Abstract
Testosterone gender affirming hormone therapy (T-GAHT) is frequently used by transgender and gender-diverse individuals assigned female at birth to establish masculinizing characteristics. Although many seek parenthood, particularly as a gestational parent or through surrogate, the current standard guidance of fertility counseling for individuals on testosterone (T) lacks clarity. At this time, individuals are typically recommended to undergo fertility preservation or stop treatment, associating T-therapy with a loss of fertility; however, there is an absence of consistent information regarding the true fertility potential for transgender and gender-diverse adults and adolescents. This review evaluates recent studies that utilize animal models of T-GAHT to relate to findings from clinical studies, with a more specific focus on fertility. Relevant literature based on murine models in post- and pre-pubertal populations has suggested reversibility of the impacts of T-GAHT, alone or following gonadotropin-releasing hormone agonist (GnRHa), on reproduction. These studies reported changes in clitoral area and ovarian morphology including corpora lutea, follicle counts and ovarian weights from T-treated mice. Future studies should aim to determine the impact of the duration of T-treatment and cessation on fertility outcomes, as well as establish animal models that are clinically representative of these outcomes with respect to gender diverse populations.
Keywords: fertility, testosterone, transgender men, gender affirming hormone therapy, animal models
In Brief:
Animal studies are needed to inform clinical guidance on the effects of testosterone gender-affirming hormone therapy (T-GAHT) on fertility. This review summarizes current animal models of T-GAHT and identifies gaps in knowledge for future study.
Introduction
In the United States, more than 1.6 million adults and adolescents identify as transgender, with the majority of transgender adults expressing desire for parenthood (Herman et al., 2022; Morong et al., 2022; Stolk et al., 2023). Many transgender and gender diverse (TGD) individuals seek gender affirming hormone therapy (GAHT) to induce secondary sex characteristics that align with their gender identity. Testosterone (T)-containing gender affirming hormone therapy (T-GAHT) is often prescribed to TGD individuals who were assigned female at birth to induce male-associated sex characteristics, such as deeper voice, bodily and facial hair growth, and desired muscle and body fat redistribution. Goals of therapy differ between individuals, but the clinical goal is often serum T levels comparable to that of eugonadal cisgender men (Hembree et al., 2017). For peripubertal TGD adolescents, gonadotropin-releasing hormone agonist (GnRHa) can be used to reversibly suppress further progression of puberty prior to GAHT initiation (de Vries et al., 2014; Stolk et al., 2023), thereby preventing the development of characteristics inconsistent with their gender identity.
GAHT is integral to the physical and mental health of many TGD individuals and should not be considered “elective” treatment (Coleman et al., 2022). Despite the clinical prevalence of GAHT use, there is a significant gap in scientific understanding of the impacts of this treatment, especially with regards to fertility. To address these potential impacts, medical professionals heavily encourage that TGD individuals seek fertility preservation options before starting GAHT or recommend a period of T-cessation prior to becoming a gestational parent, with an assumption of ameliorating teratogenic outcomes in offspring associated with prolonged T-treatment (Coleman et al., 2022). While most transgender adults express desires of parenthood, a recent survey reported that over half of these individuals do not want to pause GAHT or seek fertility preservation prior to transitioning (Alpern et al., 2022). Fertility preservation can be a burdensome experience, due to the cost, invasiveness, and uncertain fertility potential associated with cryopreserving oocytes. Additionally, patients may not be certain of their desire to have biological children prior to starting T-GAHT, raising a need to improve fertility options for patients already on T therapy (Alpern et al., 2022). Most importantly, however, the assumption of fertility loss is not appropriately supported by a sufficient amount of research specific to the reproductive health of the transgender community. Due to the lack of conclusive data on the reproductive effects of T-GAHT for transgender fertility counseling, there is an immense need for more research in this field of medicine.
Findings from animal studies have supported the need to improve clinical guidance about the impact of androgens on reproductive potential and re-assess the assumption of fertility loss associated with T-GAHT. Previous rodent studies have reported several changes to reproductive physiology following prolonged T-treatment, such as polycystic ovarian morphology, cortical stiffness, and stromal luteinization (Dela Cruz, Kinnear, et al., 2023; Kinnear et al., 2019, 2023). However, while these changes to reproductive physiology on T-GAHT are consistently observed in animal research, there is evidence to suggest that the changes do not impact fertilization rates or embryo implantation and quality (Bartels et al., 2021). Thus, there is an abundant need to standardize how trans-men are counseled with regards to fertility and to support this standardization with research relevant to transgender health. In this review, we discuss the various animal studies that have been used to address the reproductive outcomes of T-GAHT, as well as suggestions for future investigation.
Current Clinical Data on Effects of T-Treatment on Fertility in Trans-Men
Even though current clinical guidance associates T therapy with potential teratogenicity, recent studies suggest that even after T therapy, patients experience successful oocyte retrieval using assisted reproductive technology (ART) following a period of T cessation (Amir et al., 2020a; Barrero & Mockus, 2023). Some studies even report similar rates of oocyte maturity and quality from patients who have experienced T therapy compared to patients who have not (Israeli et al., 2022). While a majority of these case studies implement a period of cessation prior to oocyte retrieval, typically long enough for menstrual cycles to resume, other studies have reported retrieval of mature oocytes without menses resumption or T-cessation (Cho et al., 2020; Gale et al., 2021; Greenwald et al., 2022). Some of these studies report successful pregnancies involving the use of cryopreserved oocytes from transgender men in the uterus of their cisgender partners (Broughton & Omurtag, 2017; Moravek et al., 2023; Yaish et al., 2021). Two of these case studies did not involve a cessation of T therapy prior to oocyte retrieval, suggesting that pausing T-therapy may not be necessary for trans-men who want to use their oocytes for surrogate pregnancies (Moravek et al., 2023). Finally, several studies report live births in T-treated gestational parents using their own cryopreserved oocytes regardless of active T-treatment prior to oocyte retrieval (Adeleye et al., 2019; Leung et al., 2019; Moustakli & Tsonis, 2023). Spontaneous pregnancies without IVF have also been reported, both after a period of discontinued T-therapy and during active T-therapy (Yaish et al., 2021; Yoshida et al., 2022). These findings call into question the current fertility guidance that fertility preservation is necessary prior to T-therapy.
In addition to these important fertility data, reports on the effects of T-treatment on the reproductive organs of adults have been inconsistent. For example, amenorrhea is common in trans-men following T-therapy, which traditionally has been suggestive of inactive endometrial cyclicity. While an initial study reported a lack of endometrial proliferation associated with amenorrhea in T-treated patients (Perrone et al., 2009), studies conducted afterwards have found proliferative endometrium in trans-men during T-therapy (Grimstad et al., 2019; Loverro et al., 2016). A more recent study even observed signs of ovulation in 33% of amenorrheic participants on T, concluding that anovulation does not always occur in T-treated people who experience amenorrhea (Asseler et al., 2024). These conflicting results necessitate the need to investigate cycling in trans-men after T exposure, regardless of amenorrhea. In addition to cycling, studies focusing on follicle distribution have reported normal follicle counts in ovaries collected from individuals on T-therapy (De Roo et al., 2017; Van den Broecke et al., 2001). Other studies have documented that MII oocytes collected from trans-men after T therapy exhibit normal spindle morphology (De Roo et al., 2017; Lierman et al., 2017). However, a more recent study reported abnormal spindle morphology following T-treatment in transgender men, which was improved following spindle transfer (Christodoulaki et al., 2023). Such conflicting findings demonstrate the need for controlled studies to better inform clinical guidance given to trans-men concerning fertility.
