ABSTRACT
Malaria rapid diagnostic tests (RDTs), which detect Plasmodium falciparum (Pf)-specific histidine-rich protein-2 (HRP2), have increasing importance for the diagnosis and control of malaria, especially also in regions where routine diagnosis by microscopy is not available. HRP2-based RDTs have a similar sensitivity to expert microscopy, but their reported low specificity can lead to high false positivity rates, particularly in high-endemic areas. Despite the widespread use of RDTs, models investigating the dynamics of HRP2 clearance following Pf treatment focus rather on short-term clearance of the protein. The goal of this observational cohort study was to determine the long-term kinetic of HRP2-levels in peripheral blood after treatment of uncomplicated malaria cases with Pf mono-infection using a 3-day course of artesunate/amodiaquine. HRP2 levels were quantified at enrollment and on days 1, 2, 3, 5, 7, 12, 17, 22, and 28 post-treatment initiation. The findings reveal an unexpectedly prolonged clearance of HRP2 after parasite clearance from capillary blood. Terminal HRP2 half-life was estimated to be 9 days after parasite clearance using a pharmacokinetic two-compartmental elimination model. These results provide evidence that HRP2 clearance has generally been underestimated, as the antigen remains detectable in capillary blood for up to 28 days following successful treatment, influencing RDT-based assessment following a malaria treatment for weeks. A better understanding of the HRP2 clearance dynamics is critical for guiding the diagnosis of malaria when relying on RDTs.
IMPORTANCE
Detecting Plasmodium falciparum, the parasite responsible for the severest form of malaria, typically involves microscopy, polymerase chain reaction (PCR), or rapid diagnostic tests (RDTs) targeting the histidine-rich protein 2 or 3 (HRP2/3). While microscopy and PCR quickly turn negative after the infection is cleared, HRP2 remains detectable for a prolonged period. The exact duration of HRP2 persistence had not been well defined. Our study in Gabon tracked HRP2 levels over 4 weeks, resulting in a new model for antigen clearance. We discovered that a two-compartment model accurately predicts HRP2 levels, revealing an initial rapid reduction followed by a much slower elimination phase that can take several weeks. These findings are crucial for interpreting RDT results, as lingering HRP2 can lead to false positives, impacting malaria diagnosis and treatment decisions.
KEYWORDS: histidine-rich protein-2, malaria, Plasmodium falciparum, rapid diagnostic test, half-life, diagnosis
INTRODUCTION
Plasmodium falciparum (Pf) is still among the 10 most common leading causes of death in low-income countries, predominantly found in the Sub-Saharan region of Africa (1, 2). To this day, the main strategies in malaria control programs include insecticide-treated mosquito nets, indoor residual spraying, chemoprevention in pregnant women and children, increased use of diagnostic tests, prompt and proper treatment, and reduction in the ratio of treatments to tests (2). These interventions have resulted in a decline in malaria cases and deaths since the beginning of the century, but a plateau has been reached since about 2015, with a slight increase in disease burden since 2020 (2). Time to diagnosis is crucial for managing malaria and, therefore, an accurate diagnostic tool becomes essential. Giemsa-stained thick and thin blood smears have been the gold standard for parasite detection by microscopy for decades (3). However, in daily practice in hospitals in rural settings, this technique is not always performed well since it requires well-trained microscopists, continuous validation, and quality assurance. Otherwise, the sensitivity and specificity of this technique vary markedly between laboratories (3–5). As a result, delays may occur in initiating treatment, being especially crucial in critical cases, particularly in remote areas with limited resources (6). The development of malaria rapid diagnostic tests (RDTs) aimed to fill this gap, even though the WHO still recommends microscopic confirmation before initiating treatment (except for small children and pregnant women) (7, 8). Nevertheless, the widespread use of rapid diagnostic tests has contributed greatly in some countries to the paradigm shift of starting antimalarial treatment after obtaining a parasitological diagnosis by microscopy or rapid diagnostic tests (9).
The most common RDTs are based on the immobilization of parasite antigens by monoclonal antibodies fixed to an absorbent surface after lateral flow of a blood sample from individuals suspected of having malaria. RDTs are designed to detect one to three different antigens which are either Pf-specific (histidine-rich protein-2, HRP2) or pan-specific (pan malaria lactate dehydrogenase, pLDH, or pan malaria aldolase) (10). RDTs demand less expertise and are a time-saving alternative to microscopy.
Although RDTs in endemic areas may achieve a sensitivity similar to that of thick blood smear microscopy under optimal conditions [≥95% at ≥100 parasites per µL under routine conditions (11)], the sensitivity and specificity recommended by WHO are not always met under field conditions (5, 6, 12–15). For instance, the sensitivity of pLDH-based RDTs is often insufficient, while specificity is usually very high (>99.5%) (16, 17). Aldolase sensitivity and specificity are comparable to that of pLDH (9). The most sensitive and, therefore, most widely employed RDTs use P. falciparum HRP2 as the target antigen (5, 18). In addition, also new ultrasensitive HRP2-based RDTs have been developed, with even higher sensitivity (19, 20). The main drawback of HRP2-RDTs is their specificity (13). Based on these distinct detection capabilities, HRP2 and aldolase antigens were combined within the same RDT cassette to compensate for the specificity of one antigen with the sensitivity of the other (21).
One reason for the low specificity of HRP2-RDTs may be the persistence of the antigen in the circulation following treatment of P. falciparum malaria, as was already described in 2001 (22). This might explain the association between the specificity of the test and malaria endemicity (23, 24), which presumably contributes to the false-positive results seen in healthy individuals who have been successfully treated (12). Long-term detection of HRP2 has been proposed to be due to the persistence of sexual (25, 26) and asexual phases in the deep capillary beds (18, 27), but so far evidence is scarce. A more precise understanding of the half-life of the HRP2 parasite protein is needed due to the difficulty in determining whether antigen accumulation results from recurrent and recent infections or a constant low parasitemia only detectable by polymerase chain reaction (PCR) but not by microscopy. Reported half-lives to date were calculated from measurements taken in the first few days post-parasite treatment (24, 28, 29). However, several studies have reported positive HRP2-RDTs for more than 28 days after treatment (26, 30, 31).
In the study presented here, 27 participants with uncomplicated falciparum malaria were treated and followed up to determine the HRP2 decay over a period of 28 days (32). The false positivity rate of aldolase- and HRP2-RDTs relative to parasitemia detected by microscopy and qPCR was calculated. The half-life of HRP2 was assessed using repetitive quantitative measurements of HRP2 by ELISA, to generate a model for the decay of the parasitic protein.
MATERIALS AND METHODS
Study design
Subjects aged between 6 months and 25 years with fever in Lambaréné region (Gabon) between January and March 2009 were screened for eligibility to participate in the study. Participants were enrolled when they met the following inclusion criteria: (i) diagnosed uncomplicated Pf malaria (excluded mixed infection) and (ii) acute fever or history of fever in the last 48 hours.
Participants showing symptoms or signs of severe malaria [according to the WHO definition (7, 8)] or who had participated in any vaccine trial or had received any antimalarial in the last 28 days or who were suspected of having any other disease, were excluded and referred to the appropriate specialist if necessary. Any comorbidity was treated according to the guidelines.
Eligible participants received a 3-day oral treatment of artesunate/amodiaquine (Coarsucam, Sanofi-Aventis), namely at recruitment (day 0), day 1, and day 2 of follow-up. Since 2003, artesunate/amodiaquine has been a first-line treatment for children and adults with uncomplicated malaria caused by Pf in Gabon (33–35). The first dose of artesunate/amodiaquine was calculated according to age or weight and was administered after the first blood collection (day 0). Follow-up visits were conducted at home on days 3, 5, 7, 12, 17, 22, and 28 post-treatment initiation.
During the first 3 days of treatment and subsequent visits, 150 µL whole blood samples were collected in EDTA capillary tubes (KABE Labortechnik, Nümbrecht-Elsenroth, Germany). Capillary blood was stored at −20°C immediately after sampling and used to determine HRP2 levels and to determine parasitemia by qPCR.
Parasitemia
A Giemsa-stained thick blood smear (TBS) from the fingertip of each participant in each visit was read using the Lambaréné method (36) by two independent microscopists.
Briefly, a total of 20 microscopy fields were counted if parasitemia was ≥50 Pf parasites per field, 30 fields if parasitemia was 5–50 Pf parasites per field, and 100 fields if parasitemia was ≤5 Pf parasites per field. The slide was considered negative if no parasite was found in 100 fields. A third microscopist read the slide in case the results were divergent on positivity/negativity and when there was more than 25% discordance (lower value/upper value <0.75) in the results of asexual and sexual counts. If so, the mean parasitemia of the two closest parasite concentrations was used.
