Abstract
Background:
Seronegative elderly-onset rheumatoid arthritis (EORA)neg and polymyalgia rheumatica (PMR) have similar clinical characteristics making them difficult to distinguish based on clinical features. We hypothesized that the study of serum metabolome could identify potential biomarkers of PMR vs EORAneg.
Methods:
Arthritis in older adults (ARTIEL) is an observational prospective cohort with patients older than 60 years of age with newly diagnosed arthritis. Patients’ blood samples were compared at baseline with 18 controls. A thorough clinical examination was conducted. A Bruker Avance 600 MHz spectrometer was used to acquire Nuclear Magnetic Resonance (NMR) spectra of serum samples. Chenomx NMR suite 8.5 was used for metabolite identification and quantification. Student t-test, one-way ANOVA, binary linear regression and ROC curve, Pearson’s correlation along with pathway analyses were conducted.
Results:
Twenty-eight patients were diagnosed with EORAneg and 20 with PMR. EORAneg patients had a mean disease activity score (DAS)-Erythrocyte Sedimentation Rate (ESR) of 6.21±1.00. All PMR patients reported shoulder pain, and 90% reported pelvic pain. Fifty-eight polar metabolites were identified. Of these, 3-hydroxybutyrate, acetate, glucose, glycine, lactate, and o-acetylcholine (o-ACh), were significantly different between groups. Of interest, IL-6 correlated with different metabolites in PMR and EORAneg suggesting different inflammatory activated pathways. Finally, lactate, o-ACh, taurine, and sex (female) were identified as distinguishable factors of PMR from EORAneg with a sensitivity of 90%, specificity of 92.3%, and an AUC of 0.925 (p<0.001).
Conclusion:
These results suggest that EORAneg and PMR have different serum metabolomic profiles that might be related to their pathobiology and can be used as biomarker to discriminate between both diseases.
Keywords: Metabolomics, PMR, seronegative RA, NMR
BACKGROUND
Elderly Onset Rheumatoid Arthritis (EORA) is a geriatric rheumatic disease with a prevalence of approximately 2% (Kobak & Bes, 2018). The diagnostic is usually considered in patients who are more than 60 years old and newly diagnosed with RA(Kobak & Bes, 2018). Unlike young onset RA, EORA tends to affect larger joints such as shoulders(Aletaha et al., 2010). Even with shoulder pain being a distinguishable factor, it is still difficult to properly diagnose EORA because of the overlap with other rheumatic diseases such as polymyalgia rheumatica (PMR), which is another inflammatory and idiopathic geriatric disease, with a prevalence of 0.37–0.62%(Manzo, 2019). Women are more likely to develop PMR with a lifetime risk of 2.4% compared to 1.7% in men(Hancock et al., 2014). Symptoms of PMR include stiffness in the back, shoulders and hips, fatigue, and weight loss. Blood tests such as C-reactive Protein (CRP), erythrocyte sedimentation rate (ESR), rheumatoid factor (RF), anti-cycle citrullinated peptides (anti-CCP) or clinical parameters cannot accurately distinguish PMR from seronegative EORA (EORAneg)(Aletaha et al., 2010).
Biomarkers to differentiate between PMR and EORAneg are an unmet need. Metabolomics is an emerging field of biomedical research that can offer a better understanding of mechanisms underlying diseases and help develop new strategies for treatment. Unlike genes and proteins, whose functions are subject to epigenetic regulation and post-translational modifications, metabolites serve as direct signatures of biochemical activity that may help understand the underlying biological pathways and may be easier to correlate with phenotype (Cho et al., 2014). Variations in metabolite concentrations can also serve as diagnostic or prognostic biomarkers. A small number of metabolomics studies have been focused on identifying metabolites associated with rheumatic diseases, primarily for diagnostic purposes,(Costenbader et al., 2021; Kim et al., 2014; Kosinska et al., 2013; Luan et al., 2021; Madsen et al., 2011; Souto-Carneiro et al., 2020) but even fewer have attempted to predict response to treatment.(Kapoor et al., 2013; Medcalf et al., 2021; Murillo-Saich et al., 2021; Sweeney et al., 2016)
Metabolomic analysis can involve different analytical platforms. The most commonly utilized platforms are nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). NMR spectroscopy, although having lower sensitivity than MS, is a robust metabolomic platform with several advantages. NMR is currently the best technique for chemical structure elucidation; it requires only minimal sample preparation, and is non-destructive, inherently untargeted, highly reproducible(Dumas et al., 2006; Viant et al., 2008) and intrinsically quantitative(Viant et al., 2009; Wishart et al., 2012; Zhang et al., 2010). Researchers have used 1H-NMR to study biomarkers in different diseases such as Alzheimer’s disease(Di Costanzo et al., 2020), or the microbiome of irritable bowel syndrome(Le Gall et al., 2011) among others. 1D 1H-NMR may be a promising tool to identify patients with different types of rheumatic diseases.
Although there are prior studies defining the metabolomic profile of young onset RA patients, which successfully distinguished between healthy controls and individuals with RA(Rodríguez-Carrio et al., 2021; Teitsma et al., 2018; Young et al., 2013), there is a lack of information about the metabolomic profile in patients with EORA or PMR. The study of metabolomics could be of interest to identify significant metabolites especially in EORAneg vs PMR, and to better understand elements of inflammation pathobiology in the geriatric populations. The main objective of this study is to compare the metabolomic profiles from PMR and EORAneg patients.
METHODS
Patient selection and assessment:
This is an observational longitudinal prospective study (ARTIEL -arthritis in the elderly)(Coras et al., 2021), that enrolled elderly patients with new onset arthritis. The study was approved by the Universitat Hospital Germans Trias i Pujol Institutional Review Board and included patients older than 60 years with clinically newly diagnosed peripheral and/or rhizomelic arthritis. Patients with infections, neoplasia, dementia, immunodeficiencies, or who had received or are receiving glucocorticoids or diseases-modifying anti-rheumatic drug (DMARDs) in the last 6 months were excluded from the study. Individuals with new onset arthritis were identified by a primary care physician and then referred to a rheumatologist who prescribed treatment according to the standard of care. Additionally, 18 controls without rheumatic diseases in the same age range were recruited.
Clinical assessment included: presence/ absence of pelvic and shoulder pain, stiffness, edema, fatigue and loss of appetite, global pain using a Visual Analogue Scale (VAS - 0 to 10), evaluation of the number of tender (TJC) and swollen joints (SJC) (out of 28), functional status as assessed by Health Assessment Questionnaire (HAQ), and assessments of global disease severity by patients, and a global assessment of disease by physicians, using a VAS ranging from 0 to 10. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were also measured. Composite measures of peripheral arthritis were calculated using the above measures including Disease Assessment Score using a 28-joint count (DAS)-CRP and DAS-ESR. Blood samples were collected at baseline (first consultation in rheumatology clinics), processed immediately, and serum aliquots were stored at −80°C until analysis.
Out of 64 patients that fulfilled the inclusion criteria, 44 were diagnosed with EORA according to the ACR/EULAR 2010 criteria(Aletaha et al., 2010), 28 of them were seronegative (EORAneg), additionally, 20 were diagnosed with PMR (2012 EULAR/ACR criteria)(Dasgupta et al., 2012). From those, 28 patients with EORAneg and 20 patients with PMR were also evaluated at 3 months posttreatment. The EORAneg patients were classified in two categories: responders (good responders) and non-responders (moderate responders and null responders) according to DAS-ESR based EULAR response criteria(van Riel & Renskers, 2016).
Sample Preparation for Metabolite Extraction:
The samples were thawed at room temperature for 30 minutes, and subsequently 150 μL of each sample was transferred to a deep well plate. The plates were spun at 2000xg at 4°C for 1 min (Eppendorf 5804 R centrifuge, A-2-DWP rotor). Methanol was taken directly from a −20°C freezer and 750 μL was added into each well while shaking using an Agilent Bravo 96-channel liquid handling robot. The plates were shaken at 850 rpm, 12°C for 30 min (Eppendorf Thermomixer Comfort), and then centrifuged at 2250 x g, 4°C for 60 min. After that, 600 μL of the supernatant was transferred to a new deepwell plate with the Agilent Bravo and dried overnight (Labconco CentriVap lyophilizer set to 20°C). Each dried pellet was washed with 50 μL of methanol-d4, shaken at 850 rpm, 12°C for 10 min before drying again for 1 hr at 20°C. To finish, 200 μL of buffer (37.5 mM sodium phosphate pD 6.95, 0.02% w/v sodium azide, 0.747 mM TSP-d4) was added and it was shaken at 850 rpm, 20°C for 1 h. 180 μL of each sample was transferred to a 3 mm SampleJet NMR tube with a Bruker SamplePro Tube L robot.
NMR Acquisition and Processing:
A 600 MHz Bruker Avance III spectrometer equipped with a cooled SampleJet and a 5 mm room temperature BBI-probe was used for data acquisition. 1D 1H spectra were acquired using the pulse sequence ‘zgespe’, encompassing water suppression through excitation, sculpting, and including a perfect echo sequence. The acquisition time was 2.04s, the relaxation delay was of 2s, the receiver gain 181 and 128 scans were collected into 64k points. A 0.3Hz exponential line broadening was applied before Fourier transformation and zero filling to 128k points. Spectra were phased, baseline-corrected, and referenced to TSP-d4, all performed in TopSpin3.5pl7 (Bruker BioSpin). Samples in the SampleJet carousel were kept at 6°C before and after insertion in the magnet. A daily quality assurance procedure was performed before sample data acquisition, involving temperature calibration checks, shim and water suppression quality, and consistent quantification using a reference sample, all according to the Bruker In Vitro Diagnostics for research (IVDr) SOP.
