Abstract
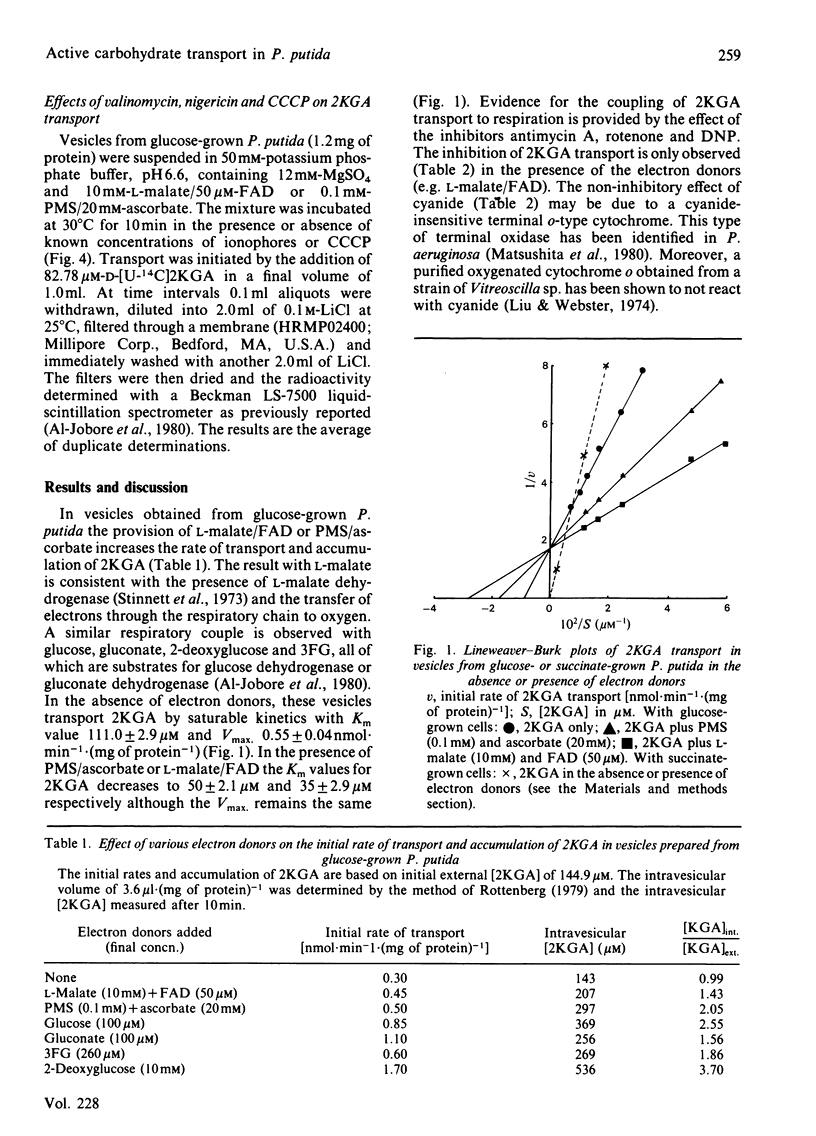
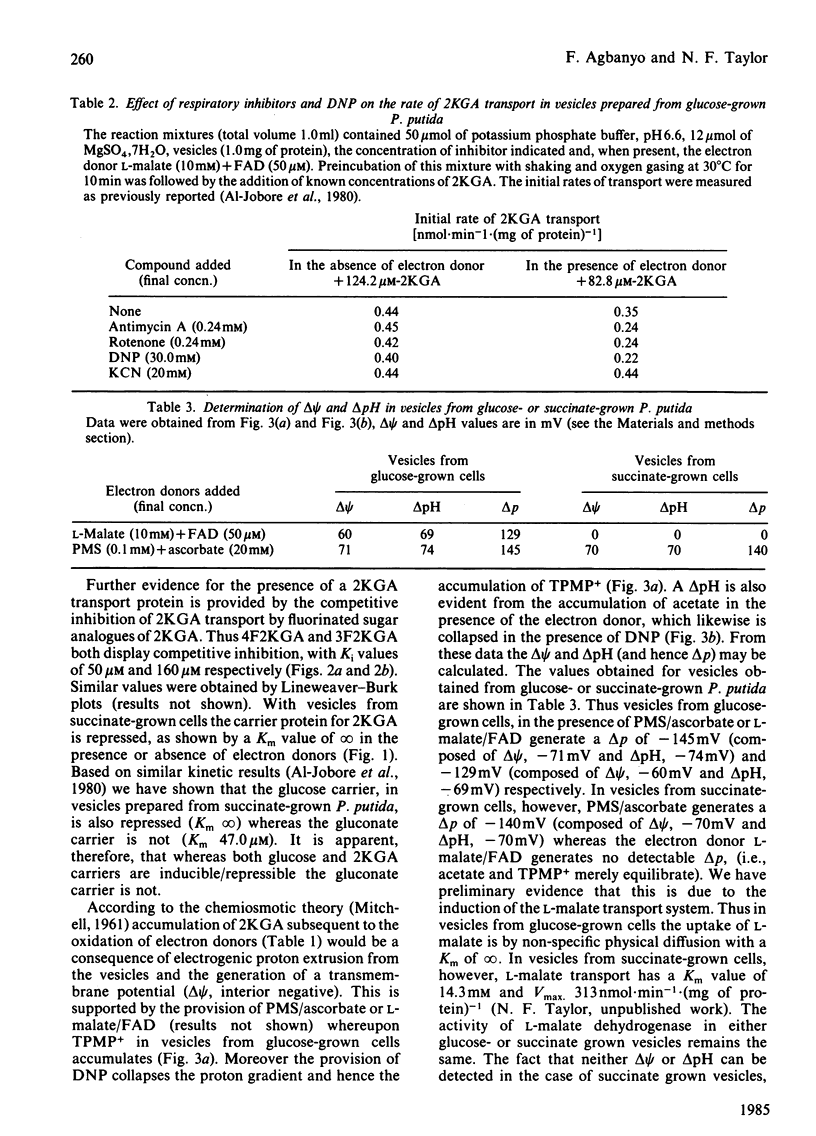
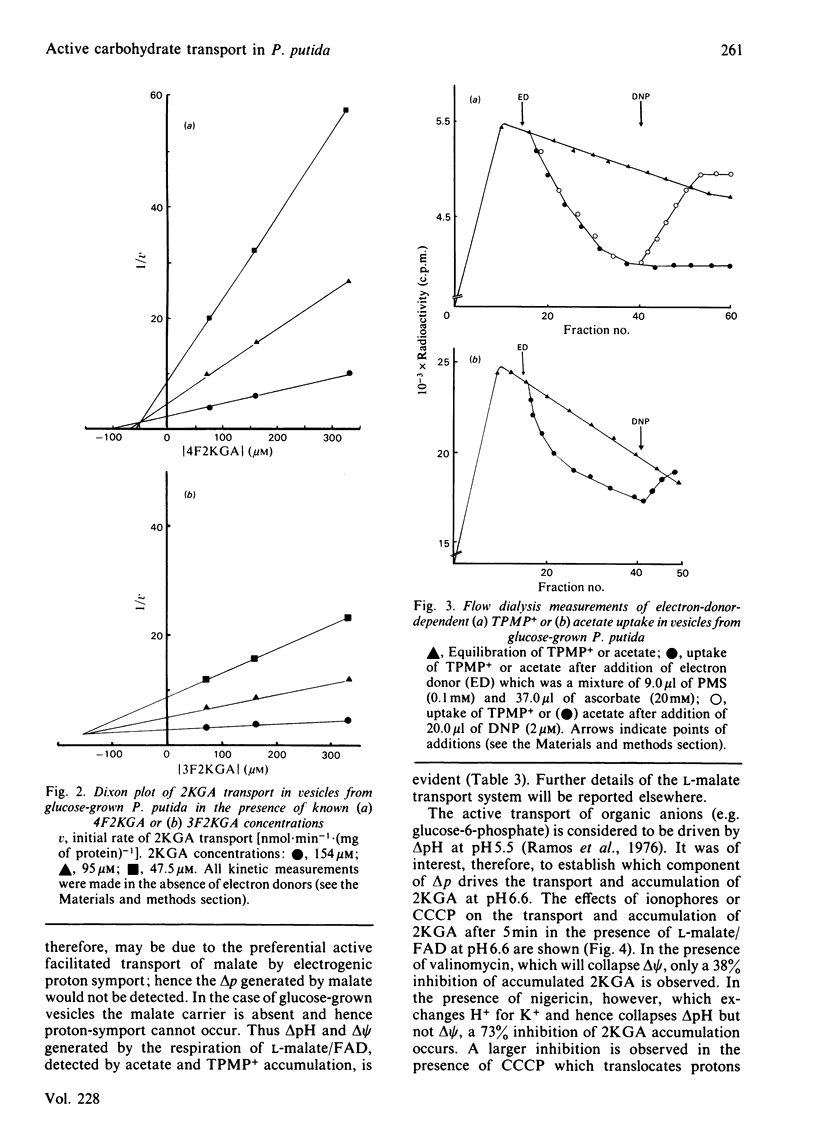
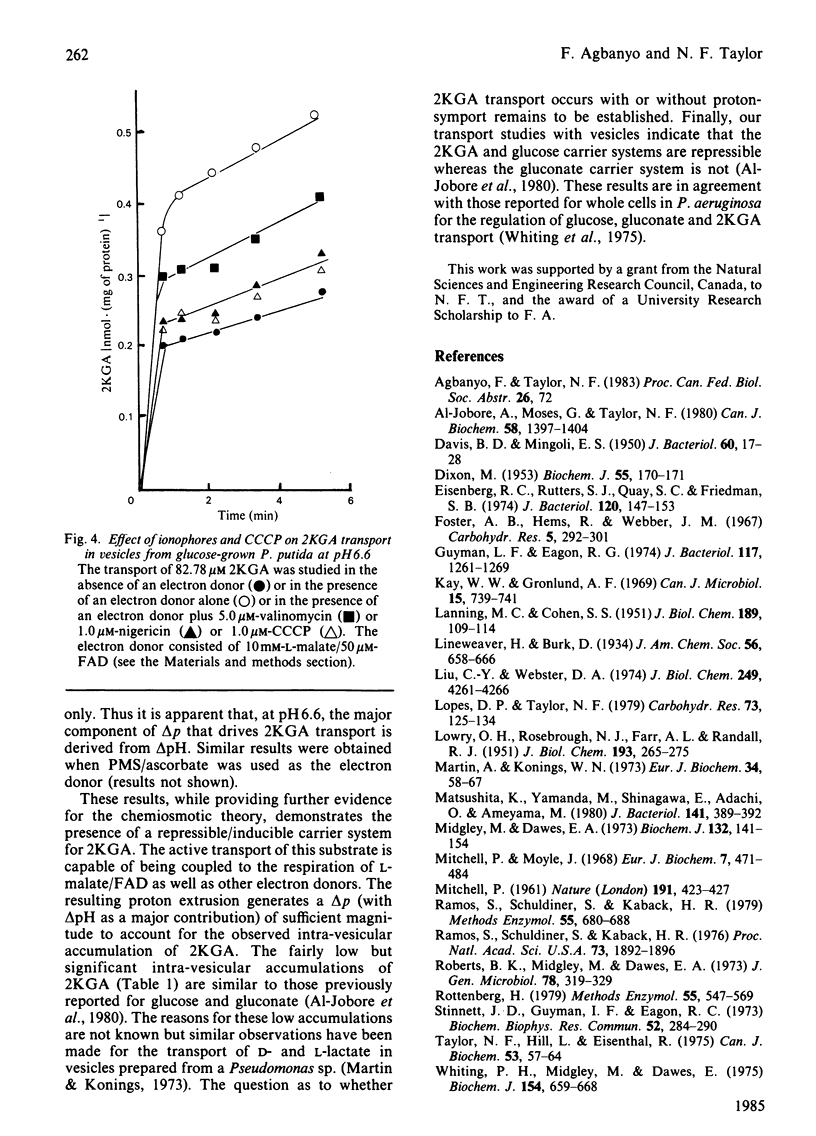
The transport of 2-keto-D-gluconate (alpha-D-arabino-2-hexulopyranosonic acid; 2KGA) in vesicles prepared from glucose-grown Pseudomonas putida occurs by a saturable process with a Km of 110.0 +/- 2.9 microM and a Vmax. of 0.55 +/- 0.04 nmol X min-1 X (mg of protein)-1. The provision of phenazine methosulphate/ascorbate or L-malate leads to an accumulation of intravescular 2KGA, a decrease in the Km value to 50 +/- 2.1 microM and 35 +/- 2.9 microM respectively and no change in the Vmax. In the presence of electron donors the transport of 2KGA is inhibited by the respiratory poisons antimycin A, rotenone and the uncoupler 2,4-dinitrophenol. 2KGA transport is also competitively inhibited by 4-deoxy-4-fluoro-2-keto- or 3-deoxy-3-fluoro-2-keto-D-gluconate with Ki values of 50 microM and 160 microM respectively. The carrier system for 2KGA is repressed in vesicles from cells grown on succinate. Such vesicles transport 2KGA by non-specific physical diffusion with a Km value of infinity in the absence or presence of electron donors. Vesicles from glucose or succinate grown cells, in the presence of phenazine methosulphate/ascorbate at pH 6.6, generate a proton-motive force (delta p) of approx. 