Abstract
Sexual reproduction in Charophyceae abounds in complex traits. Their gametangia develop as intricate structures, with oogonia spirally surrounded by envelope cells and richly pigmented antheridia. The red—probably protectant—pigmentation of antheridia is conserved across Charophyceae. Chara tomentosa is, however, unique in exhibiting this pigmentation and also in vegetative tissue. Here, we investigated the two sympatric species, C. tomentosa and Chara baltica, and compared their molecular chassis for pigmentation. Using reversed phase C30 high performance liquid chromatography (RP-C30-HPLC), we uncover that the major pigments are β-carotene, δ-carotene and γ-carotene; using headspace solid-phase microextraction coupled to gas chromatography equipped with a mass spectrometer (HS-SPME-GC-MS), we pinpoint that the unusually large carotenoid pool in C. tomentosa gives rise to diverse volatile apocarotenoids, including abundant 6-methyl-5-hepten-2-one. Based on transcriptome analyses, we uncover signatures of the unique biology of Charophycaee and genes for pigment production, including monocyclized carotenoids. The rich carotenoid pool probably serves as a substrate for diverse carotenoid-derived metabolites, signified not only by (i) the volatile apocarotenoids we detected but (ii) the high expression of a gene coding for a cytochrome P450 enzyme related to land plant proteins involved in the biosynthesis of carotenoid-derived hormones. Overall, our data shed light on a key protection strategy of sexual reproduction in the widespread group of macroalgae. The genetic underpinnings of this are shared across hundreds of millions of years of plant and algal evolution.
This article is part of the theme issue ‘The evolution of plant metabolism’.
Keywords: streptophyte algae, Charophyceae, carotenoids, apocarotenoids, sexual reproductive structures
1. Introduction
Charophyceae are a class of streptophyte macroalgae with remarkable complex body plans and biochemistry studied since the eighteenth century (for a review of Chara as a model for plant biology, see Kurtović et al. [1]). Indeed, they were long assumed to be a sister to land plants until phylogenomic studies proved that the species-rich Zygnematophyceae fill that position [2–6]; Charophyceae, Coleochaetophyceae, Zygnematophyceae and land plants form together the monophylum of Phragmoplastophyta. The remarkable features of Charophyceae include survival in brackish habitats, a trait only found in Charophyceae and land plants within the Streptophyta [7,8]. Akin to land plants, Charophyceae have intricate sexual reproduction mechanisms including complex gametangia, monoecious as well as dioecious species. Reduction of processes in sexual reproduction, including the loss of sperm motility, in Zygnematophyceae is owing to conjugation as a mode of reproduction [9]. It is thus fair to assume that molecular programmes for the development of complex reproductive structures in phragmoplastophytes share some common ancestry.
Another important feature of streptophyte evolution is that it brought forth a highly specialized metabolism [10,11]. Carotenoids, C30 to C50 polyenes, occur in any photosynthetic organism [12,13] and even in some non-photosynthetic organisms like aphids [14]. The central C5 isoprenoid building blocks of these polyenes (isopentenyl diphosphate and dimethylallyl diphosphate) are derived from the methylerythritol phosphate pathway—the source of many terpenoids including, e.g. chlorophylls, plastoquinones and aforementioned carotenoids (for a review, see Rodriguez-Concepcion et al. [15]). Carotenoid biosynthesis is committed by the synthesis of phytoene through the action of phytoene synthase [16–19]. Conversion by desaturases like phytoene desaturase [20] and the action of several isomerases yield the central compound in carotenogenesis, all-trans-lycopene [21], from which through cyclization by lycopene cyclases (LCYs) [22–25] the textbook carotenoids are produced [12]. Canonical carotenoids like β-carotene are highly conserved in Viridiplantae [12,26]. At the same time carotenoids are one of the most diverse classes of species- and lineage-specific specialized metabolites with over 1000 members currently known [27], often serving similar functions (for an elaboration on the evolutionary implications, see Dadras et al. [28]). These highly conserved functions include their central role as antioxidants and as quenchers of reactive oxygen species (ROS) producing triplet-state chlorophyll in the photosystem II (PSII) core complex (for a review, see Telfer [29]). The hydroxylation and epoxidation products of carotenes—the xanthophyll pigments—are used in nearly every known light-harvesting complex (LHC) since they enlarge the spectrum of absorbed light compared to chlorophylls alone and participate in non-photochemical quenching by heat dissipation of light energy [30].
In streptophyte algae, studies of this crucial specialized metabolism are rather rare, but still, some remarkable lineage and species-specific occurrences and mechanisms are known [31,32]. In albedo-facing Cosmarium species (Zygnematophyceae), a truncated xanthophyll cycle was detected not integrating violaxanthin and thus named the antheraxanthin-zeaxanthin cycle [32]. Most Chloroplastida use 9-cis-neoxanthin in their LHCs [33]. Surprisingly, in Mesostigma viride solely, all-trans-neoxanthin is present and thus probably slightly altered LHCs [31]. Additionally, these algae contain the rather rare siphonaxanthin and fatty acid esters of it, as well as γ-carotene [31]. Monocyclized carotenoids like γ-carotene have important functions in photosynthetic as well as non-photosynthetic organisms including green bacteria [34] and UV and psychrotolerant yeasts [35]. Especially in the latter, high levels of γ-carotene were correlated with UV tolerance [35]. Charophyceae are well known for their red antheridia, and previous studies indicated that this coloration originates from monocyclized γ-carotene [36,37]. Monocyclized carotenoids rather rarely accumulate in the green lineage despite being a central intermediate in the biosynthesis of double-cyclized α-carotene and β-carotene (for a review, see Rodriguez-Concepcion et al. and Sathasivam et al. [15,38]).
We investigated the global differential gene expression patterns, chlorophylls, carotenoids and apocarotenoids of the two brackish Charophyceaen species, Chara tomentosa L. and Chara baltica (Hartman) Bruz. Using an optimized analytical method recently published [39], we found that three carotenoids, β-carotene, δ-carotene and γ-carotene, mainly contribute to antheridia colour. Second, the characteristic reddish colour change of C. tomentosa vegetative tissue (VT) during development/seasonal changes [40] originates from the same carotenoids. Additionally, we detect volatile apocarotenoids in Charophyceae. Our data shed light on the evolution of a complex metabolite-driven phenotype based on a conserved chassis of compounds and enzymes.
2. Results and discussion
(a). Global gene expression profiles recover species and ecophysiological differences
The brackish waters that emerge from the influx of Baltic sea water into freshwater reservoirs are well known for their richness in Charophyceae [41]. During late spring/early summer, we sampled C. baltica and C. tomentosa from Michaelsdorf (54.371306° N, 12.569778° E), a lagoon in the Bodstedter Bodden of the Darss-Zingst Bodden chain (brackish; figure 1a ). These species were chosen for several reasons: first, they occur sympatrically; second, C. tomentosa appears in brackish and freshwater habitats, while C. baltica is only known from brackish habitats; third, both species have the same cortication type (diplostichous) but differ in domesticity—C. baltica is monoecious and C. tomentosa is dioecious; and lastly, and salient to the specialized metabolic focus of this study, C. tomentosa has bodies of an astonishingly red pigmentation, suggesting high levels of carotenoids.
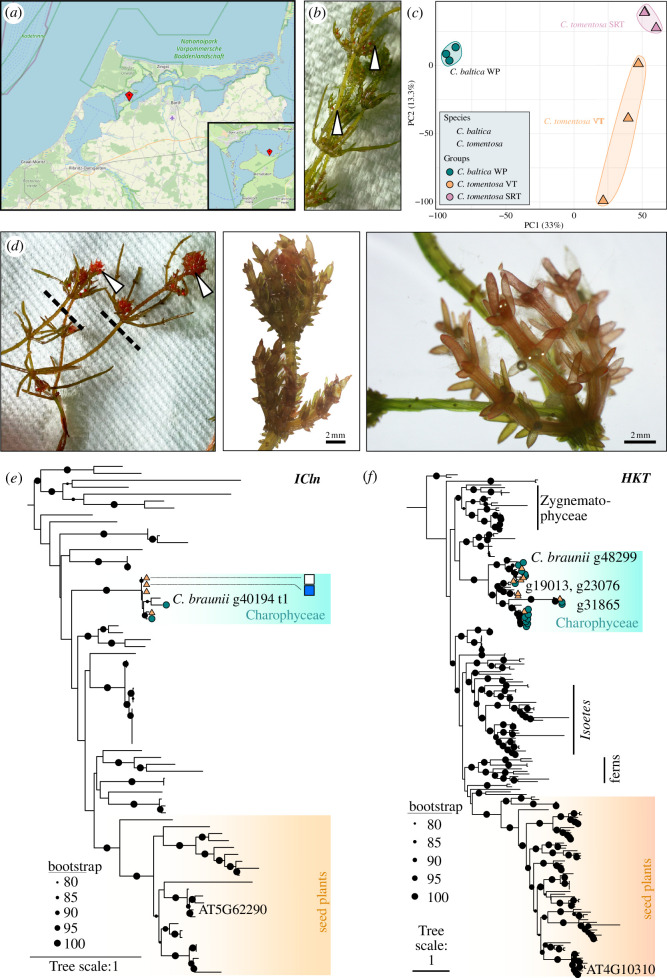
Figure 1.
Investigation of two sympatric Chara species. (a) Location of sampling near Michaelsdorf in the brackish ‘Bodstetter Bodden’ of the Darss Zingster Bodden chain. Map from www.openstreetmap.org. (d) Male C. tomentosa, dashed line indicates cutting to obtain the two respective sample groups for this organism. On the right, two pictures show a zoom-in (of a different individual) on the morphological details of the whorls; note the spotted pigmentation (the two pictures were reproduced with permission from Holzhausen [42]). (b) Chara baltica. In (b) and (d), white arrows indicate antheridia, in male C. tomentosa only in top parts, and in C. baltica distributed over the branches of the whole plant (WP). (c) Principal component analysis (PCA) of global differential gene expression of the three sample groups indicated by colour and shape as displayed on the right side. (e,f) Maximum-likelihood phylogenies of genes salient to salt tolerance. Black circles are scaled and represent the ultrafast bootstrap (UFBoot) support (see legend). Teal hues label highlight sequences from Charophyceae; C. baltica sequences are labelled with a dark turquoise circle, and C. tomentosa sequences are labelled with an orange triangle. Blue boxes indicate significant downregulation, and purple boxes indicate significant upregulation of a minimum of twofold in the comparison C. tomentosa sexual reproductive tissue (SRT) versus C. tomentosa VT.