HRP2 enzyme-linked immunosorbent assay
For quantitative detection of HRP2, a sandwich ELISA was conducted as previously described with minor modifications (37). A 96-well high-binding flat-bottom microtiter plate (Microlon 600, highbinding, F-Boden, Greiner Bio-One GmbH, Frickenhausen, Germany) was coated with 1 µg/mL anti-PfHRP2 IgM antibody (Immunology Consultants Laboratories, Inc., Newberg, USA) in PBS by overnight incubation at 4°C followed by a blocking step using 2% BSA in PBS. All subsequent washing steps were done using PBS supplemented with 0.05% Tween-20. Hemolyzed whole blood samples were incubated at room temperature for 1 hour in three different dilutions: 1:50, 1:100, and 1:200. The standard curve was prepared by twofold serial dilutions from a complete culture of P. falciparum 2% parasitemia/5% hematocrit (1:4 to 1:4,096). All samples and standard curve dilutions were plated in duplicates. After 1-hour incubation at room temperature with 0.2 µg/mL anti-PfHRP2 IgG detection antibody (Immunology Consultants Laboratories, Inc., Newberg, USA), plates were developed with TMB chromogen (Zymed Lab., Inc., San Francisco, CA, USA) and stopped with 1 M sulfuric acid. Absorption was measured at 450 nm with an ELISA reader (Asys Expert 96, anthos Mikrosysteme GmbH, Krefeld, Germany). Results were expressed as arbitrary units (AU) relative to the amount of the undiluted parasite culture used as standard.
Plasmodium spp. antigen detection by rapid diagnostic test
EDTA capillary blood was used for RDTs according to the manufacturer’s instructions (Paracheck-Pf, Orchid Biomedical Systems, Verna, India, and BinaxNowMalaria, Binax Inc., Inverness Medical, Scarborough, Maine, USA). RDTs were performed at the same time as the TBS. When a participant obtained negative RDT results at two consecutive time points from day 0, the performance of any subsequent RDT was suspended. The test was considered invalid if the control band was not stained. The RDT was repeated twice in case the test result was invalid. BinaxNow consisted of three detection bands: (i) Control, (ii) PfHRP2, and (iii) Pan-Plasmodium spp. Aldolase while Paracheck-Pf presented only two bands (i) PfHRP2 and (ii) Control. Paracheck-Pf can only determine whether participants are infected with Pf, whereas BinaxNow detects species beyond Pf, as it has the dual ability to detect the pan-Plasmodium aldolase antigen (T2 band) together with HRP2 (T1 band).
DNA extraction and 18s ribosomal gene amplification by RT-qPCR
DNA extraction was performed according to the manufacturer’s instructions with a QIAamp DNA mini kit (Qiagen, Hilden, Germany). PhHV-1 was co-extracted with each sample acting as a DNA extraction control and PCR control.
Primers and probes for amplification of the 18S rRNA gene locus and the PhHV-1 control fragments are shown in Table S1. Plasmid isolations containing the small subunit (SSU) rRNA gene sequence from either P. falciparum, P. malariae, P. ovale, or P. vivax were used as Plasmodium spp. specific controls.
Optimization of primer concentration to limit interactions was implemented. The lowest primer concentration was selected at which the Ct obtained did not show a relevant increase compared to the Ct values of higher primer concentrations.
For all amplification reactions, the Rotor-Gene 6000 PCR thermocycler (Corbett, Australia) was used with a 25 µL volume containing 1× HotstarTaq master mix PCR buffer, 3.5 mM MgCl2, 2.5 µg BSA, varying amounts of primers (see below), 100 nM Taqman probe, and 5 µL DNA sample.
DNA was denatured for 15 min at 95°C followed by 50 cycles of 15 s at 95°C and 60 s at 60°C with fluorescence data acquisition at the 60°C step. The gain of the photomultiplier was manually adjusted to 8, 10, 10, and 8 for the green (FAM fluorophore), yellow (VIC), orange (ROX), and red (Cy5) channels, respectively.
Thresholds were set within the exponential phase of the amplification curves of the PhHV-1 as well as the plasmodial (SSU) rRNA genes and adjusted for internal positive controls. A triplicate dilution series of capillary blood from a patient with microscopically confirmed Pf malaria served for the standard curve generation.
If any of the species-specific positive controls were negative or there was an amplification signal from the negative controls in any of the parasite channels, the PCR result was excluded from data analysis, and sample preparation and PCR were repeated. Samples in which the Ct value of the PhHV-1 internal control differed by more than 2 Ct values from the median in each series were omitted from the analysis and reprocessed.
Genotyping
Genotyping was based on the amplification of MSP-1, MSP-2, and GLURP polymorphic genes in those patients who presented recurrent parasitemia after antimalarial treatment, as previously described (38, 39).
Two-compartment model
The two-compartment model is composed of one central compartment (circulating plasma levels) and a second compartment (which is usually organs or peripheral tissues) in which the compound can accumulate. The system seeks an equilibrium between the two compartments regulated by constants k12, which controls the distribution of the drug to the second compartment, and a constant k21 when distribution occurs between the second and the central compartment. When there is an equilibrium between the second and the central compartment, elimination (kel) follows a first-order process. This constitutes the terminal plasma half-life, defined as the time required to reduce the plasma concentration by half after reaching a pseudo-equilibrium. Before this happens, the half-life can be misleading. This elimination model is usually applied to intravenous drugs (Fig. S1a).
The two-compartment elimination model becomes clear when a straight line (Fig. S1b and c) is plotted for the last time points on a semilogarithmic plot of the logarithm of HRP2 values (y-axis) to days (x-axis). The slope of the generated line (β) is needed to calculate what is called the terminal half-life of the protein, by using the formula:
| (1) |
The subtraction of the actual HRP2 values during the first time points and the values calculated from the freshly generated regression line (purple line, Fig. S1c and d) for the same days generate the values called “residuals.”
| (2) |
These values represent an elimination closer to reality during the initial elimination phase before equilibrium is reached in the organism. When a linear regression is calculated from these values (Fig. S1d and e), a new slope (α) is generated which helps to calculate the initial extrapolated half-life with the formula:
| (3) |
By using α and β in conjunction with the intercept on the y-axis of each regression line, the constants k21, k12, and kel can be determined through the following equations:
| (4) |
| (5) |
| (6) |
Statistics
Shapiro-Wilk test and Kolmogorov-Smirnov test were used to test for normal or log-normal distribution of the continuous data variables. All correlations were conducted using Spearman’s rank order test. The result was considered statistically significant when the P-value was below 0.05 and the absolute value of the correlation coefficient was equal to or higher than 0.5.
RESULTS
Study development
A total of 27 participants presenting with Pf mono-infection (determined microscopically and subsequently confirmed by 18S rRNA qPCR) were enrolled in the present study. All participants experienced at least fever as a symptom of malaria in the 48 hours prior to inclusion but did not show any symptoms related to severe malaria (Fig. S2a; Table S2). All but one study participant completed the follow-up period. The guardian of one child withdrew consent on day 22 but agreed to the use of the data already collected.
The median parasitemia measured by TBS at inclusion was 33,435 parasites/µL (range 449–508,200 parasites/µL; Fig. 1; Fig. S3). In the 24 hours post-administration of the first dose of treatment (day 1), nine participants became TBS negative while the median parasitemia decreased to 145 parasites/µL (range 6–35,220 parasites/µL) in those who remained positive. On day 2, only 2 out of 27 (7%) participants remained positive. Both had low parasite counts (18 and 36 parasites/µL). A higher initial parasitemia at inclusion was found in participants who still presented circulating parasites on day 1 than in those who cleared parasites 24 hours post-drug administration (median parasites/µL 71,118 and 8,525, respectively; Wilcoxon test P < 0.01).
