Abstract
Herpesvirus saimiri is capable of transforming T lymphocytes of various primate species to stable growth in culture. The interaction of the T-cellular tyrosine kinase p56lck with the transformation-associated viral protein Tip has been shown before to activate the kinase and provides one model for the T-cell-specific transformation by herpesvirus saimiri subgroup C strains. In contrast to other primate species, squirrel monkeys (Saimiri sciureus) are naturally infected with the virus without signs of lymphoma or other disease. Although the endogenous virus was regularly recovered from peripheral blood cells from squirrel monkeys, we observed that the T cells lost the virus genomes in culture. Superinfection with virus strain C488 did not induce growth transformation, in contrast to parallel experiments with T cells of other primate species. Surprisingly, p56lck was enzymatically inactive in primary T-cell lines derived from different squirrel monkeys, although the T cells reacted appropriately to stimulatory signals. The cDNA sequence revealed minor point mutations only, and transfections in COS-7 cells demonstrated that the S. sciureus lck gene codes for a functional enzyme. In S. sciureus, the tyrosine kinase p56lck was not activated after T-cell stimulation and enzymatic activity could not be induced by Tip of herpesvirus saimiri C488. However, the suppression of p56lck was partially released after administration of the phosphatase inhibitor pervanadate. This argues for unique species-specific conditions in T cells of S. sciureus which may interfere with the transforming activity and pathogenicity of herpesvirus saimiri subgroup C strains in their natural host.
Herpesvirus saimiri (HVS; saimiriine herpesvirus type 2) is a T-lymphotropic member of the gamma-2 subfamily of herpesviruses (rhadinoviruses). The natural host of HVS is the squirrel monkey (Saimiri sciureus), a New World primate species. Squirrel monkeys become infected by HVS early in life. The virus persists lifelong and can be easily isolated from peripheral blood mononuclear cells (PBMC) by cocultivation with permissive epithelial cells (owl monkey kidney [OMK] cells). As far as is known, neither acute nor persistent infection of squirrel monkeys with HVS induces any signs of disease. In contrast, the virus causes acute peripheral T-cell lymphoma after experimental infection of other New World monkeys, such as common marmosets (Callithrix jacchus), cottontop tamarins (Saguinus oedipus), and owl monkeys (Aotus trivirgatus) (reviewed in reference 16). Even in Old World monkeys, such as rhesus monkeys (Macaca mulatta) or cynomolgus monkeys (Macaca fascicularis), HVS strain C488 was shown to induce acute T-cell leukemia after experimental infection (1, 35).
HVS strains have been assigned to three subgroups (A, B, and C) based on their pathogenicity in various hosts and on their genomic sequences at one end of the nonrepetitive L-DNA segment (46, 47). Individual squirrel monkeys can be simultaneously infected with HVS strains from more than one subgroup. In comparison, subgroup B strains have the weakest transformation capability. They induce lymphomas only in certain New World monkey species but not in common marmosets. In contrast, subgroup A strains are highly oncogenic in most of the New World monkey species tested and they are able to transform T cells from marmosets and tamarins to interleukin-2 (IL-2)-independent growth in vitro. However, as stated above, neither subgroup A nor subgroup B strains transform human or rhesus monkey T cells in vitro nor do they cause lymphomas in Old World monkeys. Only subgroup C strains, especially strain C488, are able to transform human T cells to stable IL-2-dependent growth in culture (4). These findings suggest differences in the transformation mechanisms between the HVS subgroups. Accordingly, the subgroups differ in their transforming genes in the variable transformation-associated genome region. Human T cells infected with HVS strain C488 do not produce virus particles after transformation, while virus-transformed T cells from New World monkeys usually are semipermissive; i.e., they are transformed and release virus particles without synchronous cell lysis (14). Human T cells retain many of the functional features of the parental cells after transformation by HVS C488, such as functional expression of T-cell receptor and coreceptor molecules and major histocompatibility complex-restricted reactivity to their specific antigen (6, 10, 51, 61). However, the transformed cells and the parental cells differ in the following aspects. Irrespective of the cytokine profile of the parental cells, HVS-transformed T lymphocytes secrete Th1 cytokines, including gamma interferon (10). HVS-transformed human T cells are hyperresponsive to CD2 ligation (50). Similarly to human T-cell leukemia virus-transformed T cells, the Src family kinase Lyn is aberrantly expressed and enzymatically active (15, 65).
The transformation-associated genes in subgroup C viruses, stpC (saimiri transformation-associated protein) and tip (tyrosine kinase-interacting protein), are located at one end of the genomic HVS L-DNA and are transcribed into a bicistronic mRNA. These genes encode the only viral proteins known to be constitutively expressed in HVS-transformed human T cells (5, 14). While deletion of these genes does not affect the lytic replication of the virus, both stpC and tip are necessary for the transforming activity of HVS (11, 37). The protein StpC is a small cytoplasmic phosphoprotein of 102 amino acids and 21 kDa (28, 29). Rodent fibroblasts transfected with stpC form foci in culture and tumors in nude mice (31). Mice with an stpC transgene develop epithelial tumors (53). StpC was shown to directly associate with cellular Ras, leading to activation of the Ras pathway and to activation of the p42 mitogen-activated protein ERK kinase (30). Moreover, the binding of StpC to tumor necrosis factor receptor-associated factors and subsequent activation of NF-κB have been described (39). The association of StpC with both Ras and tumor necrosis factor receptor-associated factors were shown to be essential for the transforming functions (30, 39). Thus, stpC-mediated transforming activity is not specific for T cells (31, 53).
The other transformation-associated protein, Tip, is a 40-kDa phosphoprotein of 256 amino acids. Whereas the N-terminal portion is variable between different virus strains of subgroup C, two sequence motifs are well conserved which mediate the association with the T-cellular Src family tyrosine kinase p56lck: a Src homology domain 3 binding region (SH3B) and a C-terminal Src kinase homology domain (5, 15, 32, 33). A hydrophobic C-terminal domain anchors the molecule at the inside of the plasma membrane (42). Besides its interaction with p56lck, Tip has been reported to associate with the mRNA export factor Tap, a homolog of yeast Mex67p (19, 34, 66). Coexpression of Tip and Tap induced aggregation in Jurkat leukemia cells (66). Finally, phosphorylated Tip binds to and activates STAT factors (22, 43). An inducible Tip transgene caused lymphomas in mice, underlining the oncogenic potential of this molecule from subgroup C strains of HVS (62).
Lck is a 56-kDa member of the Src family of nonreceptor protein tyrosine kinases, and its expression is restricted to T cells, NK cells, and B1 cells. The p56lck sequence is highly conserved in mammals. In T-cell development and activation, p56lck is necessary for the phosphorylation of target molecules and for their recruitment to the T-cell receptor complex (60, 64). Mutations in the lck gene can cause loss of p56lck activity, followed by immunodeficiency in vivo (18). In most experimental approaches, the binding of Tip leads to the activation of p56lck and to the phosphorylation of Tip in vitro (24, 42, 65) and in vivo (43). Moreover, increased phosphorylation of other p56lck target proteins, such as Zap70 (54) and STAT1 and STAT3 (22, 43, 44), was observed. Tip-transfected Jurkat cells showed NF-κB activation (66). These facts support the concept that the Tip-Lck interaction is decisive for the T-cell-specific growth-transformation by HVS. In contrast, other observations argue against the relevance of this interaction for transformation. First, Tip-overexpressing Jurkat cells showed a reduced level of tyrosine phosphorylation (33). Second, a Tip point mutant (Y114) displayed enhanced p56lck binding and further decreased tyrosine phosphorylation (20). Finally, HVS C488 with a mutated SH3B motif of Tip was still able to transform marmoset T cells in vitro and to cause disease in vivo, whereas no Tip-Lck interaction was detectable (12). On the other hand, each of the two Lck-binding motifs of Tip, SH3B and the C-terminal Src kinase homology domain, have recently been shown to be sufficient for Lck binding and activation (23). Moreover, both Tip and StpC are necessary for the induction of IL-2 expression and NF-κB activation in Molt-4 leukemia T cells (49). Whereas the knowledge of how T cells are transformed by HVS is growing, it is not understood how the T cells of the natural host, S. sciureus, escape transformation by HVS. In this report, we describe results that may link the two questions for HVS strains of subgroup C: enzymatic p56lck activity is strongly suppressed in T cells from S. sciureus monkeys.
MATERIALS AND METHODS
Blood samples.
