Abstract
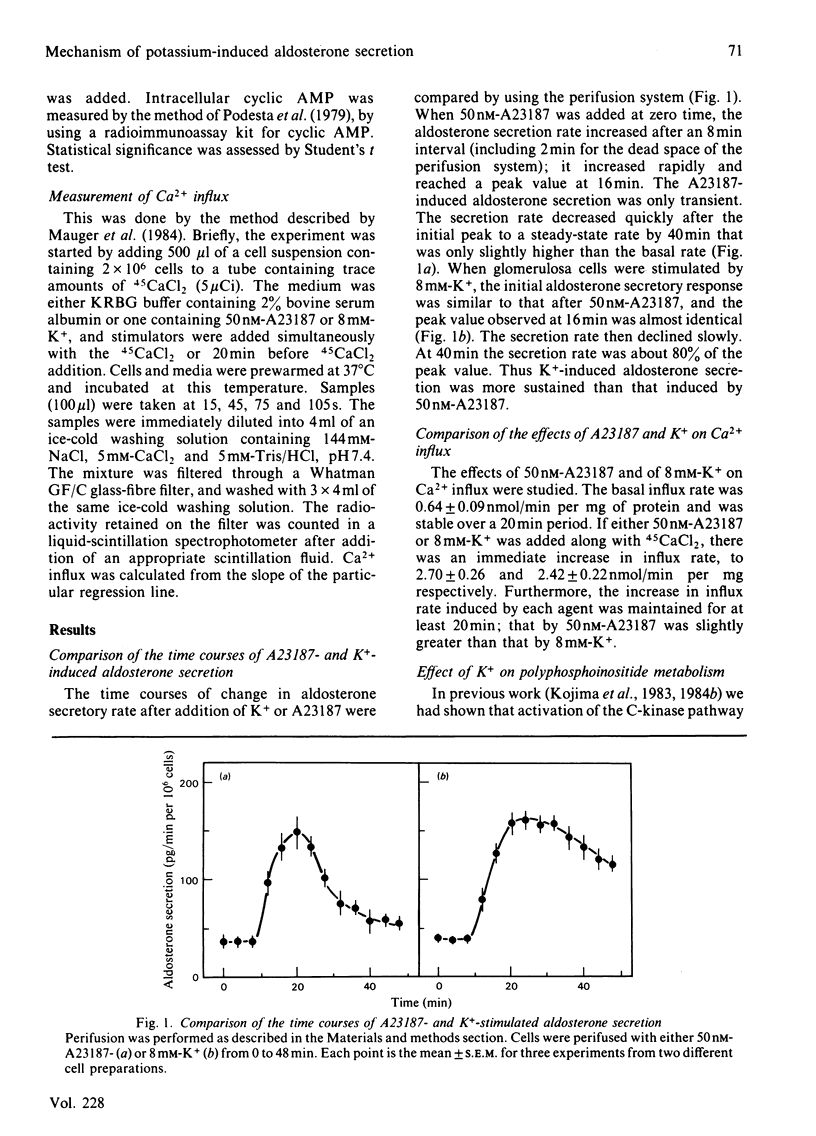
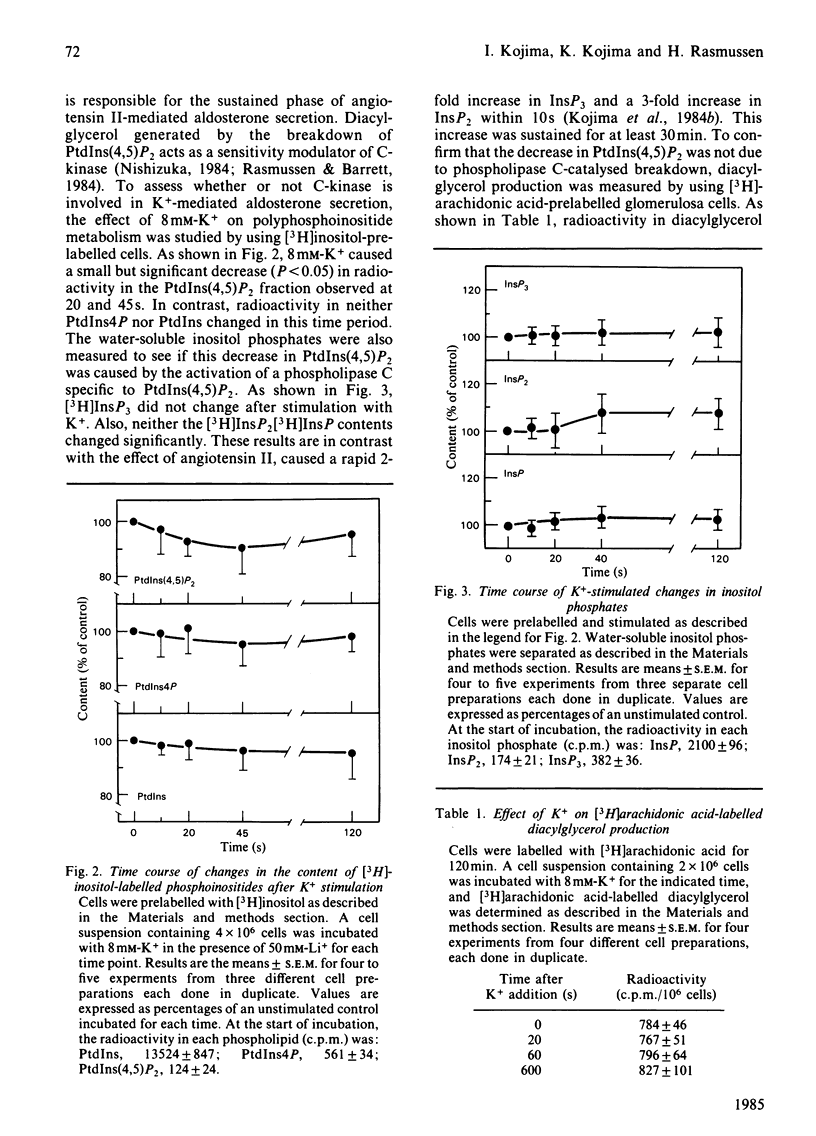
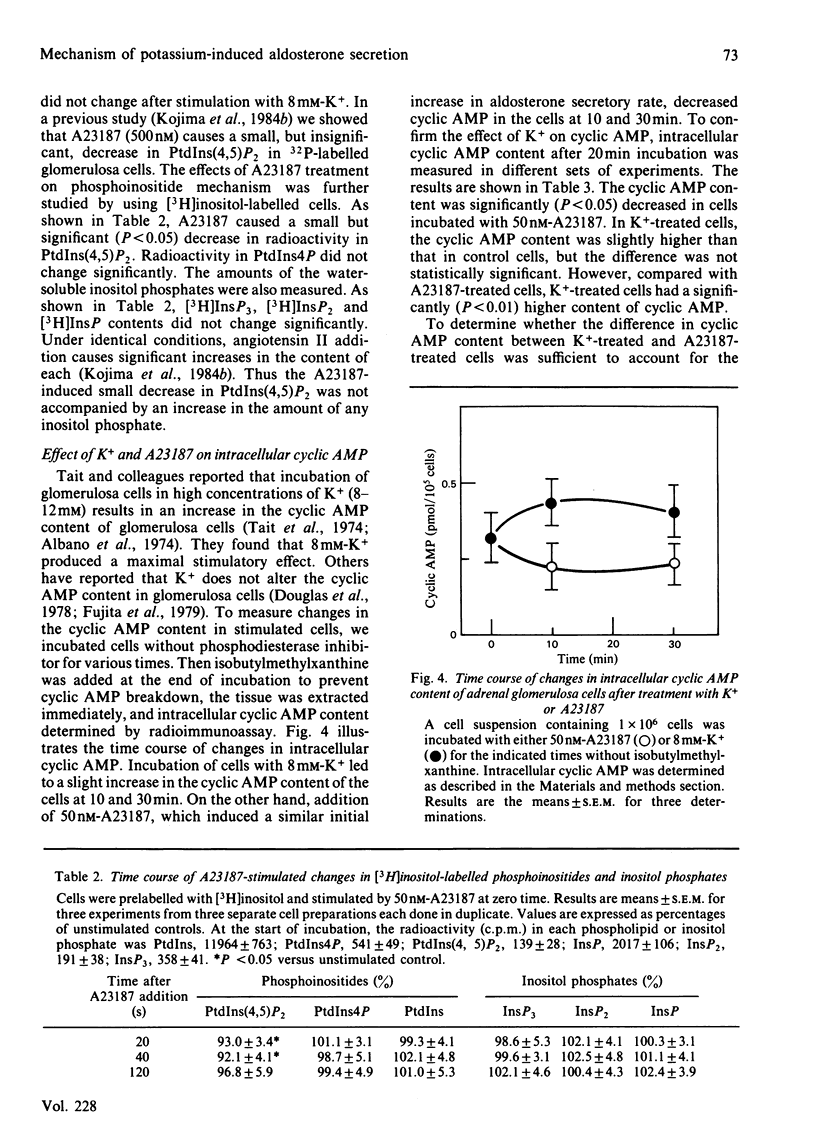
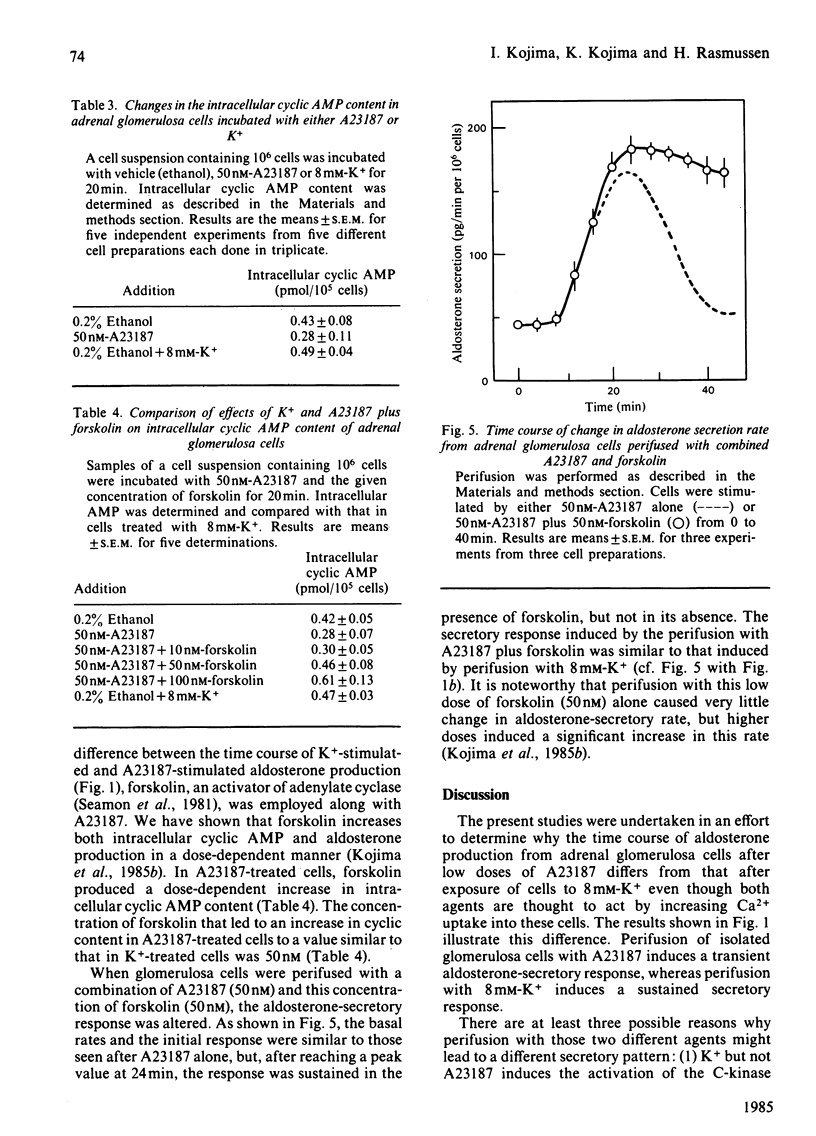
We compared the action of K+ on aldosterone secretion from isolated bovine adrenal glomerulosa cells with that of ionophore A23187. Addition of either 50 nM-A23187 or 8 mM-K+ to perifused cells induces a similar initial aldosterone-secretory responses, and a similar sustained increases in Ca2+ entry. However, K+-induced secretion is more sustained than is A23187-induced secretion, even though each agonist appears to act by increasing Ca2+ entry into the cells. When [3H]inositol-labelled cells are stimulated by 8 mM-K+, a small decrease in phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] is observed. This decrease is not accompanied by an increase in inositol trisphosphate (InsP3) concentration. Also, if [3H]arachidonic acid-labelled cells are exposed to 8 mM-K+, there is no increase in [3H]diacylglycerol production. When [3H]inositol-labelled cells are stimulated by 50 nM-A23187, a small decrease in PtdIns(4,5)P2 is observed. This decrease is not accompanied by an increase in InsP3. The cyclic AMP content of K+-treated cells was approximately twice that in A23187-treated cells. If cells are perifused simultaneously with 50 nM-forskolin and 50 nM-A23187, the initial aldosterone-secretory response is similar to that induced by A23187 alone, and the response is sustained rather than transient, and is similar to that seen during perifusion of cells with 8 mM-K+. This dose of forskolin (50 nM) causes an elevation of cyclic AMP concentration in A23187-treated cells, to a value similar to that in K+-treated cells. These results indicate that, in K+-treated cells, a rise in cyclic AMP