (Figure 1). We included 301,107 people in Cohort 1 and 934,337 people in Cohort 2.
Among Cohort 1 (pregnant people), 34,907 (11.6%) had any initial vaccination during pregnancy, another 34,538 (11.5%) had all initial vaccinations before pregnancy, and 231,662 (76.9%) did not have any COVID-19 vaccinations recorded (Table 1). The median age was around 32.0 years for pregnant people with vaccinations and 30.2 (IQR 25.0, 33.8) for those without vaccination. Among those who had any initial vaccination during pregnancy, 28,537 (81.8%) had full initial vaccinations, 8,415 (24.1%) had booster vaccination, and 25,540 (73.2%) had received a Pfizer vaccine for their first dose. Among those who had all initial vaccinations before pregnancy, 31,530 (91.6%) had full initial vaccination, 14,730 (42.6%) had booster vaccination, and 23,547 (68.2%) had received a Pfizer vaccine for their first dose.
Table 1:
Characteristics of included people in N3C cohort, December 10, 2020 - June 07, 2022
| Cohort 1 | Cohort 1∣ Cohort 2 | Cohort 1∣ Cohort 2 | Cohort 2 | |
|---|---|---|---|---|
| Non-vaccinated pregnant people (n=231,662) | Pregnant people with initial vaccination during pregnancy (n=34,907) | Pregnant people with initial vaccination before pregnancy (n=34,538) | Non-pregnant vaccinated females aged 15 - 55 years old (n= 864,892) | |
| Age (mean (IQR)) | 30.2 (25, 33.8) | 31.9 (28.0, 36.1) | 32.2 (28.2, 35.9) | 37.1 (28.1, 47.2) |
| BMI | ||||
| BMI in kg/m2, median (IQR) | 27.5 (23.3, 33.5) | 27.4 (23.3, 33.2) | 27.5 (23.4, 33.2) | 29.4 (24.3, 36.0) |
| Missing | 166,165 (69.92%) | 22,776 (65.25%) | 18,986 (54.97%) | 551,220 (63.7%) |
| Race/ethnicity | ||||
| White/non-Latinx | 118,663 (51.22%) | 18,731 (53.66%) | 20,545 (59.49%) | 514,957 (59.54%) |
| Black/non-Latinx | 42,393 (18.30%) | 4,574 (13.10%) | 3,877 (11.23%) | 120,639 (13.95%) |
| Any race/Latinx | 38,382 (16.57%) | 7,009 (20.08%) | 5,699 (16.50%) | 125,068 (14.46%) |
| Asian American | 7,880 (3.40%) | 1,850 (5.30%) | 1,770 (5.12%) | 30,391 (3.51%) |
| Other | 3,376 (1.46%) | 676 (1.94%) | 578 (1.67%) | 20,016 (2.31%) |
| Missing | 20,968 (9.05%) | 2,067 (5.92%) | 2,069 (5.99%) | 53,821 (6.22%) |
| Region | ||||
| Midwest | 32,413 (13.99%) | 2,914 (8.35%) | 2,736 (7.92%) | 63,146 (7.30%) |
| South | 34,333 (14.82%) | 8,284 (23.73%) | 8,071 (23.37%) | 159,907 (18.49%) |
| West | 24,321 (10.50%) | 3,951 (11.32%) | 5,389 (15.60%) | 102,324 (11.83%) |
| Northeast | 21,006 (9.07%) | 2,620 (7.51%) | 2,125 (6.15%) | 52,118 (6.03%) |
| Missing | 119,589 (51.62%) | 17,138 (49.10%) | 16,217 (46.95%) | 487,397 (56.35%) |
| Comorbidities | ||||
| Asthma | 30,038 (12.97%) | 4,719 (13.52%) | 4,781 (13.84%) | 112,973 (13.06%) |
| COPD | 933 (0.40%) | 144 (0.41%) | 187 (0.54%) | 12,467 (1.44%) |
| Chronic kidney disease | 1,962 (0.85%) | 353 (1.01%) | 368 (1.07%) | 17,242 (1.99%) |
| Hypertension | 2,789 (1.20%) | 424 (1.21%) | 431 (1.25%) | 10,338 (1.20%) |
| Cardiovascular disease | 24,475 (10.56%) | 3,362 (9.63%) | 3,201 (9.27%) | 89,477 (10.35%) |
| Type I diabetes | 2,632 (1.14%) | 427 (1.22%) | 394 (1.14%) | 8,743 (1.01%) |
| Type II diabetes | 9,780 (4.22%) | 1,749 (5.01%) | 1,698 (4.92%) | 73,814 (8.53%) |
| CCI Index | ||||
| CCI=0 | 43,452 (18.76%) | 6,785 (19.44%) | 6,895 (19.96%) | 165,799 (19.17%) |
| CCI=1 | 9,371 (4.05%) | 1,600 (4.58%) | 1,820 (5.27%) | 63,926 (7.39%) |
| CCI=2 | 169,671 (73.24%) | 25,068 (71.81%) | 24,391 (70.62%) | 571,002 (66.02%) |
| CCI=3+ | 9,168 (3.96%) | 1,454 (4.17%) | 1,432 (4.15%) | 64,165 (7.42%) |
| First vaccine manufacturer | ||||
| Pfizer/BioNTech | 25,540 (73.17%) | 23,547 (68.18%) | 576,921 (66.70%) | |
| Moderna | 8,026 (22.99%) | 9,130 (26.43%) | 239,928 (27.74%) | |
| Janssen | 1,195 (3.42%) | 1,773 (5.13%) | 46,919 (5.42%) | |
| Other | 146 (0.42%) | 88 (0.25%) | 1,124 (0.13%) | |
| Insurance type | ||||
| Private insurance | 32,791 (14.15%) | 6,726 (19.27%) | 7,819 (22.64%) | 160,659 (18.58%) |
