ABSTRACT
During September to December 2021, school mask policies to mitigate SARS-CoV-2 transmission varied throughout the US. We compared infection-induced seroprevalence estimates and estimated seroconversion among children residing in areas with and without school mask requirements. We estimated infection-induced seroprevalence among children in three age groups (5–17, 5–11, and 12–17 years) in areas with and without school district mask requirements for two time points: September 1–30, 2021 and December 15, 2021 to January 14, 2022. Robust Poisson regression models estimated population seroconversion over the semester among initially seronegative children. Permutation tests assessed for significant differences in the estimated population seroconversion due to incident infections by school district mask policy. Residing in an area with no school mask requirement was associated with higher infection-induced seroprevalence among children aged 5–17 years (adjusted prevalence ratio [aPR] = 1.18, 95% confidence interval [CI]: 1.10, 1.26), and those aged 5–11 years (aPR) = 1.21, 95% CI: 1.10, 1.32) and those aged 12–17 years (aPR = 1.16, 95% CI: 1.07, 1.26), compared with areas requiring masks in school. Estimated population seroconversion during the semester was also significantly higher among children in districts without mask policies than those with school mask requirements among all age groups (5–17 years: 23.7% vs 18.1%, P < 0.001; 5–11 years: 6.4% vs 4.5%, P = 0.002;12–17 years: 27.2% vs 21.0%, P < 0.001). During the U.S. Fall 2021 semester, areas with school mask requirements had lower infection-induced seroprevalence and an estimated lower proportion of seroconversion due to incident infection among school-aged children compared with areas without school mask requirements; causality cannot necessarily be inferred from these associations.
IMPORTANCE
During the U.S. Fall 2021 school semester, the estimated proportion of previously uninfected school-aged children who experienced a first infection with SARS-CoV-2 was lower in areas where public school district policies required masks for all staff and students compared with areas where the school districts had no mask requirements. Because children are more likely than adults to experience asymptomatic or mild SARS-CoV-2 infections, the presence of infection-induced antibodies is a more accurate measure of infection history than clinical testing. The proportion of children with these antibodies (i.e., seroprevalence) can improve our understanding of SARS-CoV-2 by detecting more infections and eliminating potential bias due to local testing and reporting practices. Enhanced robustness of surveillance for respiratory infections in children, including records of mitigation policies in communities and schools, as well as seroprevalence data, would establish a better evidence base for policy decisions and response measures during future respiratory outbreaks.
KEYWORDS: immunology, immunoserology, seroprevalence, SARS-CoV-2, masks, schools, pediatric infectious disease, public health
INTRODUCTION
During the COVID-19 pandemic, the U.S. educational system faced unprecedented challenges. Initially, school districts adopted virtual learning as an infection control strategy. By the start of the 2021–2022 school year (typically August or September), many U.S. school districts had reinstated in-person learning. The Delta variant of SARS-CoV-2, known to be more infectious than prior strains (1), was the predominant variant at that time. To mitigate SARS-CoV-2 spread, districts adopted varying measures, including mask requirements.
Masks have been a central mitigation measure in many settings to prevent the transmission of SARS-CoV-2 (2), which spreads through respiratory droplets and aerosols (3). Proper use of high-quality masks has been shown to reduce the level of detectable droplets and aerosols (3), lowering the risk of transmission. Mask-wearing has also been associated with a substantially reduced risk of transmission after close contact (4). In the school setting, where effective social distancing is not always possible, facemasks have been shown to help lower the risk of in-school transmission according to case report data and studies with frequent testing (5–9).
Many studies examining mask policies and COVID-19 transmission in schools are limited by their reliance on COVID-19 case report data. A lower proportion of infections in the pediatric populations are detected by case reporting than in adult populations, due in part to the increased proportion of asymptomatic or mild infections (10). Data on the proportion of the pediatric population with SARS-CoV-2 antibodies (i.e., seroprevalence) can improve understanding of the incidence of SARS-CoV-2 infection and eliminate potential bias due to local testing and reporting practices (11).
The objective of this study was to examine the effectiveness of mask requirements during the Fall 2021 semester in K-12 schools by (i) comparing infection-induced seroprevalence among school-aged children in areas where school districts required masks throughout September to November 2021 to areas where there were no school mask requirements and (ii) to compare the estimated population seroconversion of children in districts requiring masks to those in districts with no mask requirements.
MATERIALS AND METHODS
Mask policy data
Mask policy data for school districts throughout the United States were collected daily through survey results and web scraping (8, 12). Each included school district was classified by the type of school mask policy in place from September 1, 2021 to November 30, 2021 (school policy classification period). During this period, 98% of data reports from school districts showed in-person learning. For this analysis, districts with consistent school mask policies that required masks for all students and staff throughout the study period were classified as “full mask.” Those that did not implement any mask requirement during the study period were classified as “no mask.” Districts with policies that required masks for some but not all individuals, and those with policies that changed during the study period, were classified as “partial mask” districts.
Seroprevalence data
Data from the Nationwide Commercial Laboratory Survey (NCLS) were used to produce infection-induced seroprevalence estimates. NCLS partnered with three commercial laboratory networks that collected convenience samples of deidentified residual blood specimens to test for SARS-CoV-2 infection-induced antibodies throughout the United States (50 states, Washington, DC, and Puerto Rico). Specimens were originally collected for clinical screening or testing unrelated to the assessment of COVID-19 infection. All commercial laboratories tested blood specimens with the Roche Elecsys anti-nucleocapsid [anti-N] pan-immunoglobulin assay, which has an estimated mean time to sero-reversion of approximately 2 years after initial infection (13). Anti-N antibodies are only produced in response to previous infection, not in response to any vaccination authorized or approved for use in the U.S. Antibody results were reported in 4-week collection periods. Full NCLS study methods are described elsewhere (14).
