Abstract
Introduction
Breast cancer is the most common cancer and the leading cause of cancer death in women. Recent research indicates that human endogenous retroviruses (HERVs) may be linked to carcinogenesis, but the data remain controversial.
Methods
HERVs’ expression was evaluated to show the differences between breast cancer and control samples, and their associations with clinicopathological parameters. Gene expression of 12 HERVs, i.e., ERVE-4, ERVW-1, ERVFRD-1, ERVV-1, ERV3-1, ERVH48-1, ERVMER34-1, ERVK-7, ERVK13-1, ERVK11-1, ERVK3-1, and HCP5, was analyzed by qPCR and/or TCGA datasets for breast cancer.
Results
ERV3-1, ERVFRD-1, ERVH48-1, and ERVW-1 provided data to support their tumor suppressor roles in breast cancer. ERV3-1 evinced the best performing diagnostic data based on qPCR, i.e., AUC: 0.819 (p < 0.0001), sensitivity of 72.41%, and specificity of 89.66%. Lower levels of ERV3-1 were noted in advanced stage and higher grades, and significant negative association was found in relation to Ki-67 levels. Oncogenic roles may be inferred for ERVK13-1, ERVV-1, and ERVMER34-1. Data for ERVK-7, ERVE-4, ERVK11-1, and HCP5 remain inconclusive.
Conclusion
Differential HERV expression may be applicable to evaluate novel biomarkers for breast cancer. However, more research is needed to reveal their real clinical impact, the biological roles, and regulatory mechanisms in breast carcinogenesis.
Keywords: Breast cancer, Human endogenous retroviruses, ERVW-1, ERVFRD-1, ERVV-1, ERV3-1, ERVH48-1, ERVMER34-1, ERVK13-1, ERVK3-1, HCP5
Introduction
Breast cancer is a malignant disease ranking as the most common cancer worldwide and the most frequent cause of death related to cancer in women [1]. The detection of the disease remains based on the mammography and ultrasonography accompanied by magnetic resonance imaging and a variety of other examination methods [2, 3]. Routine pathological examination includes determination of tumor subtypes, hormone receptor status, and the expression of HER2 and Ki-67, eventually resulting in breast cancer subtype classification and accordingly in the relevant choice of the treatment [4]. However, there is still an urgent need to find and evaluate novel diagnostic and screening biomarkers.
Invasive breast cancer is a complex disease involving two major histological subtypes, i.e., the ductal carcinomas of no special type (NST) and lobular carcinomas. There exist several factors that increase the risk for development of breast cancer, particularly mutations in tumor suppressor genes such as BRCA1 and BRCA2 [5]. However, transcriptomic approaches have identified many genes involving noncoding RNAs such as microRNA and long noncoding RNAs dysregulated in breast cancer and participating in the processes associated with carcinogenesis known as the hallmarks of cancer [6].
Put simply, oncogenes are genes participating in the cancer promotion and may intervene in the basic cellular processes within cell cycle, senescence, apoptosis, and metabolism or may influence host immunological response to cancer cells. On the other hand, tumor suppressor genes may act in the regulation of these processes and inhibit cancer progression. Generally, many factors have been identified to affect the gene expression at different levels. For many genes, their physiological functions in carcinogenesis may vary in a context-dependent manner. This results in a more complicated and complex view of the net of mutually regulated genes and their interactions, and eventual functions in carcinogenesis and response to therapy. In breast cancer, many novel insights have been uncovered in the expression studies [7], focused on tumor tissues, cell lines, in vivo animal models [8], clinical studies focused on tissues [9], or body fluids [10]. However, this extensive exploration has brought also many new questions yet to be answered.
Human endogenous retroviruses (HERVs) belong to the genes suspected to play roles in carcinogenesis including breast cancer. Their general roles in human health and disease may be complex and yet not fully understood due to their complex genetic nature. HERVs make up over 8% of human genome [11–13] and are derived from ancestral exogenous retroviruses integrated in our germline DNA [14]. These endogenous viruses belong to the family Retroviridae, and their basic classification recognizes three classes, according to similarity to the exogenous Gammaretrovirus (= class I, HERV-H, HERV-F, HERV-W, HERV-R, HERV-P, HERV-E, HERV-I, HERV-T, ERV-FTD, ERV-FRD), Betaretrovirus (= class II, HERV-K, HML1-11), and Spumavirus (class III, HERV-L) groups.
Similarly as all the other members of the infectious exogenous Retroviridae family, HERVs (e.g., gammaretroviral subfamily) may retain several structural genes such as gag (capsid), polymerase (pol), protease (pro), and envelope (env) genes, as well as the two long terminal repeats. Additional nonstructural genes may be associated with HERVs of the betaretroviral (e.g., HERV-K) or spumaviral (e.g., HERV-L) subfamilies, and may include also lncRNAs. HERV elements have been shown to have a functional impact on cell differentiation, fusion and transformation, transcriptional regulation, and immune regulation [14, 15].
Among viruses, there are many examples of viral infections and their causative roles in the development of various cancers (e.g., HPV, EBV, etc.) [16, 17]. Therefore, HERVs have been also suspected to play roles in cancer development and many studies have supported that view showing HERVs as possible oncoproteins or autoantigens. However, their potential oncogenic functions may be complex and yet not fully determined [18]. Several studies identified HERVs (pointing particularly to HERV-K group) as potential diagnostic or prognostic markers in breast cancer [19, 20, 21] and other cancers [14, 22], but additional confirmation of HERVs as causative factors is still lacking while this issue remains controversial with respect to their real contribution to disease development, progression, or immunological properties [23].