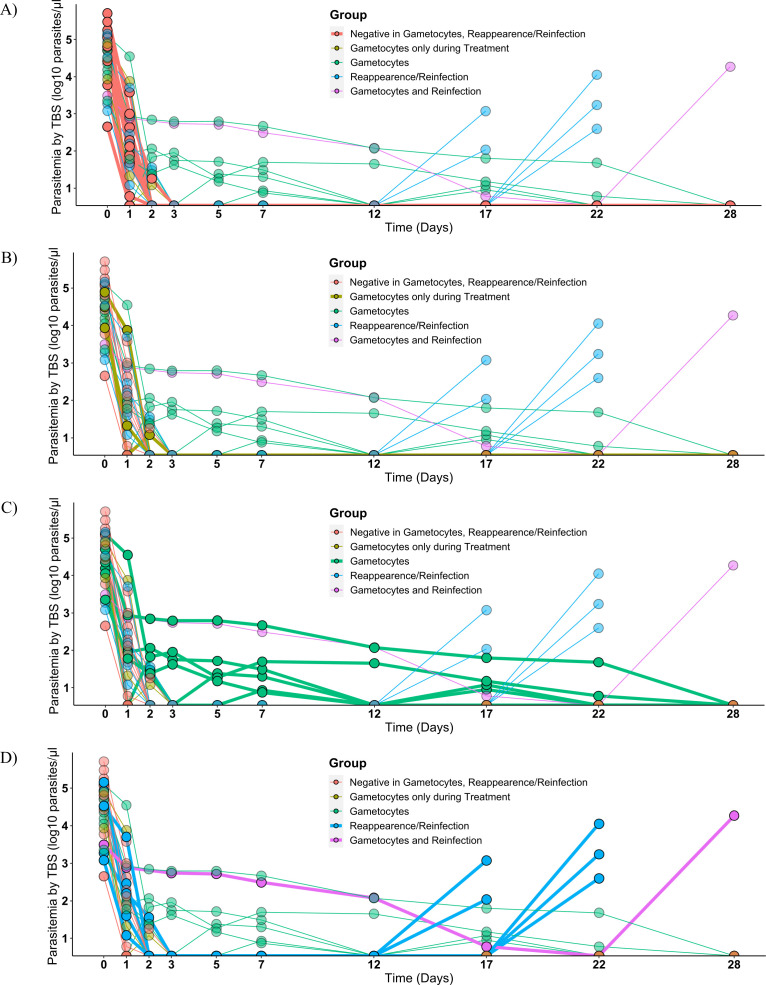
Fig 1.
Parasite clearance from first artesunate/amodiaquine administration. Parasitemia was estimated for 28 days by microscopy after drug administration (artesunate/amodiaquine) at day 0. The kinetics of the parasitemia is illustrated, with thicker lines highlighting the different groups, as estimated by TBS for the full follow-up period for participants not having gametocytes or reappearance/reinfections (A), those with only gametocytes during the treatment phase (B), gametocytes carriers during several time points after treatment, (C) and the participants that suffered reinfections and reappearances (D). Of note, one patient experienced a reappearance on day 22, five participants experienced reinfection, and from those, one also presented circulating gametocytes. In total, 10 participants showed circulating gametocytes during the trial, of those, three during the treatment phase only.
Following the completion of treatment on day 2, participants underwent follow-up on days 3, 5, 7, 12, 17, 22, and 28 after inclusion (Fig. S2b). Among the cohort of 27 participants, a subset of six individuals (6/27, 22%) exhibited reemergence of asexual parasites under microscopic examination after a span exceeding 12 days (see Fig. 1D). Specifically, this resurgence occurred in two patients on day 17, on day 22 in three patients, and day 28 in one patient. Five of these cases were classified as reinfections, while one was identified as a recrudescence of the initial infection, as evidenced by amplification-based genotyping (data not shown). This indicates a treatment failure rate of 1 out of 27 participants (4%).
Notably, the recrudescence event was observed on day 22.
Ten participants presented microscopically detectable gametocytes during follow-up, of which three showed detectable circulating sexual stages (9–21 parasites/µL) only during the treatment phase (Fig. 1B and C; Fig. S4a). The median gametocytemia across all patients during follow-up was 46 parasites/µL (range 6–1,890 gametocytes/µL) detected for up to 23 days (mean 12 days; 95% CI: 6–18).
During the first 3 days of treatment, the decreases in parasitemia measured by qPCR and microscopy were very similar. The applied qPCR approach is unable to discriminate between the asexual and sexual stages of the parasite. Thus, if all gametocyte carriers were excluded the parasitemia decreased from 32,461 parasites/µL on day 0 to 457 parasites/µL on day 1 and 45 parasites/µL on day 2 (Fig. S4b). The two participants with the lowest parasitemia in qPCR (157–197 parasites/µL) at inclusion had undetectable parasitemia from day 1 onwards. All participants were determined to be free of asexual parasites by qPCR beyond day 12, with the only exception being that gametocyte carriers after completion of treatment were still positive past this point as were those who showed reappearance/reinfections (Fig. S4b). The participants who experienced reinfections or recrudescence during the trial period were confirmed by qPCR (Fig. S4b).
HRP2
Circulating HRP2 in whole blood was quantified by ELISA. The baseline HRP2 level at inclusion for all participants was 2.48 AU (95% CI: 1.38–4.44). Initial parasitemia showed a positive correlation with HRP2 levels (Spearman P < 0.01, correlation coefficient 0.6) at day 0. The participants who presented the highest parasitemia at inclusion had early HRP2 levels above the upper limit of detection of the ELISA method. The two participants with the lowest parasitemia were below the lower limit of detection (0.01 AU) as early as day 1, rendering later half-life calculations for them impossible. Samples from two additional participants were not available to perform HRP2 ELISA. However, both participants showed gametocytes at several points following treatment. No association was found between the presence of gametocytes either at the beginning or during the study and the initial among of HRP2 (data not shown). Gametocytes could still be detected in four participants after day 12, although the HRP2 ELISA remained below the lower detection limit. Participants experiencing reinfections or recrudescence showed an identifiable increase in HRP2 levels when TBS became positive (2.3 AU 95% CI: 0.5–4.1).
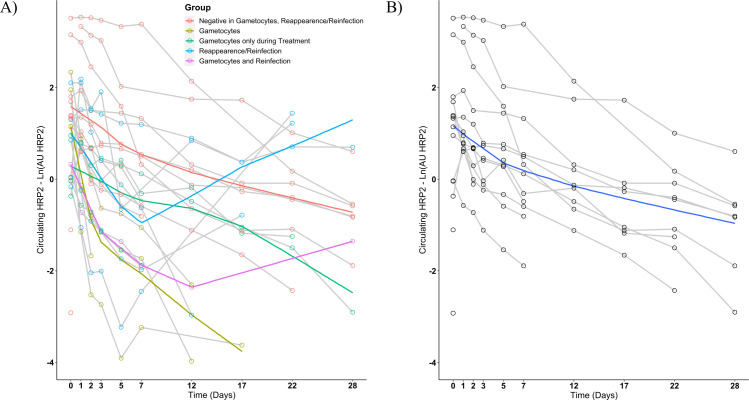
Circulating HRP2 concentration decreased more slowly during the study than parasitemia regardless of parasites being determined by microscopy or qPCR (Fig. 2). This was not explained by the cases of recrudescence or reinfections nor by the presence of gametocytes. On day 28, there were still seven positives for HRP2 that did not belong to the gametocyte-positive group and did not have recrudescence or new infections. Initial parasitemia was highly correlated with the time until a negative HRP2-ELISA test was obtained (Spearman P < 0.01, correlation coefficient 0.6) and was associated with the levels of HRP2 for each timepoint (Spearman P < 0.01, correlation coefficient D1 0.9; D2 0.9; D3 0.8; D5 0.8; D7 0.6; D12 0.8; D17 0.6; D22 0.6; and D28 0.6). One day after the start of treatment, seven participants experienced an increase in HRP2 levels despite the decrease of parasitemia in the peripheral blood as shown by TBS.
Fig 2.
HRP2 concentration during the follow-up period. HRP2 concentration in the peripheral blood is represented as a semi-logarithmic graph for the period from d0 to d28 (N = 25) including all participants (A) or excluding those with either Plasmodium spp. reappearances or reinfections and those with prolonged gametocytemia after treatment (B). Each line represents the kinetic for the HRP2 measure of a study participant. The colored lines in graph (A) represent the lowess (locally weighted scatterplot smoothing) regression for the different groups reflected on the legend. The blue line in graph (B) represents a lowess regression of the presented data set.
Half-life HRP2
Despite the steep decline in circulating parasites during the first 2–3 days, HRP2 levels only decrease slightly during this time (Fig. 2). However, there is a clear initial HRP2 curve that constitutes an initial clearance. Once parasites are no longer detected, HRP2 concentration should only increase in the case of reinfections or recrudescence. Therefore, to avoid interferences, participants undergoing new infections or recrudescence were excluded from the analysis together with those showing gametocytes from day 3 to day 28. The participants who had gametocytemia only during the treatment phase were not excluded from further analysis, as gametocytemia was low and no gametocytes were detected again.