content serves as a positive sensitivity modulator of the Ca2+ message, and plays a key role in mediating the sustained aldosterone-secretory response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Brown B. L., Ekins R. P., Tait S. A., Tait J. F. The effects of potassium, 5-hydrocytryptamine, adrenocorticotrophin and angiotensin II on the concentration of adenosine 3':5'-cyclic monophosphate in suspensions of dispersed rat adrenal zona glomerulosa and zona fasciculata cells. Biochem J. 1974 Aug;142(2):391–400. doi: 10.1042/bj1420391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banschbach M. W., Geison R. L., O'Brien J. F. Use of (1-14C) aectic anhydride to quantitate diglycerides: a new analytical procedure. Anal Biochem. 1974 Jun;59(2):617–627. doi: 10.1016/0003-2697(74)90315-7. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley L., Menachery A., Brown E., Williams G. The effects of extracellular K+ and angiotensin II on cytosolic Ca++ and steroidogenesis in adrenal glomerulosa cells. Biochem Biophys Res Commun. 1984 Sep 17;123(2):810–815. doi: 10.1016/0006-291x(84)90302-4. [DOI] [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Jornot L., Vallotton M. B. Correlation between cytosolic free Ca2+ and aldosterone production in bovine adrenal glomerulosa cells. Evidence for a difference in the mode of action of angiotensin II and potassium. J Biol Chem. 1984 Jul 25;259(14):8863–8869. [PubMed] [Google Scholar]
- Delbeke D., Kojima I., Dannies P. S., Rasmussen H. Synergistic stimulation of prolactin release by phorbol ester, A23187 and forskolin. Biochem Biophys Res Commun. 1984 Sep 17;123(2):735–741. doi: 10.1016/0006-291x(84)90291-2. [DOI] [PubMed] [Google Scholar]