Metabolites Identification and Quantification:
The Chenomx suite 8.5 professional (Chenomx Inc., Edmonton, Canada) 600 mHz, version 11 was used to identify and quantify the metabolites detected by 1D 1H-NMR by matching the compounds’ peak with its library according to the chemical shift. Metabolite’s concentration was normalized to the TSP-d4, and 58 metabolites were found in this process. The metabolites’ concentrations were reported in micromolar (μM).
ELISA:
Interleukin-6 (IL-6) and Tumor Necrosis Factor (TNF) were measured in serum according to the manufacturer’s instructions by ELISA (R&D systems).
Statistical Analysis:
The metabolites were normalized by sum of the data followed by a logarithmic transformation and scaled by mean centering using MetaboAnalyst version 5.0(Xia & Wishart, 2011) in order to achieve a comparable dataset, since some metabolites had extremely high concentrations in comparison to others, which deemed it necessary to perform a logarithmic transformation. Overall, sum normalization and logarithmic transformation helps avoid false significance(Misra, 2020). Partial Least Square Discriminant Analysis (PLS-DA) was used as a classification model between control, PMR and EORAneg according to the variable importance of projection (VIP) that identifies those metabolites which most influence the separation of groups considering a VIP>1 (Chong & Jun, 2005). Pathway analysis of control, PMR and EORAneg were evaluated with the tool of enrichment analysis available on MetaboAnalyst (Wieder et al., 2022). The 58 metabolites identified were ran through MetaboAnalyst using an open data base known as Kyoto Encyclopedia of Genes and Genomes (KEGG)(Kanehisa et al., 2016) 2019, which contains a set of 84 metabolites, including those identified in this work. Considering the algorithm proposed by G. Nyamundanda et al (Nyamundanda et al., 2013) to calculate sample size in metabolomic data, we determined that a minimum sample size of 22 patients (11 on each group) were needed to obtain 80% power and 10% of false discovery rate (FDR) assuming probabilistic principal component analysis (PPCA) as model, no covariates, 200 bins, 0.2 as expectation of proportion of significant bins and untargeted analysis calculated in R studio using “MetSizeR” package (https://cran.r-project.org/web/packages/MetSizeR/MetSizeR.pdf).
Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables as percentage. Differences in metabolite concentration between the three groups were assessed using one-way analysis of variance (ANOVA) adjusted by diabetes mellitus (DM) and sex, with Tukey Honest Significance Difference (HSD) post-hoc analysis. Additionally, quantitative variables between the three groups were analyzed using an ANOVA with a Scheffe post-hoc analysis to determine the significance of variables between groups. Heatmaps were created using R studio.v.4.0.3 to calculate Pearson’s correlations to analyze correlations between metabolite and inflammatory marker levels in PMR and EORAneg patients, and were adjusted by age, sex, DM, and BMI. A logistic regression analysis was performed to obtain a variable of prediction between EORAneg and PMR adjusted by age, sex, and DM. The variables included in the regression analyses were those with p<0.1 obtained by univariant comparisons between PMR and EORAneg. Data were assessed for multivariate outliers using Mahalanobis Distance Test (Todeschini et al., 2013) where two multivariate outliers were identified and removed for this analysis. The area under the curve (AUC) obtained from ROC analysis was used to determine the best cut-off value and predictability for PMR in base of the probability obtained from the logistic regression. The comparisons, logistic regressions and ROC curve were assessed with SPSS v.27 (IBM Corp. Released 2019 v27.0. Armonk, NY: IBM Corp.). The value p<0.05 was considered statistically significant.
Results
Patient demographics and disease characteristics
The characteristics of control and patient population are summarized in Table 1. Eighteen controls (average age: 75.38, SD 6.04, 39% males, and an average ESR level of 17.9, SD 17.03 mm/h) and 48 patients were analyzed. Of these patients, 28 were diagnosed with EORAneg with an average age of 76.75, SD 6.99, 57% males, and an average ESR level of 52.54, SD 27.20 mm/h, and 19 with PMR, with an average age of 76.40, SD 4.99, 15% were males, and an average ESR level of 57.80, SD 21.50 mm/h. There were more males in the EORAneg group (57%) compared to PMR group (15%, p=0.01), BMI was not different between groups. Comorbidities including high blood pressure (HBP), and dyslipidemia (DL), were also similar in both arthritic populations and controls, but diabetes mellitus (DM) was present in over half of EORAneg patients compared to PMR (25%) and controls (16%; p=0.02). As expected, PMR and EORAneg patients, presented with higher erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and IL-6 than controls, although TNF levels were relatively similar across all three groups.
Table 1.
Demographic and clinical characteristics between control group, PMR and EORAneg at baseline
| Variable | Control N=18 |
PMR N=20 |
EORAneg N=28 |
p |
|---|---|---|---|---|
| Female, n (%) | 11(61) | 17(85) | 12(43) *** | 0.01 |
| Age, years | 75.39±6.04 | 76.40±4.99 | 76.75±6.99 | 0.76 |
| BMI, kg/m2 | 28.84±5.56 | 27.27±4.32 | 28.82±4.76 | 0.50 |
| DM, n (%) | 3(16) * | 5(25) | 15(54) *** | 0.02 |
| HBP, n (%) | 11(61) | 17(85) | 20(71) | 0.25 |
| DL, n (%) | 9(50) | 12(60) | 19(67) | 0.48 |
| CRP mg/dL | 4.10±6.78* | 31.56±28.27 | 41.57±52.14 | 0.02 |
| ESR mm/h | 17.93±17.03* | 57.80±21.50** | 52.54±27.20 | <0.001 |
| TNF (pg/mL) | 15.05±19.86 | 13.60±6.12 | 13.86±6.68 | 0.93 |
| IL-6 (pg/mL) | 1.04±1.43* | 11.25±23.92 | 19.93±29.45 | 0.03 |
| Fatigue, n (%) | 14 (70) | 20 (71.4) | 0.92 | |
| Weight loss, n (%) | 8 (40) | 10 (36) | 0.76 | |
| Anorexia, n (%) | 7 (35) | 14 (50) | 0.30 | |
| Stiffness, n (%) | 19 (95) | 27 (96) | 0.81 | |
| Shoulder pain, n (%) | 20 (100) | 21 (75) | 0.02 | |
| Pelvis pain, n (%) | 18 (90) | 13 (64) | <0.001 | |
| Tender Joints | 2.70 ± 0.98 | 9.11 ± 5.83 | <0.001 | |
| Swollen Joints | 0.25 ± 0.64 | 11.46 ± 5.89 | <0.001 | |
| Pain | 81.25 ± 17.46 | 73.04 ± 17.81 | 0.12 | |
| HAQ | 1.50 ± 0.57 | 1.77 ± 0.80 | 0.18 | |
| DAS-CRP | 5.64±0.99 | |||
| DAS-ESR | 6.21±1.00 | |||
| NSAIDs mg | 520.00 ± 44.72 | 450.00 ± 216.35 | 0.35 | |
| NSAIDs, n (%) | 5 (25) | 11 (39.3) | 0.36 |
Continuous variables expressed in mean ± standard deviation; Categorical variables expressed as percentages. Quantitative variables between three groups were analyzed using ANOVA (Scheffe post-hoc). Student t-test was used to compare quantitative variables between variables present only in EORAneg and PMR. Chi-square analysis was used to determine the significance between groups for qualitative variables. Note. BMI: Body mass index; DM: Diabetes mellitus; HBP: high blood pressure; DL: Dyslipidemia; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; TNF: Tumor necrosis factor; IL-6: Interleukin 6.
p<0.05 Control vs EORAneg
p<0.05 Control vs PMR
p<0.05 EORAneg vs PMR
Within the arthritic population, PMR patients presented more shoulder pain (100% vs 75% in EORAneg, p=0.02), more pelvic pain (90% vs 60% in EORAneg, p<0.001), and less peripheral arthritis than EORAneg at baseline (9.11, SD 5.83 vs 2.70, SD 0.98, p<0.001 and 11.46, SD 5.89 vs 0.25, SD 0.64, p<0.0001, for tender and swollen joints in EORAneg vs PMR, respectively). We did not observe differences between PMR and EORAneg in other clinical characteristics including pain (p=0.12), HAQ (p=0.18), ESR (p=0.48), CRP (p=0.44), TNF (p=0.90) or IL-6 (p=0.28). Both PMR and EORAneg patients experienced a similar amount of pain according to the visual analog scale (VAS) (81.25, SD 17.46 and 73.04, SD 17.81, respectively), and a similar dose of non-steroidal anti-inflammatory drugs (NSAIDs) (Table 1). At baseline (first visit in rheumatology clinics), none of the patients were on steroids, or on any synthetic or biological DMARDs.