First, we looked at the global differential gene expression profiles of the following three sample groups: (i) C. tomentosa VT, (ii) antheridia-rich top parts referred to as sexual reproductive tissue (SRT; figure 1b ), and (iii) C. baltica whole plants (WP; figure 1c ). We extracted RNA from these tissues in biological triplicates and performed paired-end RNA sequencing of 150 bp length using the Illumina NovaSeq 6000 platform. We sequenced 215 491 672 reads for C. tomentosa VT, 218 432 320 of C. tomentosa apices and 191 118 580 for C. baltica. The reads were de novo assembled using the Trinity pipeline [43] (v. 2) into 9 89 447 and 1 368 348 contigs for C. tomentosa and C. baltica. Filtering for apt expression levels (≥1 transcripts per million (TPM)), we retained a reference of 8 57 835 contigs for C. tomentosa; we converted these data into predicted proteins, and after decontamination, we retained 9578 predicted proteins for C. tomentosa (23 099 for C. baltica). We mapped the reads onto our new reference assemblies using Kallisto v. 0.50.1 [44] and calculated gene expression levels using Trinity [43]; statistics were based on DESeq2 [45].
To understand the gross gene expression profiles, we conducted principal component analysis (PCA). The strongest separation was found between the two species along PC1, which described 33% of the variance in the data (see figure 1d ). Although samples of C. tomentosa VT showed the overall broadest distribution along PC2—which, however, just described 13.3% of the variance—they still clearly separated from SRT-containing samples along this component. We next turned to the specific gene expression patterns therein.
Several specific gene expression patterns correlated with the species’ morphological and ecophysiological divergence. In this regard, the lowest expression levels of chloride-exporter homologues known to play a role in volume decrease after cellular swelling with kingdom-overarching conservation [46–48] were found in C. tomentosa SRT samples including swollen end cells (figure 1e ). Consistent with studies by Phipps et al. [49], we recovered in both species homologues of a sodium transporter that were shown to be important in Charophyceaen salt resistance by their work (figure 1f ); yet, the transporter awaits classification since it has a conserved glycine that suggests it might be a potassium transporter. While it is generally assumed that C. baltica is salt-sensitive [49], we would consider the possibility that both C. baltica and C. tomentosa are salt-tolerant based on the ecology of the Chara species investigated (both from the same brackish habitat and depth) and their genetic repertoire.
(b). Accumulation of carotenoids in reproductive organs predated plant terrestrialization and probably originated at the base of Phragmoplastophyta
The red colour of the antheridia of many Charophyceaen algae is remarkable (see figure 1b,c ). Schagerl & Pichler [36] found γ-carotene to be responsible for that coloration. However, the exact function of this carotenoid accumulation remained unclear. They also found γ-carotene and β-carotene to be responsible for the reddening of C. tomentosa VT, a mechanism especially observed in late spring/early summer [36,50]. The studies of Schagerl & Pichler [36] focused on Charophyceae from freshwater habitats. We investigated the carotenoid and chlorophyll profiles of two species of the genus Chara in their sexual reproductive phase, namely C. tomentosa and C. baltica, from the brackish Darss Zingster Bodden chain and compared the pigment pools with their differential gene expression profiles to further elucidate the function of carotenoid accumulation in antheridia and VT of C. tomentosa. Furthermore, while previous investigations of Charophyceaen carotenoid profiles used reversed phase C18 (RP-C18)-systems [36] or even normal phase separation [37], a reversed phase C30 high performance liquid chromatography (RP-C30-HPLC)-based method has the capacity to resolve structurally similar carotenoids. With the RP-C30-HPLC method recently published [39], we were able to detect several carotenoids in the two Chara species (figure 2) that were not present in Zygnematophyceae and Physcomitrium patens therein (cf. [39]).
Figure 2.
(Apo)carotenogensis in the investigated Charophyceae. (a) Non-normalized chromatograms of replicate 1 of the three sample groups. Purple, C. tomentosa SRT; orange, C. tomentosa VT; teal, C. baltica WP; i, violaxanthin ; ii, 9-cis-neoxanthin; iii, antheraxanthin; iv, chlorophyll b; v, lutein; vi, zeaxanthin; vii, chlorophyll a; viii, 15-cis-β-carotene; ix, α-carotene; x, β-carotene; xi, 9-cis-β-carotene; xii, putative torulene or some cis-δ-carotene or cis-γ-carotene; xiii, δ-carotene; xiv, γ-carotene; xv, lycopene. (b) Schematic of the apocarotenoid and carotenoid pathway in the investigated species. (c) Pigment pools of the three sample groups with area relative to pool size, legend on the left. (d) Headspace solid-phase microextraction coupled to gas chromatography equipped with a mass spectrometer (HS-SPME-GC-MS) chromatogram of C. tomentosa. Data shown were derived from VT replicate 1.
Male plants of C. tomentosa were dissected to separate antheridia-containing tissue SRT from VT in order to distinguish if these carotenoids accumulate in the antheridia and whether the respective transcription patterns differ. As mentioned above, C. tomentosa is known to change the colour of VT, especially in the upper parts starting in late spring/early summer to a more reddish appearance [50]. We investigated if this originates from the same carotenoids accumulated in the antheridia. For C. baltica (monoecious), we analysed the WP including antheridia and oogonia. Besides α-carotene, β-carotene and its 9-cis-isomer (figure 2, ix–xi), two more carotenoids (figure 2, xiii and xiv) were found in all samples, but especially accumulated in the samples of C. tomentosa with the highest values in antheridia-containing tissue (see figures 1–3).
Figure 3.
Pigment levels and ratios in C. tomentosa and C. baltica. (a) Individual pigment levels or pigment ratios: purple, C. tomentosa SRT; orange, C. tomentosa VT; teal, C. baltica WP. p-values indicate results from Kruskal–Wallis test to test for significant differences between the three sample sets. For significant results, a post hoc Conover–Iman test was performed for pairwise comparisons with p1, C. tomentosa SRT versus C. tomentosa VT; p2, C. tomentosa SRT versus C. baltica; p3, C. tomentosa VT versus C. baltica WP. (b–e) Maximum-likelihood phylogenies of genes salient to carotenoid metabolism and photoprotection. Black circles are scaled and represent the ultrafast bootstrap (UFBoot) support (see legend). Teal hues label highlight sequences from Charophyceae; C. baltica sequences are labelled with a dark turquoise circle, C. tomentosa sequences are labelled with an orange triangle. Blue boxes indicate significant downregulation, and purple boxes indicate significant upregulation of a minimum of twofold in the comparison C. tomentosa SRT versus C. tomentosa VT.
The red colour of Charales antheridia thus probably originates from the following five different carotenoids: α-carotene, β-carotene, 9-cis-β-carotene and two chemically very similar carotenoids (xiii and xiv, similar retention times and absorption spectra; see figure 2). Based on their abundance, the main colour contribution results from β-carotene and the two chemically very similar carotenoids, which were present in C. tomentosa SRT at 0.0335 µmol mgDW−1, 0.0316 µmol mgDW−1 and 0.0468 µmol mgDW−1 as compared to the 0.0025 µmol mgDW−1 for α-carotene and 0.0156 µmol mgDW−1 for 9-cis-β-carotene (figure 2, xiii and xiv). This shows that carotenoid accumulation in C. tomentosa SRT is a trait not only limited to flowering plants [51,52] but also to other, about 700-million-year-divergent Phragmoplastophyta. Since algae do not rely on pollination for sexual reproduction, an attractive function of hyperaccumulated carotenoids as known for many seed plants [26,51,52] can be excluded.
(c). Hyperaccumulation of δ-carotene and γ-carotene in Chara tomentosa
Chara tomentosa stands out by having an overall highly reddish thallus—both regarding its SRT and VT. Judging by similarly high values in the reddish VT of C. tomentosa (0.0316 to 0.0468 µmol mgDW−1 in SRT as compared to 0.00999 and 0.0134 µmol mgDW−1 in VT), the same three carotenoids are also responsible for this seasonal effect of colour change (see figures 1–3). To identify the structures of these carotenoids, we respected retention time hinging on monocyclized carotenoids because of their elution between β-carotene (figure 2, x, two rings) and lycopene (figure 2, xv, no ring). We compared the absorption spectra with literature data from Gupta et al. [53] and identified them as δ-carotene and γ-carotene (see figure 2, xiii and xiv).
To scrutinize our structural prediction, we applied principles of retrosynthesis investigating the accumulation of apocarotenoids (oxidative breakdown products of carotenoids) in C. tomentosa VT by headspace solid-phase microextraction coupled to gas chromatography equipped with a mass spectrometer (HS-SPME-GC-MS; figure 2d ). The expectation is that the profiles reflect the structures of accumulated carotenoids. Indeed, we detected especially high levels of 6-methyl-5-hepten-2-one and high levels of β-cyclocitral (see figure 2) supporting the structures of δ-carotene and γ-carotene. This refines previous findings [36], which reported only β-carotene and γ-carotene in Charophyceaen antheridia and C. tomentosa VT; here, the resolving power of similar molecules by the C30 approach (used in this study) comes to bear, which separates molecules that can usually not be separated with RP-C18 systems. The strongly elevated 6-methyl-5-hepten-2-one levels (correlating with the accumulation of γ-carotene) are noteworthy. The exact functions of this apocarotenoid within photosynthetic organisms (inter- and intra-organismic) are still not known, but some interspecies/kingdom interactions have been detected including plant-insect(-plant) [54–57]. Some fishes feed on Chara spp. [58,59]. A correlation between volatile apocarotenoids and fishes on the other hand has not been studied to our current knowledge.