In this study, we selected 7 representatives of HERVs belonging to different HERV classes and analyzed their expression between malignant breast cancer samples and benign controls. We evaluated the associations with clinicopathological data and potential contributions to breast carcinogenesis. Additionally, The Cancer Genome Atlas (TCGA) datasets for breast cancer were explored resulting in a total of 12 HERVs included in this study. The investigated HERVs differed as regards their dysregulations and associations showing their diverse effects in breast cancer.
Material and Methods
Patients and Samples
Enrolled patients who were receiving treatment for NST-invasive breast carcinoma at the University Hospital in Brno (n = 29) provided tumor samples (n = 29). Benign samples (n = 29) were obtained from the same patients or from other breast cancer patients. No chemotherapy or hormonal treatments were applied at the time of sampling. All the enrolled patients were female Caucasians. The study was approved by the multicentric Ethics Committee of the General University Hospital in Prague (VFN Praha, No. 127/20 S-IV) and the Ethics Committee of the University Hospital in Brno (FN Brno) and was conducted in accordance with the Helsinki Declaration. The main clinicopathological characteristics of the patients are presented in online supplementary file 1 (online suppl. Table S1; for all online suppl. material, see https://doi.org/10.1159/000538021).
Clinical Samples: Pathological Examination, Collection, and Processing
The tumor and benign tissue samples assessed by expert pathologists were transferred to RNAlater (Thermo Fisher Scientific) liquid solution to ensure the preservation of the RNA. Total RNA was isolated using a mirVana miRNA Isolation Kit (Ambion/Thermo Fisher Scientific, cat. No. AM1560) according to the manufacturer’s instructions, and samples were stored at −80°C. The total RNA was reverse-transcribed to cDNA using a LunaScript RT SuperMix (New England Biolabs, cat. No. NEB #M3010) after an initial ezDNase (Thermo Fisher Scientific, cat. No. 11766051) treatment. The cDNA was stored at −25°C.
Selection of HERV Genes
Investigated HERV genes were selected according to literature and assay/data availability to represent different classes [14, 24, 25, 26] and HERV structure genes. We wished to survey different HERV expressions from different HERV groups and gene loci at different chromosomal locations, and also with respect to functional relevance in breast cancer. The expression levels of seven HERVs, i.e., ERVK-7, ERVK11-1, ERVK13-1, ERV3-1, ERVE-4, ERVV-1, and HCP5), and five candidate endogenous controls (actin beta, GAPDH, TBP, RPLP0, and IPO8) were analyzed using TaqMan assays (Thermo Fisher Scientific, USA) and qPCR. For details, see online supplementary file 1 (online suppl. Table S2).
Additionally, RNAseq data (TCGA Program, Illumina HiSeq) were explored and evaluated using datasets available for breast cancer [27]. Eight available HERV genes are included in these analyses: ERVK3-1, ERVFRD-1, ERVH48-1, ERVW-1, ERVMER34-1, ERV3-1, ERVV-1, and HCP5 (for details, see online suppl. file 1; Table S2). The datasets were obtained and processed as log2(RSEM+1) data via the UCSC Xena platform along with the dataset for normal controls (GTEx, patients without cancer) (TCGA TARGET GTEx dataset) and GDC TCGA Breast Cancer (BRCA) for invasive ductal carcinomas, NOS [28, 29].
Gene Expression (qPCR)
Candidate endogenous controls were further evaluated using geNorm module of qbase+, resulting in a selection of actin beta, IPO8, and RPLP0 as data normalizers. The qPCR reactions consisted of 10 μL of reaction volume per well and were run in triplicates on a CFX Connect Real-Time PCR Detection System (Bio-Rad) applying the following thermal cycler parameters: 50°C for 2 min, 95°C for 10 min, 40 cycles: 95°C for 15 s, 60°C for 30 s.
Statistical Analysis
qbase+ [30, 31] and MedCalc statistical software (Belgium) were used to analyze the expression data (log-transformed calibrated normalized relative quantity data exported from qbase+ were used in MedCalc). Nonparametric Mann-Whitney tests (according to data normality) were applied to identify differences in the expression between the pathological and control samples, and receiver operating characteristic curve analysis was used to evaluate the area under the curve (AUC), the sensitivity, and the specificity. The Kruskal-Wallis test and the Jonckheere-Terpstra trend test were used for the testing of the associations with the stages and grades. p values of <0.05 were considered significant in all the tests.
Results
Gene Expression Analyses Based on qPCR
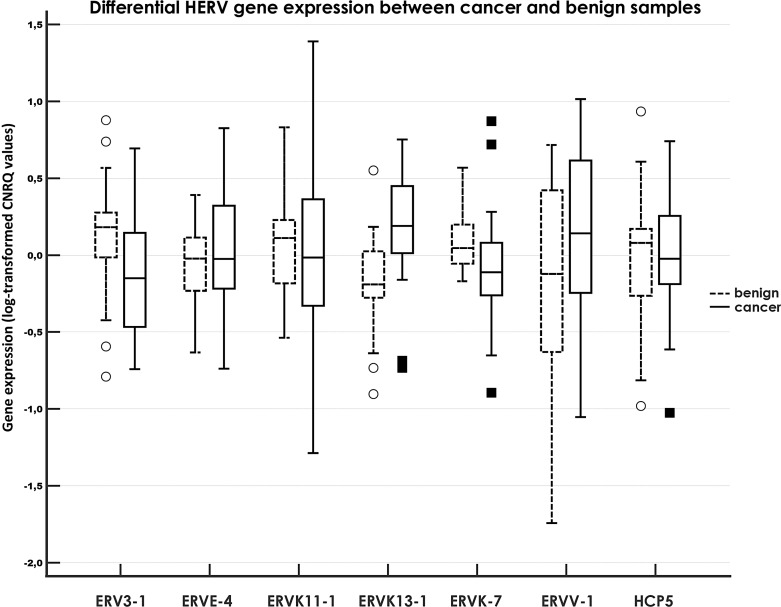
Three-directional expression pattern was observed for the tested genes; i.e., downregulated, upregulated, and unchanged expression levels were noted in comparisons of cancer (n = 29) and benign samples (n = 29). Out of the investigated targets, significant results were obtained for ERVK13-1 with elevated expression (2.3-fold), while ERVK-7 (−1.47-fold) and ERV3-1 (−1.75-fold) evinced lower expression in cancer samples. The results for remaining gene expressions were not significant, although the expression of ERVV-1 appeared to be presumably upregulated (1.99-fold, p = 0.16550). For details, see Table 1 and Figure 1.