Blood samples were obtained from 11 specimens of three different squirrel monkey (S. sciureus) colonies at the German Primate Center, Göttingen, Germany, from humans, from common marmosets (C. jacchus), from cottontop tamarins (Saguinus oedipus), and from rhesus monkeys (M. mulatta). The PBMC were prepared by direct centrifugation of EDTA-treated blood (1 to 2 ml). The erythrocytes were lysed by treatment with ACK buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA [pH 7.3]) for 5 min, followed by extensive washing in phosphate buffered saline.
Cell culture and stimulation.
Fresh PBMC were stimulated at a density of 2 × 106/ml with 4 μg of phytohemagglutinin (PHA; Murex, Groβburgwedel, Germany) or concanavalin A (ConA; Sigma, Taufkirchen, Germany) per ml. T cells were cultivated in 45% RPMI 1640 medium, 45% Panserin 401 (PAN, Aidenbach, Germany), 10% fetal bovine serum, glutamine, and gentamicin. IL-2 (Proleukin; kindly donated by Chiron, Ratingen, Germany) was added to T cells from humans and squirrel monkeys at 50 IU/ml or from cottontop marmosets at 10 IU/ml (final medium concentration). T cells of each individual monkey were cloned by limiting dilution in microtiter plates in the presence of irradiated (120 Gy) human feeder cells (105 cells/well). T-cell clones and lines were further amplified by periodic restimulation with mitogen and feeder cells every other week. Infection and transformation experiments followed published protocols (13, 48). The surface phenotype of squirrel monkey T cells was analyzed by utilizing a FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany) with monoclonal antibodies directed against CD2 (CB219 [see reference 17] and T910; Dako, Glostrup, Denmark), CD3 (LT3; a gift from A. Filatov, Moscow, Russia), CD4 (MT310; Dako), CD8 (MT1014; a gift from E. Rieber, Dresden, Germany), CD25 (2A3), and HLA-DR (L243; both from Becton Dickinson).
The T cells were allowed to rest for at least 10 days before stimulation experiments. They were then incubated at 2 × 104 to 5 × 104 cells/well in a final volume of 200 μl in 96-well round-bottom plates. Where indicated, 5 × 104 irradiated (120 Gy) human PBMC per well, 5 × 104 L428 cells per well, 2 μg of PHA per ml, or 0.1% (vol/vol) packed sheep erythrocytes were added. After 24 h, 50 μl of supernatant was removed for the determination of IL-2, and after 48 h, [3H]thymidine (1 μCi/well) was added and the mixture was incubated for a further 16 h. Cells were harvested, and thymidine incorporation was determined by liquid scintillation counting. For the determination of IL-2, 50 μl of culture supernatant was added to 100 μl of a suspension of the murine indicator cell line CTLL (2.5 × 104 cells/ml) in flat-bottom wells. After 12 to 24 h, 1 μCi of [3H]thymidine was added, the mixture was incubated for a further 24 h, and [3H]thymidine incorporation was determined.
To activate Lck, primary T cells from different species were washed three times in serum-free medium and adjusted to 107 cells/ml. The T cells from S. sciureus were documented by PCR to be free of HVS; the T cells from the other species had not been infected. Stimulation was started with the addition of preheated (37°C) serum-free medium supplemented with 10 mM pervanadate and incubation for 5 min. The reaction was stopped with ice-cold phosphate-buffered saline, followed by centrifugation. Cells were lysed immediately or shock frozen in liquid nitrogen and stored at −70°C before use.
Cells of BWδζ-neo and BWδζ-Tip, a murine thymoma cell line transfected with a control vector or a Tip expression vector, were grown in complete Iscove's medium with 10% fetal bovine serum, glutamine, and gentamicin in the presence of G418 at 1 mg/ml (65). COS-7 cells were grown in RPMI medium with 10% fetal bovine serum, glutamine, and gentamicin.
Immunoprecipitation, phosphotransferase assay, and immunoblotting.
Before biochemical analysis, S. sciureus T cells were shown to be free of HVS genomes by sensitive PCR. For immunoprecipitation, T cells were lysed in TNE buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 2 mM EDTA, 1% Triton X-100) supplemented with 1 mM sodium orthovanadate (Na3VO4), 5 mM NaF, and 10 μg each of aprotinin and leupeptin (Sigma) per ml for 30 min on ice. Lysates were cleared at 13,000 × g for 15 min, and the protein concentration in the supernatant was determined by the bicinchoninic acid assay (Pierce, Rockford, Ill.). Rabbit anti-Lck serum (kind gift of A. Tsygankov, Philadelphia, Pa.), 5 μl/mg of protein, was added, and the mixture was incubated for at least 1 h at 4°C to precipitate Lck. This was followed by incubation with 50 μl of a 10% (vol/vol) suspension of Staphylococcus aureus particles (Pansorbin; Calbiochem, Bad Soden, Germany) for 1 h. The immunoprecipitates were washed five times in TNE buffer and split for in vitro phosphotransferase assays and immunoblotting.
Cells of the transfected murine thymoma line BWδζ-Tip served as a source of the viral protein Tip. These cells, as well as those of the mock-transfected line BWδζ-neo, were lysed as described above. These cells contain only minimal levels of endogenous Lck (65), which was depleted by immunoprecipitation as described above. Since Tip is abundantly expressed by BWδζ-Tip, the coprecipitation of Tip with the few molecules of endogenous Lck did not measurably deplete Tip from the lysates. S. aureus particles with bound Lck which had been precipitated from human or monkey T-cell lysates (200 μg of protein) were added to the Lck-depleted lysates of BWδζ-Tip or BWδζ-neo cells (100 μg of protein), and the mixture was incubated for 30 min at 4°C to allow interaction of primate Lck with Tip and then washed five times in TNE buffer.
For in vitro phosphotransferase assays, the immunoprecipitates were washed once in kinase buffer (20 mM morpholinepropanesulfonic acid [MOPS; pH 7.0], 5 mM MnCl2). The pellets were then incubated for 5 min at room temperature in 25 μl of a kinase assay mixture containing 1 μM ATP (Roche Diagnostics, Mannheim, Germany) and 10 μCi of [γ-32P]ATP (Amersham Pharmacia Biotech, Freiburg, Germany). The phosphotransferase reaction was stopped with 40 μl of sample buffer (62.5 mM Tris [pH 6.8], 2% sodium dodecyl sulfate [SDS], 10% glycerol, 5% β-mercaptoethanol). Samples were then incubated for 30 min at room temperature and microcentrifuged. Supernatants were boiled for 5 min before separation by SDS–8% polyacrylamide gel electrophoresis. Gels were dried and exposed to a PhosphorImager screen (Molecular Dynamics, Sunnyvale, Calif.) or to Kodak Biomax MR film.
For immunoblots, cell lysates or immunoprecipitates were separated by SDS–8% polyacrylamide gel electrophoresis and transferred to enhanced-chemiluminescence nitrocellulose membranes (Amersham Pharmacia Biotech). Blots were incubated for 1 h at room temperature in blocking buffer (Tris-buffered saline [pH 7.4], 4% bovine serum albumin, 0.5% Tween 20), followed by incubation with an anti-Lck monoclonal antibody (3A5; Santa Cruz, Santa Cruz, Calif.) diluted in blocking buffer with 0.1% sodium azide. After washing in Tris-buffered saline containing 0.5% Tween 20, blots were incubated with goat anti-mouse immunoglobulin coupled to horseradish peroxidase (Dako) in blocking buffer for 1 h. After washing, the bands were visualized with enhanced-chemiluminescence reactions (Amersham Pharmacia Biotech).
DNA analysis and transfections.
The lck cDNA was amplified by using the primers HF633 (5′-CCA-GGG-TTC-GGG-CTC-CAG-GCT-ATT-C-3′; central position, upstream orientation), HF634 (5′-GAG-CAG-AAC-GGC-GAG-TGG-TGG-AAG-G-3′; central position, downstream orientation), HF646 (5′-ATG-GGC-TGT-GGC-TGC-AGC-TCA-3′; N terminal), and HF647 (5′-AGG-CTG-AGG-CTG-GTA-CTG-GGC-3′; C terminal). In order to clone the lck cDNA from S. sciureus T cells, polyadenylated RNA was purified with magnetic oligo(dT) beads (Dynal, Hamburg, Germany). A cDNA library was constructed with reverse transcription and second-strand synthesis using the Marathon system (BD Clontech, Heidelberg, Germany). With the primers HF633 and HF634, overlapping 5′ and 3′ products were obtained by rapid amplification of cDNA ends. The primers HF646 and HF647 were taken from a partial DNA sequence derived from the products of rapid amplification of cDNA ends and PCR. By using these primers, precisely the entire open reading frame was amplified and cloned into plasmid vectors. The entire cDNA sequence was determined from both DNA strands with the dye deoxy terminator method by primer walking using an ABI 377 sequencer.
