Abstract
We present the case of a 7-month-old female infant with a history of recurrent respiratory infections and symptoms of respiratory distress during feeding. Echocardiography isolated revealed supracardiac-type total anomalous pulmonary venous connection with a large ostium secundum atrial septal defect and severe pulmonary hypertension. Computed tomographic angiography confirmed the diagnosis and identified stenosis at the level of the venous confluence. The surgical intervention involved a novel approach using dual anastomoses between the pulmonary venous confluence and the left atrium, alongside atrial septal defect repair with a bovine pericardial patch. Postoperative recovery was uneventful, with successful weaning from mechanical ventilation on Day 9 and discharge on Day 12. The patient showed optimal venous drainage and hemodynamic stability, indicating a successful surgical outcome. This case highlights the importance of early surgical intervention in total anomalous pulmonary venous connection with complex anatomical presentations.
Keywords: Syria, congenital heart disease, total anomalous pulmonary venous connection, venous confluence stenosis, atrial septal defect, dual anastomoses
Introduction
Total anomalous pulmonary venous connection (TAPVC) represents a rare and complex congenital cardiac anomaly, characterized by the abnormal connection of all four pulmonary veins, which drain into the right atrium or its tributaries instead of the left atrium (LA) [1]. This condition, affecting ~7–9 per 100 000 live births, constitutes ~0.7%–1.5% of all congenital heart diseases and is a significant cause of cyanotic heart disease [2].
Herein, we present a rare case of supracardiac type TAPVC with successful surgical repair through two anastomoses between the confluence and LA.
Case report
A 7-month-old female infant, weighing 5500 g and born at term, presented with severe central cyanosis. The cyanosis had been observed shortly after birth and progressively worsened over the ensuing months, becoming particularly pronounced during periods of agitation or crying. In addition to cyanosis, the infant had a history of recurrent lower respiratory tract infections, beginning at 1 month of age. These episodes occurred approximately every 3–4 weeks and were characterized by cough, wheezing, tachypnea, and increased work of breathing. The infant required frequent outpatient treatments, including bronchodilators and intermittent courses of antibiotics. Additional concerning symptoms included excessive sweating during feeding, poor feeding, and failure to thrive, suggestive of an underlying cardiovascular condition. The patient was subsequently referred to our hospital for further evaluation and management.
Transthoracic echocardiography (TTE) revealed an isolated supracardiac type of TAPVC, accompanied by a large ostium secundum atrial septal defect (ASD) measuring 9 mm, exhibiting a right-to-left shunt. Furthermore, there was evidence of dilatation in the right heart cavities and severe pulmonary hypertension (PHT = 65 mmHg). The common pulmonary confluence, measuring 12 mm in diameter, drained into the left innominate vein via a vertical vein. Confirmatory computed tomographic angiography (CTA) supported the diagnosis of supracardiac type TAPVC and identified stenosis along the venous pathway between the right and left pulmonary veins (Figs 1 and 2).
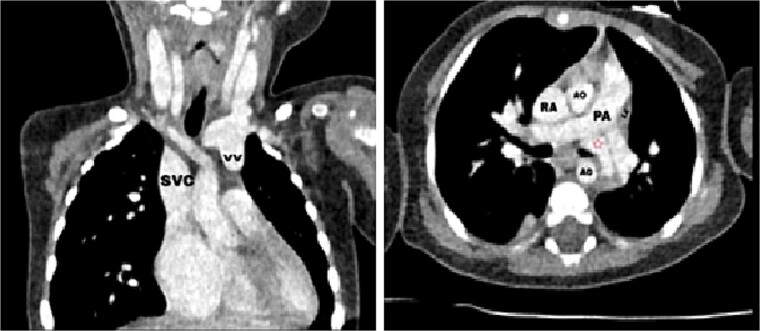
Figure 1.
Axial (right) and coronal (left) postcontrast computed tomography sections: SVC: superior vena cava, VV: vertical vein, RA: right atrium, LA: left atrium, AO: aorta, PA: pulmonary artery, red star: The confluence of PVs.
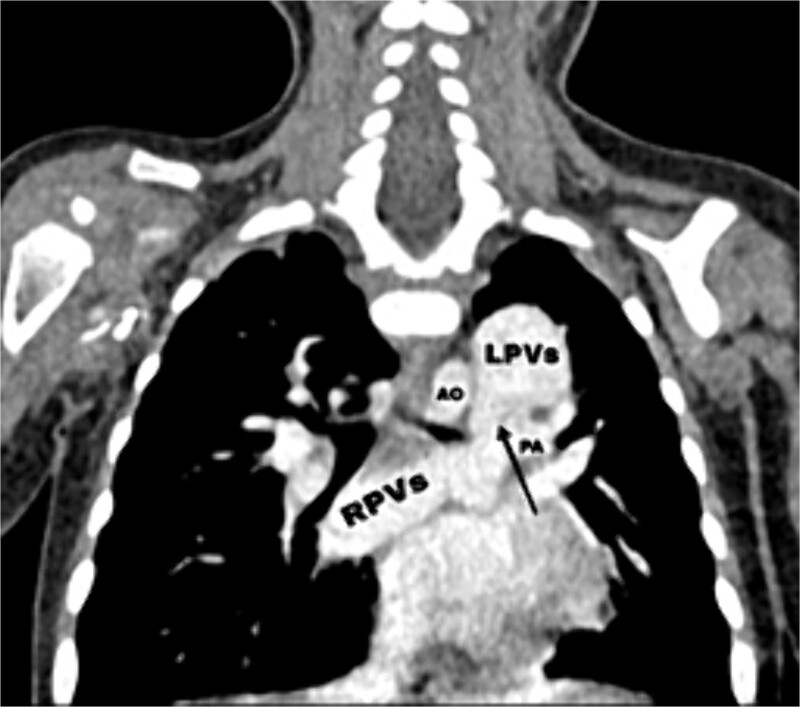
Figure 2.
