Abstract
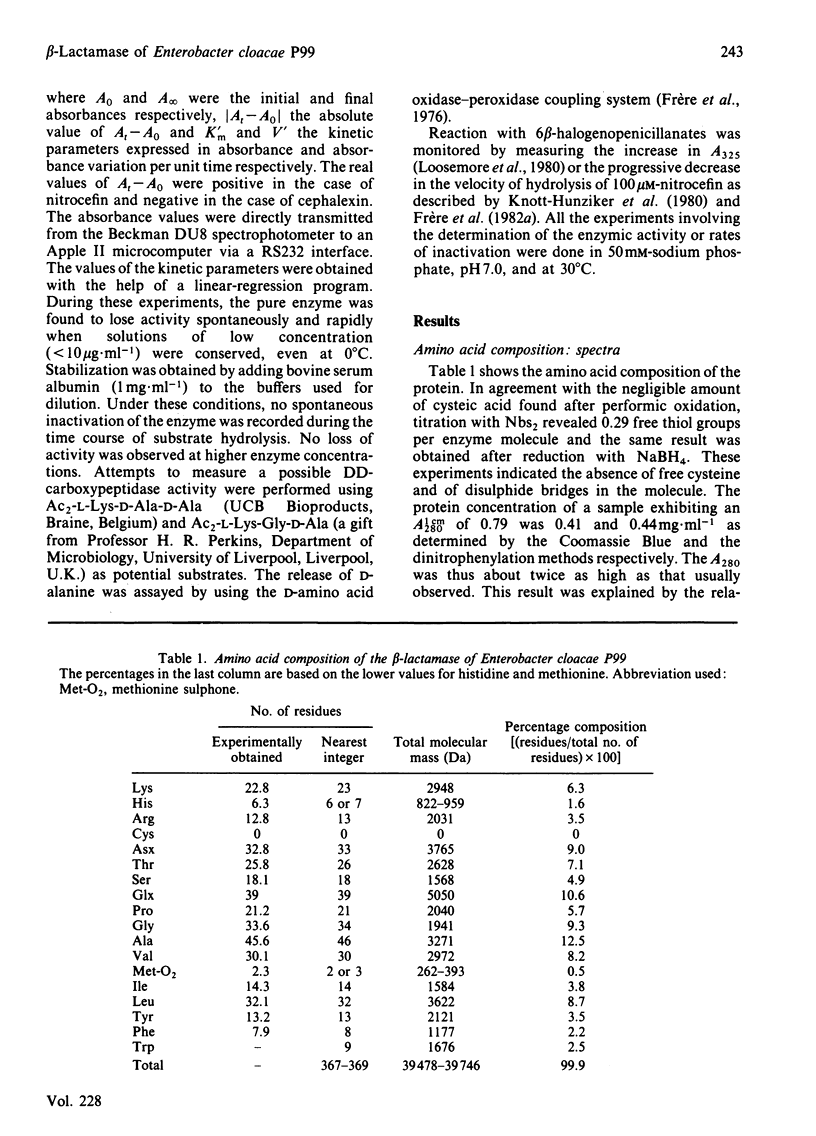
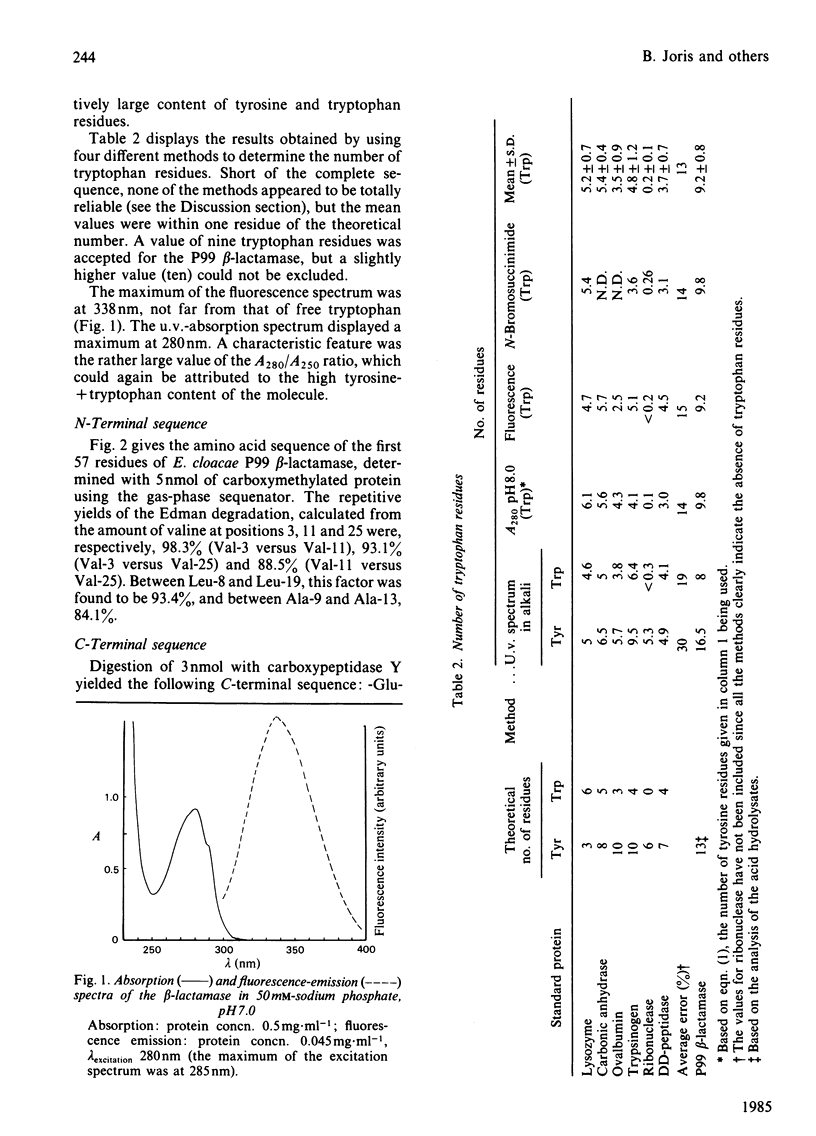
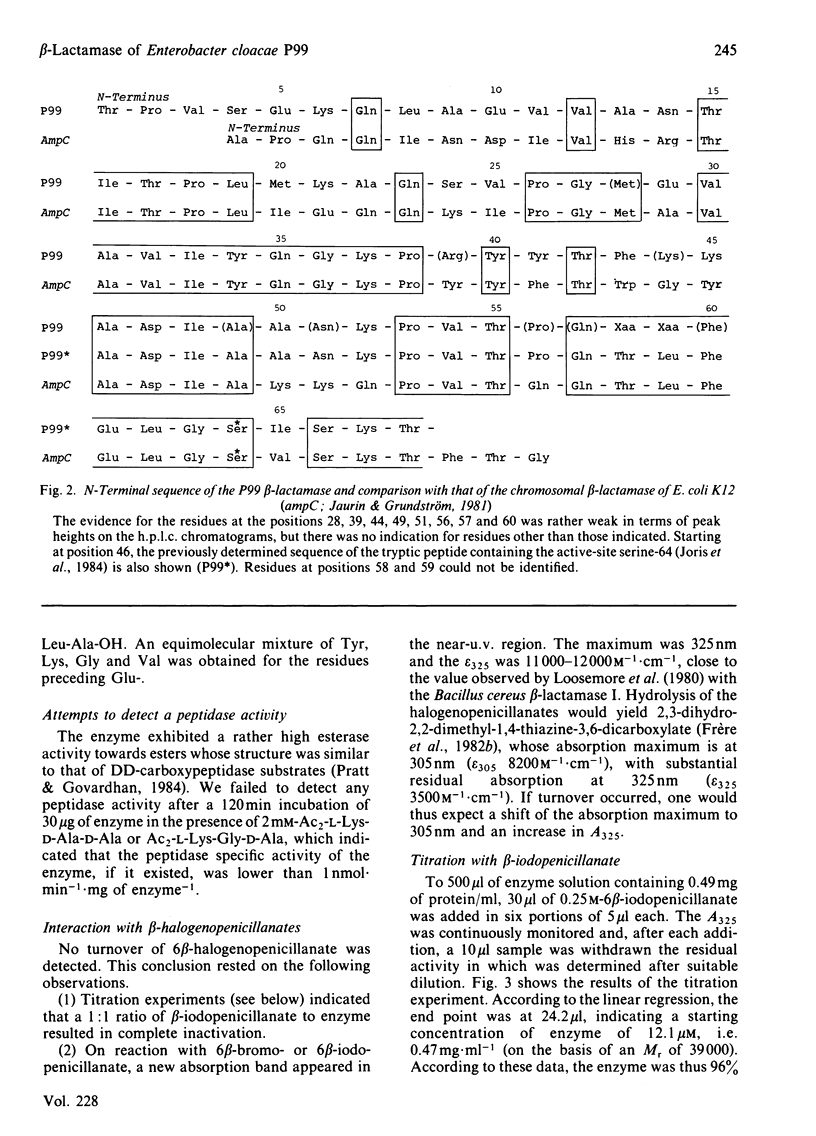
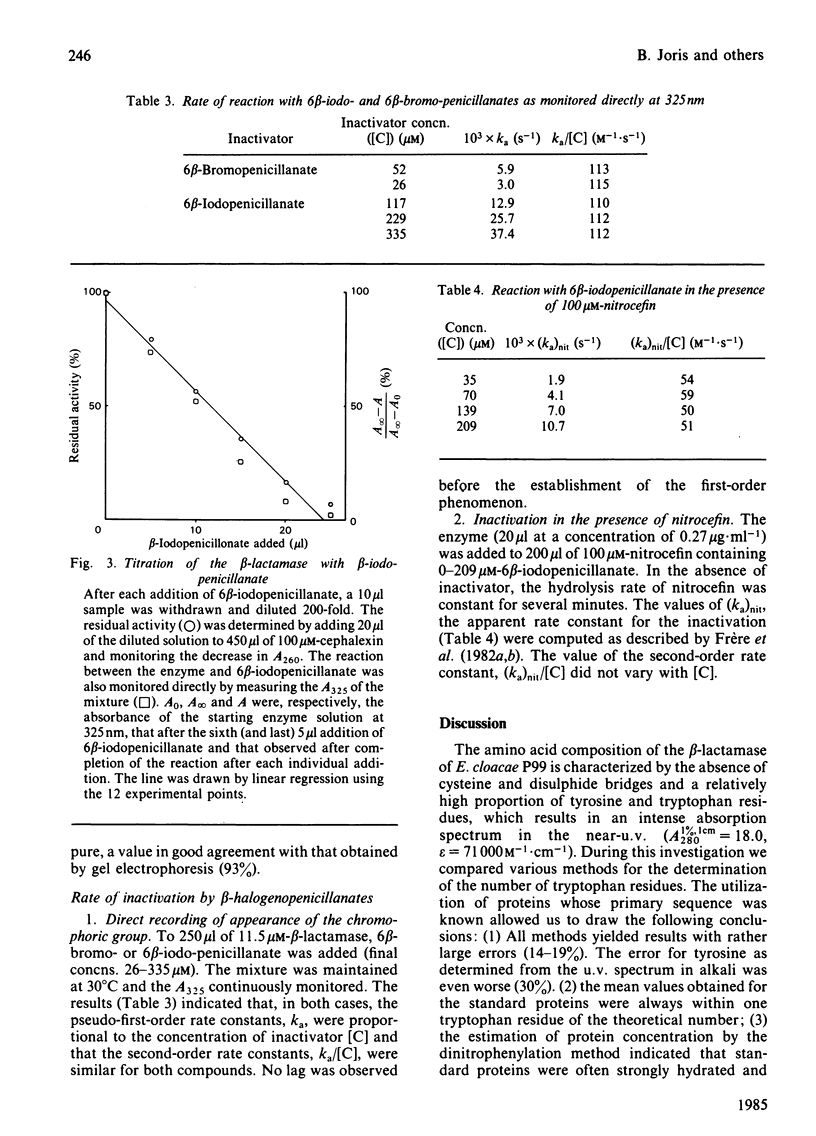
The beta-lactamase of Enterobacter cloacae P99 consists of one polypeptide chain of Mr 39000 devoid of disulphide bridges and free thiol groups. It contains an unusually high proportion of tyrosine and tryptophan. The N-terminal sequence exhibits overlaps with the tryptic peptide obtained after labelling the active site with 6 beta-iodopenicillanate. The active-site serine residue is at position 64. The homology with the chromosomal beta-lactamase of Escherichia coli K 12 (ampC gene) is lower within the 25 residues of the N-terminal portion than around the active-site serine residue. The P99 beta-lactamase is inactivated by 6 beta-bromo- and 6 beta-iodo-penicillanate, with a second-order rate constant of 110-140M-1 X s-1 at 30 degrees C and pH 7.0, a value that is much lower than that observed with class-A beta-lactamases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Bicknell R., Knott-Hunziker V., Waley S. G. The pH-dependence of class B and class C beta-lactamases. Biochem J. 1983 Jul 1;213(1):61–66. doi: 10.1042/bj2130061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright S. J., Waley S. G. Purification of beta-lactamases by affinity chromatography on phenylboronic acid-agarose. Biochem J. 1984 Jul 15;221(2):505–512. doi: 