Metabolomic Profiling in PMR and EORAneg patients
A total of 58 polar metabolites were detected in the serum of control group, PMR, and EORAneg patients. We identified several amino acids, ketone bodies, and several glycolytic intermediates among other metabolites. We performed a multivariate OPLS-DA with all the metabolites observing only a small discrimination between the groups (Figure 1a, 1b). Principal components (1–5) explained 41.9% of the variance of the 1H-NMR data. With a cutoff value of Variables Importance in Projection (VIP)>1.0 obtained from PLS-DA. However, 5 metabolites with a VIP>2 (O-acetylcholine, Hydroxyacetone, taurine, glycine and n-acetylaspartate) were the principal features driving the separation between groups. Several metabolites, such as o-acetylcholine (o-ACh), hydroxyacetone, taurine, glycine, n-acetylaspartate, methionine, glycolate, pyruvate, threonine, and creatine differentiate controls from EORAneg and PMR groups. Likewise, acetoacetate, trimethylamine, betaine, acetone, and 3-hydroxybutyrate were metabolites that better differentiate PMR patients from controls and EORAneg patients (Figure 1c).
Fig. 1. Polar metabolites’ profiling in control, PMR and EORAneg samples.
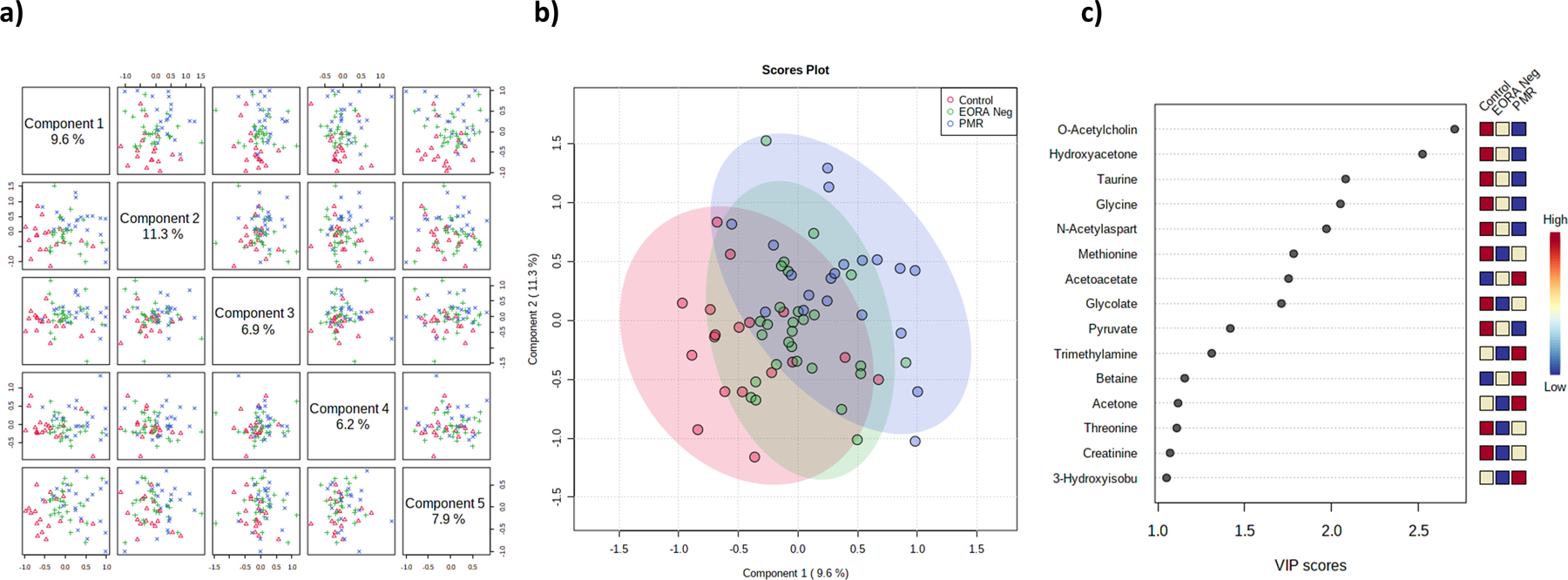
a) Shows a component analysis with a total variance of 41.9% between the 3 groups, b) PLS-DA showing PMR and control as the most different groups; c) Variance of important projection of metabolites based in component 2 (11.3%) that identified o-ACh as the most important metabolite follow by hydroxy-acetone to separate PMR from control and EORAneg. Note: o-ACh: o-acetylcholine
We then analyzed metabolomic fingerprint in the three groups at baseline. Metabolite correlation patterns is shown in Figure S1 (See Supplementary material). While metabolite clustering in PMR population was similar to controls (Figure S1b in supplementary material), it was different in EORAneg (Figure S1c in supplementary material). When the metabolites were mapped to known metabolic pathways using MetaboAnalyst V.5.0, enriched pathways were significantly different in each group. While ubiquinone metabolism, phenylalanine, and tyrosine and tryptophan metabolism were the most enriched pathways in PMR, in EORAneg galactose metabolism, starch and sucrose metabolism, and alanine, aspartate and glutamate metabolism were the most enriched. Finally, in controls, selecompound metabolism, pantothenate and CoA biosynthesis and tryptophan metabolism were the most enriched metabolic pathways (Figure S2a–c supplementary material).
Significantly different metabolites between controls, PMR and EORAneg patients
To determine the different metabolites’ concentrations between controls, PMR and EORAneg, metabolites were analyzed by using an ANOVA and adjusting variables by DM and sex. Several metabolites were significantly different between controls, PMR and EORAneg patients (Table 2 and Figure 2a). We observed that 3-hydroxybutyrate, acetate, glucose, glycine, lactate, o-Ach, and pantothenate were significantly different between the 3 groups, and some others such as phenylalanine, taurine and threonine showed tendency. Of these metabolites, phenylalanine was higher while glycine, o-Ach, and threonine were lower in PMR compared to the control group. On the contrary, we observed higher levels of 3-hydroxybutyrate, acetate, glucose, lactate, and pantothenate in the EORAneg group compared to the control group (Table S1 in supplementary material and Figure 2a). This suggests that metabolomic profiles do vary amongst controls and the diseased groups. Figure 2b shows a visual representation of Table 2 and Table S1 in supplementary material, with a picture of metabolites with higher or lower concentrations in PMR (mostly amino acids) or EORAneg (mostly glucose metabolism related metabolites) when compared with control.
Table 2:
Concentration of metabolites between controls, EORAneg and PMR patients.
| Metabolite | Control N=18 |
PMR N=20 |
EORAneg N=28 |
P Adj. |
|---|---|---|---|---|
| 3-Hydroxybutyrate | 15.61±7.60** | 24.27±21.86 | 36.40±28.26 | 0.006 |
| 3-Hydroxyisobutyrate | 8.92±4.53 | 10.37±3.04 | 9.41±3.03 | 0.38 |
| 3-Phenyllactate | 12.38±5.47 | 14.73±5.10 | 12.62±4.38 | 0.29 |
| Acetate | 23.63±5.52** | 25.53±6.35 | 31.48±8.67*** | <0.001 |
| Acetoacetate | 2.79±1.80 | 4.78±6.08 | 6.28±12.89 | 0.42 |
| Acetone | 1.37±1.09 | 3.03±5.60 | 1.63±2.44 | 0.30 |
| Adenine | 12.63±2.72 | 12.43±3.99 | 11.60±3.22 | 0.36 |
| Alanine | 130.05±31.56 | 118.65±32.44 | 134.09±36.30 | 0.27 |
| Arginine | 41.98±20.04 | 43.36±15.48 | 49.13±23.20 | 0.34 |
| Asparagine | 18.03±6.80 | 16.25±7.31 | 16.20±5.10 | 0.65 |
| Betaine | 9.11±6.04 | 10.90±7.70 | 10.68±6.08 | 0.70 |
| Butyrate | 9.49±2.68 | 8.79±2.78 | 8.26±2.23 | 0.19 |
| Choline | 12.19±2.91 | 10.71±2.58 | 10.93±2.15 | 0.09 |
| Citrate | 8.96±2.22 | 8.99±2.58 | 9.30±2.23 | 0.90 |