To assess the effects of mask policies in K-12 schools over the U.S. Fall 2021 semester, we limited the analysis to specimens drawn from persons aged 5–17 years during two time periods: initial seroprevalence assessment period (September 1–30, 2021) and end-semester assessment period (December 15 to January 14, 2022). Thirty-day time periods for seroprevalence assessment were selected to have an adequate sample size to estimate seroprevalence. The initial seroprevalence assessment period represents the earliest 30-day period of data available during the U.S. Fall 2021 academic semester. The end-semester assessment period was selected to initiate 15 days after the conclusion of the school policy classification period, since 99.5% of persons who had been infected on the last day of school policy classification period would have developed detectable anti-N antibodies by that date (15).
Patient consent statement
This activity was reviewed by the U.S. Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy (e.g., 45 C.F.R. part 46; 21 C.F.R. part 56; 42 U.S.C. §241(d), 5 U.S.C. §552 a, 44 U.S.C. §3501 et seq.). Informed consent was waived as data were de-identified and Health Insurance Portability and Accountability Act (HIPAA)-compliant.
Geographic catchment and weighting of data
School districts with at least one mask policy data point during the school policy classification period were eligible for the analysis, which included 14,368 individual school districts within 11,459 unique zip code tabulation areas (ZCTAs). School district-level mask policy data were aggregated by ZCTA since seroprevalence data by school district were not available. ZCTAs with no data for school mask policy (n = 503) were excluded. We excluded two sets of mixed or partial ZCTAs: (i) those containing more than one school district where those districts fell within differing school mask categories (n = 1,176) and (ii) those containing school districts classified in the “partial mask” group (n = 2,545).
Among 7,235 ZCTAs eligible for inclusion, 1,892 unique ZCTAs had available seroprevalence data (n = 5,214 specimens); 1,317 ZCTAs were in the full mask group and 575 ZCTAs were in the no mask group (Fig. S1). Initial seroprevalence data were available from 1,285 of these ZCTAs (n = 2,694 specimens); the median number of specimens included per ZCTA was 1 (range: 1–24). End-semester seroprevalence data were available from 1,323 ZCTAs (n = 2,520 specimens); the median number of specimens included per ZCTA was 1 (range: 1–27). Data from these ZCTAs comprised the full analytic data set (Fig. S2). We compared demographics, case rates, and vaccine rates between the analytic data set of 1,892 ZCTAs and all 33,120 ZCTAs in the United States (Table S1).
A smaller data set used for sensitivity analysis consisted of 716 ZCTAs that had data for both seroprevalence assessment time points: 529 ZCTAs where school district(s) had full mask policies (n = 2,673 specimens), and 187 ZCTAs where school districts had no mask policies (n = 841 specimens). (Fig. S1). In the sensitivity analysis, the median number of specimens included per ZCTA was two for the initial seroprevalence assessment (range: 1–27) and two for the end-semester seroprevalence assessment (range: 1–24).
Survey weights were calculated to adjust for differences in age, sex, and metro status between the analytic data set and the U.S. population. Metro status was defined as metro or non-metro based on the U.S. Department of Agriculture’s Rural-Urban Continuum Codes (metro 1–3, non-metro 4–9) (16). Weighting is performed at the national and jurisdictional levels using raking techniques (17). Weighting accounted for multiple dimensions to ensure that the weighted distributions mirror the national and state distributions even for samples selected with non-probability sampling methods.
Analysis
We first conducted a bivariate analysis to explore the relationship between mask policy group and seroprevalence. Using Pearson’s χ2 test, we compared the weighted seroprevalence between groups at the initial and end-semester assessment time periods.
Next, we applied Poisson regression models with robust variance estimation, robust Poisson regression (18–20), to examine the association between school mask policy status and seroprevalence, pooling specimens from the initial and end-of-semester assessment periods. The model employed identical survey weights to those utilized in the bivariate analysis. The outcome variable was individual-level seropositivity. Covariates included individual-level covariates (age and sex), area-level covariates (Census region, county-level urbanicity), ZCTA-level covariates (majority race/ethnicity, education, and poverty-level from the 2020 U.S. Census American Community Survey 5-year estimates), a variable representing time-period (initial or end-semester), county-level vaccination rate (21), and cumulative case incidence rate (22) for each time period according to CDC data. All covariates were categorical except for two continuous measures: vaccination rate and cumulative case incidence. Similar to a previous study, we defined the ZCTA-level main racial or ethnic group as Hispanic or Latino, non-Hispanic Black, or non-Hispanic White based on a threshold of 60% population (23). If any of these groups reach this threshold, they become the majority. If not, a combination of Hispanic and Black populations exceeding 60% defines the majority, otherwise it’s labeled as "other.” The county-level vaccination rate represents the cumulative proportion of individuals vaccinated since the onset of the pandemic; these rates were stratified by two age groups (5–11 years and 12–17 years) and two periods (September and December 2021). The county-level cumulative case incidence rate represents the cumulative proportion of reported cases accumulated across all age groups from the inception of the pandemic until September and December 2021.
Marginal means calculated from the fitted Poisson regression models were used as estimates of the proportions of seropositivity by time period and mask policy status, assuming all other covariates are the same across these strata. The estimated proportions of seropositivity were then used to estimate the proportion of individuals who underwent seroconversion between the two assessment periods by mask policy status, which is defined as the estimated proportion of people among the population that was seronegative at the initial seroprevalence assessment period who seroconverted during the study period (hereafter referred to as estimated population seroconversion) (24). The estimated population seroconversion provides valuable insights into the dynamics of seropositive transitions by accounting for variations in the initial seropositivity rates and proportion of the population still susceptible to seroconversion within both the full mask and no mask groups. The estimated population seroconversion over the study period by group was calculated by subtracting the proportion of seropositive specimens in the initial assessment period from the proportion of seropositive specimens in the end-semester assessment period and dividing this difference by the estimated proportion of seronegative specimens at the initial assessment period as seen in formula (1). In other words, this refers to the estimated proportion of initially seronegative specimens that transitioned to seropositive during the semester.
| (1) |
Finally, we employed robust (non-parametric) permutation tests (25) to assess differences in the estimated population seroconversion between the full mask and no mask groups. We chose a non-parametric test so as not to assume an approximately normal distribution of the outcome. To ensure statistical reliability, we randomly sampled (without replacement) possible permutations of mask policy status.