A series of virus genes was amplified with the following primer pairs: HVS A11 stpA, BB110 (5′-TAG-ACG-TGT-GGA-TCC-ATG-GCA-AGA-GGT-CTA-3′) and BB155 (5′-CGG-AAT-TCT-TAC-ATT-TTT-TTA-AA-3′); HVS SMHI stpB, BB170 (5′-GAG-GGC-AAA-CTA-AGC-AAC-GCT-C-3′) and BB171 (5′-GGT-CCG-CTT-CCA-GGT-GGT-TGG-G-3′); HVS C488 stpC, HF39 (5′-GAG-TTT-CCA-AAA-TGT-ACT-AAG-CTA-AC-3′) and HF40 (5′-ACT-AAT-AAA-AAG-TTC-CAC-ACA-ACT-AAC-3′); HVS orf3, HF740 (5′-CAC-AAC-ACT-GGT-ATG-TAC-CAA-TG-3′) and HF741 (5′-CTG-TGG-AGG-TAA-TGC-AGA-TAC-3′); HVS orf13, HF75 (5′-GTG-TAT-CTC-AAA-CTC-AAC-3′) and HF76 (5′-CTT-GTT-TGC-TAT-AAC-TTA-GTG-3′); HVS orf75, HF742 (5′-TGG-CTG-CTA-ACA-GGC-ATG-G-3′) and HF743 (5′-AGC-ACG-TTG-CCC-GAG-ATT-G-3′). PCR products were analyzed in 1% agarose gels. Southern blot hybridization of PCR gels was performed if weak or no signals were detectable after ethidium bromide staining. An orf13 DNA fragment served as the hybridization probe after 32P labeling.
For transfections, the human and S. sciureus lck open reading frames were cloned into the eukaryotic expression vector pcDNA3 (Invitrogen, Groningen, The Netherlands). The full-length open reading frame of the herpesvirus tip gene (5) was inserted into the eukaryotic expression vector pEFBos (52) to generate pEFBos-Tip. COS-7 cells were seeded to reach 70% confluency after overnight incubation. They were then transfected with 5 μg of each individual expression plasmid in the presence of DEAE-dextran. The total amount of DNA was adjusted to 10 μg by adding empty vector. Cells were harvested for analysis 48 h after transfection.
Nucleotide sequence accession number.
The S. sciureus lck sequence is available under accession number AJ277921 (EMBL database).
RESULTS
Squirrel monkey T cells lose herpesvirus saimiri genomes in culture.
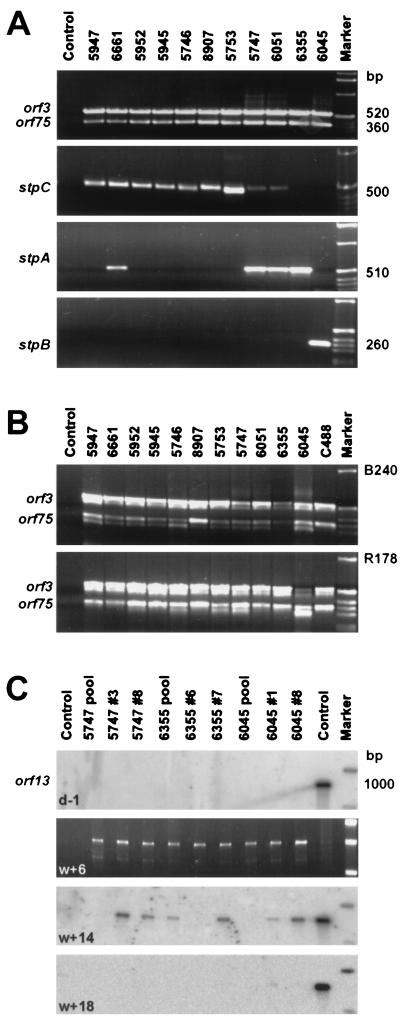
In order to study the virologic and immunologic features of T cells from squirrel monkeys, PMBC were prepared from EDTA-treated blood samples of 11 specimens from three different colonies at the German Primate Center, Göttingen, Germany. A portion of freshly isolated PBMC (approximately 106 cells) was cocultivated with permissive OMK cells, and the persisting HVS could be isolated from each individual animal. After a typical cytopathic effect had led to cell lysis, the presence of virus DNA was demonstrated by PCR for the conserved genes orf3 and orf75 (Fig. 1A). By DNA PCR for the respective transformation-associated genes stpA, stpB, and stpC, we classified nine of the primary isolates in subgroup C, four in subgroup A, and one in subgroup B (Fig. 1A; Table 1). Three of the primary isolates yielded signals for both stpA and stpC, suggesting coinfection with different virus strains in vivo.
FIG. 1.
Viruses and T cells from the PBMC of 11 squirrel monkeys. (A) HVS was isolated from PBMC of 11 monkeys by cocultivation with permissive OMK cells, as demonstrated by PCR bands for the virus genes orf3 and orf75. On the basis of subgroup-specific DNA PCR, we assigned nine of the primary isolates to subgroup C, four to subgroup A, and one to subgroup B. In three cases, coinfection with virus strains of different subgroups was detectable. (B) All of the virus isolates were capable of transforming T cells of two S. oedipus donors (cottontop tamarins B240 and R178; see references 36 and 38). In the case of subgroup B virus 6045, the growth rate of the transformed T cells was reduced in comparison with those of the parallel samples and the C488 positive control. (C) After prolonged culture with repeated restimulations using mitogen and human feeder cells, the T cells from S. sciureus lost their endogenous HVS, as demonstrated by negative PCR results for virus DNA, followed by Southern hybridization (samples were taken 1 day before infection). Subsequently, the cultures were infected with HVS C488. After 6 weeks, virus DNA was still easily detectable after PCR on ethidium bromide-stained agarose gels. After 14 weeks, only faint signals were detectable after Southern blot hybridization whereas virus-specific hybridization signals were no longer found after 18 weeks of culture. Growth-transformation was not achieved in repeated attempts. In contrast, parallel cultures of human and cottontop tamarin cells were readily transformed to stable growth by C488.
TABLE 1.
Subgroup classifications and transforming capabilities of 11 new HVS isolates
| S. sciureus donor | Source colony | HVS subgroup | T-cell transformation (S. oedipus)
|
|
|---|---|---|---|---|
| B240 | R178 | |||
| 6045 | 1 | B | Delayed | Delayed |
| 6051 | 1 | A + (C)b | + | + |
| 8907 | 1 | C | + | + |
| 5746 | 2 | C | + | + |
| 5747 | 2 | A + (C)b | + | + |
| 5753 | 2 | C | + | + |
| 5947 | 3 | C | + | + |
| 5945 | 3 | C | + | + |
| 5952 | 3 | C | + | + |
| 6661 | 1 × 2a | (A) + Cb | + | + |
| 6355 | 2 × 3a | A | + | + |
The parents were from two different colonies.
The animals were infected by viruses of two different subgroups. The less predominant subgroup is in parentheses.
The 11 virus isolates were tested for the ability to transform cottontop tamarin T cells in culture (donors B240 and R178; see references 36 and 38). All strains behaved similarly to HVS C488 with respect to simian T-cell transformation. The only exception was virus isolate 6045, which was assigned to subgroup B and yielded comparably slowly proliferating T cells. In all cases, virus DNA was clearly detectable by virus-specific DNA PCR after stable growth was established at approximately 2 months after infection (Fig. 1B; Table 1).
Further samples of fresh PBMC were stimulated with the mitogen ConA and then cultivated in the presence of recombinant human IL-2. In parallel, T cells of each animal were immediately cloned by limiting dilution with mitogen stimulation and irradiated human feeder cells. Every other week, the primary T cells were restimulated with ConA or PHA in the presence of feeder cells and amplified in the presence of recombinant IL-2. After 6 weeks in culture, all of the T-cell lines and clones showed strong signals with HVS-specific DNA PCR. The squirrel monkey T cells were further amplified by periodic restimulation for several months. After 5 months, endogenous virus DNA was no longer detectable in the squirrel monkey T-cell lines and clones (Fig. 1C, top). Subsequently, squirrel monkey T cells were infected with HVS C488 and no further restimulation was performed. At 6 weeks after infection, virus DNA was easily detectable after PCR on ethidium bromide-stained agarose gels (Fig. 1C, second from top). After 14 weeks, only faint virus DNA signals were detectable after PCR and Southern blot hybridization and no virus-specific hybridization signals were found after 18 weeks of culture (Fig. 1C, third and fourth from top). Growth transformation was not achieved in repeated attempts, whereas parallel cultures of human and cottontop tamarin T cells (B240 and R178; see references 36 and 38) were readily transformed by C488 to stable growth.
