Abstract
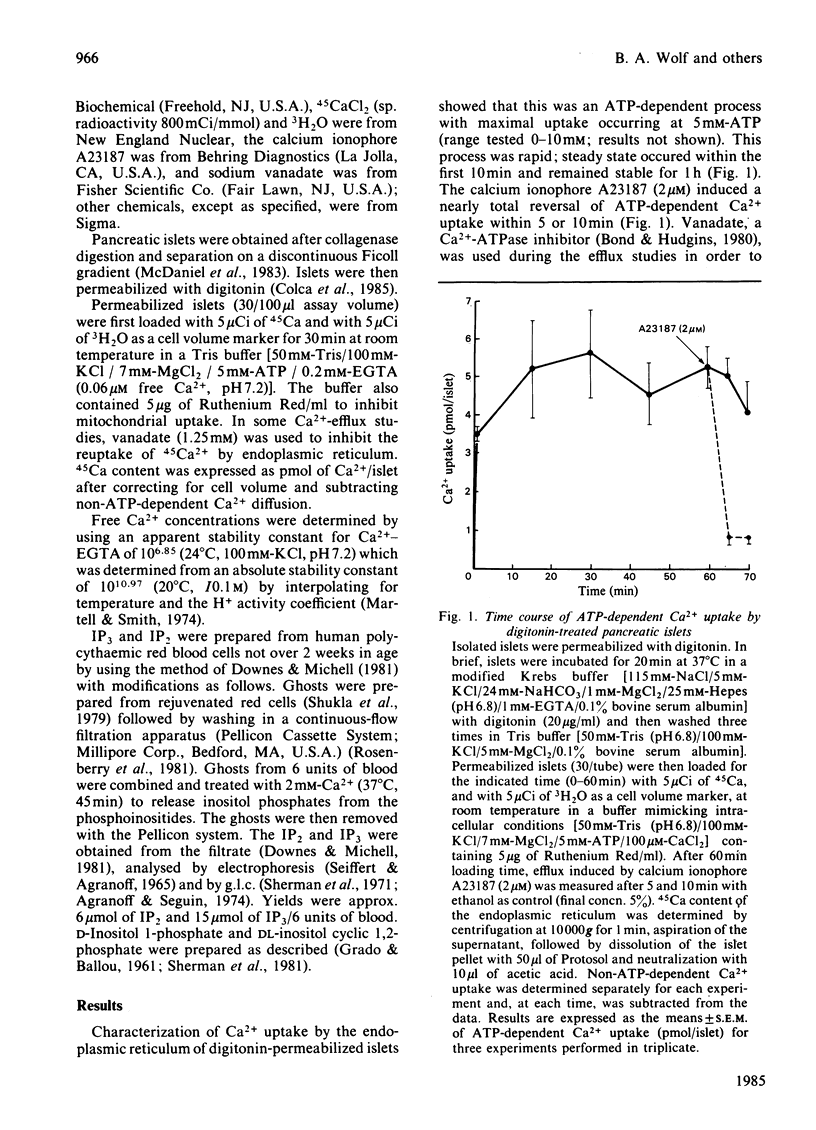
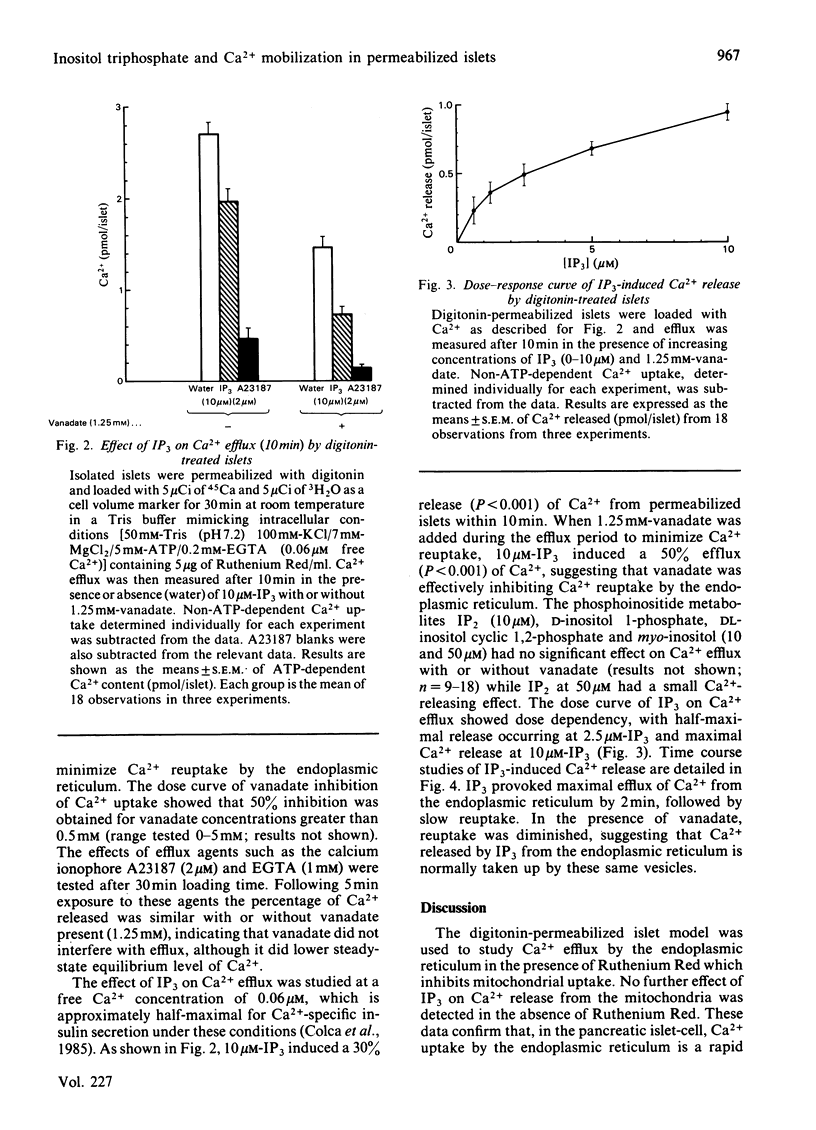
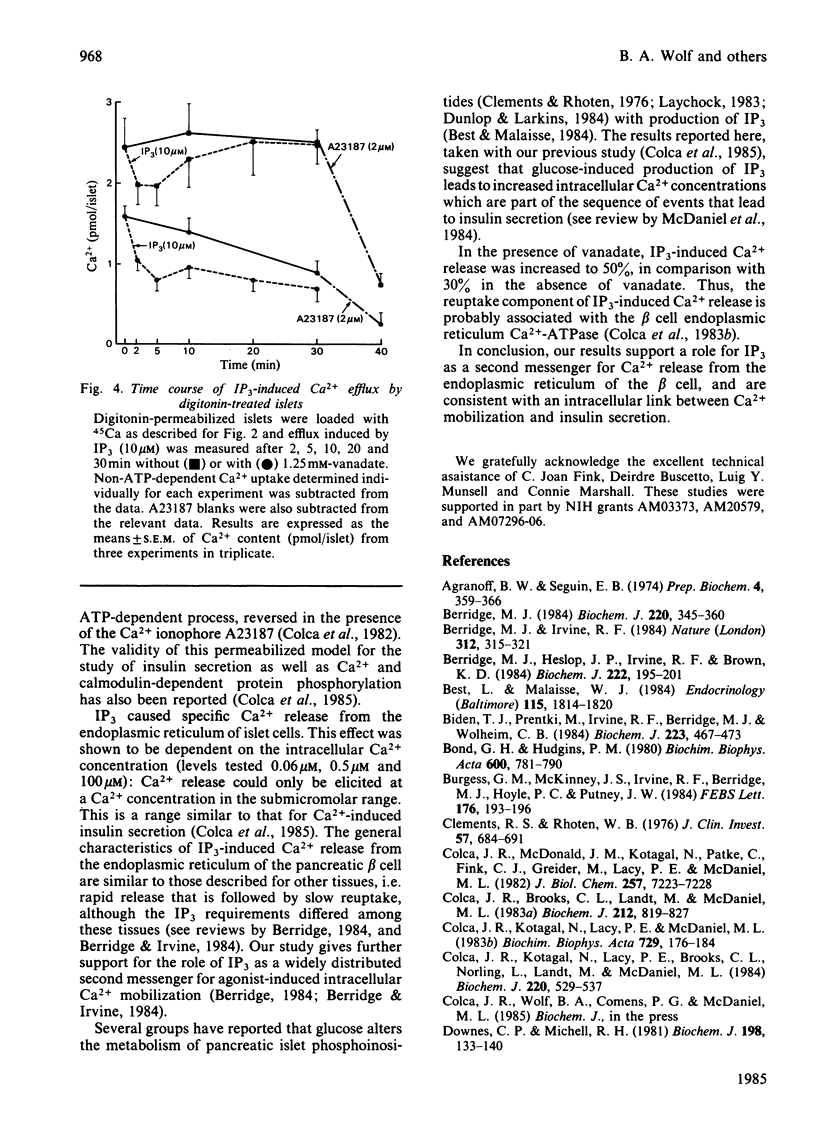
Glucose-induced insulin secretion is thought to be mediated by submicromolar increases in intracellular Ca2+, although the intracellular processes are not well understood. We have used the previously characterized digitonin-permeabilized insulin-secreting pancreatic islet model to study the role of myo-inositol 1,4,5-trisphosphate (IP3), a putative second messenger for mobilization of intracellular Ca2+. Ca2+ efflux from the endoplasmic reticulum was studied with or without vanadate present to inhibit Ca2+ reuptake. IP3 (10 microM), at a free Ca2+ level of 0.06 microM, increased Ca2+ release by 30% and, when vanadate was present, by 50%. Maximal and half-maximal Ca2+ release was observed at 10 microM- and 2.5 microM-IP3, respectively. IP3 provoked a rapid release that was followed by slow reuptake. Reuptake was diminished in the presence of vanadate. Inositol 1,4-bisphosphate, inositol 1-phosphate and other phosphoinositide metabolites did not have any significant effect. Because increases in Ca2+ levels in the submicromolar range have been previously shown to induce insulin release in digitonin-permeabilized islets, our results are consistent with the concept of IP3 serving as a second messenger for insulin secretion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Seguin E. B. Preparation of inositol triphosphate from brain: GLC of trimethylsilyl derivative. Prep Biochem. 1974;4(4):359–366. doi: 10.1080/00327487408068211. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Best L., Malaisse W. J. Nutrient and hormone-neurotransmitter stimuli induce hydrolysis of polyphosphoinositides in rat pancreatic islets. Endocrinology. 