We conducted these analyses for the combined age group (5–17 years) and separately for two sub-groups (5–11 years and 12–17 years) to assess differential effects by age. Analyses were repeated within the sensitivity data set to determine if results were robust to restriction of the data set to ZCTAs, which had seroprevalence data available at both the initial and end-semester time points.
All analyses were performed using R (Version 4.2.2, Posit). All tests of statistical significance were two-sided, and the alpha level was set at 5%.
RESULTS
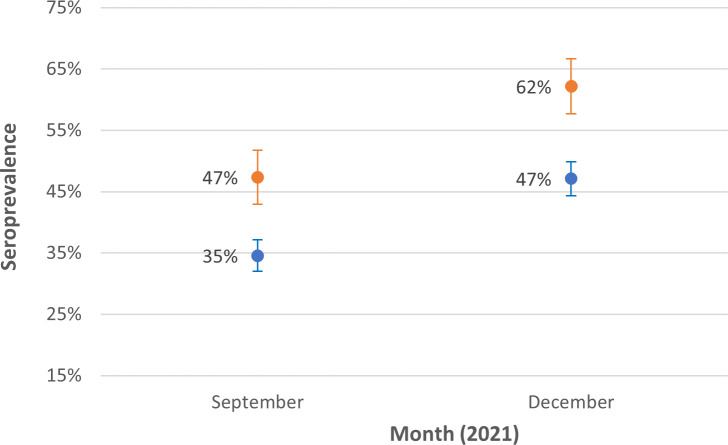
The initial estimated infection-induced seropositivity among people aged 5–17 years residing in “no mask” ZCTAs was 47%, which increased to an end-semester seroprevalence of 62%. Initial 5- to 17-year-old seropositivity in “full mask” ZCTAs was 35%, increasing to an end-semester seroprevalence of 47% (Table 1; Fig. 1). Notably, at the beginning of the study period, each group had a different proportion that was seronegative (i.e., had the potential to seroconvert). Model-based estimates in September indicated that 56% of the no mask group and 62.5% of the full mask group were seronegative (Table 2). This represents the respective proportions of people in the no mask and full mask groups who were initially at risk of infection, as they had not yet developed infection-induced antibodies against SARS-CoV-2.
TABLE 1.
SARS-CoV-2 infection-induced seroprevalence by school mask group, age group, and time period
| September 1–30, 2021 | December 15 to January 14, 2022 | Combined dates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Seropositive | P valuea | Total | Seropositive | P valuea | Total | Seropositive | P valuea | ||||
| n = 2,694b | n = 1,010 | %c | n = 2,520b | n = 1,287 | % | n = 5,214 | n = 2,297 | % | ||||
| School Mask Policy (5–17) | ||||||||||||
| Full | 1,989 | 677 | 34.6 | <0.0001 | 1,860 | 874 | 47.1 | <0.0001 | 3,849 | 1551 | 41.0 | <0.0001 |
| None | 705 | 333 | 47.3 | 660 | 413 | 62.1 | 1,365 | 746 | 54.3 | |||
| School Mask Policy (5–11) | ||||||||||||
| Full | 714 | 225 | 33.3 | 0.0007 | 608 | 289 | 46.7 | 0.0054 | 1,322 | 514 | 39.9 | <0.0001 |
| None | 210 | 100 | 47.5 | 207 | 125 | 59.4 | 417 | 225 | 53.0 | |||
| School Mask Policy (12–17) | ||||||||||||
| Full | 1,275 | 452 | 36.1 | 0.0003 | 1,252 | 585 | 47.5 | <0.0001 | 2,527 | 1,037 | 42.2 | <0.0001 |
| None | 495 | 233 | 47.2 | 453 | 288 | 64.3 | 948 | 521 | 55.3 | |||
Pearson’s χ2 test was used to examine differences in proportions between groups.
1285 ZCTAs for September 1–30, 2021 and 1,323 ZCTAs for December 15, 2021 to January 14, 2022 were represented in the analysis.
The seroprevalence is estimated with survey weights adjusting for age, sex, and metro status.
Fig 1.
SARS-CoV-2 infection-induced seroprevalence among specimens from individuals aged 5–17 years collected in ZCTAs with a full school mask policy and in ZCTAs with no school mask policy during September 1–30, 2021 and December 15, 2021 to January 14, 2022. Note: These descriptive statistics of seroprevalence is estimated with survey weights adjusting for age, sex, and metro status. Lines represent 95% confidence interval (CI).
TABLE 2.
Estimated seroconversion rates by age and mask policy, September to December 2021
| Full mask (N = 3,849) | No mask (N = 1,365) |
P
valueb (permutation) |
|||||
|---|---|---|---|---|---|---|---|
| Model estimated seroprevalencec | Estimated population seroconversiona | Model estimated seroprevalence | Estimated population seroconversiona | ||||
| September | December | September | December | ||||
| Overall | 37.46% | 48.76% | 18.07% | 44.03% | 57.31% | 23.73% | <0.001 |
| 5–11 years | 41.15% | 43.81% | 4.51% | 49.67% | 52.87% | 6.37% | 0.002 |
| 12–17 years | 40.14% | 52.69% | 20.97% | 46.50% | 61.05% | 27.19% | <0.001 |
Estimated population seroconversion was calculated by formula (1). The proportion of seronegative children in September was calculated as one minus the seroprevalence.
Permutation test evaluated whether the difference of estimated population seroconversion between full and no mask groups is significant.
Robust Poisson regression was applied to estimate marginal means of seroprevalence by full and no mask groups in two time periods. The list of variables used in the model is described in Tables 4–6.
