Abstract
Infertility, affecting one in six couples, is often related to the male partner’s congenital and/or environmental conditions or complications postsurgery. This retrospective study examines the link between orchiopexy for undescended testicles (UDT) and testicular torsion (TT) in childhood and adult fertility as assessed through sperm analysis. The study involved the analysis of semen samples from 7743 patients collected at Soroka University Medical Center (Beer Sheva, Israel) between January 2009 and December 2017. Patients were classified into two groups based on sperm concentration: those with concentrations below 5 × 106 sperm per ml (AS group) and those above (MN group). Medical records and surgical histories were reviewed, categorizing orchiopexies by surgical approach. Among 140 individuals who had undergone pediatric surgery, 83 (59.3%) were placed in the MN group and 57 (40.7%) in the AS group. A higher likelihood of being in the MN group was observed in Jewish compared to Arab patients (75.9% vs 24.1%, P = 0.006). In cases of childhood UDT, 45 (78.9%) patients exhibited sperm concentrations below 5 × 106 sperm per ml (P < 0.001), and 66 (76.7%) had undergone unilateral and 18 (20.9%) bilateral orchiopexy. Bilateral orchiopexy was significantly associated with lower sperm concentration, total motility, and progressive motility than unilateral cases (P = 0.014, P = 0.001, and P = 0.031, respectively). Multivariate analysis identified UDT as a weak risk factor for low sperm concentration (odds ratio [OR]: 2.712, P = 0.078), with bilateral UDT further increasing this risk (OR: 6.314, P = 0.012). Jewish ethnicity and TT diagnosis were associated with a reduced risk of sperm concentrations below 5 × 106 sperm per ml. The findings indicate that initial diagnosis, surgical approach, and ethnicity markedly influence male fertility outcomes following pediatric orchiopexy.
Keywords: male infertility, orchiopexy, sperm analysis, testicular torsion, undescended testicle
INTRODUCTION
Approximately 17.5% of the adult population – roughly 1 in 6 worldwide – experience infertility.1 Male infertility, which may have a role in up to half of infertility cases, has multifactorial causes, such as developmental congenital errors, environmental causes, and inadvertent injury from surgery.2,3
Surgical procedures involving the male reproductive system are common in the pediatric period4 and are designed to treat congenital disorders such as undescended testicles (UDT), as well as acquired complications such as testicular torsion (TT).
UDT, also known as cryptorchidism, is a condition in which the testicle is not properly located in the scrotum but can be found in either its normal descent path or an ectopic position.5,6 UDT occurs in 1%–3% of infants and in up to 30% of preterm infants.7
TT is a severe urological emergency primarily affecting 1 in 4000 male children and adolescents up to the age of 25 years.8 TT occurs when the testis rotates along the axis of the spermatic cord attachments, limiting blood supply to the testis and requiring surgery to restore blood flow.9 A delay in surgery may lead to recalcitrant ischemia of the testicular tissue, which can result in extensive damage or necrosis.10,11 Furthermore, reperfusion might harm the testis because of elevated testicular oxidative stress and antisperm antibodies.11,12
UDT and TT are two diagnoses leading to orchiopexy, a surgical procedure that includes testicle placement and fixation. Orchiopexy has been found to be anatomically effective in 95% of cases.13 The absence of atrophy and correct placement of the testicle is defined as surgical success.14,15 However, successful or early surgery reduces but does not eliminate the possibility of future harm, such as poor sperm quality, infertility, and testicular cancer.13,16,17 UDT is associated with 30%–60% risk of infertility in adults,13 and orchiopexy should be performed early to prevent germ cell loss.18
In accordance with the World Health Organization (WHO) guidelines, a sperm concentration of 15 × 106 per ml or less is identified as a potential indicator of fertility issues, representing the lower 5th percentile from the analysis of men who have achieved conception within 12 months without medical intervention.19,20 However, some studies suggest that lower concentrations can still lead to spontaneous pregnancies.21,22,23
In addressing male infertility and considering the use of in vitro fertilization (IVF) with intracytoplasmic sperm injection (ICSI), particularly important is the distinction in sperm concentration. ICSI is effective in severe oligozoospermia (<5 × 106 sperm per ml) in cryptozoospermia and in cases of azoospermia with the presence of testicular sperm.24,25 ICSI is more resource-intensive and carries higher risks than conventional IVF.26 Other parameters used to assess male fertility and the use of ICSI are sperm motility, progressive motility, sperm morphology, and total motile sperm count.
A semen analysis (SA) is typically used to assess these parameters and determine if male infertility may contribute to the couple’s infertility.19
The aim of the present study is to explore the impact of orchiopexy surgery and its subtypes on semen analysis, focusing on how different types of diagnoses and surgeries related to sperm parameters. We analyzed adults who underwent inguinoscrotal surgeries during childhood and, in adulthood, visited our andrology laboratory for semen analysis.
PATIENTS AND METHODS
Patients
A retrospective case–control study was conducted of 7743 patients who underwent semen analysis (SA) in the Reproductive Unit at Soroka University Medical Center (SUMC), Beer Sheva, Israel, from January 2009 to December 2017. SUMC is a tertiary medical facility where semen analysis is performed for the population of Israel’s Southern region. The study protocol received approval from the SUMC Ethics Committee (Approval No. ROS-0049-71); patient consent was waived due to the retrospective nature of the study.
Inclusion and exclusion criteria
The study comprised all semen samples obtained from patients aged 18 years or above, collected as part of the standard assessment of infertile couples in the region.
Exclusion criteria involved patients with a known cause of infertility, such as previous chemotherapy or radiotherapy, genetic abnormalities such as 47,XXY or Y-microdeletion, severe kidney or liver damage, or any severe chronic illness.
Data collection
The medical records, including surgical details of all patients, were obtained from the hospital’s information management system. The data received included general characteristics such as age, ethnicity, past medical history, diabetes mellitus, hypertension, body mass index (BMI), surgical history, and smoking history, as well as sperm analysis and endocrine evaluation, if available. Orchiopexy for TT had all been performed as open surgeries, using scrotal approach, while orchiopexy for UDT repair had all been performed as open surgery with an inguinal approach.
