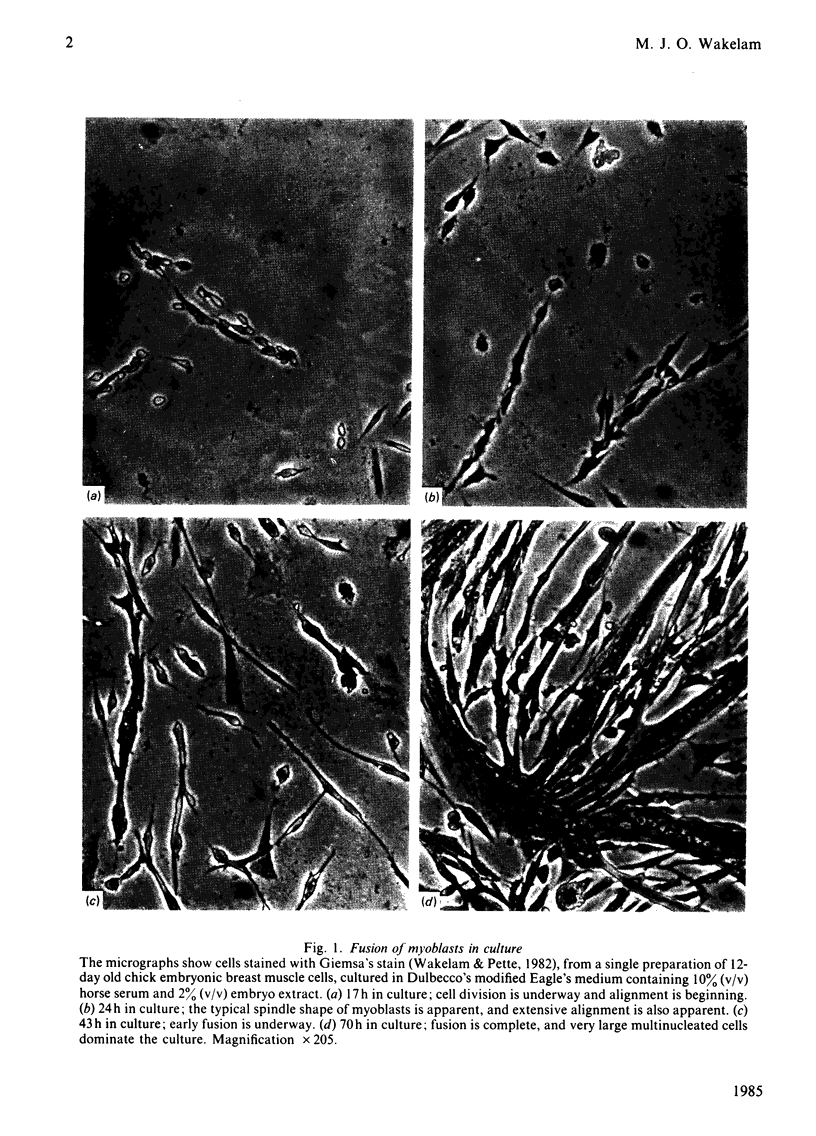
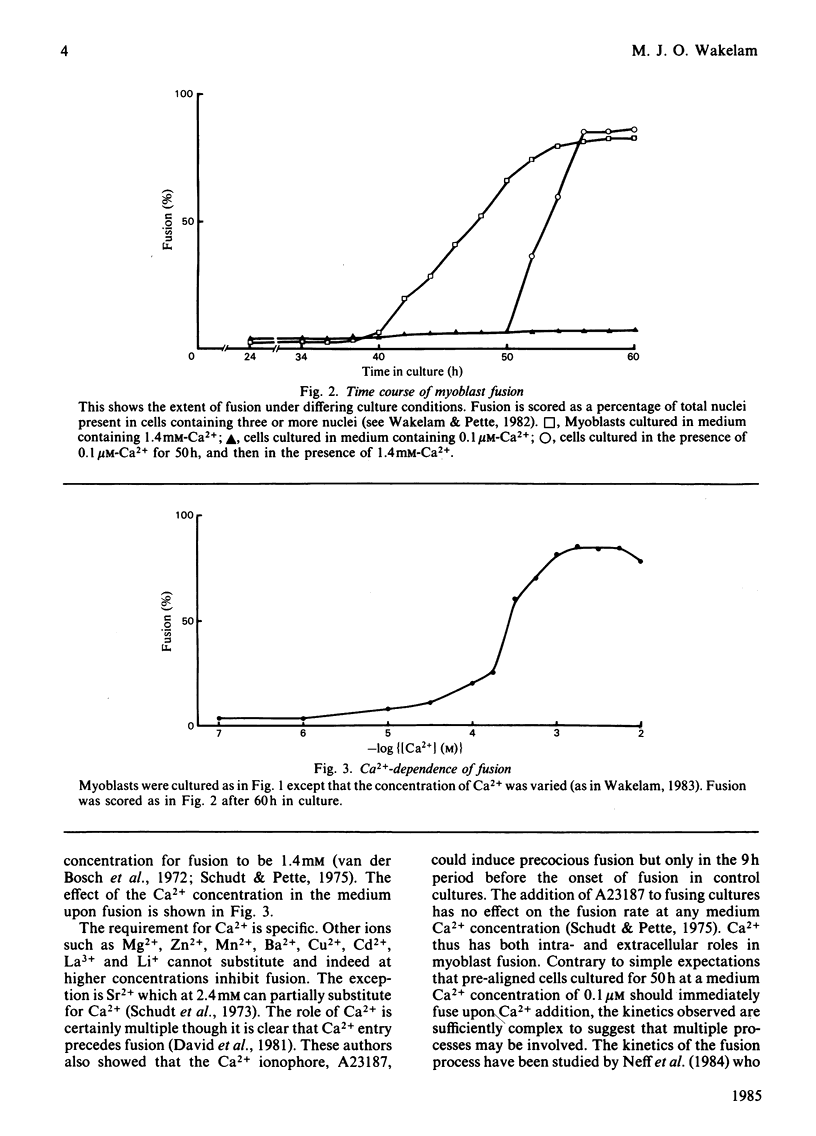
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antin P. B., Forry-Schaudies S., Friedman T. M., Tapscott S. J., Holtzer H. Taxol induces postmitotic myoblasts to assemble interdigitating microtubule-myosin arrays that exclude actin filaments. J Cell Biol. 1981 Aug;90(2):300–308. doi: 10.1083/jcb.90.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw E. J., Holt P. G., Simons P. J. Myogenesis in vitro. Enhancement by dibutyryl cAMP. Exp Cell Res. 1974 Feb;83(2):436–438. doi: 10.1016/0014-4827(74)90365-6. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Prives J. Trifluoperazine, a calmodulin antagonist, inhibits muscle cell fusion. J Cell Biol. 1983 Nov;97(5 Pt 1):1375–1380. doi: 10.1083/jcb.97.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach R. L., Popiela H., Festoff B. W. The identification of neurotrophic factor as a transferrin. FEBS Lett. 1983 May 30;156(1):151–156. doi: 10.1016/0014-5793(83)80267-1. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Chiu C. P., Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983 Apr;32(4):1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Epstein C. J. Manipulation of myogenesis in vitro: reversible inhibition by DMSO. Cell. 1979 May;17(1):95–108. doi: 10.1016/0092-8674(79)90298-8. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Webster C., Pavlath G. K. Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4856–4860. doi: 10.1073/pnas.80.15.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland R., Chyn T., Roufa D., Reyes E., Martonosi A. The lipid composition of muscle cells during development. Biochim Biophys Acta. 1977 Dec 21;489(3):349–359. doi: 10.1016/0005-2760(77)90155-2. [DOI] [PubMed] [Google Scholar]
- Burstein M., Shainberg S. Concanavalin A inhibits fusion of myoblasts and appearance of acetylcholine receptors in muscle cultures. FEBS Lett. 1979 Jul 1;103(1):33–37. doi: 10.1016/0014-5793(79)81244-2. [DOI] [PubMed] [Google Scholar]
- Cates G. A., Brickenden A. M., Sanwal B. D. Possible involvement of a cell surface glycoprotein in the differentiation of skeletal myoblasts. J Biol Chem. 1984 Feb 25;259(4):2646–2650. [PubMed] [Google Scholar]
- Cates G. A., Holland P. C. Biosynthesis of plasma-membrane proteins during myogenesis of skeletal muscle in vitro. Biochem J. 1978 Sep 15;174(3):873–881. doi: 10.1042/bj1740873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M., Eppenberger H. M., Turner D. C. Muscle morphogenesis: Evidence for an organizing function of exogenous fibronectin. Dev Biol. 1981 Dec;88(2):220–235. doi: 10.1016/0012-1606(81)90166-4. [DOI] [PubMed] [Google Scholar]