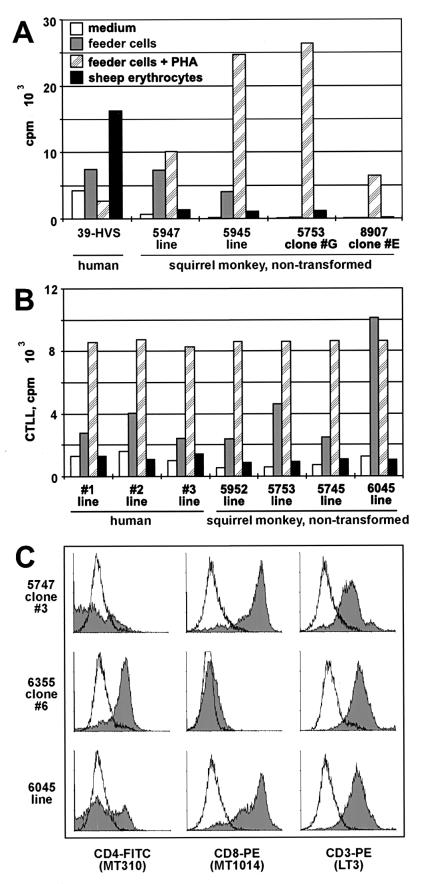
Moreover, squirrel monkey T cells were tested for their immunologic phenotype. In functional assays, S. sciureus T cells showed normal properties (Fig. 2A and B). The cells responded with proliferation (Fig. 2A) and IL-2 secretion (Fig. 2B) to the mitogen PHA in the presence of irradiated human feeder cells. Some lines, in the most pronounced form, line 6045 (Fig. 2B), responded to irradiated human PBMC without further treatment and thus appeared to be xenoreactive. Human T lymphocytes can be stimulated by sheep erythrocytes after infection with HVS C488 (Fig. 2A) but not in the absence of the virus. In contrast to allo- or xenoreactivity, which is usually caused by T-cell receptor reactivity to foreign major histocompatibility complex-peptide complexes, sheep erythrocytes stimulate through the high surface density of ovine CD58, which ligates CD2 on the T-cell surface. Human T cells are rendered hyperreactive to CD2 signals by HVS (15, 50). None of the simian T-cell lines and clones was stimulated by sheep erythrocytes, suggesting either that HVS does not induce CD2 hyperreactivity in S. sciureus T cells or that they were free of persisting virus genomes. The latter was then confirmed by DNA PCR.
FIG. 2.
Immunological phenotype of T-cell lines and clones from squirrel monkeys. (A and B) Human and squirrel monkey T-cell lines and clones, as well as a human T-cell clone transformed by HVS C488 (39-HVS), were exposed to feeder cells (A, irradiated human PBMC; B, human Hodgkin's lymphoma line L428) in the presence or absence of PHA or to sheep erythrocytes, and their proliferation (A) and IL-2 secretion (B) were determined by measurement of [3H]thymidine incorporation. Both human and squirrel monkey long-term T-cell lines, but not the T-cell clones, showed various degrees of allo- and xenoreactivity, respectively. In contrast to the HVS-transformed human T cells, none of the primary T-cell cultures proliferated or secreted IL-2 in the presence of sheep erythrocytes. White, medium; grey, feeder cells; hatched, feeder cells and PHA; black, sheep erythrocytes. (C) As an example, surface marker staining results are shown for T-cell clones 5747 #3 and 6355 #6 and for polyclonal cell line 6045. The murine monoclonal antibodies MT301 (CD4), MT1014 (CD8), and LT3 (CD3), directed against the respective human molecules, were cross-reactive against their counterparts from S. sciureus.
Flow cytometry studies are generally hampered by the fact that only a few monoclonal antibodies directed against human epitopes are cross-reactive with S. sciureus T cells. The cells were not recognized by a series of anti-CD3 antibodies, indicating that the cultures were free of contaminating human T cells. However, the CD3-specific monoclonal antibody LT3 stained squirrel monkey T cells homogeneously. The cells carried either CD8 or CD4 on their surface (Fig. 2C). Moreover, the cells were positive for CD2, CD25, and HLA-DR (data not shown).
p56lck from S. sciureus T cells is enzymatically inactive.
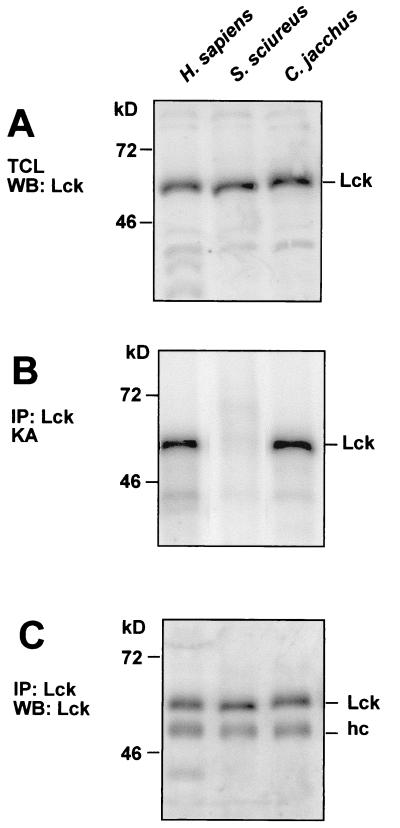
To elucidate the molecular reasons for the resistance of S. sciureus T cells to transformation by HVS, we analyzed the protein tyrosine kinase p56lck, the interaction partner of the viral protein Tip. T cells from S. sciureus and humans expressed similar amounts of p56lck protein (Fig. 3A). To measure p56lck kinase activity, we subjected Lck immunoprecipitates to in vitro phosphotransferase assays. Unexpectedly, the S. sciureus T cells showed very low levels of p56lck kinase activity, if any (Fig. 3B). This was due to a strong reduction of p56lck kinase activity, because Western blots revealed similar amounts of human and squirrel monkey Lck proteins in the precipitates (Fig. 3C). This effect was reproducibly observed in five T-cell lines from three squirrel monkeys. Measurements of p56lck activity using rabbit muscle enolase as an exogenous substrate yielded similar results (data not shown). We then determined p56lck protein levels and enzymatic activity in other New and Old World primate species, C. jacchus (common marmoset), S. oedipus (cottontop tamarin), and M. mulatta (rhesus monkey). The T cells from these species can be transformed by HVS in vivo and in vitro. T cells from all three species had normal amounts and specific activities of p56lck (Fig. 3 and data not shown).
FIG. 3.
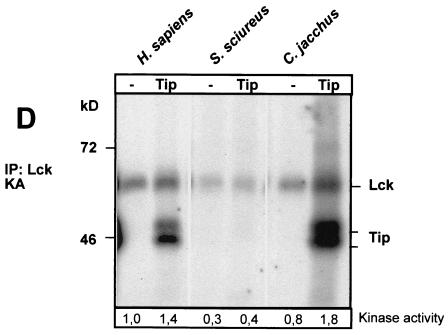
Enzymatic activity of the tyrosine kinase p56lck in T cells from S. sciureus. The tyrosine kinase p56lck was analyzed in parallel in T-cell lysates from H. sapiens, S. sciureus (squirrel monkey), and C. jacchus (common marmoset). (A) Total T-cell lysates (TCL) of all three species contained comparable amounts of p56lck protein, as demonstrated by Western blotting (WB) with a polyclonal antiserum against the unique domain of human p56lck. (B) By using in vitro kinase assays (KA), the enzymatic autophosphorylation activity of p56lck was analyzed from p56lck immunoprecipitates (IP). In contrast to that in human and marmoset T cells, Lck activity in T cells from squirrel monkeys was barely detectable. This effect was observed in five independent T-cell lines from three S. sciureus monkeys. (C) The respective immunoprecipitates yielded comparable levels of p56lck protein on Western blots. Here, the heavy chain (hc) of the precipitating antibodies was detectable as an additional band. (D) Whereas Tip from HVS C488 was efficiently transphosphorylated in kinase assays as a substrate of human or common marmoset p56lck, the addition of Tip to S. sciureus p56lck immunoprecipitates did not increase the kinase activity and Tip was not phosphorylated by S. sciureus p56lck. p56lck autophosphorylation was quantified by densitometry. Relative kinase activity is indicated at the bottom of panel D.