The data on sperm parameters were obtained from the computerized records of the Clinical Andrology Laboratory. This laboratory is subject to ongoing external quality assurance monitoring by the UK National External Quality Assessment Services (NEQAS), Sub-Fertility Laboratory at Saint Mary’s Hospital in Manchester, UK.27
Semen analysis
Before the semen analysis, patients were advised to abstain from sexual activity for 3 days.20,28 The actual duration of abstinence was documented upon their arrival at the laboratory. Samples were produced privately by masturbation into a sterile plastic container used for urine culture analysis. The specimens were delivered to the laboratory between 30 min and 60 min. All sperm samples were analyzed using the Makler counting chamber (Sefi-Medical Instruments, Haifa, Israel) by the same laboratory technician team for the following routine parameters: semen volume, total sperm count, sperm concentration, percentage of motile sperm, and percentage of normal morphology. Sperm morphology was evaluated by a laboratory technician using methodological examination according to the strict Kruger–Tygerberg criteria.29
About half of the patients underwent more than one SA. A paired t-test was employed to evaluate the differences between the initial and subsequent tests, indicating no statistically significant inequalities. Therefore, only the values obtained from the initial test were chosen for further investigation.
Categorization of patients
Patients were divided into two major groups based on their sperm concentration: AS – azoospermia or severe oligozoospermia (0–5 × 106 sperm per ml) and MN – normal sperm concentrations and nonsevere oligozoospermia (more than 5 × 106 sperm per ml). For the purpose of this study, patients whose sperm count indicated azoospermia and severe oligospermia were assigned to the AS group.30 A threshold of 5 × 106 sperm per ml was employed to identify patients with significant reproductive challenges, potentially necessitating ICSI.31 This threshold is below the WHO-recommended benchmark for potential fertility issues, facilitating a targeted approach to fertility treatment. Individuals with a sperm concentration over 5 × 106 sperm per ml were categorized as MN group, which suggests a better sperm concentration and a reduced likelihood of requiring ICSI. This division allowed a practical and meaningful statistical analysis and investigation of the impact of testicular surgery on individuals with severe fertility challenges, providing distinct and distinguishable groups for comparison.
Statistical analyses
A descriptive statistic based on demographic, laboratory, and clinical findings is provided. Continuous variables with a normal distribution are presented as mean and standard deviation (s.d.), whereas ordinal variables or continuous variables with a nonnormal distribution are presented as median with interquartile range (IQR). Categorical variables are shown as counts and percentages of all available patients.
If parametric assumptions were not met, the variable comparison was performed using analysis of variance (ANOVA) for continuous variables and Kruskal–Wallis as a nonparametric alternative. The normal plot was used to evaluate the assumptions of the metric model. The Chi-squared or Fisher’s exact test was used to compare categorical variables, as appropriate.
All statistical tests were run with a significance level of P = 0.05 (two-sided). All statistical analysis was made by the Statistical Package for the Social Sciences for Windows (version 22, released 2013; IBM Corp., Armonk, NY, USA). The data presented in the present study are available upon reasonable request from the corresponding author.
RESULTS
Of 7743 individuals initially assessed for potential inclusion in the study, 189 (2.4%) patients had a history of previous surgery in the inguinoscrotal area. Complete data were available for 147 patients. Of them, 7 patients were subsequently excluded from the analysis due to known causes of infertility. One hundred and sixteen patients had undergone orchiopexy, while 24 had other surgical interventions. Most patients (74.1%, n = 86) underwent orchiopexy for UDT and 30 (25.9%) for TT (Figure 1). Among the study population, the median abstinence time for SA was 3 (IQR: 2–5) days. Hormonal test results were available for 29 individuals (20.7%), leading to the elimination of hormonal evaluation from the fertility assessment.
Figure 1.
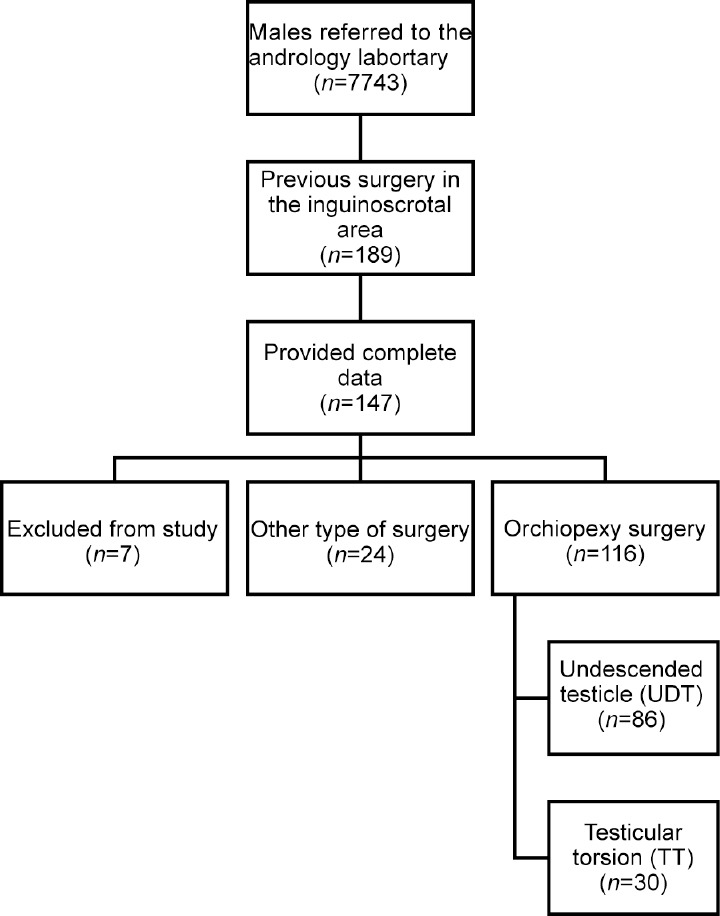
Schematic representation of the research progression illustrating patient inclusion. Each square denotes the number of patients retained at each stage. UDT: undescended testicle; TT: testicular torsion.