For six participants with lower parasitemia at inclusion, HRP2 levels were below the limit of detection already by day 12 of the study, reducing the information on the long-term behavior of the protein. Thus, the remaining nine participants were selected for the terminal half-life calculation (Fig. S5).
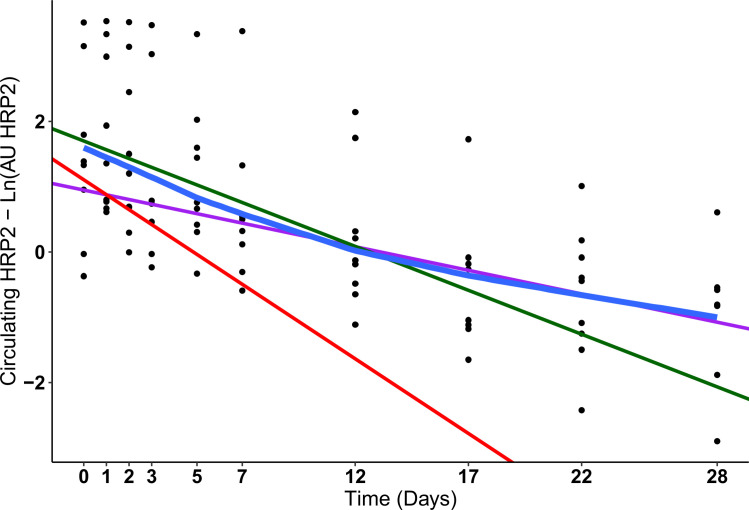
From day 12, HRP2 elimination follows a first-order elimination, since the blood-concentration–time profile is linear if plotted on a semi-logarithmic plot (Fig. 3). A linear regression analysis, represented by the purple line, was performed using the HRP2 measurements from the last four collection time points. The slope (β) derived from this regression was employed to compute the terminal half-life (Formula 1).
Fig 3.
Short- and Long-term decay of HRP2. The concentration of HRP2 in the capillary blood of patients without gametocytes after treatment, no recrudescence or reinfection, and detectable HRP2 levels for more than 7 days post-treatment initiation were selected for the generation of the long- and short-term models (N = 9). The blue line represents a lowess regression (locally weighted scatterplot smoothing). The purple line is the linear regression from the HRP2 values at day 12, day 17, day 22, and day 28 that shows the terminal elimination. The green line is the linear regression from the HRP2 values on day 0, day 1, day 2, day 3, day 5, and day 7 showing a mix of the initial parasite elimination and the terminal elimination. The red line represents the corrected initial HRP2 elimination after subtracting the contribution of the terminal elimination.
The difference between the actual values of HRP2 during the treatment phase and the values of the regression line (purple line) at those time points generate the values termed “residuals” (Formula 2).
The linear regression slope for the residual values (red line) serves to calculate the extrapolated initial half-life during the first week, which corresponds to the initial elimination phase (Formula 3).
Hence, the mean terminal half-life (slope β) for the nine participants was 9 days (95% CI: 6–12 days) and the mean extrapolated initial half-life (slope α residuals) was calculated to be 2 days (95% CI: −1 to 6 days).
Using α and β in conjunction with the intercept on the y-axis of both regression lines allowed for the determination of the constants k21, k12, and kel (Formulas 4, 5, and 6 and Fig. S1a). The resulting constant values are kel 0.23 days−1 (5.46 hours−1), k12 0.17 days−1 (4.12 hours−1), and k21 0.22 days−1 (5.27 hours−1).
HRP2-based rapid diagnostic test
Two RDTs were used for the study. Before treatment initiation, all participants showed a positive RDT for HRP2 (both RDTs), and 23 out of 27 participants were positive for Aldolase (BinaxNow T2 band). The four negative participants were among those with low parasitemia at inclusion (<6,000 parasites/µL).
Considering the complete course of the follow-up period of the study, in all measurements during the study, when aldolase (BinaxNow T2 band) was positive, HRP2 (BinaxNow T1 band and Paracheck-Pf RDT) was also positive, but never the other way around.
HRP2 ELISA results were compared with HRP2 band positivity in both RDTs. It was observed throughout the study that, with the exception of one participant, when ELISA test results crossed the lower limit of detection of the assay (at a blood dilution of 1:50 which was used for the ELISA method due to high background if used otherwise), the rapid diagnostic tests could remain positive for one or two more time points (data not shown). However, this exceptional participant, who belonged to the gametocyte group, showed a positive HRP2 ELISA on day 17, although on that day the band for both RDTs was negative and the BinaxNow T1 band had been negative since day 7.
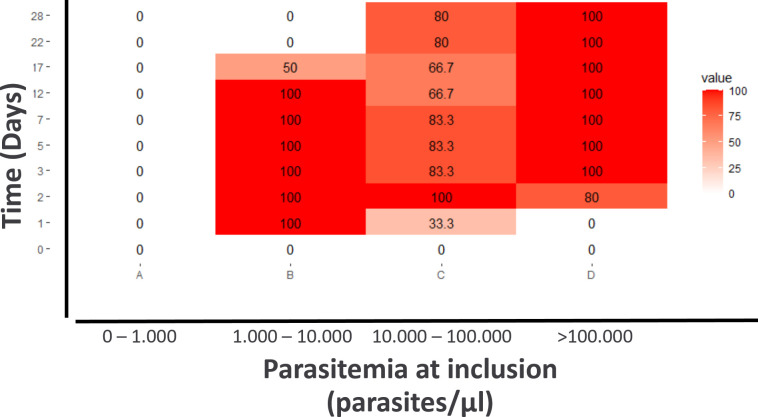
Obviously, the asexual stage parasites disappeared as determined by TBS at day 5 at the latest, whereas HRP2 levels remained positive for a long time period. The false-positive rate (FP) for HRP2-RDT results during follow-up was stratified according to groups with varying levels of thick blood smear (TBS) parasitemia prior to treatment initiation. (Fig. 4; Fig. S6). Gametocyte carriers after treatment and reappearance/reinfections were excluded from this estimation. As defined in the study inclusion criteria, participants at inclusion showed 0% FP. For participants exhibiting low parasitemia upon inclusion (<1,000 parasites per µL), the FP rate remained at 0% throughout the study period. This observation was made as these individuals attained parasite clearance, leading to consistently negative results on RDTs. Conversely, participants with higher levels of parasitemia upon inclusion (>100,000 parasites per µL) exhibited a prolonged period of 100% FP rate after parasite clearance from circulation. Consistent with the results of HRP2 ELISA, persistent long-term HRP2 positivity in RDTs was highly correlated with initial parasitemia (Paracheck-Pf RDT Spearman P < 0.001, correlation coefficient 0.6; BinaxNow T2 band Spearman P = 0.001, correlation coefficient 0.6).
Fig 4.
FP rate of the HRP2-RDT Paracheck-Pf for each time point stratified by groupings of participants who showed different ranges of initial parasitemia by TBS: (I) <1,000 parasites/µL (ii); 1,000 to 10,000 parasites/µL; (iii) 10,000 to 100,000 parasites/µL; and (iv) >100,000 parasites/µL. FP is expressed in percentage. A result of 100% FP represents a positive HRP2-RDT result while parasites are not detectable anymore. However, a 0% FP can have two interpretations, either there are detectable parasites for all participants within the group, resulting in a positive RDT test, or there are no detectable parasites in all participants in the group and the RDT test shows a negative result.
DISCUSSION
The work outlined here shows for the first time quantitative circulating HRP2 levels for 28 days after treatment of Pf mono-infections in humans from a hyper-endemic area. In our work, we propose a two-compartmental elimination model to describe the kinetics of the protein in the peripheral blood, which shows an elimination slope coincident with parasite elimination during the first 3 days and a subsequent slower terminal elimination slope until day 28.
The data suggest that the HRP2 persists in circulation long after parasite clearance, confirming previously published data (22, 26, 31, 40, 41). In acute malaria infections, the parasitemia at treatment initiation reflects the prolonged HRP2 clearance (42–46). According to reported cases, HRP2 can remain detectable for at least 7 days (45, 47) and even 32 days (44) after a successful treatment. In this study, seven participants who had no recognizable gametocytes in their blood after treatment and also had neither reinfection nor recrudescence were still positive on day 28 for HRP2. Their HRP2 values correlated well with the initial parasitemia.