A robust Poisson regression analysis, which used data from the combined study periods (September 1–30, 2021 and December 15, 2021 to January 14, 2022) and adjusted for time period and individual, area-level, and ZCTA-level covariates showed that on average, children in no mask policy ZCTAs had significantly higher infection-induced seroprevalence compared with those in full mask policy ZCTAs [adjusted prevalence ratio (aPR) = overall: 1.18, 95% CI: 1.10–1.26; 5–11 year olds: 1.21, 1.10–1.32; 12–17 years olds: 1.16, 1.07–1.26 (Tables 4–6)]. Additionally, several covariates were significantly associated with seroprevalence. For example, a 10% point increase in county-level vaccination rate was associated with lower seroprevalence (aPR = 0.97, 95% CI: 0.95–0.99) and a 10% increase in county-level cumulative case incidence was associated with higher seroprevalence (aPR = 1.24, 95% CI:1.11–1.38) (Table 4). A permutation test adjusting for individual, area-level, and ZCTA-level covariates found significant differences in estimated population seroconversion (P value < 0.001) in these groups. An estimated 23.7% of the initially seronegative population in the no mask group seroconverted by the end-semester seroprevalence assessment; an estimated 18.1% seroconverted in the full mask group (Table 2).
Differences in estimated population seroconversion were also significantly different by mask policy in both age sub-groups studied. Among children aged 5–11 years, the estimated population seroconversion in the no mask group was 6.4%, whereas that in the full mask group was 4.5%; P = 0.002) (Table 2). Children and adolescents aged 12–17 years had an estimated population seroconversion of 27.2% in “no mask” ZCTAs compared with an estimated population seroconversion of 21.0% in “full mask” areas (P < 0.001) (Table 2). In the sensitivity analysis restricted to ZCTAs that were included at both seroprevalence assessment time points (n = 716) (Table 3), the difference in estimated population seroconversion between “no mask” ZCTAs completed to “full mask” areas was significant for the entire sample and children aged 5–11 years and not significant for the those who are 12–17 years old.
TABLE 3.
Permutation test results for sensitivity analysis
| Full mask (N = 2,673) | No mask (N = 841) |
P
valueb (permutation) |
|||||
|---|---|---|---|---|---|---|---|
| Model estimated seroprevalencec | Estimated population seroconversiona | Model estimated seroprevalence | Estimated population seroconversiona | ||||
| September | December | September | December | ||||
| Overall | 36.61% | 49.77% | 20.76% | 40.19% | 54.64% | 24.16% | 0.061 |
| 5–11 yrs | 38.02% | 45.02% | 11.29% | 43.89% | 51.97% | 14.39% | 0.082 |
| 12–17 yrs | 40.63% | 56.43% | 26.62% | 43.03% | 59.76% | 29.37% | 0.339 |
Estimated population seroconversion was calculated by formula (1). The proportion of seronegative kids in September was calculated as one minus the seroprevalence.
Permutation test evaluated whether the difference of estimated population seroconversion between full and no mask groups is significant.
Robust Poisson regression was applied to estimate marginal means of seroprevalence by full and no mask groups in two time periods. The list of variables used in the model is described in Tables 4 to 6.
TABLE 4.
Covariate-adjusted associations between school mask policy and SARS-CoV-2 infection-induced seroprevalence among individuals aged 5–17 years during the combined study periods (September 1–30, 2021 and December 15, 2021 to January 14, 2022)a
| Prevalence ratios (PR) | 95% CI | ||
|---|---|---|---|
| Mask policy: No vs Full | 1.18 | 1.10 | 1.26 |
| Time period: December vs September | 1.30 | 1.21 | 1.40 |
| Gender: Female vs Male | 1.01 | 0.95 | 1.07 |
| Region: Midwest vs Northeast | 1.22 | 1.11 | 1.33 |
| Region: South vs Northeast | 1.12 | 1.01 | 1.24 |
| Region: West vs Northeast | 0.98 | 0.88 | 1.09 |
| Race: NH Black vs NH White | 1.36 | 1.23 | 1.50 |
| Race: Hispanic vs NH White | 1.04 | 0.86 | 1.26 |
| Race: Hispanic and Black vs NH White | 1.35 | 1.20 | 1.51 |
| Race: Other vs NH White | 1.10 | 1.02 | 1.18 |
| Urbanicity: Non-urban vs Urban | 0.97 | 0.91 | 1.03 |
| Age: 12–17 years vs 5–11 years | 1.18 | 1.08 | 1.30 |
| Poverty Level: Above vs Below median | 1.02 | 0.95 | 1.09 |
| Education Level: High vs Low | 0.79 | 0.73 | 0.86 |
| County-level Vaccination Rate | 0.97 | 0.95 | 0.99 |
| County-level Cumulative Case Incidence | 1.24 | 1.11 | 1.38 |
Poisson regression models with robust variance estimation model were applied to estimate the association between school mask policy status and seroprevalence, adjusting for individual-level covariates (age and sex), area-level covariates (Census region, county-level urbanicity), ZCTA-level majority race/ethnicity, education and poverty-level, time-period, and the county-level vaccination rate and cumulative case incidence. All the covariates are categorical except vaccination rate and cumulative case incidence, which were included as continuous measures.
TABLE 5.