| Creatine | 12.21±3.84 | 11.35±3.85 | 11.81±4.48 | 0.81 |
| Creatine Phosphate | 9.00±2.68 | 11.77±6.75 | 9.85±3.34 | 0.12 |
| Creatinine | 6.54±3.84 | 6.27±3.83 | 6.23±2.74 | 0.93 |
| Dimethylamine | 2.18±1.31 | 1.70±.80 | 1.72±0.95 | 0.29 |
| Formate | 13.26±4.06 | 14.35±6.14 | 12.85±3.81 | 0.50 |
| Fumarate | 2.71±1.15 | 2.84±1.70 | 2.62±1.10 | 0.83 |
| Glucose | 1142.19±273.30** | 1321.01±357.16 | 1561.20±643.18 | 0.007 |
| Glutamate | 111.29±36.57 | 123.99±35.21 | 127.46±38.66 | 0.28 |
| Glutamine | 23.14±6.62 | 27.05±9.22 | 22.35±8.02 | 0.14 |
| Glycine | 74.28±22.06 | 54.29±22.56* | 61.40±14.69 | 0.008 |
| Glycolate | 7.61±3.47 | 7.63±4.70 | 7.81±4.14 | 0.96 |
| Hydroxyacetone | 3.92±1.86 | 3.28±2.23 | 3.73±1.76 | 0.51 |
| Isobutyrate | 4.46±2.25 | 4.83±4.37 | 4.75±2.93 | 0.94 |
| Isoleucine | 27.86±7.07 | 28.44±5.65 | 31.14±8.28 | 0.24 |
| Isovalerate | 10.31±2.60 | 9.65±2.97 | 10.02±3.87 | 0.79 |
| Lactate | 697.53±199.29** | 710.61±153.32 | 894.90±256.11*** | 0.002 |
| Leucine | 39.26±13.96 | 44.07±10.89 | 45.15±18.82 | 0.47 |
| Lysine | 38.53±10.28 | 39.89±10.09 | 41.64±16.29 | 0.75 |
| Malate | 19.93±5.61 | 20.71±8.85 | 16.67±6.06 | 0.12 |
| Methionine | 2.03±1.13 | 1.95±1.14 | 2.24±1.18 | 0.61 |
| Methylamine | 2.87±1.32 | 4.00±5.65 | 2.55±0.91 | 0.32 |
| Methylsuccinate | 5.94±2.61 | 4.89±2.09 | 4.88±2.42 | 0.30 |
| N,N-Dimethylglycine | 1.16±.67 | 1.27±.81 | 1.25±0.51 | 0.86 |
| N-Acetylaspartate | 4.86±1.56 | 4.11±3.37 | 4.02±1.79 | 0.54 |
| N-Phenylacetylglycine | 8.06±3.69 | 9.13±6.03 | 8.44±5.79 | 0.81 |
| O-Acetylcholine | 4.23±2.65 | 2.36±1.59* | 4.18±2.70*** | 0.03 |
| O-Phosphocholine | 18.06±6.00 | 19.16±6.29 | 20.81±5.95 | 0.31 |
| Ornithine | 24.90±9.84 | 27.60±9.15 | 27.10±7.03 | 0.58 |
| Pantothenate | 4.51±2.58** | 6.07±3.05 | 7.06±4.07 | 0.05 |
| Phenylalanine | 12.53±3.21 | 15.68±4.49* | 14.27±4.10 | 0.06 |
| Proline | 46.99±19.09 | 58.42±38.03 | 56.74±18.34 | 0.37 |
| Pyruvate | 6.84±3.69 | 4.80±2.07 | 6.04±3.33 | 0.13 |
| Sarcosine | 6.34±3.33 | 5.65±1.67 | 5.33±2.51 | 0.61 |
| Serine | 42.07±15.79 | 49.85±13.48 | 46.41±14.70 | 0.28 |
| SG3PC | 106.86±39.32 | 135.18±43.97 | 113.23±44.72 | 0.08 |
| Succinate | 4.07±1.15 | 4.09±1.01 | 3.68±0.71 | 0.20 |
| Taurine | 49.31±33.76 | 33.44±21.42 | 54.95±33.14 | 0.06 |
| Threonine | 34.29±10.66 | 27.73±8.48 | 29.61±7.45 | 0.06 |
| Trimethylamine | 0.73±.39 | 0.69±.46 | 0.64±0.31 | 0.65 |
| TMAO | 3.21±2.92 | 4.91±6.55 | 4.08±4.93 | 0.58 |
| Tryptophan | 16.92±4.78 | 16.25±6.83 | 17.65±5.38 | 0.71 |
| Tyrosine | 21.64±5.19 | 24.10±5.39 | 23.21±4.90 | 0.36 |
| Valine | 84.84±16.07 | 91.27±17.29 | 92.96±21.40 | 0.35 |
| t-Methylhistidine | 8.87±2.26 | 9.92±3.30 | 9.89±5.74 | 0.69 |
Variables expressed in mean ± standard deviation. ANOVA (adjusted by DM and sex, with Tukey HSD post-hoc analysis) was used to determine the significance of metabolites between groups. Concentration expressed in μM. Note. TMAO: Trimethylamine N-Oxide, SG3PC: Sn-Glycero-3-Phosphocholine. P adj: P adjusted.
P<0.05 control vs PMR
p<0.05 control vs EORAneg
p<0.05 PMR vs EORAneg
Fig. 2. Metabolite concentrations in control, PMR, and EORAneg samples.
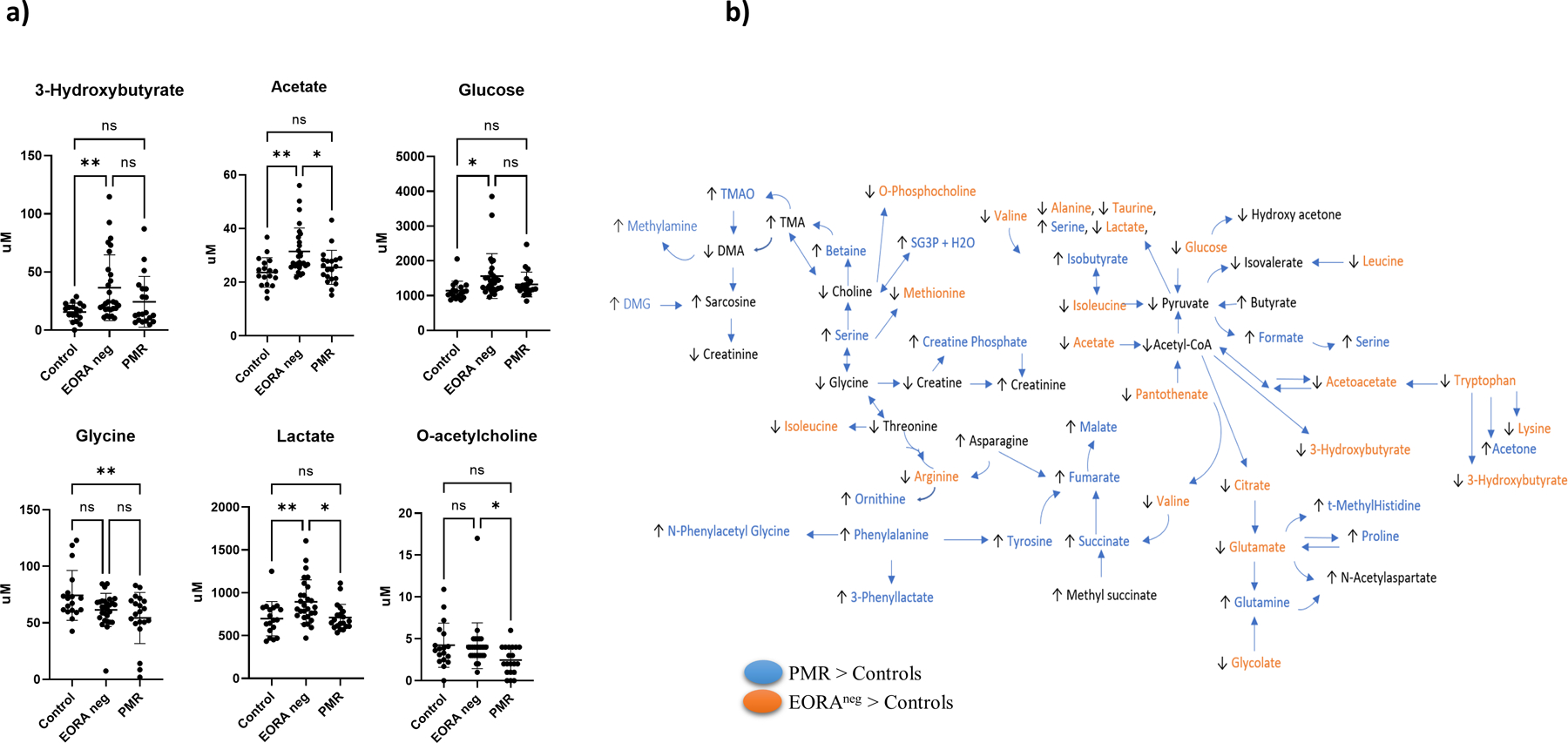
a) Comparison of concentrations of significant metabolites identified by 1H-NMR between control, EORAneg, PMR at baseline adjusted by DM and sex using Tukey HSD as post-hoc analysis. The overall ANOVA p-value is below the metabolite name, while the p-values for the post-hoc analysis are located between groups. b) Map of metabolic pathways showing metabolites with a p<0.10 obtained from Tukey HSD analysis. Metabolites different in PMR compared to controls are highlighted in blue. Metabolites different in EORAneg compared to controls are highlighted in orange. Arrows indicate whether the metabolite concentration was higher or lower than in the control group. SG3PC: Sn-Glycero-3-Phosphocholine
Association of metabolites with inflammatory markers in PMR and EORAneg
We also determined whether some of these metabolites were associated with inflammation in each disease. A Pearson correlation was performed between IL-6 levels, ESR and CRP, and metabolites’ concentration adjusted by age, BMI, DM and sex. The regression coefficients for each inflammation outcome-metabolite pair were used to form a clustered heatmap to lend insight into which clinical characteristics were correlated with which metabolites. IL-6 correlated positively with TMAO, pantothenate, O-phosphocholine, glycolate, glucose, creatine phosphate and acetate in PMR patients. While in EORAneg, IL-6 had positive correlation only with tryptophan and negative correlation with valine, isovalerate and isoleucine (Figure 3). Different metabolites also correlated with CRP and ESR in both diseases suggesting different activated metabolic pathways associated to inflammation in these diseases.