10.1042/bj2210505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier P., Dideberg O., Frère J. M., Moews P. C., Knox J. R. Crystallographic data for the beta-lactamase from Enterobacter cloacae P99. J Mol Biol. 1983 Dec 5;171(2):237–238. doi: 10.1016/s0022-2836(83)80358-1. [DOI] [PubMed] [Google Scholar]
- Claes P. J., Vanderhaeghe H., Roets E., Thomis J., De Meester F., Piette J. L. Synthesis of deuterium- and tritium-labeled 6 beta-bromopenicillanic acid. J Antibiot (Tokyo) 1985 Jan;38(1):75–82. doi: 10.7164/antibiotics.38.75. [DOI] [PubMed] [Google Scholar]
- Duez C., Frère J. M., Geurts F., Ghuysen J. M., Dierickx L., Delcambe L. The exocellular DD-carboxypeptidase-endopeptidase from Streptomyces albus G. Purification and chemical properties. Biochem J. 1978 Dec 1;175(3):793–800. doi: 10.1042/bj1750793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Dormans C., Duyckaerts C., De Graeve J. Interaction of beta-iodopenicillanate with the beta-lactamases of Streptomyces albus G and Actinomadura R39. Biochem J. 1982 Dec 1;207(3):437–444. doi: 10.1042/bj2070437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Dormans C., Lenzini V. M., Duyckaerts C. Interaction of clavulanate with the beta-lactamases of Streptomyces albus G and Actinomadura R39. Biochem J. 1982 Dec 1;207(3):429–436. doi: 10.1042/bj2070429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frére J. M., Leyh-Bouille M., Ghuysen J. M., Nieto M., Perkins H. R. Exocellular DD-carboxypeptidases-transpeptidases from Streptomyces. Methods Enzymol. 