- Cohen R., Pacifici M., Rubinstein N., Biehl J., Holtzer H. Effect of a tumour promoter on myogenesis. Nature. 1977 Apr 7;266(5602):538–540. doi: 10.1038/266538a0. [DOI] [PubMed] [Google Scholar]
- Cornell R. B., Nissley S. M., Horwitz A. F. Cholesterol availability modulates myoblast fusion. J Cell Biol. 1980 Sep;86(3):820–824. doi: 10.1083/jcb.86.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch C. B., Strittmatter W. J. Rat myoblast fusion requires metalloendoprotease activity. Cell. 1983 Jan;32(1):257–265. doi: 10.1016/0092-8674(83)90516-0. [DOI] [PubMed] [Google Scholar]
- Couch C. B., Strittmatter W. J. Specific blockers of myoblast fusion inhibit a soluble and not the membrane-associated metalloendoprotease in myoblasts. J Biol Chem. 1984 May 10;259(9):5396–5399. [PubMed] [Google Scholar]
- David J. D., Higginbotham C. A. Fusion of chick embryo skeletal myoblasts: interactions of prostaglandin E1, adenosine 3':5' monophosphate, and calcium influx. Dev Biol. 1981 Mar;82(2):308–316. doi: 10.1016/0012-1606(81)90454-1. [DOI] [PubMed] [Google Scholar]
- David J. D., See W. M., Higginbotham C. A. Fusion of chick embryo skeletal myoblasts: role of calcium influx preceding membrane union. Dev Biol. 1981 Mar;82(2):297–307. doi: 10.1016/0012-1606(81)90453-x. [DOI] [PubMed] [Google Scholar]
- Den H., Chin J. H. Endogenous lectin from chick embryo skeletal muscle is not involved in myotube formation in vitro. J Biol Chem. 1981 Aug 10;256(15):8069–8073. [PubMed] [Google Scholar]
- Den H., Malinzak D. A. Isolation and properties of beta-D-galactoside-specific lectin from chick embryo thigh muscle. J Biol Chem. 1977 Aug 10;252(15):5444–5448. [PubMed] [Google Scholar]
- Den H., Malinzak D. A., Keating H. J., Rosenberg A. Influence of Concanavalin A, wheat germ agglutinin, and soybean agglutinin on the fusion of myoblasts in vitro. J Cell Biol. 1975 Dec;67(3):826–834. doi: 10.1083/jcb.67.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den H., Malinzak D. A., Rosenberg A. Lack of evidence for the involvement of a beta-D-galactosyl-specific lectin in the fusion of chick myoblasts. Biochem Biophys Res Commun. 1976 Apr 5;69(3):621–627. doi: 10.1016/0006-291x(76)90921-9. [DOI] [PubMed] [Google Scholar]
- Easton T. G., Reich E. Muscle differentiation in cell culture. Effects of nucleoside inhibitors and Rous sarcoma virus. J Biol Chem. 1972 Oct 25;247(20):6420–6431. [PubMed] [Google Scholar]
- Ehrismann R., Chiquet M., Turner D. C. Mode of action of fibronectin in promoting chicken myoblast attachment. Mr = 60,000 gelatin-binding fragment binds native fibronectin. J Biol Chem. 1981 Apr 25;256(8):4056–4062. [PubMed] [Google Scholar]