It is also imperative to properly research the effects of T-GAHT on fertility in transgender and gender-nonconforming adolescents. Similar to clinical guidelines for adults, fertility preservation is heavily encouraged for adolescents who are interested in puberty suppression prior to T-GAHT. Studies have reported that transgender adolescents show successful ovarian stimulation response, oocyte retrieval, and maturity rates of both experimental groups, regardless of prior T exposure (Amir et al., 2020b; Chen et al., 2018; Insogna et al., 2020), suggesting that fertility preservation is a viable option for transgender adolescents who may desire biological children after medically transitioning. However, despite the demonstrated success of oocyte cryopreservation in adolescent transgender men, the number of these patients who pursue fertility preservation is reportedly low (Nahata et al., 2017). Since pausing GAHT prior to oocyte retrieval can be a traumatic experience for transgender individuals, future research should focus on methods of minimizing the resurfacing of undesired secondary sex characteristics in transgender adolescents during the oocyte cryopreservation process. One study reported success using letrozole to ensure low levels of estradiol during oocyte retrieval in a transgender male who briefly paused GnRHa treatment for purposes of fertility preservation (Martin et al., 2021). This study resulted in successful oocyte retrieval and minimal breast development and menstruation, which presents the opportunity to address the fertility desires of transgender adolescent males while ensuring a gender affirming experience. Further research in this field will increase the knowledge available to support both the fertility needs and gender affirmation of transgender individuals, decreasing the barriers to accessing reproductive healthcare for transgender adults and adolescents (Nadgauda & Butts, 2024).
Although relatively few studies have investigated the impact of T-GAHT on fertility, many studies have studied the reproductive effects of exogenous androgen administration in the context of polycystic ovarian syndrome (PCOS). PCOS is a heterogenous condition characterized by anovulation, excess androgen signaling, and decreased fertility (Dumesic et al., 2007). Common PCOS symptoms, such as reduced or irregular menstruation, multi-follicular ovarian morphology, cortex hyperplasia, increased cortical stiffness, and corpus luteum insufficiency, are often exhibited by transgender patients after androgen exposure (De Roo et al., 2019; Grynberg et al., 2010; Ikeda et al., 2013; Khalifa et al., 2019; Sheehan, 2004). However, several physiological characteristics associated with T-GAHT differ from the physiology of patients with PCOS. For example, studies have reported that patients on T-GAHT experience inconsistent presence of PCOS-like ovaries even with T levels as high as those of cisgender men, whereas PCOS patients often develop this ovarian morphology despite more variable T levels (Caanen et al., 2017; Grimstad et al., 2020; Ikeda et al., 2013; Sheehan, 2004). Similarly, vaginal ultrasounds of trans-men on T-therapy did not reveal any ovarian abnormalities associated with PCOS (Mueller et al., 2008). These studies suggest that not all trans-men on T-GAHT exhibit PCOS-like ovarian morphology; therefore, clinical PCOS data cannot replace research that focuses specifically on T-GAHT. Furthermore, the cortical and follicle distribution of T-treated patients have been shown to be similar to cisgender women (Borrás et al., 2021), indicating that these physical differences, while resembling PCOS, may not have the same detrimental impact on fertility.
Overall, the current clinical literature is insufficient to support an assumption of fertility loss following T-GAHT. The few clinical studies that exist report contradicting findings and lack consistency and replication in examined outcomes. This suggests an urgent need for further research to address the gaps in our clinical understanding of the field. As an alternative to human subjects, animal studies have served as an avenue to address a few of these gaps and present promising results for a better standard of fertility guidance for transgender patients.
Animal Models to Investigate the Effect of Androgens
Animal Models Assessing Reproductive Outcomes of Hyperandrogenism
Animal models, especially rodents, are desirable for examining reproductive effects of GAHT. Animal models are necessary because while large, controlled studies involving GAHT treatment cannot be performed ethically in humans, the similarities of their reproductive system to humans and their short reproductive cycle make rodents an ideal model (Kinnear et al., 2019). Such models of GAHT have only been developed recently, however, and prior to these studies scientists have had to rely on research that has some biological similarities to T-GAHT treatment to inform clinical practice and the development of more relevant treatment plans. Animal models of PCOS, for example, have used animals with elevated levels of T to simulate the hyperandrogenism characteristic of human PCOS patients and have evaluated potential impacts of this condition on fertility (Sun et al., 2019).
Although clinical PCOS data, as stated previously, cannot be directly applied to patients on T-GAHT, the findings of PCOS animal studies have been used to provide some direction to T-GAHT-focused research (Padmanabhan & Veiga-Lopez, 2013; Stener-Victorin, 2022). These studies assessed several aspects of fertility, such as T-exposure of the gestational parent as a potential developmental cause of PCOS in offspring or of decreased reproductive capacity in offspring (Abbott et al., 2005). Most studies with methods relevant to current animal models of T-GAHT use an established hormone delivery mechanism, such as subcutaneous injection or implant, to deliver dihydrotestosterone (DHT) or T-enanthate. One study used ovariectomized female mice treated with 10 mm subcutaneous silastic implants of DHT as a model of hyperandrogenism (Esparza et al., 2020), a method also used in mouse models of T-GAHT (Hashim et al., 2022). Increasing the androgen levels of these mice to the equivalence of control male mice resulted in sustained inhibition of the hypothalamic-pituitary-gonadal (HPG) axis, evidenced by suppression of luteinizing hormone (LH) secretion and androgen receptor (AR)-expressing neurons. DHT-treated mice also displayed lower corpora lutea counts and an increase in compromised large antral follicles, leading to decreased fertility potential as a result of androgen treatment (Esparza et al., 2020). Considering the HPG axis is vital to reproductive system regulation, studies using similar T delivery approaches can be of relevance to studying the effects of T-GAHT on reproductive function.
While these PCOS models present opportunities for potential inferences concerning T-GAHT, they are not sufficient to inform T-GAHT clinical practices. Many of these studies do not standardize variables that are physiologically relevant to humans who are on GAHT for purposes of transitioning, such as estrous cyclicity, blood serum levels of hormones, T-dosage, and time of exposure to T. Also, since PCOS is understood to be correlated with hyperandrogenism caused by dysfunction of AR signaling, PCOS murine models often use non-aromatizable DHT to control for changes in regulation of androgen production as a direct response of AR signaling alone (Aflatounian et al., 2020; Coyle et al., 2022; Esparza et al., 2020; Sun et al., 2019). However, DHT is not approved for use in GAHT; therefore, studies using aromatizable T are needed in order to more accurately replicate T-GAHT clinically (Moravek, 2019). More research, using animal models specifically designed to replicate T-GAHT, is needed to precisely determine the effects of T-therapy on reproductive outcomes and provide clinicians with evidence-supported guidelines for TGD patients.
Murine Models of Post-Pubertal Transmasculine Gender-Affirming Hormone Therapy
In recent years, investigators have developed rodent models specifically designed to study reproductive impacts of T-GAHT (Table 1). The first such model was introduced by Kinnear et al. (2019) and modeled T-GAHT with twice weekly, subcutaneous injections of T-enanthate for 6 weeks. T-enanthate is a T-variant commonly used for subcutaneous T-GAHT in humans, making this study more translatable to transgender health than previous PCOS models. The T-treated mice displayed many traits commonly observed in human T-GAHT patients, including lack of cycling (evidenced by persistent diestrus and lack of corpora lutea) and increased clitoral size. Importantly, however, these T-induced traits did not impact follicle distribution, suggesting fertility may be preserved after T therapy (Kinnear et al., 2019). Since that initial study, investigators have developed other methods of delivering T therapy in mouse models including subcutaneously implanted T enanthate pellets (Kinnear et al., 2021) or silastic tubing filled with T-enanthate (Schwartz et al., 2023). These models were compared and validated in a comparative study (Hashim et al., 2022) and are summarized in Table 1. All models produced similar T-associated reproductive outcomes including the absence of corpora lutea, increased clitoral area, and persistent diestrus for the duration of treatment, but also showed no effect on follicle distribution. These models have now made it possible to assess health effects specific to T-GAHT in controlled research investigations.
Table 1.