Divided by sperm concentration, 83 (59.3%) patients were classified as part of the MN group, whereas 57 (40.7%) patients were classified as part of the AS group.
Table 1 displays patient characteristics, initial diagnosis, and procedures categorized by MN and AS groups. A significantly higher proportion of the Jewish population versus the Arab population were classified as part of the MN group (63 [75.9%] vs 20 [24.1%]; P = 0.006). No significant differences were found between the groups in the surgery age (P = 0.279), age at semen analysis (P = 0.657), BMI (P = 0.656), smoking status (P = 0.705), or the presence of comorbidities such as diabetes mellitus, hypertension, and dyslipidemia (all P > 0.05).
Table 1.
Patient characteristics and initial diagnosis
| Characteristic | All patients (n=140) | MN (n=83; 59.3%) | AS (n=57; 40.7%) | P |
|---|---|---|---|---|
| Age at the operation (year), mean (s.d.) | 9.6 (7.8) | 10.2 (7.9) | 8.8 (7.7) | 0.279 |
| Age at SA (year), mean (s.d.) | 28.9 (6.1) | 28.8 (6.6) | 28.8 (7.0) | 0.657 |
| Jewish, n (%) | 93 (66.4) | 63 (75.9) | 30 (52.6) | 0.006 |
| BMI (kg m−2), mean (s.d.) | 26.7 (5.1) | 26.3 (4.9) | 27.4 (5.3) | 0.656 |
| Smoker, n (%) | 76 (54.2) | 44 (53.0) | 32 (56.1) | 0.705 |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 7 (5.0) | 4 (4.8) | 3 (5.3) | 1.000 |
| Hypertension | 3 (2.1) | 1 (1.2) | 2 (3.5) | 0.567 |
| Dyslipidemia | 5 (3.6) | 3 (3.6) | 2 (3.5) | 1.000 |
| Nonorchiopexy diagnoses, n (%) | 24 (17.1) | 16 (19.3) | 8 (14.0) | 0.498 |
| Undescended testis, n (%) | 86 (61.4) | 41 (49.4) | 45 (78.9) | <0.001 |
| Age at the operation (year), mean (s.d.) | 6.7 (5.0) | 5.7 (4.7) | 7.6 (5.1) | 0.091 |
| Jewish, n (%) | 57 (66.2) | 33 (80.5) | 24 (53.3) | 0.012 |
| Palpable testicle, n (%) | 79 (91.9) | 38 (92.7) | 41 (91.1) | 1.000 |
| Epididymis separation, n (%) | 13 (15.1) | 6 (14.6) | 7 (15.6) | 1.000 |
| Unilateral orchiopexy, n (%) | 66 (76.7) | 37 (90.2) | 29 (64.4) | 0.005 |
| Bilateral orchiopexy, n (%) | 18 (20.9) | 3 (7.3) | 15 (33.3) | 0.003 |
| Orchiectomy, n (%) | 2 (2.3) | 1 (2.4) | 1 (2.2) | 1.000 |
| TT, n (%) | 30 (21.4) | 26 (31.3) | 4 (7.0) | 0.001 |
| Age at the operation (year), mean (s.d.) | 18.1 (9.0) | 17.0 (8.3) | 25.1 (11.3) | 0.094 |
| Jewish, n (%) | 21 (70.0) | 18 (69.2) | 3 (75.0) | 1.000 |
| Necrotic testicle, n (%) | 5 (16.7) | 4 (15.4) | 1 (25.0) | 0.538 |
| Detorsion and bilateral orchiopexy, n (%) | 23 (76.7) | 19 (73.1) | 4 (100.0) | 0.548 |
| Orchiectomy and unilateral orchiopexy, n (%) | 7 (23.3) | 7 (26.9) | 0 (0) |
BMI: body mass index; s.d.: standard deviation; TT: testicular torsion; SA: semen analysis; AS: azoospermia or severe oligozoospermia (sperm concentration below 5 × 106 per ml); MN: normal sperm concentrations and non-severe oligozoospermia (sperm concentration above 5 × 106 per ml)
Initial diagnosis of UDT was significantly associated with the sperm count in the AS group (45/57, 78.9%, P < 0.001). However, most of the patients who underwent unilateral orchiopexy belonged to the MN group (37 [90.2%], P = 0.005), while most patients who underwent bilateral orchiopexy belonged to the AS group (15 [33.3%], P = 0.003).
Only 4 out of 57 patients with an initial diagnosis of TT were categorized in the AS group (4/57 [7.0%], P = 0.001). Notably, none of the four TT patients in the orchiectomy group were categorized in the AS group.
Undescended testicles
As presented in Table 2, the UDT group consisted of 86 patients, of whom 66 underwent unilateral orchiopexy, 18 bilateral orchiopexy, and 2 orchiectomy. The mean (s.d.) age at the time of surgery repair was 6.7 (5.0) years. The mean (s.d.) sperm concentration (×106 per ml) in the UDT group was 19.5 (29.9), mean (s.d.) total motility was 28.9% (28.4%), mean (s.d.) progressive motility was 18.3% (24.3%), and mean (s.d.) sperm morphology was 1.97% (2.3%), indicating below-reference values defined by the WHO for the motility and morphology parameters.
Table 2.