Table 1.
Differential RNA expression and FDs between breast cancer tumors and benign tissues
| HERV-related RNA (mRNA/lncRNA) | FD | 95% CI, low | 95% CI, high | p value |
|---|---|---|---|---|
| Downregulated expression | ||||
| ERV3-1 | −1.75 | −2.77 | −1.10 | 0.03639 |
| ERVK-7 | −1.47 | −2.07 | −1.05 | 0.01015 |
| Upregulated expression | ||||
| ERVK13-1 | 2.30 | 1.55 | 3.40 | 0.00021 |
| Nonsignificant results | ||||
| ERVV-1 | 1.99 | −1.04 | 4.14 | 0.16550 |
| ERVE-4 | 1.30 | −1.16 | 1.95 | 0.47441 |
| ERVK11-1 | −1.24 | −2.18 | 1.42 | 0.66534 |
| HCP5 | 1.05 | 0.65 | 1.70 | 0.79749 |
Three endogenous controls (actin beta, IPO8, and RPLP0) were used for data normalization. FD, fold difference; CI, confidential interval.
Fig. 1.
Relative expression of HERV genes compared between tumor samples of breast cancer patients and benign tissue samples. A clustered multiple-comparison graph showing RNA expression data (log-transformed CNRQ). A box of the box plot is drawn from the 1st to 3rd quartile (the 25th and 75th percentiles). A horizontal line within a box plot represents the median. Horizontal lines are drawn at the highest value and the lowest expression value. A circle/square represents an outside value. CNRQs, calibrated normalized relative quantities calculated by qbase+.
HERV expression was detected for all the tested genes in both cancer and benign sample groups. Generally, obtained Ct values were mostly acceptable (Ct < 35); however, five benign samples showed a compromised expression (Ct > 35) for ERVV-1. Nevertheless, excluding these five samples with Ct > 35 did not result in significant results for ERVV-1 (p = 0.570, fold difference: 1.23).
Next, statistical tests were performed to detect possible outside (outlier) values, potentially affecting the results as well. The log-transformed expression data revealed several outside values according to Tukey test within both benign (n = 9) and cancer sample (n = 6) sets. Therefore, an additional set of samples (verification set) was created where samples with any outside value were excluded, i.e., six cancer samples and seven benign samples. Analysis of this novel verification set (n = 23 cancer samples, n = 22 benign samples) resulted in a similar expression pattern, showing two significantly downregulated genes ERV3-1 and ERVK-7, and two significantly upregulated genes ERVK13-1 and ERVV-1, confirming the results from the original dataset also for ERVV-1 (online suppl. file 2; Table S3).
Receiver Operating Characteristics (Analysis)
We next investigated the diagnostic power of the three significantly altered RNAs with respect to their AUC, sensitivity, and specificity. Out of the three tested gene expressions (i.e., for the genes with significant deregulations), ERV3-1 evinced the best performing data, i.e., AUC: 0.819 (p < 0.0001), sensitivity of 72.41%, and specificity of 89.66%. The AUC for ERVK-7 was 0.728 (p = 0.0016), and sensitivity of 62.07% and specificity of 93.10% were noted for this gene expression. Last, AUC of 0.685 (p = 0.0110) was recorded for ERV3-1, with sensitivity of 65.52%, and specificity of 79.31%. For details, see online supplementary file 2 (online suppl. Table S4).
Associations of Gene Expressions with Clinicopathological Data
Survival Analysis
Patients providing cancer samples were divided into two subgroups with respect to the investigated gene expression levels (low- and high-expression groups). Neither overall survival nor progression-free survival did differ significantly between low- and high-expression subgroups. Although some interesting data for the survival time were observed, the results are mixed and do not allow to draw any relevant conclusions (see online suppl. file 2; Table S5).
Other Clinicopathological Data Associations
Stage and Grade
While no associations were found for the tumor stage for all tested genes, the expression of ERV3-1 differed significantly between clinical stages 1 and 2, and clinical stage 3 (the latter one with reduced expression levels, p = 0.023002). The expression of ERV3-1 was also associated with a grade, showing a significant trend of a lower gene expression in higher grades (ptrend = 0.02682), and the difference was marginally significant in Kruskal-Wallis test (p = 0.10471).
Lymph Node Metastasis and Multifocal Disease
Similarly, ERV3-1 emerged as the only one gene potentially associated with lymph node metastasis (LNM), where marginally significant association was observed for reduced expression levels in LNM-positive samples (p = 0.0546). On the other hand, both ERVK11-1 (p = 0.0849) and ERVV-1 (p = 0.1317) evinced higher expression (albeit marginally or nonsignificant) in multifocal disease samples.