(d). High levels of cis-β-carotenes in Chara tomentosa
All samples of C. tomentosa that contained high levels of β-carotene showed elevated ratios of 9-cis-β-carotene and 15-cis-β-carotene compared to C. baltica samples (see figures 2 and 3). Carotenoids can crystalize at high concentrations in plant cells as known from lycopene and β-carotene crystals in tomato chromoplasts (for a review of chromoplast functions, see Sadali et al. [60]). Simultaneous accumulation of cis-derivatives of the respective carotenoids could prevent this mechanism by disturbing packing owing to steric hindrance. Interestingly, in C. tomentosa SRT samples, the highest levels of β-carotene were detected and the lowest fluctuations in β-carotene/cis-β-carotenes ratios were observed pinpointing tight regulation.
The presence of 9-cis-neoxanthin in Charophyceae is equivocal [36,61]. Our data unequivocally detect respectable levels of 9-cis-neoxanthin in all samples and species investigated (see figure 3). The question why 9-cis-neoxanthin biosynthesis genes are not detectable in Chara braunii [61] remains to be solved in future studies.
(e). Carotenoid-derived metabolites and volatile apocarotenoids in Charophyceae
Apocarotenoids have diverse biological functions in plants, ranging from stress signalling to interspecies communication [26,62,63]. We detected the volatile apocarotenoids β-ionone and dihydroactinidiolide (figures 2 and 4). They probably completely originate from non-enzymatic cleavage since no CAROTENOID CLEAVAGE DIOXYGENASE (CCD; main enzymes of oxidative carotenoid cleavage) homologous sequences were found in the genome of C. braunii and also not in our transcriptomes. Several other carotenoids of unknown identity were detected, eluting in between β-carotene and δ-carotene (see figure 2). One of them showed slightly higher levels, comparable to α-carotene especially in C. tomentosa, and was included as ND1 (figure 2, xii) in the overall pool. The absorption spectrum was similar to δ-carotene and γ-carotene with a shift to a shorter wavelength; the absorbance spectrum included an additional peak. Based on this, the structure is most likely similar to torulene (assumed for quantification) or a cis-δ-carotene or cis-γ-carotene but needs further structural investigations.
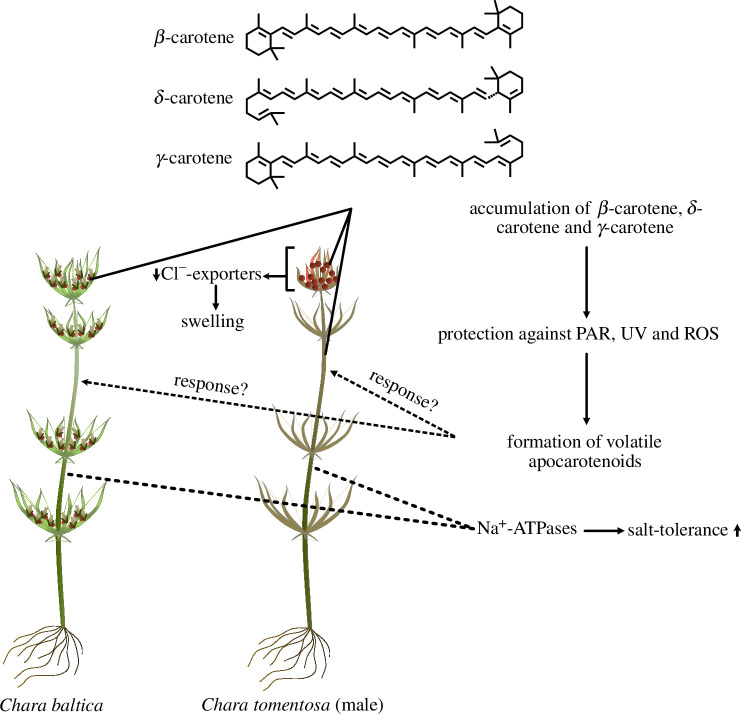
Figure 4.
Ecophysiological model for C. baltica and C. tomentosa (male). Small arrows indicate elevation or depletion.
To understand the molecular chassis for—and acting on—the large pool of carotenoids in the SRT of C. tomentosa, which also contributes to apocarotenoid abundance, we screened for homologues of genes that code for proteins involved in carotenoid biosynthesis and metabolism. Most homologues were not significantly different in their transcript levels and, if so, they were depleted (electronic supplementary material, table S1). The gene with the most strongly elevated expression in SRT versus VT was a homologue of a cytochrome P450, CYP711A1 (C. tomentosa TRINITY_DN2319_c1_g1_i17; 23.25-fold higher, Benjamini–Hochberg corrected p-value of 0.0236). CYP711A1 is also known as MORE AXILLARY BRANCHES 1 (MAX1) and is a key enzyme in strigolactone biosynthesis that converts cleavage products of carotenoids into carlactonic acid [64]. We computed a phylogeny of all homologues of MAX1 that we detected in C. tomentosa and additionally sampled homologous sequences from across the green lineage (figure 3b ). The sequences from C. tomentosa are clear co-orthologues of MAX1 (figure 3b ). Indeed, strigolactones have been reported from Charophyceae [65]—but their presence has remained enigmatic. Yet the situation is more complicated, as MAX1-independent production of strigolactone(-like) metabolites occurs in the moss P. patens [66]—which completely lacks MAX1, a lack that we also recovered in our phylogeny (figure 3b ). At the moment it appears that strigolactones have been recruited multiple times, but probably in embryophytes as ancient signalling molecules in symbiosis [67]. We interpret the MAX1 (co-)orthologues and the enriched expression of some of these in C. tomentosa SRT as a hint towards diverse carotenoid-derived molecules arising where carotenoids abound as substrates—be it strigolactone(-like) molecules or different ones. The apocarotenoids detected here are a point in case, but probably there is a diverse bouquet of carotenoid-derived metabolites emerging in Charophyceae—including by the aid of enzymes such as CYP711A1-likes.
An enrichment of certain carotenoids can not only be explained by the high expression of enzymes that yield products but also by the low expression of enzymes that use them as substrates. The altered levels of monocyclized and double-cyclized carotenoids were reflected in LCY expression levels. We computed a phylogeny of LCYs and recovered clear clades of LCYE (epsilon-ring formation) and LCYB (beta-ring formation) homologues (figure 3c ). Here, the LCYE homologue of C. tomentosa stood out as its expression levels were depleted (in some cases almost to 0) in antheridia-rich C. tomentosa SRT, compared to VT (figure 3c ). This could explain the accumulation of these ‘intermediate’ carotenoids in α-carotene and β-carotene biosynthesis. It furthermore illustrates how during evolution the same enzyme chassis can yield starkly different metabolite phenotypes.
(f). Ecological implications of the pigment pools of Chara tomentosa and Chara baltica
The overall size of the pigment pools of investigated Charales species and tissues (figure 2) reflects their appearance. Chara tomentosa changes the colour of its upper thalli to a more reddish appearance starting in late spring/early summer, while C. baltica grows in about the same depth but does not alter its colour. On carotenoid and chlorophyll levels, this is reflected by the larger overall pigment pool of C. tomentosa with the largest pool in samples containing SRT related to carotenoid hyperaccumulation detected in antheridia (see figure 2). A higher accumulation of carotenoids with absorbance shifted to longer wavelengths, namely δ-carotene and γ-carotene, was observed in C. tomentosa (see figures 2 and 3). The seasonal change of the colour of C. tomentosa VT originating from carotenoid accumulation aligns with increased photosynthetically available radiation (PAR) and longer photoperiods starting in late spring/early summer. This mechanism is probably beneficial for withstanding the characteristics of seasonal change. Chara tomentosa is found in brackish as well as freshwater habitats, while C. baltica is found only in brackish habitats. Freshwater habitats often undergo fluctuations in water depth sometimes correlated to season. As water depth increases, overall light intensity decreases, and additionally, the composition of PAR changes with the most rapid decrease in intensity of red light, followed by orange, green and blue [68].
The hyperaccumulated carotenoids detected in C. tomentosa might also help survival in differing habitats. Accumulation alone does not distinguish between a photoprotective and a light-harvesting function of carotenoids (for a review of carotenoid functions, see Nisar et al. [12]). The xanthophyll pool (Violaxanthin + Antheraxanthin + Zeaxanthin [V + A + Z]/Chlorophyll a + Chlorophyll b [Chla + Chlb], p-value = 0.066) and the de-epoxidation state (A + Z/V + A+Z, p-value = 0.061) show no significant difference between the groups. This hints at a similar amount of photo-oxidative stress in the organisms and taking the absolute range into account a small overall amount of photo-oxidative stress. Meta-analyses have shown that these values are good indicators for observed photo-oxidative stress and V + A + Z/Chla + Chlb can be seen as a light ‘memory’ [69]. Since mature antheridia are not photosynthetically active, a photoprotective function of accumulated carotenoids is way more likely. Xanthophyll pigments are usually used for light harvesting in PSII, not carotenes (for a review of carotenoid functions also see Esteban et al. [70]). This leads to the assumption that also in VT of C. tomentosa, the hyperaccumulation probably has a photoprotective function. Furthermore, carotenoids xii, xiii and xiv (figure 2) show small but significant absorbance in the range of UVB radiation (and xii even additionally in the range of UVA; see figure 2) and cis-β-carotenes in the range of UVA. Owing to the sheer immense amount of these carotenoids in antheridia of C. tomentosa SRT and C. tomentosa VT, a UV protective function is likely. This is consistent with the seasonal increase in UV radiation. Given the detrimental effect of UV and strong light on DNA, it is conceivable that the accumulation of UV-absorbing carotenoids serves as a protection mechanism of the germline in the SRT. This accumulation of carotenoids (and other antioxidants) as protectants of DNA against UV and strong light in SRT is a widely shared feature of many seed plants including high levels of lycopene in tomatoes [71] and of carotenes and lutein and zeaxanthin in maize kernels [72] to just name some examples. The question remains if this trait evolved in the last common ancestor (LCA) of all Phragmoplastophyta and was lost in some lineages (e.g. Zygnematophyceae [73]) or evolved independently in the LCA of Charophyceae and the LCA of land plants (or even specific land plant lineages).