Summary of T-GAHT Animal Models Focusing On Reproductive Outcomes. CL: corpora lutea; E: estradiol; IP: intraperitoneal; IVF: in vitro fertilization; LH: luteinizing hormone; T: testosterone; SQ: subcutaneous
| Experimental Design | T Duration | Outcomes |
|---|---|---|
| C57BL/6N adult mice, n=20, SQ injections of 0.225, 0.45 or 0.90 mg GnRHa 2x weekly (Kinnear et al., 2019) | 6 weeks/No cessation |
|
| CF-1 adult mice, n=66, SQ injections of 400 μg GnRHa weekly (Bartels et al., 2020) | 6 weeks/No cessation |
|
| C57BL/6N adult mice, n=10, SQ implanted pellets of 5 mg T (Kinnear et al., 2021) | 6 weeks/4 weeks |
|
| C57BL/6N adult mice, n=40, SQ injections of 0.9 mg T weekly (Kinnear et al., 2023) | 6 weeks/4 weeks |
|
| C57BL/6N adult mice, n=120, SQ silastic implant of 10 mg T (Schwartz et al., 2023) | 6 weeks, 12 weeks/2 weeks |
|
| C57BL/6N adolescent mice, n=80, GnRHa SQ implant, SQ injections of 0.45 mg T weekly (Dela Cruz et al., 2023) | 6 weeks/No cessation |
|
| C57BL/6N adolescent mice, n=80, GnRHa SQ implant, SQ silastic implant of 10 mg T (Dela Cruz et al., 2023) | 6 weeks/No cessation |
|
| CF-1 adolescent mice, GnRHa IP injection every 4 weeks, SQ injections of 200 μg T weekly (Godiwala et al., 2023) | 4–8 weeks/2 weeks |
|
More recent studies have modified the original experimental design to model the current clinical guidance that trans-men stop T-GAHT for at least one month or cycle prior to getting pregnant (Amir et al., 2020b). For this model, investigators assessed the fertility of animals immediately after 6 weeks of T treatment and after a washout period sufficient to return terminal T levels in serum to control female levels (Figure 1). T washout allowed the mice to recover estrous cyclicity, although these mice also developed ovarian stromal aberrations that were not observed in mice that were treated with T without a washout (Bartels et al., 2021; Kinnear et al., 2021, 2023). These aberrations were reflected as clusters of large round cells post-T-GAHT, of similar nature to those seen in aging ovaries (Briley et al., 2016). This demonstrated that impacts of active T-treatment on cycling are not permanent, but that either T treatment itself or recovery of cyclicity after treatment may be partially detrimental to ovarian health.
Figure 1. Experimental Design for Post-Pubertal T-GAHT Model with and Without Cessation.
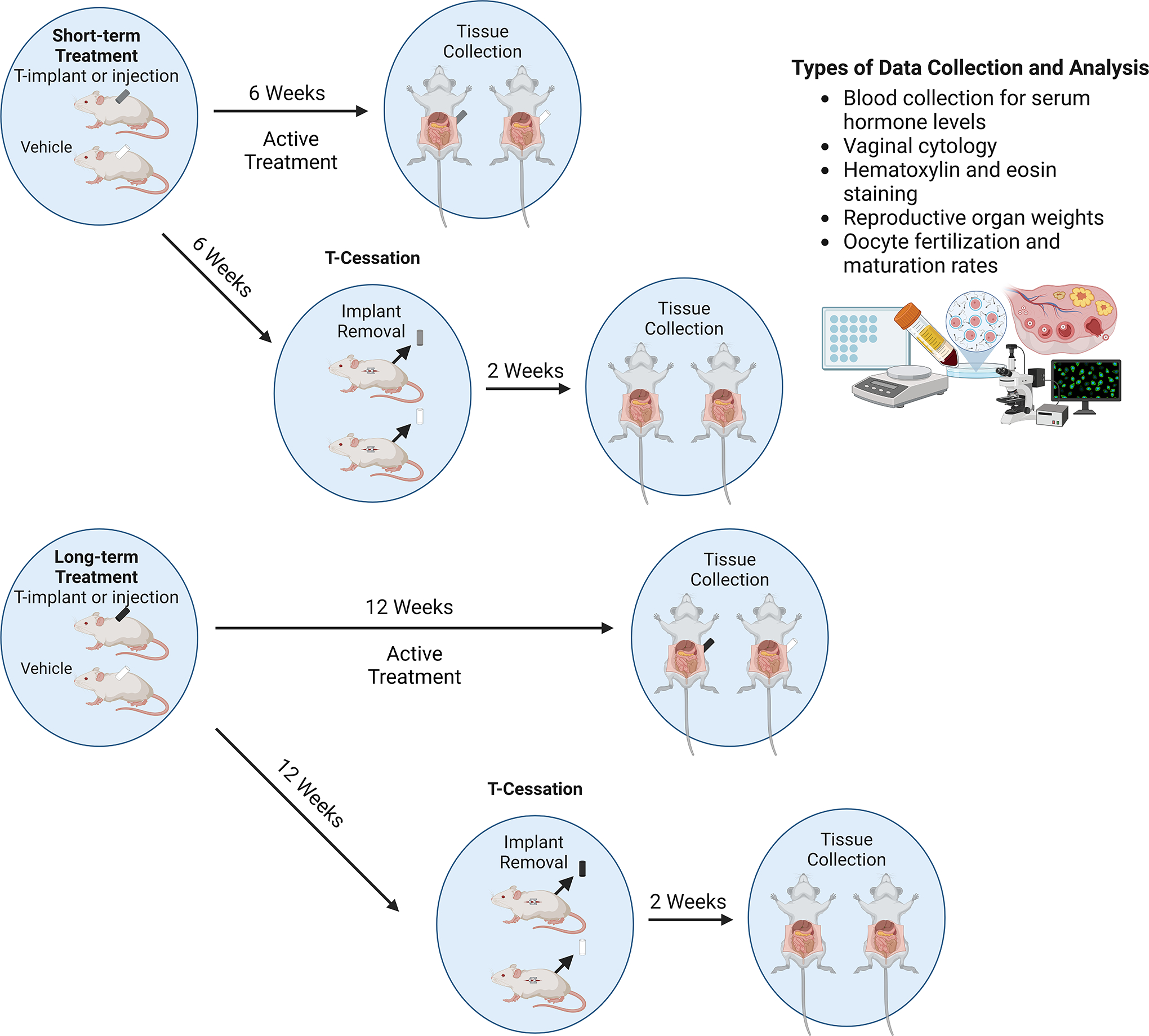
General mouse model for assessing impacts of short term (6 week) and long term (12 week) T-treatment on mice, with visual representation of T-implants. T-cessation occurs after the period of T-treatment and involves removal of T-implant or stopping T-injections for 2 weeks before assessing outcomes, as shown in the diagram. Common methods of data collection and analysis are outlined.
Using these models, researchers have begun to investigate important reproductive outcomes during and following T-GAHT. Initial studies showed that mice actively undergoing T-treatment still responded to gonadotrophin stimulation by producing and ovulating meiotically competent and fertilizable eggs similar to controls, regardless of a T-cessation period (Bartels et al., 2021). However, a later study showed that these encouraging results may depend on T-treatment length. Female mice treated with long-term T (12 weeks) displayed adverse IVF outcomes and depressed anti-Mullerian hormone (AMH) levels even after T washout (Schwartz et al., 2023). The inability of the AMH concentrations to return to baseline levels following a washout reflected potential irreversible damage to oocyte quality for the long-term treatment groups. Additional studies focusing on reproductive impacts following long-term T-GAHT are needed to clarify the extent to which this treatment may impact reproductive potential and the molecular mechanisms involved.
Altogether, these studies show that animal models of T-GAHT can provide valuable information regarding the effects of T-GAHT on fertility. Initial data shows encouraging signs that fertility may be preserved during and after T-GAHT, suggesting that the current recommendations for stopping T-GAHT prior to having biological children may be unnecessary. Considering stopping T-GAHT can be mentally and physically traumatizing for transgender individuals, challenging the current fertility guidelines may positively impact the transgender community (Coleman et al., 2022). However, significant additional research, particularly with longer T treatment duration, is still needed to provide clinicians with sound, evidence-based recommendations for transmasculine individuals seeking biological parenthood.