The plasma half-life previously described by others is 3.67 days and the half-life in whole blood is 3–5 days, which takes only a constant decay of HRP2 into account (24, 28, 29, 48). These estimates do not adequately address the reported false positivity rate of HRP2-based RDTs long after treatment. However, the terminal elimination half-life using a two-compartment model seems better suited to explain the long positivity of RDTs after cured parasitemia. Thus, the half-lives previously calculated by others might have been underestimated (22, 24, 26, 28, 29, 41, 49).
The half-life of the terminal phase is the most frequently reported parameter in pharmacokinetics when determining the appropriate dose in repetitive administrations, considering the accumulation of the drug (50). The clearance and distribution of HRP2 in the body are maintained in equilibrium between the HRP2 in plasma and HRP2 in a second body compartment. The second compartment could be explained based on recent observations that a high number of HRP2-containing red blood cells remain in the bloodstream after treatment (40). The antigen expression of these initially infected erythrocytes is perpetuated after the process of erythrocyte pitting in the spleen, in which parasites are removed from the RBC, and these once-infected RBCs return to circulation still containing HRP2 in the cytoplasm. Thus, this can be attributed, in part, to the slower clearance of HRP2 during the second phase of elimination from the erythrocyte fraction of whole blood. This might explain a high false positivity rate from blood samples once the parasites are cleared.
Interestingly, the concentration of HRP2 in the peripheral blood increased slightly after treatment. Parasitemia estimation using microscopy or qPCR only considers the non-sequestered parasites. The increase in HRP2 on the following day after treatment initiation in seven participants might be coming from previously sequestered, dying parasites, which release HRP2 into the plasma, but remain partially engulfed in pitted cells (51, 52). Assuming some of the parasites were sequestered, when treatment was initiated they were eliminated by direct parasite killing with the effect of inhibition of parasitic replication and transcription activity, as well as pitting, thus creating a sudden increase in hollow previously infected erythrocytes still containing HRP2. Interestingly, it has recently been reported that pitting is more likely to occur when artemisinin-based combination therapies are used, as we did in our study (53). Future models should incorporate the spleen’s clearance effect under different therapies to provide a more accurate representation of HRP2 kinetics, for example, by estimating the cell number of pitted erythrocytes by flow cytometry and differentially quantifying the protein both in the serum and in the erythrocytes separately.
In cases of severe malarial infection, circulating HRP2 levels have previously been stressed to represent the sequestered P. falciparum mass (54). Data collected from a previous study in Lambaréné (55) reflected that higher amounts of HRP2 were detected in children suffering from severe malarial anemia (data not shown), a complication of the disease associated in the literature with a “chronic” infection state (56). This strongly supports the hypothesis that HRP2 accumulates over time in these patients with prolonged parasitemia or repetitive infections. Thus, HRP2 levels can be regarded as a marker for cumulative parasitemia over time.
Artesunate/amodiaquine remains one of the first line of treatment in children with acute malaria in Gabon (57–59). Artesunate acts in the early stages (60) and amodiaquine is a potent schizonticide (61) and the combination has an effect on gametocytes (62, 63). As in our study, a 3-day course treatment with this combination leads to microscopically undetectable asexual parasites and treatment failure is lower than other artemisinin combination therapies (ACTs). Lambaréné is a meso- to hyper-endemic region where malaria is perennial and reinfections are usual. Due to the small study sample size, the only single treatment failure (after genotyping (38) correction) conferred a slightly higher proportion of treatment failure compared to previous reports (64–67) under the same posology. Data from Gabon gathered by others show amodiaquine resistance of 28.2% (68–70).
HRP2 has been linked to hemoglobin metabolism as a method for the parasite to digest hemoglobin as a resource for protein synthesis (18). However, late-stage gametocytes do not consume hemoglobin, and it has been suggested that especially late stages might lack HRP2 expression (25, 71). Hanssen et al. (72) showed that hemoglobin digestion stops at gametocyte stage IV as hemoglobin crystals do not change in size (72). Despite other possible factors, a correlation of HRP2 antigenemia during convalescence has previously been shown in patients presenting gametocyte-positive films (26). Circulating gametocytes influencing HRP2-RDTs results has not been well investigated to date.
Sub-detectable parasitemia has been suggested to contribute to positive RDTs (73). A model estimated that about 8 parasites/μL were necessary to maintain a positive RDT in chronic infection (45). Artemisinin dormancy phenomenon may play a role in recrudescence cases which may contribute to maintaining certain levels of HRP2 detectable by especially ultrasensitive HRP2-based RDTs (20, 74). However, evidence for the possible influence of long-term parasitemia below the usual detection threshold on HRP2-RDT positivity is scarce. Based on HRP2 levels prior to treatment and those seen at reinfection/reappearance, HRP2 accumulation reaches significant levels over several cycles of asexual growth. Thus, sequestration alone might not be the cause of HRP2 elevation. As can be intuitively seen from severe malarial anemia, repetitive or prolonged infection could also be necessary to reach elevated levels of HRP2 accumulation in the body. Most research on HRP2 kinetics has been conducted on mild malaria cases, and severe cases have not been extensively investigated until today.
One limitation of the study was to use only whole blood samples and not additional plasma. However, ELISA HRP2 and RDT results are congruent with each other. Whole blood is the sample of choice for RDTs. In our study, RDTs detected HRP2 slightly better than ELISA, as some RDTs were still positive when ELISA results were below the detection limit. The sample size for the half-life calculation was also limited, as some participants had gametocytes, recrudescence/reappearances, and the detection limit of the ELISA hampered the HRP2 measurements in some participants.
Conclusions
The HRP2 burden in the non-severe malaria cases in the present study represents well the circulating parasites before treatment. However, as reflected by the terminal half-life calculated here, HRP2 may persist for a long time in the body and, under frequent, recurrent, prolonged, and/or high parasitemia without efficient treatment, may accumulate. This might contribute to reducing the specificity of the HRP2-RDTs, which remains a concern, especially in endemic areas where children are vulnerable to malaria and other infections.
Before refining the design and calibration of RDTs to increase their sensitivity, a more precise description of the kinetics of HRP2 elimination is indispensable. This can provide valuable insights into the diagnosis and management of malaria, especially in terms of therapeutic strategies.
Thus, each case should be evaluated based on the patient’s history before proceeding with further measures. Understanding the kinetics of HRP2 clearance holds significant implications for interpreting RDT outcomes and malaria surveillance efforts.
ACKNOWLEDGMENTS
We acknowledge support from the Open Access Publication Fund of the University of Tübingen.
We would like to thank all the participants and their families who contributed to advancing science with their samples by participating in these studies. We would also like to thank all the people working at the "Centre de Recherches Médicales de Lambaréné" (Lambaréné, Gabon) for their efforts in making all this possible.
Part of the work was supported by the DFG (DFG MO 1071/4-1) from the Deutsche Forschungsgemeinschaft (http://www.dfg.de/). The funders had no role in study design, data collection and analysis, publishing decisions, or manuscript drafting.
Conceptualization, R.F., C.L.C., F.S., P.M.S., B.M., and T.R.; methodology, R.F., C.L., S.B., J.B., T.R., and AM.N.M.; software, C.L.; validation, T.R. and R.F.; formal analysis, P.M.S. and C.L.C.; investigation, R.F. and F.S.; resources, ITM AND CERMEL; data curation, C.L.C.; writing—original draft preparation, C.L.C.; writing—review and editing, R.F. and C.L.C.; visualization, C.L.C. and P.M.S.; supervision, R.F. and P.G.K.; project administration, F.S.; funding acquisition, B.M. All authors have read and agreed to the published version of the manuscript.
The present paper analyzes data on the in vivo kinetics of Plasmodium falciparum HRP2, which were obtained in the study "Time-dependent decrease of Plasmodium falciparum assessed by thick smear, rapid test, and real-time PCR after Artemisinin Combination Therapy (ACT)." Prior to the start of the study, the study protocol and related documents (information sheet, informed consent form, etc.) were reviewed and authorized by the responsible Regional Independent Ethics Committee of Lambaréné (CERIL = Comité d’Ethique Régional Indépendant de Lambaréné). The study was conducted according to the study protocol and in accordance with the guiding principles of the Declaration of Helsinki and Good Clinical Practice (GCP).
A written informed consent was obtained from all participants or their legal guardians if the participant was underage before enrolment. In addition to written informed consent by the legal tutors, assent was sought where possible. Patients and/or legal guardians had the opportunity to withdraw the participant's consent at any time during the study without expecting any negative consequences.
