Abstract
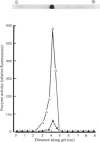
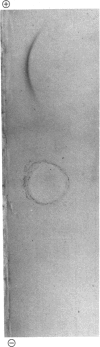
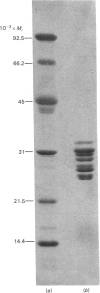
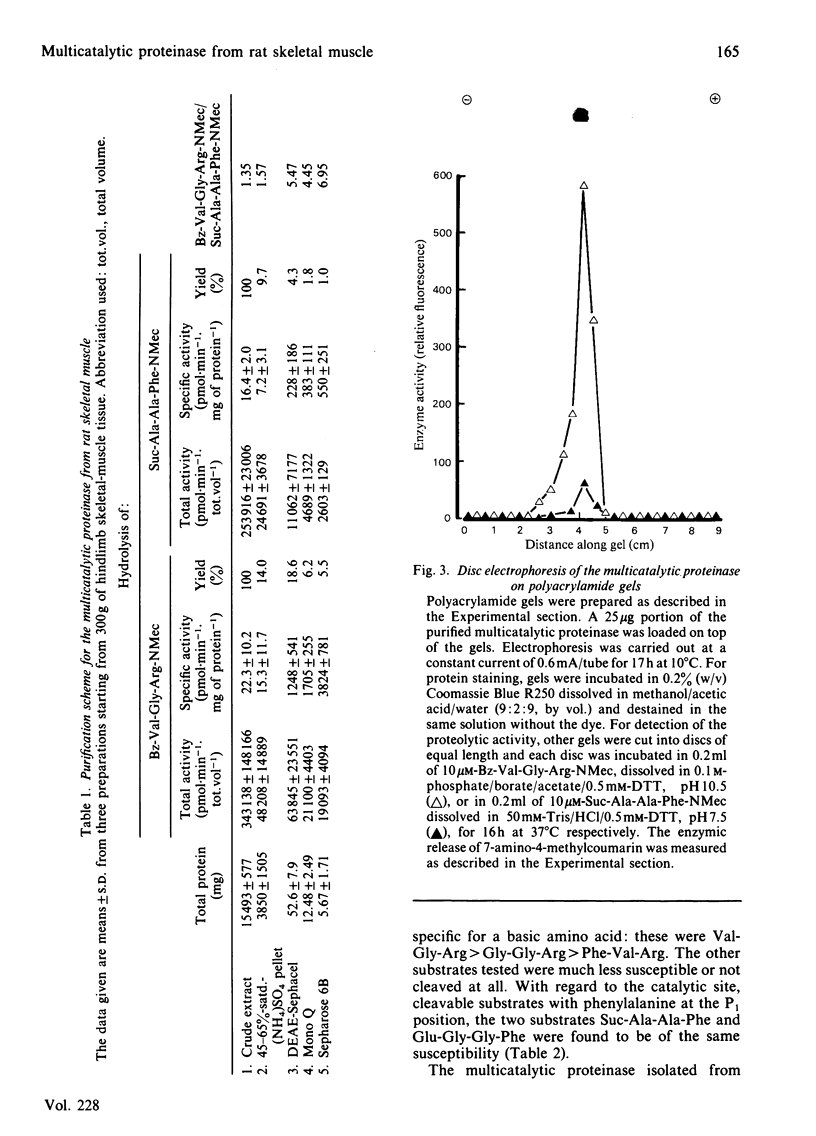
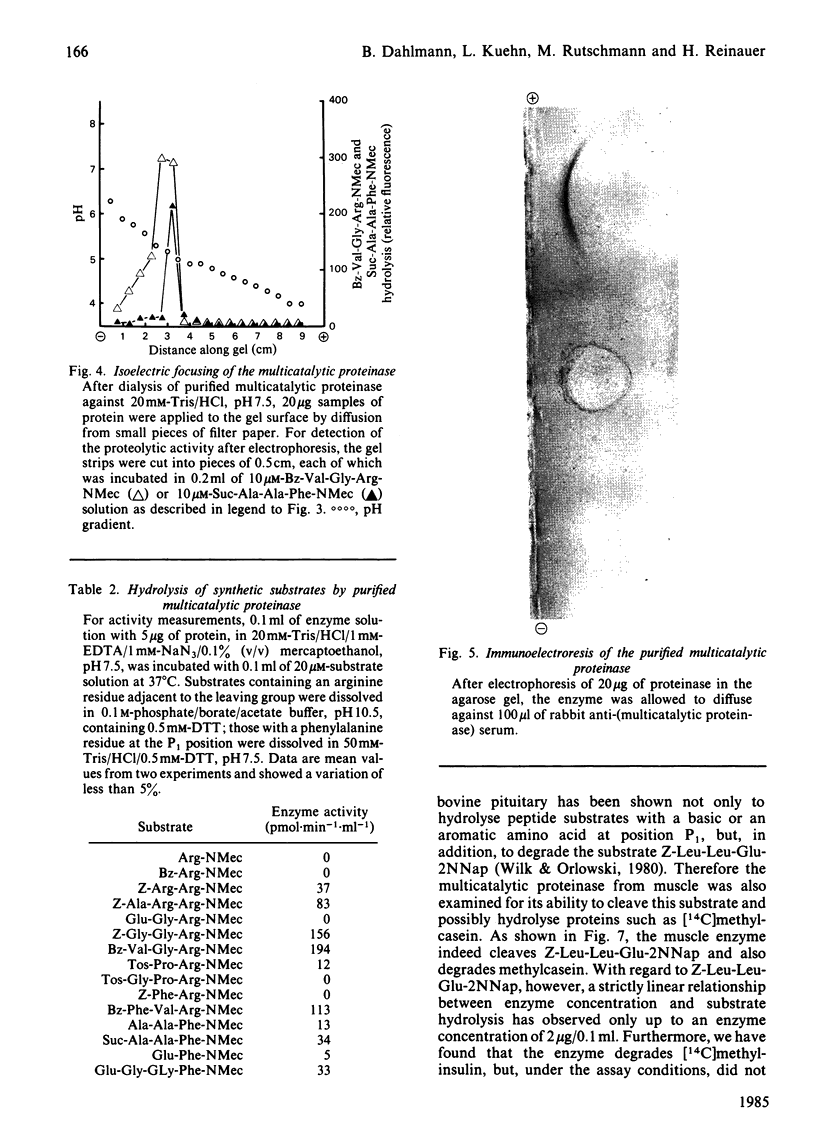
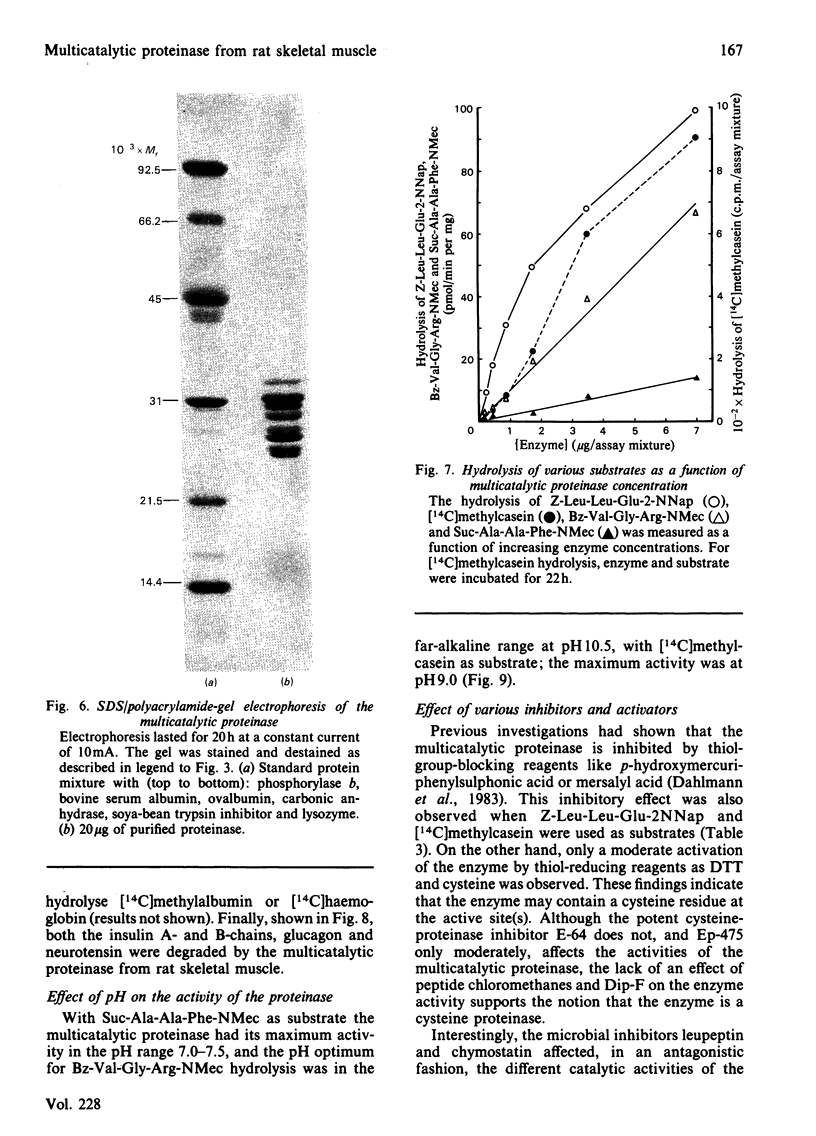
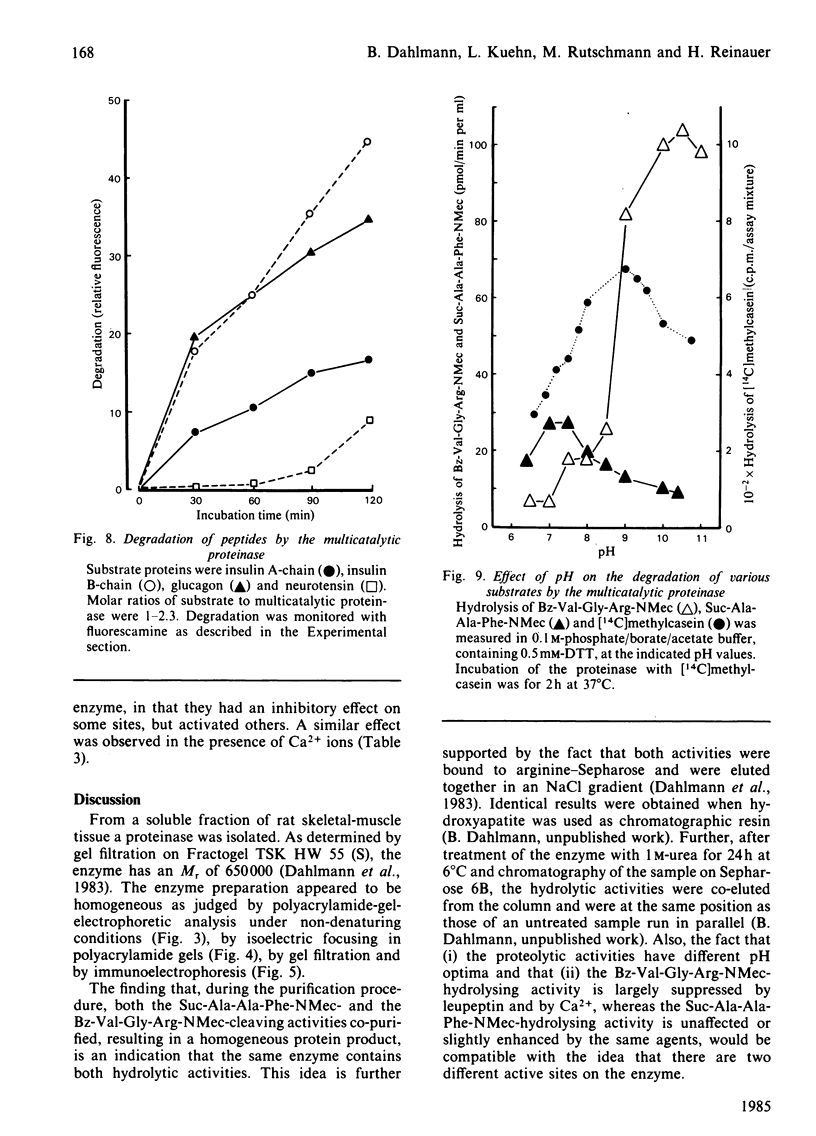
A proteolytic enzyme was purified from the post-myofibrillar fraction of rat skeletal muscle. The purification procedure consisted of fractionation of the muscle extract by (NH4)2SO4, chromatography on DEAE-Sephacel, fast protein liquid chromatography on Mono Q and gel filtration on Sepharose 6B. The enzyme preparation appeared to be homogeneous as judged by disc electrophoresis in polyacrylamide gels and by immunoelectrophoresis. The isoelectric point of the proteinase is at 5.1-5.2. The enzyme has an Mr of about 650 000 and dissociates into eight subunits of Mr 25 000-32 000 when subjected to electrophoresis in sodium dodecyl sulphate/polyacrylamide gels. The proteinase contains hydrolytic activity against N-blocked tripeptide 4-methyl-7-coumarylamide substrates with an arginine or phenylalanine residue adjacent to the leaving group. Maximum activity