Covariate-adjusted associations between school mask policy and SARS-CoV-2 infection-induced seroprevalence among individuals aged 5–11 years during the combined study periods (September 1–30, 2021 and December 15, 2021 to January 14, 2022)a
| Prevalence ratios (PR) | 95% CI | ||
|---|---|---|---|
| Mask Policy: No vs Full | 1.21 | 1.10 | 1.32 |
| Time Period: December vs September | 1.06 | 0.93 | 1.22 |
| Gender: Female vs Male | 1.01 | 0.94 | 1.10 |
| Region: Midwest vs Northeast | 1.35 | 1.18 | 1.55 |
| Region: South vs Northeast | 1.24 | 1.07 | 1.43 |
| Region: West vs Northeast | 1.12 | 0.95 | 1.32 |
| Race: NH Black vs NH White | 1.38 | 1.19 | 1.60 |
| Race: Hispanic vs NH White | 0.88 | 0.67 | 1.16 |
| Race: Hispanic and Black vs NH White | 1.38 | 1.22 | 1.57 |
| Race: Other vs NH White | 1.01 | 0.91 | 1.13 |
| Urbanicity: Non-urban vs Urban | 0.99 | 0.90 | 1.08 |
| Poverty Level: Above vs Below median | 1.02 | 0.91 | 1.14 |
| Education Level: High vs Low | 0.77 | 0.68 | 0.88 |
| County-level Vaccination Rate | 1.09 | 1.03 | 1.15 |
| County-level Cumulative Case Incidence | 1.36 | 1.15 | 1.61 |
Poisson regression models with robust variance estimation model were applied to estimate the association between school mask policy status and seroprevalence, adjusting for individual-level covariates (age and sex), area-level covariates (Census region, county-level urbanicity), ZCTA-level majority race/ethnicity, education and poverty-level, time-period, and the county-level vaccination rate and cumulative case incidence. All the covariates are categorical except vaccination rate and cumulative case incidence, which were included as continuous measures.
TABLE 6.
Covariate-adjusted associations between school mask policy and SARS-CoV-2 infection-induced seroprevalence among individuals aged 12–17 years during the combined study periods (September 1–30, 2021 and December 15, 2021 to January 14, 2022)a
| Prevalence ratios (PR) | 95% CI | ||
|---|---|---|---|
| Mask Policy: No vs Full | 1.16 | 1.07 | 1.26 |
| Time Period: December vs September | 1.31 | 1.22 | 1.42 |
| Gender: Female vs Male | 1.01 | 0.95 | 1.07 |
| Region: Midwest vs Northeast | 1.14 | 1.04 | 1.26 |
| Region: South vs Northeast | 1.05 | 0.93 | 1.18 |
| Region: West vs Northeast | 0.90 | 0.82 | 0.99 |
| Race: NH Black vs NH White | 1.34 | 1.20 | 1.49 |
| Race: Hispanic vs NH White | 1.18 | 0.98 | 1.43 |
| Race: Hispanic and Black vs NH White | 1.26 | 1.07 | 1.47 |
| Race: Other vs NH White | 1.17 | 1.07 | 1.28 |
| Urbanicity: Non-urban vs Urban | 0.96 | 0.90 | 1.02 |
| Poverty Level: Above vs Below median | 1.04 | 0.96 | 1.12 |
| Education Level: High vs Low | 0.80 | 0.74 | 0.87 |
| County-level Vaccination Rate | 0.96 | 0.93 | 0.99 |
| County-level Cumulative Case Incidence | 1.24 | 1.09 | 1.41 |
Poisson regression models with robust variance estimation model were applied to estimate the association between school mask policy status and seroprevalence, adjusting for individual-level covariates (age and sex), area-level covariates (Census region, county-level urbanicity), ZCTA-level majority race/ethnicity, education and poverty-level, time-period, and the county-level vaccination rate and cumulative case incidence. All the covariates are categorical except vaccination rate and cumulative case incidence, which were included as continuous measures.
When compared with the U.S. population, our sample exhibited a lower percentage of white individuals and a more urban composition. Furthermore, our sample was concentrated in the Northeast and South and in counties that, on average, had higher proportions of individuals under the age of 18 compared with national data. Effect sizes generally indicated small effects, even where differences were significant among the large sample size. There was a moderate effect size for COVID vaccination rates, which were higher in the analytic data set than national data (Table S1).
DISCUSSION
During the Fall 2021 school semester in the United States, ZCTAs with consistent full mask policies for all students and staff had lower seroprevalence than ZCTAs with no school mask requirement at both the initial and end-semester time points. The observed associations are likely multi-factorial, including school mask policies, current and previous mitigation measures outside of the school context, and the level of community adherence to those measures and vaccination recommendations.
Our analysis examined population seroconversion in school-age children due to incident infection during the U.S. fall 2021 semester and found greater estimated population seroconversion in children from districts without mask requirements compared to those with mask mandates for students and staff. Significant estimated seroconversion differences were also found across both age groups. The robustness of these findings is supported by the consistent findings of sensitivity analyses for the overall population studied and both age groups in a subset of ZCTAs for which more complete data over time are available.
The results from multiple studies related to SARS-CoV-2 and mask adherence or policies in schools that relied on case report data or disease incidence data (5, 26–32) are consistent with our findings that areas with mask requirements, on average, had lower rates of previous SARS-CoV-2 infection and/or lower rates of incident infections compared with community incidence or schools with no mask requirement. Studies of the effects of mask policies using seroprevalence data are important because case reports and disease incidence data are known to underestimate the actual number of COVID-19 cases (10, 33–35), especially in children. One analysis revealed an estimated 2–3 undetected infections per reported case among all ages, but an estimated 5–9 undetected infections per reported case among the pediatric population of the same states (10). There is limited literature on the use of pediatric seroprevalence data to evaluate the effectiveness of mask policies. One previous study in Sweden was consistent with our finding of lower seroprevalence in students with mask wear requirements (36).
Although full mask policies were associated with less estimated population seroconversion among the infection-naïve school-age population in our study, mask policies are one among many factors influencing the extent of COVID-19 transmission among school-age children. A study in Belgium found a statistically significant association between the detection of SARS-CoV-2 antibodies and the implementation of mitigation strategies among students and staff members. Although the suite of mitigation measures in this study included a requirement that students wear masks, the effect of masks alone could not be conclusively determined, highlighting the importance of multifaceted mitigation strategies within the school context (37). Some studies suggest that in-school transmission is overall a lower risk factor for SARS-CoV-2 outbreaks in schools than community-related transmission (38–40). Overall, the use of masks as part of a layered approach, which includes other non-pharmaceutical interventions (i.e., handwashing and social distancing), is known to be more effective at reducing the transmission of infectious diseases. Therefore, it is advisable to reference public health recommendations at the local, state, and federal levels regarding not only mask usage but also other mitigation strategies.
