Abstract
Rta, encoded primarily by open reading frame 50, is well conserved among gammaherpesviruses. It has been shown that the Rta proteins of Epstein Barr virus (EBV), Kaposi's sarcoma-associated herpesvirus (KSHV, or HHV-8), and murine gammaherpesvirus 68 (MHV-68; also referred to as γHV68) play an important role in viral reactivation from latency. However, the role of Rta during productive de novo infection has not been characterized in gammaherpesviruses. Since there are cell lines that can support efficient productive de novo infection by MHV-68 but not EBV or KSHV, we examined whether MHV-68 Rta plays a role in initiating viral lytic replication in productively infected cells. Rta, functioning as a transcriptional activator, can activate the viral promoter of early lytic genes. The amino acid sequence alignments of the Rta homologues suggest that the organizations of their functional domains are similar, with the DNA binding and dimerization domains at the N terminus and the trans-activation domain at the C terminus. We constructed two mutants of MHV-68 Rta, Rd1 and Rd2, with deletions of 112 and 243 amino acids from the C terminus, respectively. Rd1 and Rd2 could no longer trans-activate the promoter of MHV-68 gene 57, consistent with the deletions of their trans-activation domains at the C terminus. Furthermore, Rd1 and Rd2 were able to function as dominant-negative mutants, inhibiting trans-activation of wild-type Rta. To study whether Rd1 and Rd2 blocked viral lytic replication, purified virion DNA was cotransfected with Rd1 or Rd2 into fibroblasts. Expression of viral lytic proteins was greatly suppressed, and the yield of infectious viruses was reduced up to 104-fold. Stable cell lines constitutively expressing Rd2 were established and infected with MHV-68. Transcription of the immediate-early gene, rta, and the early gene, tk, of the virus was reduced in these cell lines. The presence of Rd2 also led to attenuation of viral lytic protein expression and virion production. The ability of Rta dominant-negative mutants to inhibit productive infection suggests that the trans-activation function of Rta is essential for MHV-68 lytic replication. We propose that a single viral protein, Rta, governs the initiation of MHV-68 lytic replication during both reactivation and productive de novo infection.
Gammaherpesviruses are known to establish latency in lymphocytes and are associated with tumorigenesis. Two important human pathogens in the family are Kaposi's sarcoma-associated herpesvirus (KSHV; also referred to as HHV-8) and Epstein-Barr virus (EBV). KSHV and EBV are associated with several malignancies, including B-cell lymphomas, nasopharyngeal carcinoma, and Kaposi's sarcoma. Studies of KSHV and EBV are limited by the lack of cell lines to support efficient productive infection and by their restricted host ranges. Murine gammaherpesvirus 68 (MHV-68; also referred to as γHV68) is also a member of the gammaherpesvirus family. Unlike KSHV or EBV, in vitro cell culture systems are available to study productive de novo infection by MHV-68, as well as latency and reactivation. MHV-68 forms plaques on monolayers of many cell lines, making it relatively straightforward to genetically manipulate the viral genome. MHV-68 can also establish productive and latent infections in laboratory mice (23), which allows us to pursue questions that relate to host-virus interactions (16, 17, 20, 21). Because of these advantages, MHV-68 offers an excellent model to study the biology and pathogenesis of gammaherpesviruses.
Herpesviruses have two distinct phases of their life cycle, productive infection and latency. Reactivation from latency to productive infection is essential for transmission of the virus from host to host and thus is one important aspect of herpesvirus biology. The molecular mechanisms of reactivation have been extensively studied in KSHV and EBV. Cell lines derived from KSHV- or EBV-associated lymphomas are latently infected with virus. A viral protein, Rta (replication and transcription activator) is primarily encoded by open reading frame 50 (ORF50), which is well conserved among gammaherpesviruses. EBV Rta and another viral protein, ZEBRA, function in a cooperative manner to reactivate the viral lytic cycle (2, 5, 19, 27). Although ZEBRA plays a more prominent role in inducing EBV lytic replication (4, 10, 14), Rta alone can disrupt latency in some latently infected cell lines (19, 27). KSHV Rta has been shown to be sufficient to reactivate the virus from latently infected B cells derived from KSHV-associated lymphomas (13, 22). We have previously shown that MHV-68 Rta is also able to disrupt viral latency and drive viral lytic replication to completion in a latently MHV-68-infected B-cell lymphoma line (26). These studies indicate that Rta of gammaherpesviruses plays a conserved role in virus reactivation.
The Rta protein functions as a transcriptional activator. It has been shown for several gammaherpesviruses, including EBV, KSHV, MHV-68, herpesvirus saimiri, and bovine herpesvirus 4, that Rta activates the promoters of viral early lytic genes in transient transfections and reporter assays (1, 9, 11, 18, 24). Amino acid sequence alignments of the Rta homologues revealed that the most conserved region is at the N terminus. This portion of EBV Rta was shown to mediate DNA binding and dimerization (15). The C termini of the Rta homologues are poorly conserved, but in each protein the terminus is rich in acidic amino acids, which is a characteristic of activation domains. The C-terminal portions of the EBV and KSHV Rtas, including the acidic regions, have been shown to function as potent activation domains (12, 15). Moreover, deletion of the last 131 amino acids (aa) from the C terminus of KSHV Rta not only abrogates the ability of Rta to trans-activate but also creates a dominant-negative mutant that inhibits trans-activation by wild-type Rta (12).
The role of Rta during productive de novo infection has not been well characterized. This is partially due to the lack of cell lines that can support efficient productive infection of EBV or KSHV. However, many cell lines are permissive for productive infection by MHV-68, providing a good model to study the role of Rta during de novo infection. It has been shown that after productive infection of fibroblasts, transcription of the MHV-68 rta gene is resistant to a protein synthesis inhibitor, cycloheximide, indicating that rta of MHV-68 is an immediate-early gene during productive de novo infection (11, 26). Therefore, we hypothesize that MHV-68 Rta plays a critical role in productive de novo infection, as it does in reactivation, by activating the expression of other viral lytic genes and initiating the cascade of gene expression, which ultimately leads to the production of infectious virions. In this study we investigated the role of Rta in productive infection by blocking Rta function. We constructed two Rta dominant-negative mutants by deleting portions of the C terminus of the protein. These two mutants exerted dominant inhibition on the trans-activation function of wild-type Rta. Furthermore, the dominant-negative mutants were able to suppress viral lytic replication following transfection of virion DNA or infection with virus. Our results support the hypothesis that Rta is essential for MHV-68 lytic replication during productive de novo infection.
MATERIALS AND METHODS
Viruses, cells, and plaque assays.
MHV-68 was originally obtained from the American Type Culture Collection (VR1465), and the recombinant virus tw25 was constructed by homologous recombination to contain the enhanced green fluorescence protein (EGFP) expression cassette inserted at nucleotide (nt) 1839 without disrupting any known ORF. The working virus stocks were grown by infecting BHK-21 cells (ATCC CCL-10) at a multiplicity of infection (MOI) of 0.05 PFU/cell. BHK-21 cells (a baby hamster kidney fibroblast cell line) and 293T cells (a human embryonic fibroblast cell line transfected with the E1 region of adenovirus and the simian virus 40 [SV40] T antigen) were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. Virus infection was carried out as previously described (26). Plaque assays were performed using monolayers of BHK-21 cells overlaid with 1% methylcellulose, as previously described (26).