| Medicare/Medicaid | 27,857 (12.02%) | 3,843 (11.01%) | 3,596 (63.24%) | 83,347 (9.64%) |
| Missing | 168,582 (72.77%) | 23,293 (66.73%) | 21,841 (3.71%) | 596,701 (68.99%) |
| Other | 2,432 (1.05%) | 1,045 (2.99%) | 1,282 (7.92%) | 24,185 (2.80%) |
| Prior COVID-19 positive | 4,747 (2.05%) | 2,097 (6.01%) | 1,512 (4.38%) | 64,511 (7.46%) |
| First vaccination visit type | ||||
| Outpatient visit | 17,373 (49.77%) | 15,431 (44.68%) | 345,383 (39.93%) | |
| Emergency visit | 131 (0.38%) | 97 (0.28%) | 1,729 (0.20%) | |
| Other | 153 (0.44%) | 265 (0.77%) | 6,406 (0.74%) | |
| Vaccination status | ||||
| Full initial vaccination | 28,537 (81.75%) | 31,530 (91.29%) | 765,929 (88.56%) | |
| Partial initial vaccination | 6,370 (18.25%) | 3,008 (8.71%) | 98,963 (11.44%) | |
| Booster vaccination (with full initial vaccination) | 8,415 (24.11%) | 14,730 (42.6%) | 460,967 (53.30%) | |
| Outcomes | ||||
| COVID-19 infection with hospitalization | 3,868 (1.67%) | 352 (1.01%) | 185 (0.54%) | 3,717 (0.43%) |
| Breakthrough/incident COVID-19 infection | 39,313 (16.97%) | 4,323 (12.40%) | 4,577 (13.30%) | 159,106 (18.40%) |
| Ventilation and/or ECMO | 162 (0.07%) | <20 | <20 | 118 (0.01%) |
| ICU | 52 (0.02%) | <20 | <20 | 84 (0.01%) |
Abbreviations: N3C=National COVID Cohort Collaborative; IQR= inter quantile range; CCI= Charlson Ccomorbidity Iindex; ER= emergency department; ECMO= extracorporeal membrane oxygenation; ICU= intensive care unit; COPD= Chronic obstructive pulmonary disease.
Prior COVID-19 positive was defined as whether they have prior COVID-19 diagnosis before the first vaccination date in Cohort 1 or before December.10, 2020 for unvaccinated pregnant people in Cohort 2.
COVID-19 severity outcomes include any hospitalization, including for a delivery, within 14 days before or 45 days after COVID-19 diagnosis; COVID-19-related hospitalization within 14 days before or 45 days after COVID-19 diagnosis but not within +/− 7 days of delivery date; ICU admission within 45 days after COVID-19 diagnosis; invasive ventilation or ECMO within 45 days after COVID-19 diagnosis.
Numbers in a category with number < 20 must be obfuscated to comply with the small number reporting rule for N3C.
Among Cohort 2 (vaccinated females), 864,892 (92.6%) were non-pregnant vaccinated females (Table 1). The characteristics of the vaccinated pregnant groups were the same as above for Cohort 1. Among vaccinated non-pregnant females, the median age was 37.1 (IQR 28.1, 47.2) years, 765,929 (88.6%) had full initial vaccinations, 460,967 (53.3%) had booster vaccination, and 576,921 (66.7%) had received a Pfizer vaccine for their first dose.
Cohort 1 findings: Pregnant people with initial vaccinations had a lower risk of incident COVID-19 infections compared to unvaccinated pregnant people during pre-Delta and Delta periods, and they had a lower risk of severe infections consistently throughout the pandemic stages
In Cohort 1, 4,323 (12.4%) and 4,577 (13.3%) of pregnant people with initial vaccinations during and before their pregnancy had incident COVID-19 infections across the entire study period, and 352 (1.0%) and 185 (0.5%) of them had severe COVID-19 infections, respectively. Among unvaccinated pregnant people, 39,313 (17.0%) had incident COVID-19 infections, and 3,868 (1.7%) developed severe COVID-19 infections (Table 1).
The adjusted models for incident COVID-19 infections resulted in aHRs of 0.36 (95% CI: 0.25, 0.51), 0.77 (95% CI: 0.62, 0.96), 0.88 (95% CI: 0.73, 1.07) comparing pregnant people with any initial vaccination during their pregnancy with unvaccinated pregnant people during the pre-Delta, Delta, and Omicron periods, respectively (Table 2). For severe COVID-19 infections, the aHRs were 0.65 (95% CI: 0.47, 0.90) and 0.79 (95% CI: 0.51, 1.21) for the same group during the Delta and Omicron periods, respectively. The model results for both co-primary outcomes for pregnant people with initial vaccinations before their pregnancy and unvaccinated pregnant people were similar.