Chara baltica does not accumulate carotenoids to the same extent as C. tomentosa, given that its pool is smaller than both C. tomentosa SRT and VT (figure 2c ). To dissect the possible molecular consequences of this large pool in C. tomentosa, we examined differential gene expression of light stress-related genes. Consistent with a putative photoprotective function of the accumulated carotenoids, the expression of early light-inducible proteins (ELIPs) was significantly lower expressed in SRT versus VT of C. tomentosa (figure 3d ). ELIPs are among the classical land plant light-stress-induced protective proteins by their chlorophyll a/b and LHC binding properties [74,75]. Those were also shown to be important stress responses to diverse conditions in other Phragmoplastophyta, namely Zygnematophyceae [11,39,76]. A recently emerging player in stress response, especially in high-light stress situations, and simultaneously essential for carotenogenesis are so-called plant terminal oxidases (PTOXs) [72,77]. We recovered homologues of genes probably coding for these essential enzymes; their expression was significantly enriched in SRT versus VT (figure 3e ), correlating with the observed carotenoid pool sizes. PTOXs essential role in regulating the carotenoid flux and accumulation has also been shown for maize kernels [72]. The conservation of this regulatory function can be rationalized by the need for the electron-accepting cofactor plastoquinone for the multiple desaturation steps of the carotenoid pathway (before cyclization), which is re-oxidized by PTOXs [78,79]. It is noteworthy that our phylogenetic analyses show that Charophyceae harbour a diversified set of PTOXs, which includes homologues falling into a clade of sequence homologues only found in non-seed plants and streptophyte algae (figure 3e ).
3. Conclusion
Sexual reproduction is intertwined with oxidative homeostasis. ROS are essential signalling molecules [80] in the development and function of reproductive organs [81,82]. ROS can also be culprits, e.g. emerging from photosynthesis and causing oxidative damage to the photosystem complexes [83,84]. Similarly, the reproductive organs of land plants, foremost flowers of angiosperms, are often highly pigmented, and the function of this pigmentation includes, next to pollinator attraction, also (photo-)protection [85,86]. Indeed, transcriptome analysis of the reproductive structures of C. braunii showed differential regulation in genes salient to ROS homeostasis, especially peroxidases [61].
Here, we studied two sympatric species of Chara, C. baltica and C. tomentosa (figure 4). We recovered genetic signatures of the ecophysiology of the two species, for example, swelling control by chloride exporters and enriched PTOX in C. tomentosa. We found β-carotene, δ-carotene and γ-carotene to accumulate in Chara antheridia and in C. tomentosa VT besides several other carotenoids of unknown identity. These carotenoids should be addressed in future studies. Our data suggest that the accumulated carotenoids contribute to photoprotection. However, exact molecular functions and associated mechanisms need to be further investigated. From this carotenoid pool, volatile apocarotenoids emerge, which were not reported previously in Charophyceae—with some in extensive levels hinting at a putative function in stress response or dormancy—subjects that deserve future investigation.
Overall, our work provides an example of how from a conserved chassis of enzymes and metabolites, a unique phenotype can arise: hyperaccumulation of conserved, but usually fast-metabolized carotenoids. This increased pool of carotenoids probably yields a divergent bouquet of carotenoid-derived metabolites (including apocarotenoids) that await exploration. Conceivably, this hyperaccumulation phenotype arose during evolution through minimal changes in expression patterns. At the same time, the accumulation of carotenoids in reproductive structures is a trait that is found in many streptophytes, suggesting that there is a biological programme that is shared across more than 700 million years of phragmoplastophyte evolution.
4. Material and methods
(a). Algal species
Chara tomentosa L. 1753, a dioecious and diplostichous green–greyish charophyte occurs, in brackish and freshwater (figure 1). Characteristic features of this species are the reddening of the upper thallus part during its life cycle and the swollen end cells at the branchlets [37]. The sites are usually dominated by male plants. Their antheridia can reach a diameter of up to 1425 µm [37]. Chara baltica (Hartman) Bruz. 1824 is a monoecious diplostichous to triplostichous charophyte of light to dark green colour (figure 1). This species can only be found in brackish habitats. In contrast to C. tomentosa, where gametangia are usually only formed on the uppermost segments, C. baltica develops gametangia on all segments (figure 1). Antheridia are 2–3 times smaller than those of C. tomentosa with a size of 500 to 700 µm [37].
(b). Sampling
Plant material of C. tomentosa and C. baltica from Michaelsdorf (54.371306° N, 12.569778° E), a lagoon in the Bodstedter Bodden of the Darss-Zingst Bodden chain, was used for this study. This site is characterized by a high proportion of organic suspended matter, which strongly affects the light climate and underwater vegetation. The sheltered part of the lagoon has a soft muddy bottom of up to 1 m and is covered with charophytes of C. tomentosa, C. baltica, Chara aspera and C. baltica var. liljebladii.
The plants of both species were collected by hand in the year 2023 at a depth of 50–75 cm. Male plants of C. tomentosa had developed antheridia. Harvested plants of C. baltica exhibit antheridia and oogonia but no oospores. For all following analyses, VT or top parts (antheridia + swollen end cells at the top of the plant) for C. tomentosa were used, and WP samples (VT + antheridia and oogonia) for C. baltica were used.
Physico-chemical parameters were measured during sampling as follows: temperature 16°C, salinity 4.2 ‰, conductivity 11520 μs cm−1 (measured with a commercial device for home aquaculture). Plants were transported in their native water medium.
(c). Chemicals
A detailed description of the analytical standards used for peak identification and calibration of the analytical instruments can be found in Rieseberg et al. [39]. The solvents used for the extraction of pigments and for performing the HPLC analysis are also described therein.
(d). Carotenoid and chlorophyll extraction
A detailed description of the extraction of carotenoids and pigments is published in Rieseberg et al. [39]. In short, ca 10 mg of lyophilized tissue stored at −80°C under an argon atmosphere prior to extraction was refrozen in liquid N2 and afterwards extracted with 700 μl acetone : water (80 : 20 + 0.1 wt% butylated hydroxytoluene [BHT]) and with 700 μl acetone (+ 0.1 wt% BHT) at 4°C room temperature with dimmed lights. Samples were analysed immediately after extraction.
(e). High-Performance Liquid Chromatography with Ultraviolet-Visible detection via a Diode Array Detector (HPLC–UV–Vis–DAD) measurements of pigments
The method for pigment analysis was previously described in Rieseberg et al. [39]. In short, the HPLC system Agilent 1100 series with a Ultraviolet-Visible detection via a Diode Array Detector (UV–Vis–DAD) equipped with a YMC Carotenoid C30 column (250 × 4.6 mm inner diameter [ID] S-3 µm) from YMC Europe (20°C). Eluents of the 26 min gradient were eluent A (methanol : water, 98 : 2), eluent B (methanol : water, 95 : 2) and eluent C (Methyl tert-butyl ether). For the exact gradient and the description of the quantification, see in general Rieseberg et al. [39]. ND1 (putative torulene), δ-carotene and γ-carotene were quantified at 451 nm based on β-carotene calibration since no commercial standards were at hand to include specific calibration. Identification of δ-carotene and γ-carotene was based on comparisons with absorption spectra published in Gupta et al. [53] (also see the electronic supplementary material and figure 2). Additionally, retention times and principles of retrosynthesis from detected apocarotenoids were taken into consideration.
Statistical analyses of metabolite data were performed with OriginPro 2020. If Kruskal–Wallis–ANOVA showed a significant difference among the groups (p‐value < 0.05), a post hoc Conover–Iman’s test was performed for individual comparisons.
(f). Headspace solid-phase microextraction coupled to gas chromatography equipped with a mass spectrometer measurements of apocarotenoids
The method described [39] was operated in scan mode (m/z: 40–300) to identify volatile apocarotenoids of C. tomentosa. Apocarotenoids were identified by comparison of retention times and fragmentation patterns of the commercial standards with in planta fragmentation patterns. Frozen and lyophilized tissue (20–40 mg) of C. tomentosa VT was homogenized with a sharp spatula and transferred into a brown-glass headspace vial and the analysis protocol of the GC-MS started. For details of the temperature gradient, technical compartments, etc. and the fragmentation patterns of commercial standards of the apocarotenoids from figure 2, see Rieseberg et al. [39].
(g). RNA extraction
RNA extraction was based on Dadras et al. [76] and only slightly modified. Frozen tissue of Charas was stored at –80°C prior to extraction, was refrozen in liquid N2, homogenized with a spatula and afterwards mixed with 1 ml of lysis buffer containing 2-mercaptoethanol (10 μl ml−1), vortexed and transferred for 4 min to an ultrasonic bath. Next, heat shock (56°C) for 5 min was applied. Protocol B (increased binding solution (750 μl)) of the Sigma-Aldrich Spectrum Plant Total RNA Kit was carried out as described by the vendor.
(h). RNAseq and transcriptome assembly
At Novogene (Cambridge, UK), the samples underwent quality checks using a Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA, USA), and library preparation was performed based on polyA enrichment and using directional mRNA library preparation. The libraries were quality checked and sequenced using the NovaSeq 6000 platform (Illumina) with Novogene dual adapters: 5′- AGATCGGAAGAGCGTCGTGTAGGGAAAGAGTGTAGATCTCGGTGGTCGCCGTATCATT-3′ for read 1 and 5′- GATCGGAAGAGCACACGTCTGAACTCCAGTCACGGATGACTATCTCGTATGCCGTCTTCTGCTTG-3′.
Read quality was addressed by using FastQC [87]. For this study, four different de novo assemblies were conducted using Trinity v.2.15.1 [43] after adapter trimming with Trimmomatic [88] (--trimmomatic ‘ILLUMINACLIP:novogene_adapter_sequences.fa:2:30:10:2:keepBothReads LEADING:3 TRAILING:3 MINLEN:36’). The four sets consist of the C. tomentosa sets: ‘SRT’, ‘VT’ and a combination of these two sets ‘TOR’. Another set was created based on the WP transcriptome of C. baltica called ‘BCA’. The completeness of the transcriptomes was assessed with BUSCO v5.4.3 [89] using the ‘eukaryota_odb10’ reference set. The BUSCO completeness of all newly assembled transcriptomes was on average 89.53%. Protein-coding genes were identified using Transdecoder v5.5.0 [90], using the programme’s defaults. For the differential expression analyses, we used the ‘TOR’ set assemblies and protein file.