The effect of surgery type and ethnicity on sperm parameters in patients with undescended testicle diagnosis
| Characteristics | All patients (n=86) | Unilateral orchiopexy (n=66; 76.7%) | Bilateral orchiopexy (n=18; 20.9%) | P |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age at the operation (year), mean (s.d.) | 6.7 (5.0) | 6.9 (5.2) | 6.4 (4.2) | 0.284 |
| Jewish, n (%) | 57 (66.3) | 46 (69.7) | 10 (55.6) | 0.273 |
| Sperm analysis, mean (s.d.) | ||||
| Sperm concentration (×106 per ml) | 19.5 (29.9) | 23.37 (32.0) | 4.04 (12.0) | 0.014 |
| Total sperm count (×106) | 55.1 (101.9) | 65.4 (111.5) | 12.9 (32.7) | 0.053 |
| Total motility (%) | 28.9 (28.4) | 34.4 (23.3) | 8.8 (20.7) | 0.001 |
| Progressive motility (%) | 18.3 (24.3) | 21.3 (25.2) | 7.3 (18.4) | 0.031 |
| Normal forms (%) | 2.0 (2.3) | 2.2 (2.2) | 1.2 (2.7) | 0.094 |
s.d.: standard deviation
Comparison between the unilateral and bilateral orchiopexy groups revealed significant differences in sperm concentration, motility, and progressive motility. Specifically, the unilateral surgery group exhibited a significantly higher sperm concentration (×106 per ml; mean [s.d.]) than the bilateral surgery group (23.4 [32.0] vs 4.0 [12.0], P = 0.014). Moreover, both total motility (mean [s.d.], 34.4% [23.3%] vs 8.8% [20.7%], P = 0.001) and progressive motility (mean [s.d.], 21.3% [25.2%] vs 7.3% [18.4%], P = 0.031) were significantly higher in the unilateral surgery group compared to the bilateral group. However, no significant differences were found between the two groups regarding sperm morphology, though both were found to be below WHO reference values.
The Jewish and Arab patients exhibited comparable demographic and clinical characteristics. Notably, age at the time of the procedure, BMI, and smoking prevalence were statistically similar between the two groups. Furthermore, our data revealed no significant differences in the distribution of bilateral orchiopexy between the two populations (Supplementary Table 1).
Supplementary Table 1.
Demographic and clinical characteristics of Jewish and Arab patients
| UDT patients, n=86 (%) | Jewish (n=57; 66.3%) | Arabs (n=29; 33.7%) | P |
|---|---|---|---|
| Patient characteristics | |||
| Age at the operation (year), mean (s.d.) | 6.7 (4.9) | 6.6 (4.9) | 0.809 |
| BMI, mean (s.d.) | 26.6 (5.0) | 28.3 (5.6) | 0.470 |
| Smoker | 27 (47.4) | 12 (41.4) | 0.734 |
| Initial diagnosis, n (%) | |||
| Bilateral orchiopexy | 10 (17.5) | 8 (27.6) | 0.401 |
s.d.: standard deviation
Testicular torsion
Data from the TT patient group sorted by type of procedure are presented in Table 3. Twenty-three (76.7%) patients underwent detorsion plus bilateral orchiopexy, while 7 (23.3%) underwent orchiectomy plus unilateral orchiopexy. In the SA, mean (s.d.) of progressive motility (27.3% [26.3%]) and normal forms (3.5% [1.9%]) were slightly lower than WHO reference values. However, there were no statistically significant variations between the two types of TT surgeries.
Table 3.
The effect of surgery type on demographics and sperm parameters in patients with testicular torsion diagnosis
| Characteristics | All patients (n=30) | Detorsion + bilateral orchiopexy (n=23; 76.7%) | Orchiectomy + unilateral orchiopexy (n=7; 23.3%) | P |
|---|---|---|---|---|
| Age at the operation (year), mean (s.d.) | 18.1 (9.0) | 16.6 (7.9) | 22.9 (11.3) | 0.410 |
| Jewish, n (%) | 21 (70.0) | 17 (73.9) | 4 (57.1) | 0.640 |
| Sperm analysis, mean (s.d.) | ||||
| Concentration Sperm concentration (×106 per ml) | 40.3 (30.6) | 42.8 (30.8) | 32.0 (30.5) | 0.868 |
| Total sperm count (×106) | 98.6 (92.2) | 108.6 (99.1) | 34.3 (35.1) | 0.057 |
| Total motility (%) | 49.6 (20.8) | 51.6 (21.1) | 43.1 (19.7) | 0.821 |
| Progressive motility (%) | 27.3 (26.3) | 29.7 (26.7) | 19.6 (25.3) | 0.524 |
| Normal forms (%) | 3.5 (1.9) | 3.6 (1.6) | 3.3 (2.8) | 0.173 |
s.d.: standard deviation
Multiple surgeries
Twenty-eight patients underwent more than one surgery and were categorized based on whether the interventions were performed on the same testicle or both testicles. For those whose interventions were conducted on the same testicle (n = 7), six (85.7%) patients were included in the MN group, and only one (14.3%) patient was included in the AS group. Conversely, a significant proportion of those patients who had interventions on both testicles were included in the AS group (15/21, 71.4%). The observed difference between these two groups, as determined by Fisher’s exact test, was statistically significant (P = 0.022; Supplementary Table 2).
Supplementary Table 2.
Patients undergone more than one surgery
| Patients undergone more than one surgery, n=28 (%) | MN (n=12; 42.8%) | AS (n=16; 57.1%) | P | |
|---|---|---|---|---|
| Same testicle | 7 (25.0) | 6 (50.0) | 1 (6.3) | 0.022 |
| Both testicles | 21 (75.0) | 6 (50.0) | 15 (93.3) |
AS: azoospermia or severe oligozoospermia (sperm concentration below 5 × 106 per ml); MN: normal sperm concentrations and non-severe oligozoospermia (sperm concentration above 5 × 106 per ml)
Multivariate analysis
Multivariate analysis of our study population showed that patients with UDT exhibited higher odds of belonging to the AS group, although the association was marginally and not statistically significant (odds ratio [OR]: 2.712, 95% confidence interval [CI]: 0.893–8.237, P = 0.078), indicating a potential increased risk. On the other hand, TT patients showed significantly lower odds of belonging to the AS group (OR: 0.179, 95% CI: 0.034–0.952, P = 0.044), suggesting a decreased risk of approximately 82.1% (1 − 0.179 = 0.821).
In addition, Jewish ethnicity had a significantly decreased risk (OR: 0.207, 95% CI: 0.078–0.547, P = 0.001) of being assigned to the AS group compared to Arab patients. This indicates a reduced risk by approximately 79.3% (1 − 0.207 = 0.793) for individuals of Jewish ethnicity (Table 4).