1984 Nov;115(5):1814–1820. doi: 10.1210/endo-115-5-1814. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Prentki M., Irvine R. F., Berridge M. J., Wollheim C. B. Inositol 1,4,5-trisphosphate mobilizes intracellular Ca2+ from permeabilized insulin-secreting cells. Biochem J. 1984 Oct 15;223(2):467–473. doi: 10.1042/bj2230467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond G. H., Hudgins P. M. Inhibition of red cell Ca2+-ATPase by vanadate. Biochim Biophys Acta. 1980 Aug 14;600(3):781–790. doi: 10.1016/0005-2736(80)90480-0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Berridge M. J., Hoyle P. C., Putney J. W., Jr Inositol 1,4,5-trisphosphate may be a signal for f-Met-Leu-Phe-induced intracellular Ca mobilisation in human leucocytes (HL-60 cells). FEBS Lett. 1984 Oct 15;176(1):193–196. doi: 10.1016/0014-5793(84)80939-4. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Rhoten W. B. Phosphoinositide metabolism and insulin secretion from isolated rat pancreatic islets. J Clin Invest. 1976 Mar;57(3):684–691. doi: 10.1172/JCI108325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colca J. R., Brooks C. L., Landt M., McDaniel M. L. Correlation of Ca2+-and calmodulin-dependent protein kinase activity with secretion of insulin from islets of Langerhans. Biochem J. 1983 Jun 15;212(3):819–827. doi: 10.1042/bj2120819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colca J. R., Kotagal N., Lacy P. E., Brooks C. L., Norling L., Landt M., McDaniel M. L. Glucose-stimulated protein phosphorylation in the pancreatic islet. Biochem J. 1984 Jun 1;220(2):529–537. doi: 10.1042/bj2200529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colca J. R., Kotagal N., Lacy P. E., McDaniel M. L. Comparison of the properties of active Ca2+ transport by the islet-cell endoplasmic reticulum and plasma membrane. Biochim Biophys Acta. 1983 Apr 6;729(2):176–184. doi: 10.1016/0005-2736(83)90483-2. [DOI] [PubMed] [Google Scholar]