CT scan showing the right pulmonary veins (RPVs) and left pulmonary veins (LPVs) join all together posterior to the left atrium (LA) and pulmonary artery (PA), also showing the stenosis within the venous confluence between the drain of the right pulmonary veins and the drain of the left pulmonary veins.
Upon surgical exploration by median sternotomy, the vertical vein and the pulmonary venous confluence (PVC) were located behind the pericardium, adjacent to the posterior aspect of the LA. A stenosis was recognized within the venous confluence between the drainage of the right and left pulmonary veins. The vertical vein originated from this confluence, passing behind the pulmonary artery before draining into the innominate vein.
To address the anatomical complexities and minimize stress on the anastomosis, it was decided to perform the PVC-to-LA anastomosis using two separate anastomoses. A longitudinal incision was made in both the lower wall of the PVC and the posterior wall of the LA. Subsequently, the first extended side-to-side anastomosis was created using a continuous suture technique. The bridging vertical vein was ligated at its connection to the innominate vein, followed by the construction of the second extended end-to-side anastomosis between the vertical vein and the left atrial appendage (Fig. 3). Repair of a large ASD was undertaken using a bovine pericardial patch, appropriately sized to close the defect and enlarge the LA. Subsequent stages of the operation were carried out according to standard protocols, with gradual weaning off cardiopulmonary bypass achieved without complications. Following postoperative 2D echocardiography, the patient demonstrated optimal systemic and pulmonary venous drainage without any impediments. While in the intensive care unit (ICU), the patient exhibited hemodynamic stability and successfully underwent ventilator weaning nine days post-surgery. On the 12th postoperative day, the patient was discharged from the hospital with an overall good health condition.
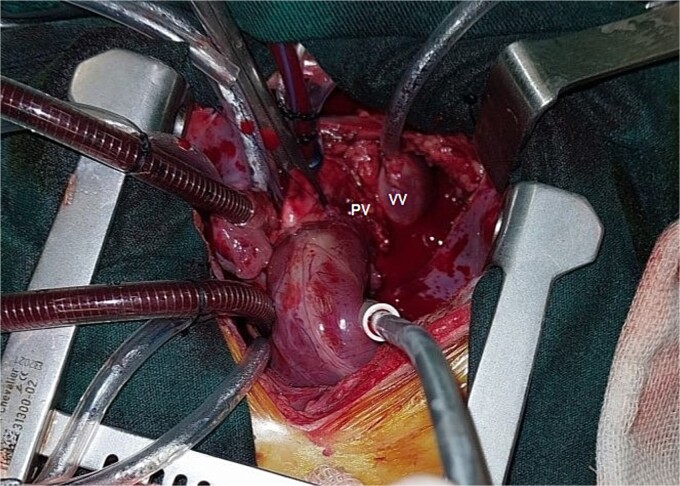
Figure 3.
Operative image showing the vertical vein (VV) and pulmonary vein (PV).
Discussion
TAPVC is a rare congenital cardiac disease, occurring in ~8 per 100 000 live births and accounting for 1.1% of all congenital heart diseases. It is the fifth most common cause of cyanotic heart disease [2]. TAPVC uniquely involves venous malformation, where all four pulmonary veins fail to connect to the LA, instead draining into the right atrium or its tributaries [1, 2].
TAPVC is categorized into four types based on pulmonary vein drainage.
Type I: supracardiac (45%–55%) drains into the superior vena cava or innominate vein.
Type II: intracardiac (20%–30%) drains into the coronary sinus or right atrium.
Type III: infracardiac (13%–25%) drains into the portal or hepatic veins, or inferior vena cava.
Type IV: mixed (10%) combines features of the other types.
Although the supracardiac type is considered the most prevalent form of TAPVC, obstruction frequently occurs in the infra-cardiac type. Typically, obstruction in cases of supra-cardiac TAPVC results from compression of the vertical vein by the left main bronchus and left pulmonary artery or from narrowing at the vertical vein’s base toward the innominate vein [3–7]. However, in our case, the patient presented with isolated supra-cardiac TAPVC associated with stenosis at the level of the venous confluence.