Estrogen Receptor, Progesterone Receptor, HER2, and Ki-67 Proliferation Marker
Regarding hormone receptors (estrogen receptor [ER], progesterone receptor [PR]), no significant associations were found. However, ERV3-1 expression showed that its higher levels (marginally significantly, p = 0.1138) may be associated with ER-positive samples (10% cut-off). Similarly, dual positivity for ER and PR (10% cut-off) was also linked nonsignificantly (p = 0.1479) with higher expression levels of ERV3-1. This pattern was similar when cut-off of 1% was applied for PR, ER, and PR/ER positivity (ER+, p = 0.1138; ER + PR+, p = 0.1196). Conversely, lower expression levels of ERVK-7 were noted for PR positivity (cut-off 10%) (marginally significantly, p = 0.0654), but not significantly when using cut-off of 1% (p = 0.1432). HER2 positivity associations could not be determined due to lack of positive samples, and also a detailed HER2 expression stratified into four categories (0–3) did not reveal any significant associations with the expression of the investigated genes.
Significant negative associations were found for ERV3-1 and the proliferation marker Ki-67, showing ERV3-1 with lower expression in Ki-67-positive samples using both cut-offs (15%, p = 0.0317; 20%, p = 0.0207) applied. Opposite (i.e., positive), but only marginally significant association (p = 0.0887) was found also for ERVV-1, showing higher expression levels in positive samples (only in case of 20% cut-off). No associations were found comparing luminal A and B samples, as well as for the age as the basic parameter.
External Validation Using TCGA Datasets
First, we used Target GTEX Breast Cancer dataset (n = 1,391) to evaluate the expression among invasive breast cancers (ductal and lobular carcinomas), metastatic samples, benign tissues, and normal tissues. The following genes had significantly downregulated expression comparing tumors with normal tissues: ERV3-1, ERVFRD-1, ERVH48-1, ERVK3-1, and ERVW-1. Significantly upregulated expression in tumors versus normal tissues was recorded for ERVV-1, ERVMER34-1, and HCP5. For details, see online supplementary file 3 (online suppl. Table S6).
Five genes revealed consistent and significant data both for comparisons of primary tumors with benign samples and normal tissue samples, respectively. It holds true for the downregulated genes ERVFRD-1, ERVH48-1, and ERVW-1, and for the upregulated genes ERVV-1 and ERVMER34-1. Controversial (but significant) data were observed for ERVK3-1, showing upregulation in primary tumors as compared with benign samples, and downregulation in primary tumors as compared with normal tissue samples. HCP5 revealed a significant upregulation in tumors as compared with normal tissues, while its downregulation in comparison with benign samples was not significant. Finally, ERV3-1 was downregulated in both comparisons of tumors, but significantly only in comparison of primary tumors with normal tissues.
Next, we analyzed the expression in the GDC TCGA Breast Cancer dataset involving invasive breast ductal carcinoma NST samples (originally n = 749). This dataset was used for the analysis of associations with tumor stage.
The expression levels of the two HERVs (out of eight investigated), i.e., ERVK3-1 (p = 0.005558, ptrend = 0.00285) and ERVFRD-1 (p = 0.048328, ptrend = 0.01237), were significantly different with lower levels in more advanced stages. ERV3-1 expression was marginally significantly different across stages (p = 0.070503, ptrend = 0.01305) showing lower expression in more advanced stages. ERVV-1 expression (p = 0.089893) showed variable expression dynamics across the stages (online suppl. file 3; Table S7).
Finally, we performed survival analyses (log-rank tests) for overall survival using GDC TCGA Breast Cancer dataset and the eight HERVs in the Xena hub. No significant associations were found, although marginally significant results were observed for ERVV-1 (p = 0.06222) and ERVFRD-1 (p = 0.09149) showing better survival in case of lower expression cases.
Comparison between the Two Studies
Three HERV genes, i.e., ERV3-1, ERVV-1, and HCP5, overlapped between the gene expression study by qPCR and the TCGA datasets. ERV3-1 was downregulated, and ERVV-1 was upregulated in both analyses (the latter not significantly in qPCR analysis) comparing primary tumors and benign samples, and in TCGA datasets also comparing tumors and the normal tissues. HCP5 was not significantly altered comparing tumors with benign samples in both analyses, and it was only upregulated in the TCGA dataset comparing tumors with normal tissues. Interestingly, ERV3-1 results of qPCR study pointed to clinical stage and other factors, similarly as it was observed in the TCGA dataset for the tumor stage.
Discussion
We performed this study to shed more light on the panel of different HERVs and their expression in breast cancer samples to reveal differentially expressed genes and alterations potentially linked with clinicopathological data. Overall, there was no uniform pattern of the HERVs’ expression in breast cancer samples compared with benign controls as downregulated, upregulated, and unchanged modes of potential expression alterations were found. Their clinical impact also differed among the investigated genes.
Out of the seven investigated genes by qPCR, three genes were found to be differentially expressed between cancer and benign samples. Two genes, i.e., ERVK-7 and ERV3-1, were downregulated, and one gene, i.e., ERVK13-1, was upregulated in this comparison. The level of the expressional alterations was relatively low ranging on average from −1.5-fold (ERVK-7) to 2.3-fold difference found for ERVK13-1. Additionally, after the exclusion of outside data, the expression of the fourth gene, i.e., ERVV-1, was significantly upregulated as well (2.7-fold). The best AUC, sensitivity, and specificity were observed for ERV3-1. ERV3-1 and ERVV-1 were included also in the TCGA datasets, and the results confirmed their altered expression and potential association with the stage in case of ERV3-1.
Our analyses showed that ERV3-1 may have presumable tumor suppressor roles. Apart from its downregulation in cancer samples, this view was further supported particularly by its significantly lower expression in advanced clinical stage 3 in comparison with early stages 1 and 2, a significant trend to a lower expression in higher grades, marginally significant negative association with LNM, significant negative association with Ki-67 levels, and partially also by higher (but nonsignificant) levels in ER+ and ER + PR + samples.