140 mV. The delta p, composed of proton gradient (delta pH) and a membrane potential (delta psi), is collapsed in the presence of dinitrophenol. Based on the results obtained with valinomycin, nigericin and carbonyl cyanide m-chlorophenylhydrazone, the active transport of 2KGA at pH 6.6 is coupled predominately to the delta pH component of delta p.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Jobore A., Moses G., Taylor N. F. D-glucose and D-gluconate transport in vesicles from Pseudomonas putida. Can J Biochem. 1980 Dec;58(12):1397–1404. doi: 10.1139/o80-189. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. C., Butters S. J., Quay S. C., Friedman S. B. Glucose uptake and phosphorylation in Pseudomonas fluorescens. J Bacteriol. 1974 Oct;120(1):147–153. doi: 10.1128/jb.120.1.147-153.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Eagon R. G. Transport of glucose, gluconate, and methyl alpha-D-glucoside by Pseudomonas aeruginosa. J Bacteriol. 1974 Mar;117(3):1261–1269. doi: 10.1128/jb.117.3.1261-1269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Carbohydrate metabolism in Pseudomonas aeruginosa: a procedure for accumulating phosphorylated intermediates. Can J Microbiol. 1969 Jul;15(7):739–741. doi: 10.1139/m69-129. [DOI] [PubMed] [Google Scholar]