- Elson H. F., Yguerabide J. Membrane dynamics of differentiating cultured embryonic chick skeletal muscle cells by fluorescence microscopy techniques. J Supramol Struct. 1979;12(1):47–61. doi: 10.1002/jss.400120106. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Miranda A. F., Babiss L. E., Pestka S., Weinstein I. B. Opposing effects of interferon produced in bacteria and of tumor promoters on myogenesis in human myoblast cultures. Proc Natl Acad Sci U S A. 1983 May;80(10):2961–2965. doi: 10.1073/pnas.80.10.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. B., Prives J., Farmer S. R., Penman S. Developmental reorganization of the skeletal framework and its surface lamina in fusing muscle cells. J Cell Biol. 1981 Oct;91(1):103–112. doi: 10.1083/jcb.91.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli G., Brigonzi A., Tachikawa T., Clementi F. Rat myoblast fusion: morphological study of membrane apposition, fusion, and fission during controlled myogenesis in vitro. J Ultrastruct Res. 1981 Apr;75(1):112–125. doi: 10.1016/s0022-5320(81)80103-7. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Podleski T. R. Evidence that a membrane bound lectin mediates fusion of L6 myoblasts. Biochem Biophys Res Commun. 1975 Dec 1;67(3):972–978. doi: 10.1016/0006-291x(75)90770-6. [DOI] [PubMed] [Google Scholar]
- Gilfix B. M., Sanwal B. D. Inhibition of myoblast fusion by tunicamycin and pantomycin. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1184–1191. doi: 10.1016/0006-291x(80)90077-7. [DOI] [PubMed] [Google Scholar]
- Grove R. I., Schimmel S. D. Effects of 12-O-tetradecanoylphorbol 13-acetate on glycerolipid metabolism in cultured myoblasts. Biochim Biophys Acta. 1982 May 13;711(2):272–280. doi: 10.1016/0005-2760(82)90036-4. [DOI] [PubMed] [Google Scholar]
- Grove R. I., Schimmel S. D. Generation of 1,2-diacylglycerol in plasma membranes of phorbol ester-treated myoblasts. Biochem Biophys Res Commun. 1981 Sep 16;102(1):158–164. doi: 10.1016/0006-291x(81)91502-3. [DOI] [PubMed] [Google Scholar]
- Herman B. A., Fernandez S. M. Changes in membrane dynamics associated with myogenic cell fusion. J Cell Physiol. 1978 Mar;94(3):253–263. doi: 10.1002/jcp.1040940303. [DOI] [PubMed] [Google Scholar]
- Herman B. A., Fernandez S. M. Dynamics and topographical distribution of surface glycoproteins during myoblast fusion: a resonance energy transfer study. Biochemistry. 1982 Jul 6;21(14):3275–3283. doi: 10.1021/bi00257a005. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A. F., Wight A., Ludwig P., Cornell R. Interrelated lipid alterations and their influence on the proliferation and fusion of cultured myogenic cells. J Cell Biol. 1978 May;77(2):334–357. doi: 10.1083/jcb.77.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii I., Kimura I., Ozawa E. A myotrophic protein from chick embryo extract: its purification, identity to transferrin, and indispensability for avian myogenesis. Dev Biol. 1982 Dec;94(2):366–377. doi: 10.1016/0012-1606(82)90354-2. [DOI] [PubMed] [Google Scholar]