1976;45:610–636. doi: 10.1016/s0076-6879(76)45054-1. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hill H. A., Sammes P. G., Waley S. G. Active sites of beta-lactamases from Bacillus cereus. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):333–344. doi: 10.1098/rstb.1980.0050. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoins by ultrasensitive gradient high-performance liquid chromatography. Methods Enzymol. 1983;91:486–493. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- Jaurin B., Grundström T. ampC cephalosporinase of Escherichia coli K-12 has a different evolutionary origin from that of beta-lactamases of the penicillinase type. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4897–4901. doi: 10.1073/pnas.78.8.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris B., Dusart J., Frere J. M., van Beeumen J., Emanuel E. L., Petursson S., Gagnon J., Waley S. G. The active site of the P99 beta-lactamase from Enterobacter cloacae. Biochem J. 1984 Oct 1;223(1):271–274. doi: 10.1042/bj2230271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris B., Van Beeumen J., Casagrande F., Gerday C., Frère J. M., Ghuysen J. M. The complete amino acid sequence of the Zn2+-containing D-alanyl-D-alanine-cleaving carboxypeptidase of streptomyces albus G. Eur J Biochem. 1983 Jan 17;130(1):53–69. doi: 10.1111/j.1432-1033.1983.tb07116.x. [DOI] [PubMed] [Google Scholar]