Table 2:
Risk of breakthrough or incident and severe COVID-19 infections among pregnant people (Cohort 1) by vaccination status and variant predominant periods in N3C Cohort, December 10, 2020- June 7, 2022
| Pre-Delta variant dominant period | Delta variant dominant period | Omicron variant dominant period | ||||
|---|---|---|---|---|---|---|
| Events/100 women- months (95% CI) |
Adjusted Model (M3) HR (95% CI)3 |
Events/100 women- months (95% CI) |
Adjusted Model (M3) HR (95% CI)3 |
Events/100 women- months (95% CI) |
Adjusted Model (M3) HR (95% CI)3 |
|
| Breakthrough or incident COVID-19 infection | ||||||
| Initial vaccinations | ||||||
| Unvaccinated pregnant people | 0.36 (0.22, 0.5) | Ref. | 1.36 (1.08, 1.63) | Ref. | 4.16 (3.59, 4.74) | Ref. |
| Vaccinated pregnant people with initial vaccinations during pregnancy | 0.12 (0, 0.42) | 0.36 (0.25, 0.51) | 0.91 (0.31, 1.51) | 0.77 (0.62, 0.96) | 3.7 (2.44, 4.96) | 0.88 (0.73, 1.07) |
| Vaccinated pregnant people with initial vaccinations before pregnancy | - | 0.07(0.04, 0.14) | 0.63 (0.16, 1.1) | 0.60 (0.53, 0.68) | 2.82 (1.79, 3.84) | 1.05 (0.95, 1.16) |
| Booster vaccinations | ||||||
| Unvaccinated pregnant people | 4.16 (3.59, 4.74) | Ref. | ||||
| Vaccinated pregnant people with booster vaccination during pregnancy | 2.55 (1.05, 6.01) | 0.52 (0.47, 0.58) | ||||
| Vaccinated pregnant people with booster vaccination before pregnancy | 3.21 (1.57, 4.85) | 0.52 (0.41, 0.64) | ||||
| Vaccinated pregnant people with initial vaccinations during pregnancya | 3.96 (0.99, 6.53) | Ref. | ||||
| Vaccinated pregnant people with booster vaccination during pregnancy | 2.55 (1.05, 6.01) | 0.64 (0.58, 0.71) | ||||
| Severe COVID-19 infection | ||||||
| Initial vaccinations | ||||||
| Unvaccinated pregnant people | 0.15 (0.06, 0.23) | Ref. | 0.37 (0.2, 0.54) | Ref. | ||
| Vaccinated pregnant people with initial vaccinations during pregnancy | 0.08 (0, 0.26) | 0.65 (0.45, 0.90) | 0.29 (0, 0.64) | 0.79 (0.51, 1.21) | ||
| Vaccinated pregnant people with initial vaccinations before pregnancy | 0.03 (0, 0.12) | 0.24(0.15, 0.39) | 0.12 (0, 0.32) | 0.39 (0.27, 0.56) | ||
| Booster vaccinations | ||||||
| Unvaccinated pregnant people | 0.45 (0.23, 0.53) | Ref | ||||
| Vaccinated pregnant people with booster vaccinations during pregnancy | 0.14 (0, 0.58) | 0.35 (0.22, 0.54) | ||||
| Vaccinated pregnant people with full initial vaccinations during pregnancya | 0.43 (0, 1.36) | Ref | ||||
| Vaccinated pregnant people with booster vaccination during pregnancy | 0.14 (0, 0.58) | 0.35 (0.24, 0.50) | ||||
Abbreviations: N3C=National COVID Cohort Collaborative; HR= hazard ratio; CI= confidence interval; Ref= reference; CCI= Charlson comorbidity index.
Model results were generated from Cox proportional hazard model with data partner site level clustered standard error stratified by pandemic variant periods.
Models were adjusted for sociodemographic variables such as age, race/ethnicity, region, clinical covariates such as comorbidities, CCI and prior COVID-19 positivity. Full model result was presented in supplementary table 2.
The number of people with records during variants periods is described in supplementary table 6.
Numbers in a category with number < 20 must be obfuscated to comply with the small number reporting rule for N3C.
We used initial vaccination date plus 90 days as the start follow up time for vaccinated pregnant people with initial vaccinations during pregnancy to compare with those who had booster vaccinations during their pregnancy. We excluded those with COVID-19 incidence/severe infections during the 90 days.
Compared with unvaccinated pregnant people, the aHR of incident COVID-19 infections for those with booster vaccinations during their pregnancy was 0.52 (95% CI: 0.47, 0.58) during the Omicron period, and the aHR of severe COVID-19 infections was 0.35 (95% CI: 0.22, 0.54). The result of the comparison between unvaccinated pregnant people and pregnant people with booster vaccinations before their pregnancy was similar. In addition, compared with pregnant people with full initial vaccinations during their pregnancy, the aHR of incident COVID-19 infections for pregnant people with booster during their pregnancy was 0.64 (95% CI: 0.58, 0.71), and the aHR of severe COVID-19 infections was 0.35 (95% CI: 0.24, 0.50).
Cohort 2 findings: Pregnant people had a lower risk of COVID-19 infections but a higher risk of severe COVID-19 infections compared to vaccinated non-pregnant people during the Delta and Omicron periods
In Cohort 2, among vaccinated non-pregnant females, 159,106 (18.4%) had breakthrough COVID-19 infections during the entire study period, of whom 3,717 (0.43%) developed severe COVID-19 infections (Table 1).
For breakthrough COVID-19 infections, the aHRs were 0.92 (95% CI: 0.61, 1.40), 0.79 (95% CI: 0.69, 0.91), and 0.61 (95% CI: 0.55, 0.69) comparing pregnant people with initial vaccinations during their pregnancy with vaccinated non-pregnant people for the pre-Delta, Delta, and Omicron periods, respectively (Table 3). For the same comparison, the aHRs for severe COVID-19 infections were 3.25 (95% CI: 1.76, 5.99) and 2.71 (95% CI: 1.31, 5.60) during the Delta and Omicron periods, respectively. The adjusted aHRs of breakthrough and severe COVID-19 infections for pregnant people with initial vaccinations before their pregnancy vs. vaccinated non-pregnant people were similar.
Table 3:
Risk of breakthrough or incident and severe COVID-19 infections among vaccinated people (Cohort 2) by pregnancy status and variant predominant periods in N3C Cohort, December 10, 2020- June 7, 2022.