- Douglas J., Saltman S., Williams C., Bartley P., Kondo T., Catt K. An examination of possible mechanisms of angiotensin II-stimulated steroidogenesis. Endocr Res Commun. 1978;5(2):173–188. doi: 10.3109/07435807809089016. [DOI] [PubMed] [Google Scholar]
- Fakunding J. L., Catt K. J. Dependence of aldosterone stimulation in adrenal glomerulosa cells on calcium uptake: effects of lanthanum nd verapamil. Endocrinology. 1980 Nov;107(5):1345–1353. doi: 10.1210/endo-107-5-1345. [DOI] [PubMed] [Google Scholar]
- Fakunding J. L., Chow R., Catt K. J. The role of calcium in the stimulation of aldosterone production by adrenocorticotropin, angiotensin II, and potassium in isolated glomerulosa cells. Endocrinology. 1979 Aug;105(2):327–333. doi: 10.1210/endo-105-2-327. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Larson R. E., Davis J. S. Rapid effects of angiotensin-II on polyphosphoinositide metabolism in the rat adrenal glomerulosa. Endocrinology. 1984 Jan;114(1):302–304. doi: 10.1210/endo-114-1-302. [DOI] [PubMed] [Google Scholar]
- Foster R., Lobo M. V., Rasmussen H., Marusic E. T. Calcium: its role in the mechanism of action of angiotensin II and potassium in aldosterone production. Endocrinology. 1981 Dec;109(6):2196–2201. doi: 10.1210/endo-109-6-2196. [DOI] [PubMed] [Google Scholar]
- Foster R., Lobo M. V., Rasmussen H., Marusic E. T. The effect of calcium on potassium-induced depolarization of adrenal glomerulosa cells. FEBS Lett. 1982 Nov 29;149(2):253–256. doi: 10.1016/0014-5793(82)81111-3. [DOI] [PubMed] [Google Scholar]
- Fraser R., Brown J. J., Lever A. F., Mason P. A., Robertson J. I. Control of aldosterone secretion. Clin Sci (Lond) 1979 May;56(5):389–399. doi: 10.1042/cs0560389. [DOI] [PubMed] [Google Scholar]
- Fujita K., Aguilera G., Catt K. J. The role of cyclic AMP in aldosterone production by isolated zona glomerulosa cells. J Biol Chem. 1979 Sep 10;254(17):8567–8574. [PubMed] [Google Scholar]