Fig. 3. Correlation between inflammatory markers and metabolites adjusted by age, sex, DM, and BMI at baseline.
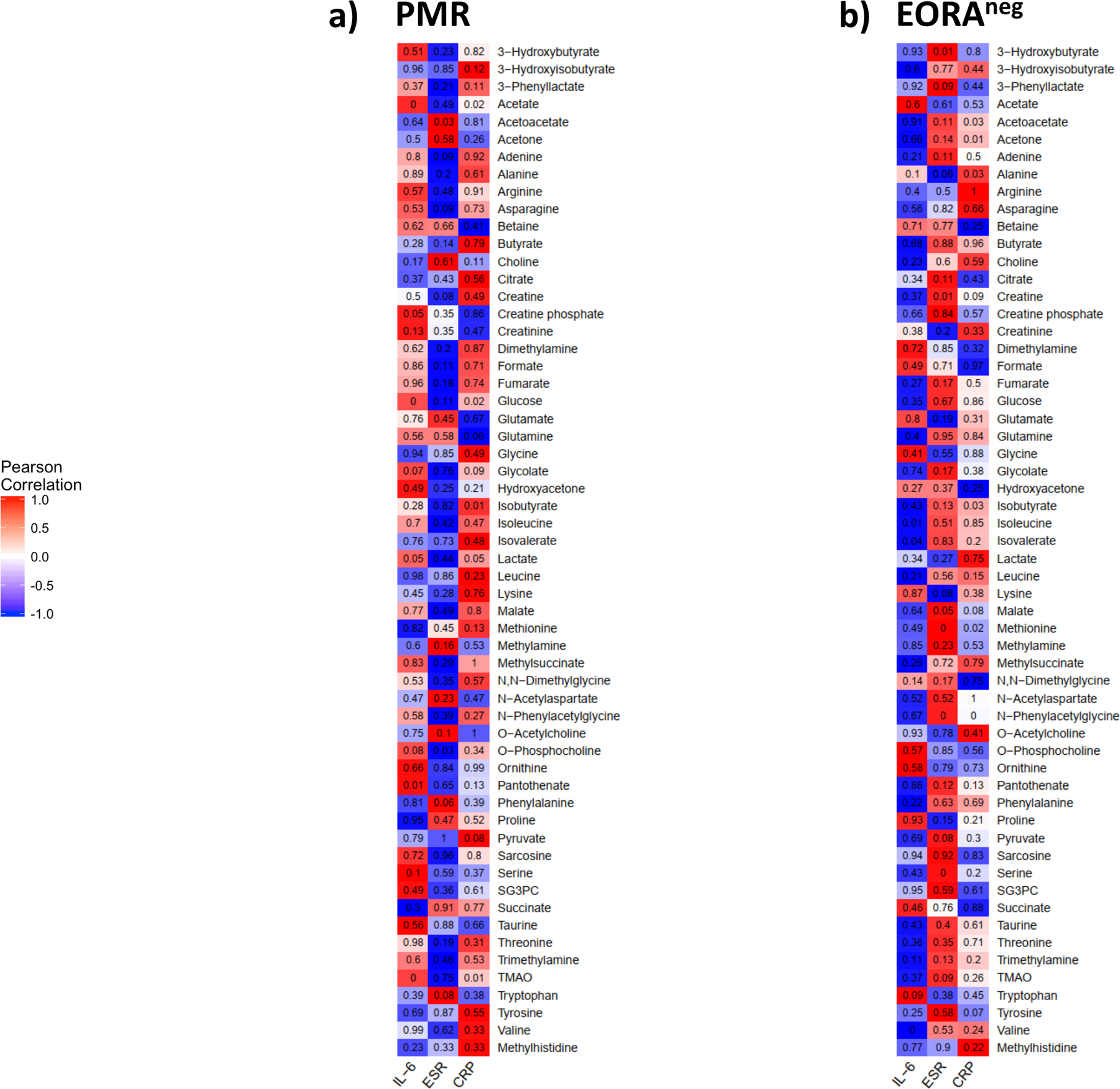
a) The strength of association of each pair of PMR were used to form a cluster heatmap to lend inside which cytokine were correlated with which group of metabolites in serum using Pearson correlation; b) The strength of association of each pair of EORAneg were used to form a cluster heatmap to lend inside which cytokine were correlated with which group of metabolites in serum using Pearson correlation. Red color indicate positive correlation while blue negative correlation. The number on each cell refers the p-value of each pair of each group.
Predictors of Classification to distinguish PMR from EORAneg
Of the 58 metabolites, it was observed that some metabolites differed between PMR and EORAneg such as acetate, lactate, taurine and o-Ach which were significantly elevated in EORAneg compared with PMR patients (Table S2 in supplementary material). Additionally, some metabolites almost reached significance, for instance glutamine, malate, succinate and SG3PC were higher in PMR than EORAneg (Table S2 in supplementary material).
Then, a binary logistic regression analysis was conducted, including the metabolites with p<0.10 in the univariate model between PMR and EORAneg (Table S2 in supplementary material) after adjusting by sex, and DM. The model showed that lactate, o-ACh, taurine, and sex (female) are predictors that classified and distinguished PMR from EORAneg. After removing the outliers from the model, the classification model showed a sensitivity of 90% and specificity of 92.3% with an AUC of 0.925 and a p<0.001 (Figure 4a). An additional logistic regression model was conducted using the same metabolites as well as oxylipins (which were recently described by our group in this cohort)(Coras et al., 2021) to determine whether or not the addition of these lipids would improve the prediction model. The new binary logistic regression analysis included lactate, o-ACh, taurine, and 16(17) EpDPE in the model. The ROC curve improved the sensitivity of the model by reaching 95% but decreasing the specificity to 88% with an AUC of 0.944 and a significance of p<0.001 (Figure 4b). Low levels of lactate, o-ACh and taurine as well as high levels of 16(17)EpDPE were important factors to predict PMR.
Fig. 4. Metabolomic prediction model for PMR classification.
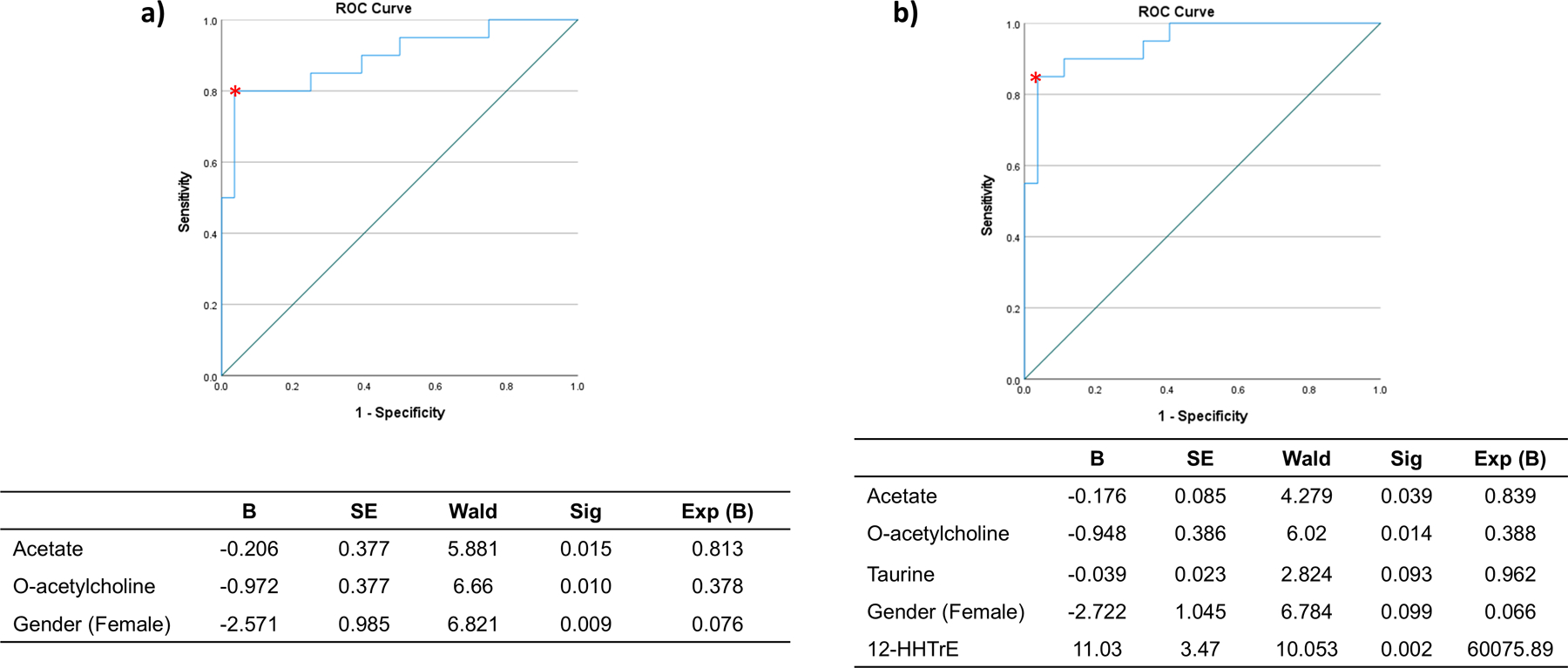
PMR was considered the principal outcome. The logistic model used metabolites that had a p value <0.10 in the binary comparison between EORAneg and PMR. a) The model showed an area under curve of 0.925 with a sensibility= 90% and specificity= 92.3%; p<0.001. b) The model showed an area under curve of 0.944 with a sensibility= 95% and specificity= 88%; p<0.001, when the oxylipins were included in the model
Metabolomic profile between responders and non-responders in EORAneg
The EORAneg patients were classified in two categories: responders (good responders) and non-responders (moderate responders and null responders) according to EULAR response criteria using DAS28-ESR at 3 months posttreatment. 21 EORAneg patients were identified as responders (R), while 7 were classified as non-responders (NR). Characteristics of R and NR are summarized in Table S3 (See supplementary material). Of the R, 24% were females, while the NR were all females (p<0.001). Both groups had a similar age and BMI (76.00, SD 6.86 vs 79.00, SD 7.44 and 27.92, SD 3.08 vs 31.52, SD 7.66), respectively. Co-morbidities between groups did not reach statistical significance, but DM was more prevalent in the NR group (86% vs 43%). Of note, DM patients did not have worse disease status than non-DM patients at baseline (Table S4 in supplementary material). Of interest, tender joints count, and pain were lower in R than NR (7.76, SD 4.55 vs 13.14, SD 7.65, p=0.12 and 69.29, SD 17.91 vs 84.29, SD 12.72, p=0.05, respectively). DAS-ESR was also lower in R than in NR (6.04, SD 0.96 vs 7.11, SD 0.89, p=0.02). Similarly, DAS-CRP was also slightly lower in R than in NR (5.46, SD 1.00 vs 6.17, SD 0.79, p=0.10). Both groups had similar CRP levels mg/dL, but ESR levels were higher in NR than in R (70.43, SD 19.37 vs 46.57, SD 27.14, p= 0.04). The cytokines levels were similar in both groups. Treatment in both groups was also similar, 28.6% of R and NR were on methotrexate. All the patients were receiving corticoids and both groups received similar doses (8.95, SD 3.32 vs 9.14, SD 3.02, ns) (Table S3 in supplementary material).