| Pre-Delta variant dominant period | Delta variant dominant period | Omicron variant dominant period | ||||
|---|---|---|---|---|---|---|
| Events/100 women-months (95% CI) |
Adjusted Model |
Events/100 women- months (95% CI) |
Adjusted Model |
Events/100 women- months (95% CI) |
Adjusted Model | |
| HR (95% CI)3 | HR (95% CI)3 | HR (95% CI)3 | ||||
| Breakthrough COVID-19 infection | ||||||
| Initial vaccinations | ||||||
| Vaccinated non-pregnant females | 0.13 (0.08, 0.18) | Ref. | 1.34 (1.2, 1.48) | Ref. | 5.19 (4.88, 5.49) | Ref. |
| Vaccinated pregnant people with initial vaccinations during pregnancy | 0.12 (0, 0.42) | 0.92 (0.61, 1.40) | 0.91 (0.31, 1.51) | 0.79 (0.69, 0.91) | 3.7 (2.44, 4.96) | 0.61 (0.55, 0.69) |
| Vaccinated pregnant people with initial vaccinations before pregnancy | - | 0.86 (0.66, 1.13) | 0.63 (0.16, 1.1) | 0.76 (0.67, 0.87) | 2.82 (1.79, 3.84) | 0.71 (0.63, 0.80) |
| Booster vaccination | ||||||
| Vaccinated non-pregnant females with booster vaccination | 4.15 (3.70, 4.6) | Ref. | ||||
| Vaccinated pregnant people with booster vaccination during pregnancy | 3.53 (1.05, 6.01) | 0.79 (0.69, 0.89) | ||||
| Vaccinated pregnant people with booster vaccination before pregnancy | 3.21 (1.57, 4.85) | 0.73 (0.64, 0.84) | ||||
| Severe COVID-19 infection | ||||||
| Initial vaccinations | ||||||
| Vaccinated non-pregnant females | 0.03 (0.01, 0.06) | Ref. | 0.11 (0.07, 0.16) | Ref. | ||
| Vaccinated pregnant people vaccinated during pregnancy | 0.08 (0, 0.26) | 3.25 (1.76, 5.99) | 0.29 (0, 0.64) | 2.71 (1.31, 5.60) | ||
| Vaccinated pregnant people vaccinated before pregnancy | 0.03 (0, 0.12) | 1.29 (0.88, 1.88) | 0.12 (0, 0.32) | 1.70 (1.08, 2.69) | ||
| Booster vaccinations | ||||||
| Vaccinated non-pregnant females with booster vaccination | 0.07 (0.01, 0.13) | Ref. | ||||
| Vaccinated pregnant people with booster vaccination during pregnancy | 0.14 (0, 0.58) | 3.43 (2.59, 4.55) | ||||
Abbreviations: N3C=National COVID Cohort Collaborative; HR= hazard ratio; CI= confidence interval; Ref= reference; CCI= Charlson comorbidity index.
Model results were generated from Cox proportional hazard model with data partner site level clustered standard error stratified by pandemic variant periods.
Models were adjusted for sociodemographic variables such as age, race/ethnicity, region, clinical covariates such as comorbidities, CCI and prior COVID-19 positivity. Full model result was presented in supplementary table 3.
The number of people with records during variants periods is described in supplementary table 6.
Numbers in a category with number < 20 must be obfuscated to comply with the small number reporting rule for N3C.
We used initial vaccination date plus 90 days as the start follow up time for vaccinated pregnant people with initial vaccinations during pregnancy to compare with those who had booster vaccinations during their pregnancy. We excluded those with COVID-19 incidence/severe infections during the 90 days.
Compared with non-pregnant people with booster vaccinations, the aHRs for breakthrough infections during the Omicron period were 0.79 (95% CI: 0.69, 0.89) and 0.73 (95% CI: 0.64, 0.84) for pregnant people with booster vaccinations during and before their pregnancy, respectively. Furthermore, the aHRs for severe COVID-19 infections was 3.43 (95% CI: 2.59, 4.55) comparing non-pregnant people with booster vaccinations and pregnant people with booster vaccinations during their pregnancy.
More descriptive results on person-time at risk distribution, the timing of vaccinations, and incident/breakthrough infections can be found in supplementary figures 2 - 7. Full primary model results can be found in supplementary tables 2 and 3. The sensitivity analyses limiting the cohort to only people with at least one recorded COVID-19 test or those vaccinated receiving only mRNA vaccines showed similar results as our main analysis (supplementary tables 4 and 5). Descriptive numbers of included people stratified by pre-Delta, Delta, and Omicron predominant periods can be found in supplementary table 6.
DISCUSSION
In the U.S. nationally sampled cohort study, pregnant people who had initial vaccinations during or before their pregnancy were at a lower risk of incident and severe COVID-19 infection compared to unvaccinated pregnant people before the Omicron period. This reduction was more pronounced for severe COVID-19 infections with greater protection during the Delta period (nearly 60% lower risk for pregnant people with initial vaccination during pregnancy). The protective effect of initial vaccinations appeared to decrease during the Omicron period. However, booster vaccinations further protected pregnant people from incident and severe COVID-19 infections during the Omicron period compared to both unvaccinated and initial series only vaccinated pregnant people. Additionally, although vaccinated pregnant people did not appear to be at higher risk for incident COVID-19 infections compared to vaccinated non-pregnant females, they did face a higher risk of severe COVID-19 infections. Our findings suggested that vaccinated pregnant people continued to be at higher risk for severe COVID-19 infections and should be considered a priority population for further booster vaccinations. We also suggest pregnant people seek medical attention if they experience symptoms of COVID-19.
COVID-19 vaccination is advantageous for various groups of people to reduce the risk of incident and severe COVID-19 infections,[2], [29], and booster vaccinations could further protect pregnant people after initial vaccinations[31], [32], [33]. These observation holds for pregnant people, as we and others have found. Simultaneously, there is reassuring data on the COVID-19 vaccine safety profile for maternal and newborn outcomes among pregnant people, as demonstrated by others and our team.[16], [17], [34] Our results suggest that vaccination during pregnancy or before the pregnancy could both be effective for preventing incident and severe COVID-19 infections. Thus, pregnant people (or those intending a pregnancy soon), their providers, and policymakers should feel confident in concluding that the currently known benefits of vaccination outweigh any known or hypothetical risks.
Our study also provides unique findings to date. We found a higher risk of severe COVID-19 infection post-COVID-19 vaccination among pregnant vs. non-pregnant people considering both initial vaccinations and booster vaccinations people across the pandemic stages. This higher risk may largely be driven by the underlying risk of COVID-19 infection severity among pregnant people, which is consistent with the findings from other studies.[22], [35] Relative to published reports, our study accounted for the potential variations in COVID-19 infection risk by the stage of the pandemic, the vaccination timing relative to the pregnancy, and vaccination status (full initial vs. booster). Though our data suggested that the monovalent boosters were less protective against incident COVID-19 infections during the Omicron predominance period than vaccinations for prior variants periods (30% vs. 60% risk reduction), our data demonstrate that booster, monovalent vaccinations still offered protection for vaccinated pregnant people. Bivalent booster vaccinations are hoped to offer even greater protection for pregnant people. We aim to better adjudicate booster vaccination effectiveness once more such records accrue in N3C.