Plasmid construction.
pCMVFLAG/Rta contains the rta genomic sequence fused to the FLAG sequence of pCMVFLAG (Kodak). The insert of the rta sequence was generated by PCR using total DNA isolated from MHV-68-infected BHK-21 cells as the template and a pair of primers, R3TR (5′-CTCTCTGAATTCTGCAGCGATGGCCTCTGAC-3′) and R4 (26). The R3TR primer contains an EcoRI site (underlined) and the translation initiation codon (boldface) of the rta gene corresponding to nt 66760 (25). The PCR product was digested with EcoRI and XbaI and cloned into pCMVFLAG. To construct pCMVFLAG/Rd1, the insert was generated by PCR using a cDNA clone of the rta gene (26) as the template and a pair of primers, R3TR and Rd1 (5′-CTCGTCTAGATTATTGCACAATATGCTGGACAG-3′). The Rd1 primer contains an XbaI site (underlined), the translation termination codon (boldface), and the sequence corresponding to nt 69037 to 69018. The PCR product was digested with EcoRI and XbaI and cloned into pCMVFLAG. The same strategy was used to construct pCMVFLAG/Rd2. The insert was generated by PCR using primers R3TR and Rd2 (5′-CTCGTCTAGATTAAGACAGTCCTGAAAAGACCA-3′). The Rd2 primer contains an XbaI site (underlined), the translation termination codon (boldface), and the sequence corresponding to nt 68622 to 68641.
Transfections.
All transfections were carried out using Lipofectamine Plus reagent (Life Technologies, Gaithersburg, Md.) according to the manufacturer's recommendations. For the luciferase reporter assays (see Fig. 2 and 3), 1.6 × 105 293T or 7 × 104 BHK-21 cells/well were seeded in 24-well plates the day before transfection. The reporter plasmid p57luc (50 ng) (11) and different amounts of the protein expression plasmids (see Fig. 2 and 3) were used for transfections. The empty vector pCMVFLAG was used to adjust the amount of total DNA transfected to 600 ng for each transfection. The constitutively active pRLCMV reporter (1 ng) was included in each transfection as an internal control for normalizing variations among transfections. For virion DNA transfection, 1.6 × 105 293T or 7 × 104 BHK-21 cells/well were seeded in 24-well plates the day before transfection. Virion DNA was isolated from tw25 virus; 0.2 μg of tw25 virion DNA and 0.2 μg of the protein expression plasmids (pCMVFLAG/Rta, Rd1, or Rd2) or pCMVFLAG was transfected in each well.
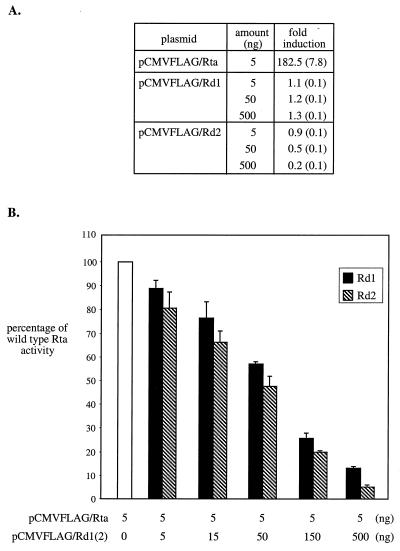
FIG. 2.
Rd1 and Rd2 function as dominant-negative mutants of Rta in 293T cells. (A) Rd1 and Rd2 do not activate the ORF57 promoter. 293T cells were cotransfected with 50 ng of the reporter construct, p57Luc, and 5 ng of pCMVFLAG/Rta, or 5 to 500 ng of pCMVFLAG/Rd1, or 5 to 500 ng of pCMVFLAG/Rd2. The total amounts of plasmid DNA were brought up to 600 ng with pCMVFLAG. The control transfection was carried out with 550 ng of pCMVFLAG and 50 ng of p57Luc. Each transfection included 1 ng of pRLCMV containing the Renilla luciferase gene driven by the constitutively active CMV promoter for nomalization of variations among transfections. At 48 h posttransfection, total cell lysates were harvested for analysis of luciferase activity. Normalized luciferase activity was calculated by dividing the level of firefly luciferase activity by the level of Renilla luciferase activity in each transfection. The fold induction was then calculated by dividing the level of normalized luciferase activity by that of the control transfection. Standard deviations derived from four experiments are shown in parentheses. (B) Rd1 and Rd2 inhibit wild-type Rta trans-activation of the ORF57 promoter. 293T cells were transfected with 50 ng of p57Luc and 5 ng of pCMVFLAG/Rta alone or with 5 to 500 ng of pCMVFLAG/Rd1 or 5 to 500 ng of pCMVFLAG/ Rd2. Total cell lysates were harvested at 48 h posttransfection, and the luciferase activity in each transfection was measured. The fold induction was calculated as described above. Wild-type Rta activity was expressed as the percentage of the fold induction relative to cotransfection of p57Luc and pCMVFLAG/Rta. Standard deviations derived from four experiments are expressed as error bars.
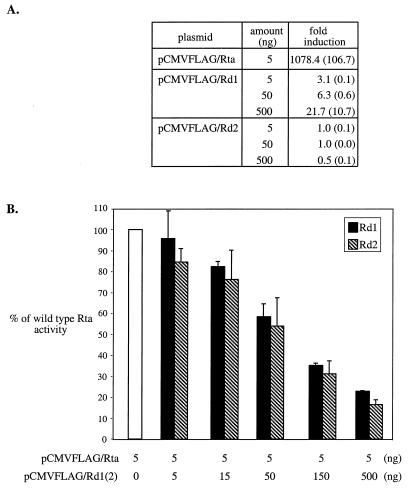
FIG. 3.
Rd1 and Rd2 function as dominant-negative mutants of Rta in BHK-21 cells. The transfections described in the legend to Fig. 2 were repeated in BHK-21 cells. (A) Rd1 and Rd2 do not activate the ORF57 promoter. (B) Rd1 and Rd2 inhibit wild-type Rta trans-activation of the ORF57 promoter.
Western blot analysis.
Cells were lysed in Laemmli buffer containing 0.25 M Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), 10% glycerol, 5% β-mercaptoethanol, and 0.002% bromophenol blue. Ten percent of the total lysates were heated to 95°C and subjected to electrophoresis on 10% polyacrylamide gels along with the broad-range prestained protein molecular weight standard (Bio-Rad, Hercules, Calif.). Proteins were electrotransferred (Bio-Rad) onto nitrocellulose membranes (Amersham Pharmacia Biotech, Arlington Heights, Ill.). To detect viral proteins, the membranes were blocked in phosphate-buffered saline plus 0.1% Tween-20 and 5% nonfat powdered milk and incubated with one of the primary antibodies, the rabbit hyperimmune serum against MHV-68-infected rabbit cells (23) or the rabbit serum against the recombinant M9 protein. The full-length MHV-68 M9 gene was cloned into pET30b(+) (Novagen, Madison, Wis.), and the His6-tagged M9 protein expressed in Escherichia coli was purified by nickel-nitrilotriacetic acid metal affinity chromatography (Qiagen, Valencia, Calif.). The purified M9 protein was injected into a rabbit for antibody production (Covance Research Products, Denver, Colo.). The membranes were washed in phosphate-buffered saline containing 0.1% Tween 20 and incubated with the secondary antibody, anti-rabbit immunoglobulin G conjugated with horseradish peroxidase (Amersham Pharmacia Biotech). The proteins were detected by the chemiluminescent detection ECL+PLUS system (Amersham Pharmacia Biotech), and the signals were detected using a STORM imaging system (Molecular Dynamics, Sunnyvale, Calif.). To reprobe with a different antibody, the membranes were first stripped in buffer containing 100 mM β-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl (pH 6.7) at 60°C for 30 min. To detect the FLAG-tagged proteins or cellular β-actin, the mouse monoclonal antibody against the FLAG epitope or β-actin (Sigma, St. Louis, Mo.) was used as the primary antibody, and anti-mouse immunoglobulin G conjugated with horseradish peroxidase (Amersham Pharmacia Biotech) was used as the secondary antibody.