- LANNING M. C., COHEN S. S. The detection and estimation of 2-ketohexonic acids. J Biol Chem. 1951 Mar;189(1):109–114. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu C. Y., Webster D. A. Spectral characteristics and interconversions of the reduced oxidized, and oxygenated forms of purified cytochrome o. J Biol Chem. 1974 Jul 10;249(13):4261–4266. [PubMed] [Google Scholar]
- Lopes D. P., Taylor N. F. An alternative synthesis of 4-deoxy-4-fluoro-D-glucose and its transport in the human erythrocyte. Carbohydr Res. 1979 Aug;73:125–134. doi: 10.1016/s0008-6215(00)85481-6. [DOI] [PubMed] [Google Scholar]
- Matin A., Konings W. N. Transport of lactate and succinate by membrane vesicles of Escherichia coli, Bacillus subtilis and a pseudomonas species. Eur J Biochem. 1973 Apr 2;34(1):58–67. doi: 10.1111/j.1432-1033.1973.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Yamada M., Shinagawa E., Adachi O., Ameyama M. Membrane-bound respiratory chain of Pseudomonas aeruginosa grown aerobically. J Bacteriol. 1980 Jan;141(1):389–392. doi: 10.1128/jb.141.1.389-392.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley M., Dawes E. A. The regulation of transport of glucose and methyl alpha-glucoside in Pseudomonas aeruginosa. Biochem J. 1973 Feb;132(2):141–154. doi: 10.1042/bj1320141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The use of flow dialysis for determinations of deltapH and active transport. Methods Enzymol. 