Table 4.
Multivariate analysis of risk for patients belonging to the AS group
| Characteristics | OR | 95% CI | P |
|---|---|---|---|
| Undescended testis | 2.712 | 0.893–8.237 | 0.078 |
| TT | 0.179 | 0.034–0.952 | 0.044 |
| Age at the operation (year) | 0.969 | 0.905–1.038 | 0.376 |
| Age at SA (year) | 0.997 | 0.991–1.003 | 0.324 |
| Jewish | 0.207 | 0.078–0.547 | 0.001 |
| BMI >30 kg m−2 | 1.580 | 0.570–4.378 | 0.080 |
| Smoker | 0.391 | 0.099–1.538 | 0.179 |
| Diabetes mellitus | 0.076 | 0.003–1.905 | 0.117 |
| Hypertension | 0.611 | 0.009–42.206 | 0.820 |
| Dyslipidemia | 6.123 | 0.287–130.490 | 0.246 |
OR: odds ratio; CI: confidence interval; BMI: body mass index; TT: testicular torsion; SA: semen analysis; AS: azoospermia or severe oligozoospermia (sperm concentration below 5 × 106 per ml)
To further explore the impact of UDT on male fertility, a separate multivariate analysis was conducted focusing solely on this subgroup (Table 5).
Table 5.
Multivariate analysis of risk for patients with a primary diagnosis of undescended testicle belonging to the AS group
| Characteristics | OR | 95% CI | P |
|---|---|---|---|
| Bilateral UDT | 6.314 | 1.497–26.638 | 0.012 |
| Age at the operation (year) | 0.929 | 0.838–1.030 | 0.163 |
| Age at SA (year) | 0.997 | 0.989–1.004 | 0.369 |
| Jewish | 0.161 | 0.044–0.587 | 0.006 |
| BMI >30 kg m−2 | 1.594 | 0.411–6.175 | 0.500 |
| Smoker | 0.342 | 0.057–2.052 | 0.241 |
| Diabetes mellitus | 0.206 | 0.001–39.341 | 0.241 |
| Dyslipidemia | 1.624 | 0.004–676.980 | 0.875 |
OR: odds ratio; CI: confidence interval; BMI: body mass index; UDT: undescended testicle; SA: semen analysis; AS: azoospermia or severe oligozoospermia (sperm concentration below 5 × 106 per ml)
The study revealed a significant correlation between bilateral surgery and categorizing in the AS group (OR: 6.314, 95% CI: 1.497–26.638, P = 0.012), indicating that patients who underwent bilateral surgery had approximately 6.314 times higher odds of having a sperm concentration lower than 5 × 106 per ml (AS group) compared to patients with unilateral surgery (Table 5).
Both analyses indicated nonsignificance in other risk variables for being categorized in the AS group.
DISCUSSION
This study explored the link between pediatric inguinoscrotal complications, subsequent surgical procedures, particularly orchiopexy, and long-term semen analysis outcomes.
It also analyzed the factors that increase the likelihood of low sperm concentrations below 5 × 106 sperm per ml, which would likely require the use of IVF with ICSI for successful fertilization rates.
Undescended testis (UDT)
Analysis of our UDT cohort revealed a discernible trend toward a reduction in sperm count. Our results supported a previous study signaling the detrimental effects of UDT on male fertility.32 As expected, abnormal sperm concentration was markedly more prevalent among UDT patients who underwent bilateral surgical interventions or had multiple surgeries on different testicles. Chilvers et al.31 indicated that sperm quality is reduced by 44%–49% in unilateral cryptorchid patients, and Kogan33 reported that 72% of treated bilateral patients displayed diminished quality when contrasted with 100% of untreated cryptorchid patients. Furthermore, David et al.,34 in their paternity study of fertility markers, reported that 67%–74% of unilateral-cryptorchid men achieved fertility, compared to only 55% of bilateral cryptorchid patients.33
Over 50% of patients with a history of unilateral UDT orchiopexy had sperm concentration below 5 × 106 per ml, with the average of 23 × 106 per ml. In our study, unilateral UDT was associated with good male reproductive prognosis as expressed in their SA, accounting for 90.2% of MN group patients with a history of unilateral UDT. These findings are consistent with previous studies that did not find differences in parity and pregnancy rates between the general population and unilateral UDT patients.34,35
In contrast to the unilateral group, the majority of bilateral UDT patients, about 83%, manifested severe oligozoospermia averaging 4 × 106 sperm cells per ml. Moreover, bilateral UDT patients exhibited an increased risk of fertility challenges, with an approximately 6.314-fold increased likelihood of sperm concentrations below 5 × 106 per ml, compared to unilateral UDT patients (P = 0.012). These outcomes echoed the findings of Fawzy et al.,36 Yavetz et al.,37 and Hutson et al.,38 further accentuating the better spermatogenic results in unilateral UDT patients over their bilateral counterparts who are likely to require assisted reproductive techniques for successful conception.39
The intrinsic differences between bilateral and unilateral UDT, reflected in these fertility outcomes, may be associated with a decrease in spermatogenic reserves. In addition, it has been suggested that bilateral UDT may have a distinct etiology. This divergence in origin dictates future fertility potential, while the mean age at surgery was very similar between these groups (mean age for unilateral was 6.4 years versus 6.9 years for bilateral in our study, P = 0.28). It remains an area of debate whether this decrement in quality is a sequela of the surgical intervention itself or of an inherent characteristic of the UDT, as postulated by a prior study.32 Considering the observed increase in sperm parameters and the higher relative risk of testicular cancer in males with a history of undescended testis – which is 2.75 times to 8 times greater than that in those without such a history – it is clear that vigilant long-term monitoring remains crucial for this patient group. Furthermore, it is important to note that testicular cancer and its treatments may impact testosterone production, potentially resulting in hypogonadism in certain individuals.