- Heisler S. Forskolin potentiates calcium-dependent amylase secretion from rat pancreatic acinar cells. Can J Physiol Pharmacol. 1983 Oct;61(10):1168–1176. doi: 10.1139/y83-174. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984 Dec 10;259(23):14448–14457. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Effects of ANG II and K+ on Ca efflux and aldosterone production in adrenal glomerulosa cells. Am J Physiol. 1985 Jan;248(1 Pt 1):E36–E43. doi: 10.1152/ajpendo.1985.248.1.E36. [DOI] [PubMed] [Google Scholar]
- Kojima I., Lippes H., Kojima K., Rasmussen H. Aldosterone secretion: effect of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Oct 31;116(2):555–562. doi: 10.1016/0006-291x(83)90559-4. [DOI] [PubMed] [Google Scholar]
- Kojima K., Kojima I., Rasmussen H. Dihydropyridine calcium agonist and antagonist effects on aldosterone secretion. Am J Physiol. 1984 Nov;247(5 Pt 1):E645–E650. doi: 10.1152/ajpendo.1984.247.5.E645. [DOI] [PubMed] [Google Scholar]
- Koletsky R. J., Brown E. M., Williams G. H. Calmodulin-like activity and calcium-dependent phosphodiesterase in purified cells of the rat zona glomerulosa and zona fasciculata. Endocrinology. 1983 Aug;113(2):485–490. doi: 10.1210/endo-113-2-485. [DOI] [PubMed] [Google Scholar]
- Litosch I., Lee H. S., Fain J. N. Phosphoinositide breakdown in blowfly salivary glands. Am J Physiol. 1984 Jan;246(1 Pt 1):C141–C147. doi: 10.1152/ajpcell.1984.246.1.C141. [DOI] [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Guesdon F., Claret M. Noradrenaline, vasopressin and angiotensin increase Ca2+ influx by opening a common pool of Ca2+ channels in isolated rat liver cells. Biochem J. 1984 Jul 1;221(1):121–127. doi: 