- Knott-Hunziker V., Orlek B. S., Sammes P. G., Waley S. G. Kinetics of inactivation of beta-lactamase I by 6 beta-bromopenicillanic acid. Biochem J. 1980 Jun 1;187(3):797–802. doi: 10.1042/bj1870797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Petursson S., Jayatilake G. S., Waley S. G., Jaurin B., Grundström T. Active sites of beta-lactamases. The chromosomal beta-lactamases of Pseudomonas aeruginosa and Escherichia coli. Biochem J. 1982 Mar 1;201(3):621–627. doi: 10.1042/bj2010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Petursson S., Waley S. G., Jaurin B., Grundström T. The acyl-enzyme mechanism of beta-lactamase action. The evidence for class C Beta-lactamases. Biochem J. 1982 Nov 1;207(2):315–322. doi: 10.1042/bj2070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Waley S. G., Orlek B. S., Sammes P. G. Penicillinase active sites: labelling of serine-44 in beta-lactamase I by 6beta-bromopenicillanic acid. FEBS Lett. 1979 Mar 1;99(1):59–61. doi: 10.1016/0014-5793(79)80248-3. [DOI] [PubMed] [Google Scholar]
- Pratt R. F., Govardhan C. P. beta-Lactamase-catalyzed hydrolysis of acyclic depsipeptides and acyl transfer to specific amino acid acceptors. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1302–1306. doi: 10.1073/pnas.81.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. W. Beta-lactamase (Enterobacter species). Methods Enzymol. 1975;43:678–687. doi: 10.1016/0076-6879(75)43133-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