Concerning the remaining investigated HERV genes, we found only marginally significant results in the qPCR study, showing higher levels in multifocal disease for ERVK11-1 and ERVV-1, and lower levels of ERVK-7 in PR + samples. Altogether, despite the fact that ERVK13-1 expression was higher in cancer samples compared with benign samples, no clinically relevant associations were found for this HERV gene within cancer samples. Its presumable and potential role as an oncogenic gene thus remains to be further elucidated. On the other hand, our data remarkably point to ERV3-1. Though being downregulated relatively modestly (−1.8-fold) in cancer samples in comparison with benign samples, this HERV was exceptionally associated with several clinicopathological parameters, supporting its view as a presumable tumor suppressor HERV in breast cancer (see above). The expression data for ERVK-7 and its lower levels in PR + samples suggest possible tumor suppressor roles, however, still not convincingly.
ERVV-1 was upregulated marginally significantly in multifocal disease samples (along with ERVK11-1) and partially in Ki-67+ samples (20% cut-off). Its expression showed increased levels (∼2-fold, but not significantly) in cancer samples compared with benign samples, but was significantly upregulated when alternative data processing was used; see above. Based on these results, ERVV-1 may be considered as a potential oncogenic gene in breast cancer. This view is also supported by the TCGA data, showing this HERV significantly upregulated comparing tumors with both benign samples and normal tissues (online suppl. file 3; Table S6). Concerning other reports, three selected HERVs (ERVK-7, ERVK11-1, and ERVK13-1) investigated in our qPCR study and ERVK3-1 analyzed in the TCGA datasets belong to the ERVK family (HML2) [15] where potential roles in cancer have been proposed previously.
Johanning et al. [19] found that HERV-K is strongly associated with the basal-like breast cancer showing higher (1.7-fold) expression in basal breast cancer compared with the other three major subtypes (i.e., luminal A, luminal B, basal, and HER2-enriched). As the basal subtype should be more aggressive resulting in poor prognosis and high frequencies of recurrence and metastasis, it would suggest HERV-K as a potential oncogenic gene. Moreover, according to the authors, HERV-K is not observed in normal cells. Curty et al. [22] reviewed HERV-K as potential biomarker and immunotherapeutic target in cancer, and the authors also point to rather oncogenic roles of this HERV family in breast cancer. The complex nature of the HERV-K expression in nine raw sequencing datasets was reported by Wei et al. [32]. The authors found that if adjacent tissues were used as controls (similarly as in our study), there were mixed clustering of the tumor and control samples, different expression levels across datasets, and increased heterogeneity in different cells and cancer types. Nevertheless, HERV-Ks were mostly increased in breast carcinomas compared with normal tissue controls [32]. Also, several other investigations support for the presumable oncogenic roles of the HERV-K in breast cancer (see [15]). Interestingly, our findings suggest that different ERVK family members may evince also different expression patterns, as downregulated ERVK-7 expression, upregulated ERVK13-1 expression, and unchanged expression of ERVK11-1 were observed. Accordingly, this may indicate also different potential roles in breast carcinogenesis. These controversies were observable also for ERVK3-1 showing different patterns when comparing tumors with benign and normal tissue samples in TCGA dataset.
ERV3-1 belongs to HERV-R family. Ko et al. [33] focused on protein levels HERV-K and HERV-R env in different cancers. In their study, HERV-K Env protein demonstrated much higher expression than HERV-R Env in breast cancer. In their survival prognosis, both HERV-K Env and HERV-R Env were downregulated (but not significantly) in the “dead group” in breast cancer. The other reports for the ERV3-1 demonstrate both potentially oncogenic (e.g., in lung cancer) and tumor suppressive functions (e.g., Hodgkin’s lymphoma cells) [15]. Concerning ERV3-1, the impact of ancestral (ethno-geographical) differences was also demonstrated that many factors may play a role within the HERV functioning, indicating that Black Americans differ in its expression from that of White Americans [34]. In our study, the results highlight possible tumor suppressor roles of ERV3-1 in breast cancer.
ERVW1 (syncytin 1) and ERVFRD-1 (syncytin 2) belong to the syncytin family genes that are expressed in human syncytiotrophoblasts, and possess clear fusogen functions in human placenta but the functions of others remain poorly understood [35]. Abnormal expression of syncytin 1 may be related to preeclampsia, infertility, and intrauterine growth restriction, as well as neuroblastoma, endometrial cancer, and endometriosis [36]. The results from the TCGA dataset clearly pointed to their underexpression in tumor samples compared with both benign samples and normal tissues, suggesting their tumor suppressor roles in breast cancer. Nevertheless, overall survival was higher (but nonsignificantly) in a low-expression group.
ERVV-1 (syn., envV1) is another member of the syncytin family implying rather opposite functions and expression pattern with higher levels in tumor samples compared with both types of controls in our study. Our results of qPCR study and TCGA dataset presumably indicate oncogenic functions of ERVV-1 in breast cancer, but further confirmation would be necessary.
Only limited information is currently available for ERVV-1 roles in cancer in the literature. One study, however, showed its significantly higher expression in endometrial cancer compared with normal tissue [37]. Interestingly, this study is also showing a discrepancy with clinicopathological data, as envV1 decreased in expression from G1 to G2 grades. This corresponds with what we have seen in our results; i.e., the mode/level of deregulations may not be always congruent or somewhat associated with clinical measures.
No significant results were obtained for ERVE-4 expression in our study. Based on two randomized trials (nivolumab versus everolimus), Ficial et al. [38] reported improved durable response rate (defined as complete response or partial response with a PFS ≥12 months) and longer PFS for high ERVE-4 expression in metastatic clear cell renal cell carcinoma in the nivolumab arm but not in the everolimus arm. It supports the role of ERVE-4 as a kidney cancer antigen and therapeutic target for immunotherapy, as previous studies indicated that antigens derived from this provirus can be immunogenic and stimulate kidney cancer cell killing by cytotoxic T cells both in vitro and in vivo [38].