Limitations
The findings in this study are subject to several limitations related to sampling, demographic and geographic adjustments, time periods, and factors for which consistent data were not available or could not be incorporated. First, our study used seroprevalence data from the NCLS, which only includes children with blood drawn during clinical visits and therefore may be more likely to include children with ready access to care and those with more frequent need for medical care. However, a previous analysis of patterns of pediatric seroprevalence within NCLS found that national estimates likely approximated seroprevalence in the U.S. pediatric population seeking well-child care (41). In addition, our geographical catchment area included only ZCTAs with the availability of both consistent school mask policy data and seroprevalence data among school-age children, which may limit generalizability. Finally, the use of non-longitudinal specimen samples during the two study periods (initial and final assessment) meant that the same children did not contribute samples at both time points; hence, population seroconversion was estimated rather than directly calculated.
Second, we could not adjust for person-level race and ethnicity, since these demographics were unavailable from commercial laboratory data. We mitigated this limitation by adjusting for county-level sociodemographic variables. Analytically, we could not adjust for all possible geographic clustering. Random effects were not included in the model because the likelihood ratio test showed no significant variation in outcome variables across ZCTA areas.
Third, some positive serology tests during the final seroprevalence assessment period could be from infections that occurred outside the school policy classification period, including during holiday gatherings or travel. However, the inclusion of school holidays in the final seroprevalence assessment period would tend to bias results toward the null, especially given the inclusion of community incidence in the model.
Fourth, some data points, not consistently available for all ZCTAs, were excluded from the analysis. This includes mask policies in private schools and the proportion of children attending them, potentially introducing bias if private and public schools differ in policy. We were also unable to include the proportion of area children who were home-schooled or electively participating in remote learning. The ecologic design of this study also did not allow us to account for other SARS-CoV-2 mitigation strategies in the community or the school, the rigor of enforcement of these measures, the level of adherence to school mask policies, or the number of individuals who may have chosen to wear masks in the school setting where there was not a requirement. Lack of data on these factors limits our ability to determine the extent to which school mask policies influenced SARS-CoV-2 transmission in the school setting. However, it is notable that both nonadherence to mask policies and voluntary mask wear in districts where it was not required would bias results toward the null. The significance of the findings despite several limitations that would bias toward the null is notable.
Finally, although the comparison of our analytic data set to the general U.S. population yielded significant results, it is crucial to acknowledge the limitations in generalizability due to the relatively small sample size. Therefore, these findings should be seen as indicative rather than definitive, emphasizing the need for further research to validate and expand upon our results.
Conclusion
During the U.S. Fall 2021 semester, blood specimens from children residing in ZCTAs with consistent full mask policies for all students and staff had lower seroprevalence than specimens in ZCTAs with no mask requirement, both initially and at the end of the semester. Additionally, an estimated lower proportion of initially seronegative children in these areas seroconverted over the semester, overall, and among both younger and older age groups. These findings contribute additional evidence that, as part of a layered strategy, masks can be an important intervention for mitigating the transmission of SARS-CoV-2 in schools (42). Mask wearing is one mitigation strategy among other priority prevention strategies, including promoting equitable access to vaccination, optimizing ventilation, physical and social distancing, staying home when sick, and enhancing hygiene practices (43). Future pediatric surveillance for respiratory infections would be enhanced by more complete records of mitigation policies in communities and schools, as well as by accounting for the limitations of surveillance based on routine clinical testing through robust seroprevalence data.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Kristie E. N. Clarke, Email: vhz9@cdc.gov.
Gabriel I. Parra, US Food and Drug Administration, Silver Spring, Maryland, USA
DATA AVAILABILITY
Federal regulations related to data privacy and re-identification of individuals based on data preclude posting of the raw data for this work. However, there are data sets that aggregate some data elements available on CDC Datahub.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.00691-24.