The authors declare no conflict of interest. The funders had no role in the study design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
AFTER EPUB
This article was published on 28 August 2024 with an error in the affiliations. The affiliations were corrected in the current version, posted on 10 September 2024.
Contributor Information
Rolf Fendel, Email: rolf.fendel@uni-tuebingen.de.
Jian Li, Hubei University of Medicine, Shiyan, Hubei Province, China.
DATA AVAILABILITY
The data supporting this study may be made available to the public upon reasonable request through the corresponding author (rolf.fendel@uni-tuebingen.de).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.00994-24.
Fig. S1 to S6; Tables S1 and S2.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Mathers CD, Loncar D. 2006. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3:e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . 2023. World malaria report 2023. Geneva, Switzerland: World Health Organization [Google Scholar]
- 3. Jonkman A, Chibwe RA, Khoromana CO, Liabunya UL, Chaponda ME, Kandiero GE, Molyneux ME, Taylor TE. 1995. Cost-saving through microscopy-based versus presumptive diagnosis of malaria in adult outpatients in Malawi. Bull World Health Organ 73:223–227. [PMC free article] [PubMed] [Google Scholar]
- 4. Cunningham J, Jones S, Gatton ML, Barnwell JW, Cheng Q, Chiodini PL, Glenn J, Incardona S, Kosack C, Luchavez J, Menard D, Nhem S, Oyibo W, Rees-Channer RR, Gonzalez I, Bell D. 2019. A review of the WHO malaria rapid diagnostic test product testing programme (2008-2018): performance, procurement and policy. Malar J 18:387. doi: 10.1186/s12936-019-3028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. 2007. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg 77:119–127. doi: 10.4269/ajtmh.2007.77.119 [DOI] [PubMed] [Google Scholar]
- 6. Moody A. 2002. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev 15:66–78. doi: 10.1128/CMR.15.1.66-78.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . 2015. Guidelines for the treatment of malaria. 3rd ed. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 8. World Health Organization . 2023. WHO guidelines for malaria
- 9. Kavanaugh MJ, Azzam SE, Rockabrand DM. 2021. Malaria rapid diagnostic tests: literary review and recommendation for a quality assurance, quality control algorithm. Diagn (Basel) 11:768. doi: 10.3390/diagnostics11050768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perkins MD, Bell DR. 2008. Working without a blindfold: the critical role of diagnostics in malaria control. Malar J 7 Suppl 1:S5. doi: 10.1186/1475-2875-7-S1-S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joanny F, Löhr SJZ, Engleitner T, Lell B, Mordmüller B. 2014. Limit of blank and limit of detection of Plasmodium falciparum thick blood smear microscopy in a routine setting in Central Africa. Malar J 13:234. doi: 10.1186/1475-2875-13-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bell D, Peeling RW, WHO-Regional Office for the Western Pacific/TDR . 2006. Evaluation of rapid diagnostic tests: malaria. Nat Rev Microbiol 4:S34–8. doi: 10.1038/nrmicro1524 [DOI] [PubMed] [Google Scholar]
- 13. Bell DR, Wilson DW, Martin LB. 2005. False-positive results of a Plasmodium falciparum histidine-rich protein 2-detecting malaria rapid diagnostic test due to high sensitivity in a community with fluctuating low parasite density. Am J Trop Med Hyg 73:199–203. doi: 10.4269/ajtmh.2005.73.199 [DOI] [PubMed] [Google Scholar]
- 14. Mboera LEG, Fanello CI, Malima RC, Talbert A, Fogliati P, Bobbio F, Molteni F. 2006. Comparison of the Paracheck-Pf test with microscopy, for the confirmation of Plasmodium falciparum malaria in Tanzania. Ann Trop Med Parasitol 100:115–122. doi: 10.1179/136485906X78571 [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization . 2009. Methods for field trials of malaria rapid diagnostic tests. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 16. Jang IK, Tyler A, Lyman C, Rek JC, Arinaitwe E, Adrama H, Murphy M, Imwong M, Proux S, Haohankhunnatham W, Barney R, Rashid A, Kalnoky M, Kahn M, Golden A, Nosten F, Greenhouse B, Gamboa D, Domingo GJ. 2020. Multiplex human malaria array: quantifying antigens for malaria rapid diagnostics. Am J Trop Med Hyg 102:1366–1369. doi: 10.4269/ajtmh.19-0763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feleke SM, Gidey B, Mohammed H, Nega D, Dillu D, Haile M, Solomon H, Parr JB, Tollera G, Tasew G, Mamo H, Petros B. 2022. Field performance of Plasmodium falciparum lactate dehydrogenase rapid diagnostic tests during a large histidine-rich protein 2 deletion survey in Ethiopia. Malar J 21:236. doi: 10.1186/s12936-022-04257-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poti KE, Sullivan DJ, Dondorp AM, Woodrow CJ. 2020. HRP2: transforming malaria diagnosis, but with caveats. Trends Parasitol 36:112–126. doi: 10.1016/j.pt.2019.12.004 [DOI] [PubMed] [Google Scholar]
- 19. Slater HC, Ding XC, Knudson S, Bridges DJ, Moonga H, Saad NJ, De Smet M, Bennett A, Dittrich S, Slutsker L, Domingo GJ. 2022. Performance and utility of more highly sensitive malaria rapid diagnostic tests. BMC Infect Dis 22:121. doi: 10.1186/s12879-021-07023-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yimam Y, Mohebali M, Abbaszadeh Afshar MJ. 2022. Comparison of diagnostic performance between conventional and ultrasensitive rapid diagnostic tests for diagnosis of malaria: a systematic review and meta-analysis. PLoS ONE 17:e0263770. doi: 10.1371/journal.pone.0263770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nkrumah B, Acquah SE, Ibrahim L, May J, Brattig N, Tannich E, Nguah SB, Adu-Sarkodie Y, Huenger F. 2011. Comparative evaluation of two rapid field tests for malaria diagnosis: partec rapid malaria test and binax now malaria rapid diagnostic test. BMC Infect Dis 11:143. doi: 10.1186/1471-2334-11-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mayxay M, Pukrittayakamee S, Chotivanich K, Looareesuwan S, White NJ. 2001. Persistence of Plasmodium falciparum HRP-2 in successfully treated acute falciparum malaria. Trans R Soc Trop Med Hyg 95:179–182. doi: 10.1016/s0035-9203(01)90156-7 [DOI] [PubMed] [Google Scholar]
- 23. Bisoffi Z, Sirima SB, Menten J, Pattaro C, Angheben A, Gobbi F, Tinto H, Lodesani C, Neya B, Gobbo M, Van den Ende J. 2010. Accuracy of a rapid diagnostic test on the diagnosis of malaria infection and of malaria-attributable fever during low and high transmission season in Burkina Faso. Malar J 9:192. doi: 10.1186/1475-2875-9-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plucinski MM, McElroy PD, Dimbu PR, Fortes F, Nace D, Halsey ES, Rogier E. 2019. Clearance dynamics of lactate dehydrogenase and aldolase following antimalarial treatment for Plasmodium falciparum infection. Parasit Vectors 12:293. doi: 10.1186/s13071-019-3549-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayward RE, Sullivan DJ, Day KP. 2000. Plasmodium falciparum: histidine-rich protein II is expressed during gametocyte development. Exp Parasitol 96:139–146. doi: 10.1006/expr.2000.4557 [DOI] [PubMed] [Google Scholar]
- 26. Tjitra E, Suprianto S, McBroom J, Currie BJ, Anstey NM. 2001. Persistent ICT malaria P.f/P.v panmalarial and HRP2 antigen reactivity after treatment of Plasmodium falciparum malaria is associated with gametocytemia and results in false-positive diagnoses of Plasmodium vivax in convalescence. J Clin Microbiol 39:1025–1031. doi: 10.1128/JCM.39.3.1025-1031.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hopkins H, Kambale W, Kamya MR, Staedke SG, Dorsey G, Rosenthal PJ. 2007. Comparison of HRP2- and pLDH-based rapid diagnostic tests for malaria with longitudinal follow-up in Kampala, Uganda. Am J Trop Med Hyg 76:1092–1097. doi: 10.4269/ajtmh.2007.76.1092 [DOI] [PubMed] [Google Scholar]