Murine Models of Pre-Pubertal Transmasculine Gender-Affirming Hormone Therapy
While adult animal models address the impacts of T on fertility, there is a major gap in understanding the effects of pre-pubertal GnRHa prior to post-pubertal T-GAHT on reproductive capacity. GnRHa therapy is vital for transgender and gender non-conforming adolescents to prevent physical manifestations of endogenous puberty before they are able to begin GAHT. Human clinical data (Coleman et al., 2022; de Vries et al., 2014; Insogna et al., 2020) and studies in rats (Guarraci et al., 2022, 2023) suggest that GnRHa treatment suppresses onset of puberty but does not result in any long-term effects on fertility if treatment is ceased. However, relatively few preclinical studies have examined GnRHa therapy followed by T-GAHT in adulthood. The first adolescent mouse model assessing the reproductive effects of pre-pubertal GnRHa treatment prior to T-GAHT was developed by Dela Cruz et al. (2023). Based on the initial T-GAHT mouse model established by Kinnear et al. (2019), this model was altered to mimic a transmasculine regimen in peripubertal transmasculine youth by subcutaneously implanting GnRHa depots into pre-pubertal female mice prior to T-GAHT (Dela Cruz et al., 2023a). This method of blocking endogenous puberty was effective for up to 21 days after implantation. Implanting adolescent mice with GnRHa capsules for 21 days followed by weekly injections of T-enanthate or subcutaneous T implants (GnRHa-T) was sufficient to suppress HPG axis function as these mice remained in diestrus for the duration of the experiment. By comparison, GnRHa-only controls resumed cycling 21 days post implantation of GnRHa, and T-only controls cycled prior to T injections but then stayed in persistent diestrus thereafter. These findings are consistent with clinical evidence that GnRHa is reversible in humans, as well as findings in previous studies focusing on the acyclicity of actively T-treated adult mice (Bartels et al., 2021; Kinnear et al., 2019; Schwartz et al., 2023).
Overall, studies employing this adolescent transmasculine model suggest that reproductive effects of GnRHa-T treatment are similar to T-GAHT alone. Both GnRHa-T and T-only controls showed lower ovarian weight, lower uterine weight, and corpora lutea absence compared to GnRHa-only and sham controls, and that these effects were not entirely rescued by T washout (Dela Cruz et al., 2023a; Dela Cruz et al., 2023b). Other studies report smaller ovarian weights following GnRHa treatment alone (Anacker et al., 2021; Godiwala et al., 2023); however, smaller ovaries are not currently understood to impact fertility. This is further supported by the fact that variables associated with fertility such as oocyte yield, fertilization rate, maturity rate, and number of developed embryos, were similarly compromised in T-only and GnRHa-T treatment groups without T-cessation. However, these outcomes were rescued in both groups following T washout (Dela Cruz et al., 2023b). Importantly, despite the smaller oocyte yield, oocytes from both GnRHa-T and T-only mice were still able to mature and undergo in vitro fertilization and blastulation similarly to controls and the meiotic spindle morphology of oocytes from these mice were unaffected following in vivo maturation (Figure 2, Bartels et al., 2021; Dela Cruz et al., 2023b; Godiwala et al., 2023). Further, eggs from GnRHa-T and T-treated mice were fertilizable and produced litters of fertile pups (Godiwala et al., 2023). Despite consistent ovarian morphological differences following puberty suppression and T-treatment, these findings suggest that fertility may not be irreversibly compromised by GAHT. More in-depth research is needed to form further conclusions on the necessity of fertility preservation prior to T-GAHT or T-cessation prior to ovarian stimulation or oocyte retrieval.
Figure 2. Comparison of Vehicle and T-treated Meiotic Spindle Morphology.
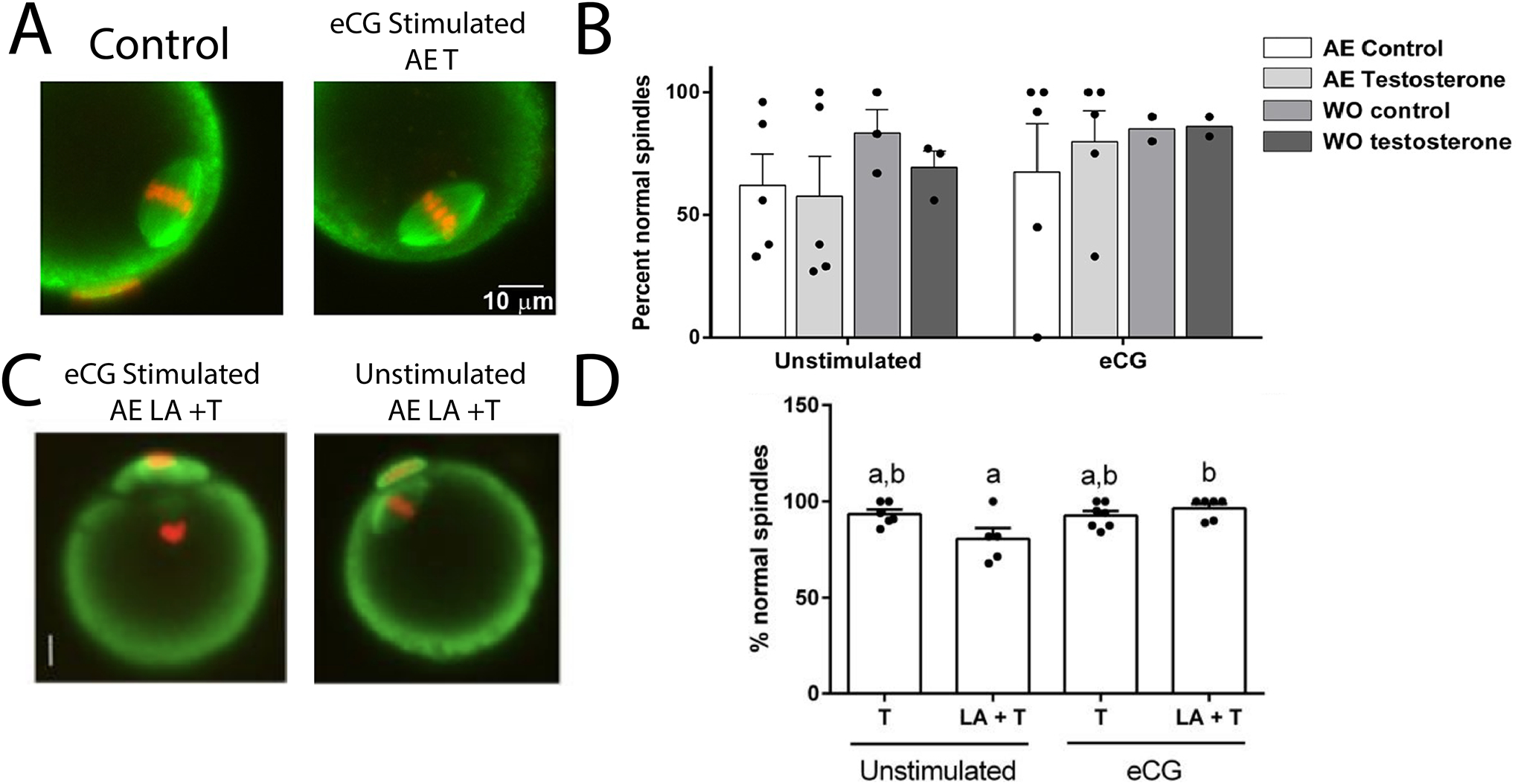
(A) Immunofluorescent stained oocytes from Bartels et al., 2020 showing normal meiotic spindle morphology in oocytes from stimulated T-treated and vehicle mice. (B) T-treated mice produce a comparable number of meiotically competent oocytes to untreated mice regardless of a washout period. (C) Immunofluorescent stained oocytes showing normal meiotic spindle formation regardless of stimulation from Godiwala et al., 2023. (D) Mice treated with T or leuprolide + T produced eggs that had morphologically normal spindles after in vitro maturation (Adapted from Bartels et al., 2020; Godiwala et al., 2023).