1979;55:680–688. doi: 10.1016/0076-6879(79)55076-9. [DOI] [PubMed] [Google Scholar]
- Roberts B. K., Midgley M., Dawes E. A. The metabolism of 2-oxogluconate by Pseudomonas aeruginosa. J Gen Microbiol. 1973 Oct;78(2):319–329. doi: 10.1099/00221287-78-2-319. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Stinnett J. D., Guymon L. F., Eagon R. G. A novel technique for the preparation of transport-active membrane vesicles from Pseudomonas aeruginosa: observations on gluconate transport. Biochem Biophys Res Commun. 1973 May 1;52(1):285–290. doi: 10.1016/0006-291x(73)90985-6. [DOI] [PubMed] [Google Scholar]
- Taylor N. F., Hill L., Eisenthal R. The specificity of oxidase and kinase preparations from Pseudomonas fluorescens towards deoxyfluoromonosaccharides. Can J Biochem. 1975 Jan;53(1):57–64. doi: 10.1139/o75-009. [DOI] [PubMed] [Google Scholar]
- Whiting P. H., Midgley M., Dawes E. A. The regulation of transport of glucose, gluconate and 2-oxogluconate and of glucose catabolism in Pseudomonas aeruginosa. Biochem J. 1976 Mar 15;154(3):659–668. doi: 10.1042/bj1540659. [DOI] [PMC free article] [PubMed] [Google Scholar]