Fig. S1 and S2; Table S1.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. El-Shabasy RM, Nayel MA, Taher MM, Abdelmonem R, Shoueir KR, Kenawy ER. 2022. Three waves changes, new variant strains, and vaccination effect against COVID-19 pandemic. Int J Biol Macromol 204:161–168. doi: 10.1016/j.ijbiomac.2022.01.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boutzoukas AE, Zimmerman KO, Inkelas M, Brookhart MA, Benjamin DK, Butteris S, Koval S, DeMuri GP, Manuel VG, Smith MJ, McGann KA, Kalu IC, Weber DJ, Falk A, Shane AL, Schuster JE, Goldman JL, Hickerson J, Benjamin V, Edwards L, Erickson TR, Benjamin DK. 2022. School masking policies and secondary SARS-CoV-2 transmission. Pediatrics 149:e2022056687. doi: 10.1542/peds.2022-056687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leung NHL, Chu DKW, Shiu EYC, Chan K-H, McDevitt JJ, Hau BJP, Yen H-L, Li Y, Ip DKM, Peiris JSM, Seto W-H, Leung GM, Milton DK, Cowling BJ. 2020. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 26:676–680. doi: 10.1038/s41591-020-0843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ju JTJ, Boisvert LN, Zuo YY. 2021. Face masks against COVID-19: standards, efficacy, testing and decontamination methods. Adv Colloid Interface Sci 292:102435. doi: 10.1016/j.cis.2021.102435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falk A, Benda A, Falk P, Steffen S, Wallace Z, Høeg TB. 2021. COVID-19 cases and transmission in 17 K–12 schools — Wood County, Wisconsin, August 31–November 29, 2020. MMWR Morb Mortal Wkly Rep 70:136–140. doi: 10.15585/mmwr.mm7004e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lam-Hine T, McCurdy SA, Santora L, Duncan L, Corbett-Detig R, Kapusinszky B, Willis M. 2021. Outbreak associated with SARS-CoV-2 B.1.617.2 (Delta) variant in an elementary school — Marin County, California, May–June 2021. MMWR Morb Mortal Wkly Rep 70:1214–1219. doi: 10.15585/mmwr.mm7035e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jehn M, McCullough JM, Dale AP, Gue M, Eller B, Cullen T, Scott SE. 2021. Association between K–12 school mask policies and school-associated COVID-19 outbreaks — Maricopa and Pima Counties, Arizona, July–August 2021. MMWR Morb Mortal Wkly Rep 70:1372–1373. doi: 10.15585/mmwr.mm7039e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Budzyn SE, Panaggio MJ, Parks SE, Papazian M, Magid J, Eng M, Barrios LC. 2021. Pediatric COVID-19 cases in counties with and without school mask requirements — United States, July 1–September 4, 2021. MMWR Morb Mortal Wkly Rep 70:1377–1378. doi: 10.15585/mmwr.mm7039e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cowger TL, Murray EJ, Clarke J, Bassett MT, Ojikutu BO, Sánchez SM, Linos N, Hall KT. 2022. Lifting universal masking in schools — Covid-19 incidence among students and staff. N Engl J Med 387:1935–1946. doi: 10.1056/NEJMoa2211029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Couture A, Lyons BC, Mehrotra ML, Sosa L, Ezike N, Ahmed FS, Brown CM, Yendell S, Azzam IA, Katić BJ, Cope A, Dickerson K, Stone J, Traxler LB, Dunn JR, Davis LB, Reed C, Clarke KEN, Flannery B, Charles MD. 2022. Severe acute respiratory syndrome coronavirus 2 seroprevalence and reported coronavirus disease 2019 cases in US Children, August 2020–May 2021. Open Forum Infect Dis 9:ofac044. doi: 10.1093/ofid/ofac044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wiegand RE, Deng Y, Deng X, Lee A, Meyer WA, Letovsky S, Charles MD, Gundlapalli AV, MacNeil A, Hall AJ, Thornburg NJ, Jones J, Iachan R, Clarke KEN. 2023. Estimated SARS-CoV-2 antibody seroprevalence trends and relationship to reported case prevalence from a repeated, cross-sectional study in the 50 states and the District of Columbia, United States-October 25, 2020-February 26, 2022. Lancet Reg Health Am 18:100403. doi: 10.1016/j.lana.2022.100403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. MCH Strategic Data . 2022. COVID-19 IMPACT: school district operational status. Available from: https://www.mchdata.com/covid19/schoolclosings. Retrieved 30 Sep 2022.
- 13. Loesche M, Karlson EW, Talabi O, Zhou G, Boutin N, Atchley R, Loevinsohn G, Chang JBP, Hasdianda MA, Okenla A, Sampson E, Schram H, Magsipoc K, Goodman K, Donahue L, MacGowan M, Novack LA, Jarolim P, Baden LR, Nilles EJ. 2022. Longitudinal SARS-CoV-2 nucleocapsid antibody kinetics, seroreversion, and implications for seroepidemiologic studies. Emerg Infect Dis 28:1859–1862. doi: 10.3201/eid2809.220729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bajema KL, Wiegand RE, Cuffe K, Patel SV, Iachan R, Lim T, Lee A, Moyse D, Havers FP, Harding L, Fry AM, Hall AJ, Martin K, Biel M, Deng Y, Meyer WA, Mathur M, Kyle T, Gundlapalli AV, Thornburg NJ, Petersen LR, Edens C. 2021. Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med 181:450–460. doi: 10.1001/jamainternmed.2020.7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Food and Drug Administration . 2022. Roche diagnostics elecsys anti-SARS-CoV-2– IFU. Instructions for Use. Available from: fda.gov. Retrieved 30 Sep 2022.
- 16. U.S. Department of Agriculture Economic Research Service . 2013. Rural-urban Continuum codes. Available from: https://www.ers.usda.gov/data-products/rural-urban-continuum-codes. Retrieved 09 Aug 2018.
- 17. Iachan R, Berman L, Kyle TM, Martin KJ, Deng Y, Moyse DN, Middleton D, Atienza AA. 2019. Weighting nonprobability and probability sample surveys in describing cancer catchment areas. Cancer Epidemiol Biomarkers Prev 28:471–477. doi: 10.1158/1055-9965.EPI-18-0797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stijnen T, Van Houwelingen HC. 1993. Relative risk, risk difference and rate difference models for sparse stratified data: a pseudo likelihood approach. Stat Med 12:2285–2303. doi: 10.1002/sim.4780122406 [DOI] [PubMed] [Google Scholar]
- 19. Barros AJD, Hirakata VN. 2003. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 3:21. doi: 10.1186/1471-2288-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou G. 2004. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159:702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention . Total cumulative number of vaccinated people, percentage of vaccinated people. Available from: https://data.cdc.gov/Vaccinations/COVID-19-Vaccinations-in-the-United-States-County/8xkx-amqh
- 22. Centers for Disease Control and Prevention . United States COVID-19 county level of community transmission historical changes. Available from: https://data.cdc.gov/Public-Health-Surveillance/United-States-COVID-19-County-Level-of-Community-T/nra9-vzzn
- 23. Anand S, Montez-Rath M, Han J, Bozeman J, Kerschmann R, Beyer P, Parsonnet J, Chertow GM. 2020. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet 396:1335–1344. doi: 10.1016/S0140-6736(20)32009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brookmeyer R. 2010. Measuring the HIV/AIDS epidemic: approaches and challenges. Epidemiol Rev 32:26–37. doi: 10.1093/epirev/mxq002 [DOI] [PubMed] [Google Scholar]