- Kalderon N., Gilula N. B. Membrane events involved in myoblast fusion. J Cell Biol. 1979 May;81(2):411–425. doi: 10.1083/jcb.81.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman S. J., Lawless M. L. Thiodigalactoside binding lectin and skeletal myogenesis. Differentiation. 1980 Feb;16(1):41–48. doi: 10.1111/j.1432-0436.1980.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Kaur H., Sanwal B. D. Regulation of the activity of a calcium-activated neutral protease during differentiation of skeletal myoblasts. Can J Biochem. 1981 Sep;59(9):743–747. doi: 10.1139/o81-103. [DOI] [PubMed] [Google Scholar]
- Kent C. Inhibition of myoblast fusion by lysosomotropic amines. Dev Biol. 1982 Mar;90(1):91–98. doi: 10.1016/0012-1606(82)90214-7. [DOI] [PubMed] [Google Scholar]
- Kent C., Schimmel S. D., Vagelos P. R. Lipid composition of plasma membranes from developing chick muscle cells in culture. Biochim Biophys Acta. 1974 Sep 19;360(3):312–321. doi: 10.1016/0005-2760(74)90061-7. [DOI] [PubMed] [Google Scholar]
- Kent C. Stimulation of phospholipid metabolism in embryonic muscle cells treated with phospholipase C. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4474–4478. doi: 10.1073/pnas.76.9.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K. A., Horwitz A. F. Tandem events in myoblast fusion. Dev Biol. 1977 Jul 15;58(2):328–338. doi: 10.1016/0012-1606(77)90095-1. [DOI] [PubMed] [Google Scholar]
- Knudsen K. A., Horwitz A. F. Toward a mechanism of myoblast fusion. Prog Clin Biol Res. 1978;23:563–568. [PubMed] [Google Scholar]
- Kolb H. A., Wakelam M. J. Transmitter-like action of ATP on patched membranes of cultured myoblasts and myotubes. Nature. 1983 Jun 16;303(5918):621–623. doi: 10.1038/303621a0. [DOI] [PubMed] [Google Scholar]
- Linkhart T. A., Clegg C. H., Hauschika S. D. Myogenic differentiation in permanent clonal mouse myoblast cell lines: regulation by macromolecular growth factors in the culture medium. Dev Biol. 1981 Aug;86(1):19–30. doi: 10.1016/0012-1606(81)90311-0. [DOI] [PubMed] [Google Scholar]
- Lipton B. H., Konigsberg I. R. A fine-structural analysis of the fusion of myogenic cells. J Cell Biol. 1972 May;53(2):348–364. doi: 10.1083/jcb.53.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey C. H., Horwitz A. F. Effect of inhibitors of cholesterol synthesis on muscle differentiation. Biochim Biophys Acta. 1982 Aug 18;712(2):430–432. doi: 10.1016/0005-2760(82)90364-2. [DOI] [PubMed] [Google Scholar]
- Lucy J. A. Do hydrophobic sequences cleaved from cellular polypeptides induce membrane fusion reactions in vivo? FEBS Lett. 1984 Jan 30;166(2):223–231. doi: 10.1016/0014-5793(84)80085-x. [DOI] [PubMed] [Google Scholar]