Models Of T-GAHT for Other Health Outcomes
Reproductive impacts are not the only poorly understood outcomes of T-GAHT in transgender patients. Recent studies have also begun to investigate a wide variety of other health outcomes that may be experienced by individuals on T-GAHT. These studies (summarized in Table 2) show that T-GAHT may be associated with a number of adverse health outcomes such as increased risk of cardiovascular disease (Goetz et al., 2019; Santos et al., 2022), overstimulated adaptive immune response (Santos et al., 2022), decreased trabecular bone density (Goetz et al., 2017), impaired wound healing (Reiche et al., 2022), decreased brain cortical volume (Perez-Laso et al., 2018), and impaired insulin response (Kothmann et al., 2021). However, not all studies report consistent results. For example, Dubois et al. (2023) report increased bone density in a mouse model simulating T-GAHT in adolescents, contrary to an earlier study (Goetz et al., 2017). It is likely that the differences in outcomes result from the differing methods used to model T-GAHT, which vary widely between studies (Table 2). As transgender individuals also utilize a diversity of treatment paradigms, more research is needed to clarify whether different treatment options may result in differing health outcomes. Some studies have already begun to investigate methods to improve T delivery and limit undesirable side-effects, optimizing dosages and developing long-term delivery methods (Mahabir & Newell-Fugate, 2024; Tassinari & Maranghi, 2021). Interestingly, some of the undesirable outcomes associated with T-therapy were rescued by adding a low dose of estrogen during T treatment (Goetz et al., 2017; Reiche et al., 2022). This suggests that adding a low dose of estrogen to gender affirming treatments for trans-men could prevent unwanted health complications while still fulfilling their desires of medically transitioning. Although these findings present interesting and exciting possibilities, many of these studies are the only such studies in their field and require much additional investigation to come to an informed conclusion, let alone translation to clinical practice. Altogether, these studies highlight just how limited our current understanding of the clinical outcomes of GAHT is and emphasize the urgent need for further research to responsibly inform transgender patients and health care providers.
Table 2.
Summary of Examined Health Outcomes in T-GAHT Animal Models. E: estradiol; F: female; GnRHa: gonadotropin-releasing hormone agonist; IM: intramuscular; M: male; OVX: ovariectomy; SQ: subcutaneous; T: testosterone; XHT: hormone therapy
| Experimental Design | Outcomes |
|---|---|
| C57BL/6 adult or adolescent F mice, n=31, SQ injections of 31 μg T and 6.4 μg E benzoate weekly for 6 or 10 weeks OVX/XHT (Goetz et al., 2017) |
|
| ApoE−/− C57BL/6 adolescent F mice, n=24, SQ injections of 31 μg T and 6.4 μg E benzoate weekly for 6 or 10 weeks OVX/XHT (Goetz et al., 2018) |
|
| Adult F Wistar rats, n=20, SQ injections of 40 mg/kg T-propionate weekly for 29 days (Perez-Laso et al., 2018) |
|
| C57BL/6J adolescent M and F mice, n=35–40, SQ injections of 20 μg GnRHa - leuprolide daily for 6 weeks (Anacker et al., 2021) |
|
| Adult M and F Wistar rats, n=5–10/group, IM injections of T-cypionate 3.0 mg/kg every 10 days for 4 months (Lichtenecker et al., 2021) |
|
| Adult F Landrace pigs, n=5/group, IM injections of 50 mg T-cypionate every 6 days for 13 days (Kothmann et al., 2021) |
|
| C57BL/6J adult F mice, n=3–9/group, IM injections of 24 or 48 mg/kg T-cypionate weekly for 8 or 24 weeks (Santos et al., 2022) |
|
| C57BL/6J adult F mice, n=75, injections of 0.6 mg T-cypionate mono or bi-weekly for 3 weeks (Reiche et al., 2022) |
|
| Adult M and F Sprague-Dawley rats, n=16, SQ injections of 0.45, 0.95 and 2.05 mg T enanthate 2x weekly for 2 weeks (Tassinari et al., 2022) |
|
| C57BL/6J adolescent F mice, n=12, SQ injections of 25 mg/kg GnRHa degarelix, silastic implants of 5 mg/kg T for 12 weeks (Dubois et al., 2023) |
|
| Adult F Yorkshire/Hampshire pigs, n=2, SQ silastic implants of 5–8 ng/ml T-testosterone enanthate for 30 days (Mahabir et al., 2024) |
|
Future Research Implications
To date, studies of the impacts of T-GAHT on fertility have strong implications for challenging the current standards for fertility counseling in trans-men. However, these studies identify several gaps in scientific literature that can be addressed by further investigation. Future studies should continue assessing the reproductive outcomes associated with various durations of T-treatment (Hashim et al., 2022; Kinnear et al., 2019). Published mouse studies generally focus on 6-week or 12-week treatment, but this is not fully indicative of the wide range of T-GAHT treatment durations that each individual may have experienced prior to desiring biological children. Additionally, research should further address the impacts of varying periods of treatment cessation on the reversibility of T-induced fertility outcomes (Kinnear et al., 2019; Schwartz et al., 2023). This will assess whether or not the reproductive outcomes associated with T-GAHT are significant enough for clinicians to continue recommending a period of T-cessation prior to becoming pregnant. So far, the only observed detrimental effects of T-treatment that were not reversed by a period of cessation have been decreased ovarian weight and stromal changes (Borrás et al., 2021; Dela Cruz et al., 2023a; Kinnear et al., 2021; Schwartz et al., 2023). However, even with lower ovarian weight, the maturation and fertilization rates of oocytes from T-treated mice were not compromised (Dela Cruz et al., 2023b; Godiwala et al., 2023), suggesting that prior T-treatment may not be detrimental to fertility. If future data suggests an impact of lower ovarian weight on fertility, further research with varying washout durations following T-GAHT would be needed to confirm that these changes are irreversible. So far, only two studies using animal models have addressed the effects of GnRHa and T-GAHT on oocyte quality, aneuploidy rates, pregnancy outcomes, and live birth rates (Dela Cruz et al., 2023b; Schwartz et al., 2023). Future research on IVF outcomes after puberty suppression and T-GAHT can provide more information on the functional impacts of T induced fertility outcomes (Kinnear & Moravek, 2023). Additionally, future studies should focus on health and reproductive outcomes of pups generated from T-treated dams, either from IVF or natural breeding after T cessation. As animal models of PCOS show detrimental reproductive effects in female offspring (Abbott et al., 2005; Padmanabhan & Veiga-Lopez, 2013), it is important to discover whether T-GAHT may have similar multi-generational effects.
Implications for future studies can also be used to address outcomes beyond fertility in trans-men, as presented in Table 1. This research could be improved by transitioning from using C57BL/6N mice to outbred strains of mice, such as CD-1 (Hashim et al., 2022). Outbred strains would increase the clinical relevance of data collected from these studies, since these populations are more genetically diverse and therefore more representative of human genetic diversity. In addition to addressing gaps in existing mouse models, employing different animal models in future studies can also provide a broader understanding of how puberty suppression and T-GAHT may impact fertility and other health outcomes for trans-men. One option would be to use transgenic mouse models that replicate androgen-induced health outcomes. For example, Doxycycline (Dox) treated 17α-hydroxylase/17,20-desmolase (TC17) mice were genetically modified by Secchi et al. (2021) to assess PCOS-like outcomes, which may be an avenue to explore with respect to T-GAHT. Additionally, non-murine models such as rats, monkeys, and sheep have all have been used for PCOS studies (Padmanabhan & Veiga-Lopez, 2013; Stener-Victorin, 2022) and can be used to address T-GAHT, as previously discussed. As T-GAHT research develops further, standardizing aspects of these paradigms across various species, such as T dose, treatment duration, and delivery mechanism, should also be prioritized in future studies in order to enhance clinical relevance of these animal models.