- 25. Good PI. 2005. Permutation, parametric and bootstrap tests of hypotheses. 3rd ed. Springer. [Google Scholar]
- 26. Donovan CV, Rose C, Lewis KN, Vang K, Stanley N, Motley M, Brown CC, Gray FJ, Thompson JW, Amick BC, Williams ML, Thomas E, Neatherlin J, Zohoori N, Porter A, Cima M. 2022. SARS-CoV-2 incidence in K–12 school districts with mask-required versus mask-optional policies — Arkansas, August–October 2021. MMWR Morb Mortal Wkly Rep 71:384–389. doi: 10.15585/mmwr.mm7110e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boutzoukas AE, Zimmerman KO, Benjamin DK. 2021. School safety, masking, and the delta variant. Pediatrics 149:e2021054396. doi: 10.1542/peds.2021-054396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yan L, Talic S, Wild H, Gasevic D, Gasević D, Ilic D, Deppeler J, Corrigan D, Martinez-Maldonado R, Trauer J. 2022. Transmission of SARS-CoV-2 in a primary school setting with and without public health measures using real-world contact data: a modelling study. J Glob Health 12:05034. doi: 10.7189/jogh.12.05034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hershow RB, Wu K, Lewis NM, Milne AT, Currie D, Smith AR, Lloyd S, Orleans B, Young EL, Freeman B, et al. 2021. Low SARS-CoV-2 transmission in elementary schools — Salt Lake County, Utah, December 3, 2020–January 31, 2021. MMWR Morb Mortal Wkly Rep 70:442–448. doi: 10.15585/mmwr.mm7012e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dawson P, Worrell MC, Malone S, Tinker SC, Fritz S, Maricque B, Junaidi S, Purnell G, Lai AM, Neidich JA, et al. 2020. Pilot investigation of SARS-CoV-2 secondary transmission in Kindergarten through grade 12 schools implementing mitigation strategies — St. Louis County and City of Springfield, Missouri, December 2020. MMWR 70:449–455. doi: 10.15585/mmwr.mm7012e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sombetzki M, Lücker P, Ehmke M, Bock S, Littmann M, Reisinger EC, Hoffmann W, Kästner A. 2021. Impact of changes in infection control measures on the dynamics of COVID-19 infections in schools and pre-schools. Front Public Health 9:780039. doi: 10.3389/fpubh.2021.780039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gettings J, Czarnik M, Morris E, Haller E, Thompson-Paul AM, Rasberry C, Lanzieri TM, Smith-Grant J, Aholou TM, Thomas E, Drenzek C, MacKellar D. 2021. Mask use and ventilation improvements to reduce COVID-19 incidence in elementary schools — Georgia, November 16–December 11, 2020. MMWR Morb Mortal Wkly Rep 70:779–784. doi: 10.15585/mmwr.mm7021e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bergeri I, Whelan MG, Ware H, Subissi L, Nardone A, Lewis HC, Li Z, Ma X, Valenciano M, Cheng B, et al. 2022. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: a systematic review and meta-analysis of standardized population-based studies. PLoS Med 19:e1004107. doi: 10.1371/journal.pmed.1004107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clarke KEN, Jones JM, Deng Y, Nycz E, Lee A, Iachan R, Gundlapalli AV, Hall AJ, MacNeil A. 2022. Seroprevalence of infection-induced SARS-CoV-2 antibodies — United States, September 2021–February 2022. MMWR Morb Mortal Wkly Rep 71:606–608. doi: 10.15585/mmwr.mm7117e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Byambasuren O, Dobler CC, Bell K, Rojas DP, Clark J, McLaws ML, Glasziou P. 2021. Comparison of seroprevalence of SARS-CoV-2 infections with cumulative and imputed COVID-19 cases: systematic review. PLoS One 16:e0248946. doi: 10.1371/journal.pone.0248946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ulyte A, Radtke T, Abela IA, Haile SR, Ammann P, Berger C, Trkola A, Fehr J, Puhan MA, Kriemler S. 2021. Evolution of SARS-CoV-2 seroprevalence and clusters in school children from June 2020 to April 2021: prospective cohort study Ciao Corona. Swiss Med Wkly 151:w30092. doi: 10.4414/smw.2021.w30092 [DOI] [PubMed] [Google Scholar]
- 37. Callies M, Kabouche I, Desombere I, Merckx J, Roelants M, Vermeulen M, Duysburgh E. 2023. SARS-CoV-2 infection prevention and control measures in Belgian schools between December 2020 and June 2021 and their association with seroprevalence: a cross-sectional analysis of a prospective cohort study. BMC Public Health 23:898. doi: 10.1186/s12889-023-15806-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ismail SA, Saliba V, Lopez Bernal J, Ramsay ME, Ladhani SN. 2021. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis 21:344–353. doi: 10.1016/S1473-3099(20)30882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boey L, Roelants M, Merckx J, Hens N, Desombere I, Duysburgh E, Vandermeulen C. 2022. Age-dependent seroprevalence of SARS-CoV-2 antibodies in school-aged children from areas with low and high community transmission. Eur J Pediatr 181:571–578. doi: 10.1007/s00431-021-04222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Götzinger F, Strenger V. 2022. The role of children and young people in the transmission of SARS-CoV-2. Pediatr Infect Dis J 41:e172–e174. doi: 10.1097/INF.0000000000003497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clarke KEN, Kim Y, Jones J, Lee A, Deng Y, Nycz E, Iachan R, Gundlapalli AV, MacNeil A, Hall A. 2023. Pediatric infection-induced SARS-CoV-2 seroprevalence increases and seroprevalence by type of clinical care—September 2021 to February 2022. J Infect Dis 227:364–370. doi: 10.1093/infdis/jiac423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Centers for Disease Control and Prevention . 2023. Operational guidance for K-12 schools and early care and education programs to support safe in-person learning. Available from: https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/k-12-childcare-guidance.html#anchor_1648820793946. Retrieved 27 Sep 2023.
- 43. Centers for Disease Control and Prevention . United States COVID-19 how to protect yourself and others. Available from: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2; Table S1.
Data Availability Statement
Federal regulations related to data privacy and re-identification of individuals based on data preclude posting of the raw data for this work. However, there are data sets that aggregate some data elements available on CDC Datahub.