Despite being the largest and first study of its kind, our study faces several limitations. 1) As with any dataset composed of EHR data, the risk of misclassification and the rate of missingness is high. We believe that the vaccination rates are likely underreported in N3C,[24] a non-differential misclassification biasing our results towards the null. By excluding data partner sites that reported vaccination rates less than the lowest quartile, we hope to have minimized potential bias from this misclassification. 2) Pregnant people may have received more frequent testing or more frequent monitoring for incident or severe COVID-19 infections than non-pregnant people. We attempted to ascertain this potential source of bias via a sensitivity analysis limiting the analytic group to only people with at least one COVID-19 test result recorded, and the attenuated point estimates still suggested similar findings. 3) Hospitalization rates are high within our pregnant cohorts, likely due to confounding of hospitalization due to delivery. To address this issue, we defined severe COVID-19 infections as any COVID-19-related hospitalization but not within 7 days of delivery. Relatedly, the overall rates of other severe COVID-19 outcomes are, fortunately, rare in this dataset, which could be secondary to underreporting. 4) As with all N3C data, source validation is not possible; our attempts at phenotyping the study population, healthcare sites, exposures, and outcomes have been established in other validated studies or published previously with N3C data.[2], [23] 5) As this is a retrospective cohort study, we are not able to obtain information about the way that COVID-19 testing was implemented. To address the potential bias in COVID-19 testing, we conducted a sensitivity analysis by limiting our dataset to only those with at least one COVID-19 test record. Notwithstanding these limitations, our work presents some of the most compelling data to date on understanding the real-world COVID-19 vaccine effectiveness through various variant periods among pregnant people.
Conclusion
In conclusion, we find that pregnant people who are vaccinated during or before their pregnancy had a lower risk of developing incident or severe COVID-19 infection compared to unvaccinated pregnant people, and booster vaccinations could provide further protection for pregnant people during the Omicron period after the initial series of vaccinations. However, vaccinated pregnant people still face a higher risk of severe COVID-19 infection post-vaccination compared to vaccinated non-pregnant females. Thus, pregnant people should continue to get vaccinated to protect against COVID-19 infections and should be considered a priority population for booster vaccinations. With increasingly reassuring data of no known adverse maternal or infant outcomes associated with COVID-19 vaccinations, pregnant people and their providers should feel confident about pursuing vaccinations during pregnancy.
Supplementary Material
Key messages:
-
What is already known on this topic
Pregnant people are at higher risk of severe COVID-19 outcomes and adverse pregnancy outcomes (e.g., preeclampsia/eclampsia, preterm birth, or fetal death).
COVID-19 vaccination can reduce the risk of COVID-19 infection and severe COVID-19 outcomes and is safe for use among pregnant people.
-
What this study adds
The protective effect of initial COVID-19 vaccines (i.e., two doses for mRNA or other vaccines and one dose for the Janssen vaccine) waned down during the Omicron period, but booster vaccines further protected pregnant people from incident and severe COVID-19 infection during the Omicron period.
Vaccinated pregnant people still had a higher risk of severe COVID-19 infections compared to vaccinated non-pregnant people during Delta and Omicron periods.
-
How this study might affect research, practice, or policy
Pregnant people should continue to get booster COVID-19 vaccinations, and be considered as a priority group to get protection interventions during public health crises
Acknowledgements
This research was possible because of the patients whose information is included within the data, the organizations, and the scientists who have contributed to the ongoing development of this community resource.
N3C Attribution
The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave covid.cd2h.org/enclave and supported by CD2H - The National COVID Cohort Collaborative (N3C) IDeA CTR Collaboration 3U24TR002306-04S2 NCATS U24 TR002306. This research was possible because of the patients whose information is included within the data from participating organizations (covid.cd2h.org/dtas) and the organizations and scientists (covid.cd2h.org/duas) who have contributed to the on-going development of this community resource (cite this https://doi.org/10.1093/jamia/ocaa196).
Individual Acknowledgements For Core Contributors
We gratefully acknowledge the following core contributors to N3C:
Adam B. Wilcox, Adam M. Lee, Alexis Graves, Alfred (Jerrod) Anzalone, Amin Manna, Amit Saha, Amy Olex, Andrea Zhou, Andrew E. Williams, Andrew Southerland, Andrew T. Girvin, Anita Walden, Anjali A. Sharathkumar, Benjamin Amor, Benjamin Bates, Brian Hendricks, Brijesh Patel, Caleb Alexander, Caroline Signore, Carolyn Bramante, Cavin Ward-Caviness, Charisse Madlock-Brown, Christine Suver, Christopher Chute, Christopher Dillon, Chunlei Wu, Clare Schmitt, Cliff Takemoto, Dan Housman, Davera Gabriel, David A. Eichmann, Diego Mazzotti, Don Brown, Eilis Boudreau, Elaine Hill, Elizabeth Zampino, Emily Carlson Marti, Emily R. Pfaff, Evan French, Farrukh M Koraishy, Federico Mariona, Fred Prior, George Sokos, Greg Martin, Harold Lehmann, Heidi Spratt, Hemalkumar Mehta, Hongfang Liu, Hythem Sidky, J.W. Awori Hayanga, Jami Pincavitch, Jaylyn Clark, Jeremy Richard Harper, Jessica Islam, Jin Ge, Joel Gagnier, Joel H. Saltz, Joel Saltz, Johanna Loomba, John Buse, Jomol Mathew, Joni L. Rutter, Julie A. McMurry, Justin Guinney, Justin Starren, Karen Crowley, Katie Rebecca Bradwell, Kellie M. Walters, Ken Wilkins, Kenneth R. Gersing, Kenrick Dwain Cato, Kimberly Murray, Kristin Kostka, Lavance Northington, Lee Allan Pyles, Leonie Misquitta, Lesley Cottrell, Lili Portilla, Mariam Deacy, Mark M. Bissell, Marshall Clark, Mary Emmett, Mary Morrison Saltz, Matvey B. Palchuk, Melissa A. Haendel, Meredith Adams, Meredith Temple-O’Connor, Michael G. Kurilla, Michele Morris, Nabeel Qureshi, Nasia Safdar, Nicole Garbarini, Noha Sharafeldin, Ofer Sadan, Patricia A. Francis, Penny Wung Burgoon, Peter Robinson, Philip R.O. Payne, Rafael Fuentes, Randeep Jawa, Rebecca Erwin-Cohen, Rena Patel, Richard A. Moffitt, Richard L. Zhu, Rishi Kamaleswaran, Robert Hurley, Robert T. Miller, Saiju Pyarajan, Sam G. Michael, Samuel Bozzette, Sandeep Mallipattu, Satyanarayana Vedula, Scott Chapman, Shawn T. O’Neil, Soko Setoguchi, Stephanie S. Hong, Steve Johnson, Tellen D. Bennett, Tiffany Callahan, Umit Topaloglu, Usman Sheikh, Valery Gordon, Vignesh Subbian, Warren A. Kibbe, Wenndy Hernandez, Will Beasley, Will Cooper, William Hillegass, Xiaohan Tanner Zhang. Details of contributions available at covid.cd2h.org/core-contributors
Data Partners with Released Data
The following institutions whose data is released or pending:
Available: Advocate Health Care Network — UL1TR002389: The Institute for Translational Medicine (ITM) • Boston University Medical Campus — UL1TR001430: Boston University Clinical and Translational Science Institute • Brown University — U54GM115677: Advance Clinical Translational Research (Advance-CTR) • Carilion Clinic — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • Charleston Area Medical Center — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • Children’s Hospital Colorado — UL1TR002535: Colorado Clinical and Translational Sciences Institute • Columbia University Irving Medical Center — UL1TR001873: Irving Institute for Clinical and Translational Research • Duke University — UL1TR002553: Duke Clinical and Translational Science Institute • George Washington Children’s Research Institute — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • George Washington University — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • Indiana University School of Medicine — UL1TR002529: Indiana Clinical and Translational Science Institute • Johns Hopkins University — UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • Loyola Medicine — Loyola University Medical Center • Loyola University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Maine Medical Center — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Massachusetts General Brigham — UL1TR002541: Harvard Catalyst • Mayo Clinic Rochester — UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Medical University of South Carolina — UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • Montefiore Medical Center — UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore • Nemours — U54GM104941: Delaware CTR ACCEL Program • NorthShore University HealthSystem — UL1TR002389: The Institute for Translational Medicine (ITM) • Northwestern University at Chicago — UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • OCHIN — INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks • Oregon Health & Science University — UL1TR002369: Oregon Clinical and Translational Research Institute • Penn State Health Milton S. Hershey Medical Center — UL1TR002014: Penn State Clinical and Translational Science Institute • Rush University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Rutgers, The State University of New Jersey — UL1TR003017: New Jersey Alliance for Clinical and Translational Science • Stony Brook University — U24TR002306 • The Ohio State University — UL1TR002733: Center for Clinical and Translational Science • The State University of New York at Buffalo — UL1TR001412: Clinical and Translational Science Institute • The University of Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Iowa — UL1TR002537: Institute for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine — UL1TR002736: University of Miami Clinical and Translational Science Institute • The University of Michigan at Ann Arbor — UL1TR002240: Michigan Institute for Clinical and Health Research • The University of Texas Health Science Center at Houston — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • The University of Texas Medical Branch at Galveston — UL1TR001439: The Institute for Translational Sciences • The University of Utah — UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center — UL1TR002544: Tufts Clinical and Translational Science Institute • Tulane University — UL1TR003096: Center for Clinical and Translational Science • University Medical Center New Orleans — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • University of Alabama at Birmingham — UL1TR003096: Center for Clinical and Translational Science • University of Arkansas for Medical Sciences — UL1TR003107: UAMS Translational Research Institute • University of Cincinnati — UL1TR001425: Center for Clinical and Translational Science and Training • University of Colorado Denver, Anschutz Medical Campus — UL1TR002535: Colorado Clinical and Translational Sciences Institute • University of Illinois at Chicago — UL1TR002003: UIC Center for Clinical and Translational Science • University of Kansas Medical Center — UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute • University of Kentucky — UL1TR001998: UK Center for Clinical and Translational Science • University of Massachusetts Medical School Worcester — UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University of Minnesota — UL1TR002494: Clinical and Translational Science Institute • University of Mississippi Medical Center — U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center — U54GM115458: Great Plains IDeA-Clinical & Translational Research • University of North Carolina at Chapel Hill — UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Oklahoma Health Sciences Center — U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • University of Rochester — UL1TR002001: UR Clinical & Translational Science Institute • University of Southern California — UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • University of Vermont — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • University of Virginia — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • University of Washington — UL1TR002319: Institute of Translational Health Sciences • University of Wisconsin-Madison — UL1TR002373: UW Institute for Clinical and Translational Research • Vanderbilt University Medical Center — UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • Virginia Commonwealth University — UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • Wake Forest University Health Sciences — UL1TR001420: Wake Forest Clinical and Translational Science Institute • Washington University in St. Louis — UL1TR002345: Institute of Clinical and Translational Sciences • Weill Medical College of Cornell University — UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center • West Virginia University — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI)
Submitted: Icahn School of Medicine at Mount Sinai — UL1TR001433: ConduITS Institute for Translational Sciences • The University of Texas Health Science Center at Tyler — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • University of California, Davis — UL1TR001860: UCDavis Health Clinical and Translational Science Center • University of California, Irvine — UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS) • University of California, Los Angeles — UL1TR001881: UCLA Clinical Translational Science Institute • University of California, San Diego — UL1TR001442: Altman Clinical and Translational Research Institute • University of California, San Francisco — UL1TR001872: UCSF Clinical and Translational Science Institute
Pending: Arkansas Children’s Hospital — UL1TR003107: UAMS Translational Research Institute • Baylor College of Medicine — None (Voluntary) • Children’s Hospital of Philadelphia — UL1TR001878: Institute for Translational Medicine and Therapeutics • Cincinnati Children’s Hospital Medical Center — UL1TR001425: Center for Clinical and Translational Science and Training • Emory University — UL1TR002378: Georgia Clinical and Translational Science Alliance • HonorHealth — None (Voluntary) • Loyola University Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • Medical College of Wisconsin — UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin • MedStar Health Research Institute — UL1TR001409: The Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) • MetroHealth — None (Voluntary) • Montana State University — U54GM115371: American Indian/Alaska Native CTR • NYU Langone Medical Center — UL1TR001445: Langone Health’s Clinical and Translational Science Institute • Ochsner Medical Center — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • Regenstrief Institute — UL1TR002529: Indiana Clinical and Translational Science Institute • Sanford Research — None (Voluntary) • Stanford University — UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education • The Rockefeller University — UL1TR001866: Center for Clinical and Translational Science • The Scripps Research Institute — UL1TR002550: Scripps Research Translational Institute • University of Florida — UL1TR001427: UF Clinical and Translational Science Institute • University of New Mexico Health Sciences Center — UL1TR001449: University of New Mexico Clinical and Translational Science Center • University of Texas Health Science Center at San Antonio — UL1TR002645: Institute for Integration of Medicine and Science • Yale New Haven Hospital — UL1TR001863: Yale Center for Clinical Investigation
Role of the funding source
The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave https://covid.cd2h.org and N3C Attribution and Publication Policy v 1.2-2020-08-25b supported by NCATS U23 TR002306. Individual authors were supported by the following funding sources: NIMH R01131542 (Rena C. Patel), NICHD R21105304 (Anup P. Challa). The funding sources or study sponsors had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Transparency statement:
The lead author, Qiuyuan Qin, affirms that this manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned (and if relevant, registered) have been explained.