(i). Decontamination
Because the samples were not from an axenic origin, we wanted to remove potential decontaminants. We conducted this by doing sequence similarity searches against a comprehensive database that included proteins from various sources. These sources include the C. braunii S276 genome [61] as well as potential contaminants such as RefSeq [91] representative bacterial genomes (210 042 521 proteins), fungi (4 990 228 proteins), viruses (644 246), invertebrate (8 686 952), mitochondrion (240 117), plasmid (2 080 798), plastid (1 064 130) and protozoa (1 164 439). We employed MMseqs2 [92] for the search, using an iterative approach with increasing sensitivities and maintaining a maximum of 10 hits (--start-sens 1 --sens-steps 3 -s 7 --alignment-mode 3 --max-seqs 10). To ensure stringent decontamination, we retained, with the help of Get Positive DataSet (GPDS) [93].
(j). Differential expression analyses
To understand the profile of the three different sets (C. tomentosa SRT, C. tomentosa VT, and C. baltica), a PCA was performed according to the methods described by Dadras et al. [76]. We followed this up by doing a quantification of the transcript and gene abundance. We started by running the Trinity ‘align_and_estimate_abundance.pl’ and using Kallisto [44] as its alignment-free quantification method. Abundances were estimated with the Trinity script ‘abundance_estimates_to_matrix.pl’. Additionally, we wanted to know how many number of expressed transcripts or genes there were, per TPM value. That is why we followed up with the two additional Trinity scripts. ‘count_matrix_features_given_MIN_TPM_threshold.pl’ and ‘count_matrix_features_given_MIN_TPM_threshold.pl’.
This resulted in 989 207 possible transcripts for C. tomentosa and 1 367 824 for C. baltica. Since this was an unlikely high number for meaningful transcripts in a transcriptome, we conducted several filtering steps based on the minimum expression levels of 1, 3 and 8 TPM with the Trinity script ‘filter_low_expr_transcripts.pl’ followed by ‘get_Trinity_gene_to_trans_map.pl’. For these three new fasta files proteins were identified with Transdecoder and decontaminated, this was followed up by transcript quantification as previously described. Differential expression analyses were done with the Trinity script ‘run_DE_analysis.pl’ and the differential expression analyses method of DESeq2 [45]. Additionally, we did a transcriptome functional annotation analysis with Trinotate [94]. For this, we conducted a BLASTp and blastx against the UniProtKB/Swiss-Prot database [95], Infernal [96], HHMer [97] with the Pfam Database [98] and eggnog-mapper V2 [99]. The results were loaded into a Trinotate database, and a Trinotate report was generated. We then used DEMC (de novo tool) to extract several files (several trinotate-derived Kyoto Encyclopedia of Genes and Genomes (KEGG)-related files [pathway, module, KEGG Orthology {KO}], differential expressed transcripts and proteins in fasta format and a differential expressed relevant Trinotate output). With the help of KEGGCounter (de novo tool), we filtered out irrelevant pathways and created a KEGG matrix file.
(k). Phylogenetic analysis
To understand the evolutionary history of several genes of interest, we used: (i) the homologues we detected in the predicted proteomes of the two Chara species investigated here (C. tomentosa and C. baltica), and (ii) well-characterized Arabidopsis proteins as a query in a BLASTp search against a protein database inferred from the genomes of: Arabidopsis thaliana (Lamesch et al. [100]), Arabidopsis lyrata (V2.1; [101,102]), Brassica oleracea [103], Brassica rapa (FPsc v1.3; [104]), Capsella grandiflora [105], Triticum aestivum [106], Carica papaya [107], Oryza sativa (v7.0; [108]), Brachipodium distachyon (v3.2; [109]), Amborella trichopoda (v1.0; [110]), Gossypium hirsutum [111], Theobroma cacao [112], Solanum lycopersicum [113], Nicotiana tabacum [114], Gnetum montanum [115], Picea abies [116], Azolla filiculoides [117], Isoetes taiwaniensis [118], Selanigella moellendorffi [119], P. patens [120], Sphagnum fallax [121], Marchantia polymorphya [122], Anthoceros punctatus [123], Anthoceros agrestis [123], Mesotaenium endlicherianum (v1 [124] and v2 [76]), Penium margaritaceum [125], Spirogloea muscicola [124], C. braunii [61], Klebsormidium nitens (v1.1; [126]), Chlorokybus melkonianii ([127]—for naming, see Irisarri et al. [128]), M. viride [127], Micromonas pusilla (v3.0; [129]), Ostreococcus lucimarinus (v2.0; [130]), Ulva mutabilis [131], Chlamydomonas reinhardtii (v5.6; [132]) and Chlorella variabilis (Blanc et al. [133])—as well as against the two proteomes predicted for C. tomentosa and C. baltica. All significant hits (e-value cut-off of 10−5; in the case of larger gene families, we applied a bit score cut-off of 100) were aligned with MAFFT v7.490 [134] (L-INS-I). We computed maximum-likelihood trees using IQ-Tree v.1.5.5 [135] with 1000 ultrafast bootstrap pseudo-replicates [136]. The best models for protein evolution were determined by ModelFinder [137], and the best model according to the Bayesian information criterion was used.
Acknowledgements
We thank René Heise, Sabine Freitag, and Malte Bürsing for excellent technical support.
Contributor Information
Tim P. Rieseberg, Email: timphilipp.rieseberg@uni-goettingen.de.
Anja Holzhausen, Email: anja.holzhausen@landw.uni-halle.de.
Maaike J. Bierenbroodspot, Email: maaikejacobine.bierenbroodspot@uni-goettingen.de.
Wanchen Zhang, Email: wanchen.zhang@stud.uni-goettingen.de.
Ilka N. Abreu, Email: ilkanacif.deabreu@uni-goettingen.de.
Jan de Vries, Email: devries.jan@uni-goettingen.de.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
All RNA sequencing data have been uploaded to NCBI SRA under Bioproject PRJNA1088485. The predicted proteins and data on the phylogenetic analyses are available on Zenodo [138]. The computational analyses are outlined under [139].
Supplementary material is available online [140].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
T.P.R.: conceptualization, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; A.H.: conceptualization, investigation, resources, visualization, writing—review and editing; M.J.B.: data curation, formal analysis, investigation, methodology, software, visualization, writing—review and editing; W.Z.: data curation, formal analysis; I.N.A.: data curation, formal analysis, investigation, resources; J.d.V.: conceptualization, formal analysis, funding acquisition, investigation, project administration, supervision, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interests
We declare we have no competing interests.
Funding
J.d.V. thanks the European Research Council for funding under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 852725; ERC-StG ‘TerreStriAL’). J.d.V. is grateful for support through the German Research Foundation (DFG) on the grant Klebsome (509535047; VR132/11-1) and within the framework of the Priority Programme ‘MAdLand – Molecular Adaptation to Land: Plant Evolution to Change’ (SPP 2237; 440231723 VR 132/4-1; VR 132/4-2), in which T.P.R. is a PhD student. M.J.B. is grateful for being supported through the International Max Planck Research School (IMPRS) for Genome Science. T.P.R. gratefully acknowledges support by the PhD programme ‘Microbiology and Biochemistry’ within the framework of the ‘Göttingen Graduate Center for Neurosciences, Biophysics, and Molecular Biosciences’ (GGNB) at the University of Goettingen.