TT
Our study did not find a significant correlation between a history of TT and disrupted fertility based on sperm concentration analysis. Only 7.0% of our participants with a past TT diagnosis were included in the AS group, with no cases of azoospermia recorded postorchiectomy. In line with the literature, these data supported favorable fertility outcomes in TT patients.10,40,41
Our findings indicate a slight reduction in progressive motility and sperm morphology in TT patients, but no significant decrease in total sperm count was observed. This aligns with the report of similar sperm concentrations in TT patients compared to healthy controls.42 Conversely, several studies have linked TT with reduced sperm motility and overall sperm count.12,43,44 Additionally, Almekaty et al.44 reported occurrences of azoospermia in postpubertal TT patients.
These results can be influenced by parameters such as time to hospital and surgery, recovery process, and testicle damage. Testicular conditions during semen analysis, including location, volume, and texture, may also contribute to these disparities. In our study, none of the patients experienced testicular necrosis, atrophy, or required secondary surgery during the follow-up period. Nonetheless, further research is needed to evaluate the effect of post-TT testicle conditions on SA.
The influence of demographic disparities on semen parameters
In our analysis, Jewish and Arab cohorts displayed similar demographic and clinical characteristics. There were no significant disparities in age at the time of surgical procedure, BMI, smoking habits, or even the proportion of bilateral orchiopexy cases, which inherently carry a heightened risk of fertility complications. Despite these congruences, the findings of our study revealed distinct ethnic disparities in postorchiopexy fertility outcomes, especially in UDT patients. Jewish individuals diagnosed with UDT demonstrated a substantially reduced risk, with an 83.9% lower likelihood of presenting with sperm counts below 5 × 106 sperm per ml than Arab individuals (P = 0.006). To note, the health-care system in Israel provides public health insurance, ensuring that all patients receive equal and consistent quality of care.
This marked difference alludes to possible genetic or environmental factors influencing fertility. The Arab group, predominantly composed of the Bedouin Arab population, is characterized by a high prevalence of consanguineous marriage and genetic diseases.45,46,47,48 Prior investigations by our group identified several genes associated with severe oligozoospermic or azoospermic outcomes, specifically within the Bedouin demographic.49,50,51,52 Nevertheless, the insufficient data on testicular function and morphology during SA necessitate further research to assess the correlation between ethnicity and infertility efficacy.
Multiple interventions
There is a consensus that preserving at least one healthy testicle is crucial for maintaining an optimal fertility rate.53 As expected, we found that among patients who underwent multiple surgical procedures, those with interventions on the same testicle only 6.9% were categorized in the AS group. In contrast, individuals who underwent surgery on two different testicles exhibited significantly impaired fertility outcomes (93.7%) in the AS group, emphasizing the need for cautious surgical planning.
Limitations
While our study provides valuable insights into the fertility implications of pediatric orchiopexy as expressed by SA, it is not without limitations. First, our research exclusively relies on SA for evaluating male fertility, without assessment of the couple’s overall infertility investigation. Ideally, the pregnancy and/or delivery rates should serve as a definitive metric for evaluating a couple’s fertility; however, due to the absence of detailed information about the female partner and pregnancy status, this study’s focus was confined to SA. Additionally, as a tertiary center receiving samples from across Southern Israel, not all medical files offered complete essential patient data such as hormonal profiles and testicular characteristics (size, texture, and firmness), which are pivotal in assessing testicular health and function at the time of SA. Moreover, the lack of detailed postoperative evaluation information, such as the success of fertility-related surgeries and immediate postoperative testicular function, presents another constraint.
Moreover, our research is based on a single-center experience in Israel, and thus, the findings might not be entirely generalizable to other populations or health-care settings. Our sample size, although substantial, might not have provided enough statistical power to detect minor differences between the groups, especially in less common conditions or outcomes. Finally, the retrospective nature of this study could introduce selection bias, given that it relied on preexisting medical records and cannot fully control for confounding factors such as genetic predispositions, lifestyle choices, or other medical conditions that could impact fertility.
CONCLUSIONS
This research illuminates the multifaceted relationship between pediatric orchiopexy, initial diagnoses, and long-term SA outcomes. Our findings demonstrated that the benefits and challenges associated with orchiopexy greatly depend on the initial condition. Testicular torsion patients typically experience improved outcomes, while those with undescended testicles, especially bilateral, may face fertility problems later in life. Interestingly, our study uncovered ethnic disparities in fertility risk postorchiopexy, potentially hinting at underlying genetic or environmental factors that warrant further exploration. The results also accentuated the importance of accurate and timely initial diagnosis and strategic surgical planning, especially when multiple interventions are required, due to their potentially detrimental impact on fertility. These insights enhance our understanding of the fertility implications of pediatric orchiopexy, emphasizing the crucial role of careful diagnosis, personalized surgical planning, and diligent postoperative monitoring in optimizing long-term outcomes.
AUTHOR CONTRIBUTIONS
NHN developed the protocol/project, interpreted the data, and wrote the manuscript. IF contributed to the development of the protocol/project, gathered and analyzed the data, conducted the statistical analysis, interpreted the data, and wrote the manuscript. EL was involved in the development of the protocol/project, interpreted the data, and edited the manuscript. AZ and ZA focused on interpreting the data and editing the manuscript. Finally, IHV was responsible for the editing of the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.World Health Organization. Infertility Prevalence Estimates 1990-2021. [[Last accessed on 2023 April 03]]. Available from: https://www.who.int/publications/i/item/978920068315 .