- Colca J. R., McDonald J. M., Kotagal N., Patke C., Fink C. J., Greider M. H., Lacy P. E., McDaniel M. L. Active calcium uptake by islet-cell endoplasmic reticulum. J Biol Chem. 1982 Jun 25;257(12):7223–7228. [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop M. E., Larkins R. G. The role of calcium in phospholipid turnover following glucose stimulation in neonatal rat cultured islets. J Biol Chem. 1984 Jul 10;259(13):8407–8411. [PubMed] [Google Scholar]
- GRADO C., BALLOU C. E. Myo-inositol phosphates obtained by alkaline hydrolysis of beef brain phosphoinositide. J Biol Chem. 1961 Jan;236:54–60. [PubMed] [Google Scholar]
- Gershengorn M. C., Geras E., Purrello V. S., Rebecchi M. J. Inositol trisphosphate mediates thyrotropin-releasing hormone mobilization of nonmitochondrial calcium in rat mammotropic pituitary cells. J Biol Chem. 1984 Sep 10;259(17):10675–10681. [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Hirata M., Suematsu E., Hashimoto T., Hamachi T., Koga T. Release of Ca2+ from a non-mitochondrial store site in peritoneal macrophages treated with saponin by inositol 1,4,5-trisphosphate. Biochem J. 1984 Oct 1;223(1):229–236. doi: 10.1042/bj2230229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Brown K. D., Berridge M. J. Specificity of inositol trisphosphate-induced calcium release from permeabilized Swiss-mouse 3T3 cells. Biochem J. 1984 Aug 15;222(1):269–272. doi: 10.1042/bj2220269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Williams R. J., Corkey B. E., Matschinsky F. M., Williamson J. R. The effect of inositol trisphosphate on Ca2+ fluxes in insulin-secreting tumor cells. J Biol Chem. 1984 Nov 10;259(21):12952–12955. [PubMed] [Google Scholar]