with the first group of substrates was at pH 10.5, and this activity was inhibited by leupeptin, chymostatin and Ca2+. Maximum activity with the latter group of substrates was at pH 7.5, and was also inhibited by the two microbial inhibitors, but was activated by Ca2+ ions. By using [14C]methylcasein as a substrate, maximum activity was observed at pH9.0, and this proteolytic activity was not affected by leupeptin, was enhanced by chymostatin and inhibited by Ca2+. Similar effects were observed when benzyloxycarbonyl-Leu-Leu-Glu 2-naphthylamide was used as a substrate. These enzymic activities were abolished by p-hydroxymercuribenzenesulphonic acid or mersalyl acid, whereas a small activation was observed with cysteine or dithiothreitol.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J. Intracellular protein degradation. Essays Biochem. 1977;13:1–37. [PubMed] [Google Scholar]
- Dahlmann B., Block I., Kuehn L., Rutschmann M., Reinauer H. Immunological evidence for the identity of three proteinases from rat skeletal muscle. FEBS Lett. 1982 Feb 8;138(1):88–90. doi: 10.1016/0014-5793(82)80401-8. [DOI] [PubMed] [Google Scholar]
- Dahlmann B., Kuehn L., Reinauer H. Identification of three high molecular mass cysteine proteinases from rat skeletal muscle. FEBS Lett. 1983 Aug 22;160(1-2):243–247. doi: 10.1016/0014-5793(83)80975-2. [DOI] [PubMed] [Google Scholar]
- Dahlmann B., Rutschmann M., Kuehn L., Reinauer H. Activation of the multicatalytic proteinase from rat skeletal muscle by fatty acids or sodium dodecyl sulphate. Biochem J. 1985 May 15;228(1):171–177. doi: 10.1042/bj2280171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartino G. N. Identification of a high molecular weight alkaline protease in rat heart. J Mol Cell Cardiol. 1983 Jan;15(1):17–29. doi: 10.1016/0022-2828(83)90304-8. [DOI] [PubMed] [Google Scholar]
- GRABAR P., WILLIAMS C. A. Méthode permettant l'étude conjuguée des proprietés électrophorétiques et immunochimiques d'un mélange de protéines; application au sérum sanguin. Biochim Biophys Acta. 1953 Jan;10(1):193–194. doi: 10.1016/0006-3002(53)90233-9. [DOI] [PubMed] [Google Scholar]
- Garesse R., Castell J. V., Vallejo C. G., Marco R. A fluorescamine-based sensitive method for the assay of proteinases, capable of detecting the initial cleavage steps of a protein. Eur J Biochem. 