In summary, recent animal models have provided data to better inform current clinical guidance on fertility outcomes with respect to T-GAHT for transgender adults and adolescents. These studies have used various T-treatment methods and durations with and without periods of cessation, and consistently observed changes in reproductive physiology including altered ovarian morphology, decreased corpora lutea counts, and stromal aberrations. Importantly, fertility outcomes from these studies, such as fertility, maturation, and blastulation rates, suggest that prior GnRHa or T therapy may not prevent biological parenthood. However, these critical fertility outcomes have not been reported in as many studies or with the same level of consistency. Further research is needed to assess these effects in other, non-rodent models, with a focus on outcomes more specifically associated with fertility such as oocyte quality and live birth rates, if we are to develop sound, evidence-based reproductive health guidelines for the transgender community.
Acknowledgements
Figures were constructed using BioRender.com.
Funding
This work was supported by the National Institutes of Health (NIH) R01-HD098233 (to M.M.); and National Institute of Diabetes and Digestive and Kidney Diseases Institutional Training Grant No. T32 DK071212 (to R.E.K.).
Footnotes
Declaration of interest
Vasantha Padmanabhan is review editor of Reproduction. Vasantha Padmanabhan was not involved in the review or editorial process for this paper, on which she is listed as an author. The other authors have nothing to disclose.
References
- Abbott DH, Barnett DK, Bruns CM, & Dumesic DA (2005). Androgen excess fetal programming of female reproduction: A developmental aetiology for polycystic ovary syndrome? Human Reproduction Update, 11(4), 357–374. 10.1093/humupd/dmi013 [DOI] [PubMed] [Google Scholar]
- Adeleye AJ, Cedars MI, Smith J, & Mok-Lin E (2019). Ovarian stimulation for fertility preservation or family building in a cohort of transgender men. Journal of Assisted Reproduction and Genetics, 36(10), 2155–2161. 10.1007/s10815-019-01558-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aflatounian A, Edwards MC, Paris VR, Bertoldo MJ, Desai R, Gilchrist RB, Ledger WL, Handelsman DJ, & Walters KA (2020). Androgen signaling pathways driving reproductive and metabolic phenotypes in a PCOS mouse model. Journal of Endocrinology, 245(3), 381–395. 10.1530/JOE-19-0530 [DOI] [PubMed] [Google Scholar]
- Alpern S, Yaish I, Wagner-Kolasko G, Greenman Y, Sofer Y, Paltiel Lifshitz D, Groutz A, Azem F, & Amir H (2022). Why fertility preservation rates of transgender men are much lower than those of transgender women. Reproductive BioMedicine Online, 44(5), 943–950. 10.1016/j.rbmo.2022.01.003 [DOI] [PubMed] [Google Scholar]
- Amir H, Oren A, Klochendler Frishman E, Sapir O, Shufaro Y, Segev Becker A, Azem F, & Ben-Haroush A (2020). Oocyte retrieval outcomes among adolescent transgender males. Journal of Assisted Reproduction and Genetics, 37(7), 1737–1744. 10.1007/s10815-020-01815-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir H, Yaish I, Samara N, Hasson J, Groutz A, & Azem F (2020). Ovarian stimulation outcomes among transgender men compared with fertile cisgender women. Journal of Assisted Reproduction and Genetics, 37(10), 2463–2472. 10.1007/s10815-020-01902-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Sydnor E, Chen BK, LaGamma CC, McGowan JC, Mastrodonato A, Hunsberger HC, Shores R, Dixon RS, McEwen BS, Byne W, Meyer-Bahlburg HFL, Bockting W, Ehrhardt AA, & Denny CA (2021). Behavioral and neurobiological effects of GnRH agonist treatment in mice—potential implications for puberty suppression in transgender individuals. Neuropsychopharmacology, 46(5), 882–890. 10.1038/s41386-020-00826-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseler JD, del Valle JS, Chuva de Sousa Lopes SM, Verhoeven MO, Goddijn M, Huirne JAF, & van Mello NM (2024). One-third of amenorrheic transmasculine people on testosterone ovulate. Cell Reports Medicine, 5(3), 101440. 10.1016/j.xcrm.2024.101440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero JA, & Mockus I (2023). Preservation of Fertility in Transgender Men on Long-Term Testosterone Therapy: A Systematic Review of Oocyte Retrieval Outcomes During and After Exogenous Androgen Exposure. Transgender Health, 8(5), 408–419. 10.1089/trgh.2022.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels CB, Uliasz TF, Lestz L, & Mehlmann LM (2021). Short-term testosterone use in female mice does not impair fertilizability of eggs: Implications for the fertility care of transgender males. Human Reproduction, 36(1), 189–198. 10.1093/humrep/deaa282 [DOI] [PubMed] [Google Scholar]
- Borrás A, Manau MD, Fabregues F, Casals G, Saco A, Halperin I, Mora M, Goday A, Barral Y, & Carmona F (2021). Endocrinological and ovarian histological investigations in assigned female at birth transgender people undergoing testosterone therapy. Reproductive BioMedicine Online, 43(2), 289–297. 10.1016/j.rbmo.2021.05.010 [DOI] [PubMed] [Google Scholar]
- Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT, & Duncan FE (2016). Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction, 152(3), 245–260. 10.1530/REP-16-0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton D, & Omurtag K (2017). Care of the transgender or gender-nonconforming patient undergoing in vitro fertilization. International Journal of Transgenderism, 18(4), 372–375. 10.1080/15532739.2017.1352554 [DOI] [Google Scholar]
- Chen D, Bernardi LA, Pavone ME, Feinberg EC, & Moravek MB (2018). Oocyte cryopreservation among transmasculine youth: a case series. Journal of Assisted Reproduction and Genetics, 35(11), 2057–2061. 10.1007/s10815-018-1292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K, Harjee R, Roberts J, & Dunne C (2020). Fertility Preservation in a Transgender Man Without Prolonged Discontinuation of Testosterone: a Case Report and Literature Review. Fertility and Sterility, 114(3), e198–e199. 10.1016/j.fertnstert.2020.08.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulaki A, He H, Zhou M, Barberán AC, De Roo C, De Sousa Lopes SMC, Baetens M, Menten B, Van Soom A, De Sutter P, Weyers S, Boel A, Stoop D, & Heindryckx B (2023). Characterization of ovarian tissue oocytes from transgender men reveals poor calcium release and embryo development, which might be overcome by spindle transfer. Human Reproduction, 38(6), 1135–1150. 10.1093/humrep/dead068 [DOI] [PubMed] [Google Scholar]
- Coleman E, Radix AE, Bouman WP, Brown GR, de Vries ALC, Deutsch MB, Ettner R, Fraser L, Goodman M, Green J, Hancock AB, Johnson TW, Karasic DH, Knudson GA, Leibowitz SF, Meyer-Bahlburg HFL, Monstrey SJ, Motmans J, Nahata L, … Arcelus J (2022). Standards of Care for the Health of Transgender and Gender Diverse People, Version 8. International Journal of Transgender Health, 23(S1), S1–S259. 10.1080/26895269.2022.2100644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CS, Prescott M, Handelsman DJ, Walters KA, & Campbell RE (2022). Chronic androgen excess in female mice does not impact luteinizing hormone pulse frequency or putative GABAergic inputs to GnRH neurons. Journal of Neuroendocrinology, 34(4), 1–13. 10.1111/jne.13110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roo C, Lierman S, Tilleman K, Peynshaert K, Braeckmans K, Caanen M, Lambalk CB, Weyers S, T’Sjoen G, Cornelissen R, & De Sutter P (2017). Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reproductive BioMedicine Online, 34(6), 557–566. 10.1016/j.rbmo.2017.03.008 [DOI] [PubMed] [Google Scholar]