No other detailed reports are available in the literature for ERVE-4 and cancer, although one previous study [39] using microarrays to specifically detect the long terminal repeat-containing transcripts including HERV-E family demonstrated some HERV-E probesets to be overexpressed in ovary, uterus, and prostate tumor tissues, while some were downregulated in the uterus tumor. However, the results were not conclusive due to an inefficient design of the probes observed and a heterogeneous behavior of the HERV-E probesets across different sample types.
Two other HERVs, i.e., ERVH48-1 and ERVMER34-1, belong to class I (Gammaretrovirus), and their expression was analyzed in TCGA datasets. ERVH48-1 evinced significant underexpression and ERVMER34-1 significant overexpression in tumor samples of invasive breast cancer compared with both benign and normal tissue samples. In literature, there is a lack of studies concerning ERVH48-1 and breast cancer. Recently [40], ERVH48-1 designated as “lncRNA” has been shown to promote proliferation, invasion, and migration and also inhibited apoptosis in prostate cancer. In another report focused on lung adenocarcinoma, Zhan et al. [41] have identified ERVH48-1 expression to be elevated in patients with distant metastasis and may be upregulated in lung cancer cells. Interestingly, this finding is in a contradiction with TCGA Target GTEX dataset, where ERVH48-1 is downregulated in tumor samples (lung cancer) compared with normal tissues and benign samples (data not shown). Kasperek et al. [42] in their TCGA dataset analyses of ERVMER34-1 revealed that HEMO (a synonym) is highly activated in head and neck, lung, breast, endometrium, cervix, esophagus, and bladder tumors, showing an oncogenic potential of ERVMER34-1 in those cancers.
HCP5 was selected (along with ERVK13-1) as another representative of the lncRNA class of noncoding RNAs associated with HERVs. HCP5 revealed no significant results as regards deregulations or associations in our qPCR study. Similarly, the results were not convincing for the TCGA data as a different pattern was observed for HCP5 (only its upregulation in tumors compared with normal tissue was significant).
According to literature, HCP5 should have different relations to breast cancer. Li et al. [43] based on their data mining reported that elevated expression of HCP5 may be beneficial for BC patients (HR = 0.88). Ghafouri-Fard et al. [44] found HCP5 with significantly lower expression (−5.9-fold) in malignant tissues of invasive ductal carcinoma of breast compared with adjacent noncancerous tissues. On the other hand, Wu et al. [45] reported that overexpression of HCP5 may contribute to cisplatin (DDP) resistance in triple-negative breast cancer (TNBC). Similarly, Wang et al. [26] suggested that HCP5 is upregulated in TNBC (cell lines and specimens) and its knockdown may induce cell apoptosis and inhibit cell proliferation and orthotopic xenograft tumor growth. Recently, Tong et al. [46] found that lncRNA HCP5 encodes a 132-amino acid sequence (HCP5-132aa) enhancing the growth of TNBC cells by regulating ferroptosis, and patients with high levels of HCP5-132aa may have poorer prognosis.
Concerning other cancers and HCP5, Hu et al. [47] provided a meta-analysis of the published studies showing HCP5 as oncogenic gene promoting carcinogenesis in a variety of cancers such as non-small cell lung cancer, colorectal cancer, oral squamous cell carcinoma, clear cell renal cell carcinoma, gastric cancer, and osteosarcoma. In those cancers, higher HCP5 levels were associated with a poor overall survival. On the contrary, HCP5 was reported to function as the tumor suppressor only in skin cutaneous melanoma (see [47]). To note some examples, Qin et al. [48] demonstrated higher expression level of HCP5 in the serum of gastric cancer patients as compared with healthy controls. Bai et al. [49] have shown higher levels of HCP5 in colorectal cancer samples compared with controls and knockdown of HCP5 inhibited viabilities, migration, and invasion, while inducing apoptosis in SW480 and HCT-116 cells.
Taken altogether, reports on HCP5 functioning in cancers possibly suggest oncogenic functions. In breast cancer, however, the reports are mixed while the results may depend also on the heterogeneity of different subtypes of breast cancer. In our study, we did not find any overwhelming support for oncogenic functioning of HCP5 in breast cancer.
Conclusion
In this study, we evaluated expression of 7 selected HERVs in breast cancer and tumor adjacent benign samples in a Czech population sample. To the best of our knowledge, this is the first report on determination of their expression for this population. We evaluated the expression of three of the already included HERVs and additional five HERVs using TCGA datasets, increasing the number of investigated HERVs to 12 genes. To sum up, ERV3-1, ERVFRD-1, ERVH48-1, and ERVW-1 were identified as potential tumor suppressors, while ERVK13-1, ERVV-1, and ERVMER34-1 may act to support oncogenic roles in breast cancer. Data for ERVK-7, ERVE-4, ERVK11-1, and HCP5 remain inconclusive.
The mode of the investigated HERV expressions implies variation in their activation, and further examination elucidating the genetic and epigenetic basis of these differences would be necessary. Therefore, further research is warranted before considering treatments based, for example, on HERV inhibitors or antibodies, to shed more light on the detailed roles of HERVs in breast carcinogenesis and the impact on clinical outcomes for patients.
Acknowledgments
This research was supported by the Cooperatio Program, research area Oncology and Haematology (Charles University, Prague), and Ministry of Health of the Czech Republic (MH CZ-DRO-VFN 64165, MH CZ-DRO FNBr 65269705). We would like to thank ČEPS, a.s. (1410003016, 1410003317), for their kind support of our research.