10.1042/bj2210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Podesta E. J., Milani A., Steffen H., Neher R. Steroidogenesis in isolated adrenocortical cells. Correlation with receptor-bound adenosine e 3':5'-cyclic monophosphate. Biochem J. 1979 May 15;180(2):355–363. doi: 10.1042/bj1800355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E., Horne W. C. Ionomycin can elevate intraplatelet Ca2+ and activate phospholipase A without activating phospholipase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):393–397. doi: 10.1016/0006-291x(84)90426-1. [DOI] [PubMed] [Google Scholar]
- Saruta T., Cook R., Kaplan N. M. Adrenocortical steroidogenesis: studies on the mechanism of action of angiotensin and electrolytes. J Clin Invest. 1972 Sep;51(9):2239–2245. doi: 10.1172/JCI107032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff O., Foder B., Skibsted U. Hysteretic activation of the Ca2+ pump revealed by calcium transients in human red cells. Biochim Biophys Acta. 1983 May 5;730(2):295–305. doi: 10.1016/0005-2736(83)90346-2. [DOI] [PubMed] [Google Scholar]
- Schiffrin E. L., Lis M., Gutkowska J., Genest J. Role of Ca2+ in response of adrenal glomerulosa cells to angiotensin II, ACTH, K+, and ouabain. Am J Physiol. 1981 Jul;241(1):E42–E46. doi: 10.1152/ajpendo.1981.241.1.E42. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh N. A., Palmer F. B. Phosphoinositide kinases in chick brain and sciatic nerve, a developmental study. J Neurochem. 1977 Feb;28(2):395–402. doi: 10.1111/j.1471-4159.1977.tb07760.x. [DOI] [PubMed] [Google Scholar]
- Zawalich W., Brown C., Rasmussen H. Insulin secretion: combined effects of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Dec 16;117(2):448–455. doi: 10.1016/0006-291x(83)91221-4. [DOI] [PubMed] [Google Scholar]