Isolation of cell lines expressing Rd2.
293T cells (5 × 106) were seeded in a 10-cm-diameter dish the day before transfection. The cells were cotransfected with 10 μg of pCMVFLAG/Rd2 and 1 μg of pLTRpuro (kindly provided by David Rawlings) by the calcium phosphate method. At 48 h posttransfection, the cells were split 1:100 and grown in the medium containing 1 μg of puromycin/ml. Individual colonies were picked, expanded, and tested for expression of Rd2 by Western blot analysis using the antibody against the FLAG epitope (Sigma). Two cell clones, designated 45-5 and V-30, displaying the highest expression levels of Rd2, were chosen for further study.
RNA extraction and Northern blot analysis.
Total RNA was extracted from 293T cells, using the guanidinium-acid phenol method as described by Chomczynski and Sacchi (3). One-third of the total RNA was treated with a mixture of 1 M glyoxal and 50% (vol/vol) dimethyl sulfoxide at 50°C for 30 min (7). The glyoxalated RNAs were then separated on 1% agarose gels in circulating 10 mM sodium phosphate buffer (pH 6.8). A 1-kb ladder (Life Techonologies) was 5′-end labeled with [γ-32P]dATP, glyoxalated, and loaded onto the gels as the size standard. The RNAs were transferred onto charged nylon membranes (Amersham Pharmacia Biotech). The membranes were UV cross-linked and deglyoxalated at 80°C in 20 mM Tris-HCl (pH 8). Prehybridization and hybridization were carried out at 65°C in 0.5 M K2HPO4 (pH 6.8) containing 7% SDS and 1% bovine serum albumin. Probes were synthesized by the random-priming method in the presence of [α-32P]dCTP using viral DNA fragments generated by PCR as templates. The membranes were washed at 65°C in 40 mM sodium phosphate (pH 6.8) buffer containing 5% SDS and 0.5% bovine serum albumin, then washed with 2X SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.1% SDS, and finally washed with 0.1X SSC containing 0.1% SDS. Radioactivity was detected using a STORM imaging system. Before rehybridization with a different probe, the membranes were stripped at 80°C in 10 mM Tris-HCl (pH 8) containing 1% SDS.
RESULTS
Construction of the Rta deletion mutants.
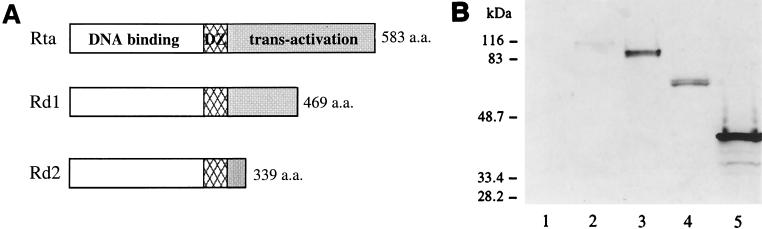
We constructed potential Rta dominant-negative mutants that retained the ability to bind DNA but not to activate transcription, allowing the mutants to compete with wild-type Rta for the target promoter and interfere with the function of MHV-68 Rta. Although functional domains of MHV-68 Rta have not been defined, based on the amino acid sequence homology to EBV and HHV-8 Rta, it is likely that the N terminus of MHV-68 Rta contains DNA binding and dimerization domains while the activation domain is located at the C terminus. Therefore, we deleted either 112 or 243 aa from the C terminus of the Rta protein. The truncated Rta sequences were generated by PCR, and the PCR products were cloned downstream of the FLAG epitope sequence in pCMVFLAG. The resulting clones, termed pCMVFLAG/Rd1 (aa 1 to 471) and pCMVFLAG/Rd2 (aa 1 to 340), together with the wild-type pCMVFLAGRta (aa 1 to 583) were individually transfected into 293T cells to examine protein expression. At 24 h posttransfection, total protein was harvested and analyzed by Western blot analysis, using monoclonal antibody against the FLAG epitope (Fig. 1B). Although the predicted size of wild-type MHV-68 Rta is 64 kDa, the protein expressed from pCMVFLAG/Rta showed an apparent mobility of 90 kDa (Fig. 1B, lane 3). Likewise, the KSHV Rta protein expressed from pCMVFLAG/Rta/KSHV exhibited a mobility of 100 kDa (Fig. 1B, lane 2) despite the predicted size of 76 kDa. Others have made a similar observation about KSHV Rta, and they attributed this size difference to phosphorylation (12). The truncated protein expressed from pCMVFLAG/Rd1 was ≈70 kDa with a predicted size of 52 kDa (Fig. 1B, lane 4), and that expressed from pCMVFLAG /Rd2 was ≈45 kDa with a predicted size of 37 kDa (Fig. 1B, lane 5).
FIG. 1.
Construction and expression of wild-type and mutant Rta proteins. (A) The structures of the wild-type and mutant Rta proteins are shown, with the open boxes representing the DNA binding domains, the hatched boxes representing the dimerization domains (DZ), and the shaded boxes representing the activation domains. The size of each protein is indicated at the right. (B) Wild-type and mutant Rta proteins are expressed in 293T cells. The coding sequences of Rta, Rd1, and Rd2 were individualy cloned into pCMVFLAG. The cells were transfected with the empty vector, pCMVFLAG (lane 1), pCMVFLAG/Rta/KSHV (lane 2), pCMVFLAG/Rta (lane 3), pCMVFLAG/Rd1 (lane 4), or pCMVFLAG /Rd2 (lane 5). Ten percent of the total cell lysates was loaded onto a 10% denaturing polyacrylamide gel. Western blot analysis was carried out using the monoclonal antibody against the FLAG epitope. The masses of individual proteins in the molecular weight standard are indicated at the left.
The Rta mutants fail to trans-activate the ORF57 promoter and block trans-activation by wild-type Rta.
To determine whether Rd1 and Rd2 lost the ability to activate transcription, each protein expression plasmid was transfected with a reporter construct, p57Luc, containing the firefly luciferase gene driven by the MHV-68 ORF57 promoter. This viral promoter has been shown to be responsive to wild-type MHV-68 Rta (11). The results obtained from transfections in 293T cells are summarized in Fig. 2A. There was ≈180-fold induction of ORF57 promoter activity by the wild-type Rta. However, neither Rd1 nor Rd2 activated the ORF57 promoter, nor did transfections of increasing amounts of either protein expression plasmid (up to 100-fold).
Since neither Rd1 nor Rd2 trans-activated the ORF57 promoter, the mutant protein expression plasmids were individually cotransfected with the wild-type Rta expression plasmid at different ratios to determine whether they inhibited trans-activation of wild-type Rta. Increasing amounts of pCMVFLAG/Rd1 or pCMVFLAG/Rd2 (5 to 500 ng) were cotransfected with constant amounts of pCMVFLAG/Rta (5 ng) and p57Luc (5 ng). As the ratio of Rd1 or Rd2 to Rta increased, the percentages of wild-type activity were gradually reduced (Fig. 2B). In cotransfections of 100-fold excess (500 ng) of either pCMVFLAG/Rd1 or pCMVFLAG/Rd2 with pCMVFLAG/Rta (5 ng), wild-type Rta activity was reduced by 87 and 95%, respectively.