Statement of Ethics
The research was conducted in accordance with the 1964 Helsinki Declaration. Written informed consent was obtained from all individual participants included in the study. The study was approved by the multicentric Ethics Committee of the General University Hospital in Prague (VFN Praha, No. 127/20 S-IV).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
L.Z. and M.K. received financial support from Charles University, Prague (Cooperatio Program, research area Oncology and Haematology). L.Z. received research grants from ČEPS, a.s. (1410003016, 1410003317). V.W. and L.M. obtained financial research support from Ministry of Health of the Czech Republic (MH CZ-DRO FNBr 65269705). L.Z. and O.S. received financial research support from Ministry of Health of the Czech Republic (MH CZ-DRO-VFN 64165). None of the funding sources influenced the processing and preparation of the study.
Author Contributions
Conception and preparation of the manuscript: Luděk Záveský. Interpretation or analysis of data: Luděk Záveský, Eva Jandáková, Vít Weinberger, Luboš Minář, Ondřej Slanař, and Milada Kohoutová. Histopathological examination: Eva Jandáková. Revision for important intellectual content: Luděk Záveský, Ondřej Slanař, and Milada Kohoutová. Supervision: Luděk Záveský, Ondřej Slanař, and Eva Jandáková.
Funding Statement
L.Z. and M.K. received financial support from Charles University, Prague (Cooperatio Program, research area Oncology and Haematology). L.Z. received research grants from ČEPS, a.s. (1410003016, 1410003317). V.W. and L.M. obtained financial research support from Ministry of Health of the Czech Republic (MH CZ-DRO FNBr 65269705). L.Z. and O.S. received financial research support from Ministry of Health of the Czech Republic (MH CZ-DRO-VFN 64165). None of the funding sources influenced the processing and preparation of the study.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material.
Supplementary Material.
Supplementary Material.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Tadesse GF, Tegaw EM, Abdisa EK. Diagnostic performance of mammography and ultrasound in breast cancer: a systematic review and meta-analysis. J Ultrasound. 2023;26(2):355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lother D, Robert M, Elwood E, Smith S, Tunariu N, Johnston SRD, et al. Imaging in metastatic breast cancer, CT, PET/CT, MRI, WB-DWI, CCA: review and new perspectives. Cancer Imag. 2023;23(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Najjar S, Allison KH. Updates on breast biomarkers. Virchows Arch. 2022;480(1):163–76. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong N, Ryder S, Forbes C, Ross J, Quek RGW. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin Epidemiol. 2019;11:543–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. [DOI] [PubMed] [Google Scholar]
- 7. Ouyang YF, Lu WX, Wang Y, Wang BT, Li FY, Li XH, et al. Integrated analysis of mRNA and extrachromosomal circular DNA profiles to identify the potential mRNA biomarkers in breast cancer. Gene. 2023;857:147174. [DOI] [PubMed] [Google Scholar]
- 8. Ortiz MMO, Andrechek ER. Molecular characterization and landscape of breast cancer models from a multi-omics perspective. J Mammary Gland Biol Neoplasia. 2023;28(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Volovat SR, Volovat C, Hordila I, Hordila DA, Mirestean CC, Miron OT, et al. MiRNA and LncRNA as potential biomarkers in triple-negative breast cancer: a review. Front Oncol. 2020;10:526850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Padroni L, De Marco L, Dansero L, Fiano V, Milani L, Vasapolli P, et al. An epidemiological systematic review with meta-analysis on biomarker role of circulating MicroRNAs in breast cancer incidence. Int J Mol Sci. 2023;24(4):3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bannert N, Kurth R. Retroelements and the human genome: new perspectives on an old relation. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hohn O, Hanke K, Bannert N. HERV-K(HML-2), the best preserved family of HERVs: endogenization, expression, and implications in health and disease. Front Oncol. 2013;3:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grandi N, Tramontano E. Type W Human Endogenous Retrovirus (HERV-W) integrations and their mobilization by L1 machinery: contribution to the human transcriptome and impact on the host physiopathology. Viruses. 2017;9(7):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petrizzo A, Ragone C, Cavalluzzo B, Mauriello A, Manolio C, Tagliamonte M, et al. Human endogenous retrovirus reactivation: implications for cancer immunotherapy. Cancers. 2021;13(9):1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stricker E, Peckham-Gregory EC, Scheurer ME. HERVs and cancer-A comprehensive review of the relationship of human endogenous retroviruses and human cancers. Biomedicines. 2023;11(3):936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zapatka M, Borozan I, Brewer DS, Iskar M, Grundhoff A, Alawi M, et al. The landscape of viral associations in human cancers. Nat Genet. 2020;52(3):320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulherkar TH, Gomez DJ, Sandel G, Jain P. Co-infection and cancer: host-pathogen interaction between dendritic cells and HIV-1, HTLV-1, and other oncogenic viruses. Viruses. 2022;14(9):2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kitsou K, Lagiou P, Magiorkinis G. Human endogenous retroviruses in cancer: oncogenesis mechanisms and clinical implications. J Med Virol. 2023;95(1):e28350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johanning GL, Malouf GG, Zheng XF, Esteva FJ, Weinstein JN, Wang-Johanning F, et al. Expression of human endogenous retrovirus-K is strongly associated with the basal-like breast cancer phenotype. Sci Rep. 2017;7:41960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tavakolian S, Goudarzi H, Faghihloo E. Evaluating the expression level of HERV-K env, np9, rec and gag in breast tissue. Infect Agent Cancer. 2019;14(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tourang M, Fang L, Zhong Y, Suthar RS. Association between Human Endogenous Retrovirus K gene expression and breast cancer. Cell Mol Biomed Rep. 2021;1(1):7–13. [Google Scholar]
- 22. Curty G, Marston JL, de Mulder Rougvie M, Leal FE, Nixon DF, Soares MA. Human endogenous retrovirus K in cancer: a potential biomarker and immunotherapeutic target. Viruses. 2020;12(7):726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xue B, Sechi LA, Kelvin DJ. Human endogenous retrovirus K (HML-2) in health and disease. Front Microbiol. 2020;11:1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vargiu L, Rodriguez-Tome P, Sperber GO, Cadeddu M, Grandi N, Blikstad V, et al. Classification and characterization of human endogenous retroviruses; mosaic forms are common. Retrovirology. 2016;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kulski JK. Long noncoding RNA HCP5, a hybrid HLA class I endogenous retroviral gene: structure, expression, and disease associations. Cells. 2019;8(5):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang LH, Luan T, Zhou SH, Lin J, Yang Y, Liu W, et al. LncRNA HCP5 promotes triple negative breast cancer progression as a ceRNA to regulate BIRC3 by sponging miR-219a-5p. Cancer Med. 2019;8(9):4389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Cancer Genome Atlas Program (TCGA). Available from: https://www.cancer.gov/tcga.