After comparing the metabolomic profile at baseline of both groups adjusted by sex and DM, 3-hydroxybutyrate, adenine, glucose, taurine, and TMAO, were statistically higher in the NR group, while glutamate was higher in R group (Table S5 in supplementary material). The OPLS-DA showed a clear separation between R and NR (Figure S3a, b in supplementary material). Furthermore, the VIP scores showed that several metabolites, with TMAO as an outstanding feature driving the separation with a VIP >3.5. Other metabolites including betaine, glycine, glutamate, lysine, n-acetylaspartate, acetone, dimethylamine, ornithine, threonine, proline, o-ACh, and tryptophan were also driving the separation between R from NR (Figure S3c in supplementary material). Additionally, we compared metabolites concentrations from R or NR vs control, and it was observed that 3-hydroxybutyrate, acetate, glucose, lactate, pantothenate and taurine showed significantly higher concentrations in NR compared to control while glycine had lower levels in NR compared to control (Figure S3d and Table S6 in supplementary material).
DISCUSSION
Prior studies have tried to differentiate EORA and PMR. One study described the use of physical exam paired with blood tests such as alanine aminotransferase, alkaline phosphatase, and glutamyl transferase, but the study was unable to fully separate and properly diagnose all enrolled participants(Caporali et al., 2001). Cytokine level measurements such as TNF, IL-1Ra and IL-6 along with steroidal hormone level measurements were also used to distinguish the two diseases(Cutolo et al., 2006). In this study, cytokine and steroidal hormone patterns suggested that patients with PMR and those with EORA with PMR-like onset had higher levels of cytokines and overall inflammation(Cutolo et al., 2006). Another way that researchers have tried to distinguish these diseases is using Fluorodeoxyglucose (FDG) Positron Emission Tomography (PET), which evaluates glucose metabolism in organs and tissues. Of interest, FDG accumulation was much higher in PMR patients at the 9 study sites compared to EORA and suggested that enthesitis in addition to bursitis was more present in PMR(Wakura et al., 2016).
In our study, we have described different metabolomic profiles between PMR and EORAneg that might be useful to better understand and classify these 2 diseases. Several metabolites were significantly different or almost reached significance between diseases suggesting that metabolomics can be a useful tool to understand their pathobiology and help in biomarker identification. For instance, concentrations of amino acids such as glycine and phenylalanine were different in the PMR group compared to the control, whereas some glucose-related metabolites were different in the EORAneg group compared to the control. Of note, in addition to the metabolites detected by NMR, we also included oxylipins from our previous study(Coras et al., 2021), which helped improve the classification of PMR from EORAneg patients. Our results suggest that the combination of multiple biomarkers may lead to promising results to understand the pathobiology of different rheumatic diseases and improve diagnosis.
O-ACh is recognized to be a neurotransmitter but also can be secreted by several immune cells, which helps maintain cell function through proliferation and differentiation, among others(Wessler & Kirkpatrick, 2008). In addition, o-ACh can modify immune responses and it was described to have an important role in the production of antibodies(Wessler & Kirkpatrick, 2008). O-ACh is also released by macrophages(Wang et al., 2004) and T cells(Rosas-Ballina et al., 2011) and has an anti-inflammatory effect. In our study, serum concentration of o-ACh in EORAneg and control were remarkably similar, but lower in PMR. Given that several stimuli are responsible for an increase of o-ACh receptor in cells(Chiba et al., 2019), one possible explanation is that it is consumed locally, due to the higher expression of their receptors. Of interest, glycine also appears to play an anti-inflammatory role and maintains homeostasis of the immune system(Zhong et al., 2003). PMR patients had also significantly lower levels of glycine compared to controls and EORAneg patients.
Acetate, which was significantly higher in EORAneg patients when compared to PMR and control, regulates inflammation in the body. Abnormal levels of acetate can lead to many inflammatory disorders, such as inflammatory bowel disease, Crohn’s disease, and ulcerative colitis(Parada Venegas et al., 2019; Xu et al., 2019). Acetate, together with other significant metabolites such as pyruvate, glucose and lactate are related to glycolysis, which has been associated with inflammatory diseases including RA(Biniecka et al., 2016; Bustamante et al., 2018; de Oliveira et al., 2019; Falconer et al., 2018; Garcia-Carbonell et al., 2016). Although another study suggested that in the presence of inflammation, glucose levels drop while lactate levels increase(Holmberg et al., 2017), in our study both metabolites were elevated, especially in EORAneg patients. 3-hydroxybutyrate was another metabolite that had a higher concentration in the EORAneg group than controls and PMR patients. Based on previous studies, this metabolite has been known to be elevated in RA patients (11, 35). Phenylalanine was increased in PMR. Some studies have found that phenylalanine is effective in treating chronic pain and inflammation in diseases such as arthritis which can explain why in this study, phenylalanine is increased in patients with PMR when compared to the controls, suggesting a compensatory increased in order to regulate the inflammation(Ehrenpreis, 1982).
In our study, we also determined metabolites associated to response. Several studies have succeeded in identifying metabolites associated to response in RA(Cuppen et al., 2016; Murillo-Saich et al., 2021; Sweeney et al., 2016; Tatar et al., 2016). Since all the PMR patients tend to respond on prednisone, we focused on EORAneg patients. 3-hydroxybutyrate, adenine glucose, glutamate, TMAO, and taurine were the significant metabolites between R and NR, although some other metabolites almost reached significance such as acetate or citrate (Table S5). Of interest, the proportion of DM was elevated in NR. The association between DM and response may be critical in older adults, since prior studies did not described a worse response in young RA patients with DM, in fact, one study suggested that methotrexate may decrease the risk of developing DM in RA patients(Baghdadi, 2020), yet, the same proportion of R and NR were taking methotrexate in this cohort.
This study suggests that some distinct metabolic pathways can be involved in the pathogenesis of EORAneg and PMR, that can be used to differentiate between these clinically similar diseases. Although our findings are promising, this study is not without its limitations. Since this is an exploratory study, the size of the cohort is small, and we lack validation cohort, which limits the generalization of our findings. The use of non-fasting samples is another limitation of our study, since diet can affect the concentrations of serum metabolites. It would also be of interest to study local metabolic changes in the inflamed synovial and periarticular tissue to evaluate the relation between local and circulating metabolites.
CONCLUSION
This exploratory study yielded very promising and significant results to help understand these diseases’ pathobiology in these geriatric populations. In this study, we identified three metabolites that helped to predict PMR from EORAneg patients. The use of 1D 1H-NMR can be a promising tool to distinguished between EORA and PMR.
Supplementary Material
Funding
This work was supported by the National Institutes of Health (R01AR073324 to M.G., T32AR064194 to JDM-S and RC).