The same transfections were also carried out in a hamster cell line, BHK-21 (Fig. 3). The level of induction by wild-type Rta was ≈6-fold higher in BHK-21 cells than in 293T cells. This was due to a lower basal activity of p57luc in BHK-21 cells than in 293T cells. In BHK-21 cells, low levels of trans-activation were detected for Rd1. This Rd1 activity was dose dependent, with induction up to 21.7-fold when 500 ng of plasmid DNA was transfected; however, this was only ≈2% of wild-type Rta activity (Fig. 3A). In contrast, no trans-activation was detected for Rd2. The results of cotransfection studies in BHK-21 cells (Fig. 3B) show a trend of inhibition of wild-type Rta activity similar to that seen in 293T cells (Fig. 2). Cotransfections of 100-fold pCMVFLAG/Rd1 or pCMVFLAG/Rd2 with pCMVFLAG/Rta resulted in a reduction in wild-type Rta activity by 77 and 83%, respectively.
MHV-68 Rta dominant-negative mutants inhibit viral lytic replication.
Transfection of purified MHV-68 virion DNA into fibroblasts leads to expression of viral lytic proteins and production of infectious viruses. Since Rd1 and Rd2 were able to inhibit trans-activation of wild-type Rta, we examined whether introducing these two Rta dominant-negative mutants could affect lytic replication initiated from transfected virion DNA. The virion DNA used in this study was isolated from a recombinant virus constructed in our laboratory, tw25, which contains the insertion of the EGFP expression cassette driven by the cytomegalovirus (CMV) promoter at the left end of the viral genome. The tw25 virus exhibits a growth curve similar to that of wild-type MHV-68 and allows us to monitor the infected cells, which fluoresce green upon exposure to UV light (data not shown).
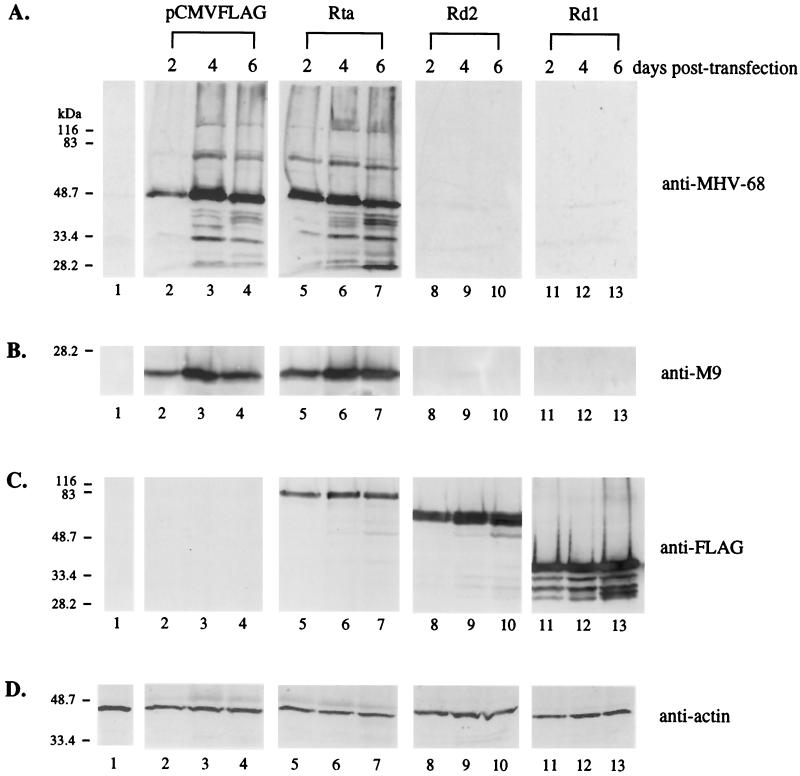
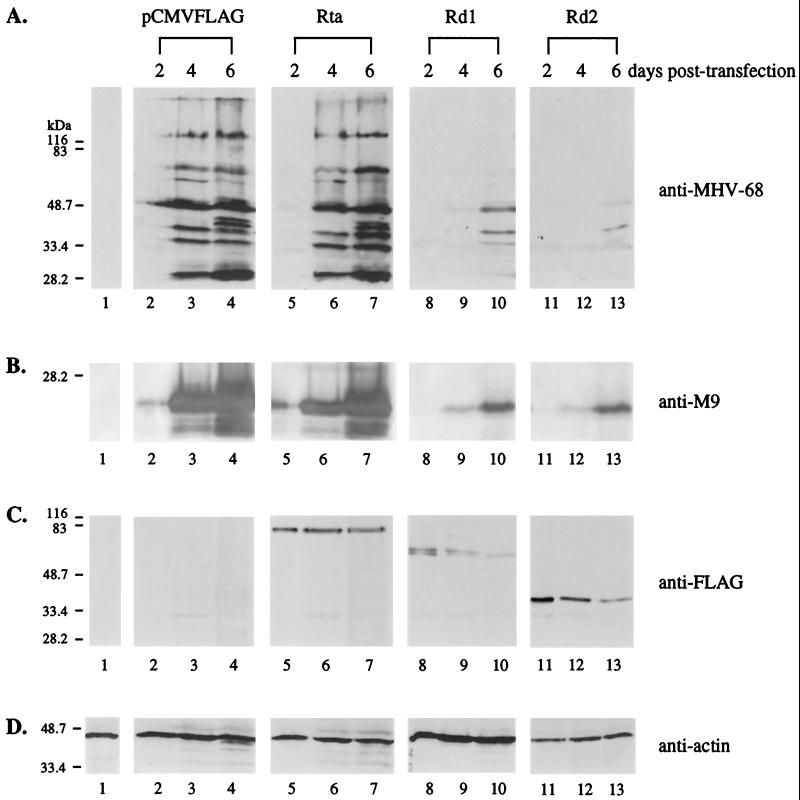
293T cells were cotransfected with equal amounts (0.2 μg each) of tw25 virion DNA and empty vector (pCMVFLAG) or the vector containing the expression cassette of wild-type Rta, Rd1, or Rd2. At 24 h posttransfection, the percentages of EGFP-positive cells were similar, indicating no difference in transfection efficiency. Expression of viral proteins was analyzed by Western blotting using anti-MHV-68 polyclonal serum, which recognizes multiple lytic antigens (Fig. 4A). At day 2 posttransfection, synthesis of viral proteins was detected in cells cotransfected with virion DNA and empty vector (Fig. 4A, lane 2); however, more viral proteins were produced in cells receiving virion DNA and pCMVFLAG/Rta (Fig. 4A, lane 5). The results suggest that wild-type Rta might promote and accelerate viral gene expression from transfected virion DNA. Expression of viral proteins was more pronounced at day 4 (Fig. 4A, lanes 3 and 6) and day 6 (Fig. 4A, lanes 4 and 7) posttransfection. In contrast, expression of viral lytic proteins was not detected at any time point in cells cotransfected with virion DNA and pCMVFLAG/Rd1 (Fig. 4A, lanes 8 to 10) or pCMVFLAG/Rd2 (Fig. 4A, lanes 11 to 13). During the course of the 6-day transfection experiment, no cell death was observed for the EGFP-positive cells (data not shown), indicating that inhibition of viral protein expression could not be attributed to apoptosis caused by overexpression of Rd1 or Rd2. We used a rabbit polyclonal antibody against the full-length MHV-68 M9 protein for Western blots. The M9 gene of MHV-68 has homology to a capsid gene (ORF65) of HHV-8 and has been shown to be expressed as a late gene (26). Rd1 and Rd2 also inhibited the expression of the M9 protein from transfected virion DNA (Fig. 4B, lanes 8 to 13). The expression of wild-type Rta, Rd1, and Rd2 was examined using the monoclonal antibody against the FLAG epitope (Fig. 4C). Although the same amounts of protein expression plasmid DNA were transfected, lower levels of wild-type Rta were detected relative to Rd1 or Rd2, suggesting that Rd1 and Rd2 might be more stable than wild-type Rta. The expression levels of all three proteins did not significantly change over time.