Conflicts of Interest and Disclaimers
APC helped to conceptualize this study while at Vanderbilt University. Following this contribution, APC joined the staff of AstraZeneca Pharmaceuticals, LP (“AZ”), which markets a COVID-19 vaccine in direct competition to other products considered in this manuscript. After joining AZ, APC did not participate in the conduct of this research, with his subsequent contributions limited to ad hoc consultation on methods for real-world data management, as part of routine meetings of the N3C Pregnancy Domain Team for which he is an advisor. APC did not provide any guidance on product-specific or advocacy topics. Similarly, AZ did not provide any data, funding, or scientific input towards the results herein.
The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health, AZ, or the N3C program.
Data sharing
Concept IDs, templates, or other specified data tools may be made available to others requesting them upon communication with the corresponding author, demonstration of appropriate ethic reviews (IRB approval), and establishment of data sharing agreements with N3C.
References
- [1].Johnson AG, “COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence — 25 U.S. Jurisdictions, April 4-December 25, 2021,” MMWR Morb. Mortal. Wkly. Rep, vol. 71, 2022, doi: 10.15585/mmwr.mm7104e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sun J et al. , “Association Between Immune Dysfunction and COVID-19 Breakthrough Infection After SARS-CoV-2 Vaccination in the US,” JAMA Intern. Med, vol. 182, no. 2, pp. 153–162, Feb. 2022, doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Agrawal U et al. , “Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales,” The Lancet, vol. 400, no. 10360, pp. 1305–1320, Oct. 2022, doi: 10.1016/S0140-6736(22)01656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dashraath P et al. , “Coronavirus disease 2019 (COVID-19) pandemic and pregnancy,” Am. J. Obstet. Gynecol, vol. 222, no. 6, pp. 521–531, Jun. 2020, doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].CDC, “Pregnant and Recently Pregnant People,” Centers for Disease Control and Prevention. Accessed: May 04, 2022. [Online]. Available: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnant-people.html [Google Scholar]
- [6].Lv D et al. , “Exploring the Immunopathogenesis of Pregnancy With COVID-19 at the Vaccination Era,” Front. Immunol, vol. 12, 2021, Accessed: May 04, 2022. [Online]. Available: https://www.frontiersin.org/article/10.3389/fimmu.2021.683440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McClymont E et al. , “Association of SARS-CoV-2 Infection During Pregnancy With Maternal and Perinatal Outcomes,” JAMA, May 2022, doi: 10.1001/jama.2022.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gurol-Urganci I et al. , “Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study,” Am. J. Obstet. Gynecol, vol. 225, no. 5, p. 522.e1–522.e11, Nov. 2021, doi: 10.1016/j.ajog.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Edlow AG, Castro VM, Shook LL, Kaimal AJ, and Perlis RH, “Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy,” JAMA Netw. Open, vol. 5, no. 6, p. e2215787, Jun. 2022, doi: 10.1001/jamanetworkopen.2022.15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jeong S, Jang EJ, Jo J, and Jang S, “Effects of maternal influenza vaccination on adverse birth outcomes: A systematic review and Bayesian meta-analysis,” PLoS ONE, vol. 14, no. 8, p. e0220910, Aug. 2019, doi: 10.1371/journal.pone.0220910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ohfuji S et al. , “Safety of influenza vaccination on adverse birth outcomes among pregnant women: A prospective cohort study in Japan,” Int. J. Infect. Dis, vol. 93, pp. 68–76, Apr. 2020, doi: 10.1016/j.ijid.2020.01.033. [DOI] [PubMed] [Google Scholar]
- [12].Jarvis JR, Dorey RB, Warricker FDM, Alwan NA, and Jones CE, “The effectiveness of influenza vaccination in pregnancy in relation to child health outcomes: Systematic review and meta-analysis,” Vaccine, vol. 38, no. 7, pp. 1601–1613, Feb. 2020, doi: 10.1016/j.vaccine.2019.12.056. [DOI] [PubMed] [Google Scholar]
- [13].Kharbanda EO and Vazquez-Benitez G, “COVID-19 mRNA Vaccines During Pregnancy: New Evidence to Help Address Vaccine Hesitancy,” JAMA, vol. 327, no. 15, pp. 1451–1453, Apr. 2022, doi: 10.1001/jama.2022.2459. [DOI] [PubMed] [Google Scholar]
- [14].Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, and Håberg SE, “Covid-19 Vaccination during Pregnancy and First-Trimester Miscarriage,” N. Engl. J. Med, vol. 385, no. 21, pp. 2008–2010, Nov. 2021, doi: 10.1056/NEJMc2114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lipkind HS et al. , “Receipt of COVID-19 Vaccine During Pregnancy and Preterm or Small-for-Gestational-Age at Birth - Eight Integrated Health Care Organizations, United States, December 15, 2020-July 22, 2021,” MMWR Morb. Mortal. Wkly. Rep, vol. 71, no. 1, pp. 26–30, Jan. 2022, doi: 10.15585/mmwr.mm7101e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Goldshtein I et al. , “Association of BNT162b2 COVID-19 Vaccination During Pregnancy With Neonatal and Early Infant Outcomes,” JAMA Pediatr, vol. 176, no. 5, pp. 470–477, May 2022, doi: 10.1001/jamapediatrics.2022.