- 2.Male Infertility Best Practice Policy Committee of the American Urological Association;Practice Committee of the American Society for Reproductive Medicine. Report on optimal evaluation of the infertile male. Fertil Steril. 2004;82(Suppl 1):S123–30. doi: 10.1016/j.fertnstert.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 3.Practice Committee of American Society for Reproductive Medicine in Collaboration with Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility. Fertil Steril. 2008;90(Suppl 5):S1–6. doi: 10.1016/j.fertnstert.2008.08.122. [DOI] [PubMed] [Google Scholar]
- 4.Marte A, Caldamone AA, Aguiar LM. The history of the pediatric inguinal hernia repair. J Pediatr Urol. 2021;17:485–91. doi: 10.1016/j.jpurol.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Suede SH, Malik A, Sapra A. StatPearls [Internet] Treasure Island: StatPearls Publishing; 2024. Histology, Spermatogenesis [Updated 2023 Mar 6] Available from: https://www.ncbi.nlm.nih.gov/books/NBK553142/ [PubMed] [Google Scholar]
- 6.Kolon TF, Patel RP, Huff DS. Cryptorchidism:diagnosis, treatment, and long-term prognosis. Urol Clin North Am. 2004;31:469–80. doi: 10.1016/j.ucl.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Chung E, Brock GB. Cryptorchidism and its impact on male fertility:a state of art review of current literature. Can Urol Assoc J. 2011;5:210–4. doi: 10.5489/cuaj.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shunmugam M, Goldman RD. Testicular torsion in children. Can Fam Physician. 2021;67:669–71. doi: 10.46747/cfp.6709669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kylat RI. Perinatal testicular torsion. Arch Pediatr. 2021;28:75–9. doi: 10.1016/j.arcped.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Gielchinsky I, Suraqui E, Hidas G, Zuaiter M, Landau E, et al. Pregnancy rates after testicular torsion. J Urol. 2016;196:852–5. doi: 10.1016/j.juro.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 11.Arap MA, Vicentini FC, Cocuzza M, Hallak J, Athayde K, et al. Late hormonal levels, semen parameters, and presence of antisperm antibodies in patients treated for testicular torsion. J Androl. 2007;28:528–32. doi: 10.2164/jandrol.106.002097. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen FM, Rudlang TM, Fode M, Østergren PB, Sønksen J, et al. The impact of testicular torsion on testicular function. World J Mens Health. 2019;37:298–307. doi: 10.5534/wjmh.190037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritzén EM. Undescended testes:a consensus on management. Eur J Endocrinol. 2008;159:87–90. doi: 10.1530/EJE-08-0181. [DOI] [PubMed] [Google Scholar]
- 14.Niedzielski JK, Oszukowska E, Słowikowska Hilczer J. Undescended testis –current trends and guidelines:a review of the literature. Arch Med Sci. 2016;12:667–77. doi: 10.5114/aoms.2016.59940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geuvbashian G, Jednak R, Capolicchio JP, El Sherbiny M. Outcome of surgical management of non-palpable testes. Urol Ann. 2013;5:273–6. doi: 10.4103/0974-7796.120306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadziselimovic F, Herzog B. The importance of both an early orchidopexy and germ cell maturation for fertility. Lancet. 2001;358:1156–7. doi: 10.1016/S0140-6736(01)06274-2. [DOI] [PubMed] [Google Scholar]
- 17.Misseri R. Association between male genital anomalies and adult male reproductive disorders:a population-based data linkage study spanning more than 40 years. J Pediatr Urol. 2020;16:504–5. doi: 10.1016/j.jpurol.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Hadziselimovic F, Höcht B, Herzog B, Buser MW. Infertility in cryptorchidism is linked to the stage of germ cell development at orchidopexy. Horm Res. 2007;68:46–52. doi: 10.1159/000100874. [DOI] [PubMed] [Google Scholar]
- 19.Boitrelle F, Shah R, Saleh R, Henkel R, Kandil H, et al. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life. 2021;11:1368. doi: 10.3390/life11121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 6th edition. Geneva: World Health Organization; 2021. pp. 9–20. [Google Scholar]
- 21.Guzick DS, Overstreet JW, Factor Litvak P, Brazil CK, Nakajima ST, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–93. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 22.Bonde JP, Ernst E, Jensen TK, Hjollund NH, Kolstad H, et al. Relation between semen quality and fertility:a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–7. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- 23.Slama R, Eustache F, Ducot B, Jensen TK, Jørgensen N, et al. Time to pregnancy and semen parameters:a cross-sectional study among fertile couples from four European cities. Hum Reprod. 2002;17:503–15. doi: 10.1093/humrep/17.2.503. [DOI] [PubMed] [Google Scholar]
- 24.Palermo G, Neri Q, Rosenwaks Z. To ICSI or not to ICSI. Semin Reprod Med. 2015;33:92–102. doi: 10.1055/s-0035-1546825. [DOI] [PubMed] [Google Scholar]
- 25.O'Neill CL, Chow S, Rosenwaks Z, Palermo GD. Development of ICSI. Reproduction. 2018;156:F51–8. doi: 10.1530/REP-18-0011. [DOI] [PubMed] [Google Scholar]
- 26.Jones J, Horne G, Fitzgerald C. Who needs ICSI? A nationwide UK survey on ICSI use. Hum Fertil (Camb) 2012;15:144–9. doi: 10.3109/14647273.2012.720051. [DOI] [PubMed] [Google Scholar]
- 27.Shalev H, Mizrakli Y, Zeadna A, Harlev A, Levitas E, et al. Does methylphenidate use affect sperm parameters in patients undergoing infertility investigation? A retrospective analysis of 9769 semen samples. Arch Gynecol Obstet. 2021;304:539–46. doi: 10.1007/s00404-020-05938-z. [DOI] [PubMed] [Google Scholar]
- 28.Levitas E, Lunenfeld E, Weiss N, Friger M, Har Vardi I, et al. Relationship between the duration of sexual abstinence and semen quality:analysis of 9,489 semen samples. Fertil Steril. 2005;83:1680–6. doi: 10.1016/j.fertnstert.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 29.Menkveld R, Kruger TF. Advantages of strict (Tygerberg) criteria for evaluation of sperm morphology. Int J Androl. 1995;18(Suppl 2):36–42. [PubMed] [Google Scholar]