FIG. 4.
Rd1 and Rd2 inhibit MHV-68 lytic protein expression from transfected virion DNA in 293T cells. pCMVFLAG (0.2 μg; lanes 2 to 4), pCMVFLAG/Rta (0.2 μg; lanes 5 to 7), pCMVFLAG/Rd1 (0.2 μg; lanes 8 to 10), or pCMVFLAG/Rd2 (0.2 μg; lanes 11 to 13) was cotransfected into 293T cells with 0.2 μg of virion DNA derived from the recombinant green fluorescent protein-expressing virus tw25. Total cell lysates were harvested at 2, 4, and 6 days posttransfection (as indicated above the lanes), and 10% of each lysate, including a negative control of untransfected cells (lanes 1), was used for Western blot analysis. (A) Expression of MHV-68 lytic proteins is suppressed by Rd1 and Rd2. The membrane was probed with the polyclonal rabbit serum against the MHV-68-infected cell lysates (anti-MHV-68). (B) Expression of MHV-68 M9 protein is suppressed by Rd1 and Rd2. The membrane was probed with anti-M9 polyclonal rabbit serum. (C) Wild-type and mutant Rta proteins are expressed in 293T cells. The membrane was probed with the monoclonal antibody against the FLAG epitope (anti-FLAG). (D) The levels of cellular β-actin were examined (anti-actin). The membrane was probed with monoclonal antibody against cellular β-actin.
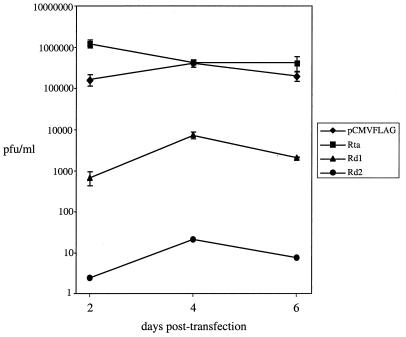
The viral titers in the supernatants from transfections were measured by plaque assays (Fig. 5). At day 2 posttransfection, there was ≈7.5-fold increase in the level of infectious viruses produced from transfections of wild-type Rta with virion DNA compared to the control transfection of empty vector with virion DNA. This result is consistent with the Western blot analysis data showing that more viral proteins were synthesized when wild-type Rta was cotransfected with virion DNA (Fig. 4A and B, lanes 2 to 7). However, when pCMVFLAG/Rd1 was cotransfected with virion DNA, the level of infectious viruses was reduced 240-fold compared to the control transfection of empty vector with virion DNA. Cotransfections of pCMVFLAG/Rd2 with virion DNA led to even further reduction in virus production (≈7 × 104-fold). Although the level of infectious viruses produced from cotransfections of virion DNA and pCMV/FLAG/Rd1 or pCMVFLAG/Rd2 increased 10-fold between day 2 and day 4 posttransfection, it was still much lower than in cotransfections of virion DNA and empty vector. No further increase was detected at day 6 posttransfection. The results are consistent with the Western blot analysis results (Fig. 4A and B, lanes 8 to 13), indicating that Rd1 and Rd2 inhibit virus lytic replication after transfection of virion DNA.
FIG. 5.
Rd1 and Rd2 inhibit the production of infectious viruses after transfection of virion DNA in 293T cells. The supernatants from the transfections described in the legend to Fig. 4 were harvested, and the viral titers were determined using plaque assays. The assays were repeated three times for each transfection. Standard deviations are expressed as error bars.
The same cotransfections were repeated in BHK-21 cells. The transfection efficiencies based on examining the EGFP-positive cells were similar in all samples. Inhibition by Rd1 and Rd2 of viral lytic protein expression from transfected virion DNA was also detected (Fig. 6). At days 4 and 6 posttransfection, high levels of viral proteins were synthesized in cells cotransfected with virion DNA and pCMVFLAG (Fig. 6A, lanes 3 and 4) or pCMVFLAG/Rta (Fig. 6A, lanes 6 and 7). Very little viral protein was detected in cotransfections of virion DNA with pCMVFLAG/Rd1 (Fig. 6A, lanes 8 and 9) or pCMVFLAG/Rd2 (Fig. 6A, lanes 11 and 12) at days 2 and 4 posttransfection. However, the amounts of viral proteins were increased from day 4 to day 6 posttransfection (Fig. 6A, lanes 10 and 13) but were still much less than in the control transfections (Fig. 6A, lanes 3 and 4). Rd1 and Rd2 also inhibited the expression of the MHV-68 M9 protein (Fig. 6B, lanes 8 to 13). The levels of the three FLAG-tagged Rta proteins in BHK-21 cells, unlike expression in 293T cells (Fig. 4C), decreased over time (Fig. 6C). At day 6 posttransfection, expression of Rd1 and Rd2 was barely detectable (Fig. 6C, lanes 10 and 13). The level of inhibition by Rd1 and Rd2 mirrored the expression level of the proteins, indicating that Rd1 and Rd2 suppressed expression of viral lytic proteins from transfected virion DNA.
FIG. 6.
Rd1 and Rd2 inhibit MHV-68 lytic protein expression from transfected virion DNA in BHK-21 cells. The transfections described in the legend to Fig. 4 were repeated in BHK-21 cells. Total cell lysates were harvested at 2, 4, and 6 days posttransfection (as indicated above the lanes), and 10% of each lysate, including a negative control of untransfected cells (lanes 1), was used for Western blot analysis. (A) Expression of MHV-68 lytic proteins is suppressed by Rd1 and Rd2. The membrane was probed with the polyclonal serum against MHV-68-infected cell lysates (anti-MHV-68). (B) Expression of MHV-68 M9 protein is suppressed by Rd1 and Rd2. The membrane was probed with a rabbit serum against the MHV-68 M9 protein (anti-M9). (C) Wild-type and mutant Rta proteins are expressed in BHK-21 cells. The membrane was probed with the monoclonal antibody against the FLAG epitope (anti-FLAG). (D) The levels of cellular β-actin were examined. The membrane was probed with monoclonal antibody against cellular β-actin (anti-actin).
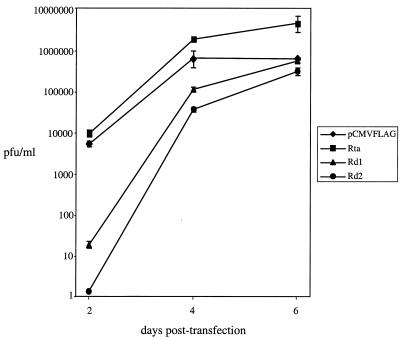
The production of infectious viruses from transfected BHK-21 cells was examined (Fig. 7). Wild-type Rta also enhanced the production of infectious viruses by ≈2- to 3-fold at day 4 and ≈7-fold at day 6 posttransfection, consistent with the data obtained from 293T cells. The maximal amount of infectious viruses produced from BHK-21 cells cotransfected with virion DNA and empty vector (Fig. 7) was similar to that from transfected 293T cells (Fig. 5), but the kinetics of virus production were slower. At day 2 posttransfection, virus production from cotransfection of virion DNA and pCMVFLAG/Rd1 or pCMVFLAG/Rd2 was reduced 290- (Rd1) and 4,000-fold (Rd2) compared to cotransfection of virion DNA and pCMVFLAG. The virus yield from cotransfections of virion DNA and pCMVFLAG/Rd1 or pCMVFLAG/Rd2 was greatly increased from day 2 to day 4 posttransfection, when the levels of Rd1 and Rd2 were declining (Fig. 6C). The viral titers were further increased from day 4 to day 6 posttransfection and were comparable to or slightly (twofold) lower than the maximal viral titer obtained in the control cotransfection of virion DNA and pCMVFLAG. The data indicate that Rd1 and Rd2 block virus production in BHK-21 cells and that this block is alleviated when the levels of Rd1 and Rd2 are reduced.