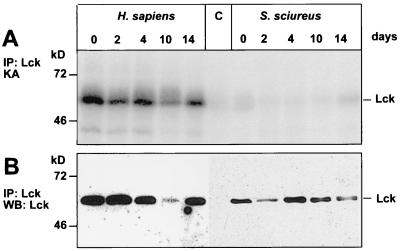
The viral transformation-associated protein Tip is a substrate for p56lck, and binding of Tip to the enzyme increased its specific activity in several previous studies (24, 42, 43, 65). We tested if this is also the case with S. sciureus p56lck. However, in contrast to the Homo sapiens and C. jacchus p56lck proteins, the addition of Tip to S. sciureus p56lck did not enhance the kinase activity and Tip was not phosphorylated by S. sciureus p56lck (Fig. 3D). p56lck activity is usually not altered during restimulation. To exclude the possibility that the specific p56lck activity had been downregulated in response to restimulation of the T cells, we measured the enzymatic activity at different time points after restimulation. But unlike p56lck from similarly treated human T cells, S. sciureus p56lck was inactive during the entire time course experiment (Fig. 4). Thus, the very low specific autophosphorylation level appears to be a constant feature of S. sciureus p56lck.
FIG. 4.
p56lck activity after restimulation of T cells. The activity and abundance of p56lck from human or squirrel monkey T cells were determined at different time points after restimulation (days 0 to 14). (A) Whereas p56lck activity was stable after stimulation of human T cells, the S. sciureus p56lck autophosphorylation signals were faint in all samples. (B) Western blotting (WB) revealed constant amounts of protein during the time course after immunoprecipitation (IP). KA, kinase activity.
The p56lck proteins of humans and S. sciureus are highly conserved.
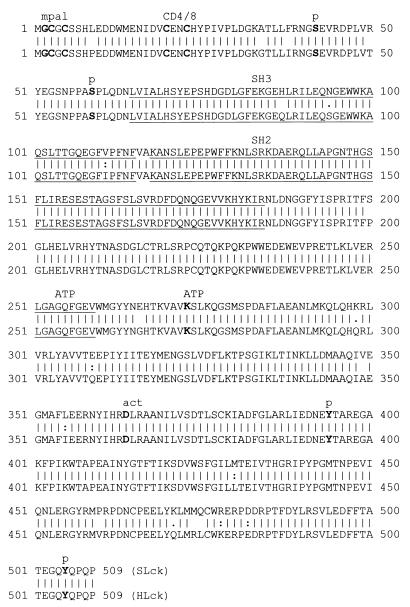
To obtain more information about the p56lck protein in S. sciureus, we determined the cDNA sequence of the squirrel monkey gene. The human and squirrel monkey p56lck proteins are highly conserved, with 96% amino acid identity (Fig. 5). In human p56lck, amino acids essential for the localization, regulation, and activity of the kinase are known. The autophosphorylation site Y394 and the ATP binding site K273 are important for catalytic activity. Y505 regulates enzyme activity by intramolecular binding to the SH2 domain. None of these key amino acids have diverged during H. sapiens and S. sciureus evolution. The myristoylation and palmitoylation anchor residues are conserved, as well as the CD4 or CD8 binding motif and the serine phosphorylation sites (S42 and S59).
FIG. 5.
Sequence comparison of p56lck proteins from humans and squirrel monkeys. The S. sciureus p56lck sequence (SLck) was aligned with the human counterpart (pir1:jq0152; HLck) by using the GCG gap program. The p56lck sequences are highly conserved, with 96% amino acid identity. The major functional sites are not altered (myristoylation and palmitoylation [mpal], G2, C3, and C5; CD4 or CD8 binding motif, C20 and C23; serine phosphorylation sites, S42 and S59; ATP binding, positions 251 to 259, K273; active center [act], D364; autophosphorylation, Y394; intramolecular binding to the SH2 domain, Y505). p, phosphorylation site.
Recombinant S. sciureus p56lck is a functional enzyme.
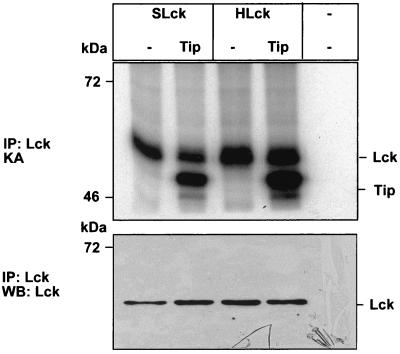
In order to analyze whether the minor sequence differences between the human and squirrel monkey p56lck proteins are responsible for the lack of enzymatic activity in monkey T cells, we cloned the S. sciureus and human p56lck coding regions into expression vector pcDNA3 and transfected COS-7 cells. In vitro phosphotransferase assays of lysates of transfected cells revealed that the recombinant S. sciureus and H. sapiens p56lck proteins had similar activities (Fig. 6). Cotransfected Tip was coprecipitated with p56lck and served as a substrate for the recombinant p56lck enzymes from S. sciureus and H. sapiens. In COS-7 cells, activation of Lck by cotransfected Tip was not always observed. Thus, our results show that the S. sciureus lck gene codes for a functional enzyme that, in principle, is able to interact with Tip in the same way as that described for human p56lck.
FIG. 6.
Enzymatic activity of recombinant S. sciureus p56lck. The p56lck enzymes from humans (HLck) and squirrel monkeys (SLck) were transiently expressed in COS-7 cells. In vitro kinase assays (KA) revealed that the recombinant p56lck enzymes of S. sciureus and H. sapiens had similar activities after immunoprecipitation (IP). Coexpressed Tip from HVS C488 was coprecipitated with p56lck and served equally well as a substrate for the recombinant p56lck proteins of S. sciureus and H. sapiens. Thus, the S. sciureus lck gene codes for a functional enzyme. WB, Western blot.
Pervanadate stimulates S. sciureus p56lck.
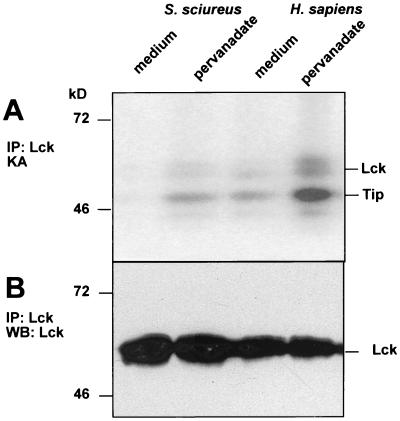
To study whether the inactive state of p56lck in S. sciureus T cells is reversible, we treated the cells with pervanadate, a potent inhibitor of tyrosine phosphatases. The treatment induced p56lck kinase activity (Fig. 7) in human T cells and also in S. sciureus T cells. This suggests that p56lck activity is suppressed by phosphatases in S. sciureus T cells.
FIG. 7.
p56lck activity from squirrel monkey T cells stimulated by phosphatase inhibition. S. sciureus and human T cells were treated with pervanadate (10 mM), a potent inhibitor of tyrosine phosphatases, for 5 min in vitro. This treatment induced Lck kinase activity in S. sciureus T cells and strongly increased it in the human counterparts (A). Western blots (WB) show equal amounts of Lck protein in the immunoprecipitates (IP) (B). KA, kinase assay.
DISCUSSION
We report here that squirrel monkey T cells lack p56lck activity, which is in sharp contrast to all of the other primate species tested. However, the immunological phenotype of squirrel monkey T cells was comparable to that of normal human T-cell lines and clones. Peripheral blood cells were isolated from squirrel monkeys and could be cultivated under standard conditions. The T cells could be stimulated with mitogen to proliferation and cytokine production and expressed surface molecules which are typical for T cells (Fig. 2).
Although the persisting virus could be easily isolated from fresh peripheral blood cells, we observed a reproducible tendency of the cultivated squirrel monkey T cells to lose the HVS genomes (Fig. 1). This is in contrast to the stable episomal persistence of this virus in T cells of a series of closely related New World monkey and distantly related Old World primate species. These in vitro culture results raise the question of whether T cells are really the main reservoir of HVS in squirrel monkeys. If they are, it is unclear why T cells invariably lose the viral genomes in vitro. The loss of HVS genomes in long-term culture could reflect an absence of a virus-induced growth advantage for virus-infected T cells. Theoretically, this could be the result of the inactivation of HVS C488 transformation-associated functions in S. sciureus T cells.