- Lucy J. A. Fusogenic mechanisms. Ciba Found Symp. 1984;103:28–44. doi: 10.1002/9780470720844.ch3. [DOI] [PubMed] [Google Scholar]
- MacBride R. G., Przybylski R. J. Purified lectin from skeletal muscle inhibits myotube formation in vitro. J Cell Biol. 1980 Jun;85(3):617–625. doi: 10.1083/jcb.85.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Induction of membrane fusion by polysialogangliosides. FEBS Lett. 1978 Jun 1;90(1):149–152. doi: 10.1016/0014-5793(78)80318-4. [DOI] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Molecular behaviour of glycosphingolipids in interfaces. Possible participation in some properties of nerve membranes. Biochim Biophys Acta. 1981 Dec;650(2-3):69–87. doi: 10.1016/0304-4157(81)90001-0. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Miranda A. F., Babiss L. E., Fisher P. B. Transformation of human skeletal muscle cells by simian virus 40. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6581–6585. doi: 10.1073/pnas.80.21.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss M., Norris J. S., Peck E. J., Jr, Schwartz R. J. Alterations in iodinated cell surface proteins during myogenesis. Exp Cell Res. 1978 May;113(2):445–450. doi: 10.1016/0014-4827(78)90388-9. [DOI] [PubMed] [Google Scholar]
- Nakornchai S., Falconer A. R., Fisher D., Goodall A. H., Hallinan T., Lucy J. A. Effects of retinol, fatty acids and glycerol monooleate on the fusion of chick embryo myoblasts in vitro. Biochim Biophys Acta. 1981 Apr 22;643(1):152–160. doi: 10.1016/0005-2736(81)90227-3. [DOI] [PubMed] [Google Scholar]
- Nameroff M., Trotter J. A., Keller J. M., Munar E. Inhibition of cellular differentiation by phospholipase C. I. Effects of the enzyme on myogenesis and chondrogenesis in vitro. J Cell Biol. 1973 Jul;58(1):107–118. doi: 10.1083/jcb.58.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff N. T., Horwitz A. F. A rapid assay for fusion of embryonic chick myoblasts. Exp Cell Res. 1982 Aug;140(2):479–483. doi: 10.1016/0014-4827(82)90146-x. [DOI] [PubMed] [Google Scholar]
- Neff N., Decker C., Horwitz A. The kinetics of myoblast fusion. Exp Cell Res. 1984 Jul;153(1):25–31. doi: 10.1016/0014-4827(84)90444-0. [DOI] [PubMed] [Google Scholar]
- Nowak T. P., Kobiler D., Roel L. E., Barondes S. H. Developmentally regulated lectin from embryonic chick pectoral muscle. Purification by affinity chromatography. J Biol Chem. 1977 Sep 10;252(17):6026–6030. [PubMed] [Google Scholar]