- 28. Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, Newton PN, Pitisuttithum P, Smithyman AM, White NJ, Day NPJ. 2005. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med 2:e204. doi: 10.1371/journal.pmed.0020204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Plucinski MM, Dimbu PR, Fortes F, Abdulla S, Ahmed S, Gutman J, Kachur SP, Badiane A, Ndiaye D, Talundzic E, Lucchi N, Aidoo M, Udhayakumar V, Halsey E, Rogier E. 2018. Posttreatment HRP2 clearance in patients with uncomplicated Plasmodium falciparum malaria. J Infect Dis 217:685–692. doi: 10.1093/infdis/jix622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Humar A, Ohrt C, Harrington MA, Pillai D, Kain KC. 1997. Parasight F test compared with the polymerase chain reaction and microscopy for the diagnosis of Plasmodium falciparum malaria in travelers. Am J Trop Med Hyg 56:44–48. doi: 10.4269/ajtmh.1997.56.44 [DOI] [PubMed] [Google Scholar]
- 31. Swarthout TD, Counihan H, Senga RKK, van den Broek I. 2007. Paracheck-Pf accuracy and recently treated Plasmodium falciparum infections: is there a risk of over-diagnosis? Malar J 6:58. doi: 10.1186/1475-2875-6-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization . 2009. Methods for surveillance of antimalarial drug efficacy. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 33. Karl S, Laman M, Moore BR, Benjamin J, Koleala T, Ibam C, Kasian B, Siba PM, Waltmann A, Mueller I, Woodward RC, St Pierre TG, Davis TME. 2015. Gametocyte clearance kinetics determined by quantitative magnetic fractionation in melanesian children with uncomplicated malaria treated with artemisinin combination therapy. Antimicrob Agents Chemother 59:4489–4496. doi: 10.1128/AAC.00136-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Visser BJ, Meerveld-Gerrits J, Kroon D, Mougoula J, Vingerling R, Bache E, Boersma J, van Vugt M, Agnandji ST, Kaur H, Grobusch MP. 2015. Assessing the quality of anti-malarial drugs from Gabonese pharmacies using the MiniLab: a field study. Malar J 14:273. doi: 10.1186/s12936-015-0795-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Visser BJ, van Vugt M, Grobusch MP. 2014. Malaria: an update on current chemotherapy. Expert Opin Pharmacother 15:2219–2254. doi: 10.1517/14656566.2014.944499 [DOI] [PubMed] [Google Scholar]
- 36. Planche T, Krishna S, Kombila M, Engel K, Faucher JF, Ngou-Milama E, Kremsner PG. 2001. Comparison of methods for the rapid laboratory assessment of children with malaria. Am J Trop Med Hyg 65:599–602. doi: 10.4269/ajtmh.2001.65.599 [DOI] [PubMed] [Google Scholar]
- 37. Noedl H, Bronnert J, Yingyuen K, Attlmayr B, Kollaritsch H, Fukuda M. 2005. Simple histidine-rich protein 2 double-site sandwich enzyme-linked immunosorbent assay for use in malaria drug sensitivity testing. Antimicrob Agents Chemother 49:3575–3577. doi: 10.1128/AAC.49.8.3575-3577.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Medicines for Malaria Venture . 2008. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations: informal consultation organized by the medicines for malaria venture and cosponsored by the World Health Organization. World Health Organization, Amsterdam, The Netherlands. [Google Scholar]
- 39. Ranford-Cartwright LC, Taylor J, Umasunthar T, Taylor LH, Babiker HA, Lell B, Schmidt-Ott JR, Lehman LG, Walliker D, Kremsner PG. 1997. Molecular analysis of recrudescent parasites in a Plasmodium falciparum drug efficacy trial in Gabon. Trans R Soc Trop Med Hyg 91:719–724. doi: 10.1016/s0035-9203(97)90539-3 [DOI] [PubMed] [Google Scholar]
- 40. Poti KE, Balaban AE, Pal P, Kobayashi T, Goldberg DE, Sinnis P, Sullivan DJ. 2019. In vivo compartmental kinetics of Plasmodium falciparum histidine-rich protein II in the blood of humans and in BALB/c mice infected with a transgenic Plasmodium berghei parasite expressing histidine-rich protein II. Malar J 18:78. doi: 10.1186/s12936-019-2712-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iqbal J, Siddique A, Jameel M, Hira PR. 2004. Persistent histidine-rich protein 2, parasite lactate dehydrogenase, and panmalarial antigen reactivity after clearance of Plasmodium falciparum monoinfection. J Clin Microbiol 42:4237–4241. doi: 10.1128/JCM.42.9.4237-4241.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Desakorn V, Silamut K, Angus B, Sahassananda D, Chotivanich K, Suntharasamai P, Simpson J, White NJ. 1997. Semi-quantitative measurement of Plasmodium falciparum antigen PfHRP2 in blood and plasma. Trans R Soc Trop Med Hyg 91:479–483. doi: 10.1016/s0035-9203(97)90292-3 [DOI] [PubMed] [Google Scholar]
- 43. Ghanchi NK, Beg MA, Hussain R. 2009. Estimation of parasite load using rapid diagnostic test ICT now malaria P.f/P.v in Plasmodium falciparum malaria. Scand J Infect Dis 41:597–601. doi: 10.1080/00365540903022832 [DOI] [PubMed] [Google Scholar]
- 44. Kyabayinze DJ, Tibenderana JK, Odong GW, Rwakimari JB, Counihan H. 2008. Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for Plasmodium falciparum malaria in a hyperendemic region of Uganda. Malar J 7:221. doi: 10.1186/1475-2875-7-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marquart L, Butterworth A, McCarthy JS, Gatton ML. 2012. Modelling the dynamics of Plasmodium falciparum histidine-rich protein 2 in human malaria to better understand malaria rapid diagnostic test performance. Malar J 11:74. doi: 10.1186/1475-2875-11-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martin SK, Rajasekariah GH, Awinda G, Waitumbi J, Kifude C. 2009. Unified parasite lactate dehydrogenase and histidine-rich protein ELISA for quantification of Plasmodium falciparum. Am J Trop Med Hyg 80:516–522. doi: 10.4269/ajtmh.2009.80.516 [DOI] [PubMed] [Google Scholar]
- 47. Biswas S, Tomar D, Rao DN. 2005. Investigation of the kinetics of histidine-rich protein 2 and of the antibody responses to this antigen, in a group of malaria patients from India. Ann Trop Med Parasitol 99:553–562. doi: 10.1179/136485905X51463 [DOI] [PubMed] [Google Scholar]
- 48. Marquart L, Webb L, O’Rourke P, Gatton ML, Hsiang MS, Kalnoky M, Jang IK, Ntuku H, Mumbengegwi DR, Domingo GJ, McCarthy JS, Britton S. 2022. The in-vivo dynamics of Plasmodium falciparum HRP2: implications for the use of rapid diagnostic tests in malaria elimination. Malar J 21:233. doi: 10.1186/s12936-022-04245-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shiff CJ, Premji Z, Minjas JN. 1993. The rapid manual ParaSight-F test. a new diagnostic tool for Plasmodium falciparum infection. Trans R Soc Trop Med Hyg 87:646–648. doi: 10.1016/0035-9203(93)90273-s [DOI] [PubMed] [Google Scholar]
- 50. Toutain PL, Bousquet-Mélou A. 2004. Plasma terminal half-life. J Vet Pharmacol Ther 27:427–439. doi: 10.1111/j.1365-2885.2004.00600.x [DOI] [PubMed] [Google Scholar]
- 51. Shelby JP, White J, Ganesan K, Rathod PK, Chiu DT. 2003. A microfluidic model for single-cell capillary obstruction by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A 100:14618–14622. doi: 10.1073/pnas.2433968100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ndour PA, Larréché S, Mouri O, Argy N, Gay F, Roussel C, Jauréguiberry S, Perillaud C, Langui D, Biligui S, et al. 2017. Measuring the Plasmodium falciparum HRP2 protein in blood from artesunate-treated malaria patients predicts post-artesunate delayed hemolysis. Sci Transl Med 9:eaaf9377. doi: 10.1126/scitranslmed.aaf9377 [DOI] [PubMed] [Google Scholar]
- 53. Wojnarski M, Mouri O, Chambrion C, Roussel C, Chartrel N, Smith B, Smith P, Thellier M, Buffet P, Ndour PA. 2019. Plasmodium falciparum clearance is pitting-dependent with artemisinin-based drugs but pitting-independent with atovaquone-proguanil or mefloquine. J Infect Dis 220:535–539. doi: 10.1093/infdis/jiz115 [DOI] [PubMed] [Google Scholar]