FIG. 7.
Rd1 and Rd2 inhibit the production of infectious viruses after transfection of virion DNA in BHK-21 cells. The supernatants from the transfections described in the legend to Fig. 6 were harvested, and the viral titers were determined using plaque assays. The assays were repeated three times for each transfection. Standard deviations are expressed as error bars.
Viral replication is suppressed in cell lines constitutively expressing Rd2.
In transient cotransfections of virion DNA and the plasmids expressing Rd1 and Rd2, the Rta dominant-negative mutants inhibit expression of viral lytic proteins and production of infectious viruses. Since Rd2 exhibited more prominent inhibitory effects than Rd1 (Fig. 2 to 7), we established stable 293T cell lines constitutively expressing Rd2 to examine how productive de novo infection was affected in the presence of Rd2. Two cell clones with the highest expression levels of Rd2, 45-5 and V-30, were used for MHV-68 infection.
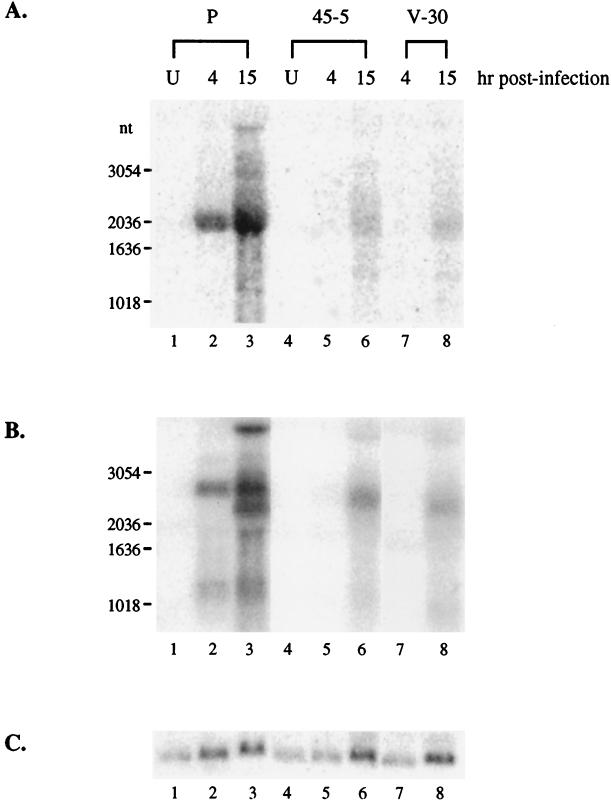
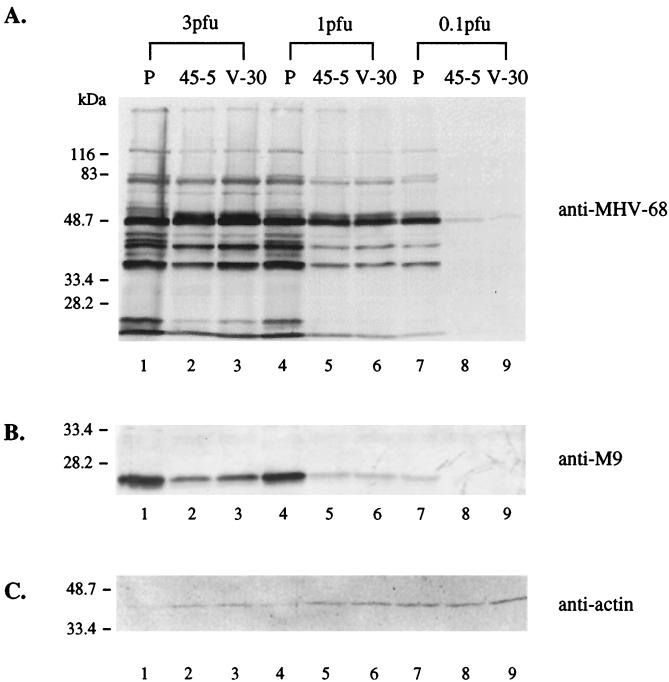
We first determined the stage of MHV-68 infection at which lytic replication was affected by examining the transcription of viral genes. A high multiplicity of infection (MOI) was used to detect the less abundant rta transcript. The three cell lines, parental 293T, 45-5, and V-30, were infected at 3 PFU/cell, and total RNA was harvested at 4 and 15 h postinfection for Northern blot analysis. To study transcription of the rta gene, we used a probe derived from the 0.7-kb region at the 3′ end of the rta gene, so the probe was not hybridized to the transcript generated from pCMVFLAG/Rd2 (Fig. 8A, lane 4). It has been shown that the rta gene of MHV-68 is expressed as an immediate-early gene during productive de novo infection (11, 26). In parental 293T cells, a major 2-kb band was detected as early as 4 h, and the intensity was greater at 15 h postinfection (Fig. 8A, lanes 2 and 3). The size of the rta transcript in 293T cells is consistent with that in BHK-21 cells (26). In 45-5 and V-30 cells, the 2-kb rta transcript was not detected until 15 h postinfection, and the intensity was much lower than that in parental 293T cells. These results indicate that transcription of the rta gene is inhibited in the presence of Rd2. Similar inhibitory effects were obtained when analyzing transcription of a viral early gene, the thymidine kinase gene (tk), which was greatly reduced in 45-5 and V-30 cells compared to 293T parental cells. These findings suggest that expression of viral lytic genes is impaired at a very early stage following MHV-68 infection of Rd2-expressing cells. This is not due to 45-5 and V-30 cells becoming less infectible by MHV-68. We infected parental 293T, 45-5, and V-30 cells with a recombinant MHV-68 containing the expression cassette of the β-galactocidase marker driven by the CMV immediate-early promoter. At 18 h postinfection, the percentages of β-galactosidase-positive cells were similar in the three cell lines, indicating that there were no differences in their susceptibilities to MHV-68 infection (data not shown).
FIG. 8.
Transcription of tk and rta genes is suppressed in stable cell lines expressing Rd2. Two cell lines, 45-5 and V-30, stably transfected with pCMVFLAG/Rd2 and the parental 293T cells (P) were infected with wild-type MHV-68 (3 PFU/cell). Total RNA from uninfected (U) or infected cells was harvested at 4 or 15 h postinfection, and 30% of each RNA sample was used for Northern blot analysis. (A) Transcription of rta is reduced in 45-5 and V-30 cells. The probe was derived from the rta gene (nt 68651 to 69378). (B) Transcription of tk is reduced in 45-5 and V-30 cells. The same membrane was stripped and rehybridized with a probe derived from the tk gene (nt 32879 to 34813). (C) The RNA loadings were examined by rehybridizing with a probe derived from the cellular GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene.
We next examined the expression of viral proteins in cells infected at different MOIs. Cell lysates were harvested at day 2 postinfection and analyzed by Western blotting, using anti-MHV-68 polyclonal rabbit serum (Fig. 9A) or a polyclonal antibody against the recombinant MHV-68 M9 protein (Fig. 9B). The expression of viral proteins, including M9, was reduced in 45-5 and V-30 cells compared to parental 293T cells at the MOIs examined. However, the reduction was more profound at lower MOIs.