- de Vries ALC, McGuire JK, Steensma TD, Wagenaar ECF, Doreleijers TAH, & Cohen-Kettenis PT (2014). Young Adult Psychological Outcome After Puberty Suppression and Gender Reassignment. American Academy of Pediatrics, 134(4), 696–704. [DOI] [PubMed] [Google Scholar]
- Dela Cruz C, Kinnear HM, Hashim PH, Wandoff A, Nimmagadda L, Chang FL, Padmanabhan V, Shikanov A, & Moravek MB (2023). A mouse model mimicking gender-affirming treatment with pubertal suppression followed by testosterone in transmasculine youth. Human Reproduction (Oxford, England), 38(2), 256–265. 10.1093/humrep/deac257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Cruz C, Wandoff A, Brunette M, Padmanabhan V, Shikanov A, & Moravek MB (2023). In vitro fertilization outcomes in a mouse model of gender-affirming hormone therapy in transmasculine youth. F and S Science, 4(4), 302–310. 10.1016/j.xfss.2023.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois V, Ciancia S, Doms S, El Kharraz S, Sommers V, Kim NR, David K, Van Dijck J, Valle-Tenney R, Maes C, Antonio L, Decallonne B, Carmeliet G, Claessens F, Cools M, & Vanderschueren D (2023). Testosterone Restores Body Composition, Bone Mass, and Bone Strength Following Early Puberty Suppression in a Mouse Model Mimicking the Clinical Strategy in Trans Boys. Journal of Bone and Mineral Research, 38(10), 1497–1508. 10.1002/jbmr.4832 [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, & Padmanabhan V (2007). Polycystic ovary syndrome and its developmental origins. Reviews in Endocrine and Metabolic Disorders, 8(2), 127–141. 10.1007/s11154-007-9046-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza LA, Terasaka T, Lawson MA, & Kauffman AS (2020). Androgen Suppresses in Vivo and in Vitro LH Pulse Secretion and Neural Kiss1 and Tac2 Gene Expression in Female Mice. Endocrinology, 161(12), 1–16. 10.1210/endocr/bqaa191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale J, Magee B, Forsyth-Greig A, Visram H, & Jackson A (2021). Oocyte cryopreservation in a transgender man on long-term testosterone therapy: a case report. F and S Reports, 2(2), 249–251. 10.1016/j.xfre.2021.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godiwala P, Uliasz TF, Lowther KM, Kaback D, & Mehlmann LM (2023). Puberty Suppression Followed by Testosterone Therapy Does Not Impair Reproductive Potential in Female Mice. Endocrinology, 164(11), 1–10. 10.1210/endocr/bqad145 [DOI] [PubMed] [Google Scholar]
- Goetz LG, Mamillapalli R, Devlin MJ, Robbins AE, Majidi-Zolbin M, & Taylor HS (2017). Cross-sex testosterone therapy in ovariectomized mice: Addition of low-dose estrogen preserves bone architecture. American Journal of Physiology - Endocrinology and Metabolism, 313(5), E540–E551. 10.1152/ajpendo.00161.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz LG, Mamillapalli R, Sahin C, Majidi-Zolbin M, Ge G, Mani A, & Taylor HS (2019). Addition of Estradiol to Cross-Sex Testosterone Therapy Reduces Atherosclerosis Plaque Formation in Female ApoE−/− Mice. Endocrinology, 159(2), 754–762. 10.1210/en.2017-00884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald P, Dubois B, Lekovich J, Pang JH, & Safer J (2022). Successful In Vitro Fertilization in a Cisgender Female Carrier Using Oocytes Retrieved From a Transgender Man Maintained on Testosterone. AACE Clinical Case Reports, 8(1), 19–21. 10.1016/j.aace.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimstad FW, Fowler KG, New EP, Ferrando CA, Pollard RR, Chapman G, Gomez-Lobo V, & Gray M (2019). Uterine pathology in transmasculine persons on testosterone: a retrospective multicenter case series. American Journal of Obstetrics and Gynecology, 220(3), 257.e1–257.e7. 10.1016/j.ajog.2018.12.021 [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Avendano L, Kelly M, Estoesta C, Frohock B, Candelario I, Davis LK, Oevermann M, Sencherey B, Toro E, Valdivia HS, & Gore AC (2022). Daily GnRH agonist treatment effectively delayed puberty in female rats without long-term effects on sexual behavior or estrous cyclicity. Physiology and Behavior, 254, 1–7. 10.1016/j.physbeh.2022.113879 [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Avendano L, Kelly M, Estoesta C, Sencherey B, Valdivia HS, Gale A, Yepez L, Belfield JB, Carter KM, Williams N, & Gore AC (2023). Chronic periadolescent leuprolide exposure affects the development of reproductive physiology and behavior of female and male rats differently, but both mature after treatment termination. Biology of Sex Differences, 14(1), 1–14. 10.1186/s13293-022-00485-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim PH, Kinnear HM, Dela Cruz C, Padmanabhan V, Moravek MB, & Shikanov A (2022). Pharmacokinetic comparison of three delivery systems for subcutaneous testosterone administration in female mice. General and Comparative Endocrinology, 327, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, & T’Sjoen GG (2017). Endocrine treatment of gender-dysphoric/ gender-incongruent persons: An endocrine society*clinical practice guideline. Journal of Clinical Endocrinology and Metabolism, 102(11), 3869–3903. 10.1210/jc.2017-01658 [DOI] [PubMed] [Google Scholar]
- Herman JL, Flores AR, & O’Neill KK (2022). How many adults and youth identify as transgender in the United States. UCLA School of Law: The Williams Institute, June, 1–26. [Google Scholar]
- Insogna IG, Ginsburg E, & Srouji S (2020). Fertility Preservation for Adolescent Transgender Male Patients: A Case Series. Journal of Adolescent Health, 66(6), 750–753. 10.1016/j.jadohealth.2019.12.004 [DOI] [PubMed] [Google Scholar]
- Israeli T, Preisler L, Kalma Y, Samara N, Levi S, Groutz A, Azem F, & Amir H (2022). Similar fertilization rates and preimplantation embryo development among testosterone-treated transgender men and cisgender women. Reproductive BioMedicine Online, 45(3), 448–456. 10.1016/j.rbmo.2022.04.016 [DOI] [PubMed] [Google Scholar]
- Kinnear HM, Constance ES, David A, Marsh EE, Padmanabhan V, Shikanov A, & Moravek MB (2019). A mouse model to investigate the impact of testosterone therapy on reproduction in transgender men. Human Reproduction, 34(10), 2009–2017. 10.1093/humrep/dez177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear HM, Hashim PH, Dela Cruz C, Chang AL, Rubenstein G, Nimmagadda L, Ramamoorthi Elangovan V, Jones A, Brunette MA, Hannum DF, Li JZ, Padmanabhan V, Moravek MB, & Shikanov A (2023). Presence of ovarian stromal aberrations after cessation of testosterone therapy in a transgender mouse model. Biology of Reproduction, 108(5), 802–813. 10.1093/biolre/ioad019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear HM, Hashim PH, Dela Cruz C, Rubenstein G, Chang FL, Nimmagadda L, Brunette MA, Padmanabhan V, Shikanov A, & Moravek MB (2021). Reversibility of Testosterone-Induced Acyclicity after Testosterone Cessation in a Transgender Mouse Model. F&S Science, 2(2), 116–123. 10.1016/j.xfss.2021.01.008.Reversibility [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear HM, & Moravek MB (2023). Reproductive capacity after gender-affirming testosterone therapy. Human Reproduction, 38(10), 1872–1880. 10.1093/humrep/dead158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothmann KH, Jacobsen V, Laffitte E, Bromfield C, Grizzaffi M, Jarboe M, Braundmeier-Fleming AG, Bahr JM, Nowak RA, & Newell-Fugate AE (2021). Virilizing doses of testosterone decrease circulating insulin levels and differentially regulate insulin signaling in liver and adipose tissue of females. American Journal of Physiology - Endocrinology and Metabolism, 320(6), E1107–E1118. 10.1152/AJPENDO.00281.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Sakkas D, Pang S, Thornton K, & Resetkova N (2019). Assisted reproductive technology outcomes in female-to-male transgender patients compared with cisgender patients: a new frontier in reproductive medicine. Fertility and Sterility, 112(5), 858–865. 10.1016/j.fertnstert.2019.07.014 [DOI] [PubMed] [Google Scholar]