LIST OF ABBREVIATION
- PMR
Polymyalgia Rheumatica
- EORAneg
Seronegative Elderly Onset Rheumatoid Arthritis
- TMAO
Trimethylamine N-Oxide
- SG3PC
Sn-Glycero-3-Phosphocholine
- R
Responder
- NR
Non-Responder
- DM
Diabetes Mellitus
- HTA
Hypertension
- DLP
Dyslipidemia
- BMI
Body Mass Index
- ESR
Erythrocyte Sedimentation Rate
- CRP
C-Reactive protein
- IL-6
Interleukin-6
- CRP
C-Reactive Protein
- NSAIDs
Non-Steroidal Anti-Inflammatory Drugs
- HAQ
Health Assessment Questionnaire
- ANOVA
Analysis of Variance
- O-PLSDA
Orthogonal Partial Least-Squares Discriminant Analysis
- VIP
Variable Importance in Projection
- AUC
Area Under the Curve
- 1H-NMR
Hydrogen-Nuclear Magnetic Resonance
Footnotes
DECLARATIONS
Ethics approval and consent to participate
The study was approved by the Institutional Board Review at Clinic of the Hospital Universitari Germans Trias i Pujol with the number IP-13-001. All procedures performed in this study were in accordance with the ethical standards of the Institutional Board Review at Clinic of the Hospital Universitari Germans Trias i Pujol and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable
Competing interest
All the authors declare that they have no competing interest.
Availability of data and materials
The datasets of metabolites generated during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article and its supplementary information files.
REFERENCES
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, & Hawker G (2010). 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum, 62(9), 2569–2581. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- Anderson JR, Chokesuwattanaskul S, Phelan MM, Welting TJM, Lian LY, Peffers MJ, & Wright HL (2018). (1)H NMR Metabolomics Identifies Underlying Inflammatory Pathology in Osteoarthritis and Rheumatoid Arthritis Synovial Joints. J Proteome Res, 17(11), 3780–3790. 10.1021/acs.jproteome.8b00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghdadi LR (2020). Effect of methotrexate use on the development of type 2 diabetes in rheumatoid arthritis patients: A systematic review and meta-analysis. PLoS One, 15(7), e0235637. 10.1371/journal.pone.0235637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biniecka M, Canavan M, McGarry T, Gao W, McCormick J, Cregan S, Gallagher L, Smith T, Phelan JJ, Ryan J, O’Sullivan J, Ng CT, Veale DJ, & Fearon U (2016). Dysregulated bioenergetics: a key regulator of joint inflammation. Ann Rheum Dis, 75(12), 2192–2200. 10.1136/annrheumdis-2015-208476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante MF, Oliveira PG, Garcia-Carbonell R, Croft AP, Smith JM, Serrano RL, Sanchez-Lopez E, Liu X, Kisseleva T, Hay N, Buckley CD, Firestein GS, Murphy AN, Miyamoto S, & Guma M (2018). Hexokinase 2 as a novel selective metabolic target for rheumatoid arthritis. Ann Rheum Dis, 77(11), 1636–1643. 10.1136/annrheumdis-2018-213103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporali R, Montecucco C, Epis O, Bobbio-Pallavicini F, Maio T, & Cimmino MA (2001). Presenting features of polymyalgia rheumatica (PMR) and rheumatoid arthritis with PMR-like onset: a prospective study. Annals of the Rheumatic Diseases, 60(11), 1021. 10.1136/ard.60.11.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham JC, & Blackband SJ (2001). Nuclear Magnetic Resonance Spectroscopy and Imaging in Animal Research. ILAR Journal, 42(3), 189–208. 10.1093/ilar.42.3.189 [DOI] [PubMed] [Google Scholar]
- Chiba T, Sakuma K, Komatsu T, Cao X, Aimoto M, Nagasawa Y, Shimizu K, Takahashi M, Hori Y, Shirai K, & Takahara A (2019). Physiological role of nitric oxide for regulation of arterial stiffness in anesthetized rabbits. Journal of Pharmacological Sciences, 139(1), 42–45. 10.1016/j.jphs.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Cho K, Mahieu NG, Johnson SL, & Patti GJ (2014). After the feature presentation: technologies bridging untargeted metabolomics and biology. Curr Opin Biotechnol, 28, 143–148. 10.1016/j.copbio.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong I-G, & Jun C-H (2005). Performance of some variable selection methods when multicollinearity is present. Chemometrics and Intelligent Laboratory Systems, 78(1), 103–112. 10.1016/j.chemolab.2004.12.011 [DOI] [Google Scholar]
- Coras R, Pedersen B, Narasimhan R, Brandy A, Mateo L, Prior-Español A, Kavanaugh A, Armando AM, Jain M, Quehenberger O, Martínez-Morillo M, & Guma M (2021). Imbalance Between Omega-6- and Omega-3-Derived Bioactive Lipids in Arthritis in Older Adults. J Gerontol A Biol Sci Med Sci, 76(3), 415–425. 10.1093/gerona/glaa113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costenbader KH, DiIorio M, Chu SH, Cui J, Sparks JA, Lu B, Moss L, Kelmenson L, Feser M, Edison J, Clish C, Lasky-Su J, Deane KD, & Karlson EW (2021). Circulating blood metabolite trajectories and risk of rheumatoid arthritis among military personnel in the Department of Defense Biorepository. Ann Rheum Dis. 10.1136/annrheumdis-2020-219682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppen BV, Fu J, van Wietmarschen HA, Harms AC, Koval S, Marijnissen AC, Peeters JJ, Bijlsma JW, Tekstra J, van Laar JM, Hankemeier T, Lafeber FP, & van der Greef J (2016). Exploring the Inflammatory Metabolomic Profile to Predict Response to TNF-α Inhibitors in Rheumatoid Arthritis. PLoS One, 11(9), e0163087. 10.1371/journal.pone.0163087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Montecucco CM, Cavagna L, Caporali R, Capellino S, Montagna P, Fazzuoli L, Villaggio B, Seriolo B, & Sulli A (2006). Serum cytokines and steroidal hormones in polymyalgia rheumatica and elderly-onset rheumatoid arthritis. Annals of the Rheumatic Diseases, 65(11), 1438. 10.1136/ard.2006.051979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Cimmino MA, Maradit-Kremers H, Schmidt WA, Schirmer M, Salvarani C, Bachta A, Dejaco C, Duftner C, Jensen HS, Duhaut P, Poór G, Kaposi NP, Mandl P, Balint PV, Schmidt Z, Iagnocco A, Nannini C, Cantini F, Macchioni P, Pipitone N, Amo MD, Espígol-Frigolé G, Cid MC, Martínez-Taboada VM, Nordborg E, Direskeneli H, Aydin SZ, Ahmed K, Hazleman B, Silverman B, Pease C, Wakefield RJ, Luqmani R, Abril A, Michet CJ, Marcus R, Gonter NJ, Maz M, Carter RE, Crowson CS, & Matteson EL (2012). 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis, 71(4), 484–492. 10.1136/annrheumdis-2011-200329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira PG, Farinon M, Sanchez-Lopez E, Miyamoto S, & Guma M (2019). Fibroblast-Like Synoviocytes Glucose Metabolism as a Therapeutic Target in Rheumatoid Arthritis. Front Immunol, 10, 1743. 10.3389/fimmu.2019.01743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Costanzo A, Paris D, Melck D, Angiolillo A, Corso G, Maniscalco M, & Motta A (2020). Blood biomarkers indicate that the preclinical stages of Alzheimer’s disease present overlapping molecular features. Sci Rep, 10(1), 15612. 10.1038/s41598-020-71832-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas ME, Maibaum EC, Teague C, Ueshima H, Zhou B, Lindon JC, Nicholson JK, Stamler J, Elliott P, Chan Q, & Holmes E (2006). Assessment of analytical reproducibility of 1H NMR spectroscopy based metabonomics for large-scale epidemiological research: the INTERMAP Study. Analytical chemistry, 78(7), 2199–2208. 10.1021/ac0517085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenpreis S (1982). D-phenylalanine and other enkephalinase inhibitors as pharmacological agents: implications for some important therapeutic application. Acupunct Electrother Res, 7(2–3), 157–172. 10.3727/036012982816952099 [DOI] [PubMed] [Google Scholar]
- Falconer J, Murphy AN, Young SP, Clark AR, Tiziani S, Guma M, & Buckley CD (2018). Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis. Arthritis Rheumatol, 70(7), 984–999. 10.1002/art.40504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Carbonell R, Divakaruni AS, Lodi A, Vicente-Suarez I, Saha A, Cheroutre H, Boss GR, Tiziani S, Murphy AN, & Guma M (2016). Critical Role of Glucose Metabolism in Rheumatoid Arthritis Fibroblast-like Synoviocytes. Arthritis Rheumatol, 68(7), 1614–1626. 10.1002/art.39608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock AT, Mallen CD, Muller S, Belcher J, Roddy E, Helliwell T, & Hider SL (2014). Risk of vascular events in patients with polymyalgia rheumatica. Cmaj, 186(13), E495–501. 10.1503/cmaj.140266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg CN, Åstrand A, Wingren C, Garnett JP, Mayer G, Taylor JD, Baker EH, & Baines DL (2017). Differential Effect of LPS on Glucose, Lactate and Inflammatory Markers in the Lungs of Normal and Diabetic Mice. J Pulm Respir Med, 2017(1). [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Sato Y, Kawashima M, Furumichi M, & Tanabe M (2016). KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res, 44(D1), D457–462. 10.1093/nar/gkv1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor SR, Filer A, Fitzpatrick MA, Fisher BA, Taylor PC, Buckley CD, McInnes IB, Raza K, & Young SP (2013). Metabolic profiling predicts response to anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis [Multicenter Study Research Support, Non-U.S. Gov’t]. Arthritis and rheumatism, 65(6), 1448–1456. 10.1002/art.37921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Hwang J, Xuan J, Jung YH, Cha HS, & Kim KH (2014). Global metabolite profiling of synovial fluid for the specific diagnosis of rheumatoid arthritis from other inflammatory arthritis [Research Support, Non-U.S. Gov’t]. PLoS One, 9(6), e97501. 10.1371/journal.pone.0097501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobak S, & Bes C (2018). An autumn tale: geriatric rheumatoid arthritis. Ther Adv Musculoskelet Dis, 10(1), 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinska MK, Liebisch G, Lochnit G, Wilhelm J, Klein H, Kaesser U, Lasczkowski G, Rickert M, Schmitz G, & Steinmeyer J (2013). A lipidomic study of phospholipid classes and species in human synovial fluid [Research Support, Non-U.S. Gov’t]. Arthritis and rheumatism, 65(9), 2323–2333. 10.1002/art.38053 [DOI] [PubMed] [Google Scholar]