- 30.Koşar PA, Ozçelik N, Koşar AC. Cytogenetic abnormalities detected in patients with non-obstructive azoospermia and severe oligozoospermia. J Assist Reprod Genet. 2010;27:17–21. doi: 10.1007/s10815-009-9366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chilvers C, Dudley NE, Gough MH, Jackson MB, Pike MC. Undescended testis:the effect of treatment on subsequent risk of subfertility and malignancy. J Pediatr Surg. 1986;21:691–6. doi: 10.1016/s0022-3468(86)80389-x. [DOI] [PubMed] [Google Scholar]
- 32.Virtanen HE, Toppari J. Cryptorchidism and Fertility. Endocrinol Metab Clin North Am. 2015;44:751–60. doi: 10.1016/j.ecl.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Kogan SJ. Fertility in cryptorchidism an overview in 1987. Eur J Pediatr. 1987;146(Suppl 2):S21–4. doi: 10.1007/BF00452863. [DOI] [PubMed] [Google Scholar]
- 34.David G, Jouannet P, Martin Boyce A, Spira A, Schwartz D. Sperm counts in fertile and infertile men. Fertil Steril. 1979;31:453–5. [PubMed] [Google Scholar]
- 35.Virtanen HE, Bjerknes R, Cortes D, Jørgensen N, Rajpert De Meyts E, et al. Cryptorchidism:classification, prevalence and long-term consequences. Acta Paediatr. 2007;96:611–6. doi: 10.1111/j.1651-2227.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 36.Fawzy F, Hussein A, Eid MM, Kashash El AM, Salem HK. Cryptorchidism and fertility. Clin Med Insights Reprod Health. 2015;9:39–43. doi: 10.4137/CMRH.S25056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yavetz H, Harash B, Paz G, Yogev L, Jaffa AJ, et al. Cryptorchidism:incidence and sperm quality in infertile men. Andrologia. 1992;24:293–7. doi: 10.1111/j.1439-0272.1992.tb02655.x. [DOI] [PubMed] [Google Scholar]
- 38.Hutson JM, Balic A, Nation T, Southwell B. Cryptorchidism. Semin Pediatr Surg. 2010;19:215–24. doi: 10.1053/j.sempedsurg.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Cendron KM, Huff DS, Koop CE, Snyder HM, 3rd, Duckett JW. Cryptorchidism, orchiopexy and infertility:a critical long-term retrospective analysis. J Urol. 1989;142:559–62. doi: 10.1016/s0022-5347(17)38815-8. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Zhang J, Cai Z, Wang X, Lu W, et al. Effect of unilateral testicular torsion at different ages on male fertility. J Int Med Res. 2020;48:300060520918792. doi: 10.1177/0300060520918792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Törzsök P, Steiner C, Pallauf M, Abenhardt M, Milinovic L, et al. Long-term follow-up after testicular torsion:prospective evaluation of endocrine and exocrine testicular function, fertility, oxidative stress and erectile function. J Clin Med. 2022;11:6507. doi: 10.3390/jcm11216507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen AH, Priskorn L, Hansen LS, Carlsen E, Joensen UN, et al. Testicular torsion and subsequent testicular function in young men from the general population. Hum Reprod. 2023;38:216–24. doi: 10.1093/humrep/deac271. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal D, Parmar K, Sharma AP, Tyagi S, Kumar S, et al. Long-term impact of testicular torsion and its salvage on semen parameters and gonadal function. Indian J Urol. 2022;38:135–9. doi: 10.4103/iju.iju_328_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almekaty K, Zahran MH, Eid A, Ralph D, Rashed AA. Azoospermia and sperm retrieval in post-pubertal testicular torsion;benefits and limitations. Urology. 2023;171:121–6. doi: 10.1016/j.urology.2022.09.019. [DOI] [PubMed] [Google Scholar]
- 45.Rudnitzky A, Abu Ras T. The Bedouin Population in the Negev. Tel Aviv: Abraham Fund Initiatives; 2012. [Google Scholar]
- 46.Stavsky M, Robinson R, Sade MY, Krymko H, Zalstein E, et al. Elevated birth prevalence of conotruncal heart defects in a population with high consanguinity rate. Cardiol Young. 2017;27:109–16. doi: 10.1017/S1047951116000202. [DOI] [PubMed] [Google Scholar]
- 47.Tadmouri GO, Nair P, Obeid T, Al Ali MT, Al Khaja N, et al. Consanguinity and reproductive health among Arabs. Reprod Health. 2009;6:17. doi: 10.1186/1742-4755-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singer S, Davidovitch N, Abu Fraiha Y, Abu Freha N. Consanguinity and genetic diseases among the Bedouin population in the Negev. J Community Genet. 2020;11:13–9. doi: 10.1007/s12687-019-00433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arafat M, Harlev A, Har Vardi I, Levitas E, Priel T, et al. Mutation in CATIP (C2orf62) causes oligoteratoasthenozoospermia by affecting actin dynamics. J Med Genet. 2021;58:106–15. doi: 10.1136/jmedgenet-2019-106825. [DOI] [PubMed] [Google Scholar]
- 50.Arafat M, Har Vardi I, Harlev A, Levitas E, Zeadna A, et al. Mutation in TDRD9 causes non-obstructive azoospermia in infertile men. J Med Genet. 2017;54:633–9. doi: 10.1136/jmedgenet-2017-104514. [DOI] [PubMed] [Google Scholar]
- 51.Arafat M, Kleiman SE, AbuMadighem A, Zeadna A, Levitas E, et al. Pathogenic variations in Germ Cell Nuclear Acidic Peptidase (GCNA) are associated with human male infertility. Eur J Hum Genet. 2021;29:1781–8. doi: 10.1038/s41431-021-00946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeadna A, Har Vardi I, Khateeb N, Steiner N, Berkovitz A, et al. Ethnic origin as a parameter in the prediction of successful testicular sperm extraction in patients with non-obstructive azoospermia. Obstet Gynecol Res. 2021;04:12–20. [Google Scholar]
- 53.Gerstl B, Bertoldo MJ, Sullivan E, Volckmar X, Kerr A, et al. Fatherhood following treatment for testicular cancer:a systematic review and meta-analyses. J Adolesc Young Adult Oncol. 2020;9:341–53. doi: 10.1089/jayao.2019.0164. [DOI] [PubMed] [Google Scholar]