Immunological mechanisms that control the virus in vivo and may also operate in vitro cannot explain the phenomenon for the following reasons. First, the S. sciureus cells were extensively washed initially and then cultured in medium containing fetal bovine serum. Therefore, antiviral antibodies or other simian serum components cannot play a role. Second, T cells, which were cloned by limiting dilution immediately after isolation, also lost the virus with time (Fig. 1C), and these virus-free T-cell clones could not be transformed by HVS C488. In contrast, their growth remained fully dependent on periodic restimulation with the mitogen PHA in the presence of irradiated human PBMC as feeder cells, as well as on exogenous IL-2. The limiting-dilution procedure excludes the possibility that simian NK cells or HVS-specific cytotoxic T cells were carried over into the culture from the host animal and caused the loss of the viral genomes by selectively eliminating the virus-infected T cells. Consequently, cell-autonomous mechanisms might confer resistance to S. sciureus T cells against HVS persistence and transformation. Squirrel monkeys are the natural host of HVS and tolerate the infection without developing pathological symptoms (16). Also, squirrel monkey T cells are the only known primate T cells which are not transformed in vitro by HVS and which, remarkably, lack Lck tyrosine kinase activity.
The transformation-associated protein Tip of HVS had been shown to bind to and activate the T-cellular tyrosine kinase p56lck (5, 32, 43, 65). The two Tip sequence motifs required for Lck binding are well conserved among different subgroup C virus strains (15, 32, 43). This suggested that the direct Tip-Lck association might contribute to T-cell transformation by HVS. However, this hypothesis has been controversially discussed because recombinant HVS subgroup C strains expressing a mutant Tip protein that neither interacts with nor activates cellular Lck was still able to induce T-cell lymphomas in New World primates (12), while it did not transform human T cells in vitro. Some other virus mutants have also been reported to be able to transform marmoset but not human T cells (21, 39). There is no information about the behavior of HVS subgroup C strains in squirrel monkeys. In the natural host, the T cells of which cannot be transformed by HVS, Tip had no effect on p56lck activity. In contrast to other primate species, such as humans, macaques, and common marmosets, p56lck had poor or no activity in squirrel monkey T cells and the enzyme activity could not be rescued by addition of exogenous Tip protein (Fig. 3). This observation was made in all of the tested T-cell cultures from different squirrel monkeys from three colonies. The lack of p56lck activity in squirrel monkey T cells provides a possible explanation for the resistance of squirrel monkeys to T-cell transformation and pathogenicity by HVS strains of subgroup C.
The lack of p56lck activity could be caused either by the lack of a functional enzyme or by the downregulation of p56lck. Splice mutations of lck which block p56lck protein expression are described in the Jurkat derivative JCaM1 and recently also in an immunodeficient patient (18, 57). However, normal amounts of p56lck protein were detected in squirrel monkey T cells (Fig. 3), which also excludes a transcriptional defect. Other known lck mutations, such as translocations, deletions, or insertions, led to constitutive activation of p56lck, resulting in a transforming phenotype (7, 45, 59). Sequencing of the lck open reading frame from squirrel monkey T cells predicted a protein which was almost identical to its human counterpart (Fig. 5). All of the major functionally characterized residues were preserved (56, 63). As expected from the high degree of sequence conservation, the recombinant S. sciureus p56lck protein showed normal enzyme activity after transfection into COS-7 cells (Fig. 6). Thus, p56lck of squirrel monkeys is a fully functional protein similar to that of other primate species, including humans, the T cells of which are easily transformed by HVS C488. This suggests that in the absence of causative genetic mutations, interaction with or enzymatic activation by Tip is functionally blocked in p56lck from squirrel monkey T cells.
Therefore, we investigated whether the p56lck activity is subject to cellular downregulation specifically in squirrel monkey T cells. Restimulation of S. sciureus T cells with PHA and feeder cells induced proliferation and cytokine production but did not rescue p56lck activity either in the short term (data not shown) or over a 14-day stimulation cycle (Fig. 4). In addition, we tested the influence of pervanadate, a known inducer of human and murine p56lck activity that inhibits tyrosine phosphatases. Pervanadate led to p56lck activation in human T cells and also in S. sciureus T cells (Fig. 7). Two phosphatases are known to dephosphorylate p56lck on Y394 and to deactivate the enzyme, CD45 and the Csk-associated phosphatase PEP (9, 27, 58). In addition, Csk phosphorylates the regulatory residue Y505, resulting in enzymatic inactivation (58). The tyrosine phosphatase CD45 could not be analyzed because none of a series of monoclonal antibodies against human CD45 was cross-reactive to S. sciureus CD45 in flow cytometry and Western blot analysis. Csk expression levels of humans and squirrel monkeys were similar, as tested by Western blotting (data not shown). Thus, Csk is probably not the reason for the lack of p56lck activity in squirrel monkey T cells.
The lack of p56lck enzymatic activity in immunocompetent animals with functional T cells appears to be unique to squirrel monkeys. In other species, p56lck has a pivotal role in T-cell development and signal transduction. Inactivation of Lck in transgenic or inducible knockout mice results in an early block in T-cell development and in severe functional impairment of the few mature T cells (3, 25, 41). Generally, low p56lck activity is associated with a loss of T-cell function. In anergic TH1 and TH2 cells, as well as in tumor patients, small amounts and low enzyme activity of p56lck were described (2, 40, 55). Loss of p56lck protein was also seen in patients under immunosuppression (8). Finally, a lack of specific p56lck kinase activity has been recently described in one patient with idiopathic CD4+ lymphopenia. This patient had normal numbers of CD8+ T cells but only few CD4+ helper cells (26). Thus, it remains an open question how squirrel monkey T cells can function without p56lck enzyme activity and it is also not known whether p56lck is active in squirrel monkey thymocytes. The observed lack of specific p56lck activity in T cells of S. sciureus that can be reversed by phosphatase inhibition suggests a novel regulatory mechanism for p56lck.
ACKNOWLEDGMENTS
T. Greve and G. Tamgüney contributed equally to this work.
We thank Susanne Rensing (Göttingen, Germany) for kindly providing samples of squirrel monkey blood, Alexander Tsygankov (Philadelphia, Pa.) for anti-Lck serum, Armin Ensser (Erlangen, Germany) and Brigitte Biesinger (Munich, Germany) for providing PCR primer sequences specific for HVS subgroups A and B, Anja Schmitt (Heidelberg, Germany) for generating the pEFBos-Tip expression vector, Ulricke Klauenberg (Hamburg, Germany) and Sabine Wittmann (Erlangen, Germany) for expert technical assistance, and Bernhard Fleckenstein (Erlangen, Germany) for continuous support. We are grateful to Peter M. Lydyard (London, England) for helpful comments on the manuscript.
This project was supported in part by grants from the Deutsche Forschungsgemeinschaft to B. M. Bröker (Br 952/4-3) and from the Bayerische Forschungsstiftung, the Deutsche Forschungsgemeinschaft (SFB 466), and the Wilhelm Sander-Stiftung to H. Fickenscher. G. Tamgüney is a postgraduate fellow of the Boehringer Ingelheim Fonds.