Most patients with TAPVC develop symptoms within their first year of life, and without surgical repair, 80% die before their first birthday [5]. The key characteristics of TAPVC are low oxygen saturation, pulmonary hypertension, unstable blood pressure, cyanosis, breathing difficulties, rapid breathing, feeding challenges, poor growth, and frequent lung infections [6].
TTE serves as the principal diagnostic tool; however, CTA is invaluable in such cases, providing critical anatomical details, especially regarding the site of any obstruction [6].
The surgical management of TAPVC depends on the anatomic type. While unobstructed TAPVC can be addressed electively, it is typically operated on soon after diagnosis [8]. Although recent surgical advancements improved the outcomes, early mortality rates remain 10%–20% [9].
For supracardiac TAPVC, surgical intervention typically consists of lifting the heart to the right and constructing a side-to-side anastomosis between the PVC and the LA [4]. However, in our case, a second procedure was executed to minimize stress on the anastomosis as there was an obstruction in the coinfluence. This involved performing end-to-side anastomosis of the vertical vein to the left atrial appendage.
Factors that increase the risk of morbidity and mortality include undergoing surgery at a younger age and having associated cardiac lesions such as hypoplastic pulmonary veins, postoperative pulmonary hypertension, and postoperative pulmonary venous obstruction (PVO) [10, 11].
Contributor Information
Ghaith Hasan, Department of Surgery, Faculty of Medicine, Damascus University, Damascus, Syria; Syrian Medical Research Group, Damascus, Syria.
Abdulrahman Almjersah, Department of Surgery, Faculty of Medicine, Damascus University, Damascus, Syria; Syrian Medical Research Group, Damascus, Syria.
Mohamed Younes, Department of Pediatric Cardiac Surgery, Children's University Hospital, Damascus University, Damascus, Syria.
Author contributions
Ghaith Hasan (Conceptualization, Writing—original draft), Abdulrahman Almjersah (Writing—review & editing), and Mohamed Younes (Validation, Visualization, Supervision, Writing—review & editing)
All authors have read and approved the final version of the manuscript.
Conflict of interest statement
All authors have no competing interests, financial or non-financial.
Funding
None declared.
Data availability
The data used to support the findings of this study are included in the article.
Patient consent statement
Written informed consent was obtained from the patient’s guardian for publication of this case report and accompanying images. A copy of the consent form is available for review by the Editor-in-Chief of this journal.
References
- 1. Murillo H, Cutalo M, Jones R, et al. . Pulmonary circulation imaging: embryology and normal anatomy. Semin Ultrasound CT MR 2012;33:473–84. 10.1053/j.sult.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 2. Kao CC, Hsieh CC, Cheng PJ, et al. . Total anomalous pulmonary venous connection: from embryology to a prenatal ultrasound diagnostic update. J Med Ultrasound 2017;25:130. 10.1016/j.jmu.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maurya A, Rodge H, Kurian B, et al. . Total anomalous of pulmonary venous connection - a case report. J Pharm Res Int 2021;33:226–30. 10.9734/jpri/2021/v33i39A32164. [DOI] [Google Scholar]
- 4. Seale AN, Uemura H, Webber SA, et al. . British Congenital Cardiac Association. Total anomalous pulmonary venous connection: morphology and outcome from an international population-based study. Circulation 2010;122:2718–26. 10.1161/CIRCULATIONAHA.110.940825. [DOI] [PubMed] [Google Scholar]
- 5. Chowdhury UK, Airan B, Malhotra A, et al. . Mixed total anomalous pulmonary venous connection: anatomic variations, surgical approach, techniques, and results. J Thorac Cardiovasc Surg 2008;135:106–16, 116.e1-5. 10.1016/j.jtcvs.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 6. Files MD, Morray B. Total anomalous pulmonary venous connection: preoperative anatomy, physiology, imaging, and interventional Management of Postoperative Pulmonary Venous Obstruction. Semin Cardiothorac Vasc Anesth 2017;21:123–31. 10.1177/1089253216672442. [DOI] [PubMed] [Google Scholar]
- 7. Mulia EPB, Rahman MA. Treatment considerations in total anomalous pulmonary venous connection. KK Hospital Review 2023;32:1–7. 10.1177/20101058231188865. [DOI] [Google Scholar]
- 8. Kanter KR. Surgical repair of total anomalous pulmonary venous connection. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2006;9:40–4. 10.1053/j.pcsu.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 9. Singh N, Singh R, Aga P, et al. . Cardiac type of total anomalous pulmonary venous connection: diagnosis and demonstration by multidetector CT angiography. Case Reports 2013;2013:bcr2012007994. 10.1136/bcr-2012-007994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang C, Xie X, Zhuang H, et al. . Successful surgical repair in an older adult with supracardiac total anomalous pulmonary venous connection: a case report. Front Cardiovasc Med 2023;10:1121037. 10.3389/fcvm.2023.1121037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hancock C, Zurakowski D, Thiagarajan RR, et al. . Total anomalous pulmonary venous connection: an analysis of current management strategies in a single institution. Ann Thorac Surg 2005;79:596–606. 10.1016/j.athoracsur.2004.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included in the article.