- Laychock S. G. Identification and metabolism of polyphosphoinositides in isolated islets of Langerhans. Biochem J. 1983 Oct 15;216(1):101–106. doi: 10.1042/bj2160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel M. L., Colca J. R., Kotagal N., Lacy P. E. A subcellular fractionation approach for studying insulin release mechanisms and calcium metabolism in islets of Langerhans. Methods Enzymol. 1983;98:182–200. doi: 10.1016/0076-6879(83)98149-1. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Chen J. F., Lee M. M., Moulton T. A., Onigman P. Large scale isolation of human erythrocyte membranes by high volume molecular filtration. J Biochem Biophys Methods. 1981 Jan;4(1):39–48. doi: 10.1016/0165-022x(81)90004-x. [DOI] [PubMed] [Google Scholar]
- Seiffert U. B., Agranoff B. W. Isolation and separation of inositol phosphates from hydrolysates of rat tissues. Biochim Biophys Acta. 1965 Jun 1;98(3):574–581. doi: 10.1016/0005-2760(65)90154-2. [DOI] [PubMed] [Google Scholar]
- Sherman W. R., Leavitt A. L., Honchar M. P., Hallcher L. M., Phillips B. E. Evidence that lithium alters phosphoinositide metabolism: chronic administration elevates primarily D-myo-inositol-1-phosphate in cerebral cortex of the rat. J Neurochem. 1981 Jun;36(6):1947–1951. doi: 10.1111/j.1471-4159.1981.tb10819.x. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Coleman R., Finean J. B., Michell R. H. Are polyphosphoinositides associated with glycophorin in human erythrocyte membranes? Biochem J. 1979 May 1;179(2):441–444. doi: 10.1042/bj1790441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]