- 54. Cunnington AJ, Riley EM, Walther M. 2013. Stuck in a rut? Reconsidering the role of parasite sequestration in severe malaria syndromes. Trends Parasitol 29:585–592. doi: 10.1016/j.pt.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fendel R, Brandts C, Rudat A, Kreidenweiss A, Steur C, Appelmann I, Ruehe B, Schröder P, Berdel WE, Kremsner PG, Mordmüller B. 2010. Hemolysis is associated with low reticulocyte production index and predicts blood transfusion in severe malarial anemia. PLoS ONE 5:e10038. doi: 10.1371/journal.pone.0010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perkins DJ, Were T, Davenport GC, Kempaiah P, Hittner JB, Ong’echa JM. 2011. Severe malarial anemia: innate immunity and pathogenesis. Int J Biol Sci 7:1427–1442. doi: 10.7150/ijbs.7.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Adegbite BR, Edoa JR, Honkpehedji YJ, Zinsou FJ, Dejon-Agobe JC, Mbong-Ngwese M, Lotola-Mougueni F, Koehne E, Lalremruata A, Kreidenweiss A, Nguyen TT, Kun J, Agnandji ST, Lell B, Safiou AR, Obone Atome FA, Mombo-Ngoma G, Ramharter M, Velavan TP, Mordmüller B, Kremsner PG, Adegnika AA. 2019. Monitoring of efficacy, tolerability and safety of artemether-lumefantrine and artesunate-amodiaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Lambaréné, Gabon: an open-label clinical trial. Malar J 18:424. doi: 10.1186/s12936-019-3015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Oyakhirome S, Pötschke M, Schwarz NG, Dörnemann J, Laengin M, Salazar CO, Lell B, Kun JFJ, Kremsner PG, Grobusch MP. 2007. Artesunate--amodiaquine combination therapy for falciparum malaria in young Gabonese children. Malar J 6:29. doi: 10.1186/1475-2875-6-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ndong Ngomo JM, Ondzagha Megnie GJ, Moutombi Ditombi B, Koumba Lengongo JV, M’Bondoukwé NP, Offouga CL, Mawili-Mboumba DP, Lekana-Douki JB, Ringwald P, Fandeur T, Bouyou-Akotet MK. 2019. Persistence of high In vivo efficacy and safety of artesunate-amodiaquine and artemether-lumefantrine as the first- and second-line treatments for uncomplicated Plasmodium falciparum malaria 10 years after their implementation in Gabon. Acta Parasitol 64:898–902. doi: 10.2478/s11686-019-00115-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Held J, Gebru T, Kalesse M, Jansen R, Gerth K, Müller R, Mordmüller B. 2014. Antimalarial activity of the myxobacterial macrolide chlorotonil A. Antimicrob Agents Chemother 58:6378–6384. doi: 10.1128/AAC.03326-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Olliaro P, Mussano P. 2003. Amodiaquine for treating malaria. Cochrane Database Syst Rev:CD000016. doi: 10.1002/14651858.CD000016 [DOI] [PubMed] [Google Scholar]
- 62. Dorkenoo AM, Yehadji D, Agbo YM, Layibo Y, Agbeko F, Adjeloh P, Yakpa K, Sossou E, Awokou F, Ringwald P. 2016. Therapeutic efficacy trial of artemisinin-based combination therapy for the treatment of uncomplicated malaria and investigation of mutations in k13 propeller domain in Togo, 2012-2013. Malar J 15:331. doi: 10.1186/s12936-016-1381-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lingani M, Bonkian LN, Yerbanga I, Kazienga A, Valéa I, Sorgho H, Ouédraogo JB, Mens PF, Schallig HDFH, Ravinetto R, d’Alessandro U, Tinto H. 2020. In vivo/ex vivo efficacy of artemether-lumefantrine and artesunate-amodiaquine as first-line treatment for uncomplicated falciparum malaria in children: an open label randomized controlled trial in Burkina Faso. Malar J 19:8. doi: 10.1186/s12936-019-3089-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Wit M, Funk AL, Moussally K, Nkuba DA, Siddiqui R, Bil K, Piriou E, Bart A, Bahizi Bizoza P, Bousema T. 2016. In vivo efficacy of artesunate-amodiaquine and artemether-lumefantrine for the treatment of uncomplicated falciparum malaria: an open-randomized, non-inferiority clinical trial in South Kivu, Democratic Republic of Congo. Malar J 15:455. doi: 10.1186/s12936-016-1444-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ndiaye JL, Randrianarivelojosia M, Sagara I, Brasseur P, Ndiaye I, Faye B, Randrianasolo L, Ratsimbasoa A, Forlemu D, Moor VA, Traore A, Dicko Y, Dara N, Lameyre V, Diallo M, Djimde A, Same-Ekobo A, Gaye O. 2009. Randomized, multicentre assessment of the efficacy and safety of ASAQ--a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria. Malar J 8:125. doi: 10.1186/1475-2875-8-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schramm B, Valeh P, Baudin E, Mazinda CS, Smith R, Pinoges L, Dhorda M, Boum Y, Sundaygar T, Zolia YM, Jones JJ, Comte E, Houzé P, Jullien V, Carn G, Kiechel J-R, Ashley EA, Guérin PJ. 2013. Efficacy of artesunate-amodiaquine and artemether-lumefantrine fixed-dose combinations for the treatment of uncomplicated Plasmodium falciparum malaria among children aged six to 59 months in Nimba County, Liberia: an open-label randomized non-inferiority trial. Malar J 12:251. doi: 10.1186/1475-2875-12-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yeka A, Lameyre V, Afizi K, Fredrick M, Lukwago R, Kamya MR, Talisuna AO. 2014. Efficacy and safety of fixed-dose artesunate-amodiaquine vs. artemether-lumefantrine for repeated treatment of uncomplicated malaria in Ugandan children. PLoS ONE 9:e113311. doi: 10.1371/journal.pone.0113311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aubouy A, Bakary M, Keundjian A, Mbomat B, Makita JR, Migot-Nabias F, Cot M, Le Bras J, Deloron P. 2003. Combination of drug level measurement and parasite genotyping data for improved assessment of amodiaquine and sulfadoxine-pyrimethamine efficacies in treating Plasmodium falciparum malaria in Gabonese children. Antimicrob Agents Chemother 47:231–237. doi: 10.1128/AAC.47.1.231-237.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mawili-Mboumba DP, Borrmann S, Cavanagh DR, McBride JS, Matsiegui PB, Missinou MA, Kremsner PG, Ntoumi F. 2003. Antibody responses to Plasmodium falciparum merozoite surface protein-1 and efficacy of amodiaquine in Gabonese children with P. falciparum malaria. J Infect Dis 187:1137–1141. doi: 10.1086/368414 [DOI] [PubMed] [Google Scholar]
- 70. Nsimba B, Guiyedi V, Mabika-Mamfoumbi M, Mourou-Mbina JR, Ngoungou E, Bouyou-Akotet M, Loembet R, Durand R, Le Bras J, Kombila M. 2008. Sulphadoxine/pyrimethamine versus amodiaquine for treating uncomplicated childhood malaria in Gabon: a randomized trial to guide national policy. Malar J 7:31. doi: 10.1186/1475-2875-7-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Oulton T, Mahamar A, Sanogo K, Diallo M, Youssouf A, Niambele SM, Samaké S, Keita S, Sinaba Y, Sacko A, Traore SF, Lanke K, Collins KA, Bradley J, Drakeley C, Stone WJR, Dicko A. 2022. Persistence of Plasmodium falciparum HRP-2 antigenaemia after artemisinin combination therapy is not associated with gametocytes. Malar J 21:372. doi: 10.1186/s12936-022-04387-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hanssen E, Knoechel C, Dearnley M, Dixon MWA, Le Gros M, Larabell C, Tilley L. 2012. Soft X-ray microscopy analysis of cell volume and hemoglobin content in erythrocytes infected with asexual and sexual stages of Plasmodium falciparum. J Struct Biol 177:224–232. doi: 10.1016/j.jsb.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Michael OS, Orimadegun AE, Falade CO. 2021. Persistence of Plasmodium falciparum HRP2 antigen after effective antimalarial therapy. Ann Ib Postgrad Med 19:15–21. doi: 10.1186/s12936-022-04387-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wellems TE, Sá JM, Su X-Z, Connelly SV, Ellis AC. 2020. “Artemisinin resistance”: something new or old? Something of a misnomer? Trends Parasitol 36:735–744. doi: 10.1016/j.pt.2020.05.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S6; Tables S1 and S2.
Data Availability Statement
The data supporting this study may be made available to the public upon reasonable request through the corresponding author (rolf.fendel@uni-tuebingen.de).