- 28. Goldman MJ, Craft B, Hastie M, Repecka K, McDade F, Kamath A, et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38(6):675–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xena UCSC. Available From: https://xena.ucsc.edu/.
- 30. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of realtime quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8(2):R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei YZ, Wei HL, Wei YF, Tan AH, Chen XY, Liao XQ, et al. Screening and identification of human endogenous retrovirus-K mRNAs for breast cancer through integrative analysis of multiple datasets. Front Oncol. 2022;12:820883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ko EJ, Song KS, Ock MS, Choi YH, Kim S, Kim HS, et al. Expression profiles of human endogenous retrovirus (HERV)-K and HERV-R Env proteins in various cancers. BMB Rep. 2021;54(7):368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kumar V, McClelland M, Nguyen J, De Robles G, Ittmann M, Castro P, et al. Expression of endogenous retroviral RNA in prostate tumors has prognostic value and shows differences among Americans of african versus European/middle eastern ancestry. Cancers. 2021;13(24):6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roberts RM, Ezashi T, Schulz LC, Sugimoto J, Schust DJ, Khan T, et al. Syncytins expressed in human placental trophoblast. Placenta. 2021;113:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang QQ, Shi Y, Bian Q, Zhang NB, Wang M, Wang JN, et al. Molecular mechanisms of syncytin-1 in tumors and placental development related diseases. Discov Oncol. 2023;14(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strissel PL, Ruebner M, Thiel F, Wachter D, Ekici AB, Wolf F, et al. Reactivation of codogenic endogenous retroviral (ERV) envelope genes in human endometrial carcinoma and prestages: emergence of new molecular targets. Oncotarget. 2012;3(10):1204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ficial M, Jegede OA, Sant’Angelo M, Hou Y, Flaifel A, Pignon JC, et al. Expression of T-cell exhaustion molecules and human endogenous retroviruses as predictive biomarkers for response to nivolumab in metastatic clear cell renal cell carcinoma. Clin Cancer Res. 2021;27(5):1371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gimenez J, Montgiraud C, Pichon JP, Bonnaud B, Arsac M, Ruel K, et al. Custom human endogenous retroviruses dedicated microarray identifies self-induced HERV-W family elements reactivated in testicular cancer upon methylation control. Nucleic Acids Res. 2010;38(7):2229–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen BS, Xu K, Zhang YM, Xu P, Li CM, Liu J, et al. LncRNA ERVH48-1 contributes to the drug resistance of prostate cancer and proliferation through sponging of miR-4784 to the activation of the wnt/β-catenin pathway. Cancers. 2023;15(6):1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhan X, Feng S, Zhou X, Liao W, Zhao B, Yang Q, et al. Immunotherapy response and microenvironment provide biomarkers of immunotherapy options for patients with lung adenocarcinoma. Front Genet. 2022;13:1047435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kasperek A, Beguin A, Bawa O, De Azevedo K, Job B, Massard C, et al. Therapeutic potential of the human endogenous retroviral envelope protein HEMO: a pan-cancer analysis. Mol Oncol. 2022;16(7):1451–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li J, Gao CD, Liu C, Zhou C, Ma XR, Li HY, et al. Four lncRNAs associated with breast cancer prognosis identified by coexpression network analysis. J Cell Physiol. 2019;234(8):14019–30. [DOI] [PubMed] [Google Scholar]
- 44. Ghafouri-Fard S, Taherian-Esfahani Z, Dashti S, Kholghi Oskooei V, Taheri M, Samsami M. Gene expression of indoleamine and tryptophan dioxygenases and three long non-coding RNAs in breast cancer. Exp Mol Pathol. 2020;114:104415. [DOI] [PubMed] [Google Scholar]
- 45. Wu JJ, Chen H, Ye MN, Wang B, Zhang YZ, Sheng JY, et al. Long noncoding RNA HCP5 contributes to cisplatin resistance in human triple-negative breast cancer via regulation of PTEN expression. Biomed Pharmacother. 2019;115:108869. [DOI] [PubMed] [Google Scholar]
- 46. Tong X, Yu ZL, Xing JN, Liu HZ, Zhou SH, Huang YE, et al. LncRNA HCP5-encoded protein regulates ferroptosis to promote the progression of triple-negative breast cancer. Cancers. 2023;15(6):1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu SP, Ge MX, Gao L, Jiang M, Hu KW. LncRNA HCP5 as a potential therapeutic target and prognostic biomarker for various cancers: a meta-analysis and bioinformatics analysis. Cancer Cell Int. 2021;21(1):686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qin SY, Yang L, Kong S, Xu YH, Liang B, Ju SQ. LncRNA HCP5: a potential biomarker for diagnosing gastric cancer. Front Oncol. 2021;11:684531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bai N, Ma Y, Zhao J, Li B. Knockdown of lncRNA HCP5 suppresses the progression of colorectal cancer by miR-299-3p/PFN1/AKT Axis. Cancer Manag Res. 2020;12:4747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.