References
- 1. Kurtović K, Schmidt V, Nehasilová M, Vosolsobě S, Petrášek J. 2024. Rediscovering Chara as a model organism for molecular and evo-devo studies. Protoplasma 261 , 183–196. ( 10.1007/s00709-023-01900-3) [DOI] [PubMed] [Google Scholar]
- 2. Wodniok S, Brinkmann H, Glöckner G, Heidel AJ, Philippe H, Melkonian M, Becker B. 2011. Origin of land plants: do conjugating green algae hold the key? BMC Evol. Biol. 11 , 104. ( 10.1186/1471-2148-11-104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wickett NJ, et al. 2014. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc. Natl Acad. Sci. USA 111 , E4859–E4868. ( 10.1073/pnas.1323926111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Puttick MN, et al. 2018. The interrelationships of land plants and the nature of the ancestral embryophyte. Curr. Biol. 28 , 733–745.( 10.1016/j.cub.2018.01.063) [DOI] [PubMed] [Google Scholar]
- 5. One Thousand Plant Transcriptomes Initiative . 2019. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574 , 679–685. ( 10.1038/s41586-019-1693-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hess S, et al. 2022. A phylogenomically informed five-order system for the closest relatives of land plants. Curr. Biol. 32 , 4473–4482.( 10.1016/j.cub.2022.08.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fürst-Jansen JMR, de Vries S, de Vries J. 2020. Evo-physio: on stress responses and the earliest land plants. J. Exp. Bot. 71 , 3254–3269. ( 10.1093/jxb/eraa007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu L, et al. 2023. Ocean current patterns drive the worldwide colonization of eelgrass (Zostera marina). Nat. Plants 9 , 1207–1220. ( 10.1038/s41477-023-01464-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higo A, et al. 2018. Transcription factor DUO1 generated by neo-functionalization is associated with evolution of sperm differentiation in plants. Nat. Commun. 9 , 5283. ( 10.1038/s41467-018-07728-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maeda HA, Fernie AR. 2021. Evolutionary history of plant metabolism. Annu. Rev. Plant Biol. 72 , 185–216. ( 10.1146/annurev-arplant-080620-031054) [DOI] [PubMed] [Google Scholar]
- 11. Rieseberg TP, Dadras A, Fürst-Jansen JMR, Dhabalia Ashok A, Darienko T, de Vries S, Irisarri I, de Vries J. 2023. Crossroads in the evolution of plant specialized metabolism. Semin. Cell Dev. Biol. 134 , 37–58. ( 10.1016/j.semcdb.2022.03.004) [DOI] [PubMed] [Google Scholar]
- 12. Nisar N, Li L, Lu S, Khin NC, Pogson BJ. 2015. Carotenoid metabolism in plants. Mol. Plant 8 , 68–82. ( 10.1016/j.molp.2014.12.007) [DOI] [PubMed] [Google Scholar]
- 13. Sandmann G. 2021. Diversity and origin of carotenoid biosynthesis: its history of coevolution towards plant photosynthesis. New Phytol. 232 , 479–493. ( 10.1111/nph.17655) [DOI] [PubMed] [Google Scholar]
- 14. Takemura M, Maoka T, Koyanagi T, Kawase N, Nishida R, Tsuchida T, Hironaka M, Ueda T, Misawa N. 2021. Elucidation of the whole carotenoid biosynthetic pathway of aphids at the gene level and arthropodal food chain involving aphids and the red dragonfly. BMC Zool. 6 , 19. ( 10.1186/s40850-021-00082-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodriguez-Concepcion M, et al. 2018. A global perspective on carotenoids: metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 70 , 62–93. ( 10.1016/j.plipres.2018.04.004) [DOI] [PubMed] [Google Scholar]
- 16. Dogbo O, Laferriére A, D’Harlingue A, Camara B. 1988. Carotenoid biosynthesis: isolation and characterization of a bifunctional enzyme catalyzing the synthesis of phytoene. Proc. Natl Acad. Sci. USA 85 , 7054–7058. ( 10.1073/pnas.85.19.7054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bird CR, Ray JA, Fletcher JD, Boniwell JM, Bird AS, Teulieres C, Blain I, Bramley PM, Schuch W. 1991. Using antisense RNA to study gene function: inhibition of carotenoid biosynthesis in transgenic tomatoes. Nat. Biotechnol. 9 , 635–639. ( 10.1038/nbt0791-635) [DOI] [Google Scholar]
- 18. Bartley GE, Viitanen PV, Bacot KO, Scolnik PA. 1992. A tomato gene expressed during fruit ripening encodes an enzyme of the carotenoid biosynthesis pathway. J. Biol. Chem. 267 , 5036–5039. ( 10.1016/s0021-9258(18)42724-x) [DOI] [PubMed] [Google Scholar]
- 19. Schledz M, al-Babili S, von Lintig J, Haubruck H, Rabbani S, Kleinig H, Beyer P. 1996. Phytoene synthase from Narcissus pseudonarcissus: functional expression, galactolipid requirement, topological distribution in chromoplasts and induction during flowering. Plant J. 10 , 781–792. ( 10.1046/j.1365-313x.1996.10050781.x) [DOI] [PubMed] [Google Scholar]
- 20. Bartley GE, Scolnik PA, Beyer P. 1999. Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and zeta-carotene desaturase, expressed in Escherichia coli, catalyze a poly-cis pathway to yield pro-lycopene. Eur. J. Biochem. 259 , 396–403. ( 10.1046/j.1432-1327.1999.00051.x) [DOI] [PubMed] [Google Scholar]
- 21. Fantini E, Falcone G, Frusciante S, Giliberto L, Giuliano G. 2013. Dissection of tomato lycopene biosynthesis through virus-induced gene silencing. Plant Physiol. 163 , 986–998. ( 10.1104/pp.113.224733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu Q, Schaub P, Ghisla S, Al-Babili S, Krieger-Liszkay A, Beyer P. 2010. The lycopene cyclase crty from Pantoea ananatis (formerly Erwinia uredovora) catalyzes an fadred-dependent non-redox reaction. J. Biol. Chem. 285 , 12109–12120. ( 10.1074/jbc.M109.091843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alquézar B, Zacarías L, Rodrigo MJ. 2009. Molecular and functional characterization of a novel chromoplast-specific lycopene beta-cyclase from Citrus and its relation to lycopene accumulation. J. Exp. Bot. 60 , 1783–1797. ( 10.1093/jxb/erp048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cunningham FX, Sun Z, Chamovitz D, Hirschberg J, Gantt E. 1994. Molecular structure and enzymatic function of lycopene cyclase from the cyanobacterium Synechococcus sp strain PCC7942. Plant Cell 6 , 1107–1121. ( 10.1105/tpc.6.8.1107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cunningham FX, Gantt E. 2001. One ring or two? Determination of ring number in carotenoids by lycopene epsilon-cyclases. Proc. Natl Acad. Sci. USA 98 , 2905–2910. ( 10.1073/pnas.051618398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moreno JC, Mi J, Alagoz Y, Al-Babili S. 2021. Plant apocarotenoids: from retrograde signaling to interspecific communication. Plant J. 105 , 351–375. ( 10.1111/tpj.15102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yabuzaki J. 2017. Carotenoids database: structures, chemical fingerprints and distribution among organisms. Database (Oxf.) 2017 , bax004. ( 10.1093/database/bax004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dadras A, Rieseberg TP, Zegers JMS, Fürst-Jansen JMR, Irisarri I, de Vries J, de Vries S. 2023. Accessible versatility underpins the deep evolution of plant specialized metabolism. Phytochem. Rev. ( 10.1007/s11101-023-09863-2) [DOI] [Google Scholar]
- 29. Telfer A. 2014. Singlet oxygen production by PSII under light stress: mechanism, detection and the protective role of beta-carotene. Plant Cell Physiol. 55 , 1216–1223. ( 10.1093/pcp/pcu040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Correa-Galvis V, Poschmann G, Melzer M, Stühler K, Jahns P. 2016. PsbS interactions involved in the activation of energy dissipation in Arabidopsis. Nat. Plants 2 , 15225. ( 10.1038/nplants.2015.225) [DOI] [PubMed] [Google Scholar]
- 31. Yoshii Y, Takaichi S, Maoka T, Inouye I. 2003. Photosynthetic pigment composition in the primitive green alga Mesostigma viride (Prasinophyceae): phylogenetic and evolutionary implications 1. J. Phycol. 39 , 570–576. ( 10.1046/j.1529-8817.2003.02098.x) [DOI] [Google Scholar]
- 32. Stamenković M, Bischof K, Hanelt D. 2014. Xanthophyll cycle pool size and composition in several Cosmarium strains (Zygnematophyceae, Streptophyta) are related to their geographic distribution patterns. Protist 165 , 14–30. ( 10.1016/j.protis.2013.10.002) [DOI] [PubMed] [Google Scholar]
- 33. Liguori N, Periole X, Marrink SJ, Croce R. 2015. From light-harvesting to photoprotection: structural basis of the dynamic switch of the major antenna complex of plants (LHCII). Sci. Rep. 5 , 15661. ( 10.1038/srep15661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Melø TB, Frigaard NU, Matsuura K, Razi Naqvi K. 2000. Electronic energy transfer involving carotenoid pigments in chlorosomes of two green bacteria: Chlorobium tepidum and Chloroflexus aurantiacus. Spectrochim. Acta A Mol. Biomol. Spectrosc. 56 , 2001–2010. ( 10.1016/S1386-1425(00)00289-4) [DOI] [PubMed] [Google Scholar]
- 35. Villarreal P, Carrasco M, Barahona S, Alcaíno J, Cifuentes V, Baeza M. 2016. Tolerance to ultraviolet radiation of psychrotolerant yeasts and analysis of their carotenoid, mycosporine, and ergosterol content. Curr. Microbiol. 72 , 94–101. ( 10.1007/s00284-015-0928-1) [DOI] [PubMed] [Google Scholar]
- 36. Schagerl M, Pichler C. 2000. Pigment composition of freshwater charophyceae. Aquat. Bot. 67 , 117–129. ( 10.1016/S0304-3770(99)00095-9) [DOI] [Google Scholar]
- 37. Stransky H, Hager A. 1970. Das carotinoidmuster und die verbreitung des lichtinduzierten Xanthophyllcyclus in verschiedenen Algenklassen. Archiv. Mikrobiol. 71 , 164–190. ( 10.1007/BF00417740) [DOI] [PubMed] [Google Scholar]
- 38. Sathasivam R, Radhakrishnan R, Kim JK, Park SU. 2021. An update on biosynthesis and regulation of carotenoids in plants. S. Afr. J. Bot. 140 , 290–302. ( 10.1016/j.sajb.2020.05.015) [DOI] [Google Scholar]
- 39. Rieseberg TP, et al. 2024. Time-resolved oxidative signal convergence across the algae–embryophyte divide. bioRxiv. ( 10.1101/2024.03.11.584470) [DOI]
- 40. Deutschlands AC. 2016. Armleuchteralgen: die characeen deutschlands, 1st edn. Heidelberg, Germany: Springer-Verlag. [Google Scholar]
- 41. Schubert H, Blindow I. 2004. Charophytes of the baltic sea. Ruggell, Principality of Liechtenstein: Gantner Verlag. [Google Scholar]
- 42. Holzhausen A. 2018. Resilienz aquatischer Ökosysteme – Vitalitätsabschätzungen von Diasporenbanken. Rostock, Germany: Universität Rostock. [Google Scholar]