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Faherty EAG et al. , “Pregnancy Outcomes among Pregnant Persons after COVID-19 Vaccination: Assessing Vaccine Safety in Retrospective Cohort Analysis of U.S. National COVID Cohort Collaborative (N3C),” Vaccines, vol. 12, no. 3, Art. no. 3, Mar. 2024, doi: 10.3390/vaccines12030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pratama NR, Wafa IA, Budi DS, Putra M, Wardhana MP, and Wungu CDK, “mRNA Covid-19 vaccines in pregnancy: A systematic review,” PLOS ONE, vol. 17, no. 2, p. e0261350, Feb. 2022, doi: 10.1371/journal.pone.0261350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burd I, Kino T, and Segars J, “The Israeli study of Pfizer BNT162b2 vaccine in pregnancy: considering maternal and neonatal benefits,” J. Clin. Invest, vol. 131, no. 13, p. 150790, Jul. 2021, doi: 10.1172/JCI150790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, and Swift M, “Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy,” Am. J. Obstet. Gynecol. Mfm, vol. 3, no. 6, p. 100467, Nov. 2021, doi: 10.1016/j.ajogmf.2021.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Goldshtein I et al. , “Association Between BNT162b2 Vaccination and Incidence of SARS-CoV-2 Infection in Pregnant Women,” JAMA, vol. 326, no. 8, pp. 728–735, Aug. 2021, doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].MD DL, Barkley E, Kibitel J, PharmD NC, and McNitt J, “Which Comorbidities Increase the Risk of a COVID-19 Breakthrough Infection?,” Epic Research. Accessed: May 04, 2022. [Online]. Available: https://epicresearch.org/articles/which-comorbidities-increase-the-risk-of-a-covid-19-breakthrough-infection [Google Scholar]
- [23].Jones SE et al. , “Who is pregnant? Defining real-world data-based pregnancy episodes in the National COVID Cohort Collaborative (N3C),” JAMIA Open, vol. 6, no. 3, p. ooad067, Aug. 2023, doi: 10.1093/jamiaopen/ooad067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bennett TD et al. , “Clinical Characterization and Prediction of Clinical Severity of SARS-CoV-2 Infection Among US Adults Using Data From the US National COVID Cohort Collaborative,” JAMA Netw. Open, vol. 4, no. 7, p. e2116901, Jul. 2021, doi: 10.1001/jamanetworkopen.2021.16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Haendel MA et al. , “The National COVID Cohort Collaborative (N3C): Rationale, design, infrastructure, and deployment,” J. Am. Med. Inform. Assoc. JAMIA, vol. 28, no. 3, pp. 427–443, Mar. 2021, doi: 10.1093/jamia/ocaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].“Clinical Guidance for COVID-19 Vaccination ∣ CDC.” Accessed: Oct. 02, 2023. [Online]. Available: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
- [27].Lambrou AS, “Genomic Surveillance for SARS-CoV-2 Variants: Predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants — United States, June 2021–January 2022,” MMWR Morb. Mortal. Wkly. Rep, vol. 71, 2022, doi: 10.15585/mmwr.mm7106a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].“Trends in Length of Stay for Hospital Deliveries -- United States, 1970–1992.” Accessed: Oct. 02, 2023. [Online]. Available: https://www.cdc.gov/mmwr/preview/mmwrhtml/00036988.htm
- [29].Fendler A et al. , “COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety,” Nat. Rev. Clin. Oncol, vol. 19, no. 6, Art. no. 6, Jun. 2022, doi: 10.1038/s41571-022-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mirtaleb MS et al. , “An insight overview on COVID-19 mRNA vaccines: Advantageous, pharmacology, mechanism of action, and prospective considerations,” Int. Immunopharmacol, vol. 117, p. 109934, Apr. 2023, doi: 10.1016/j.intimp.2023.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wan J et al. , “Booster vaccination protection against SARS-CoV-2 infections in young adults during an Omicron BA.1-predominant period: A retrospective cohort study,” PLOS Med, vol. 20, no. 1, p. e1004153, Jan. 2023, doi: 10.1371/journal.pmed.1004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chemaitelly H et al. , “Long-term COVID-19 booster effectiveness by infection history and clinical vulnerability and immune imprinting: a retrospective population-based cohort study,” Lancet Infect. Dis, vol. 23, no. 7, pp. 816–827, Jul. 2023, doi: 10.1016/S1473-3099(23)00058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Plumb ID et al. , “Effectiveness of a Messenger RNA Vaccine Booster Dose Against Coronavirus Disease 2019 Among US Healthcare Personnel, October 2021-July 2022,” Open Forum Infect. Dis, vol. 10, no. 10, p. ofad457, Oct. 2023, doi: 10.1093/ofid/ofad457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ellington S and Olson CK, “Safety of mRNA COVID-19 vaccines during pregnancy,” Lancet Infect. Dis, vol. 22, no. 11, pp. 1514–1515, Nov. 2022, doi: 10.1016/S1473-3099(22)00443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Holland C, Hammond C, and Richmond MM, “COVID-19 and Pregnancy: Risks and Outcomes,” Nurs. Womens Health, vol. 27, no. 1, pp. 31–41, Feb. 2023, doi: 10.1016/j.nwh.2022.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Concept IDs, templates, or other specified data tools may be made available to others requesting them upon communication with the corresponding author, demonstration of appropriate ethic reviews (IRB approval), and establishment of data sharing agreements with N3C.