- Le Gall G, Noor SO, Ridgway K, Scovell L, Jamieson C, Johnson IT, Colquhoun IJ, Kemsley EK, & Narbad A (2011). Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome. J Proteome Res, 10(9), 4208–4218. 10.1021/pr2003598 [DOI] [PubMed] [Google Scholar]
- Luan H, Gu W, Li H, Wang Z, Lu L, Ke M, Lu J, Chen W, Lan Z, Xiao Y, Xu J, Zhang Y, Cai Z, Liu S, & Zhang W (2021). Serum metabolomic and lipidomic profiling identifies diagnostic biomarkers for seropositive and seronegative rheumatoid arthritis patients. J Transl Med, 19(1), 500. 10.1186/s12967-021-03169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen RK, Lundstedt T, Gabrielsson J, Sennbro CJ, Alenius GM, Moritz T, Rantapaa-Dahlqvist S, & Trygg J (2011). Diagnostic properties of metabolic perturbations in rheumatoid arthritis [Research Support, Non-U.S. Gov’t Validation Studies]. Arthritis research & therapy, 13(1), R19. 10.1186/ar3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo C (2019). Incidence and Prevalence of Polymyalgia Rheumatica (PMR): The Importance of the Epidemiological Context. The Italian Case. Med Sci (Basel), 7(9). 10.3390/medsci7090092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf MR, Bhadbhade P, Mikuls TR, O’Dell JR, Gundry RL, & Funk RS (2021). Plasma Metabolome Normalization in Rheumatoid Arthritis Following Initiation of Methotrexate and the Identification of Metabolic Biomarkers of Efficacy. Metabolites, 11(12). 10.3390/metabo11120824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra BB (2020). Data normalization strategies in metabolomics: Current challenges, approaches, and tools. European Journal of Mass Spectrometry, 26(3), 165–174. 10.1177/1469066720918446 [DOI] [PubMed] [Google Scholar]
- Murillo-Saich JD, Diaz-Torne C, Ortiz MA, Coras R, Gil-Alabarse P, Pedersen A, Corominas H, Vidal S, & Guma M (2021). Metabolomics profiling predicts outcome of tocilizumab in rheumatoid arthritis: an exploratory study. Metabolomics, 17(9), 74. 10.1007/s11306-021-01822-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyamundanda G, Gormley IC, Fan Y et al. MetSizeR: selecting the optimal sample size for metabolomic studies using an analysis based approach. BMC Bioinformatics 14, 338 (2013). 10.1186/1471-2105-14-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, & Hermoso MA (2019). Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases [Review]. Frontiers in Immunology, 10(277). 10.3389/fimmu.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Carrio J, Coras R, Alperi-López M, López P, Ulloa C, Ballina-García FJ, Armando AM, Quehenberger O, Guma M, & Suárez A (2021). Profiling of Serum Oxylipins During the Earliest Stages of Rheumatoid Arthritis. Arthritis & Rheumatology, 73(3), 401–413. 10.1002/art.41537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, & Tracey KJ (2011). Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science, 334(6052), 98–101. 10.1126/science.1209985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto-Carneiro M, Toth L, Behnisch R, Urbach K, Klika KD, Carvalho RA, & Lorenz HM (2020). Differences in the serum metabolome and lipidome identify potential biomarkers for seronegative rheumatoid arthritis versus psoriatic arthritis. Ann Rheum Dis, 79(4), 499–506. 10.1136/annrheumdis-2019-216374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney SR, Kavanaugh A, Lodi A, Wang B, Boyle D, Tiziani S, & Guma M (2016). Metabolomic profiling predicts outcome of rituximab therapy in rheumatoid arthritis. RMD open, 2(2), e000289. 10.1136/rmdopen-2016-000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar Z, Migne C, Petera M, Gaudin P, Lequerre T, Marotte H, Tebib J, Pujos Guillot E, & Soubrier M (2016). Variations in the metabolome in response to disease activity of rheumatoid arthritis. BMC Musculoskelet Disord, 17(1), 353. 10.1186/s12891-016-1214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitsma XM, Yang W, Jacobs JWG, Pethö-Schramm A, Borm MEA, Harms AC, Hankemeier T, van Laar JM, Bijlsma JWJ, & Lafeber F (2018). Baseline metabolic profiles of early rheumatoid arthritis patients achieving sustained drug-free remission after initiating treat-to-target tocilizumab, methotrexate, or the combination: insights from systems biology. Arthritis Res Ther, 20(1), 230. 10.1186/s13075-018-1729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todeschini R, Ballabio D, Consonni V, Sahigara F, & Filzmoser P (2013). Locally centred Mahalanobis distance: A new distance measure with salient features towards outlier detection. Analytica Chimica Acta, 787, 1–9. 10.1016/j.aca.2013.04.034 [DOI] [PubMed] [Google Scholar]
- van Riel PL, & Renskers L (2016). The Disease Activity Score (DAS) and the Disease Activity Score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin Exp Rheumatol, 34(5 Suppl 101), S40–s44. [PubMed] [Google Scholar]
- Viant MR, Bearden DW, Bundy JG, Burton IW, Collette TW, Ekman DR, Ezernieks V, Karakach TK, Lin CY, & Rochfort S (2008). International NMR-based environmental metabolomics intercomparison exercise. Environmental science & technology, 43(1), 219–225. [DOI] [PubMed] [Google Scholar]
- Viant MR, Bearden DW, Bundy JG, Burton IW, Collette TW, Ekman DR, Ezernieks V, Karakach TK, Lin CY, Rochfort S, de Ropp JS, Teng Q, Tjeerdema RS, Walter JA, & Wu H (2009). International NMR-based environmental metabolomics intercomparison exercise. Environ Sci Technol, 43(1), 219–225. 10.1021/es802198z [DOI] [PubMed] [Google Scholar]
- Wakura D, Kotani T, Takeuchi T, Komori T, Yoshida S, Makino S, & Hanafusa T (2016). Differentiation between Polymyalgia Rheumatica (PMR) and Elderly-Onset Rheumatoid Arthritis Using 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography: Is Enthesitis a New Pathological Lesion in PMR? PLoS One, 11(7), e0158509. 10.1371/journal.pone.0158509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, & Ulloa L (2004). Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med, 10(11), 1216–1221. 10.1038/nm1124 [DOI] [PubMed] [Google Scholar]
- Wessler I, & Kirkpatrick CJ (2008). Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol, 154(8), 1558–1571. 10.1038/bjp.2008.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieder C, Bundy JG, Frainay C, Poupin N, Rodríguez-Mier P, Vinson F, Cooke J, Lai RPJ, Jourdan F, & Ebbels TMD (2022). Avoiding the Misuse of Pathway Analysis Tools in Environmental Metabolomics. Environmental Science & Technology, 56(20), 14219–14222. 10.1021/acs.est.2c05588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, & Dong E (2012). HMDB 3.0—the human metabolome database in 2013. Nucleic acids research, gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, & Wishart DS (2011). Metabolomic data processing, analysis, and interpretation using MetaboAnalyst. Curr Protoc Bioinformatics, Chapter 14, Unit 14.10. 10.1002/0471250953.bi1410s34 [DOI] [PubMed] [Google Scholar]
- Xu M, Jiang Z, Wang C, Li N, Bo L, Zha Y, Bian J, Zhang Y, & Deng X (2019). Acetate attenuates inflammasome activation through GPR43-mediated Ca2+-dependent NLRP3 ubiquitination. Experimental & Molecular Medicine, 51(7), 1–13. 10.1038/s12276-019-0276-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SP, Kapoor SR, Viant MR, Byrne JJ, Filer A, Buckley CD, Kitas GD, & Raza K (2013). The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis Rheum, 65(8), 2015–2023. 10.1002/art.38021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Nagana Gowda GA, Ye T, & Raftery D (2010). Advances in NMR-based biofluid analysis and metabolite profiling. Analyst, 135(7), 1490–1498. 10.1039/c000091d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, & Lemasters JJ (2003). L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care, 6(2), 229–240. 10.1097/00075197-200303000-00013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets of metabolites generated during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article and its supplementary information files.