FIG. 9.
Viral protein expression is inhibited in stable cell lines expressing Rd2. 45-5, V-30, and the parental 293T (P) cells were infected with wild-type MHV-68 at the different MOIs (PFU/cell) indicated above the panel. Total cell lysates were harvested at day 2 postinfection, and 10% of each lysate was used for Western blot analyses. (A) Expression of viral lytic proteins is reduced in 45-5 and V-30 cells. The membrane was probed with polyclonal serum against MHV-68-infected cell lysates. (B) Expression of M9 (a viral late protein) is reduced in 45-5 and V-30 cells. The membrane was probed with polyclonal antibody against the recombinant MHV-68 M9 protein. (C) The protein loadings were examined by reprobing with monoclonal antibody against cellular β-actin.
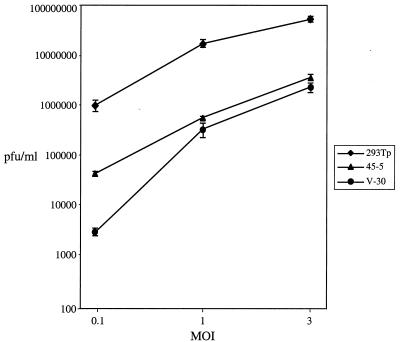
The viral titers in the supernatants collected at day 2 postinfection from infected cells were measured by plaque assay (Fig. 10). At a low MOI of 0.1 PFU/cell, virus production was reduced ≈24-fold in 45-5 cells and ≈360-fold in V-30 cells relative to that in parental 293T cells. When the cells were infected at a high MOI of 3 PFU/cell, there was a 15-fold reduction in virus production from 45-5 cells and a 23-fold reduction from V-30 cells relative to that produced from parental 293T cells.
FIG. 10.
The yield of infectious viruses is reduced in cell lines expressing Rd2. The supernatants were harvested from the infections described in the legend to Fig. 9, and viral titers were determined using plaque assays. The assays were performed three times for each infection, and standard deviations are expressed as error bars.
DISCUSSION
Our previous studies have shown that MHV-68 Rta is sufficient to reactivate MHV-68 and drive the lytic cycle to completion in latently infected B cells (26). In this study, the function of Rta during productive infection of permissive 293T and BHK-21 cells was examined. We constructed two mutants of MHV-68 Rta, Rd1 and Rd2, with 112 (Rd1) and 243 (Rd2) aa deleted from the C terminus, where the putative trans-activation domain is located. Rd1 and Rd2 were unable to activate the MHV-68 ORF57 promoter and functioned as dominant-negative mutants to inhibit trans-activation of wild-type Rta. Cotransfection of the Rd1 or Rd2 expression plasmid with MHV-68 virion DNA suppressed the synthesis of viral proteins and the production of infectious virions. Furthermore, viral lytic replication was inhibited in cells constitutively expressing Rd2. Therefore, these results provide strong evidence supporting the pivotal role of Rta during productive infection of permissive cells by MHV-68 in addition to viral reactivation in latently infected B cells.
MHV-68 Rta was previously demonstrated to activate the ORF57 promoter (11). Our study shows that C-terminal deletions in Rta abrogated its ability to trans-activate, consistent with the hypothesis that a trans-activation domain resides at the C terminus. In the presence of Rd1 or Rd2, activation of the ORF57 promoter by wild-type Rta was reduced. Thus, Rd1 and Rd2 exhibit dominant inhibitory effects on Rta trans-activation. This is not due to nonspecific inhibition by high levels of Rd1 or Rd2, since the activity of the internal control containing the Renilla luciferase reporter driven by the CMV promoter was not significantly reduced (data not shown). However, further investigation will be required to elucidate the mechanism by which the dominant-negative mutants disrupt the function of wild-type Rta. One possibility is that the mutants dimerize with wild-type Rta and these heterodimers are no longer able to activate transcription of the reporter gene. Although the N terminus of MHV-68 Rta shares significant homology with EBV Rta, which was shown to dimerize, it has yet to be proven whether MHV-68 Rta or the mutants can form homo- or heterodimers. Another possibility is that the dominant-negative mutants compete for binding to the promoter, thereby preventing wild-type Rta from binding and activating the promoter.
The results from this study clearly suggest that Rta trans-activation is essential for MHV-68 lytic replication in permissive cells. When MHV-68 lytic replication was initiated by transfection of purified virion DNA into fibroblasts, cotransfection of either Rd1 or Rd2 expression plasmid suppressed expression of viral lytic proteins and virus production (Fig. 4 to 7). Productive de novo infection was severely compromised in cell lines constitutively expressing Rd2 (Fig. 8 to 10). The inhibitory effects of the Rta dominant-negative mutants are most likely due to their ability to inhibit the trans-activation function of wild-type Rta synthesized from the viral genome. Moreover, Rd2 was more effective than Rd1 in suppressing viral lytic replication in cotransfection studies (Fig. 4 to 7), which correlates with the greater inhibition of wild-type Rta trans-activation by Rd2 (Fig. 2 and 3). We hypothesize that Rd2 exhibits more prominent dominant inhibitory effects because Rd2 has a larger truncation and thus has less residual trans-activation activity than Rd1.
The inhibitory effects of the dominant-negative mutants on viral lytic replication were consistently more pronounced in 293T cells than in BHK-21 cells (Fig. 5 versus Fig. 7). One explanation for this result is differential protein expression in the two cell lines. The expression levels of the Rta dominant-negative mutants decreased with time in BHK-21 cells compared to 293T cells (Fig. 4 versus Fig. 6). The transcripts for Rd1 and Rd2 were expressed from pCMVFLAG-based vectors, which contain the SV40 replication origin. Therefore, we would expect the protein expression levels to be greater and more sustained in 293T cells, which constitutively express the SV40 T antigen.
Upon infection of Rd2-expressing stable cell lines (45-5 and V-30), viral replication was significantly impaired, with the greatest inhibitory effects observed at the lowest MOI of 0.1 PFU/cell compared to 1 or 3 (Fig. 8 to 10). Our interpretation of this result is that cells infected at higher MOIs contain greater numbers of viral genomes and thus express higher levels of wild-type Rta, overcoming the inhibition of Rd2. As the MOI of MHV-68 increases, the ratio of Rd2 to wild-type Rta synthesized from the virus decreases, resulting in less repression of Rta function and viral lytic replication. Although 45-5 and V-30 cells express high levels of Rd2, it remains to be determined whether these levels are sufficient to completely block wild-type Rta function and viral lytic replication.
We have previously proposed a model for the function of MHV-68 Rta (26). In this model, Rta is the central viral factor determining the outcome of MHV-68 infection, lytic replication or latency. During productive de novo infection of permissive cells, Rta is one of the earliest viral proteins to be expressed and functions as a transcriptional activator to induce the cascade of viral lytic gene expression, which ultimately leads to viral DNA replication and production of infectious virions. Upon infection of nonpermissive cells, Rta expression is suppressed, preventing the initiation of lytic replication, and the virus establishes a latent infection. In response to certain stimuli, Rta expression is activated or derepressed, latency is disrupted, and the virus undergoes reactivation, entering the productive phase. This model is supported by our previous finding that ectopic Rta expression in latently infected cells activates the entire viral lytic cycle (26) and the results from this study, which demonstrate that the inhibition of Rta trans-activation blocks viral lytic replication in permissive cells. Moreover, expression of viral proteins and production of infectious viruses is enhanced by overexpression of Rta (Fig. 4 to 7). Therefore, Rta plays a key role in initiating viral lytic replication, not only during reactivation in latently infected nonpermissive cells but also during de novo infection of permissive cells. This study also suggests that the function of Rta is most likely mediated through its ability to trans-activate. Our preliminary data showed that the Rta dominant-negative mutants, Rd1 and Rd2, failed to reactivate the virus in latently infected cells and interfered with wild-type Rta reactivation of latent viruses (data not shown).