- Olden K., Law J., Hunter V. A., Romain R., Parent J. B. Inhibition of fusion of embryonic muscle cells in culture by tunicamycin is prevented by leupeptin. J Cell Biol. 1981 Jan;88(1):199–204. doi: 10.1083/jcb.88.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfett C. L., Jamieson J. C., Wright J. A. A correlation between loss of fusion potential and defective formation of mannose-linked lipid intermediates in independent concanavalin A-resistant myoblast cell lines. Exp Cell Res. 1981 Nov;136(1):1–14. doi: 10.1016/0014-4827(81)90032-x. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Rand R. P., Gingell D. Lessons for the study of membrane fusion from membrane interactions in phospholipid systems. Ciba Found Symp. 1984;103:9–27. doi: 10.1002/9780470720844.ch2. [DOI] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin synthesis in cultures of differentiating chicken embryo skeletal muscle. Dev Biol. 1972 Oct;29(2):113–138. doi: 10.1016/0012-1606(72)90050-4. [DOI] [PubMed] [Google Scholar]
- Pauw P. G., David J. D. Alterations in surface proteins during myogenesis of a rat myoblast cell line. Dev Biol. 1979 May;70(1):27–38. doi: 10.1016/0012-1606(79)90004-6. [DOI] [PubMed] [Google Scholar]
- Podleski T. R., Greenberg I. Distribution and activity of endogenous lectin during myogenesis as measured with antilectin antibody. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1054–1058. doi: 10.1073/pnas.77.2.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popiela H., Ellis S., Festoff B. W. Dose-dependent initiation of myogenesis by neurotrophic factor. J Neurosci Res. 1982;8(2-3):547–567. doi: 10.1002/jnr.490080240. [DOI] [PubMed] [Google Scholar]
- Prives J., Shinitzky M. Increased membrane fluidity precedes fusion of muscle cells. Nature. 1977 Aug 25;268(5622):761–763. doi: 10.1038/268761a0. [DOI] [PubMed] [Google Scholar]
- Quinn C. A., Goodfellow P. N., Povey S., Walsh F. S. Human--rat muscle somatic cell hybrids form myotubes and express human muscle gene products. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5031–5035. doi: 10.1073/pnas.78.8.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash J. E., Fambrough D. Ultrastructural and electrophysiological correlates of cell coupling and cytoplasmic fusion during myogenesis in vitro. Dev Biol. 1973 Jan;30(1):166–186. doi: 10.1016/0012-1606(73)90055-9. [DOI] [PubMed] [Google Scholar]
- Rash J. E., Staehelin L. A. Freeze-cleave demonstration of gap junctions between skeletal myogenic cells in vivo. Dev Biol. 1974 Feb;36(2):455–461. doi: 10.1016/0012-1606(74)90066-9. [DOI] [PubMed] [Google Scholar]
- Sandra A., Leon M. A., Przybylski R. J. Suppression of myoblast fusion by concanavalin A: possible involvement of membrane fluidity. J Cell Sci. 1977 Dec;28:251–272. doi: 10.1242/jcs.28.1.251. [DOI] [PubMed] [Google Scholar]
- Schudt C., van der Bosch J., Pette D. Inhibition of muscle cell fusion in vitro by Mg2+ and K+ ions. FEBS Lett. 1973 Jun 1;32(2):296–298. doi: 10.1016/0014-5793(73)80857-9. [DOI] [PubMed] [Google Scholar]