- 43. Haas BJ, et al. 2013. De novo transcript sequence reconstruction from RNA-seq using the trinity platform for reference generation and analysis. Nat. Protoc. 8 , 1494–1512. ( 10.1038/nprot.2013.084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bray NL, Pimentel H, Melsted P, Pachter L. 2016. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34 , 525–527. ( 10.1038/nbt.3519) [DOI] [PubMed] [Google Scholar]
- 45. Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 , 550. ( 10.1186/s13059-014-0550-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brumós J, Talón M, Bouhlal R, Colmenero-Flores JM. 2010. Cl- homeostasis in includer and excluder citrus rootstocks: transport mechanisms and identification of candidate genes. Plant Cell Environ. 33 , 2012–2027. ( 10.1111/j.1365-3040.2010.02202.x) [DOI] [PubMed] [Google Scholar]
- 47. Tamma G, Procino G, Strafino A, Bononi E, Meyer G, Paulmichl M, Formoso V, Svelto M, Valenti G. 2007. Hypotonicity induces aquaporin-2 internalization and cytosol-to-membrane translocation of ICln in renal cells. Endocrinology 148 , 1118–1130. ( 10.1210/en.2006-1277) [DOI] [PubMed] [Google Scholar]
- 48. Fürst J, et al. 2006. The ICln interactome. Acta Physiol. 187 , 43–49. ( 10.1111/j.1748-1716.2006.01549.x) [DOI] [PubMed] [Google Scholar]
- 49. Phipps S, Delwiche CF, Bisson MA. 2021. Salinity-induced changes in gene expression in the streptophyte alga Chara: the critical role of a rare Na(+) -ATPase. J. Phycol. 57 , 1004–1013. ( 10.1111/jpy.13166) [DOI] [PubMed] [Google Scholar]
- 50. Moore JA. 1986. Charophytes of great britain and ireland. London, UK: Botanical Society of the British Isles. [Google Scholar]
- 51. Ohmiya A. 2013. Qualitative and quantitative control of carotenoid accumulation in flower petals. Sci. Hortic. 163 , 10–19. ( 10.1016/j.scienta.2013.06.018) [DOI] [Google Scholar]
- 52. Han Y, Wang X, Chen W, Dong M, Yuan W, Liu X, Shang F. 2014. Differential expression of carotenoid-related genes determines diversified carotenoid coloration in flower petal of Osmanthus fragrans. Tree Genet. Genom. 10 , 329–338. ( 10.1007/s11295-013-0687-8) [DOI] [Google Scholar]
- 53. Gupta P, Sreelakshmi Y, Sharma R. 2015. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 11 , 5. ( 10.1186/s13007-015-0051-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karlsson MF, Birgersson G, Cotes Prado AM, Bosa F, Bengtsson M, Witzgall P. 2009. Plant odor analysis of potato: response of Guatemalan moth to above- and belowground potato volatiles. J. Agric. Food Chem. 57 , 5903–5909. ( 10.1021/jf803730h) [DOI] [PubMed] [Google Scholar]
- 55. Schiestl FP. 2010. The evolution of floral scent and insect chemical communication. Ecol. Lett. 13 , 643–656. ( 10.1111/j.1461-0248.2010.01451.x) [DOI] [PubMed] [Google Scholar]
- 56. Dekel A, Yakir E, Bohbot JD. 2019. The sulcatone receptor of the strict nectar-feeding mosquito Toxorhynchites amboinensis. Insect Biochem. Mol. Biol. 111 , 103174. ( 10.1016/j.ibmb.2019.05.009) [DOI] [PubMed] [Google Scholar]
- 57. Cascone P, Vuts J, Birkett MA, Rasmann S, Pickett JA, Guerrieri E. 2023. Small volatile lipophilic molecules induced belowground by aphid attack elicit a defensive response in neighbouring un-infested plants. Front. Plant Sci. 14 , 1154587. ( 10.3389/fpls.2023.1154587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dugdale TM, Hicks BJ, De Winton M, Taumoepeau A. 2006. Fish exclosures versus intensive fishing to restore charophytes in a shallow New Zealand lake. Aquat. Conserv. 16 , 193–202. ( 10.1002/aqc.711) [DOI] [Google Scholar]
- 59. Yu J, et al. 2016. Submerged macrophytes facilitate dominance of omnivorous fish in a subtropical shallow lake: implications for lake restoration. Hydrobiologia 775 , 97–107. ( 10.1007/s10750-016-2717-7) [DOI] [Google Scholar]
- 60. Sadali NM, Sowden RG, Ling Q, Jarvis RP. 2019. Differentiation of chromoplasts and other plastids in plants. Plant Cell Rep. 38 , 803–818. ( 10.1007/s00299-019-02420-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nishiyama T, et al. 2018. The Chara genome: secondary complexity and implications for plant terrestrialization. Cell 174 , 448–464.( 10.1016/j.cell.2018.06.033) [DOI] [PubMed] [Google Scholar]
- 62. Walter MH, Floss DS, Strack D. 2010. Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles. Planta 232 , 1–17. ( 10.1007/s00425-010-1156-3) [DOI] [PubMed] [Google Scholar]
- 63. Ahrazem O, Rubio-Moraga A, López RC, Gómez-Gómez L. 2010. The expression of a chromoplast-specific lycopene beta cyclase gene is involved in the high production of saffron’s apocarotenoid precursors. J. Exp. Bot. 61 , 105–119. ( 10.1093/jxb/erp283) [DOI] [PubMed] [Google Scholar]
- 64. Abe S, et al. 2014. Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc. Natl Acad. Sci. USA 111 , 18084–18089. ( 10.1073/pnas.1410801111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Delaux PM, et al. 2012. Origin of strigolactones in the green lineage. New Phytol. 195 , 857–871. ( 10.1111/j.1469-8137.2012.04209.x) [DOI] [PubMed] [Google Scholar]
- 66. Decker EL, et al. 2017. Strigolactone biosynthesis is evolutionarily conserved, regulated by phosphate starvation and contributes to resistance against phytopathogenic fungi in a moss, Physcomitrella patens. New Phytol. 216 , 455–468. ( 10.1111/nph.14506) [DOI] [PubMed] [Google Scholar]
- 67. Kodama K, et al. 2022. An ancestral function of strigolactones as symbiotic rhizosphere signals. Nat. Commun. 13 , 3974. ( 10.1038/s41467-022-31708-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Abdelrhman M. 2016. Modeling water clarity and light quality in oceans. J. Mar. Sci. Eng. 4 , 80. ( 10.3390/jmse4040080) [DOI] [Google Scholar]
- 69. Esteban R, Barrutia O, Artetxe U, Fernández-Marín B, Hernández A, García-Plazaola JI. 2015. Internal and external factors affecting photosynthetic pigment composition in plants: a meta-analytical approach. New Phytol. 206 , 268–280. ( 10.1111/nph.13186) [DOI] [PubMed] [Google Scholar]
- 70. Esteban R, Moran JF, Becerril JM, García-Plazaola JI. 2015. Versatility of carotenoids: an integrated view on diversity, evolution, functional roles and environmental interactions. Environ. Exp. Bot. 119 , 63–75. ( 10.1016/j.envexpbot.2015.04.009) [DOI] [Google Scholar]
- 71. Lin CH, Chen BH. 2003. Determination of carotenoids in tomato juice by liquid chromatography. J. Chromatogr. A 1012 , 103–109. ( 10.1016/s0021-9673(03)01138-5) [DOI] [PubMed] [Google Scholar]
- 72. Nie Y, et al. 2024. The maize plastid terminal oxidase (PTOX) locus controls the carotenoid content of kernels. Plant J. 118 , 457–468. ( 10.1111/tpj.16618) [DOI] [PubMed] [Google Scholar]
- 73. Permann C, Pichrtová M, Šoljaková T, Herburger K, Jouneau PH, Uwizeye C, Falconet D, Marechal E, Holzinger A. 2023. 3D-reconstructions of zygospores in Zygnema vaginatum (Charophyta) reveal details of cell wall formation, suggesting adaptations to extreme habitats. Physiol. Plant. 175 , e13988. ( 10.1111/ppl.13988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hutin C, Nussaume L, Moise N, Moya I, Kloppstech K, Havaux M. 2003. Early light-induced proteins protect Arabidopsis from photooxidative stress. Proc. Natl Acad. Sci. USA 100 , 4921–4926. ( 10.1073/pnas.0736939100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rossini S, Casazza AP, Engelmann ECM, Havaux M, Jennings RC, Soave C. 2006. Suppression of both ELIP1 and ELIP2 in Arabidopsis does not affect tolerance to photoinhibition and photooxidative stress. Plant Physiol. 141 , 1264–1273. ( 10.1104/pp.106.083055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dadras A, et al. 2023. Environmental gradients reveal stress hubs pre-dating plant terrestrialization. Nat. Plants 9 , 1419–1438. ( 10.1038/s41477-023-01491-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Calzadilla PI, Song J, Gallois P, Johnson GN. 2024. Proximity to photosystem II is necessary for activation of plastid terminal oxidase (PTOX) for photoprotection. Nat. Commun. 15 , 287. ( 10.1038/s41467-023-44454-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Carol P, Kuntz M. 2001. A plastid terminal oxidase comes to light: implications for carotenoid biosynthesis and chlororespiration. Trends Plant Sci. 6 , 31–36. ( 10.1016/s1360-1385(00)01811-2) [DOI] [PubMed] [Google Scholar]
- 79. Joet T, Genty B, Josse EM, Kuntz M, Cournac L, Peltier G. 2002. Involvement of a plastid terminal oxidase in plastoquinone oxidation as evidenced by expression of the Arabidopsis thaliana enzyme in tobacco. J. Biol. Chem. 277 , 31623–31630. ( 10.1074/jbc.M203538200) [DOI] [PubMed] [Google Scholar]
- 80. Mittler R. 2017. ROS are good. Trends Plant Sci. 22 , 11–19. ( 10.1016/j.tplants.2016.08.002) [DOI] [PubMed] [Google Scholar]
- 81. Zafra A, Rodríguez-García MI, Alché J de D. 2010. Cellular localization of ROS and NO in olive reproductive tissues during flower development. BMC Plant Biol. 10 , 36. ( 10.1186/1471-2229-10-36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ali MF, Muday GK. 2024. Reactive oxygen species are signaling molecules that modulate plant reproduction. Plant Cell Environ. 47 , 1592–1605. ( 10.1111/pce.14837) [DOI] [PubMed] [Google Scholar]
- 83. Aro EM, Virgin I, Andersson B. 1993. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143 , 113–134. ( 10.1016/0005-2728(93)90134-2) [DOI] [PubMed] [Google Scholar]
- 84. Foyer CH. 2018. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 154 , 134–142. ( 10.1016/j.envexpbot.2018.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhu C, Bai C, Sanahuja G, Yuan D, Farré G, Naqvi S, Shi L, Capell T, Christou P. 2010. The regulation of carotenoid pigmentation in flowers. Arch. Biochem. Biophys. 504 , 132–141. ( 10.1016/j.abb.2010.07.028) [DOI] [PubMed] [Google Scholar]
- 86. Miller R, Owens SJ, Rørslett B. 2011. Plants and colour: flowers and pollination. Opt. Laser Technol. 43 , 282–294. ( 10.1016/j.optlastec.2008.12.018) [DOI] [Google Scholar]
- 87. Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. Cambridge, UK: Babraham Bioinformatics, Babraham Institute. [Google Scholar]
- 88. Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30 , 2114–2120. ( 10.1093/bioinformatics/btu170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Seppey M, Manni M, Zdobnov EM. 2019. BUSCO: assessing genome assembly and annotation completeness. Gene Pred. Meth. Prot. 227–245. ( 10.1007/978-1-4939-9173-0_14) [DOI] [PubMed] [Google Scholar]
- 90. Haas BJ. 2018. Transdecoder. See https://github.com/TransDecoder.