In the alpha and beta subfamilies of herpesviruses, more than one immediate-early gene is expressed and controls the initiation of lytic gene expression. We do not know whether gammaherpesvirus MHV-68 encodes other immediate-early proteins in addition to Rta. EBV encodes two immediate-early proteins, ZEBRA and Rta, and both are important in activating the viral lytic cycle in latently infected cells. KSHV Rta is an immediate-early protein and is sufficient to induce viral lytic replication. KSHV also encodes a homologue of ZEBRA, but KSHV ZEBRA is an early lytic protein following reactivation and is incapable of reactivating latent virus. It appears that MHV-68 does not encode a ZEBRA homologue. Moreover, since MHV-68 Rta alone can reactivate latent virus and inhibition of Rta function is sufficient to block viral lytic replication at an early stage in permissive cells, other unidentified immediate-early genes, if there are any, may not play a major role in activating the cascade of viral gene expression.
The regulation of Rta expression plays a primary role in determining the outcome of MHV-68 infection and controlling the switch from latency to lytic replication. However, the mechanisms by which Rta expression is regulated are not clear. Cellular factors are likely involved in activating expression of immediate-early genes, since transfection of purified virion MHV-68 DNA is sufficient to initiate viral lytic replication. Another possible regulator of Rta expression is Rta itself. Autoactivation of the rta gene during reactivation has been demonstrated for KSHV and has been proposed to be an important strategy to enhance Rta expression and maximally activate the viral lytic cycle (6, 8). Our results show that transcription of MHV-68 rta following productive virus infection was repressed in Rd2-expressing cells, consistent with the interpretation that Rd2 interferes with Rta autoactivation.
Using MHV-68, we can manipulate Rta expression to create a virus that constitutively enters the lytic cycle or a virus that is quiescent even in permissive cells. These recombinant viruses will be valuable reagents to examine the role of viral latency and lytic replication in viral pathogenesis, using infection of mice as a model.
ACKNOWLEDGMENTS
We thank Helen Brown, Tonia Symensma, and Iglika Pavlova for critical comments and Wendy Aft for editing the manuscript.
This work is supported by a Frontiers of Science Award, a Stein Oppenheimer Award, and a Stop Cancer Career Development Award. T.-T.W. is supported by a fellowship from the Cancer Research Institute.
REFERENCES
- 1.Buisson M, Manet E, Trescol-Biemont M C, Gruffat H, Durand B, Sergeant A. The Epstein-Barr virus (EBV) early protein EB2 is a posttranscriptional activator expressed under the control of EBV transcription factors EB1 and R. J Virol. 1989;63:5276–5284. doi: 10.1128/jvi.63.12.5276-5284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chevallier-Greco A, Manet E, Chavrier P, Mosnier C, Daillie J, Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Countryman J, Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci USA. 1985;82:4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox M A, Leahy J, Hardwick J M. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J Virol. 1990;64:313–321. doi: 10.1128/jvi.64.1.313-321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng H, Young A, Sun R. Auto-activation of the rta gene of human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus. J Gen Virol. 2000;81:3043–3048. doi: 10.1099/0022-1317-81-12-3043. [DOI] [PubMed] [Google Scholar]
- 7.Farrell R E J. RNA methodologies. San Diego, Calif: Academic Press Inc.; 1993. pp. 135–138. [Google Scholar]
- 8.Gradoville L, Gerlach J, Grogan E, Shedd D, Nikiforow S, Metroka C, Miller G. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J Virol. 2000;74:6207–6212. doi: 10.1128/jvi.74.13.6207-6212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenney S, Holley-Guthrie E, Mar E C, Smith M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J Virol. 1989;63:3878–3883. doi: 10.1128/jvi.63.9.3878-3883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieberman P M, Hardwick J M, Sample J, Hayward G S, Hayward S D. The zta transactivator involved in induction of lytic cycle gene expression in Epstein-Barr virus-infected lymphocytes binds to both AP-1 and ZRE sites in target promoter and enhancer regions. J Virol. 1990;64:1143–1155. doi: 10.1128/jvi.64.3.1143-1155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Pavlova I V, Virgin IV H W, Speck S H. Characterization of gammaherpesvirus 68 gene 50 transcription. J Virol. 2000;74:2029–2037. doi: 10.1128/jvi.74.4.2029-2037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukac D M, Kirshner J R, Ganem D. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol. 1999;73:9348–9361. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukac D M, Renne R, Kirshner J R, Ganem D. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- 14.Manet E, Gruffat H, Trescol B M C, Moreno N, Chambard P, Giot J F, Sergeant A. Epstein-Barr virus bicistronic mRNAs generated by facultative splicing code for two transcriptional trans-activators. EMBO J. 1989;8:1819–1826. doi: 10.1002/j.1460-2075.1989.tb03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manet E, Rigolet A, Gruffat H, Giot J F, Sergeant A. Domains of the Epstein-Barr virus (EBV) transcription factor R required for dimerization, DNA binding and activation. Nucleic Acids Res. 1991;19:2661–2667. doi: 10.1093/nar/19.10.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nash A A, Sunil-Chandra N P. Interactions of the murine gammaherpesvirus with the immune system. Curr Opin Immunol. 1994;6:560–563. doi: 10.1016/0952-7915(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 17.Nash A A, Usherwood E J, Stewart J P. Immunological features of murine gammaherpesvirus infection. Semin Virol. 1996;7:125–130. [Google Scholar]
- 18.Nicholas J, Coles L S, Newman C, Honess R W. Regulation of the herpesvirus saimiri (HVS) delayed-early 110-kilodalton promoter by HVS immediate-early gene products and a homolog of the Epstein-Barr virus R trans activator. J Virol. 1991;65:2457–2466. doi: 10.1128/jvi.65.5.2457-2466.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ragoczy T, Heston L, Miller G. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J Virol. 1998;72:7978–7984. doi: 10.1128/jvi.72.10.7978-7984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarawar S R, Cardin R D, Brooks J W, Mehrpooya M, Tripp R A, Doherty P C. Cytokine production in the immune response to murine gammaherpesvirus 68. J Virol. 1996;70:3264–3268. doi: 10.1128/jvi.70.5.3264-3268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson P G, Doherty P C. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J Virol. 1998;72:943–949. doi: 10.1128/jvi.72.2.943-949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun R, Lin S F, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sunil-Chandra N P, Efstathiou S, Arno J, Nash A A. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J Gen Virol. 1992;73:2347–2356. doi: 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- 24.van Santen V L. Characterization of a bovine herpesvirus 4 immediate-early RNA encoding a homolog of the Epstein-Barr virus R transactivator. J Virol. 1993;67:773–784. doi: 10.1128/jvi.67.2.773-784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virgin H W, IV, Latreille P, Wamsley P, Hallsworth K, Weck K E, Dal Canto A J, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu T T, Usherwood E J, Stewart J P, Nash A A, Sun R. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J Virol. 2000;74:3659–3667. doi: 10.1128/jvi.74.8.3659-3667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zalani S, Holley-Guthrie E, Kenney S. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc Natl Acad Sci USA. 1996;93:9194–9199. doi: 10.1073/pnas.93.17.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]