REFERENCES
- 1.Alexander L, Du Z, Rosenzweig M, Jung J U, Desrosiers R C. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 Nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Ramadi B K, Nakamura T, Leitenberg D, Bothwell A L. Deficient expression of p56(lck) in Th2 cells leads to partial TCR signaling and a dysregulation in lymphokine mRNA levels. J Immunol. 1996;157:4751–4761. [PubMed] [Google Scholar]
- 3.Anderson S J, Levin S D, Perlmutter R M. Involvement of the protein tyrosine kinase p56lck in T cell signaling and thymocyte development. Adv Immunol. 1994;56:151–178. doi: 10.1016/s0065-2776(08)60451-4. [DOI] [PubMed] [Google Scholar]
- 4.Biesinger B, Müller-Fleckenstein I, Simmer B, Lang G, Wittmann S, Platzer E, Desrosiers R C, Fleckenstein B. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci USA. 1992;89:3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biesinger B, Tsygankov A Y, Fickenscher H, Emmrich F, Fleckenstein B, Bolen J B, Bröker B M. The product of the herpesvirus saimiri open reading frame 1 (tip) interacts with T cell-specific kinase p56lck in transformed cells. J Biol Chem. 1995;270:4729–4734. doi: 10.1074/jbc.270.9.4729. [DOI] [PubMed] [Google Scholar]
- 6.Bröker B M, Tsygankov A Y, Müller-Fleckenstein I, Guse A H, Chitaev N A, Biesinger B, Fleckenstein B, Emmrich F. Immortalization of human T cell clones by herpesvirus saimiri. Signal transduction analysis reveals functional CD3, CD4, and IL-2 receptors. J Immunol. 1993;151:1184–1192. [PubMed] [Google Scholar]
- 7.Burnett R C, Thirman M J, Rowley J D, Diaz M O. Molecular analysis of the T-cell acute lymphoblastic leukemia-associated t(1;7)(p34;q34) that fuses LCK and TCRB. Blood. 1994;84:1232–1236. [PubMed] [Google Scholar]
- 8.Cayota A, Vuillier F, Siciliano J, Dighiero G. Defective protein tyrosine phosphorylation and altered levels of p59fyn and p56lck in CD4 T cells from HIV-1 infected patients. Int Immunol. 1994;6:611–621. doi: 10.1093/intimm/6.4.611. [DOI] [PubMed] [Google Scholar]
- 9.Cloutier J F, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. 1999;189:111–121. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Carli M, Berthold S, Fickenscher H, Müller-Fleckenstein I, D'Elios M M, Gao Q, Biagiotti R, Giudizi M G, Kalden J R, Fleckenstein B, Romagnani S, Del Prete G. Immortalization with herpesvirus saimiri modulates the cytokine secretion profile of established Th1 and Th2 human T cell clones. J Immunol. 1993;151:5022–5030. [PubMed] [Google Scholar]
- 11.Duboise S M, Guo J, Czajak S, Desrosiers R C, Jung J U. STP and Tip are essential for herpesvirus saimiri oncogenicity. J Virol. 1998;72:1308–1313. doi: 10.1128/jvi.72.2.1308-1313.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duboise S M, Lee H, Guo J, Choi J K, Czajak S, Simon M, Desrosiers R C, Jung J U. Mutation of the Lck binding motif of Tip enhances lymphoid cell activation by herpesvirus saimiri. J Virol. 1998;72:2607–2614. doi: 10.1128/jvi.72.4.2607-2614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fickenscher H, Fleckenstein B. Growth-transformation of human T cells. Methods Microbiol. 1998;25:573–602. [Google Scholar]
- 14.Fickenscher H, Biesinger B, Knappe A, Wittmann S, Fleckenstein B. Regulation of the herpesvirus saimiri oncogene stpC, similar to that of T-cell activation genes, in growth-transformed human T lymphocytes. J Virol. 1996;70:6012–6019. doi: 10.1128/jvi.70.9.6012-6019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fickenscher H, Bökel C, Knappe A, Biesinger B, Meinl E, Fleischer B, Fleckenstein B, Bröker B M. Functional phenotype of transformed human αβ and γδ T cells determined by different subgroup C strains of herpesvirus saimiri. J Virol. 1997;71:2252–2263. doi: 10.1128/jvi.71.3.2252-2263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleckenstein B, Desrosiers R C. Herpesvirus saimiri and herpesvirus ateles. In: Roizman B, editor. The herpesviruses. Vol. 1. New York, N.Y: Plenum Press; 1982. pp. 253–322. [Google Scholar]
- 17.Fleischer B. A novel pathway of human T lymphocyte activation via a 103 kd T cell activation antigen. J Immunol. 1987;138:1346–1350. [PubMed] [Google Scholar]
- 18.Goldman F D, Ballas Z K, Schutte B C, Kemp J, Hollenback C, Noraz N, Taylor N. Defective expression of p56lck in an infant with severe combined immunodeficiency. J Clin Investig. 1998;102:421–429. doi: 10.1172/JCI3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grüter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber B K, Izaurralde E. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol Cell. 1998;1:649–659. doi: 10.1016/s1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- 20.Guo J, Duboise M, Lee H, Li M, Choi J K, Rosenzweig M, Jung J U. Enhanced downregulation of Lck-mediated signal transduction by a Y114 mutation of herpesvirus saimiri Tip. J Virol. 1997;71:7092–7096. doi: 10.1128/jvi.71.9.7092-7096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo J, Williams K, Duboise S M, Alexander L, Veazey R, Jung J U. Substitution of ras for the herpesvirus saimiri STP oncogene in lymphocyte transformation. J Virol. 1998;72:3698–3704. doi: 10.1128/jvi.72.5.3698-3704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartley D A, Cooper G M. Direct binding and activation of STAT transcription factors by the herpesvirus saimiri protein Tip. J Biol Chem. 2000;275:16925–16932. doi: 10.1074/jbc.M000709200. [DOI] [PubMed] [Google Scholar]
- 23.Hartley D A, Amdjadi K, Hurley T R, Lund T C, Medveczky P G, Sefton B M. Activation of the Lck tyrosine protein kinase by the herpesvirus saimiri tip protein involves two binding interactions. Virology. 2000;276:339–348. doi: 10.1006/viro.2000.0570. [DOI] [PubMed] [Google Scholar]
- 24.Hartley D A, Hurley T R, Hardwick J S, Lund T C, Medveczky P G, Sefton B M. Activation of the Lck tyrosine protein kinase by the binding of the Tip protein of herpesvirus saimiri in the absence of regulatory tyrosine phosphorylation. J Biol Chem. 1999;274:20056–20059. doi: 10.1074/jbc.274.29.20056. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto K, Sohn S J, Levin S D, Tada T, Perlmutter R M, Nakayama T. Requirement for p56lck tyrosine kinase activation in T cell receptor-mediated thymic selection. J Exp Med. 1996;184:931–943. doi: 10.1084/jem.184.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubert P, Bergeron F, Ferreira V, Seligmann M, Oksenhendler E, Debre P, Autran B. Defective p56(Lck) activity in T cells from an adult patient with idiopathic CD4(+) lymphocytopenia. Int Immunol. 2000;12:449–457. doi: 10.1093/intimm/12.4.449. [DOI] [PubMed] [Google Scholar]
- 27.Imbert V, Farahifar D, Auberger P, Mary D, Rossi B, Peyron J F. Stimulation of the T-cell antigen receptor-CD3 complex signaling pathway by the tyrosine phosphatase inhibitor pervanadate is mediated by inhibition of CD45: evidence for two interconnected Lck/Fyn- or zap-70-dependent signaling pathways. J Inflamm. 1996;46:65–77. [PubMed] [Google Scholar]
- 28.Jung J U, Desrosiers R C. Identification and characterization of the herpesvirus saimiri oncoprotein STP-C488. J Virol. 1991;65:6953–6960. doi: 10.1128/jvi.65.12.6953-6960.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung J U, Desrosiers R C. Herpesvirus saimiri oncogene STP-C488 encodes a phosphoprotein. J Virol. 1992;66:1777–1780. doi: 10.1128/jvi.66.3.1777-1780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung J U, Desrosiers R C. Association of the viral oncoprotein STP-C488 with cellular ras. Mol Cell Biol. 1995;15:6506–6512. doi: 10.1128/mcb.15.12.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung J U, Trimble J J, King N W, Biesinger B, Fleckenstein B W, Desrosiers R C. Identification of transforming genes of subgroup A and C strains of herpesvirus saimiri. Proc Natl Acad Sci USA. 1991;88:7051–7055. doi: 10.1073/pnas.88.16.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung J U, Lang S M, Friedrich U, Jun T, Roberts T M, Desrosiers R C, Biesinger B. Identification of Lck-binding elements in tip of herpesvirus saimiri. J Biol Chem. 1995;270:20660–20667. doi: 10.1074/jbc.270.35.20660. [DOI] [PubMed] [Google Scholar]