- 91. O’Leary NA, et al. 2016. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44 , D733–D745. ( 10.1093/nar/gkv1189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Steinegger M, Soding J. 2017. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 35 , 1026–1028. ( 10.1038/nbt.3988) [DOI] [PubMed] [Google Scholar]
- 93. Bierenbroodspot MJ, Darienko T, de Vries S, Fürst-Jansen JMR, Buschmann H, Pröschold T, Irisarri I, de Vries J. 2024. Phylogenomic insights into the first multicellular streptophyte. Curr. Biol. 34 , 670–681.( 10.1016/j.cub.2023.12.070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bryant DM, et al. 2017. A tissue-mapped axolotl de novo transcriptome enables identification of limb regeneration factors. Cell Rep. 18 , 762–776. ( 10.1016/j.celrep.2016.12.063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Boutet E, Lieberherr D, Tognolli M, Schneider M, Bansal P, Bridge AJ, Poux S, Bougueleret L, Xenarios I. 2016. UniProtKB/Swiss-Prot, the manually annotated section of the UniProt knowledgebase: how to use the entry view. Methods Mol. Biol. 1374 , 23–54. ( 10.1007/978-1-4939-3167-5_2) [DOI] [PubMed] [Google Scholar]
- 96. Nawrocki EP, Eddy SR. 2013. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 29 , 2933–2935. ( 10.1093/bioinformatics/btt509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Eddy SR. 2009. A new generation of homology search tools based on probabilistic inference. Genome Inform. 23 , 205–211. ( 10.1142/9781848165632_0019) [DOI] [PubMed] [Google Scholar]
- 98. Mistry J, et al. 2021. Pfam: the protein families database in 2021. Nucleic Acids Res. 49 , D412–D419. ( 10.1093/nar/gkaa913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cantalapiedra CP, Hernández-Plaza A, Letunic I, Bork P, Huerta-Cepas J. 2021. eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 38 , 5825–5829. ( 10.1093/molbev/msab293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lamesch P, et al. 2012. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 40 , D1202–10. ( 10.1093/nar/gkr1090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hu TT, et al. 2011. The Arabidopsis lyrata genome sequence and the basis of rapid genome size change. Nat. Genet. 43 , 476–481. ( 10.1038/ng.807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rawat V, Abdelsamad A, Pietzenuk B, Seymour DK, Koenig D, Weigel D, Pecinka A, Schneeberger K. 2015. Improving the annotation of Arabidopsis lyrata using RNA-seq data. PLoS One 10 , e0137391. ( 10.1371/journal.pone.0137391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liu S, et al. 2014. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 5 , 3930. ( 10.1038/ncomms4930) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Goodstein DM, et al. 2012. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40 , D1178–D1186. ( 10.1093/nar/gkr944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Slotte T, et al. 2013. The Capsella rubella genome and the genomic consequences of rapid mating system evolution. Nat. Genet. 45 , 831–835. ( 10.1038/ng.2669) [DOI] [PubMed] [Google Scholar]
- 106. Appels R, et al. 2018. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361 , eaar7191. ( 10.1126/science.aar7191) [DOI] [PubMed] [Google Scholar]
- 107. Ming R, et al. 2008. The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature 452 , 991–996. ( 10.1038/nature06856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kawahara Y, et al. 2013. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 6 , 1–10. ( 10.1186/1939-8433-6-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Initiative IB. 2010. Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 463 , 763–768. ( 10.1038/nature08747) [DOI] [PubMed] [Google Scholar]
- 110. Amborella Genome Project . 2013. The Amborella genome and the evolution of flowering plants. Science 342 , 1241089. ( 10.1126/science.1241089) [DOI] [PubMed] [Google Scholar]
- 111. Li F, et al. 2015. Genome sequence of cultivated upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat. Biotechnol. 33 , 524–530. ( 10.1038/nbt.3208) [DOI] [PubMed] [Google Scholar]
- 112. Argout X, et al. 2011. The genome of Theobroma cacao. Nat. Genet. 43 , 101–108. ( 10.1038/ng.736) [DOI] [PubMed] [Google Scholar]
- 113. Tomato Genome Consortium . 2012. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485 , 635–641. ( 10.1038/nature11119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sierro N, Battey JND, Ouadi S, Bakaher N, Bovet L, Willig A, Goepfert S, Peitsch MC, Ivanov NV. 2014. The tobacco genome sequence and its comparison with those of tomato and potato. Nat. Commun. 5 , 3833. ( 10.1038/ncomms4833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wan T, et al. 2018. A genome for gnetophytes and early evolution of seed plants. Nat. Plants 4 , 82–89. ( 10.1038/s41477-017-0097-2) [DOI] [PubMed] [Google Scholar]
- 116. Nystedt B, et al. 2013. The Norway spruce genome sequence and conifer genome evolution. Nature 497 , 579–584. ( 10.1038/nature12211) [DOI] [PubMed] [Google Scholar]
- 117. Li FW, et al. 2018. Fern genomes elucidate land plant evolution and cyanobacterial symbioses. Nat. Plants. 4 , 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wickell D, et al. 2021. Underwater CAM photosynthesis elucidated by Isoetes genome. Nat. Commun. 12 , 6348. ( 10.1038/s41467-021-26644-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Banks JA, et al. 2011. The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science 332 , 960–963. ( 10.1126/science.1203810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lang D, et al. 2018. The Physcomitrella patens chromosome‐scale assembly reveals moss genome structure and evolution. Plant J. 93 , 515–533. ( 10.1111/tpj.13801) [DOI] [PubMed] [Google Scholar]
- 121. Healey AL, et al. 2023. Newly identified sex chromosomes in the Sphagnum (peat moss) genome alter carbon sequestration and ecosystem dynamics. Nat. Plants 9 , 238–254. ( 10.1038/s41477-022-01333-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bowman JL, et al. 2017. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171 , 287–304.( 10.1016/j.cell.2017.09.030) [DOI] [PubMed] [Google Scholar]
- 123. Li FW, et al. 2020. Anthoceros genomes illuminate the origin of land plants and the unique biology of hornworts. Nat. Plants 6 , 259–272. ( 10.1038/s41477-020-0618-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cheng S, et al. 2019. Genomes of subaerial zygnematophyceae provide insights into land plant evolution. Cell 179 , 1057–1067.( 10.1016/j.cell.2019.10.019) [DOI] [PubMed] [Google Scholar]
- 125. Jiao C, et al. 2020. The Penium margaritaceum genome: hallmarks of the origins of land plants. Cell 181 , 1097–1111.( 10.1016/j.cell.2020.04.019) [DOI] [PubMed] [Google Scholar]
- 126. Hori K, et al. 2014. Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nat. Commun. 5 , 3978. ( 10.1038/ncomms4978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wang S, et al. 2020. Genomes of early-diverging streptophyte algae shed light on plant terrestrialization. Nat. Plants 6 , 95–106. ( 10.1038/s41477-019-0560-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Irisarri I, Darienko T, Proschold T, Furst-Jansen JMR, Jamy M, de Vries J. 2021. Unexpected cryptic species among streptophyte algae most distant to land plants. Proc. R. Soc. B 288 , 20212168. ( 10.1098/rspb.2021.2168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Worden AZ, et al. 2009. Green evolution and dynamic adaptations revealed by genomes of the marine picoeukaryotes Micromonas. Science 324 , 268–272. ( 10.1126/science.1167222) [DOI] [PubMed] [Google Scholar]
- 130. Palenik B, et al. 2007. The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proc. Natl Acad. Sci. USA 104 , 7705–7710. ( 10.1073/pnas.0611046104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. De Clerck O, et al. 2018. Insights into the evolution of multicellularity from the sea lettuce genome. Curr. Biol. 28 , 2921–2933.( 10.1016/j.cub.2018.08.015) [DOI] [PubMed] [Google Scholar]
- 132. Merchant SS, et al. 2007. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318 , 245–250. ( 10.1126/science.1143609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Blanc G, et al. 2010. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 22 , 2943–2955. ( 10.1105/tpc.110.076406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30 , 772–780. ( 10.1093/molbev/mst010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32 , 268–274. ( 10.1093/molbev/msu300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Minh BQ, Nguyen MAT, von Haeseler A. 2013. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 30 , 1188–1195. ( 10.1093/molbev/mst024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14 , 587–589. ( 10.1038/nmeth.4285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Bierenbroodspot M, de Vries J. 2024. Assemblies and phylogenetic analyses for the comparative analysis of Chara tomentosa and Chara baltica . Zenodo. ( 10.5281/zenodo.10822046) [DOI]
- 139. Bierenbroodspot MJ. 2024. Differential expression charaphyceae. GitHub. See https://github.com/mjbieren/Differential_Expression_Charaphyceae.
- 140. Rieseberg T, Holzhausen A, Bierenbroodspot M, Zhang W, Abreu I, de Vries J. 2024. Data from: Conserved carotenoid pigmentation in reproductive organs of Charophyceae. Figshare. ( 10.6084/m9.figshare.c.7440866) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All RNA sequencing data have been uploaded to NCBI SRA under Bioproject PRJNA1088485. The predicted proteins and data on the phylogenetic analyses are available on Zenodo [138]. The computational analyses are outlined under [139].
Supplementary material is available online [140].