- Schudt O., Pette D. Influence of the ionophore A 23 187 on myogenic cell fusion. FEBS Lett. 1975 Nov 1;59(1):36–38. doi: 10.1016/0014-5793(75)80335-8. [DOI] [PubMed] [Google Scholar]
- Schützle U. B., Wakelam M. J., Pette D. Prostaglandins and cyclic AMP stimulate creatine kinase synthesis but not fusion in cultured embryonic chick muscle cells. Biochim Biophys Acta. 1984 Oct 12;805(2):204–210. doi: 10.1016/0167-4889(84)90169-1. [DOI] [PubMed] [Google Scholar]
- Sessions A., Horwitz A. F. Differentiation-related differences in the plasma membrane phospholipid asymmetry of myogenic and fibrogenic cells. Biochim Biophys Acta. 1983 Feb 9;728(1):103–111. doi: 10.1016/0005-2736(83)90442-x. [DOI] [PubMed] [Google Scholar]
- Sessions A., Horwitz A. F. Myoblast aminophospholipid asymmetry differs from that of fibroblasts. FEBS Lett. 1981 Nov 2;134(1):75–78. doi: 10.1016/0014-5793(81)80554-6. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Control of myogenesis in vitro by Ca 2 + concentration in nutritional medium. Exp Cell Res. 1969 Nov;58(1):163–167. doi: 10.1016/0014-4827(69)90127-x. [DOI] [PubMed] [Google Scholar]
- Sundler R., Wijkander J. Protein-mediated intermembrane contact specifically enhances Ca2+-induced fusion of phosphatidate-containing membranes. Biochim Biophys Acta. 1983 May 5;730(2):391–394. doi: 10.1016/0005-2736(83)90357-7. [DOI] [PubMed] [Google Scholar]
- Sénéchal H., Pichard A. L., Delain D., Schapira G., Wahrmann J. P. Changes in plasma membrane phosphoproteins during differentiation of an established myogenic cell line and a non-fusing alpha-amanitin resistant mutant. FEBS Lett. 1982 Mar 22;139(2):209–213. doi: 10.1016/0014-5793(82)80853-3. [DOI] [PubMed] [Google Scholar]
- Tautu C., Jasmin G. Atypical myogenesis in hamster hereditary polymyopathy. An in vitro study. J Neuropathol Exp Neurol. 1980 Mar;39(2):173–180. doi: 10.1097/00005072-198003000-00006. [DOI] [PubMed] [Google Scholar]
- Thayer A. M., Kohler S. J. Phosphorus-31 nuclear magnetic resonance spectra characteristic of hexagonal and isotropic phospholipid phases generated from phosphatidylethanolamine in the bilayer phase. Biochemistry. 1981 Nov 24;20(24):6831–6834. doi: 10.1021/bi00527a014. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Lawton J., Dollenmeier P., Ehrismann R., Chiquet M. Guidance of myogenic cell migration by oriented deposits of fibronectin. Dev Biol. 1983 Feb;95(2):497–504. doi: 10.1016/0012-1606(83)90052-0. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Leunissen-Bijvelt J., de Kruijff B., Hope M., Cullis P. R. Non-bilayer structures in membrane fusion. Ciba Found Symp. 1984;103:45–59. doi: 10.1002/9780470720844.ch4. [DOI] [PubMed] [Google Scholar]