- 33.Jung J U, Lang S M, Jun T, Roberts T M, Veillette A, Desrosiers R C. Downregulation of Lck-mediated signal transduction by tip of herpesvirus saimiri. J Virol. 1995;69:7814–7822. doi: 10.1128/jvi.69.12.7814-7822.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katahira J, Strasser K, Podtelejnikov A, Mann M, Jung J U, Hurt E. The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J. 1999;18:2593–2609. doi: 10.1093/emboj/18.9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knappe A, Feldmann G, Dittmer U, Meinl E, Nisslein T, Wittmann S, Mätz-Rensing K, Kirchner T, Bodemer W, Fickenscher H. Herpesvirus saimiri-transformed macaque T cells are tolerated and do not cause lymphoma after autologous reinfusion. Blood. 2000;95:3256–3261. [PubMed] [Google Scholar]
- 36.Knappe A, Hiller C, Niphuis H, Fossiez F, Thurau M, Wittmann S, Kuhn E M, Lebecque S, Banchereau J, Rosenwirth B, Fleckenstein B, Heeney J, Fickenscher H. The interleukin-17 gene of herpesvirus saimiri. J Virol. 1998;72:5797–5801. doi: 10.1128/jvi.72.7.5797-5801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knappe A, Hiller C, Thurau M, Wittmann S, Hofmann H, Fleckenstein B, Fickenscher H. The superantigen-homologous viral immediate-early gene ie14/vsag in herpesvirus saimiri-transformed human T cells. J Virol. 1997;71:9124–9133. doi: 10.1128/jvi.71.12.9124-9133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knappe A, Thurau M, Niphuis H, Hiller C, Wittmann S, Kuhn E-M, Rosenwirth B, Fleckenstein B, Heeney J, Fickenscher H. T-cell lymphoma caused by herpesvirus saimiri C488 independently of ie14/vsag, a viral gene with superantigen homology. J Virol. 1998;72:3469–3471. doi: 10.1128/jvi.72.4.3469-3471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee H, Choi J-K, Li M, Kaye K, Kieff E, Jung J U. Role of cellular tumor necrosis factor receptor-associated factors in NF-κB activation and lymphocyte transformation by herpesvirus saimiri STP. J Virol. 1999;73:3913–3919. doi: 10.1128/jvi.73.5.3913-3919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J E, Cossoy M B, Chau L A, Singh B, Madrenas J. Inactivation of lck and loss of TCR-mediated signaling upon persistent engagement with complexes of peptide:MHC molecules. J Immunol. 1997;159:61–69. [PubMed] [Google Scholar]
- 41.Legname G, Seddon B, Lovatt M, Tomlinson P, Sarner N, Tolaini M, Williams K, Norton T, Kioussis D, Zamoyska R. Inducible expression of a p56Lck transgene reveals a central role for Lck in the differentiation of CD4 SP thymocytes. Immunity. 2000;12:537–546. doi: 10.1016/s1074-7613(00)80205-8. [DOI] [PubMed] [Google Scholar]
- 42.Lund T, Medveczky M M, Neame P J, Medveczky P G. A herpesvirus saimiri membrane protein required for interleukin-2 independence forms a stable complex with p56Lck. J Virol. 1996;70:600–606. doi: 10.1128/jvi.70.1.600-606.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lund T C, Garcia R, Medveczky M M, Jove R, Medveczky P G. Activation of STAT transcription factors by herpesvirus saimiri Tip-484 requires p56Lck. J Virol. 1997;71:6677–6682. doi: 10.1128/jvi.71.9.6677-6682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lund T C, Coleman C, Horvath E, Sefton B M, Jove R, Medveczky M M, Medvezky P G. The Src family kinase Lck can induce STAT3 phosphorylation and DNA binding activity. Cell Signal. 1999;11:789–796. doi: 10.1016/s0898-6568(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 45.Marth J D, Overell R W, Meier K E, Krebs E G, Perlmutter R M. Translational activation of the lck proto-oncogene. Nature. 1988;332:171–173. doi: 10.1038/332171a0. [DOI] [PubMed] [Google Scholar]
- 46.Medveczky P, Szomolanyi E, Desrosiers R C, Mulder C. Classification of herpesvirus saimiri into three groups based on extreme variation in a DNA region required for oncogenicity. J Virol. 1984;52:938–944. doi: 10.1128/jvi.52.3.938-944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medveczky M M, Szomolanyi E, Hesselton R, DeGrand D, Geck P, Medveczky P G. Herpesvirus saimiri strains from three DNA subgroups have different oncogenic potentials in New Zealand White rabbits. J Virol. 1989;63:3601–3611. doi: 10.1128/jvi.63.9.3601-3611.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meinl E, Fickenscher H. Viral transformation of lymphocytes. In: Rowland-Jones S, McMichael A, editors. Lymphocytes—a practical approach. Oxford, England: Oxford University Press; 2000. pp. 55–74. [Google Scholar]
- 49.Merlo J J, Tsygankov A Y. Herpesvirus saimiri oncoproteins Tip and StpC synergistically stimulate NF-kappaB activity and interleukin-2 gene expression. Virology. 2001;279:325–338. doi: 10.1006/viro.2000.0714. [DOI] [PubMed] [Google Scholar]
- 50.Mittrücker H W, Müller-Fleckenstein I, Fleckenstein B, Fleischer B. CD2-mediated autocrine growth of herpesvirus saimiri-transformed human T lymphocytes. J Exp Med. 1992;176:909–913. doi: 10.1084/jem.176.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mittrücker H W, Müller-Fleckenstein I, Fleckenstein B, Fleischer B. Herpesvirus saimiri-transformed human T lymphocytes: normal functional phenotype and preserved T cell receptor signalling. Int Immunol. 1993;5:985–990. doi: 10.1093/intimm/5.8.985. [DOI] [PubMed] [Google Scholar]
- 52.Mizushima S, Nagata S. pEF-Bos, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy C, Kretschmer C, Biesinger B, Beckers J, Jung J, Desrosiers R C, Müller-Hermelink H K, Fleckenstein B W, Rüther U. Epithelial tumours induced by a herpesvirus oncogene in transgenic mice. Oncogene. 1994;9:221–226. [PubMed] [Google Scholar]
- 54.Noraz N, Saha K, Ottones F, Smith S, Taylor N. Constitutive activation of TCR signaling molecules in IL-2-independent herpesvirus saimiri-transformed T cells. J Immunol. 1998;160:2042–2045. [PubMed] [Google Scholar]
- 55.Quill H, Riley M P, Cho E A, Casnellie J E, Reed J C, Torigoe T. Anergic Th1 cells express altered levels of the protein tyrosine kinases p56lck and p59fyn. J Immunol. 1992;149:2887–2893. [PubMed] [Google Scholar]
- 56.Shaw A S, Amrein K E, Hammond C, Stern D F, Sefton B M, Rose J K. The lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell. 1989;59:627–636. doi: 10.1016/0092-8674(89)90008-1. [DOI] [PubMed] [Google Scholar]
- 57.Straus D B, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 58.Thomas M L, Brown E J. Positive and negative regulation of Src-family membrane kinases by CD45. Immunol Today. 1999;20:406–411. doi: 10.1016/s0167-5699(99)01506-6. [DOI] [PubMed] [Google Scholar]
- 59.Tycko B, Smith S D, Sklar J. Chromosomal translocations joining LCK and TCRB loci in human T cell leukemia. J Exp Med. 1991;174:867–873. doi: 10.1084/jem.174.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veillette A, Abraham N, Caron L, Davidson D. The lymphocyte-specific tyrosine protein kinase p56lck. Semin Immunol. 1991;3:143–152. [PubMed] [Google Scholar]
- 61.Weber F, Meinl E, Drexler K, Czlonkowska A, Huber S, Fickenscher H, Müller-Fleckenstein I, Fleckenstein B, Wekerle H, Hohlfeld R. Herpesvirus saimiri-transformed human T cell lines expressing functional receptor for myelin basic protein. Proc Natl Acad Sci USA. 1993;90:11049–11054. doi: 10.1073/pnas.90.23.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wehner L E, Schröder N, Kamino K, Friedrich U, Biesinger B, Rüther U. Herpesvirus saimiri Tip gene causes T-cell lymphomas in transgenic mice. DNA Cell Biol. 2001;20:81–88. doi: 10.1089/104454901750070283. [DOI] [PubMed] [Google Scholar]
- 63.Weil R, Cloutier J F, Fournel M, Veillette A. Regulation of Zap-70 by Src family tyrosine protein kinases in an antigen-specific T-cell line. J Biol Chem. 1995;270:2791–2799. doi: 10.1074/jbc.270.6.2791. [DOI] [PubMed] [Google Scholar]
- 64.Weil R, Veillette A. Signal transduction by the lymphocyte-specific tyrosine protein kinase p56lck. Curr Top Microbiol Immunol. 1996;205:63–87. doi: 10.1007/978-3-642-79798-9_4. [DOI] [PubMed] [Google Scholar]
- 65.Wiese N, Tsygankov A Y, Klauenberg U, Bolen J B, Fleischer B, Bröker B M. Selective activation of T cell kinase p56lck by herpesvirus saimiri protein Tip. J Biol Chem. 1996;271:847–852. doi: 10.1074/jbc.271.2.847. [DOI] [PubMed] [Google Scholar]
- 66.Yoon D W, Lee H, Seol W, DeMaria M, Rosenzweig M, Jung J U. Tap: a novel cellular protein that interacts with tip of herpesvirus saimiri and induces lymphocyte aggregation. Immunity. 1997;6:571–582. doi: 10.1016/s1074-7613(00)80345-3. [DOI] [PubMed] [Google Scholar]