1979 Sep;99(2):253–259. doi: 10.1111/j.1432-1033.1979.tb13252.x. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Ismail F., Gevers W. A high-molecular-weight cysteine endopeptidase from rat skeletal muscle. Biochim Biophys Acta. 1983 Jan 26;742(2):399–408. doi: 10.1016/0167-4838(83)90327-8. [DOI] [PubMed] [Google Scholar]
- Kay J. Intracellular protein degradation. Biochem Soc Trans. 1978;6(4):789–797. doi: 10.1042/bst0060789. [DOI] [PubMed] [Google Scholar]
- Roth J. S., Losty T., Wierbicki E. Assay of proteolytic enzyme activity using a 14C-labeled hemoglobin. Anal Biochem. 1971 Jul;42(1):214–221. doi: 10.1016/0003-2697(71)90029-7. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. Cation-sensitive neutral endopeptidase: isolation and specificity of the bovine pituitary enzyme. J Neurochem. 1980 Nov;35(5):1172–1182. doi: 10.1111/j.1471-4159.1980.tb07873.x. [DOI] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. Evidence that pituitary cation-sensitive neutral endopeptidase is a multicatalytic protease complex. J Neurochem. 1983 Mar;40(3):842–849. doi: 10.1111/j.1471-4159.1983.tb08056.x. [DOI] [PubMed] [Google Scholar]