- Lierman S, Tilleman K, Braeckmans K, Peynshaert K, Weyers S, T’Sjoen G, & De Sutter P (2017). Fertility preservation for trans men: frozen-thawed in vitro matured oocytes collected at the time of ovarian tissue processing exhibit normal meiotic spindles. Journal of Assisted Reproduction and Genetics, 34(11), 1449–1456. 10.1007/s10815-017-0976-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loverro G, Resta L, Dellino M, Edoardo DN, Cascarano MA, Loverro M, & Mastrolia SA (2016). Uterine and ovarian changes during testosterone administration in young female-to-male transsexuals. Taiwanese Journal of Obstetrics and Gynecology, 55(5), 686–691. 10.1016/j.tjog.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Mahabir N, & Newell-Fugate AE (2024). Fabrication of silastic testosterone enanthate implants to achieve virilizing levels of serum testosterone in swine. MethodsX, 12(September 2023), 102549. 10.1016/j.mex.2024.102549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CE, Lewis C, & Omurtag K (2021). Successful oocyte cryopreservation using letrozole as an adjunct to stimulation in a transgender adolescent after GnRH agonist suppression. Fertility and Sterility, 116(2), 522–527. 10.1016/j.fertnstert.2021.02.025 [DOI] [PubMed] [Google Scholar]
- Moravek MB (2019). Fertility preservation options for transgender and gender-nonconforming individuals. Current Opinion in Obstetrics and Gynecology, 31(3), 170–176. 10.1097/GCO.0000000000000537 [DOI] [PubMed] [Google Scholar]
- Moravek MB, Dixon M, Pena SM, & Obedin-Maliver J (2023). Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Human Reproduction (Oxford, England), 38(3), 482–488. 10.1093/humrep/dead003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morong JJ, Class QA, Zamah AM, & Hinz E (2022). Parenting intentions in transgender and gender-nonconforming adults. International Journal of Gynecology and Obstetrics, 159(2), 557–562. 10.1002/ijgo.14194 [DOI] [PubMed] [Google Scholar]
- Moustakli E, & Tsonis O (2023). Exploring Hormone Therapy Effects on Reproduction and Health in Transgender Individuals. Medicina (Lithuania), 59(12). 10.3390/medicina59122094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A, Gooren LJ, Naton-Schötz S, Cupisti S, Beckmann MW, & Dittrich R (2008). Prevalence of polycystic ovary syndrome and hyperandrogenemia in female-to-male transsexuals. Journal of Clinical Endocrinology and Metabolism, 93(4), 1408–1411. 10.1210/jc.2007-2808 [DOI] [PubMed] [Google Scholar]
- Nadgauda AS, & Butts S (2024). Barriers to fertility preservation access in transgender and gender diverse adolescents: a narrative review. Therapeutic Advances in Reproductive Health, 18, 1–9. 10.1177/26334941231222120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahata L, Tishelman AC, Caltabellotta NM, & Quinn GP (2017). Low Fertility Preservation Utilization Among Transgender Youth. Journal of Adolescent Health, 61(1), 40–44. 10.1016/j.jadohealth.2016.12.012 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, & Veiga-Lopez A (2013). Animal models of the polycystic ovary syndrome phenotype. Steroids, 78(8), 734–740. 10.1016/j.steroids.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Laso C, Cerdan S, Junque C, Gómez Á, Ortega E, Mora M, Avendaño C, Gómez-Gil E, Del Cerro MCR, & Guillamon A (2018). Effects of adult female rat androgenization on brain morphology and metabolomic profile. Cerebral Cortex, 28(8), 2846–2853. 10.1093/cercor/bhx163 [DOI] [PubMed] [Google Scholar]
- Perrone AM, Cerpolini S, Maria Salfi NC, Ceccarelli C, De Giorgi LB, Formelli G, Casadio P, Ghi T, Pelusi G, Pelusi C, & Meriggiola MC (2009). Effect of long-term testosterone administration on the endometrium of female-to-male (FtM) transsexuals. Journal of Sexual Medicine, 6(11), 3193–3200. 10.1111/j.1743-6109.2009.01380.x [DOI] [PubMed] [Google Scholar]
- Reiche E, Tan Y, Louis MR, Keller PR, Soares V, Schuster CR, Lu T, & O’Brien Coon D (2022). A Novel Mouse Model for Investigating the Effects of Gender-affirming Hormone Therapy on Surgical Healing. Plastic and Reconstructive Surgery - Global Open, 10(11), E4688. 10.1097/GOX.0000000000004688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JD, Oliveira-Neto JT, Barros PR, Damasceno LEA, Lautherbach N, Assis AP, Silva CAA, Sorgi CA, Faccioli LH, Kettelhut IC, Salgado HC, Carneiro FS, Alves-Filho JC, & Tostes RC (2022). Th17 cell-linked mechanisms mediate vascular dysfunction induced by testosterone in a mouse model of gender-affirming hormone therapy. American Journal of Physiology - Heart and Circulatory Physiology, 323(2), H322–H335. 10.1152/AJPHEART.00182.2022 [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Xu M, Henderson NC, Dela Cruz C, Pfau D, Padmanabhan V, Shikanov A, & Moravek MB (2023). Impaired in vitro fertilization outcomes following testosterone treatment improve with washout in a mouse model of gender-affirming hormone treatment. American Journal of Obstetrics and Gynecology, 229(4), 419.e1–419.e10. 10.1016/j.ajog.2023.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stener-Victorin E (2022). Update on Animal Models of Polycystic Ovary Syndrome. Endocrinology, 163(12), 1–11. 10.1210/endocr/bqac164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk THR, Asseler JD, Huirne JAF, van den Boogaard E, & van Mello NM (2023). Desire for children and fertility preservation in transgender and gender-diverse people: A systematic review. Best Practice and Research: Clinical Obstetrics and Gynaecology, 87, 102312. 10.1016/j.bpobgyn.2023.102312 [DOI] [PubMed] [Google Scholar]
- Sun L-F, Yang Y-L, Xiao T-X, Li M-X, & Zhang JV (2019). Removal of DHT can relieve polycystic ovarian but not metabolic abnormalities in DHT-induced hyperandrogenism in mice. Reproduction, Fertility, and Development, 31(10), 1597–1606. 10.1071/RD18459 [DOI] [PubMed] [Google Scholar]
- Tassinari R, & Maranghi F (2021). Rodent model of gender-affirming hormone therapies as specific tool for identifying susceptibility and vulnerability of transgender people and future applications for risk assessment. International Journal of Environmental Research and Public Health, 18(23). 10.3390/ijerph182312640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broecke R, Van der Elst J, Liu J, Hovatta O, & Dhont M (2001). The female-to-male transsexual patient: a source of human ovarian cortical tissue for experimental use. Human Reproduction, 16(1), 145–147. [DOI] [PubMed] [Google Scholar]
- Yaish I, Tordjman K, Amir H, Malinger G, Salemnick Y, Shefer G, Serebro M, Azem F, Golani N, Sofer Y, Stern N, & Greenman Y (2021). Functional ovarian reserve in transgender men receiving testosterone therapy: Evidence for preserved anti-Müllerian hormone and antral follicle count under prolonged treatment. Human Reproduction, 36(10), 2753–2760. 10.1093/humrep/deab169 [DOI] [PubMed] [Google Scholar]
- Yoshida A, Kaji T, Imaizumi J, Shirakawa A, Suga K, Nakagawa R, Maeda K, Irahara M, & Iwasa T (2022). Transgender man receiving testosterone treatment became pregnant and delivered a girl: A case report. Journal of Obstetrics and Gynaecology Research, 48(3), 866–868. 10.1111/jog.15145 [DOI] [PubMed] [Google Scholar]