- Wahrmann J. P., Winand R., Luzzati D. Effect of cyclic AMP on growth and morphological differentiation of an established myogenic cell line. Nat New Biol. 1973 Sep 26;245(143):112–113. doi: 10.1038/newbio245112a0. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J. Inositol phospholipid metabolism and myoblast fusion. Biochem J. 1983 Jul 15;214(1):77–82. doi: 10.1042/bj2140077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelam M. J., Pette D. The breakdown of phosphatidylinositol in myoblasts stimulated to fuse by the addition of Ca2+. Biochem J. 1982 Mar 15;202(3):723–729. doi: 10.1042/bj2020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Phillips E. Specific changes in cellular glycoproteins and surface proteins during myogenesis in clonal muscle cells. Dev Biol. 1981 Jan 30;81(2):229–237. doi: 10.1016/0012-1606(81)90286-4. [DOI] [PubMed] [Google Scholar]
- Weidekamm E., Schudt C., Brdiczka D. Physical properties of muscle cell membranes during fusion. A fluorescence polarization study with the ionophore A23187. Biochim Biophys Acta. 1976 Aug 16;443(2):169–180. doi: 10.1016/0005-2736(76)90500-9. [DOI] [PubMed] [Google Scholar]
- Whatley R., Ng S. K., Rogers J., McMurray W. C., Sanwal B. D. Developmental changes in gangliosides during myogenesis of a rat myoblast cell line and its drug resistant variants. Biochem Biophys Res Commun. 1976 May 3;70(1):180–185. doi: 10.1016/0006-291x(76)91125-6. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7(3):159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]
- Yoshioka M., Sueoka N. Cell surface proteins of rat myoblasts. Exp Cell Res. 1983 Jun;146(1):219–223. doi: 10.1016/0014-4827(83)90342-7. [DOI] [PubMed] [Google Scholar]
- Zalin R. J., Leaver R. The effect of a transient increase in intracellular cyclic AMP upon muscle cell fusion. FEBS Lett. 1975 Apr 15;53(1):33–36. doi: 10.1016/0014-5793(75)80675-2. [DOI] [PubMed] [Google Scholar]
- Zalin R. J., Montague W. Changes in adenylate cyclase, cyclic AMP, and protein kinase levels in chick myoblasts, and their relationship to differentiation. Cell. 1974 Jun;2(2):103–108. doi: 10.1016/0092-8674(74)90098-1. [DOI] [PubMed] [Google Scholar]
- Zalin R. The cell cycle, myoblast differentiation and prostaglandin as a developmental signal. Dev Biol. 1979 Aug;71(2):274–288. doi: 10.1016/0012-1606(79)90169-6. [DOI] [PubMed] [Google Scholar]
- van der Bosch J., Schudt C., Pette D. Influence of temperature, cholesterol, dipalmitoyllecithin and Ca2+ on the rate of muscle cell fusion. Exp Cell Res. 1973 Dec;82(2):433–438. doi: 10.1016/0014-4827(73)90362-5. [DOI] [PubMed] [Google Scholar]
- van der Bosch J., Schudt C., Pette D. Quantitative investigation on Ca++-and pH-dependence of muscle cell fusion in vitro. Biochem Biophys Res Commun. 1972 Jul 25;48(2):326–332. doi: 10.1016/s0006-291x(72)80054-8. [DOI] [PubMed] [Google Scholar]